The spread of diseases such as ischaemia–reperfusion (IR), atherosclerosis and diabetes is a great concern for developed countries. Oxidative stress has been suggested to be responsible, at least partially, for the onset and progression of these diseases(Reference Brownlee1, Reference Furukawa, Fujita and Shimabukuro2). Alleviation of oxidative stress is therefore considered an effective measure for the prevention of these diseases. Many researchers have investigated the effectiveness of various antioxidants, such as ascorbic acid, tocopherols, polyphenols and carotenoids against these diseases.
Dietary fibre and resistant starch (RS), which are not digested by digestive enzymes, are metabolised by intestinal microbiota in the large intestine to form SCFA and gases including H2(Reference Bond and Levitt3–Reference Muir, Lu and Young5). Rackis et al. (Reference Rackis, Honig and Sessa6) previously reported that H2 accounts for 30 % of the flatus for participants administered soyabean oligosaccharides, raffinose and verbascose. Furthermore, breath H2 excretion is known to increase when dietary fibre and lactose are administered to healthy and lactose-intolerant participants, respectively(Reference Bond and Levitt7–Reference Savaiano, AbouElAnouar and Smith10). Change in breath H2 concentration has been used to determine the transit time required for indigestible materials to reach the large intestine as well as to diagnose lactose intolerance, although the effect of H2 in vivo is not known.
In a chemical reaction, H2 acts as a strong reducing agent under the co-existence of catalysts such as platinum, nickel and palladium. It has long been said but never proved that the redox potential of H2 is considerably lower than that of glutathione or ascorbic acid, and it has strong reducing capability in vivo. In 2007, Ohsawa et al. (Reference Ohsawa, Ishikawa and Takahashi11) found that H2 selectively eliminates reactive oxygen species (ROS), especially those that are highly oxidative such as the hydroxyl radical and peroxynitrite, in the brain of IR rats, and, consequently reduces cerebral infarction. The present study is the first to demonstrate the antioxidative effect of H2 in vivo. Furthermore, antioxidative effects of H2 on other organs (e.g. heart(Reference Hayashida, Sano and Ohsawa12), liver(Reference Fukuda, Asoh and Ishikawa13), retina(Reference Oharazawa, Igarashi and Yokota14), intestine(Reference Buchholz, Kaczorowski and Sugimoto15, Reference Chen, Sun and Hu16) and kidney(Reference Cardinal, Zhan and Wang17)) have been observed in IR-treated rodents administered H2 via the inhalation of H2 gas or administration of H2 water. It is clear from these studies that H2 is an effective antioxidant in vivo. Therefore, we speculated that the H2 formed during large-bowel fermentation of dietary fibre can also effectively eliminate ROS generated in vivo, and, consequently can contribute in alleviating oxidative stress in vivo. We mention in the present study that Neale(Reference Neale18) had earlier proposed a similar hypothesis; however, as far as we know, his hypothesis was never proved. The present study proposes another function for dietary fibre and RS in nutritional science.
While fermentation substrates are supplied, H2 will continuously be generated by large-bowel fermentation, and hence, H2 concentration in vivo will be maintained at a stationary high level. We believe that consuming foods containing fermentation substrates daily will relieve oxidative stress in vivo. Our suggestion to supply the body with H2 daily is significant, since it is not easy to do so by conventional means (i.e. via the inhalation of H2 gas or the administration of H2 water) in a continuous manner.
In the present study, to determine the suppressive effect of dietary fibre and RS on hepatic IR injury, we examined the dose–response relationship between portal H2 concentration and the amount of pectin (Pec) or high-amylose maize starch (HAS) supplementation. Simultaneously, we examined the effects of Pec and HAS on liver damage in a rat hepatic IR model.
Materials and methods
Samples
Pec (Z4A-618, non-sugar type) and HAS (Hi-maize 1043) were kindly supplied by Taiyo Kagaku Company Limited (Mie, Japan) and Nippon NSC Limited (Tokyo, Japan), respectively.
Animals and diets
The study was approved by the Nayoro City University Animal Use Committee, and the animals were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals, Nayoro City University. Male Sprague–Dawley rats (8 weeks old), weighing 260–280 g, were obtained from Japan SLC (Haruno colony; Shizuoka, Japan). They were housed in individual cages with screen bottoms made of stainless steel in a room maintained at 23 ± 1°C with humidity ranging from 50 to 70 % under lighting conditions with 12 h light–12 h darkness (07.00–19.00 hours) daily. The rats were acclimatised by feeding a 25 % casein control (C) diet (Table 1)(Reference Reeves, Nielsen and Fahey19) for 3 d in Expts 1, 2 and 3 and a laboratory chow diet (CE-2; Japan Clea, Tokyo, Japan) for 3–5 d in Expts 4 and 5 in order to select high H2-generating (HG) rats before being subjected to the experiments. The composition of the chow diet was as follows: protein, 251 g/kg; fat, 48 g/kg; dietary fibre, 42 g/kg; ash, 67 g/kg; N-free extract, 500 g/kg; moisture, 9·3 g/kg.
Table 1 Composition of the control diet
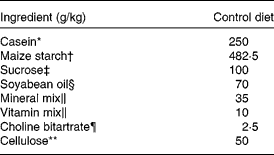
* Acid casein purchased from Murray Goulburn Co-operative Company (Melbourne, Australia).
† Maize starch W purchased from Nihon Shokuhin Kako Company Limited (Tokyo, Japan).
‡ Supplied from Nippon Beet Sugar Manufacturing Company Limited (Obihiro, Japan).
§ Purchased from Ajinomoto Company (Tokyo, Japan)
∥ Mineral mixture and vitamin mixture are identical to AIN-93G-MX and AIN-93-VX, as reported by Reeves et al. (Reference Reeves, Nielsen and Fahey19), respectively. These were purchased from Nihon Nosan Kogyo Company (Tokyo, Japan).
¶ Purchased from Wako Pure Chemical Industries Company (Tokyo, Japan).
** Purchased from Oriental Yeast Company (Tokyo, Japan).
Expt 1
To determine the dose–response relationship between Pec and H2 generation in the large intestine, we examined the changes that occurred in portal H2 concentration in rats fed a diet containing different amounts of Pec. After the acclimatisation period, thirty-six rats were assigned into six groups (n 6) based on body weight and were given a diet supplemented with or without 10, 20, 30, 40 and 50 g of Pec/kg diet for 7 d. The supplement of Pec was done by the replacement of an equal weight of cellulose in the C diet. On the last day of the experiment, portal H2 concentration was measured in rats after anaesthesia (pentobarbital 50 mg/kg body weight) using a hydrogen sensor (H2-100; Unisense A/S, Aarhus, Denmark) placed directly in the portal veins, and a picoampere meter (PA2000; Unisense A/S) was used to measure the current values. The sensor was calibrated using H2 water with H2 concentration in a range of 0–30 μmol/l.
Expt 2
To determine the dose–response relationship between HAS and H2 generation in the large intestine, we examined the changes that occurred in portal H2 concentration in rats fed a diet containing different amounts of HAS. After the acclimatisation period, thirty-six rats were assigned into six groups (n 6) based on body weight and were given a diet supplemented with or without 50, 100, 150, 200 and 300 g of HAS/kg diet for 7 d. The supplement of HAS was done by the replacement of an equal weight of maize starch in the C diet. On the last day of the experiment, portal H2 concentration was measured as in Expt 1.
Expt 3
To determine whether Pec, which enhances H2 production by fermentation, can alleviate hepatic oxidative IR injury, we examined the time course of change in plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities in IR-Pec (IR rats fed a diet containing Pec). After the acclimatisation period, twenty-one rats were assigned into three groups (n 7) based on body weight. Of the three groups, two were administered the C diet and the remaining was administered the 5 % Pec diet for 7 d. On the last day of the experiment, rats from one of the C diet groups and the Pec diet group received IR treatment (mentioned later). The rats from the remaining C diet group were sham operated.
Expt 4
To determine H2 productivity and its alleviating effect on hepatic IR injury with Pec supplementation, H2 productivity, plasma ALT and AST activities, and hepatic glutathione redox status were examined in HG rats fed the Pec diet. After the acclimatisation period, net H2 excretion per 5 min was measured by placing the rats inside a sealed polypropylene chamber for 5 min. GC (Biogas analyser BAS-1000; Mitleben, Osaka, Japan) was used to determine the total H2 excretion from expired air and flatus. A total of thirty-nine rats with net H2 excretion of more than 0·13 μmol/5 min were considered as HG rats and were selected for the experiment. These rats were further assigned into three groups (n 13) based on net H2 excretion and body weight. As in Expt 3, two groups were administered the C diet and the remaining group was administered the 5 % Pec diet for 7 d. On the last day of the experiment, the rats from one of the C diet groups and the Pec diet group underwent IR treatment (mentioned later). Rats from the remaining C diet group were sham operated.
Expt 5
To determine H2 productivity and its alleviating effect on hepatic IR injury with HAS supplementation, H2 productivity, plasma ALT and AST activities, and hepatic glutathione redox status were examined in HG rats fed a diet containing HAS. As in Expt 4, after the acclimatisation period, net H2 excretion/5 min was measured. A total of thirty rats with net H2 excretion of more than 0·14 μmol/5 min were considered as HG rats and were selected for the experiment. These rats were further assigned into four groups (n 7–8) based on net H2 excretion and body weight. Of the four groups, two were administered the C diet and the remaining two were administered the 20 % HAS diet for 7 d. On the last day of the experiment, rats from one of each C and HAS diet groups underwent IR treatment (mentioned later). Rats from the remaining C and HAS diet groups were sham operated.
Ischaemia–reperfusion treatment
Under pentobarbital sodium anaesthesia (70 mg/kg intraperitoneal), a midline laparotomy incision was performed. Next, the hepatic artery and portal vein to the left lateral and median lobe was occluded (70 % of the liver) using a bulldog clamp to interrupt blood supply to the liver for 30 min while allowing blood flow through the remaining sections. The clamps were removed 30 min after ischaemia, and hepatic reperfusion was initiated. The rats were killed at 60 (Expt 3) or 45 min (Expts 4 and 5) after reperfusion for sampling. Sham-operated rats were prepared in a similar manner except without vascular occlusion. During surgery, the abdominal incision site was wrapped in a plastic wrap to prevent tissues from drying out. The rats were placed over an isothermal pad (with a layer of cloth between the animal and pad) to maintain their body temperature at 37°C.
Sampling
In Expt 3, 100 μl of blood from the tail vein of the rats under anaesthesia was collected into heparin tubes at the following times: before ischaemia, 30 min after ischaemia, and 15, 30, 45 and 60 min after reperfusion. In Expts 4 and 5, 1 ml of blood from the portal vein was successively collected into sealed heparin vials and microtubes for H2 analysis and plasma preparation. A 1 ml sample of the gaseous phase was withdrawn using a gas-tight syringe, and H2 concentration was determined with GC (Biogas analyser BAS-1000; Mitleben). The remaining blood sample was separated by centrifugation (1200 g for 20 min at 4°C), and plasma samples were stored at − 80°C until ALT and AST analyses. The liver was perfused immediately after blood withdrawal with 12 ml cold saline at 4°C via the portal vein. Immediately after perfusion, the median lobe (ischaemic area) was removed and a portion was rapidly frozen in liquid N2, and the samples were stored at − 80°C until ALT and AST analyses. The remaining tissue was fixed with 4 % paraformaldehyde in 0·1 mol/l phosphate buffer at pH 7·4 and then embedded in paraffin for histological evaluation.
Assessment of oxidative stress in the liver
Hepatic reduced glutathione (GSH) and oxidised glutathione (GSSG) levels were determined by the method of Anderson(Reference Anderson20). Briefly, 1 volume of liver tissue was homogenised in 9 volumes of 5 % 5-sulfosalicylic acid and centrifuged (10 000 g for 5 min at 4°C). The supernatant was used for the 5,5′-dithiobis(2-nitrobenzoic acid)-glutathione reductase recycling assay(Reference Anderson20) to determine the total glutathione and GSSG concentrations. GSH concentration was calculated from the difference between total glutathione and GSSG. Malondialdehyde concentration in the liver was measured according to the procedure of Ohkawa et al. (Reference Ohkawa, Ohishi and Yagi21). In brief, 1 volume of liver tissue was homogenised in 9 volume of 1·15 % KCl, and the homogenate was used to measure malondialdehyde concentration.
Qualitative and quantitative assessment of liver injury
Plasma ALT and AST activities were measured using a commercial kit, Transaminase CII-test (Wako Pure Chemical Industries, Tokyo, Japan). Haematoxylin–eosin-stained liver sections (4 μm) were used for histological evaluation of liver injury. The liver sections were evaluated for the presence of congestion, cellular degenerative changes, cytoplasmic vacuolisation and leucocyte infiltration.
Statistical analysis
Values obtained from the experiments were expressed as means with their standard errors. Data were subjected to Bartlett's test for homogeneity of variances, and unequal variances were stabilised by log transformation. For samples with equal variances, one-way ANOVA was used, followed by the Tukey–Kramer post hoc test for multiple comparisons between individual group means. If sample variances were still unequal after log transformation, we used the Steel–Dwass test (plasma ALT and AST activities, net H2 excretion and portal H2 concentration). Furthermore, to examine the possible role of caecal H2 in alleviating oxidative stress, we used Student's t test using only IR rat data for plasma ALT and AST activity analyses. The Tukey–Kramer test and Student's t test were performed using SAS JMP software (version 8.0.1; SAS Institute, Tokyo, Japan), and the Steel–Dwass test was performed using KyPlot software (version 5.0; KyensLab, Inc., Tokyo, Japan). Significance was defined as P < 0·05.
Results
Expts 1 and 2
Body weight gain and food intake did not differ among the groups (data not shown). Portal H2 concentration dose-dependently increased with the amount of Pec or HAS supplemented, and reached a plateau at 2 % Pec and a peak at 20 % HAS (Fig. 1).

Fig. 1 (A) Dose–response of pectin (Pec) and (B) high-amylose maize starch (HAS) for portal H2 concentration in rats. Values are means, with their standard errors represented by vertical bars, (n 6). a,b Mean values with unlike letters were significantly different (P < 0·05). C, control.
Expt 3
Body weight gain and food intake did not differ among the groups (data not shown). Both plasma ALT and AST activities in the IR-C group (IR rats fed the C diet) increased with time after reperfusion and was six-fold higher after 60 min (Fig. 2). Values remained at low levels at all times in sham-operated rats. For the IR-Pec group, values increased by 50 % compared with the IR-C group. Among the IR rat groups, plasma ALT activity after 15 min and plasma AST activity after 30 min of reperfusion were significantly lower in the IR-Pec group than in the IR-C group. Liver histology (Fig. 3) revealed hepatic sinusoids that had a normal appearance in the sham group, while a degree of occlusion was observed in the IR-C group. Samples from the IR-Pec group were similar to those in the sham group.

Fig. 2 (A) Time course of plasma alanine aminotransferase (ALT) and (B) aspartate aminotransferase (AST) activities in ischaemia–reperfusion (IR) and sham-operated rats fed the control (C) and pectin (Pec) diets. Values are means, with their standard errors represented by vertical bars, (n 6 or 7). a,b Mean values with unlike letters were significantly different (P < 0·05). Sham-C, sham-operated rats fed the C diet; IR-C, IR rats fed the C diet; IR-Pec, IR rats fed the 5 % Pec diet.

Fig. 3 Protective effect of pectin (Pec) on hepatic injury in ischaemia–reperfusion (IR) rats. Representative liver sections on sinusoids (original magnification × 100 and × 200; bars indicate 200 and 50 μm, respectively) from sham-operated rats fed the control (C) diet (Sham-C), IR rats fed the C diet (IR-C) and IR rats fed the 5 % Pec diet (IR-Pec) rats are illustrated. The rats were subjected to 30 min of ischaemia followed by 60 min of reperfusion. After reperfusion, the livers were perfused with 12 ml cold saline to remove blood and fixed with 4 % paraformaldehyde in 0·1 mol/l phosphate buffer at pH 7·4.
Expt 4
Body weight gain and food intake did not differ among the groups (data not shown). Net H2 excretion per 5 min and portal H2 concentration in the IR-Pec group were 20- and 3·5-fold higher than those in the IR-C group, respectively (Table 2). Plasma ALT and AST activities in the IR-C group were significantly higher than those in the sham group. Among the IR rat groups, plasma ALT activity in the IR-Pec group was significantly lower than that in the IR-C group. Furthermore, compared with the IR-C group, liver GSSG concentration did not differ in the IR-Pec group (Table 2). However, total glutathione and GSH concentrations and the GSH:GSSG ratio in the IR-Pec group were significantly higher than those in the IR-C group. Liver malondialdehyde concentration did not differ among the groups.
Table 2 Effect of pectin (Pec) on net H2 excretion, portal H2 concentration and plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities in ischaemia–reperfusion (IR)-treated and sham-operated rats†
(Mean values with their standard errors, n 13)

Sham-C, sham-operated rats fed the control (C) diet; IR-C, IR rats fed the C diet; IR-Pec, IR rats fed the 5 % Pec diet; GSH, reduced glutathione; GSSG, oxidised glutathione; MDA, malondialdehyde.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Mean values were significantly different from those of IR-C groups (P < 0·05).
† Data were analysed with one-way ANOVA and the Tukey–Kramer post hoc test, or non-parametric multiple test (Steel–Dwass test). High H2-generating rats, which we selected, were used.
‡ After 30 min of ischaemia, 45 min of reperfusion was performed in IR rats.
§ Values of plasma ALT activities in both IR groups were analysed by Student's t test as variances were too different between sham-operated and IR-treated groups.
Expt 5
Body weight gain and food intake did not differ among the groups (data not shown). Net H2 excretion per 5 min and portal H2 concentration in the IR-HAS group (IR rats fed the HAS diet) were twenty-four- and thirteen-fold higher than those in the IR-C group, respectively (Table 3). Plasma ALT and AST activities were significantly higher in the IR-C group compared with the sham group. Within the IR groups, plasma ALT and AST activities in the IR-HAS group showed tendencies to decrease compared with the IR-C group. Moreover, liver GSSG concentration in IR rats was significantly higher than that in sham rats (Table 3). Regardless of whether the IR treatment was performed, rats fed the HAS diet had significantly decreased liver GSSG concentration and GSSG:total glutathione ratio compared with the C diet groups, but significantly increased GSH:GSSG and GSH:total glutathione ratios. Liver malondialdehyde concentration did not differ among the groups.
Table 3 Effect of high amylose maize starch on net H2 excretion, portal H2 concentration, plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities, and hepatic glutathione status in ischaemia–reperfusion (IR)-treated and sham-operated rats†
(Mean values with their standard errors, n 7, 8)

Sham-C, sham-operated rats fed the control (C) diet; sham-HAS, sham-operated rats fed the high amylose maize starch (HAS) diet; IR-C, IR rats fed the C diet; IR-HAS, IR rats fed the HAS diet; GSH, reduced glutathione; GSSG, oxidised glutathione; MDA, malondialdehyde.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Mean values were significantly different from those of IR-C groups (P < 0·05).
† Data were analysed with two-way ANOVA and the Tukey–Kramer post hoc test, or non-parametric multiple test (Steel–Dwass test). High H2-generating rats, which we selected, were used.
‡ After 30 min of ischaemia, 45 min of reperfusion was performed in IR rats.
§ Values of plasma AST activities in both IR groups was analysed by Student's t test as variances were too different between sham-operated and IR-treated groups.
Discussion
IR injury in tissues is associated with ROS production and reactive nitrogen species, especially hydroxyl radical and peroxynitrite(Reference Cuzzocrea, Riley and Caputi22). Hydroxyl radicals have a high redox potential and are capable of oxidising tissue components such as lipids, proteins and DNA. Ohsawa et al. (Reference Ohsawa, Ishikawa and Takahashi11) have recently shown that an inhalation of 2–4 % H2 gas induces selective removal of hydroxyl radicals and peroxynitrite from the brain of the IR rats, and consequently reduces cerebral infarction. They were the first to show the ability of H2 to suppress oxidation in vivo. Studies demonstrating the antioxidative effect of inhaled H2 in organs such as liver(Reference Fukuda, Asoh and Ishikawa13), retina(Reference Oharazawa, Igarashi and Yokota14), heart(Reference Hayashida, Sano and Ohsawa12) and intestine(Reference Buchholz, Kaczorowski and Sugimoto15) followed the research of Ohsawa et al. Furthermore, a similar effect was shown for the myocardium and the intestine(Reference Mao, Zheng and Cai23, Reference Sun, Kang and Cai24) via the administration of H2-rich saline. In these studies, blood H2 concentration in H2-administered rats and mice reached 5–10 μmol/l in vivo. There is a high possibility that H2 may exhibit a role in alleviating oxidative stress.
In the present experiments, we observed high portal H2 concentrations (4·5–11 μmol/l) in rats fed an IR-Pec or IR-HAS diet. These concentrations are comparable with those that were shown to relieve oxidative stress in rats inhaling H2 gas(Reference Ohsawa, Ishikawa and Takahashi11). To our knowledge, few studies have directly examined portal H2 concentrations in animals fed dietary fibre and RS. Pec and HAS feedings result in the delivery of a large amount of carbohydrate to the large intestine, which then potentiates H2 production. As Levitt(Reference Levitt25) reported, 14 % of H2 generated in the large intestine readily diffuses into the portal circulation. Because the liver is the first organ to receive H2 via the portal vein, it is more likely to be exposed to a higher H2 concentration than the other organs, and hence, one might expect to be reduced. As shown in Tables 2 and 3, and Fig. 3, we found that hepatic injuries in the IR-Pec or IR-HAS groups tended to be lighter than those in the IR-C group. Statistical analysis of the IR rats alone showed that enzymatic activities were significantly reduced in HAS- and Pec-fed groups. This suggests that in addition to the H2 gas inhaled or consumed with water, H2 generated by fermentation in the large intestine plays some part in alleviating hepatic IR injury. Similar results have been reproducibly observed in IR rats (see Fig. S1 of the supplementary material, available online http://www.journals.cambridge.org/bjn). In 1988, Neale(Reference Neale18) proposed a similar hypothesis that H2 generated during large-bowel fermentation of dietary fibres may serve as an antioxidant, although, as far as we know, his hypothesis has never been proved. Kajiya et al. (Reference Kajiya, Sato and Silva26) reported that suppression of the intestinal microbiota by antibiotics increased concanavalin A-induced liver damage, while administration of a H2-producing Escherichia coli reduced liver inflammation in mice. However, it is unclear whether this reduction is caused by H2, because changes in the intestinal H2 concentration and production were not measured. Moreover, they gave no dietary fibre sources and fermentable substrates.
We estimated the human portal H2 concentration using the breath H2 excretion curve in participants fed 20 g of lactose in a study by Savaiano et al. (Reference Savaiano, AbouElAnouar and Smith10) and also general physiological data such as minute ventilation, 8–9 litres/min; systolic output, 5 litres/min; and portal blood flow (about 28 % of systolic output), 80 litres/h(Reference Flindt27). As a result, the average human portal H2 concentration of a subject fed 20 g of lactose was estimated to be 6·4–7·2 μmol/l. Although the speed of fermentation is presumed to affect this value, our estimation is comparable with portal H2 concentrations in the IR-HAS group and also with those of rodents that inhaled H2 gas(Reference Ohsawa, Ishikawa and Takahashi11) or were orally administrated H2 water(Reference Nakashima-Kamimura, Mori and Ohsawa28, Reference Nagata, Nakashima-Kamimura and Mikami29), for which an antioxidative effect of H2 was observed. We presume from this evidence that H2 produced in the large intestine can also exhibit an antioxidative effect in the liver and play a role in controlling oxidative stress in human subjects. In the present experiments, portal H2 concentrations obtained in the IR-Pec or IR-HAS groups ranged from 4·5 to 11 μmol/l. This suggests that H2 concentrations in the portal vein depend greatly on the quantity and quality of fermentation substrates supplied. Furthermore, the solubility of H2 in water is about 700 μmol/l (37°C, 1 atm). Aortic blood, which is supplied continuously to the large intestine, is low in H2 and thus could conceivably diffuse to portal the large amounts of H2 produced by fermentation.
The maximum portal H2 concentration was observed in rats fed a 5 % Pec and a 20 % HAS diet (Expts 1 and 2, respectively). Digestibility of HAS used in the present study was about 40 %, so that the dietary level of RS is roughly 12 %. Since more fermentation substrates were supplied to the large intestine in rats fed the 20 % HAS diet than those fed the 5 % Pec diet, a higher portal H2 concentration was observed. Also, an appropriate carbohydrate:N ratio is required to promote fermentation by bacteria(Reference Macfarlane and Macfarlane30). Therefore, H2 does not increase simply proportionally to the amount of fermentation substrate entered into the caecum as observed in rats fed a 30 % HAS diet. On the other hand, fermentation is affected not only by substrates but also by the intestinal microbiota. Since the microbiota of laboratory animals differ among breeders and breeding colonies(Reference Ohashi, Hiraguchi and Ushida31), their fermentation patterns may vary even with the administration of the same fermentation substrate. During our preliminary experiments, we came across rats whose portal H2 concentration increased only slightly, even after the administration of Pec and HAS. These rats were assumed to be low H2 generators. With these rats, we could not obtain clear H2 effects on hepatic injury (data not shown). In Expts 4 and 5, we preferentially selected HG rats. These HG rats were better able to show the suppressive ability of generated H2 on hepatic IR injury due to the production of sufficient amounts of H2. However, in our studies, portal H2 concentrations in Expts 4 and 5 were lower than in Expts 1 and 2; this discrepancy is due to the different apparatus applied.
Glutathione, an antioxidant, helps protect cells against ROS such as free radicals and peroxides(Reference Pompella, Visvikis and Paolicchi32), and hepatic IR treatment decreases GSH and increases hepatic GSSG concentrations in the liver, resulting in high oxidative stress(Reference Hasegawa, Malle and Farhood33). In the present study, we observed statistically higher GSSG:total glutathione ratios and lower GSH:total glutathione ratios in IR-C rat livers. In IR-Pec and IR-HAS livers, these ratios turned out to be similar to sham groups. Because the condition (short reperfusion) of hepatic IR in the present study is milder than that in many studies, the difference in glutathione level between the control and test groups, although statistically significant, appears relatively small(Reference Fukuda, Asoh and Ishikawa13, Reference Hasegawa, Malle and Farhood33, Reference Acquaviva, Lanteri and Li Destri34). Although it remains unclear whether the changes in these ratios have biological significance, a higher level of GSH helps, in part, to alleviate oxidative stress. Therefore, even if the differences are small, the long-term accumulated effect is large. Because the redox potential of H2 is approximately twice as low as that of GSH(Reference Neale18), it would take precedence over GSH in the reduction of ROS, if sufficient quantities are available.
Inhalation of H2 gas and administration of H2 water are tools to introduce H2 into the body; however, the former requires an adequate hospital and costly medical facilities, and the latter requires the preservation of highly concentrated H2 in its stable state for a long time, which is difficult from a packaging point of view. Furthermore, it is not easy to supply a large amount of highly concentrated H2 into our bodies in a continuous manner using these tools in everyday situations. On the other hand, H2 production via large-bowel fermentation is a much more continuous means of supplying H2 in vivo. As expired air and flatus are the only excretion routes for H2 produced in the large intestine, this may limit the organs in which H2 is capable of exerting an antioxidative effect to the intestine and the liver. The products of fermentation are various, therefore further investigation is required to evaluate the unique role of H2 derived from dietary fibre and RS.
We found in the present study that portal H2 concentration increased by Pec or HAS administration and hepatic IR injuries were suppressed. This may be due to the antioxidative effect of H2. This would represent further insight into the biological functions of dietary fibre and RS. The onset and progression of many diseases are attributed to oxidative stress, and these dietary components are believed to provide therapeutic and preventive effects against common diseases. Establishing better conditions for colonic fermentation by the selection of fermentation substrates, appropriate microbiota, eating habits, etc., will give us more means to protect against and cure various oxidative disorders.
Acknowledgements
The present study was supported in part by a Grant-in-Aid for Scientific Research (C) (21500781) from Japan Society for the Promotion of Science, Japan. We thank Crimson Interactive Private Limited (Ulatus, Mumbai, India) for their assistance in the translation and editing of the manuscript. Also, we are grateful to Martin Meadows, for checking the English. N. N. and S. K. conducted the study, analysed the dataset and wrote the manuscript. N. N., H. T., Y. S., Y. M., M. O., S. Y. and M. A. conducted the biological analysis. M. O. and T. Y. performed the histological analysis. All authors were involved in designing the study, reviewing and interpreting the results, and drafting the manuscript. The authors have no conflict of interest associated with the present study.








