The question of whether people with schizophrenia are at greater risk of developing type 2 diabetes than the general population has been puzzling researchers and clinicians since the early 1900s – long before the introduction of antipsychotic medications. As far back as 1897, the eminent Victorian psychiatrist Henry Maudsley (1835–1918) noted, ‘Diabetes is a disease which often shows itself in families in which insanity prevails’ (Reference MaudsleyMaudsley, 1979). More than a century later, and after increasingly sophisticated research into the issue, Maudsley's intuitive observations about the association between severe psychiatric disorders and diabetes still appear to hold true.
Although early naturalistic approaches to the study of diabetes in patients with severe mental illness provided clues that an association did exist, it has been the more recent cross-sectional epidemiological studies assessing the prevalence of diabetes in populations of people with schizophrenia that have established beyond reasonable doubt that such people are at increased risk of developing diabetes, and that schizophrenia should now be considered to be an independent risk factor for the condition.
METHOD
Review of current literature.
Confirming the association: research challenges
Studies in the pre-neuroleptic era clearly suggested that schizophrenia might be a risk factor for the development of diabetes (Reference KooyKooy, 1919; Reference LorenzLorenz, 1922; Reference Henry and ManganHenry & Mangan, 1925; Reference Freeman, Rodnick and ShakowFreeman et al, 1944; Reference Braceland, Meduna and VaichulisBraceland et al, 1945; Reference FreemanFreeman, 1946). However, many different criteria were used to define both schizophrenia and diabetes mellitus in these studies, and the ‘pre-diabetic’ state of impaired glucose tolerance has only relatively recently been defined (World Health Organization, 1999). Today, standardisation of criteria for the diagnosis of diabetes, impaired glucose tolerance and schizophrenia make accurate and reproducible diagnoses possible, and allow epidemiological comparisons to be made across populations.
In order to establish definitively an association between schizophrenia and diabetes, it would be necessary for researchers to show that the prevalence of diabetes is higher among people with schizophrenia than in reference populations using modern research techniques and standardised disease definitions. Unfortunately, establishing the true prevalence of impaired glucose tolerance and/or diabetes in any population is fraught with difficulty. Diabetes has a gradual and insidious onset and is frequently asymptomatic, thereby explaining in part why up to half of all individuals with diabetes are thought to remain undiagnosed in the community (Reference Harris, Hadden and KnowlerHarris et al, 1987; Reference Mykkänen, Laasko and UusitupaMykkänen et al, 1990; Reference HarrisHarris, 1993).
Prospective population-based studies in which there is universal screening for impaired glucose tolerance and diabetes using recommended blood glucose testing methods and established diagnostic criteria (World Health Organization, 1999) provide the best estimates of diabetes prevalence in the general population. Unfortunately, few such studies have ever been conducted, and because of the marked differences in diabetes rates between countries, social classes and age groups, it is difficult to extrapolate their estimates to other populations. Establishing the true prevalence of impaired glucose tolerance and diabetes in patients with schizophrenia is even more challenging. Difficulties with the identification and diagnosis of patients with diabetes are further compounded by inconsistencies in the diagnosis of schizophrenia. Some diabetes prevalence studies such as that by Cohen et al (Reference Cohen, Puite and Dekker2003) have evaluated heterogeneous populations that have included individuals with schizoaffective disorder, bipolar disorder and personality disorders, as well as those with schizophrenia, and although these studies provide much valuable information, they must be interpreted with care, since diabetes prevalence estimates do differ between these psychiatric conditions (Reference Regenold, Thapar and MaranoRegenold et al, 2002).
Patients with schizophrenia are often reluctant to give consent to participate in epidemiological studies – especially ones that require blood samples to be taken (Reference Cohen, Puite and DekkerCohen et al, 2003). This may result in prevalence estimates that are based on a self-selecting population and are therefore not necessarily an accurate reflection on the true picture. Even when patients consent to participate in diabetes prevalence studies, many investigators struggle to successfully complete oral glucose tolerance testing (Reference Hagg, Joelsson and MjorndalHägg et al, 1998), or even to obtain fasting blood glucose levels in their patients. This can lead to high study drop-out rates, or the use of less reliable indicators of glucose tolerance such as non-fasting blood glucose levels (Reference Mukherjee, Decina and BocolaMukherjee et al, 1996; Reference Cohen, Puite and DekkerCohen et al, 2003). Clearly, failure to perform fasting blood glucose tests could lead to an overestimate of diabetes prevalence.
Finally, in order to test the hypothesis that people with schizophrenia are at greater risk of developing diabetes than the general population, it is important that comparator populations are well matched for geographical region, age, gender, ethnicity and body mass index, and that as many other potential confounding factors are controlled for. Ideally, neither population should be taking medications that might alter the natural course of diabetes.
RESULTS
Prevalence of diabetes in the general population
Notwithstanding the methodological challenges outlined above, it is now clear that the prevalence of diabetes in the general population is high and increasing year by year (Reference Mokdad, Ford and BowmanMokdad et al, 2001). Studies in the USA have found rates of known diabetes in the general population of between 1.2% (for people aged 18–44 years) and 6.3% (for people aged 45–64 years) (Reference Adams and MaranoAdams & Marano, 1995), with the highest prevalence rates reported in African Americans, Native Americans and Mexican Americans (Reference Adams and MaranoAdams & Marano, 1995; Reference HarrisHarris, 1998).
The Isle of Ely Diabetes Project in the UK, which was a prospective population-based study, evaluated over a thousand individuals aged 40–65 years who were not known to have diabetes (Reference Williams, Wareham and BrownWilliams et al 1995). Individuals underwent a standard 75 g oral glucose tolerance test (OGTT) and were classified according to World Health Organization (WHO) criteria. This study found that 4.5% of participants had undiagnosed diabetes and 16.7% had impaired glucose tolerance (Reference Williams, Wareham and BrownWilliams et al, 1995). A similar large, population-based screening study in Australia (Reference Dunstan, Zimmet and WelbornDunstan et al, 2002) found an overall prevalence of diabetes of 7.4%, and identified an additional 16.4% of the population with impaired glucose tolerance or impaired fasting blood glucose, according to WHO criteria.
Studies in Japan (Reference Tabata, Kikuoka and KikuokaTabata et al, 1987) and Singapore (Singapore Ministry of Health, 1998) show prevalence rates of diabetes to be around 5% and 9%, respectively, and confirm that twice as many people in the general population may have impaired glucose tolerance as have diabetes.
Prevalence of diabetes in schizophrenia
Studies assessing the prevalence of type 2 diabetes in people with schizophrenia broadly agree that the condition may be at least two to four times more prevalent than in the general population (Reference Keskiner, El Toumi and BousquetKeskiner et al, 1973; Reference Mukherjee, Decina and BocolaMukherjee et al, 1996; Reference Dixon, Weiden and DelahantyDixon et al, 2000; Reference Sernyak, Leslie and AlarconSernyak et al, 2002; Reference Lindenmayer, Czobor and VolavkaLindenmayer et al, 2003; Reference Subramaniam, Chong and PekSubramaniam et al, 2003). There is, however, significant variability in the reported prevalence rates from different studies. One major confounder when attempting to establish a true prevalence figure in schizophrenia and other populations is the number of people who have been actively screened. Citrome et al (Reference Citrome, Jaffe and Levine2003) have recently reported that, in a retrospective epidemiological study evaluating the incidence of newly diagnosed diabetes mellitus in patients commencing antipsychotic treatment, some of the apparent differences in risk between individual agents could be attributed to the greater degree of glucose monitoring in patients receiving clozapine compared with those receiving other antipsychotics.
Another important factor that might influence prevalence figures reported in schizophrenia studies is the design of the study. Some studies require glucose screening (and possible exclusion for glycaemic abnormalities) prior to patient enrolment; others are cross-sectional in nature. In the latter case, all known confounders (such as population age, ethnicity and geography) must be accounted for. Even the year of the study and the type of schizophrenia diagnosed in the patients enrolled may affect prevalence estimates. In 2003, for example, improved awareness of the possibility of blood glucose abnormalities meant that more patients taking antipsychotic medications might have been screened for diabetes, which might account for the apparently higher prevalence rates being reported compared with 10 years ago.
Patients with treatment-resistant schizophrenia may be more likely to be screened for glucose abnormalities than other people with schizophrenia, and those that showed positive would probably be excluded from prevalence trials, a fact illustrated clearly by Lindenmayer et al (Reference Lindenmayer, Czobor and Volavka2003), who found a prevalence of diabetes mellitus of only 7% in their cohort of patients with treatment-resistant schizophrenia prior to study entry.
Mukherjee et al (Reference Mukherjee, Decina and Bocola1996) found an overall prevalence of known diabetes of 15.8% in a cohort of patients with schizophrenia admitted to a long-term care facility in Italy; diabetes was significantly more common in patients not receiving antipsychotics than in those who were. This overall prevalence rate is substantially higher than the known prevalence of diabetes in the general population of Italy, which is estimated to be 2–3% (Reference Verrillo, De Teresa and La RoccaVerrillo et al, 1985; Reference Bruno, Barbero and VuoloBruno et al, 1992). The prevalence of known diabetes in Mukherjee's patient cohort increased with age, from 0% in individuals younger than 50 years, to 12.9% in the 50–59 years age group, and to 18.9% in the 60–69 years age group (Reference Mukherjee, Decina and BocolaMukherjee et al, 1996). The prevalence of diabetes decreased to 16.7% in patients aged 70–74 years and to 9.4% in those aged over 75 years, which has been interpreted by the investigators to indicate selective mortality due to coronary heart disease (Reference Mukherjee, Decina and BocolaMukherjee et al, 1996).
Dixon et al (Reference Dixon, Weiden and Delahanty2000) used a large national database of patients receiving treatment for schizophrenia compiled by the Schizophrenia Patient Outcomes Research Team (PORT) in the USA to assess the prevalence and clinical correlates of known diabetes in this population. Much of the data were collected before the widespread use of novel antipsychotic medications. The lifetime prevalence of known diabetes reported for the people with schizophrenia in this study was 15%, with increasing age, being female and being of African American or ‘other’ non-White racial origin increasing the likelihood of having diabetes (Reference Dixon, Weiden and DelahantyDixon et al, 2000). The 15% prevalence of diabetes found in this study far exceeded rates typically reported in age-matched general populations, but this probably reflects the exhaustive interviewing techniques used in the study.
Sernyak et al (Reference Sernyak, Leslie and Alarcon2002) reported an overall prevalence of diagnosed diabetes of 18% in a large sample of people with schizophrenia being treated as out-patients with typical and atypical antipsychotic drugs. Prevalence increased with age, with the highest prevalence (>25%) being seen in patients aged 60–69 years. Comparing the prevalence of known diabetes across age groups, no difference was found between patients who received an atypical antipsychotic and patients who received a typical antipsychotic (Reference Sernyak, Leslie and AlarconSernyak et al, 2002).
Subramaniam et al (Reference Subramaniam, Chong and Pek2003) conducted a chart review of 607 people with schizophrenia resident in a long-stay ward in Singapore. None of the patients had received atypical antipsychotic medication either in the past or at the time of the study. A prevalence of known diabetes of 4.9% was reported, and patients with this diagnosis were excluded from further study. Informed consent was then obtained to screen 194 of the remaining patients, and 16% of these were found to have diabetes, taking the total diabetes prevalence rate to around 21%. Prevalence rates were found to be double those seen in the general population of Singapore in patients under the age of 60 years (Table 1), but lower than the general population in those aged 60 years and above. In this study, the highest rate of diabetes (50%) was found in patients aged 50–59 years (Reference Subramaniam, Chong and PekSubramaniam et al, 2003). Gupta et al (Reference Gupta, Steinmeyer and Frank2003) reported a prevalence rate of 17% for diabetes in a chart review of 208 patients with psychotic disorders who were receiving antipsychotic medication. Fasting blood glucose levels were used to establish the diagnosis of diabetes in this study (Reference Gupta, Steinmeyer and FrankGupta et al, 2003).
Table 1 Age-specific prevalence rates of diabetes mellitus in a group of people with schizophrenia compared with the general population in Singapore
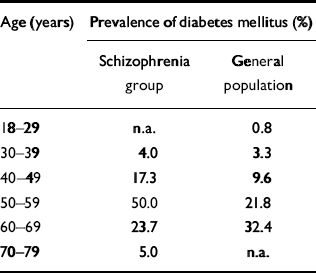
| Age (years) | Prevalence of diabetes mellitus (%) | |
|---|---|---|
| Schizophrenia group | General population | |
| 18-29 | n.a. | 0.8 |
| 30-39 | 4.0 | 3.3 |
| 40-49 | 17.3 | 9.6 |
| 50-59 | 50.0 | 21.8 |
| 60-69 | 23.7 | 32.4 |
| 70-79 | 5.0 | n.a. |
Subramaniam et al (Reference Subramaniam, Chong and Pek2003) report the prevalence of impaired glucose tolerance in people with schizophrenia is less well defined, but screening studies suggest that it may be almost twice as high as the prevalence of diabetes. In their screening study, the overall prevalence of diabetes was found to be 21% but that of impaired glucose tolerance was 31%. The study has also highlighted the substantial problem of underrecognition of diabetes and impaired glucose tolerance in people with schizophrenia. Although 21% of patients were identified as having diabetes once they were actively screened, only 4.9% of patients had previously received a diagnosis (Reference Subramaniam, Chong and PekSubramaniam et al, 2003). Another study (Reference Cohn, Wolever and ZipurskyCohn et al, 2002) found that active screening for impaired glucose tolerance and diabetes in another cohort of 153 patients who had been taking antipsychotic monotherapy for at least 3 months found that 31% of individuals had either diabetes or impaired glucose tolerance, and that in the majority (67%) the condition was previously unidentified (Reference Cohn, Wolever and ZipurskyCohn et al, 2002). Taylor also describes similar findings in 607 patients with schizophrenia at the Maudsley Hospital in London (Reference Taylor, Young and MahomedTaylor et al, 2003; D. Taylor, personal communication, 2003). An apparent prevalence rate of glycaemic abnormalities (diabetes and impaired glucose tolerance) of 8.6% rose to 19.4% after prospective blood glucose testing.
Ryan and colleagues reported results from a landmark study involving young (mean age 33.6 years) patients with first-episode schizophrenia. All patients were drug-naïve, allowing a separation of the effects of the disease from the potential confounding effects of any antipsychotic medication. Despite being young and never having been exposed to neuroleptics, more than 15% of the individuals in this study had already developed impaired fasting glucose tolerance, compared with none of the matched healthy control groups (Reference Ryan, Collins and ThakoreRyan et al, 2003). This provides some of the clearest evidence that schizophrenia itself may be an independent risk factor for impaired glucose tolerance, which is a known risk factor for the development of type 2 diabetes (Reference AlbertiAlberti, 1996). Ryan et al (Reference Ryan, Collins and Thakore2003) contrast the prevalence of impaired glucose tolerance found in their study with that recently reported from a general population study in three regions of France (Reference Gourdy, Ruidavets and FerrieresGourdy et al, 2001). The mean age of the Ryan cohort was 33 years, and the age-adjusted (35–64 years) prevalence of impaired glucose tolerance found in the French study was 11.8% in men and 5.2% in women. The prevalence of impaired glucose tolerance in the population with schizophrenia was therefore found to be twice that in a general population (Reference Ryan, Collins and ThakoreRyan et al, 2003).
Prevalence of diabetes in other psychiatric conditions
Schizophrenia is not the only severe mental illness associated with an apparent increase in the risk of diabetes. Regenold et al (Reference Regenold, Thapar and Marano2002) assessed the medical records of 243 in-patients, aged 50–74 years, with a diagnosis of major depression, bipolar I disorder, schizoaffective disorder, schizophrenia or dementia, and compared their rates of diabetes with rates in the US general population matched for age, gender and race. The prevalence of diabetes was found to be significantly higher than national norms in patients with bipolar I disorder (26%) and schizoaffective disorder (50%), and was independent of psychotropic drug use (Reference Regenold, Thapar and MaranoRegenold et al, 2002). This study, and the work of others (Reference Cassidy, Ahearn and CarrollCassidy et al, 1999), indicate that the prevalence of diabetes in patients with bipolar disorder may be two to three times higher than that found in the general population.
Schizophrenia: an independent risk factor for diabetes?
The interaction between diabetes and schizophrenia, although relatively well established, is far from simple. The mechanisms behind the interaction are likely to be multifactorial, and to include genetic and environmental factors, as well as the possible effects of antipsychotic medications.
Genetic factors
Genetic factors appear to have a key role in the association between schizophrenia and diabetes, since it has been reported that up to 50% of individuals with schizophrenia have a family history of type 2 diabetes, compared with just 4.6% of healthy adult controls (Reference DynesDynes, 1969; Reference Mukherjee, Schnur and ReddyMukherjee et al 1989; Reference Cheta, Dumitrescu and GeorgescuCheta et al, 1990; Reference Lamberti, Crilly and MaharajLamberti et al, 2003; Reference Shiloah, Witz and AbramovichShiloah et al, 2003). In one of the largest chart reviews ever conducted of schizophrenia, Lamberti et al (Reference Lamberti, Crilly and Maharaj2003) found a family history of type 2 diabetes in 17% of the total cohort of 436 patients. Importantly, in the cohort of patients who had a positive family history of diabetes, the prevalence of diabetes mellitus was 33%. In those with a negative family history of diabetes, its prevalence was just 10%. These data-sets suggest that genetic factors may explain to some extent the higher diabetes prevalence figures found in patients with schizophrenia compared with the general population.
Environmental factors
Many people with schizophrenia exhibit poor health behaviours that may also contribute to them developing diabetes; these include having less healthy diets (generally high in fat and low in fibre), exercising less and smoking more than normal controls (Reference Brown, Birtwistle and RoeBrown et al, 1999). Poverty, unstable living conditions and lower than expected educational attainment are all associated with schizophrenia, and increase the risk of obesity and other adverse medical sequelae (Reference Dixon, Weiden and DelahantyDixon et al, 2000). Additional factors thought to predispose individuals with schizophrenia to developing diabetes include ethnicity, a history of glucose dysregulation, and pre-existing hypertension (Reference Lindenmayer, Nathan and SmithLindenmayer et al, 2001).
The role of antipsychotic medications in the development of diabetes is far from well defined. Evidence from studies published before the introduction of neuroleptic drugs showed a strong association between severe mental illness and abnormal glucose metabolism (Reference KooyKooy, 1919; Reference LorenzLorenz, 1922; Reference Henry and ManganHenry & Mangan, 1925; Reference Freeman, Rodnick and ShakowFreeman et al, 1944; Reference FreemanFreeman, 1946; Reference Braceland, Meduna and VaichulisBraceland et al, 1945), and although this evidence must be interpreted with care because of the definitions used in the studies, it suggests that schizophrenia itself might be an independent risk factor for the development of diabetes. Reports of impaired glucose tolerance in young, drug-naïve individuals with first-episode schizophrenia (Reference Ryan, Collins and ThakoreRyan et al, 2003) add further weight to the argument that people with schizophrenia may be naturally predisposed to developing diabetes, and should therefore be considered to be a high-risk group.
DISCUSSION
People with schizophrenia and other serious psychiatric conditions appear to be at significant risk of developing impaired glucose tolerance and diabetes, regardless of whether they are receiving antipsychotic medication. Many of these individuals at risk are currently unrecognised. Cardiovascular risk factors such as diabetes, hypertension and dyslipidaemia commonly coexist (Reference Mukherjee, Decina and BocolaMukherjee et al, 1996), so it seems likely that these ‘missing millions’ of people with impaired glucose tolerance and diabetes may also have comorbidities that further increase their risk of cardiovascular disease and premature death (Reference Subramaniam, Chong and PekSubramaniam et al, 2003).
Natural causes are responsible for two-thirds of the excess mortality associated with schizophrenia (Reference Brown, Barraclough and InskipBrown et al, 2000). In one record-linkage study, ischaemic heart disease was found to be the most common cause of death in both men and women with schizophrenia (Reference Herman, Baldwin and ChristieHerman et al, 1983).
Age, ethnic origin and geographical location all appear to alter the prevalence of diabetes mellitus and impaired glucose tolerance. Early identification of these conditions in high-risk groups is now a priority in many countries. Diabetes UK currently recommends routine screening for diabetes in White people over the age of 40 years and in people from Black, Asian and minority ethnic groups aged over 25 years if they have a first-degree family history of diabetes, and/or are overweight and have a sedentary lifestyle, and/or have cardiovascular disease (Diabetes UK, 2002).
The strength of the association between schizophrenia and diabetes is such that timely screening and effective management of diabetes risk factors in all our patients is now recommended. Early detection of impaired glucose metabolism and effective education about healthy living should help to reduce the risk of patients developing diabetes and its complications, and may ultimately help to improve long-term outcomes.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Individuals with schizophrenia and other severe psychiatric conditions are at greater risk of developing diabetes mellitus and impaired glucose tolerance than the general population.
-
▪ Approximately 15% of patients with schizophrenia may have diabetes, and a similar percentage may have impaired glucose tolerance. Increasing age is associated with increased risk.
-
▪ Routine screening and the development of pragmatic pathways for diabetes risk management in all patients with schizophrenia are recommended.
LIMITATIONS
-
▪ The true prevalence of diabetes and impaired glucose tolerance in all populations may still be grossly underestimated because of the silent nature of the disease.
-
▪ The association between schizophrenia and diabetes has been established on the basis primarily of early case reports and evidence from retrospective studies.
-
▪ Only large-scale, prospective, longitudinal studies in which all potential confounders are controlled for will establish the precise nature of the association.




eLetters
No eLetters have been published for this article.