Patients with schizophrenia are often seen as irrational because their beliefs or actions are irrational at first glance. Cognitive science distinguishes between two kinds of rationality: instrumental and epistemic. Instrumental rationality concerns the extent to which decisions and actions approximate the best ways to achieve appropriate goals. Epistemic rationality concerns the extent to which beliefs and thought processes are formed in coherence with logical rules – and correspond to the structure of the world as it is intersubjectively assumed Reference Stanovich1 . Thus, rationality is a normative concept, which means that it corresponds to a certain ideal of adequacy and efficiency. It is not clear how the flagrant irrationality of psychosis relates to possible irrationality as described by cognitive science.
Dual process theory of rationality
The most widely acknowledged neuroscientific view on rationality is the so-called ‘dual process theory’. This theory proposes two parallel processes that are active in both epistemic and instrumental rationality: analytic v. heuristic or explicit-tacit v. reflective-automatic, etc. Reference Stanovich1 Sometimes, it is appropriate to be analytic and reflect systematically to reach a strategy or an understanding; on other occasions, it is more appropriate to use heuristic strategies to make fast choices and take immediate action. Which style dominates depends on the situational demands. Reference Gigerenzer and Goldstein2 For instance, when writing a manuscript most people apply analytic processes to evaluate every word and sentence to make the text logical and easy to understand. However, it would not make sense to engage in deep reflection when deciding to take a left turn in a complex traffic situation. Normally, automatised behavioural patterns, sedimented from experience are mobilised to make a heuristically determined move. However, in many situations both analytic and heuristic processes are activated; it is more a matter of relative dominance in any given situation. Reference Stanovich1,Reference Gigerenzer and Goldstein2
Rationality and irrationality
In cognitive tests, rationality is assessed and defined by the level of efficiency in solving test items. As efficiency is a matter of degree, being less rational does not imply irrationality. Irrationality – at least in a common understanding – is moving beyond a critical point of inefficiency on a continuum. An irrational person displays behaviour, thoughts and beliefs that do not benefit the person's goals (lack of instrumental rationality), nor do they fit the structure of the world as it is commonly accepted to be (lack of epistemic rationality). Reference Stanovich1
Testing irrationality in schizophrenia
Cognitive science has studied rationality and irrationality by employing various tests of reasoning. Among those are syllogisms. Reference Goel, Bartolo, St Clair and Venneri3–Reference Owen, Cutting and David7
A syllogism is a certain argument structure, where the validity of the conclusion depends on the validity of the preceding premises (i.e. the truth of the conclusion depends on the truth of the premises). Reference Forbes8 To illustrate, the following content concerning water, ice and temperature has been structured as both a valid and an invalid syllogism. Valid: All ice (P) is water (Q). All ice (P) is frozen (R); therefore some water (Q) is frozen (R). Invalid: All ice (P) is water (Q). Some water (Q) is frozen (R); therefore, ice (P) is frozen (R). Although it may be difficult to understand at first glance, the syllogism reflects a certain logical structure or a blueprint, which can be applied to any verbal content. Formally, it is like an equation applicable to sentences, but it is likely that the error rate is affected by the semantic embodiment (content) of the sentences. Such embodiment may be ordinary or unusual. Ordinary content refers to the instance where the content of the syllogism is congruent with what would be typically familiar to us from experience. Thus, some syllogisms may be based on completely ordinary content such as ‘the sun rises in the east’, or as in the syllogism concerning water above, Reference Owen, Cutting and David7 whereas other syllogisms have unusual presentations such as ‘blue concepts’ or ‘buildings that sing’.
Validity judgements about syllogisms have been documented to be influenced by the nature of their content (ordinary v. unusual). Reference Goel and Dolan4,Reference Evans9,Reference Wilkins10 Thus if the participant finds the presented content as being unusual, a ‘false’-response will be more easily activated. Conversely, if the participant finds the presentation content ordinary, it will more likely stimulate a ‘true’-response. Reference Mirian, Heinrichs and McDermid Vaz5,Reference Wilkins10
In general, research based on syllogisms has suggested that patients with schizophrenia show impaired performance in syllogism tests of rationality. Reference Goel, Bartolo, St Clair and Venneri3,Reference Mujica-Parodi, Malaspina and Sackeim11,Reference Speechley, Murray, McKay, Munz and Ngan12 However, performance on syllogism tests of rationality is to some extent associated with intelligence Reference Mirian, Heinrichs and McDermid Vaz5 and consequently, rationality deficits in schizophrenia may simply reflect the lower average IQ which is observed in patients with schizophrenia. Reference Urfer-Parnas, Mortensen, Saebye and Parnas13 Goel et al Reference Goel, Bartolo, St Clair and Venneri3 found that patients performed equally poorly across all content types and controls performed better in general. However, only controls showed better performance as a result of ordinary presentation. Williams Reference Williams6 and Mirian et al Reference Mirian, Heinrichs and McDermid Vaz5 found no differences between diagnostic groups when controlling for IQ. They suggested that the underperformance of patients could be attributed to a general cognitive dysfunction that characterises schizophrenia. Mirian et al Reference Mirian, Heinrichs and McDermid Vaz5 also found that both patients and controls performed better on certain syllogisms, i.e. valid syllogism with ordinary content and invalid syllogisms with unusual content. However, the sample size in this study was small and IQ was the only covariate.
Contradicting all expectations, Owen et al Reference Owen, Cutting and David7 found that when the study groups were matched for IQ, patients with schizophrenia outperformed healthy controls on valid syllogisms with unusual presentation content or invalid syllogisms with ordinary presentation content. These results were interpreted as being consistent with phenomenological observations on cognition in schizophrenia: many patients with schizophrenia have an intact ability to analytic-reflective reasoning and are often even hyper-reflective, but are in general impaired in a pre-reflective, context-sensitive understanding of the world. Reference Owen, Cutting and David7,Reference Minkowski, Cutting and Shepherd14 These phenomena manifest in tendencies towards being hyper-logical and hyper-reflective and in lacking the mastery of more contextually adapted, fluid aspects of life, governed more by ‘the logic of the world rather than the logic of the logicians’. Reference Minkowski, Cutting and Shepherd14 Therefore, the results of Owen et al Reference Owen, Cutting and David7 carried a substantial significance and novelty. However, their study did not include syllogisms that were invalid with unusual presentation content nor valid with ordinary presentation content and the study was also limited by a small sample size, only group level IQ matching, and the lack of comprehensive neuropsychological data.
The aim of the present study was to investigate performance of patients with schizophrenia on syllogism tests of rationality, including analyses controlling for intelligence and neuropsychological test performance. An additional aim was to replicate the theoretically very important results of Owen et al by investigating whether patients with schizophrenias reason relatively more logically when syllogisms are presented through unusual and strange content.
Method
The study was carried out at Psychiatric Center Hvidovre, a department of the University of Copenhagen providing psychiatric service to a population of 150 000 in a catchment area of the city of Copenhagen. Data collection took place over 18 months starting from September 2011. To be included, all participants had to give informed consent and be considered capable of lengthy testing.
Participants
We recruited 38 patients diagnosed with the DSM-IV schizophrenia from a sample of 100 first-admitted, diagnostically heterogeneous, consecutive patients who had participated in a larger diagnostic project, which involved very lengthy psychiatric interviews. Reference Nordgaard, Revsbech, Saebye and Parnas15,Reference Nordgaard and Parnas16 Severely psychotic, aggressive, forcibly admitted or legal patients, and patients with primary or clinically significant alcohol/drug abuse were excluded (all these categories constitute a substantial proportion of first, acute admissions), resulting in a patient sample comprising 20 women and 18 men. The details of diagnostic assessment are published elsewhere. Reference Nordgaard, Revsbech, Saebye and Parnas15 Briefly, all patients were interviewed with the Structured Clinical Interview for DSM-IV (SCID)-I and the schizotypal personality disorder module from the SCID-II, the Operational Criteria Checklist scale expanded with the additional items from the Schedule for Affective Disorders and Schizophrenia-Lifetime and Bonner Skala Für die Beurteilung von Basissymptomen and a checklist of the First Rank Symptom continua, and a Mental Status Examination, listing a variety of abnormal expressive features. Reference Parnas, Cannon, Jacobsen, Schulsinger, Schulsinger and Mednick17,Reference Vaever, Licht, Moller, Perlt, Jorgensen and Handest19 The DSM-IV schizophrenia diagnosis was allocated according to best lifetime consensus estimate between two senior clinicians (J.N. and J.P.).
The 38 healthy control participants were recruited as volunteers from the greater Copenhagen area, mainly among staff and medical students at the Psychiatric Center Hvidovre. It was required that the controls had no psychiatric history, no abuse, no current mental problems, and had not suffered from organic brain damage or a neurological disease. The healthy control participants were screened (by R.R.) for eligibility with a brief, semi-structured interview addressing social and psychiatric history, evidence of abuse and current possible psychiatric complaints and a mental state evaluation. Six potential volunteers for the control group had to be excluded due to self-reported problems that met the exclusion criteria.
The final study sample comprised 38 healthy control participants and 38 patients meeting the DSM-IV criteria for schizophrenia.
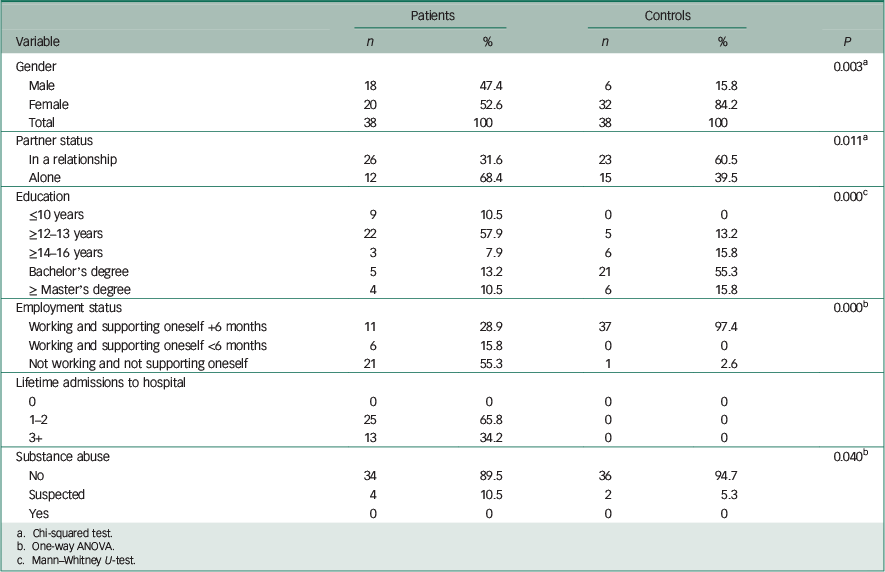
Descriptive information of the demographic data is presented in Table 1.
Table 1 Demographic characteristics of sample
| Patients | Controls | ||||
|---|---|---|---|---|---|
| Variable | n | % | n | % | P |
| Gender | 0.003 a | ||||
| Male | 18 | 47.4 | 6 | 15.8 | |
| Female | 20 | 52.6 | 32 | 84.2 | |
| Total | 38 | 100 | 38 | 100 | |
| Partner status | 0.011 a | ||||
| In a relationship | 26 | 31.6 | 23 | 60.5 | |
| Alone | 12 | 68.4 | 15 | 39.5 | |
| Education | 0.000 c | ||||
| ≤10 years | 9 | 10.5 | 0 | 0 | |
| ≥12–13 years | 22 | 57.9 | 5 | 13.2 | |
| ≥14–16 years | 3 | 7.9 | 6 | 15.8 | |
| Bachelor's degree | 5 | 13.2 | 21 | 55.3 | |
| ≥ Master's degree | 4 | 10.5 | 6 | 15.8 | |
| Employment status | 0.000 b | ||||
| Working and supporting oneself +6 months | 11 | 28.9 | 37 | 97.4 | |
| Working and supporting oneself <6 months | 6 | 15.8 | 0 | 0 | |
| Not working and not supporting oneself | 21 | 55.3 | 1 | 2.6 | |
| Lifetime admissions to hospital | |||||
| 0 | 0 | 0 | 0 | 0 | |
| 1–2 | 25 | 65.8 | 0 | 0 | |
| 3+ | 13 | 34.2 | 0 | 0 | |
| Substance abuse | 0.040 b | ||||
| No | 34 | 89.5 | 36 | 94.7 | |
| Suspected | 4 | 10.5 | 2 | 5.3 | |
| Yes | 0 | 0 | 0 | 0 | |
a Chi-squared test.
b One-way ANOVA.
c Mann–Whitney U-test.
Assessments
Psychometric testing of healthy controls as well as patients was undertaken by an MA in psychology with experience in neuropsychology (R.R.).
Rationality was measured using syllogisms. The syllogisms comprised two premises and a conclusion varying in two dimensions: ordinary v. unusual presentation content and valid v. invalid syllogisms. The syllogisms were presented on paper, where the participant was instructed to respond by marking each syllogism as either ‘True’ or ‘False’. Participants were given standardised verbal and written instructions that had been formulated in collaboration with Owen et al Reference Owen, Cutting and David7 to make the instructions as similar as possible to those used in the original study by Owen et al.
The task included 29 syllogisms divided into four subgroups:
-
7 Valid syllogisms with Ordinary Presentations (VOP) (e.g. if one steals, one is not liked; all thieves steal; therefore, all thieves are not liked).
-
7 Invalid syllogisms with Ordinary Presentations (IOP) (e.g. if the sun rises, the sun is in the east; the sun is in the east; therefore, the sun rises).
-
8 Valid syllogisms with Unusual Presentations (VUP) (e.g. all buildings speak loudly; a hospital does not speak loudly; therefore, a hospital is not a building).
-
7 Invalid syllogisms with Unusual Presentations (IUP) (e.g. the sun emits radio waves; radio waves emit Madonna; therefore, the sun emits Madonna). The IUP are strictly logically speaking, not proper syllogisms. However, we added this group to achieve a certain symmetrical balance in the test material.
The syllogisms were numbered and randomised using a number generator in SPSS. The randomised order was then used as the standard order of the syllogisms throughout the entire study to avoid noise related to different item orders. The IOP and VUP syllogisms were kindly provided by the research group of Owen et al. Reference Owen, Cutting and David7 The two additional subgroups of syllogisms (VOP and IUP) were designed according to guidelines from Forbes. Reference Forbes8
In this manner, complementing Owen et al's Reference Owen, Cutting and David7 IOP and VUP with the additional VOP and IUP syllogisms, resulted in the entire matrix of constellations between unusual and ordinary presentations and valid and invalid syllogisms.
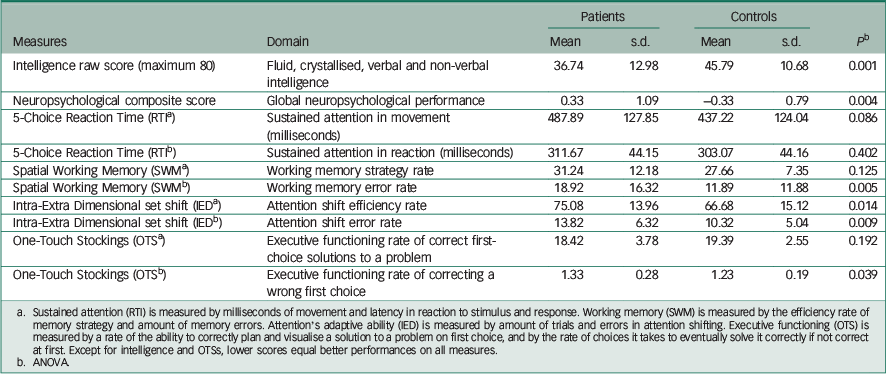
Intelligence (IQ) was derived from a sum score of four IST-2000-R computerised subtests. Reference Liepmann, Beauducel, Brocke and Amthauer20 The IQ battery comprised Sentence Completion, Verbal Analogies, Number Series and Matrices. The selected IQ subtests represent both verbal and non-verbal IQ domains. Reference Liepmann, Beauducel, Brocke and Amthauer20 The possible maximum was 80 because each of the four subtests had a maximum score of 20. Means and standard errors of intelligence and each neuropsychological measure are presented in Table 2.
Table 2 Mean scores, standard deviations (s.d.) and group difference significances for intelligence and neuropsychological measures and their composite score a
| Patients | Controls | |||||
|---|---|---|---|---|---|---|
| Measures | Domain | Mean | s.d. | Mean | s.d. | P b |
| Intelligence raw score (maximum 80) | Fluid, crystallised, verbal and non-verbal intelligence | 36.74 | 12.98 | 45.79 | 10.68 | 0.001 |
| Neuropsychological composite score | Global neuropsychological performance | 0.33 | 1.09 | −0.33 | 0.79 | 0.004 |
| 5-Choice Reaction Time (RTI a ) | Sustained attention in movement (milliseconds) | 487.89 | 127.85 | 437.22 | 124.04 | 0.086 |
| 5-Choice Reaction Time (RTI b ) | Sustained attention in reaction (milliseconds) | 311.67 | 44.15 | 303.07 | 44.16 | 0.402 |
| Spatial Working Memory (SWM a ) | Working memory strategy rate | 31.24 | 12.18 | 27.66 | 7.35 | 0.125 |
| Spatial Working Memory (SWM b ) | Working memory error rate | 18.92 | 16.32 | 11.89 | 11.88 | 0.005 |
| Intra-Extra Dimensional set shift (IED a ) | Attention shift efficiency rate | 75.08 | 13.96 | 66.68 | 15.12 | 0.014 |
| Intra-Extra Dimensional set shift (IED b ) | Attention shift error rate | 13.82 | 6.32 | 10.32 | 5.04 | 0.009 |
| One-Touch Stockings (OTS a ) | Executive functioning rate of correct first-choice solutions to a problem | 18.42 | 3.78 | 19.39 | 2.55 | 0.192 |
| One-Touch Stockings (OTS b ) | Executive functioning rate of correcting a wrong first choice | 1.33 | 0.28 | 1.23 | 0.19 | 0.039 |
a Sustained attention (RTI) is measured by milliseconds of movement and latency in reaction to stimulus and response. Working memory (SWM) is measured by the efficiency rate of memory strategy and amount of memory errors. Attention's adaptive ability (IED) is measured by amount of trials and errors in attention shifting. Executive functioning (OTS) is measured by a rate of the ability to correctly plan and visualise a solution to a problem on first choice, and by the rate of choices it takes to eventually solve it correctly if not correct at first. Except for intelligence and OTSs, lower scores equal better performances on all measures.
b ANOVA.
Neuropsychological performance was measured with four PC-implemented subtests from Cambridge Neuropsychological Test Automated Battery (CANTAB). This CANTAB-derived battery consisted of the Reaction Time (RTI) test, Reference Sahakian, Owen, Morant, Eagger, Boddington and Crayton21,Reference Riekkinen, Laakso and Jakala22 the Spatial Working Memory (SWM) test, Reference Morris, Downes, Sahakian, Evenden, Heald and Robbins23,Reference Owen, Iddon, Hodges, Summers and Robbins24 the Intra-Extra Dimensional Set Shift (IED) test Reference Downes, Roberts, Sahakian, Evenden, Morris and Robbins25 and the One-Touch Stockings (OTS) test. Reference Coull, Middleton, Robbins and Sahakian26,Reference Elliott, Sahakian, Herrod, Robbins and Paykel27 The selected tests represent a continuum from simple to more complex cognitive functioning, which put demands on frontal lobe-dependent functioning. A composite score was extracted from the neuropsychological battery by principle components analysis.
Data analysis
Statistical analyses were conducted in SPSS version 20. We compared the groups on IQ and the neuropsychological battery using ANOVA. Owing to non-normal distributions the scores of two groups on the four types of syllogism were compared using both the t-test and the non-parametric rank-sum test. Furthermore, the scores of the two groups were compared by analysis of covariance (ANCOVA) adjusting for intelligence and the composite score of neuropsychological measures. The assumption of parallel regression in the two groups between covariates and syllogism outcomes was tested in preliminary analyses and revealed only minor violations, and ANCOVA with robust variance estimation showed essentially the same results.
Ethical issues
The Institutional Review Board of the University of Copenhagen, the Mental Health Services – Capital Region of Denmark, the Danish Data Protection Agency and the National Committee on Health Research Ethics approved the study.
Results
Table 1 shows the demographic characteristics of the two study groups. There were significant differences between the two groups with respect to gender (P=0.003), educational level (P<0.001), employment and income status (P<0.001). These differences reflect the fact that the control group mainly consisted of well-educated persons, whereas patients with schizophrenia had little education and were often unemployed. Reference Saraceno, Levav and Kohn28
Table 2 presents the means, standard deviations and main effects of groups for intelligence and the neuropsychological measures. There were significant group differences in intelligence (P=0.001), the ability to correct memory mistakes in SWM (P=0.005) and OTS (prefrontal functioning flexibility) (P=0.039), and IED (attention flexibility) (P=0.009).
In models adjusting for intelligence, the composite neuropsychological score, and group (patient v. controls), gender was not significantly associated with performance on any of the four types of syllogism and therefore not adjusted for in the subsequent comparisons.
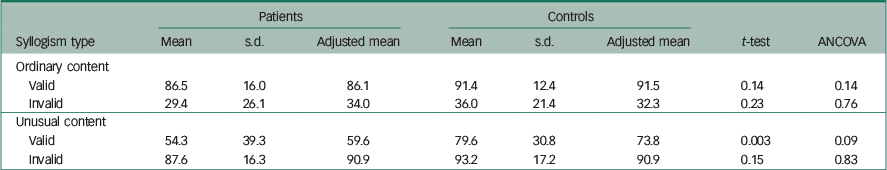
Table 3 presents the means and standard deviations of the percentage correct answers for the four types of syllogisms. Several of these distributions showed considerable negative skewness, probably reflecting ceiling effects. Both patients and controls obtained the lowest percentage of correct answers on invalid syllogism presented with ordinary content and the highest percentage of correct answers on invalid syllogisms presented with unusual content. The controls outperformed the patients on both types of syllogism, but the mean group differences were small and non-significant by a t-test.
Table 3 Unadjusted and adjusted mean group differences for percentage rates of correct responses on the four syllogism types (i.e. VOP, IOP, VUP and IUP)
| Patients | Controls | |||||||
|---|---|---|---|---|---|---|---|---|
| Syllogism type | Mean | s.d. | Adjusted mean | Mean | s.d. | Adjusted mean | t-test | ANCOVA |
| Ordinary content | ||||||||
| Valid | 86.5 | 16.0 | 86.1 | 91.4 | 12.4 | 91.5 | 0.14 | 0.14 |
| Invalid | 29.4 | 26.1 | 34.0 | 36.0 | 21.4 | 32.3 | 0.23 | 0.76 |
| Unusual content | ||||||||
| Valid | 54.3 | 39.3 | 59.6 | 79.6 | 30.8 | 73.8 | 0.003 | 0.09 |
| Invalid | 87.6 | 16.3 | 90.9 | 93.2 | 17.2 | 90.9 | 0.15 | 0.83 |
For the valid syllogisms both groups obtained the highest percentage correct when the syllogisms were presented with ordinary content. The controls outperformed the patients, but the difference between the two groups was small and non-significant. The largest group difference was obtained for valid syllogisms presented with unusual content. For these syllogisms, both a t-test and a rank-sum test showed a significant group difference (P<0.01).
For the combined sample, the correlations between the IQ score and the four types of syllogism were 0.38, 0.03, 0.42 and 0.44 for the VUP-, VOP-, IUP- and IOP syllogisms (the corresponding correlation for the composite neuropsychological measure were 0.45, 0.07, 0.21 and 0.24). Thus, correlations were significant except for the valid ordinary syllogism, and the non-significant correlations for this syllogism type may reflect a ceiling effect.
Table 3 shows that when ANCOVA was conducted with intelligence and the composite neuropsychological score as covariates, all group differences on the four types of syllogism became non-significant.
Finally, within the patient group no correlation was significant between the neurocognitive measures, IQ, and syllogism test performance on the one hand and the summary scores of positive and negative symptom scores. Reference Nordgaard and Parnas16
Discussion
In this cross-sectional, case–control study investigating performance of patients with schizophrenia on four types of syllogism, we found that the patients performed non-significantly lower than healthy controls on three types of syllogism, whereas the group difference was significant on valid syllogism presented with unusual content. However, this group difference also became non-significant when adjusted for intelligence and a composite score on a neuropsychological battery.
The patient and control groups differed by about 0.7 s.d. on both the measure of intelligence and the composite neuropsychological score. It is therefore remarkable that intelligence and the neuropsychological score explained most variance (24%) on valid syllogisms with unusual content, which was the type of syllogism for which the largest group difference was observed. The fact that the group difference became non-significant when intelligence and the neuropsychological score were controlled in ANCOVA suggests the observed group differences are a consequence of global cognitive levels of performance in the two groups. For the combined sample both intelligence and the composite neuropsychological score correlated significantly with syllogism scores except for the valid ordinary syllogism, and the non-significant correlations for this syllogism type may reflect a ceiling effect. Thus, the correlations also suggest that the demands of syllogism tests of rationality are closely related to verbal intelligence, widely documented to be impaired in patients with schizophrenia. Reference Urfer-Parnas, Mortensen, Saebye and Parnas13,Reference Burgess, Curtis-Downes and Gibson29,Reference Woodberry, Giuliano and Seidman30 In contrast, the irrationality that is clinically characteristic of psychosis and schizophrenia in particular appears in this study to be unrelated to the cognitive processes assessed by syllogism tests of rationality.
Mirian et al Reference Mirian, Heinrichs and McDermid Vaz5 found similar results and suggested that reasoning measured by syllogisms in patients with schizophrenia simply mirror the broad range of cognitive dysfunctions and lower IQ. This interpretation is likely in the light of schizophrenia's well documented broad range of cognitive dysfunction Reference Heinrichs and Zakzanis31,Reference Vohringer, Barroilhet, Amerio, Reale, Alvear and Vergne32 as well as the nature of Mirian et al's findings. Reference Mirian, Heinrichs and McDermid Vaz5 Thus, tests of syllogistic reasoning do not appear to be a sensitive method for assessing irrationality relevant to psychosis.
In sum, we were not able to replicate the findings of Owen et al. Reference Owen, Cutting and David7 Since our study included the same test items, possible explanations for the discrepant results should be considered. Relevant methodological factors may be adjusting for IQ by matching v. ANCOVA and adjusting for IQ v. for both IQ and neuropsychological measures. However, a more likely explanation is that the discrepant results reflect the differences between the included patient and control study samples. The groups in Owen et al's study were only about half our sample size and rather homogeneous (with a smaller variance in IQ in both cases and controls), whereas the groups in the present study had a larger variance in IQ among both cases and controls. A limitation of our study was the relatively small sample size and the relatively large differences between patients and controls with regard to IQ and neuropsychological performance. Adjusting for IQ and the composite neuropsychological score in ANCOVA should theoretically account for these group differences, but the distributions of the outcome on syllogisms were skewed and the ANCOVA may not fully adjust for the relatively large group differences. Furthermore, the possibility of ceiling effects could indicate that the valid ordinary syllogisms and invalid unusual syllogisms were too easy to solve.
Finally, there is one notable methodological difference between the two studies with respect to administration of the syllogisms. In the study by Owen et al, a researcher administered the syllogisms and the participants read the syllogisms out aloud. To eliminate the risk of a possible Rosenthal effect (i.e. the administrator involuntarily and subliminally signalling right or wrong answers) we made sure that the patient had understood the principles and left all participants to solve the syllogisms on their own.
The results of this study shed light on certain forms of rationality and irrationality in schizophrenia by corroborating one previous study Reference Mirian, Heinrichs and McDermid Vaz5 and contradicting another. Reference Owen, Cutting and David7 The adjusted results suggest that rational thinking is more normal in patients with schizophrenia than usually assumed, and that patients with schizophrenia, despite psychosis, do not perform qualitatively different from controls in syllogism tests of rationality. However, the unadjusted results suggest deficits in rationality, and the substantial effects of adjusting for IQ and neuropsychological performance indicate that the observed rationality deficits in schizophrenia to some extent reflect deficits in cognitive and neuropsychological functions. It remains an open question whether schizophrenia is associated with rationality deficits that are specific to the disease, and the common assumption that healthy people and people with schizophrenia can be differentiated by the presence or non-presence of test-measurable rationality is questionable and needs further exploration. Moreover, it leaves important questions as to whether the metacognitive ‘top-down’ deficits in psychoticpatients Reference Bruno, Sachs, Demily, Franck and Pacherie33,Reference Riggs, Grant, Perivoliotis and Beck34 are, in fact, responsible for delusion formation. As mentioned above, we found no correlation between psychometric measures of the severity of the psychosis and syllogism tests, IQ or neurocognition.
In conclusion, rationality and irrationality in schizophrenia may not be sufficiently illuminated by the use of syllogisms. To explore rationality more closely, we need a variety of clearly defined, ecologically valid tests, as well as tests of neurocognition that has unequivocal relevance for real, daily life functioning (including social cognition). Future research in rationality and schizophrenia could probably benefit from the research in neuro-economical decision bias (see, for instance, Stanovich & West Reference Stanovich and West35 ) to search for similarities and discrepancies between healthy and pathological rationality. We also need to explore the relations between specific dimensions of psychopathology, such as delusionality, formal thought disorder and subjective anomalies in self-awareness and world- and social relations.
Funding
This study was supported by the University of Copenhagen's Faculty of Health Sciences and by the Mental Health Services – Capital Region of Denmark.
Acknowledgements
The authors thank all the study participants, Dr John Cutting, Louis Sass and the staff from Hvidovre Psychiatric Center, University of Copenhagen.







eLetters
No eLetters have been published for this article.