Dietary risks, including high Na intake and low consumption of fruits, vegetables and whole grains, have been identified among the most important risk factors for the global burden of disease( Reference Forouzanfar, Alexander and Anderson 1 ). Thus, higher adherence to a healthy diet has the potential to prevent disease and prolong life span and is emphasised in current dietary guidelines( 2 , 3 ). Healthy dietary patterns comprise different food combinations with common core foods influenced by regional food culture( Reference Jacobs and Tapsell 4 , Reference Koch, Nothlings and Lieb 5 ). Diet scores or empirically derived food patterns are used to study healthy dietary patterns in a population, both with strengths and weaknesses( Reference Wirfalt, Drake and Wallstrom 6 ). Diet scores are often influenced by official dietary recommendations – for example, the Diet Quality Index and the Healthy Eating Index( Reference Moeller, Reedy and Millen 7 ) – but also the regional context( Reference Waijers, Feskens and Ocké 8 ). The Mediterranean diet is the most investigated healthy dietary pattern, and a high adherence to such a diet has been linked to less chronic metabolic disease, longer survival and favourable cardiometabolic risk markers in observational studies( Reference Anderson and Nieman 9 , Reference Martinez-Gonzalez, Salas-Salvado and Estruch 10 ). The Lyon diet heart (secondary prevention)( Reference de Lorgeril, Salen and Martin 11 ) and the Prevencion con Dieta Mediterranea (PREDIMED) (primary prevention)( Reference Estruch, Ros and Salas-Salvado 12 ) trials have also showed beneficial effects of a Mediterranean diet on cardiovascular end points. A recent systematic review and meta-analysis on clinical trials supported favourable effects of the Mediterranean diet on cardiovascular outcomes, although the investigators raised a concern that the ‘quantity and quality of the available evidence is relatively limited’( Reference Liyanage, Ninomiya and Wang 13 ). Many observational studies investigating Mediterranean diet scores and disease risk have been conducted in non-Mediterranean countries with other food cultures, which influence the level of intakes and thus scoring of components and classification of adherent participants. This may influence association with disease and comparability between studies( Reference Waijers, Feskens and Ocké 8 ). However, this does not necessarily disqualify the generation of a Mediterranean type of diet score in a non-Mediterranean context( Reference Martinez-Gonzalez and Martin-Calvo 14 ), but is one way of assessing a prudent diet in line with contemporary dietary recommendations( 2 , 3 ). Alternately, a regional-specific dietary pattern that is more reflective of foods from the Nordic countries might be better to use in a Swedish context. The Nordic diet has been linked to amelioration of cardiovascular risk factors such as inflammatory markers and lipids in intervention studies( Reference Adamsson, Cederholm and Vessby 15 , Reference Uusitupa, Hermansen and Savolainen 16 ). Different scores based on Nordic foods have been developed: the Healthy Nordic Food Index (HNFI) that was developed in Denmark( Reference Olsen, Egeberg and Halkjaer 17 ) and adapted for a Swedish population( Reference Roswall, Eriksson and Sandin 18 ), the Baltic Sea Diet Score (BSDS) from Finland( Reference Kanerva, Kaartinen and Schwab 19 ) and the New Nordic Diet score (NNDS) from Norway( Reference Hillesund, Bere and Haugen 20 ). The only score that is purely based on food items is the HNFI. BSDS includes information on fat intake, whereas the NNDS takes meal patterns into account. In cohort studies, high adherence to different Nordic diet scores has been associated with a general healthy diet composition( Reference Kanerva, Kaartinen and Schwab 19 , Reference Bjornara, Overby and Stea 21 ) and positive health outcomes( Reference Olsen, Egeberg and Halkjaer 17 , Reference Roswall, Eriksson and Sandin 18 , Reference Roswall, Sandin and Löf 22 ). However, not all reported associations have been favourable; the HNFI was not associated with CVD in one analysis( Reference Roswall, Sandin and Scragg 23 ) and the association between BSDS and cardiometabolic risks was deemed unclear( Reference Kanerva, Kaartinen and Rissanen 24 ).
In the present cohort study in women, we aim to study the association of two healthy diet scores: a modified Mediterranean diet score (mMED) adapted to be suitable in a non-Mediterranean context and the HNFI, as well as the joint effect of the two scores with all-cause and cause-specific mortality in a Swedish setting. A high adherence to each score is reflective of adherence to a healthy diet, although defined in different ways. Because of the large number of outcomes, it will, for the first time, be possible to investigate mortality in women highly adherent to one diet but not to the other.
Methods
Study population
The Swedish Mammography Cohort was established in 1987–1990. All 90 303 women residing in two Swedish counties, born between 1914 and 1948, were invited to a mammography screening. Enclosed with this invitation was a questionnaire covering diet and lifestyle, which was completed by 74 % of the women. In late 1997, a second expanded questionnaire was sent to all participating women still living (n 56 030; 70 % of those eligible) in the study. The study sample with exclusions has been described previously( Reference Larsson, Bergkvist and Wolk 25 ). For the present analysis, the 1997 investigation was considered as baseline. To define the study sample (Fig. 1), we excluded individuals with implausible energy intakes ±3 sd from the ln-transformed mean total energy intake (n 521) and thirty-five individuals who completed the questionnaire but died before 1 January 1998, leaving 38 428 for analysis. Those individuals with missing values of one or more components of the diet score were kept in the data set and treated as null reporting, but were excluded in sensitivity analysis. Missing values were <5 % for all food groups used to define the scores, except for oatmeal porridge, which had 14 % missing values. In the analysis of cause-specific mortality, participants who had been diagnosed with cancer and CVD (n 5087) before the investigation in 1997 were excluded, leaving 33 341 for analysis. The study was approved by the regional ethics committees at Uppsala University, Uppsala, and Karolinska Institutet, Stockholm, Sweden.
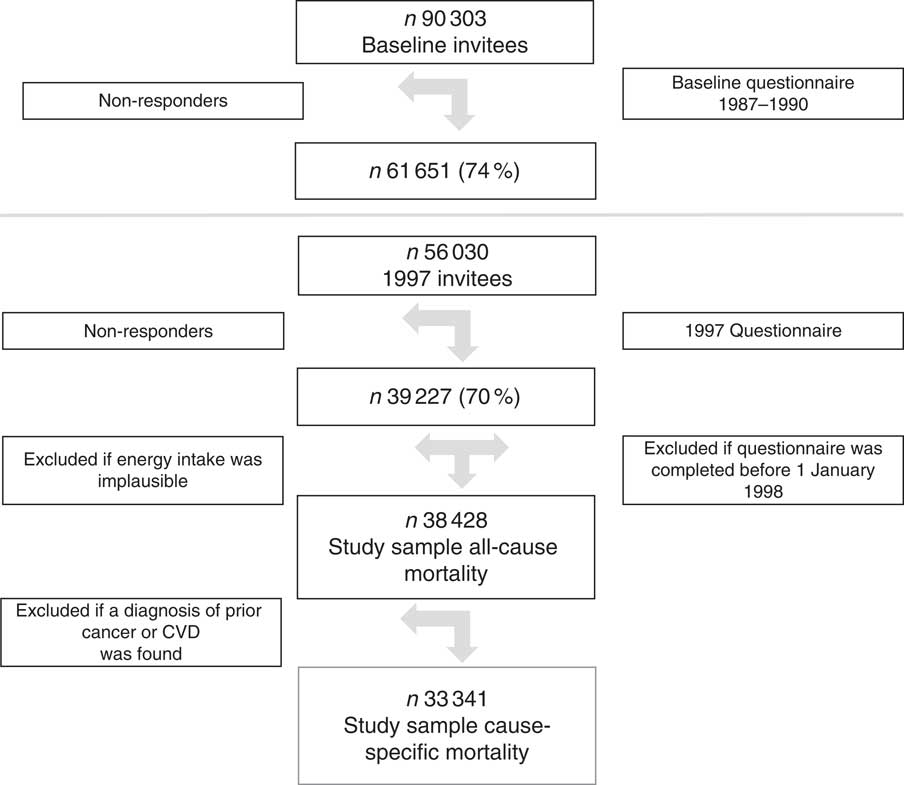
Fig. 1 The flow chart depicts the study sample with exclusions. The baseline for the present analyses was the 1997 investigation and the study sample used in the analysis with all-cause mortality was 38 428 and 33 341 for cause-specific mortality.
Dietary and covariate assessments
The FFQ has been described in more detail previously( Reference Tektonidis, Åkesson and Gigante 26 ). In short, the FFQ included ninety-six food items and the participants indicated in the FFQ how often, on average, they had consumed different food items during the past year and chose from eight pre-defined frequency categories ranging from ‘never/seldom’ to ‘3 or more times per day’. Frequently consumed foods such as dairy products and bread slices were additionally reported as number of servings per day. Information on fat type used in cooking and as salad dressing was also reported. Total amount of alcohol consumed per day was derived from the FFQ by multiplying the reported frequency with the reported amount on a single occasion. Energy and nutrient intakes were estimated by multiplying the consumption frequency of each food item with the nutrient content (including energy) of age-specific portion sizes. Nutrient values were obtained from the Swedish food composition database, National Food Agency. Nutrient intakes were adjusted for total energy intake using the residual method( Reference Willett, Howe and Kushi 27 ). The covariates collected in the 1997 questionnaire included educational level, living alone, physical activity, smoking habits, weight (kg) and height (m). Physical activity had five pre-defined levels ranging from 1 h to more than 5 h/week, and this question has been found to be valid compared with activity records and accelerometer data( Reference Orsini, Bellocco and Bottai 28 ). Energy intake and Charlson’s comorbidity index were also included as covariates. Charlson’s weighted comorbidity index( Reference Charlson, Pompei and Ales 29 , Reference Quan, Sundararajan and Halfon 30 ) was defined by ICD diagnosis codes (versions 8, 9 and 10) from in-patient care registered in the National Patient Register. BMI was calculated as weight divided by height squared.
Dietary exposures
Modified Mediterranean diet score
Adapted from the Mediterranean diet scale by Trichopoulou et al.( Reference Trichopoulou, Costacou and Bamia 31 ), a mMED (range 0–8 points) was calculated by use of previously defined food items( Reference Tektonidis, Åkesson and Gigante 26 ). One point was given for intakes above the median of each of the following components: fruit and vegetables (apple, banana, berry, orange/citrus and other fruit; carrot, beetroot, broccoli, cabbage, cauliflower, lettuce, onion, garlic, pepper, spinach, tomato and other vegetables), legumes (peas, lentils, beans and pea soup) and nuts, non-refined or high-fibre grains (whole meal bread, crisp bread, oatmeal and bran of wheat), fermented dairy products (sour milk, yogurt and cheese) and fish (excluding shellfish). In addition, one point was given for intakes below the median of red and processed meat. Any use of olive or rapeseed oil for cooking or as dressing and moderate alcohol consumption (intake range 5–15 g ethanol/d) also rendered 1 point. Median intakes and distribution of the components are presented in Table 1. A three-level categorical diet score was formed, with 0–2 points indicating low adherence, 3–5 points indicating medium adherence and 6–8 points indicating high adherence, as previously suggested( Reference Bellavia, Tektonidis and Orsini 32 ).
Table 1 Distribution and percentages for foods, food groups, the Healthy Nordic Food Index and the modified Mediterranean diet score in the Swedish Mammography Cohort (Mean values and 25th (p25), 50th (p50) and 75th (p75) percentiles; n 38 428)
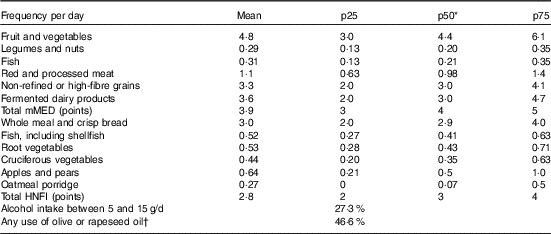
* The median intake as presented above was the cut-off used for the scoring but without rounding.
† Used in cooking and for dressings.
Healthy Nordic Food Index
The HNFI (range 0–6 points) was calculated as previously done in a different Swedish population( Reference Roswall, Eriksson and Sandin 18 ), but using the frequency per day of the different foods rather than grams per day to be comparable with the mMED. The foods in the HNFI were originally chosen because of expected beneficial health effects and that the foods were originating from Nordic nature, as well as being quantitative important foods in the Nordic diet( Reference Olsen, Egeberg and Halkjaer 17 ). The median intakes and distribution of the six components are presented in Table 1. One point was given for intakes above the median of each of the following foods: apples and pears, root vegetables (carrot and beet root), cruciferous vegetables (broccoli, cabbage and cauliflower), whole-grain bread (whole meal bread, crisp bread of rye), oatmeal porridge and fish (salmon, mackerel, herring and white fish such as cod and shellfish). A three-level categorical diet score was formed from the HNFI, with 0–1 points indicating low adherence, 2–4 points indicating medium adherence and 5–6 points indicating high adherence, making the low and the high categories approximately similar in size. As a sensitivity analysis, the HNFI was also generated with grams per day. The generated three-level categorical variables ranked participants exactly the same using either frequencies or grams per day.
Outcomes
We considered mortality collected from the Swedish cause of death registry between study baseline 1 January 1998 and 31 December 2014. A complete linkage with the register is possible as all Swedish residents have a personal identity number. Since 1952, the National Board of Health and Welfare has collected information on the causes of death for all Swedish residents in the cause of death registry. We used the underlying cause of death to define mortality from all causes, CVD (International Classification of Diseases, 10th revision (ICD-10) codes I00–I99), ischaemic heart disease (ICD-10 codes I20–I25) and cancer (ICD-10 C-codes).
Statistical analysis
For each participant, follow-up time was accrued from 1 January 1998 until date of death or the end of the study period (31 December 2014). The associations between mMED and HNFI on the one hand and all-cause mortality and cause-specific mortality on the other were estimated as age- and multivariable-adjusted hazard ratios (HR) and 95 % CI by Cox proportional hazards regression using age as the primary time scale. Both of the diet scores – mMED and HNFI – were initially treated as continuous variables, to assess how every one-point increment in each score was related to mortality. The scores were then treated as categorical variables to assess how high, moderate and low adherence to respective dietary score was associated with mortality. The low and high categories roughly reflected the lowest and the highest quintiles. We also investigated the trend over categories. The combination of mMED and HNFI was thereafter used to jointly classify study participants into nine categories. The high mMED/high HNFI level was the reference category in the analyses. The combined analysis was performed for all-cause mortality but not for cause-specific death because of limited number of cases in some of the cells. Covariates for the present analyses were chosen using directed acyclic graphs( Reference VanderWeele, Hernan and Robins 33 ), and the multivariable model I included educational level (≤9, 10–12, >12 years, other), living alone (yes or no), physical activity (five categories), energy intake (continuous), smoking habits (current, former, never) and Charlson’s weighted comorbidity index (continuous; 1–16). We further included the other diet score (HNFI or mMED) in an additional model (model II) and stratified the analysis of one diet score on every category of the other. Missing data on covariates were imputed using Stata’s ‘mi’ package (chained multiple imputation) and twenty imputations were done to reduce sampling error. The multiple imputation takes into account model variables and produces twenty separate data sets. The Cox analysis is subsequently run on all the separate data sets and the results are combined. We imputed missing observations for physical activity level, living alone and smoking status (missing values <14 %). The proportional hazard assumption in the Cox models was confirmed graphically by log-log plots of survival. As a sensitivity analysis, the main analysis was restricted to individuals with complete case data. We further re-ran the analysis excluding participants with missing data on any of the components of respective diet score. BMI was not primarily considered as a confounder but rather an intermediate, but was added to model I as a sensitivity analysis. Non-fermented milk intake has previously been positively associated with mortality in the present cohort( Reference Michaelsson, Wolk and Langenskiold 34 ) and was also added to model I as a sensitivity analysis. All analyses were carried out in Stata version 12.0 (StataCorp LP).
Results
Baseline characteristics
The baseline characteristics among the study participants stratified by adherence to mMED and HNFI are presented in Table 2. Despite modest absolute differences, mean BMI and age were the lowest in the high-adherence group of mMED, whereas an opposite pattern was observed for HNFI. Energy intake was the highest in both high-adherence groups, and there were more individuals classified as highly adherent with the mMED – 6965 individuals compared with 5527 individuals for HNFI. Nutrient intakes increased with higher adherence to either diet scores. The intake of red and processed meat was lowest in the high mMED adherence group and highest in the high HNFI adherence group. The alcohol intake levels between adherence groups of the two diet scores were opposite, with the highest mean intake in the highest mMED adherence group, whereas the lowest mean intake in the high HNFI adherence group. Further, educational level did not differ much between the different adherence categories of HNFI, whereas there was a gradient across the three adherence categories of mMED with the highest proportion of high educational attainment in the high-adherence category. For smoking status and physical activity level there was a similar tendency across the adherence categories of both mMED and HNFI.
Table 2 Characteristics of study participants according to adherence category of the modified Mediterranean diet score (mMED) and the Healthy Nordic Food Index (HNFI) taking part of the Swedish Mammography Cohort (Numbers and percentages; mean values and standard deviations)
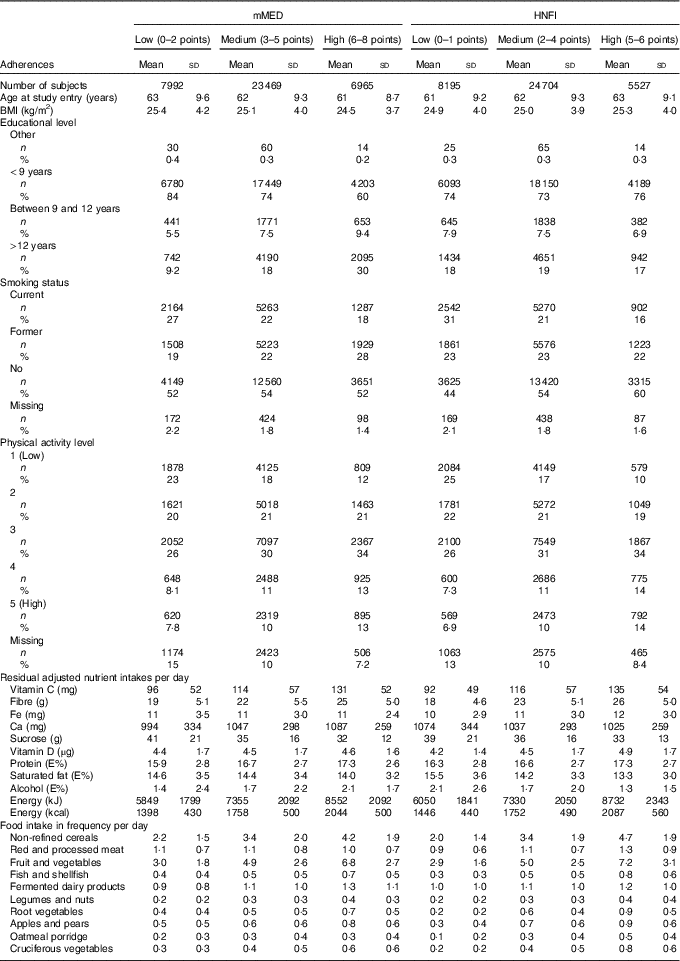
E%, percentage of energy intake.
Diet scores and mortality
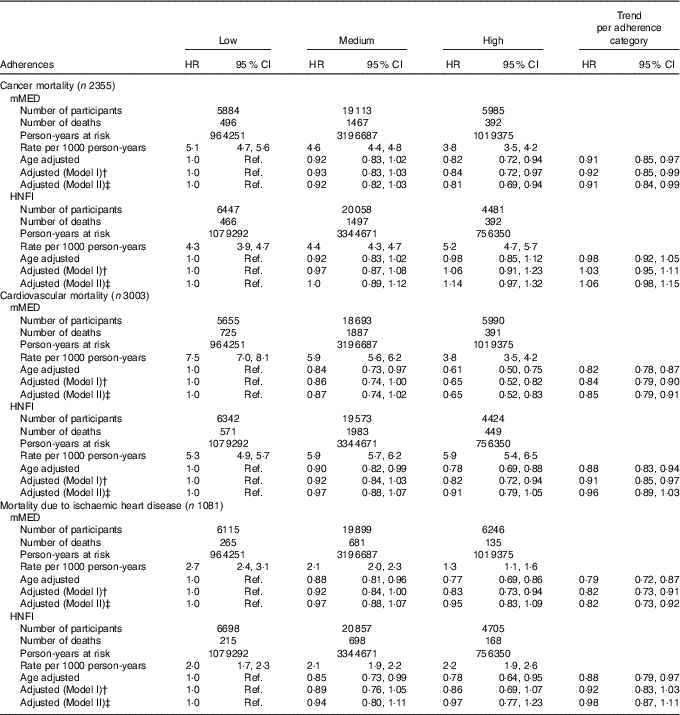
During a median of 17 years of follow-up and 583 826 person-years at risk, 10 478 women died among the 38 428 women who entered the study. After exclusion of individuals with prior cancer or CVD, 33 341 women remained to follow-up for cause-specific death; of those, 2355 died from cancer, 3003 from cardiovascular causes and 1081 from ischaemic heart disease, with a total of 518 031 person-years at risk.
Age- and multivariable-adjusted HR for all-cause and cause-specific mortality are presented in Tables 3 and 4, respectively. For all-cause mortality, those classified in the highest compared with the lowest category of the two diet scores had lower mortality rates. The multivariable-adjusted HR (model I) for all-cause mortality with a high adherence of mMED, compared with low adherence, was 0·76 (95 % CI 0·70, 0·81). Comparing high adherence of HNFI with low adherence, the HR for all-cause mortality was 0·89 (95 % CI 0·83, 0·96). Although we detected an educational gradient across mMED categories, we obtained similar estimates stratified on educational attainment: 0·76 (95 % CI 0·70, 0·83) among those with ≤9 years of schooling and 0·70 (95 % CI 0·56, 0·86) among those with >9 years of schooling in post hoc analysis. Further, to examine whether the detected differences in certain food intakes over adherence categories of HNFI influenced the estimates, we added the intake of red and processed meat and alcohol to model I, and this did not attenuate the estimates.
Table 3 All-cause mortality stratified by adherence to respective diet (modified Mediterranean diet score (mMED) and Healthy Nordic Food Index (HNFI)) score in the Swedish Mammography Cohort (Age- and multivariable-adjusted hazard ratios (HR)* and 95 % confidence intervals; n 38 428)
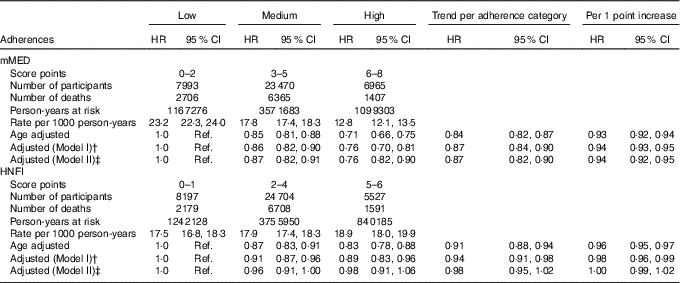
Ref., referent values.
* HR were determined in Cox proportional hazard analysis. † Model I was adjusted for educational level (≤9, 10–12, >12 years, other), living alone (yes or no), physical activity (five categories), energy intake (continuous), smoking habits (current, former, never) and Charlson’s comorbidity index (continuous; 1–16). ‡ Model II was adjusted for model I and the other diet score (mMED or HNFI).
Table 4 Cause-specific death due to cancer, CVD and ischaemic heart disease stratified by adherence to modified Mediterranean diet score (mMED) and Healthy Nordic Food Index (HNFI) in the Swedish Mammography Cohort (Age- and multivariable-adjusted hazard ratios (HR)Footnote * and 95 % CI; n 33 341)
Ref., referent values.
* HR were determined in Cox proportional hazard analysis.
† Model I was adjusted for educational level (≤9, 10–12, >12 years, other), living alone (yes or no), physical activity (five categories), energy intake (continuous), smoking habits (current, former, never) and Charlson’s comorbidity index (continuous; 1–16).
‡ Model II was adjusted for model I and the other diet score (mMED or HNFI).
After mutual adjustment for the other diet score (model II), the inverse association remained for mMED (0·76; 95 % CI 0·82, 0·90), comparing the highest with the lowest adherence categories, whereas it was attenuated for HNFI. This was also confirmed when the analysis with respective diet score was stratified on every category of the other diet score (Table 5). The strongest inverse association between mMED and all-cause mortality was observed in the low adherence category of HNFI. Fig. 2 illustrates the combined exposure of mMED and HNFI on mortality and shows that higher adherence to the mMED was associated with lower all-cause mortality in each stratum of the HNFI.
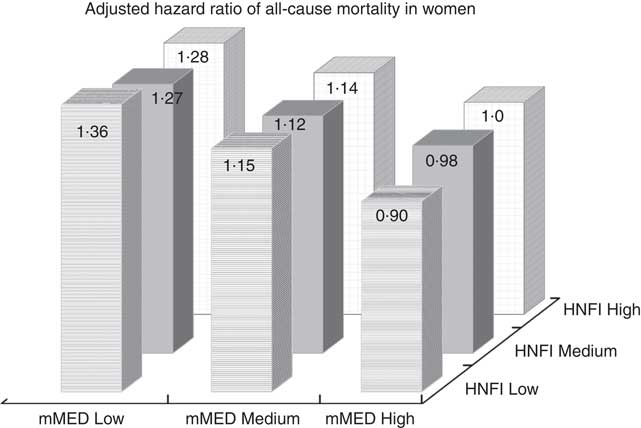
Fig. 2 Multivariable-adjusted hazard ratio of mortality in women across nine strata formed by the combined categories of modified Mediterranean diet score (mMED) and Healthy Nordic Food Index (HNFI). The high mMED/high HNFI adherence category was used as the reference category. The model was adjusted for educational level (≤9, 10–12, >12 years, other), living alone (yes or no), physical activity (five categories), energy intake (continuous), smoking habits (current, former, never) and Charlson’s weighted comorbidity index (continuous; 1–16).
Table 5 All-cause mortality in association with adherence to modified Mediterranean diet score (mMED) and Healthy Nordic Food Index (HNFI) stratified on each adherence category of the other diet score in the Swedish Mammography Cohort (Numbers and percentages; age- and multivariable-adjusted hazard ratios (HR)Footnote * and 95 % CI; n 38 428)
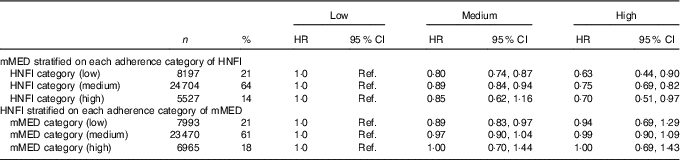
Ref, referent values.
* HR were determined in Cox proportional hazard analysis.
For cause-specific mortality, the associations with mMED were in general stronger than with HNFI. Cancer mortality was not independently associated with HNFI, whereas higher adherence of the mMED was associated with lower cancer mortality in a dose–response manner by adherence category (model I). For mortality due to CVD, there was an inverse association for both diet scores, but the association was abolished for HNFI when adjusting for mMED. The inverse age-adjusted association between the HNFI and mortality due to ischaemic heart disease was attenuated after adjustment for covariates, while the association remained for mMED.
Complete case analysis (all-cause mortality cohort 29 415 and 7811 deaths; cause-specific death cohort 25 754 and 1829 cancer deaths, 2276 cardiovascular deaths and 812 ischaemic heart disease deaths) confirmed the reported associations for all-cause and cause-specific mortality (data not shown). When excluding individuals with missing data on any of the score components of the diet scores, the estimates did not materially change for neither all-cause mortality nor cause-specific mortality. Adding BMI or non-fermented milk intake to model I did not change estimates either.
Discussion
In the present study, two scores reflective of a healthy diet, mMED and HNFI, were inversely associated with all-cause and cardiovascular mortality, although the strength of the associations differed between the scores. Those women who were classified in the highest compared with the lowest category of the mMED had a 24 % lower multivariable-adjusted all-cause mortality, whereas the corresponding estimate for HNFI was 11 %. Thus, a high scoring (high adherence) of the mMED compared with a high scoring of the HNFI in this cohort of Swedish women seems to be associated with an advantage in terms of survival. This was further confirmed in the analysis with combined exposures; a higher adherence to the mMED was associated with lower mortality in each stratum of the HNFI, whereas results for HNFI were not independent from mMED. It was previously noted that the HNFI may lack important features of a healthy Nordic diet( Reference Riserus 35 ), including the use of rapeseed oil, and that a Nordic diet could be more widely defined and include more items( Reference Kanerva, Kaartinen and Schwab 19 , Reference Adamsson, Reumark and Cederholm 36 ) than those included in the proposed HNFI( Reference Olsen, Egeberg and Halkjaer 17 ). These factors may indeed have influenced the findings in the present study.
To our knowledge, this is the first study to classify participants in joint exposure strata reflecting the combined adherence to the mMED and the HNFI. This analysis makes it possible to discern whether there may be an advantage to be adherent to either the HNFI or the mMED in the present cohort. As the HNFI was developed to be specific to the Nordic region, it could have been expected that high adherence to the HNFI, compared with a high adherence to mMED, would be an advantage in this Swedish population. However, this was not the case – an observation that was strengthened by the analysis across the nine joint strata as presented in the Fig. 2. Moreover, in the multivariable analysis including the other diet score, the association with mortality remained for mMED but the association between HNFI and mortality was attenuated comparing the high- and the low-adherence categories. The scores are correlated with each other (r 0·53) but they do not reflect the same dietary components. The combination of components of the HNFI did not associate with mortality independent of the mMED score. However, this does not necessarily mean that a healthy Nordic diet per se is less healthy than a Mediterranean type of diet; rather, it means that the index may not capture the full potential of a healthy Nordic diet and may need refinement.
The HNFI was originally developed in Denmark, a country with some similar but also some different features of food culture than Sweden( Reference Amcoff, Edberg and Enghardt Barbieri 37 , Reference Pedersen, Christensen and Matthiessen 38 ). The HNFI comprised six different foods and food groups – apples and pears, root vegetables, cruciferous vegetables, whole-grain bread, oatmeal porridge and fish – which are all healthy foods that will provide fibre, whole grains, micronutrients such as folate, vitamin C, K and Fe, n-3 fatty acids and other bioactive compounds, which may help prevent chronic metabolic disease( 2 ). In the original HNFI rye bread was included, as rye bread consumption is an important feature of the Danish diet( Reference Olsen, Egeberg and Halkjaer 17 ). However, rye bread is not as commonly consumed in Sweden and was exchanged with whole-grain bread when adapted for a Swedish cohort( Reference Roswall, Eriksson and Sandin 18 ). In this study, we included high-fibre and whole-grain breads, as well as crisp bread.
The mMED is a more diversified diet score and is less reliant on single foods, which may have affected the association between HNFI and mortality. The HNFI was generated by the use of fourteen food items from the FFQ, whereas the mMED was based on forty-one food items. The scoring of the mMED was also dependent on a lower intake of red and processed meat, as well as on a moderate alcohol intake. The scoring of the HNFI relied only on high intakes of a limited number of healthy foods and may thus permit individuals with a less healthy total diet in the high-adherence group. Indeed, those who were highly adherent to HNFI had the highest intake of red and processed meat, as well as the lowest intake of alcohol; these factors may have influenced the results, but adjustment for these food groups did not influence estimates. However, high adherence to the mMED was more common than high adherence of HNFI. Further, a high adherence to mMED was associated with a higher educational level, but stratifying the analysis on an educational level (shorter or longer than 9 years of schooling) did not influence the point estimates in the present study.
Previously, the HNFI has been related to lower total mortality in Denmark( Reference Olsen, Egeberg and Halkjaer 17 ), with a 4 % lower rate for each one-point higher score in men and women, comparable to the 2 % lower rate per point among women in the present study. Further, several studies based on the Swedish Women’s Lifestyle and Health cohort have been published with the HNFI as the exposure. In that cohort, the HNFI was related to a healthier lifestyle( Reference Roswall, Eriksson and Sandin 18 ), whereas not to the risk of CVD( Reference Roswall, Sandin and Scragg 23 ), but presented a 6 % lower total mortality per 1 point increment, which was confined to cancer and non-CVD causes( Reference Roswall, Sandin and Löf 22 ). This is seemingly in contrast to our results, where the HNFI was independently associated with lower mortality due to CVD, but not to cancer death. The differences in results may be because of a larger number of CVD cases in the present cohort and that the women were older at follow-up and had a higher baseline BMI and thus consequently were also at a higher risk for CVD( Reference McKibben, Al Rifai and Mathews 39 ). In addition, median intake levels of the score components were higher in the present cohort, and our score may be reflective of a more nutrient-dense diet. Moreover, a recent study from Denmark reported an inverse association between high adherence to the HNFI and risk of myocardial infarction( Reference Gunge, Andersen and Kyro 40 ) over 13·6 years. The fact that we failed to see an inverse adjusted association with mortality due to ischaemic heart disease may be explained by fewer cases and that the HNFI failed to account for important features of a healthy diet, especially in a Swedish setting, important in the prevention of ischaemic heart disease, as previously suggested( Reference Riserus 35 ). This may relate to the content of foods rich in phytochemicals, such as carotenoids and polyphenols, which are abundant in plant foods and have antioxidant activities( Reference Anderson and Nieman 9 ) or some other component of a Nordic diet such as specific types of dairy products, berries and moderate intakes of meat( Reference Adamsson, Reumark and Cederholm 36 ).
The inverse association between a Mediterranean type of diet and CVD and mortality has been reported previously( Reference Martinez-Gonzalez, Salas-Salvado and Estruch 10 , Reference Martinez-Gonzalez and Martin-Calvo 14 , Reference Trichopoulou, Costacou and Bamia 31 , Reference Martinez-Gonzalez 41 , Reference Martinez-Gonzalez, Garcia-Lopez and Bes-Rastrollo 42 ) and includes studies from non-Mediterranean countries( Reference Martinez-Gonzalez and Martin-Calvo 14 , Reference Tektonidis, Åkesson and Gigante 26 , Reference Bellavia, Tektonidis and Orsini 32 , Reference Tektonidis, Akesson and Gigante 43 , Reference Tong, Wareham and Khaw 44 ). A recent study from the UK evaluated associations between four different Mediterranean diet scores and CVD and mortality and reported inverse associations for three of the scores, but not for the score using sex-specific medians. This score was seen as too crude and not sensitive enough to evaluate adherence to a Mediterranean diet in a non-Mediterranean context( Reference Tong, Wareham and Khaw 44 ). In the present study, mMED was median-derived and was sensitive enough to classify participants into distinct adherence categories, and a higher adherence was inversely associated with all-cause and cause-specific death. This may have been influenced by the wide exposure range of the different components of the mMED, as well as our large sample size. The beneficial effects of a Mediterranean type of diet have been ascribed to high-quality fat and carbohydrates, as well as a high intake of key micronutrients and other bioactive compounds such as polyphenols with antioxidant capacity( Reference Anderson and Nieman 9 , Reference Martinez-Gonzalez, Salas-Salvado and Estruch 10 ), factors known to influence lipid levels, insulin resistance, oxidative stress and inflammatory processes, which in turn are involved in the pathophysiology of CVD( Reference Grosso, Mistretta and Frigiola 45 , Reference Schwingshackl and Hoffmann 46 ). There has been criticism regarding the use of the term Mediterranean diet in a non-Mediterranean context( Reference Bere and Brug 47 ); however, this view is not shared by all nutrition researchers, and recent systematic reviews support beneficial associations between Mediterranean diet scores used in diverse populations and different health outcomes( Reference Martinez-Gonzalez 41 , Reference Rosato, Temple and La Vecchia 48 ).
The strengths of the present study include the long follow-up time with proportional hazards and the large number of deaths ascertained through individual linkage to the national death registry. Although we adjusted for important covariates as proxies for socio-economic status, there may be an influence of residual or unmeasured confounding. As with any self-reported exposure, reporting of diet is connected to random and systematic measurement errors, and further to difficulties in estimating portion sizes and inadequacies in food composition tables. These problems are partially compensated for by exclusion of participants with the most extreme energy intakes and by adjustment for total energy intake rendering adherence to the isoenergetic principle of the exposure categories( Reference Rhee, Sampson and Cho 49 ). Furthermore, the use of only one diet assessment in a study with long follow-up time is a weakness, which may also lead to attenuated estimates, as previously discussed( Reference Paeratakul, Popkin and Kohlmeier 50 ). The study questionnaire has the ability to rank participants and has been found to be valid and reproducible, and the large study size compensates for random misclassification. These results might not apply to other ethnicities or men.
In conclusion, a higher adherence to a healthy diet, reflected by the mMED and the HNFI, was independently and inversely associated with lower total all-cause and mortality owing to cardiovascular causes, but with different strengths. The rate of cancer death and death due to ischaemic heart disease was only inversely and independently associated with mMED. There seems to be an advantage to be adherent to the mMED, rather than HNFI, in terms of survival in this cohort of Swedish women. Further, we conclude that HNFI may not necessarily capture the full potential of a Nordic diet related to health outcomes, at least in the present cohort, and may need further development.
Acknowledgements
The study was supported by the Swedish Research Council (research grant nos 2015-02302 and 2015-03527).
E. W. L. and K. M. designed the research; E. W. L., L. B., A. W. and K. M. conducted the research; E. W. L. performed statistical analysis, drafted the paper and had primary responsibility for final content; and L. B., A. W. and K. M. participated in the interpretation of data and in finalising the paper. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.









