Introduction and Background
The management of post-craniotomy pain is an important yet generally overlooked area of research. This is in part due to the assumption that post-craniotomy patients experience less pain than other surgical patients.Reference Dunbar, Visco and Lam1 However, more recent studies suggest that up to two-thirds of post-craniotomy patients experience moderate to severe pain,Reference De Benedittis, Lorenzetti, Migliore, Spagnoli, Tiberio and Villani2 which is often poorly managed.
Opioids are the most commonly used agents for treating moderate to severe post-surgical pain. In the neurosurgical population, opioids have the potential to interfere with neurological monitoring, can cause respiratory depression leading to hypercapnia, increased cerebral blood flow and ultimately increased intracranial pressure, and can mask early signs of intracranial complications.Reference Gottschalk and Yaster3 Furthermore, opioids are associated with several known adverse effects. Undertreatment of post-craniotomy pain can also lead to sympathetic stimulation leading to hypertension and intracranial hemorrhage.Reference Basali, Mascha, Kalfas and Schubert4 As a consequence of these conflicting scenarios and emerging interest in avoiding opioids, there is greater emphasis on non-opioid alternatives, as well as growing interest in the use of opioid-free anesthesia and perioperative analgesia.Reference Brandal, Keller and Lee5,Reference White6 This concept has been applied to other surgical areas such as cholecystectomies, bariatric surgery, head and neck surgeries, and others.Reference Gottschalk and Yaster3,Reference Bakan, Umutoglu and Topuz7–Reference Ziemann-Gimmel, Hensel, Koppman and Marema9 However, the available published studies surrounding the management of post-craniotomy pain have yielded inconsistent results.Reference Haldar, Kaushal, Gupta, Srivastava and Singh10 Hence, we decided to focus our review on opioid-free postoperative analgesia, which could include the use of regional blockade as well as other non-opioid analgesics. In our study we chose to focus on supratentorial craniotomies since these are a relatively homogenous patient population with perhaps the least severe post-operative pain. If we could eliminate the need for opiates for these patient it would help improve post-operative neurological examination significantly and hopefully decrease the number of investigations (e.g. computed tomography [CT] scans) due to more reliable clinical examination.Reference Flexman, Ng and Gelb11–Reference Dolmatova, Imaev and Lubnin14 Studies show that when looking at the different sites for craniotomies, the muscle dissection involved in posterior fossa craniotomies leads to a relatively higher post-operative pain experience.Reference Flexman, Ng and Gelb11–Reference Thibault, Girard, Moumdjian, Chouinard, Boudreault and Ruel13 Similarly, patients with vascular malformations and/or cerebral aneurysms were excluded as they tend to require more postoperative analgesia to achieve pain control compared with their tumor counterparts.Reference Glisic, Gardiner and Josti15 Associated cerebral vasospasm and hydrocephalus may also introduce considerable heterogeneity.Reference Chen, Luo, Reis, Manaenko and Zhang16–Reference Roos, de Haan, Beenen, Groen, Albrecht and Vermeulen19 Thus, the objectives of this systematic review were: (1) to compare the effectiveness of non-opioid and opioid analgesia in the treatment of post-craniotomy pain and (2) to identify available options for opioid-free analgesia post-craniotomy and consider clinical pathways for their effective use. Our secondary objective was to compare adverse effects between opioid and non-opioid analgesia groups.
Methods
Design
We undertook a systematic review of randomized controlled trials (RCTs) and constructed a narrative synthesis of results and available options.
Search Strategy
We conducted a comprehensive search of MEDLINE, Cochrane, and EMBASE databases from their inception to February 14, 2017. We used relevant keywords and medical subheadings to look for RCTs comparing opioid versus non-opioid post-operative analgesia for supratentorial craniotomies for elective tumor resections. We chose to focus on supratentorial craniotomies specifically as they result in relatively less pain compared to other craniotomy sites and therefore have the highest potential for completely opioid-free techniques. The full search terms and strategy are provided in the Supplementary Appendix. The search strategy was developed by input from physicians and further refined for each database in consultation with an experienced librarian. Using paired reviewers screening independently (DD and MS) and in duplicate, study selection was performed in two stages. Titles and abstracts were screened in the first stage, mainly looking for articles to exclude based on incorrect study design (i.e. non-RCT), language (i.e. non-English), and population. Any articles which were deemed to have incomplete information based on title and abstract or those that may fulfill at least the minimum criteria for inclusion were further screened in the second stage. The second stage involved full-text screening of potentially eligible citations, looking for articles that had comparators of interest (opioid vs non-opioid) and surgical site of interest. Disagreements were resolved by consensus between the two reviewers, and any unresolved decisions were decided by HS. A Cohen’s kappa statistic on the full article final decision was estimated as a measure of interobserver agreement. A final search through the gray literature was performed by searching through Google Scholar, Web of Science, and manually screening referenced articles in each of the RCTs included in this review.
Study Selection
We searched for English-language RCTs comparing opioid to non-opioid post-operative analgesia for supratentorial craniotomies. For the non-opioid group, studies were included if they used only short-acting opioid agents for intraoperative analgesia and excluded if they used long-acting agents or administered any opioid after extubation. We also excluded observational studies, quasi-randomized studies, and studies in which the study population included infratentorial pathology, aneurysms or vascular malformations, or emergency surgeries. Whenever full reports were not available, authors were contacted.
Data Collection
The same pair of reviewers extracted the data independently and in duplicate, using data extraction forms created in Microsoft Excel (Ver 15.28) that were piloted between the reviewers for consistency and accuracy. Data items extracted from each study included study characteristics, risk of bias (RoB) items, demographic information, participant disposition through the study, and our review outcomes in continuous and binary measures.
RoB in Individual Studies
RoB was assessed using the Cochrane Collaboration’s tool for assessing RoB in randomized trialsReference Higgins, Altman and Gotzsche20 to capture the components of random sequence generation; allocation concealment; blinding of participants; blinding of outcome assessment; and analysis of incomplete outcome data. No attempt was made to contact authors for clarification on the RoB items. Selective outcome reporting was judged based on the outcomes described in the Methods section but not reported in the Results section.
Outcomes
We were interested in studies that provided opioid-free analgesia post-operatively, with or without the use of regional blockade. The effectiveness of opioid-free analgesia was assessed by comparing either (1) use of rescue analgesia or (2) post-operative pain scores. In one study, pain scores were presented in a graphical format; therefore, we used online software (https://apps.automeris.io/wpd/) to extract data points. We decided a priori that we would combine studies (meta-analysis) if there were two or more studies using similar modalities to avoid opioid use. Our secondary objective included comparison of adverse effects including post-operative nausea and vomiting (PONV), sedation, itching, respiratory depression, and hemodynamic parameters as well as potential irritation at the site of the scalp blocks.
Data Analysis and Reporting
Considering the limited number of studies and associated heterogeneity, we decided to not pool data; hence, the outcomes are reported as tables and relevant outcomes for each study are summarized. Comparative effectiveness is reported either as proportion of patients with successful outcomes or as mean or median scores. Precision was reported using 95% confidence intervals (CI) around the effect estimate.
Narrative Synthesis of Available Options
Based on the existing literature, we give a brief description of individual options that could be used in various phases of surgery, as a multimodal concept, to allow for opioid-free anesthesia or analgesia. We summarize the individual agents and their possible considerations for clinical use.
Results
Study Characteristics
A total of 467 citations were identified through the literature search (Figure 1), of which 53 studies were selected for full-text review (kappa = 0.64; CI = 0.54, 0.75). Subsequently, four studies (Table 1) were ultimately selected for inclusion in the systematic review (kappa = 0.88; CI = 0.64, 1.11). Two studies compared opioid to non-opioid drugsReference Hassani, Mahoori, Sane and Tolumehr21,Reference Turgut, Turkmen, Ali and Altan22 and two studies compared opioids to regional scalp blocks (RSB).Reference Ayoub, Girard, Boudreault, Chouinard, Ruel and Moumdjian23,Reference Biswas and Bithal24 In three of the four studies, patients reported pain using a visual analog scale score;Reference Hassani, Mahoori, Sane and Tolumehr21,Reference Turgut, Turkmen, Ali and Altan22,Reference Biswas and Bithal24 one study used the numerical rating score to quantify pain.Reference Dunbar, Visco and Lam1 Both scoring systems ranged from 0 to 10.
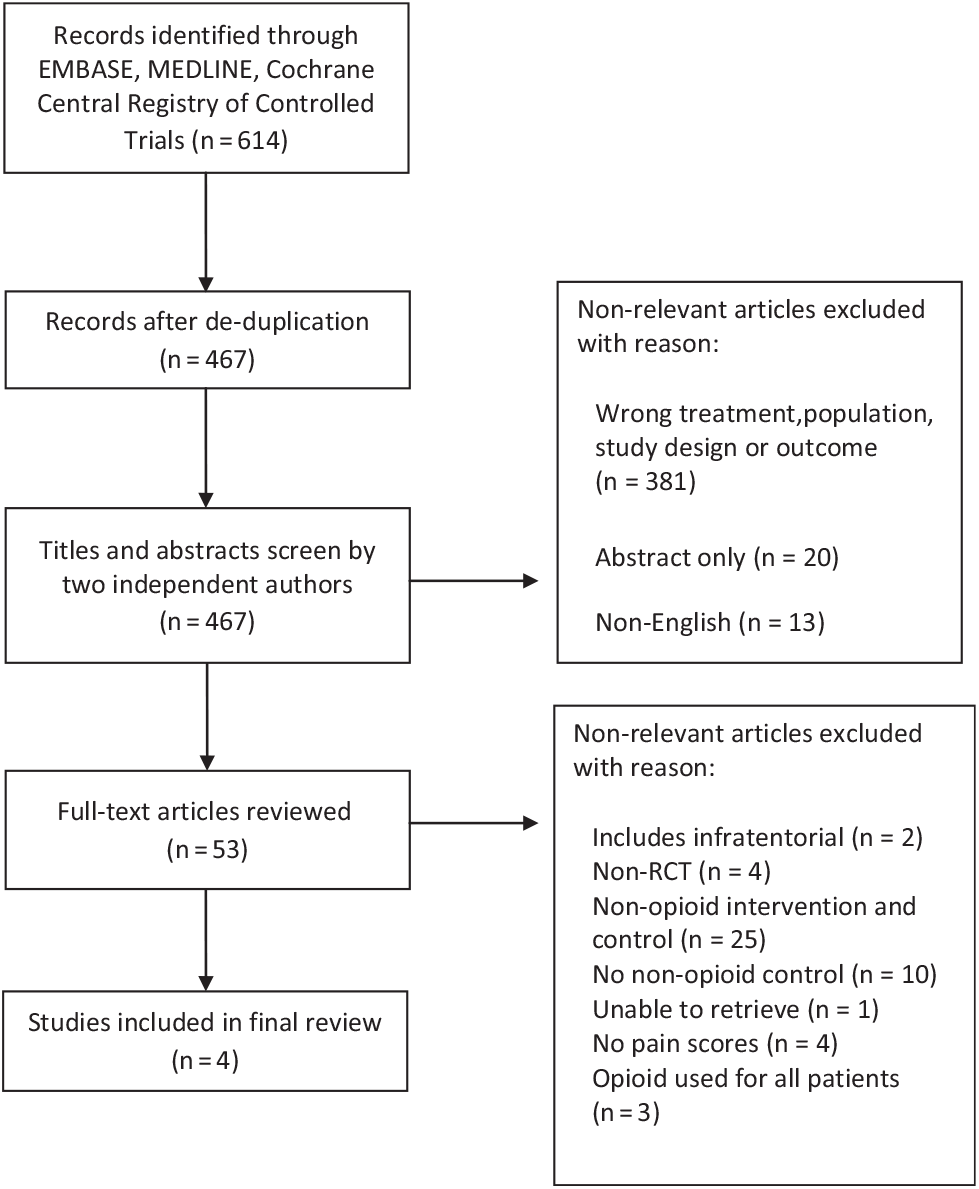
Figure 1: Study flow chart indicating study selection.
Table 1: Characteristics of included trials
Methodological Quality
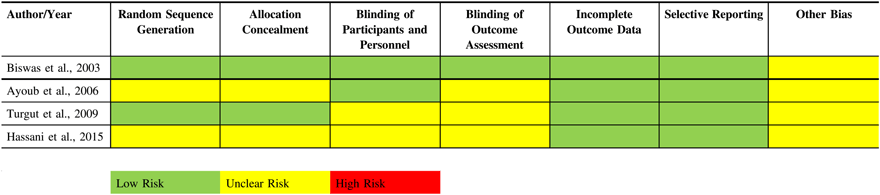
The RoB assessment is presented in Table 2. Reporting of methodological details of the trials was generally incomplete. All included trials were randomized; however, for all but one study,Reference Biswas and Bithal24 it was unclear how randomization was achieved. Only two studies commented on how allocation concealment was achieved.Reference Turgut, Turkmen, Ali and Altan22,Reference Biswas and Bithal24 In two studies,Reference Hassani, Mahoori, Sane and Tolumehr21,Reference Turgut, Turkmen, Ali and Altan22 blinding of participants and personnel was unclear and in three studies, blinding of outcome assessment was unclear.Reference Hassani, Mahoori, Sane and Tolumehr21–Reference Ayoub, Girard, Boudreault, Chouinard, Ruel and Moumdjian23 Outcome data collection was generally complete and no loss to follow-up was observed in any of the studies.
Table 2: Risk of bias assessment
Pain Scores
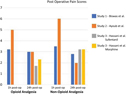
Table 3 shows outcomes of interest in all included studies. In one of the studies,Reference Turgut, Turkmen, Ali and Altan22 pain scores were not reported at 1-h and 24-h post-operatively. Furthermore, Biswal et al.Reference Biswas and Bithal24 showed pain scores in the form of a graph only; thus, numbers were extrapolated for this study as the original authors no longer had access to their original data. For studies that reported pain scores (ranging from 0 to 10), none had a statistically significant difference between opioid and non-opioid analgesia for post-craniotomy pain when compared at 1-h and 24-h post-operatively (Table 3 and Figure 2).
Table 3: Comparison of study outcomes
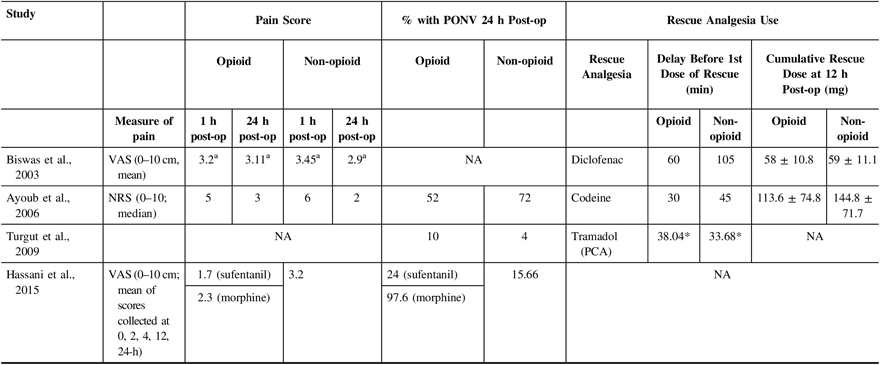
NA = not applicable; NRS = Numerical Rating Score; VAS = Visual Analog Score; PCA = patient-controlled analgesia; PONV = post-operative nausea and vomiting
a numbers extrapolated from graph.
* p < 0.05.
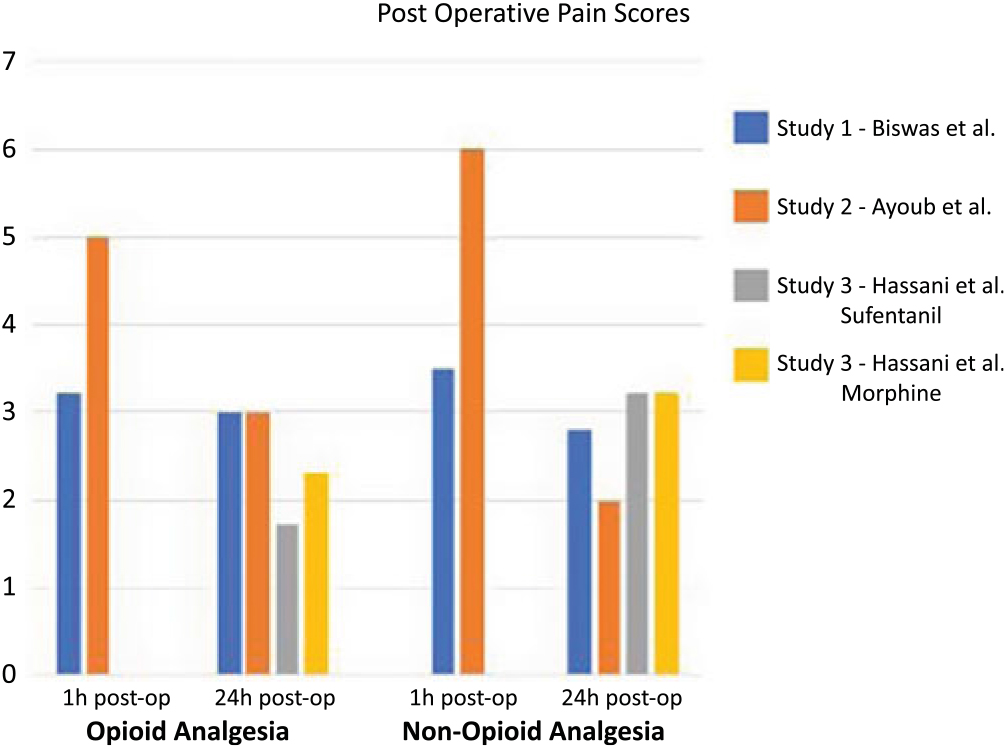
Figure 2: This figure illustrates a comparison between opioid and non-opioid analgesia used in three studies identified in this review. The studies by Biswas et al. and Ayoub et al. compared opioids versus non-opioids within 1 h from surgery and 24-h post-surgery. They found no significant difference in pain control between both studies. The study by Hassani et al. compared two different opioids (sufentanil and morphine) with non-opioid analgesia 24-h post-operatively and found no difference between opioids and non-opioids.
Analgesia Requirements
Use of supplemental analgesia was reported for three of the four included trialsReference Turgut, Turkmen, Ali and Altan22–Reference Biswas and Bithal24 (Table 3). In two of the three studies, no statistically significant difference in the time to rescue analgesia or the amount of rescue analgesia was noted between patients who were allocated to receive opioids and those who received non-opioid treatments. One studyReference Turgut, Turkmen, Ali and Altan22 found a statistically significant delay in the time to rescue analgesia among patients who were given opioids.
Adverse Events
Post-Operative Nausea and Vomiting
The incidence of PONV was reported in all but one study.Reference Biswas and Bithal24 This is shown in Table 3. Two of the three studies that compared the incidence of PONV in opioid versus non-opioid groups did observe a trend toward a decrease in PONV among patients who did not receive opioids, although the differences were not statistically significant.Reference Hassani, Mahoori, Sane and Tolumehr21,Reference Turgut, Turkmen, Ali and Altan22
Adverse Events Related to Scalp Blocks
Biswas et al.Reference Biswas and Bithal24 observed no incidence of hematoma formation due to unsatisfactory hemostasis. Ayoub et al.Reference Ayoub, Girard, Boudreault, Chouinard, Ruel and Moumdjian23 did not report any adverse events related to scalp blocks.
Discussion
In this systematic review, we compared the effect of opioid versus non-opioid analgesia in the treatment of post-craniotomy pain. We identified four RCTs, of which two compared opioids to RSB. The other two studies compared acetaminophen or dexmedetomidine as non-opioid analgesics and did not show any superiority over the opioid group. As opioids are known to be superior to other agents for pain control, we expected studies to be designed for testing equivalency or non-inferiority. However, we did not find any such studies.
Pain Scores
The findings of this systematic review indicate that pain scores did not significantly differ between patients who received opioid analgesia and those who received non-opioid agents. Similarly, there was no difference in the incidence of PONV between those who were administered opioids and those who were given non-opioid medications. However, it is worth noting that Turgut et al.Reference Turgut, Turkmen, Ali and Altan22 observed a statistically significant delay in the need for rescue analgesia for patients who received opioids compared to those who did not.
To the best of our knowledge, this is the first systematic review to compare the use of opioid versus non-opioid analgesia for post-craniotomy pain. A meta-analysis on the use of RSB for post-craniotomy analgesia demonstrated significant reductions in pain scores in patients who received RSB.Reference Guilfoyle, Helmy, Duane and Hutchinson25 Furthermore, RSB was not associated with adverse events. However, unlike our review, that review included any study that compared regional blockade with non-regional blockade. Similarly, dexmedetomidine has been investigated and found to reduce post-operative opioid consumption and the incidence of PONV when used as an anesthetic adjuvant in intracranial procedures.Reference Peng, Jin, Liu and Ji26,Reference Song, Ji, Sun, Gao, Liu and Li27
Multimodal Analgesia
It is widely known that multimodal analgesia improves pain while decreasing opioid requirements in a variety of post-surgical populations.Reference Horlocker, Hebl, Kinney and Cabanela28–Reference Ng, Leong and Tan30 Minimizing the use of opioids reduces their side effects, which can range from pruritus and gastrointestinal disturbances to significant respiratory depression. More importantly, in the setting of craniotomies, any reduction in the use of opioids can help to optimize post-surgical neurologic monitoring. Multimodal analgesia can comprise regional and neuraxial techniques, and non-opioid medications. In another center, a multimodal, interdepartmental, standardized analgesia protocol has been demonstrated to reduce post-operative pain among neurosurgical patients, improve documentation, and increase use of multimodal analgesia, including use of non-steroidal anti-inflammatory drugs and gabapentin.Reference Titsworth, Abram and Guin31 Furthermore, adherence to protocol was associated with a significant reduction in monthly use of naloxone, indicating a safety improvement. Other multimodal analgesia protocols have similarly been implemented in various surgical populations with good success.Reference Lee, Min, Bae, Cho and Kwon29,Reference Ng, Leong and Tan30,Reference Shi and Dang32 Table 4 summarizes the commonly used non-opioid analgesic agents that have been described in the literature as part of multimodal analgesia.
Table 4: Non-opioid medications that can be considered for use in a multimodal analgesic pathway
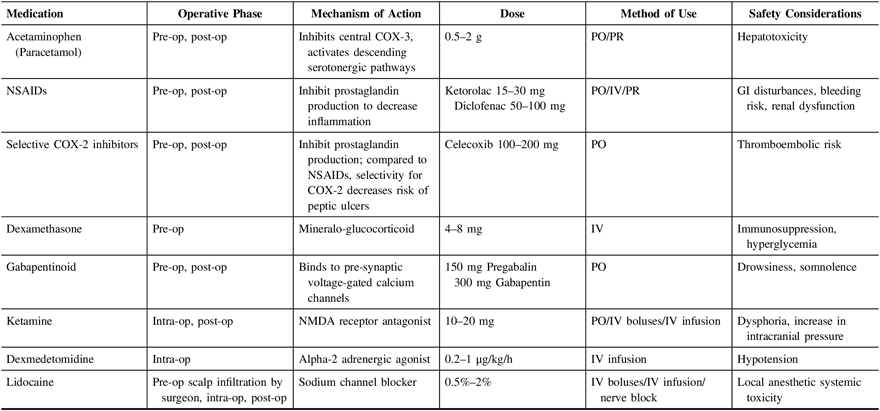
COX = cyclooxygenase; GI = gastrointestinal; Intra-op = intraoperatively; IV = intravenous; NMDA = N-methyl-D-aspartate; NSAIDs = non-steroidal anti-inflammatory drugs; Pre-op = pre-operative; PO = per os; Post-op = post-operative; PR = per rectum.
The use of multimodal analgesia has also become increasingly popular with the concurrent evolution of the opioid epidemic.Reference Kelly33, Reference Koepke, Manning, Miller, Ganesh, Williams and Manning34 It is well known that opioid prescription following surgery can potentially lead to long-term use and can be a significant source of misuse and abuse. This is especially true for a few subsets of the population, including acute post-surgical patients.Reference Noble, Treadwell and Tregear35 Therefore, opioid-sparing techniques can have positive public health implications.
In an era when hospital length of stay continues to decrease despite an increasing number of more complex surgical procedures, self-administration of opioids at home is now a routine practice. The safety of such practice is questionable as opioids are associated with many adverse effects, including breathing impairment and constipation. These adverse events can lead to the need for admission to hospital and thereby augment the economic burden associated with their management.Reference Kane-Gill, Rubin, Smithburger, Buckley and Dasta36,Reference Wan, Corman, Gao, Liu, Patel and Mody37 Further, surplus unconsumed opioids can become a source for misuse by patients or others, and problems with non-medical use.
Limitations
Our review has several limitations. The scope of this review was restricted only to post-operative analgesia, yet the use of intraoperative opioids may have a bearing on the development of PONV and other perioperative complications. Additionally, our review was based on a limited number of studies, most of which had inherent biases.
Conclusions
In summary, our review suggests that both opioids and non-opioid options, such as dexmedetomidine and RSB, can provide at least comparative pain relief with similar side effect profiles. As opioids can profoundly interfere with neurologic monitoring post-operatively, the use of multimodal analgesia is attractive in the neurosurgical patient. Nevertheless, given the small sample sizes of available RCTs, there is insufficient evidence to conclude that these opioid-sparing strategies are effective at this point in time. Further research with large sample sizes are required to determine the efficacy of these techniques. Future studies should also look to identify whether using alternate modalities of analgesia (non-opioid alternatives) would reduce hospital length of stay and the use of unnecessary CT scans.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2019.57
Acknowledgments
We thank Sara Miller M.Sc., Scientific Editor, Department of Anesthesia – Research Office, McMaster University for editing this manuscript.
Funding
HS is supported by the Canadian Anesthesia Research Foundation through the Career Scientist Award, 2018–2020.
Disclosures
The authors report no conflicts of interest.
Statement of Authorship
DD: Analysis and Interpretation of Data, Drafting the Article, Critically Revising the Article, Reviewed the submitted version of manuscript.
MS: Analysis and Interpretation of Data, Drafting the Article, Critically Revising the Article, Reviewed the submitted version of manuscript.
RC: Acquisition of Data, Review of the manuscript.
SK: Critically Revising the Article.
KKVR: Conception and Design, Critically Revising the Article, Reviewed the submitted version of manuscript, Study supervision.
HS: Conception and Design, Critically Revising the Article, Reviewed the submitted version of manuscript, Study supervision. and support including additional material or technical support.
All authors have read and approved the final version of the manuscript.