INTRODUCTION
In the summer of 2009, an outbreak of verocytotoxigenic Escherichia coli serogroup O157 (VTEC O157) was identified in visitors to a large petting farm in South East England. This subsequently proved to be the largest documented outbreak of VTEC O157 infection linked to animal contact ever reported in England and Wales to date. It attracted widespread media attention and led to an independent review (http://www.griffininvestigation.org.uk/).
Verocytotoxin-producing E. coli strains of serogroup O157 have been linked to disease outbreaks in many parts of the world [Reference Michino1–Reference Rangel3]. Although most reported outbreaks have been foodborne, investigators have demonstrated that infection can be transmitted from person to person or by animal contact when the organism is ingested by a host after it is excreted in the environment by humans (the faecal–oral route) [Reference O'Brien, Adak and Gilham4] or animals; the latter being particularly implicated in sporadic cases [Reference Locking5, Reference Kassenborg6]. The risk of disease from VTEC O157 can be high even at very low doses [Reference Tuttle7]; counts as low as 50 bacteria have been reported in foodborne outbreaks [8]. VTEC O157 infection is potentially life-threatening, particularly in children aged <5 years who develop haemolytic uraemic syndrome (HUS) [9–Reference Lynn12].
Petting or ‘open’ farms have become an important growth area in recent years. They are a significant part of the tourism and leisure industries and also have a valuable educational role. There were an estimated 13 million visitors to open farms in England and Wales in 1998 [Reference Pritchard13] and recent figures are likely to be similar or greater. Visiting open farms has been identified as a significant risk factor for VTEC O157 infections in humans [Reference Locking5] and many studies have shown that VTEC O157 is common in farming environments, particularly cattle farms [Reference Paiba14]. VTEC O157 was confirmed in one or more species in 61% of 31 public amenity premises with animals in England and Wales investigated between 1997 and 2007 because of putative associations with cases in visitors [Reference Pritchard15]. Three independent case-control studies of VTEC O157 infection in England, Scotland, and Wales conducted in the late 1990s all demonstrated significant associations between visiting farms and acquiring infection [Reference Pritchard13, Reference Howie16–Reference Payne18]. Until this outbreak most animal-associated outbreaks in the UK involved fairly small numbers of cases [Reference Pritchard13] compared to large foodborne outbreaks [Reference Salmon19]. The largest reported outbreak linked to animal contact prior to this one involved 23 cases [Reference Payne18]. In the USA, however, outbreaks of VTEC O157 linked to petting farms and state fairs affecting over one hundred people have been reported [9].
On 28 August 2009, a cluster of three cases of VTEC O157 infection was identified. All had visited a large petting farm in South East England. This farm was a very popular attraction and, during the summer holiday period, hosted 1500–2000 visitors daily. The farm kept a range of animals in fields and petting barns and permitted varying degrees of contact, including animal feeding and an area where children could climb in and play with lambs in the main barn. There was also potential contact with animal faecal matter from bedding contaminating the walkways used by the public. Initial control measures included strengthening hygiene precautions, restricting access to the lambs on 28 August and subsequently to all animal barns on 4 September and ultimately complete voluntary closure of the farm on 12 September. There were no further cases among visitors after 4 September (Fig. 1). We carried out epidemiological and microbiological investigations to describe the outbreak, determine the sources of infection and define the risk factors.
METHODS
Definitions for descriptive analysis
Case-finding was done by alerting local public health departments, general practitioners and microbiologists. A press statement was also issued to alert members of the general public who may have visited the farm during the outbreak period. Cases were described in terms of time, place, person and spectrum of associated illness. Confirmed cases were patients with confirmed VTEC O157 culture-positive stool samples; or with serological evidence of recent E. coli O157 infection. Primary cases were defined as confirmed cases with a date of onset of symptoms within 14 days of visiting the farm, while secondary cases were confirmed cases who belonged to a household with a primary case, but had not visited the farm within 14 days of becoming ill or having a positive stool sample (for asymptomatic cases). Asymptomatic cases were laboratory-confirmed cases (detected by screening of asymptomatic household contacts) without symptoms. The descriptive analysis guided the development of a case-control study to investigate the risk factors for acquiring VTEC O157 at the farm.
Ethical approval was not considered to be necessary for this study as this was part of an outbreak investigation undertaken by the HPA.
Definitions for case-control study
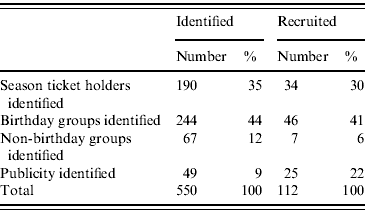
The population at risk was defined as those that visited the petting farm between 23 August and 4 September 2009. Findings from the descriptive analysis indicated that most cases (85% of primary cases) were children aged <11 years. For the case-control study, cases were therefore limited to primary cases aged <11 years whose date of onset of illness was within 6 days of visiting the farm (a tighter incubation period of 6 days was used in the analytical study to reduce the likelihood of mis-classification of cases). A control was defined as ‘a child aged <11 years from the population at risk without reported gastrointestinal symptoms who also visited the farm within the same period’. There was no comprehensive list of farm visitors, controls were therefore identified from three sources; a list of group bookings, a list of season ticket holders and following local media announcements asking anyone who visited the farm during the period of interest to contact their local Health Protection Unit. Leaders of group bookings and parents of season ticket holders were contacted and the details of potential controls collected from them. Residences of controls were uniquely coded so that non-independence of these observations could be taken into account in the analysis by adjusting the standard errors in logistic regression models and using Wald test P values to assess degree of significance. In the event that there was more than one potential control identified in a family, we used a non-restrictive control selection rule: using the initial of first names of the children and selecting the child within the household with initial of first name closest to ‘A’. We estimated this to be unrelated to age or sex.
Sample size calculations
To detect an odds ratio (OR) of 3 with 80% power and a significance level of 5%, with 50% of controls exposed we planned to recruit 45 cases with at least three controls each. Due to the clustering within control groups, we sought to recruit six controls per case.
Exposures of interest
The descriptive analysis showed that the farm was the common exposure setting for all the primary cases; therefore this was the main focus of our investigation. Information was collected by a questionnaire on demography, illness status, contact with different areas of the farm, duration of farm visit, contact with different types of animals, sources of any food and drink consumed on the farm, personal hygiene practices while on the farm, and the number of previous visits to a petting farm within the last year.
Data collection
Questionnaires were administered by telephone interviews to the carers who visited the farm with the children. The interviews were conducted by nine public health-trained interviewers who had received training on administering the questionnaire. Parental consent was obtained for all interviews.
Statistical analysis
Statistical analysis was conducted using Stata v. 11 (StataCorp., USA). A hierarchical approach was taken to the analyses, bearing in mind the biological plausibility and the ecology of a farm visit. Variables were divided into groups pertaining to: person, behaviour, premises visited, activities and food or water exposure (Fig. 2). Within each exposure group, the effect of each variable on being a case or control was examined by applying logistic regression while controlling for day of visit, being part of a group, age and gender. Where a specific farm area was associated with an increased risk of being a case, the individual animal exposures within these areas were examined further by the same means. Multivariable logistic regression was used to examine potential confounding between variables positively associated (or negatively associated for putative protective factors such as handwashing) with being a case at P⩽0·05. Within-premises effects were ‘promoted’ to primary variables by recoding to zero where there was no exposure to the respective premises; these were examined first. Variables were added to a single model using the likelihood ratio test; each variable under consideration was dropped sequentially and tested for significance. Primary variables from the other premises and/or pathways were then added and the process repeated until only significant variables remained in the model. Variables for day of visit, being part of a group visit, age and gender were retained in the model throughout. Differences in proportions were assessed using the χ2 test and the χ2 test for trend respectively for binary and categorical variables. Differences in medians were assessed using the non-parametric test for equality of medians.
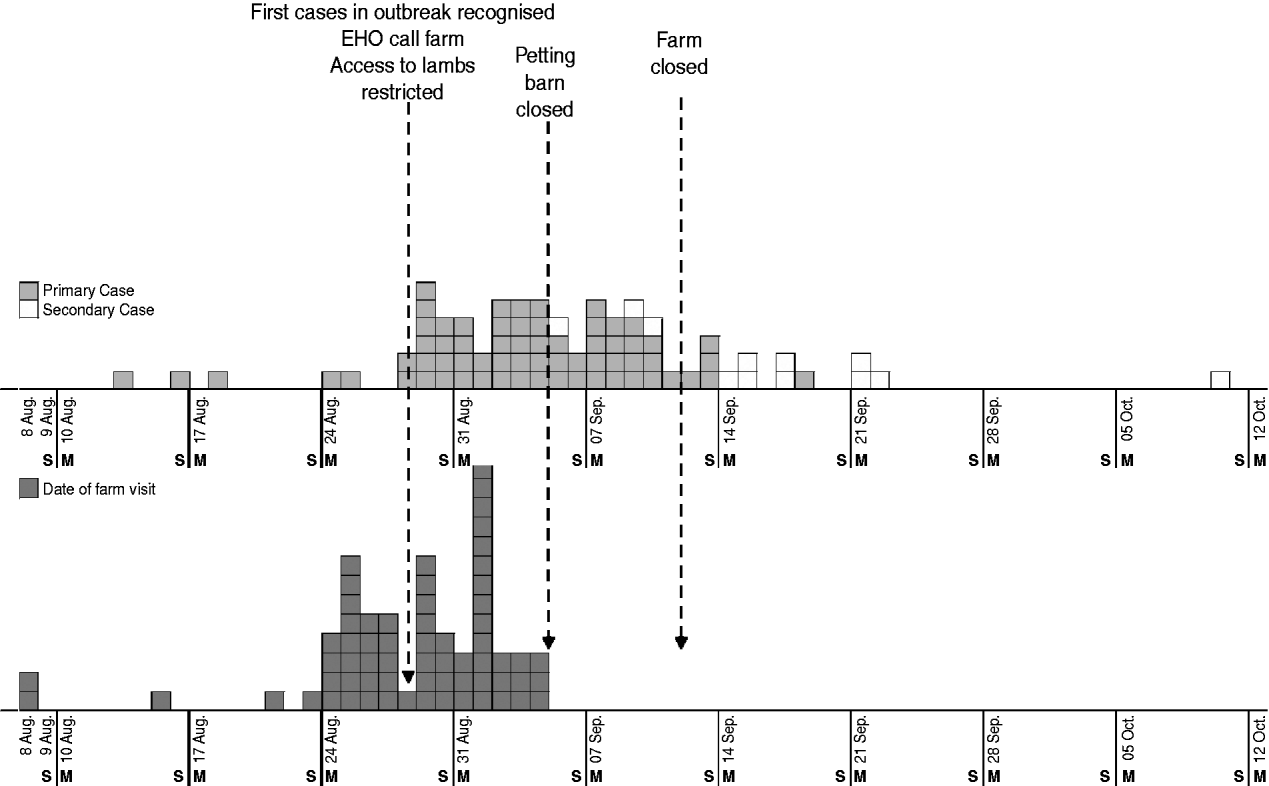
Fig. 1. Epidemic curve of VTEC cases in visitors to a petting farm in South East England in 2009.
Microbiological investigations
Human microbiology
Stool samples for all cases were tested for E. coli O157 by local microbiology laboratories to identify presumptive VTEC O157 infection. The presumptive isolates were sent to the Laboratory of Gastrointestinal Infections (LGI) at the HPA's Centre for Infection (the Reference Laboratory for England and Wales) for confirmation. Isolates were tested for Verocytotoxin (VT)-encoding genes 1 and 2, identified biochemically, serotyped and phage-typed [Reference Willshaw20]. Sera were tested for evidence of E. coli O157 infections [Reference Chart and Cheasty21].
Animal microbiology and environmental samples
A visit to the premises was undertaken by a veterinary investigation officer of the Veterinary Laboratories Agency (VLA), Winchester, on 7 September 2009 to collect samples from animals for O157 cultures and to advise on veterinary aspects of VTEC O157 infection; two other veterinary sampling visits were undertaken to follow-up some initial findings. Most of the animals present on the farm during the exposure period were sampled; fresh faecal (floor) samples were taken from animal pens including those which could be readily accessed by the public. Animals sampled included: cattle (9 samples), sheep (15), goats (29), pigs (24), equines (7), llamas (4), poultry (7), pet rabbits (6) and a fox (1). Sample selection and collection used a well established veterinary approach which ensured the detection of at least one positive sample in an epidemiological group of animals with 95% confidence if the prevalence was at least 10% [Reference Pritchard15]. Environmental samples from around the farm were variously collected by environmental health officers (EHOs) of the local authority.
Animal samples were cultured for E. coli O157 at VLA Bury St Edmunds as described previously [Reference Pritchard15]; environmental samples collected by EHOs were cultured by the local public health laboratory using similar methodology as for human isolates. All presumptive VTEC O157 isolates from animals or the environment were examined by the LGI as for human isolates.
Human and non-human isolates were compared by two independent DNA-based techniques. All strains were tested by multilocus variable number tandem repeat (VNTR) typing [Reference Hyytia-Trees22] and a selection from different sources were also examined by pulsed-field gel electrophoresis (PFGE) [Reference Willshaw20].
RESULTS
Descriptive analysis
Ninety-three people with VTEC O157 with dates of onset between 13 August and 18 September were detected in this outbreak; 65 primary and 28 secondary cases. Of these, 15 were asymptomatic cases, detected through screening of household contacts; 13 of these cases were classified as secondary cases, and two were classified as co-primary cases because they also visited the farm during the at-risk period. All the primary cases visited the affected farm between 8 August and 4 September 2009 with dates of onset between 13 August and 18 September (Fig. 1). The incubation periods for the primary cases ranged from 1 to 14 days (median 5 days). Cases were aged between 8 months and 36 years (median 4 years). Eighty-two percent (76/93) of all cases were aged <10 years; 47 (51%) were males. Of the 78 symptomatic cases, 27 (35%) were hospitalized and 17 (22%) were diagnosed with HUS. Ninety percent of hospital admissions and all cases with HUS were children aged <10 years.
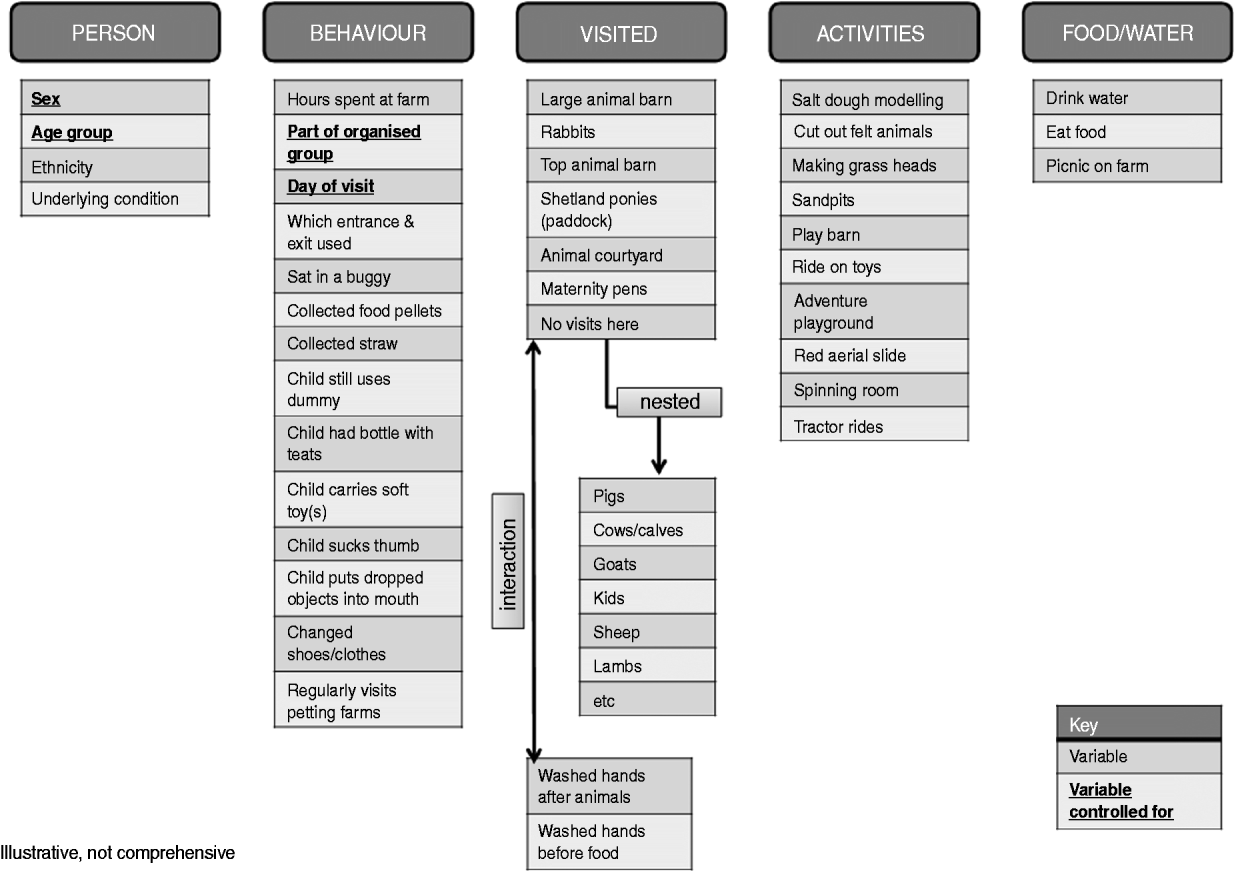
Fig. 2. Hierarchical structure of biologically plausible variables included in analytical approach.
Access to the lambs ceased on 28 August and the two barns housing animals for petting were voluntarily closed by the farm owner at the end of the day on 4 September. The farm subsequently completely closed to public admissions on 12 September. Figure 1 shows the dates of onset and farm visit for primary and secondary cases.
Daily attack rates for the entire visitor population were also calculated as well as for those aged <2 years for the period 8 August to 4 September 2009, the period during which all primary cases visited the farm. Peak attack rates occurred on 1 September (0·58%). The highest attack rates were seen in those aged <2 years, with a maximum of 1·56% on specific days.
Information about household contacts was available for 60 of the 65 primary cases. Of these, 16 were classified as co-primary cases (i.e. cases that were simultaneously exposed to the farm, mostly siblings who visited together). From the remaining 44 primary cases 123 household contacts were identified. The secondary attack rate for symptomatic cases among household contacts was 10·6% (n=13); the other two asymptomatic cases were co-primary cases. The secondary attack rate for both symptomatic and asymptomatic cases among household contacts was 21·1% (n=26).
Case-control study
We conducted a case-control study to investigate risk factors for acquiring VTEC O157 on the farm. At the time of initiating the study, we identified 38 cases and 294 controls that satisfied our case definition. Nine cases were either too ill at the time of the investigation or declined to participate. Six further cases were excluded due to their incubation period being outside the 0–6 days range. Of the 294 controls recruited, 154 were randomly selected; of these 34 could not be contacted, three declined and five were excluded as they were outside the age range (one control) or outside the date of visit range (four controls). The analysis was therefore based on 23 cases and 112 controls (Fig. 3, Table 1).
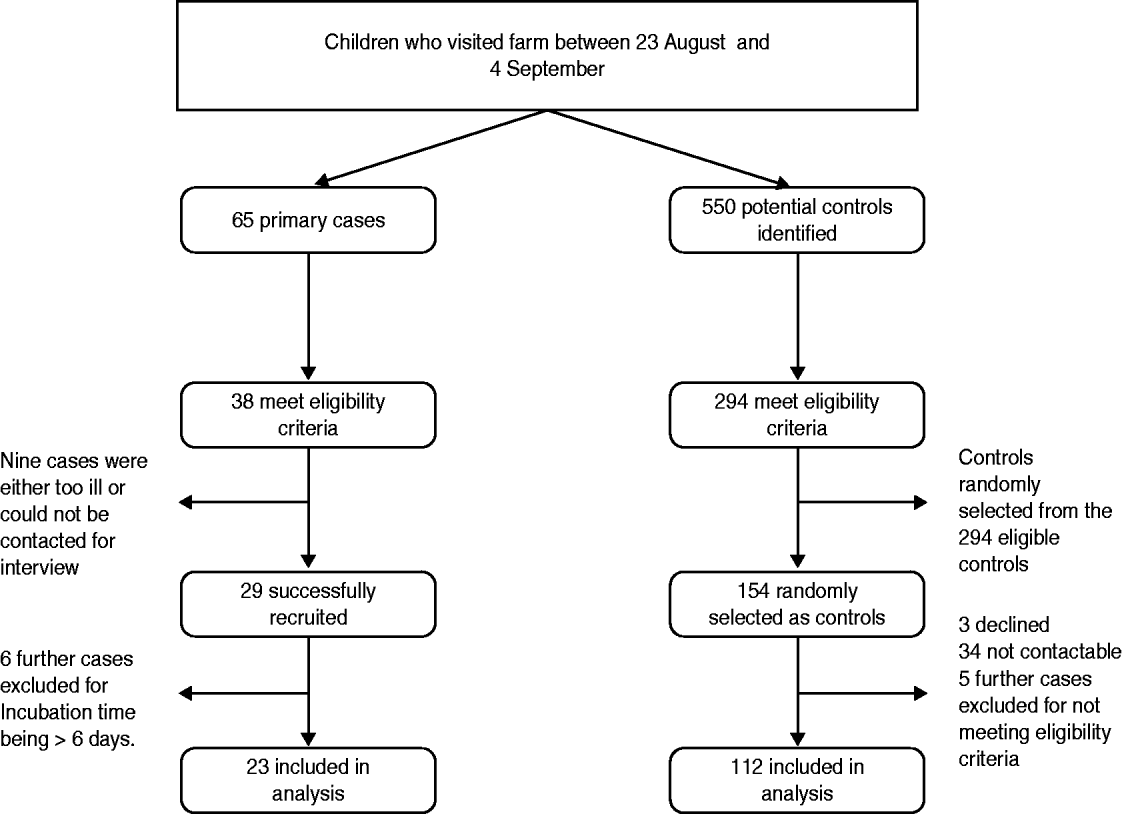
Fig. 3. Case and control recruitment process.
Table 1. Identification, eligibility and recruitment of controls
Controls were broadly similar to cases with regard to age, gender, ethnicity, underlying morbidity and week of visit (Table 2). The period from farm visit to interview was similar. The weekly periodicity of farm visits was different for cases and controls, with more cases visiting on Tuesdays and Thursdays. Controls were also more likely than cases to attend the farm as part of an organized group, reflecting the methods of control recruitment employed. Adjustment was made in the analysis for the day of visit, being part of group, age and sex.
Table 2. Demographic and other differences between cases and controls
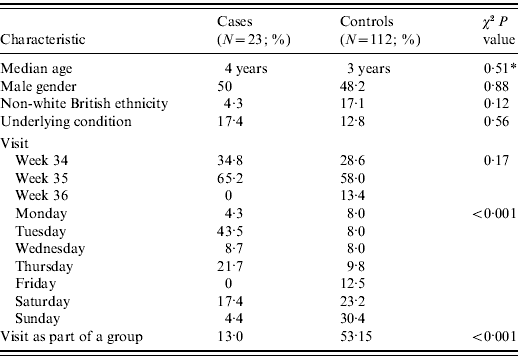
* Non-parametric test for the equality of medians.
A detailed single variable analysis is presented in Tables 3 and 4. Cases were more likely than controls to have entered or left the farm via a specific entrance/exit and to have stayed for a prolonged period (>5 h), but were less likely to have made more than five farm visits in the previous 12 months. Cases were more likely to have hand-fed animals, visited the large animal barn and the climb-in rabbit enclosure and eaten ice-lollies bought at the farm. Within the large animal barn, cases were more likely than controls to have fed the adult goats and petted the adult sheep.
Table 3. Single variable analysis controlling for age, gender, day of visit and being in a group
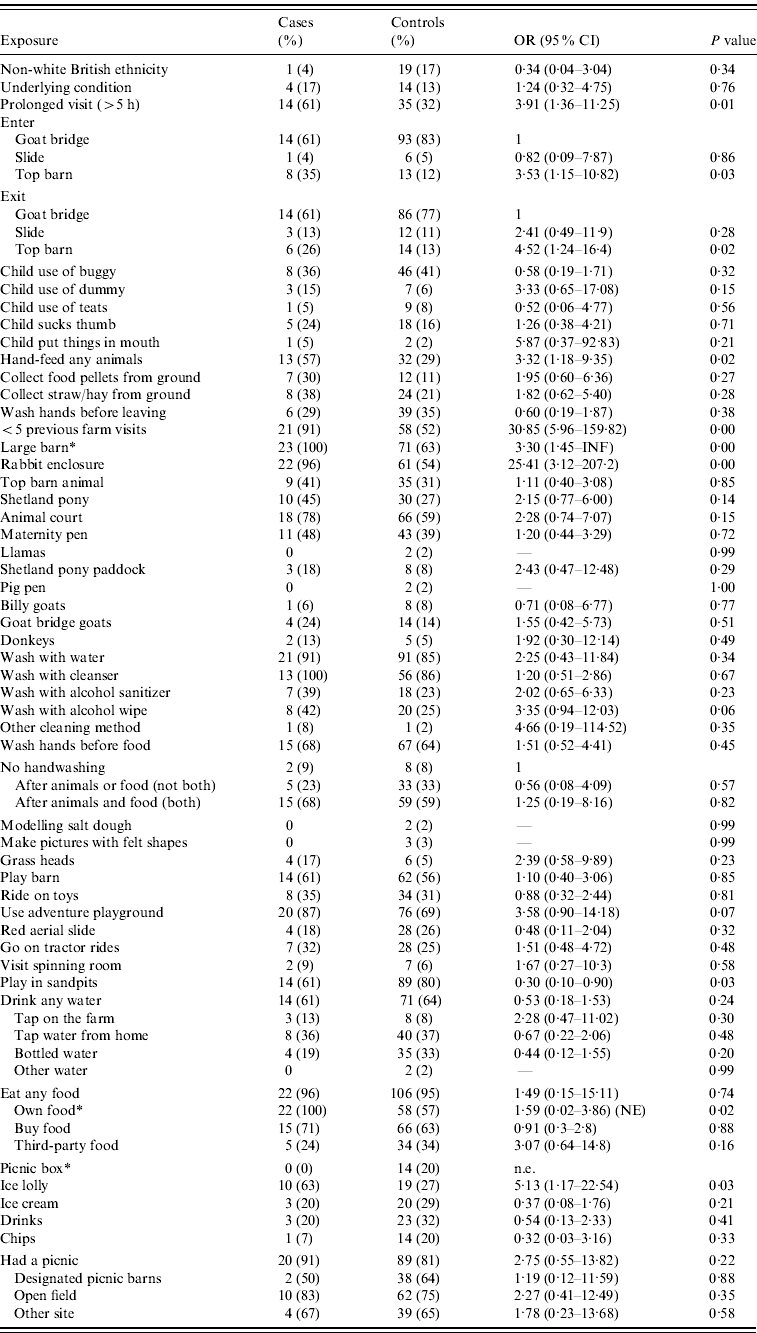
OR, Odds ratio; CI, confidence interval; n.e., too few observations to estimate.
* Median unbiased estimates.
There is some variability in denominators resulting from variability in the amount of persons with information by exposure.
Table 4. Within-premises exposures for high-risk premises. Single variable analysis controlling for age, gender, day of visit and being in a group
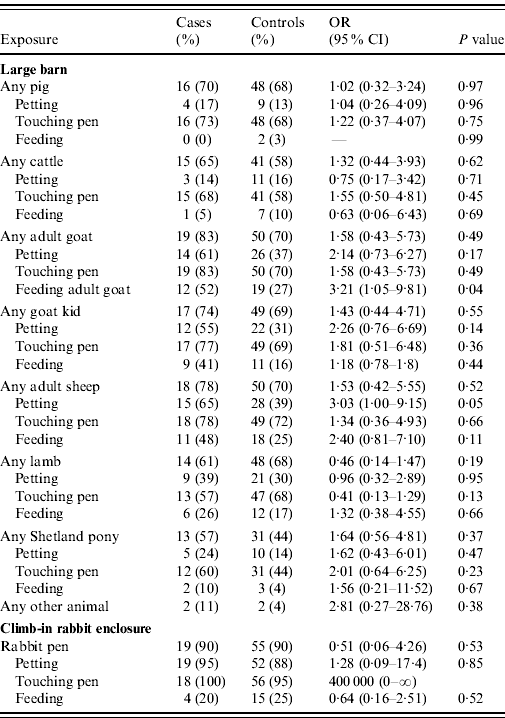
OR, Odds ratio; CI, confidence interval.
The final logistic regression model is shown in Table 5. Our study demonstrated an association between acquiring infection and visiting the large barn (P<0·001, OR not estimable), and in those who reported petting adult sheep in the large barn (P<0·001, OR not estimable). Cases were more likely than controls to have visited for a prolonged period (OR 5·93) and more likely to be infrequent visitors to open/petting farms (OR 39·26). No associations with the consumption of foods (including water) or visiting particular sites to eat were identified. Handwashing conferred no demonstrable protective effect. The day of visit, the method of entrance to or exit from the site were not statistically significant, nor was hand-feeding the animals, visiting the climb-in rabbit enclosure or eating ice-lollies. There were no significant interactions between visiting specific premises and handwashing.
Table 5. Final multivariable logistic regression model (131 observations)
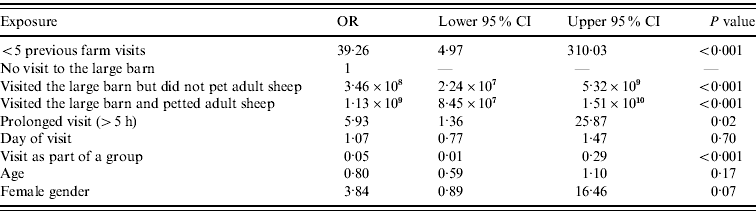
OR, Odds ratio; CI, confidence interval.
Human microbiology results
Isolates from 91 cases were confirmed as E. coli belonging to serogroup O157; they possessed genes for VT2 but not VT1 and were all phage typed (PT) as 21/28. One case had evidence of E. coli O157 infection diagnosed only by the presence of antibodies to the O157 lipopolysaccharide antigen. One case was diagnosed as presumptive E. coli O157 by the local laboratory but an isolate was not available for confirmation and typing by the LGI.
Animal microbiology results
A total of 102 faecal samples were collected from animals at the visit on 7 September. These comprised: cattle (9 samples), sheep (15), goats (29), pigs (24), equines (7), llamas (4), poultry (7), pet rabbits (6) and a fox (1). Of these, E. coli O157 (subsequently confirmed as VTEC O157 PT21/28 VT2) was isolated from 33 (32·3%) of the samples, all but four of which were from the large and top barns. Most positives were from sheep and goats. The details of all the positive animal samples are presented in Table 6.
Table 6. Isolations of verocytoxigenic Escherichia coli O157 from sampling visit on 7 September 2009
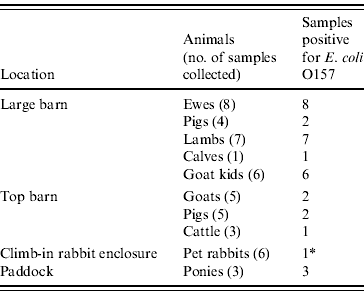
* Negative on extensive resampling.
One of the samples from the pet rabbits was unexpectedly positive for VTEC O157. VTEC O157 was not isolated from a further 115 samples collected from the rabbits and various small mammals and birds on the farm.
Environmental microbiology results
Five of 45 environmental samples were positive for VTEC O157. These were from bark chippings in the adventure playground and from the straw bedding, dust and metal railings on the pig and goat pen and two birds' nest samples, all collected from the large barn.
Molecular profiles of VTEC O157 isolates from people, animals and environment
VNTR typing of human and non-human VTEC O157 isolates identified two predominant outbreak profiles that differed at one locus by a single tandem repeat. These profiles were found in 84 (92%) human isolates and 32 (78%) strains from non-human sources. All the remaining isolates differed from either of these two predominant ‘outbreak profiles’ at a single locus. In total, six single locus variant profiles were found in non-human strains, four in isolates from cases and one profile in both human and animal isolates. PFGE on a sample of isolates showed four profiles with ⩾90% relatedness among 52 human isolates and two of these were also found in 15 non-human strains tested. The most common profile was found in 79% of human and 73% of non-human isolates. There was no correlation between possession of a specific VNTR type and particular PFGE profile.
DISCUSSION
This is the largest documented outbreak of VTEC O157 reported in England and Wales associated with animal contact to date. The causative organism was VTEC O157 PT21/28 with genes for the production of VT2. VTEC O157 PT21/28 has been the type most commonly reported in England and Wales since 1999; comprising 33% of human isolates in 2008 [23]. However, neither the PFGE nor VNTR profiles of the outbreak strain had been identified in previous outbreaks in England and Wales (VNTR a more rapid method than PFGE, has been performed in England since mid-2008). Profile variation as seen with this outbreak strain, is probably a reflection of the large number of isolates available for testing. This was seen in both methods but both gave equal support to the phenotypic typing and epidemiological conclusions indicating that the animal and human isolates belonged in the same outbreak group.
The epidemiological investigation showed that the large barn was the area of the farm where most of the transmission occurred. Cases were more likely to have spent >5 h on the farm. The high proportion of animals excreting VTEC O157 in the large barn, together with the isolation of the outbreak strain from multiple locations in the farm environment suggests that people were exposed through contact with infected animals or faecally contaminated bedding or fixtures. The outbreak was controlled by restriction of visitors to the animal barns.
The results from the epidemiological and microbiological investigations provided good evidence to indicate that individuals became infected with VTEC O157 PT21/28 through direct and/or indirect contact with infected animals, mainly in the large barn of the farm.
The results of our investigations are consistent with those of previous reports [Reference Shukla24, Reference Trevena25] showing direct transmission of VTEC O157 to humans as well as transmission through contact with the farming environment [Reference O'Brien, Adak and Gilham4]. A review of 55 outbreaks of enteric disease associated with animals in public settings in the USA identified risk factors for indirect transmission through contact with contaminated shoes and clothing and environmental contamination [Reference Steinmuller26].
On this farm, deep straw litter was used for animals in the heavily infected barns and at the time of the outbreak it had been down for at least 2 months and was likely to have been heavily contaminated with animal faeces and urine [Reference Davis27]. It was observed that public walkways could become contaminated with bedding spilling over from the animal pens. Although we can speculate on the role of this practice in the causal pathway of the outbreak, the considered veterinary view was that this bedding management system was unremarkable compared to practice encountered on open farms elsewhere (G. C. Pritchard, personal communication).
We also found that cases were more likely to be infrequent visitors to open farms. The most likely explanation, however, is a selection bias in our control selection, as one source of controls was from a list of regular farm visitors. Other possible explanations could be that frequent visitors are more adept with infection control precautions or have underlying immunity from previous exposures. In a review of outbreaks in the USA, between 1991 and 2005 [Reference Steinmuller26] people with enteric illness were less likely to own farm animals. It is also possible that frequent visitors, especially children, may have been more interested in the non-animal attractions and therefore evaded infection.
No associations were identified with the consumption of food or water, or visiting particular eating areas. Handwashing conferred no demonstrable protective effect. This was a surprising finding and is at variance with findings from other outbreaks; Crump et al. report a protective effect of handwashing in a similar setting [Reference Crump28]. The intense publicity associated with this outbreak may have resulted in reporting and recall bias with regard to handwashing behaviour. Handwashing facilities were found in all the toilets and at three other locations around the barns on the farm.
Animals carrying VTEC O157 do not show clinical signs of illness following infection with this organism and shedding is intermittent and transient [Reference Keen29–Reference Wang, Zhao and Doyle31]. Shedding has also been shown to be seasonal, with excretion rates peaking in the summer and early autumn [Reference Synge32]. Although the main reservoirs of VTEC O157 infection are cattle and sheep, the infection has also been demonstrated in a very wide range of species on open farms including pigs, equines, camelids [Reference Pritchard15] and wild rabbits [Reference Pritchard33]. Significantly, in previous studies [Reference Pritchard15], VTEC O157 has not been found in small mammals such as guinea pigs and rabbits making the isolation of VTEC O157 from one of six pet rabbit samples in this investigation unexpected. Extensive re-sampling did not confirm the initial finding and it was concluded that environmental contamination from the nearby heavily contaminated large barn was the most likely explanation.
It is notable that the farm had a very high throughput of visitors during August 2009. Attendance at this farm was well above the average visitor attendance of open farms investigated by the VLA between 1997 and 2007 (R. P. Smith, personal communication). Therefore, large numbers of people were potentially exposed to VTEC O157 during the outbreak period. Despite the large population at risk, it remains unclear why infection occurred in such a high proportion of people who visited the farm (especially the large barn) during the specific period of 24 August to 4 September. It is tempting to speculate that there were larger numbers of bacteria present in the environment and a higher rate of animal shedding at this time, but there are no quantitative data available to support this theory. The ‘normal’ quantity of bacteria or rate of shedding is unknown. However, restriction of access to the animal barns which occurred 8 days before the closure of the farm controlled the outbreak. Larger open farm premises (such as that described here) have been shown to be more likely to be contaminated with VTEC O157 than smaller premises [Reference Paiba14].
The widespread contamination of this farm environment by VTEC O157 and a high attack rate in visitors, leading to the large outbreak described by our investigation suggests that broad and often non-specific outbreak control measures are indicated even on the detection of a few cases linked to a farm venue. The objective should be the restriction of contact with animals and to the physical environment where animals are housed until the outbreak is understood and controlled. Moreover, from the findings of many previous published studies, it must be assumed that all petting or open farms are potentially high-risk environments for the acquisition of VTEC O157 infection. Control and prevention should therefore be based on risk management procedures such as those advised by the Health and Safety Executive [34] to prevent animal faecal–human oral contact rather than attempting to eliminate or monitor infection; the guidance has been updated in 2011 to explicitly state that ‘You should not allow the public to enter animal pens’ [34]. Open farm operators should ensure that visitor contact with animal faeces is minimized or eliminated and that there is full compliance with current advice [34].
This study has some limitations. The absence of a complete sampling frame made the random selection of controls impossible, leading to a probable selection bias. Given the publicity associated with the outbreak, individuals, who did not visit the barn may have been more likely to agree to act as controls leading to an overestimation of the effect of the barn exposure. Alternatively, individuals who were more diligent with infection control procedures may also have been more likely to volunteer as controls, obscuring the likely increased risk associated with poor hand hygiene, as reported by other studies. The wide publicity this outbreak generated is also likely to have led to reporting biases especially regarding personal hygiene behaviour, as cases may have been less likely to admit to activities that may be perceived to have put their children at increased risk of infection. These are common limitations in field epidemiology where ideal study conditions are rarely found. The method used to select variables for inclusion in the multivariable analysis may have allowed some variables to be excluded that may have influenced the outcome, although with the number of variables investigated, a less stringent cut-off for selection may have increased the chance for variables to appear significant that did not have a true effect on disease outcome. To ensure against the subjective nature of variable selection in a statistical regression model an independent analysis was performed. The results from the two analyses were compared, and no major differences were observed in the inferences made regarding risk factors.
This outbreak is the largest documented outbreak of VTEC O157 associated with a farm in the UK. Likely explanations include: the high percentage of animals on the farm excreting VTEC O157; a strain previously uncharacterized in the UK; the large number of farm visitors as well as the duration of contact between visitors and the farm animals or a combination of all these factors. The outbreak strain was of the phage and VT type most common in the UK, although the VNTR and PFGE profiles had not been seen in previous outbreaks. More research is required into shedding patterns of VTEC O157 in farm settings, particularly those with large numbers of visitors. The relatively high proportion of clinical cases who developed HUS (22%) also warrants further study. The investigation brought together expertise from various organizations and demonstrated the value of collaboration between human and veterinary medical disciplines during outbreaks of zoonotic disease.
DECLARATION OF INTEREST
None.
ACKNOWLEDGEMENTS
We are grateful to Neil Palmer at VLA Winchester for collecting faecal samples from the animals and providing veterinary information about the farm. Charlotte Featherstone, VLA Thirsk, provided valuable veterinary advice to the Outbreak Control Team. Laboratory staff at VLA Bury St Edmunds undertook the culture of animal samples for E. coli O157. Funding for the veterinary part of the investigation was provided by Defra through surveillance project FZ2100. This investigation was funded by the participating agencies, HPA and VLA. No external funding was required.











