Introduction
Sweat bees in the subgenus Lasioglossum (Dialictus) are one of the most diverse, abundant, and taxonomically challenging bee taxa in the Nearctic region (Moure and Hurd Reference Moure and Hurd1987; Eickwort Reference Eickwort1988; Gibbs Reference Gibbs2018b; Ascher and Pickering Reference Ascher and Pickering2020; Portman et al. Reference Portman, Brunninga-Socolar and Cariveau2020). Recent taxonomic studies have improved understanding of these bees in parts of North America (Gibbs Reference Gibbs2010, Reference Gibbs2011, Reference Gibbs2016, Reference Gibbs2018a; Gardner and Gibbs Reference Gardner and Gibbs2020), but much work remains to be done. These revisions have supported subsequent pollinator inventory and monitoring (Wolf and Ascher Reference Wolf and Ascher2009; Scott et al. Reference Scott, Ascher, Griswold and Nufio2011; Dibble et al. Reference Dibble, Drummond, Stubbs, Veit and Ascher2017; Gibbs et al. Reference Gibbs, Ascher, Rightmyer and Isaacs2017; Kilpatrick et al. Reference Kilpatrick, Gibbs, Mikulas, Spichiger, Ostiguy, Biddinger and López-Uribe2020; Portman et al. Reference Portman, Brunninga-Socolar and Cariveau2020), ecological studies (Burkle et al. Reference Burkle, Marlin and Knight2013; Park et al. Reference Park, Blitzer, Gibbs, Losey and Danforth2015; Winfree et al. Reference Winfree, Reilly, Bartomeus, Cariveau, Williams and Gibbs2018; De Luca et al. Reference De Luca, Buchmann, Galen, Mason and Vallejo-Marín2019; Grab et al. Reference Grab, Branstetter, Amon, Urban-Mead, Park and Gibbs2019), and evolutionary studies (Schwarz et al. Reference Schwarz, Richards and Danforth2007; Gibbs et al. Reference Gibbs, Albert and Packer2012a, Reference Gibbs, Brady, Kanda and Danforth2012b).
The first thorough and comprehensive revision of Lasioglossum (Dialictus) was for the species of Canada (Gibbs Reference Gibbs2010), in which 84 species were recorded. As is often the case, taxonomic revision facilitated additional discoveries in the same group. Since 2010, additional taxonomic and nomenclatural changes to the subgenus have been proposed (Gibbs Reference Gibbs2011, Reference Gibbs2012). Two species were transferred back to L. (Dialictus) after briefly being considered L. (Evylaeus) (Gibbs Reference Gibbs2011), and three species were newly discovered to occur in Canada (Gibbs Reference Gibbs2011; Heron and Sheffield Reference Heron and Sheffield2015; Onuferko et al. Reference Onuferko, Kutby and Richards2015). In the course of ongoing taxonomic work on the L. (Dialictus) of the western United States of America and Mexico, two new species and three new Canadian distribution records were discovered, bringing the total number of known L. (Dialictus) species in Canada to 94.
The discovery of new species of Lasioglossum (Dialictus) in Canada is taken as an opportunity to provide an updated key to species that incorporates other recent taxonomic changes to the fauna, including recently described species, sex associations, and synonyms. The purpose of this paper is to bring knowledge of the L. (Dialictus) of Canada up to date and emend the key in Gibbs (Reference Gibbs2010), so that nonspecialist researchers can accurately identify Canadian specimens. A checklist of all the bees of Canada is in progress, because many new discoveries have been made since the first such checklist (Canadian Endangered Species Conservation Council 2016). Now is an opportune time to comprehensively update knowledge of Canadian L. (Dialictus) so that the next list of the bees of Canada can be as accurate as possible for this group.
Methods
The taxon concept for L. (Dialictus) follows the classification in Gibbs (Reference Gibbs2018a). This concept differs from the one used by Gibbs (Reference Gibbs2010) in that the “black Dialictus” (= Hemihalictus sensu Gibbs et al. (Reference Gibbs, Packer, Dumesh and Danforth2013)) are excluded and L. rufulipes (Cockerell, 1938) and L. testaceum (Robertson, 1897) are included. Species were delimited using an evolutionary species concept (Wiley and Mayden Reference Wiley and Mayden2000) and integrative taxonomy (DeSalle et al. Reference DeSalle, Egan and Siddall2005), that is, species were considered as independent evolutionary lineages and were tested in a hypothesis-driven approach using multiple sources of data. Specimen examination, format and style of descriptions, and imaging methods follow that of Gardner and Gibbs (Reference Gardner and Gibbs2020). The DNA barcodes were generated following protocols outlined in Gibbs (Reference Gibbs2009) or Gardner and Gibbs (Reference Gardner and Gibbs2020) and uploaded to the Barcode of Life Data System (BOLD; http://www.boldsystems.org; Ratnasingham and Hebert Reference Ratnasingham and Hebert2007).
The following abbreviations are used below in diagnoses, descriptions, and keys: IS = interspaces (between punctures), PD = puncture diameter, OD = median ocellus diameter, IAD = interantennal distance, and AOD = antennocular distance. Care must be taken to distinguish head length and face length. Gibbs (Reference Gibbs2010) measured total head length from the lower margin of the clypeus to the highest point of the vertex, but more recent work (Gardner and Gibbs Reference Gardner and Gibbs2020) measured face length only up to the lower margin of the median ocellus. The latter measurement is more reliable because the top of the head is often obscured by hair and changes in focal depth as it curves towards the occiput. Generally, the revised key in this paper still uses head length, but new species descriptions use face length. Both values are given when direct comparisons between new and previously described species are required.
Spelling of some species names differs from previous usage based on the recognition that some specific epithets are nouns and therefore nondeclinable (International Committee on Zoological Nomenclature Article 31.2.2). These epithets include heterognathus, leucocomus, lionotus, pavonotus, platyparius, and zephyrus.
Plant names recorded on specimen labels were updated to current taxonomy using the World Flora Online database (World Flora Online Consortium 2020) for floral host lists. Distribution maps were generated in Maxent, version 3.4.1 (Phillips et al., no date) using the 19 bioclimatic variables, solar radiation, wind speed, and elevation data from WorldClim (Fick and Hijmans Reference Fick and Hijmans2017), soil classifications from the Digital Soil Map of the World (Food and Agriculture Organisation of the United Nations 2007), auto features, regularisation multiplier of 1, and record pruning procedures described in Gardner and Gibbs (Reference Gardner and Gibbs2020).
Specimens examined
Type material and other specimens from the following institutions and individuals were examined. Abbreviations used throughout the rest of the text are given first, with the full name and curator/lender (if applicable) given in parentheses afterward.
-
AMNH – American Museum of Natural History, New York, New York, United States of America (Jerome G. Rozen)
-
ASUHIC – Arizona State University, Hasbrouck Insect Collection, Tempe, Arizona, United States of America (Desirée Narango)
-
BBSL – United States Department of Agriculture Agricultural Research Service Pollinating Insect Research Unit, Utah State University, Logan, Utah, United States of America (H. Ikerd, Terry Griswold, Lauren Ponisio)
-
CAS – California Academy of Sciences, San Francisco, California, United States of America (Chris Grinter)
-
CMNC – Canadian Museum of Nature, Ottawa, Ontario, Canada (Thomas Onuferko)
-
CNC – Canadian National Collection of Insects, Arachnids, and Nematodes, Ottawa, Ontario, Canada (Andrew Bennett)
-
CUIC – Cornell University Insect Collection, Ithaca, New York, United States of America (Jason Dombroskie)
-
FWSE – United States Fish and Wildlife Service, Vancouver, Washington, United States of America (Joe Engler)
-
LRF – Personal collection, Monterrey, Nuevo Leon, Mexico (Liliana Ramírez Freire)
-
NMSU – New Mexico State University Arthropod Museum, Las Cruces, New Mexico, United States of America (Helen Vessels)
-
PCYU – Packer collection at York University, York, Ontario, Canada (Laurence Packer)
-
PMAE – Royal Alberta Museum, Edmonton, Alberta, Canada (Matthias Buck)
-
RLM – Personal collection, Rochester, New York, United States of America (Robert L. Minckley, University of Rochester)
-
SEMC – University of Kansas Biodiversity Institute and Natural History Museum, Lawrence, Kansas, United States of America (Jennifer Thomas, Michael Engel)
-
TAMU – Texas A&M University Insect Collection, College Station, Texas, United States of America (Karen Wright)
-
UCDC – University of California-Davis, R.M. Bohart Museum of Entomology, Davis, California, United States of America (Neal Williams lab)
-
UCRC – University of California-Riverside Entomology Research Museum, Riverside, California, United States of America (Doug Yanega)
-
UMSP – University of Minnesota Insect Collection, St. Paul, Minnesota, United States of America (Robin Thomson)
-
USNM – Smithsonian Institution National Museum of Natural History, Washington, D. C., United States of America (Seán Brady, Brian Harris)
-
WRME – J.B. Wallis/R.E. Roughley Museum of Entomology, Winnipeg, Manitoba, Canada (Jason Gibbs)
Results
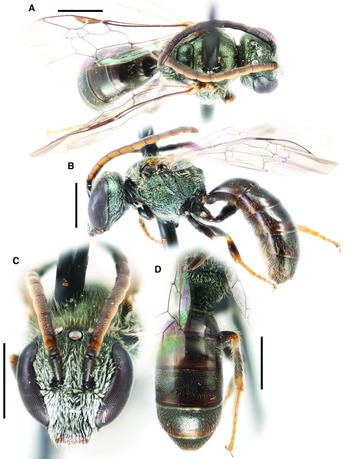
Lasioglossum (Dialictus) absimile (Sandhouse, 1924)
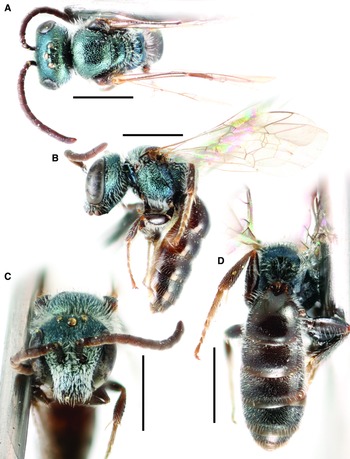
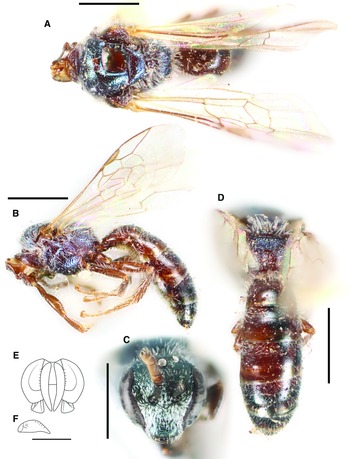
Figure 1.

Fig. 1. Lasioglossum absimile (Sandhouse, 1924) (holotype ♀): A, lateral habitus; B, face, mesoscutum, and T1 anterior slope; and C, propodeum and metasoma. Scale bars: 1 mm.
Halictus (Chloralictus) absimilis Sandhouse Reference Sandhouse1924: 21 (holotype, ♀, deposited in USNM, examined)
Lasioglossum (Chloralictus) absimile – Michener Reference Michener1951: 1111 (catalogue)
Dialictus absimilis – Hurd Reference Hurd1979: 1963 (catalogue) – Moure and Hurd Reference Moure and Hurd1987: 87 (catalogue)
Diagnosis. Females of Lasioglossum absimile can be distinguished from similar species in the L. viridatum species complex defined in Gibbs (Reference Gibbs2010), especially L. pacatum (Sandhouse, 1924), L. admirandum (Sandhouse, 1924), L. sagax (Sandhouse, 1924), and L. ephialtum Gibbs, 2010, by the combination of mesepisternum very smooth and imbricate to tessellate (rugulose dorsally in L. admirandum, L. ephialtum, and L. sagax), mesoscutum shiny except sometimes dull anteromedially (dull tessellate except sometimes shiny posteriorly in L. admirandum, L. pacatum, and L. sagax), head short (head length/width ratio = 0.93; face length/width ratio = 0.79) (usually longer (length/width ratio = 0.94–1.01) in L. admirandum, L. ephialtum, and L. sagax), T1 disc very sparsely punctate (IS = 2–6 PD medially) (more densely punctate medially (IS = 1–2 PD) in L. admirandum), and T2–T4 with abundant tomentum and dense apical fringes (less extensive tomentum and weaker apical fringes in L. ephialtum and L. pacatum).
Comments. Lasioglossum absimile is included based on material from Manitoba and British Columbia, Canada. Due to taxonomic difficulties in the L. viridatum species complex, the identification of these specimens is tentative, pending a more thorough revision of this species complex. It is included here on a preliminary basis because some specimens are liable to cause confusion otherwise.
Lasioglossum absimile is very similar to Lasioglossum caducum (Sandhouse, 1924), described from New Mexico, and diagnostic characters distinguishing the two are not yet known.
Lasioglossum (Dialictus) ascheri Gibbs, 2011
Lasioglossum (Dialictus) ascheri Gibbs Reference Gibbs2011: 57 (holotype, ♀, deposited in AMNH; examined)
Lasioglossum (Dialictus) curculum Gibbs Reference Gibbs2011: 80 (holotype, ♀, deposited in CNC; examined) new synonymy
Comments. Gibbs (Reference Gibbs2011) described L. ascheri and L. curculum each from two females. They are remarkably similar but differ in the absence and presence of a preapical mandibular tooth, respectively. A series of two females with and two without a preapical tooth on the mandible were examined from four sites within a 5.5-km radius in Algonquin Provincial Park, Ontario, Canada in 2011. It seems unlikely that two such rare species would occur together so near to the same place and time. No other known morphological characters separate the two putative species except the length of the branches of the inner metatibial spur, which is extremely subtle and may also be variable. No DNA barcodes are available for either species. With the lack of geographic separation and dubious utility of morphology, there is no longer sufficient evidence to support a two-species hypothesis. Lasioglossum ascheri is chosen as the valid name in the absence of publication priority.
Lasioglossum ascheri is a newly confirmed record for Canada. The four specimens examined were included in a Master’s thesis by Nardone (Reference Nardone2013) as L. cf. ascheri and are now deposited in WRME.
Lasioglossum (Dialictus) cephalotes (Dalla Torre, 1896)
Comments. Lasioglossum cephalotes has not been confirmed for Canada, but is believed to be a parasite of L. zephyrus (Smith, 1853) (Robertson Reference Robertson1926), a relatively common species in Canada. Lasioglossum cephalotes was recorded from southern Ontario (MacKay and Knerer Reference MacKay and Knerer1979), but the specimens were re-examined and identified as L. lionotus (Sandhouse, 1924) (Gibbs Reference Gibbs2010, Reference Gibbs2011). The name L. cephalotes was used incorrectly by Gibbs (Reference Gibbs2010) to refer to L. rozeni (Gibbs Reference Gibbs2011).
Lasioglossum (Dialictus) furunculum Gibbs, 2011
Comments. Lasioglossum furunculum is a rare parasite, described from a single female collected in Massachusetts and later recorded from the Niagara region of Ontario (Onuferko et al. Reference Onuferko, Kutby and Richards2015). It appears closely related to L. izawsum Gibbs, 2011, another rare parasite, and the names may represent variation within a single species (Gibbs Reference Gibbs2011).
Lasioglossum (Dialictus) georgeickworti Gibbs, 2011
Comments. Lasioglossum georgeickworti is evidently restricted to coastal areas in eastern North America. It was described from material ranging from Virginia to Massachusetts, United States of America (Gibbs Reference Gibbs2011). A single specimen from Prince Edward Island, Canada was also examined and tentatively identified as this species.
Lasioglossum (Dialictus) helianthi (Cockerell, 1916) new combination
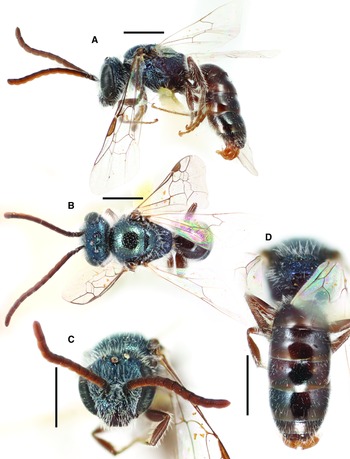
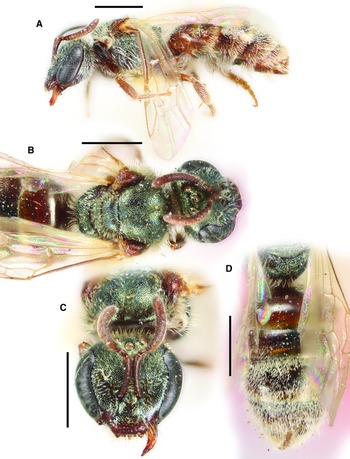
Figure 2
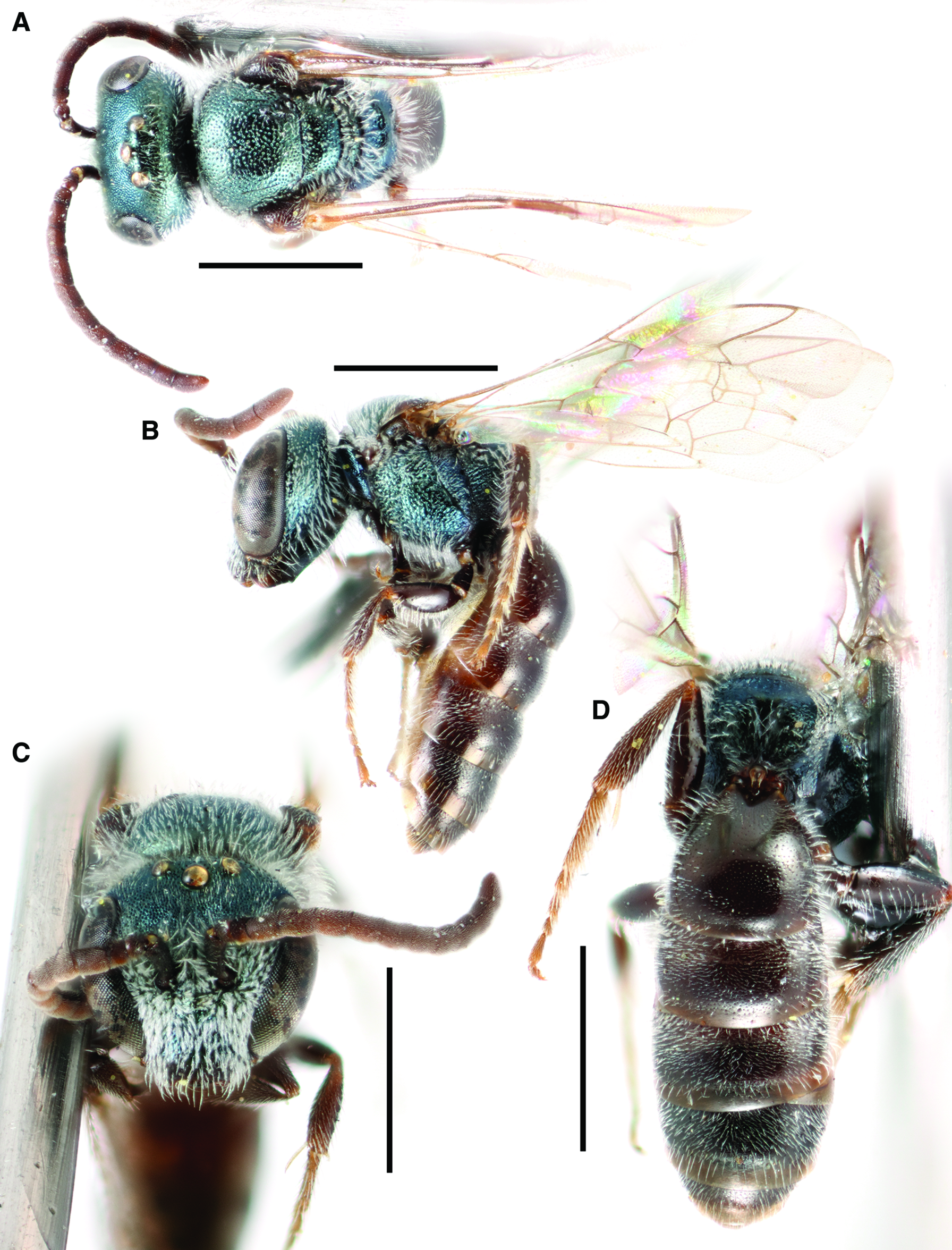
Fig. 2. Lasioglossum helianthi (Cockerell, 1916) (♂): A, dorsal habitus; B, lateral habitus; C, face; and D, metasoma. Scale bars: 1 mm.
Halictus helianthi Cockerell Reference Cockerell1916: 77 (holotype, ♀, deposited in USNM; examined)
Lasioglossum (Dialictus) imbrex Gibbs Reference Gibbs2010: 153 (holotype, ♀, deposited in PCYU; examined) new synonymy
Diagnosis. Males of L. helianthi can be recognised by the combination of tegula enlarged (reaching but not exceeding posterior margin of mesoscutum in dorsal view), with inner posterior margin weakly concave, coming to a narrow point posteriorly, and usually densely punctate (IS ≤ 1 PD); mesoscutum densely punctate (IS ≤ 1 PD) especially laterad of parapsidal line (IS < 0.5 PD); metapostnotum shiny with strong anastomosing rugae; and discs of T1–T2 deeply and densely punctate in basal half (IS ≤ 1 PD). They are most similar to L. tegulariforme and L. ellisiae. Males of L. tegulariforme have the tegula larger (clearly exceeding posterior margin of mesoscutum in dorsal view) with a broadly rounded projection posteriorly, and discs of T1–T2 (especially T1 medially) more sparsely punctate (IS = 1–3 PD). Males of L. ellisiae have the metapostnotum with parallel or subparallel rugae and are also found east of the Rocky Mountains and therefore are very unlikely to occur sympatrically with L. helianthi.
Comments. Lasioglossum helianthi is herein resurrected from synonymy with L. tegulariforme (Crawford, 1907), and L. imbrex Gibbs, 2010 is considered a junior subjective synonym (see comments on L. tegulariforme for details).
Lasioglossum (Dialictus) hitchensi Gibbs, 2012
Comments. This species was treated by Gibbs (Reference Gibbs2010) using the name L. mitchelli. Subsequently, this was discovered to be a secondary homonym with Halictus mitchelli Cockerell, 1906, and L. hitchensi was introduced as a replacement name in Gibbs (Reference Gibbs2012).
Lasioglossum (Dialictus) immigrans new species
ZooBank Registration # urn:lsid:zoobank.org:act:472373E0-2D3E-4C09-961C-DE937C034C71

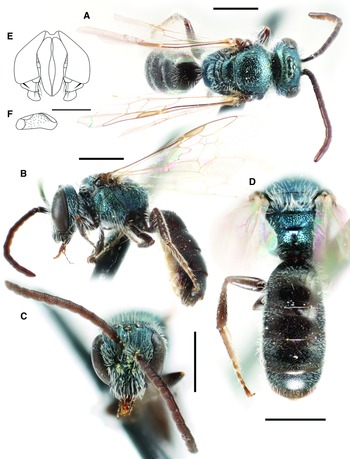
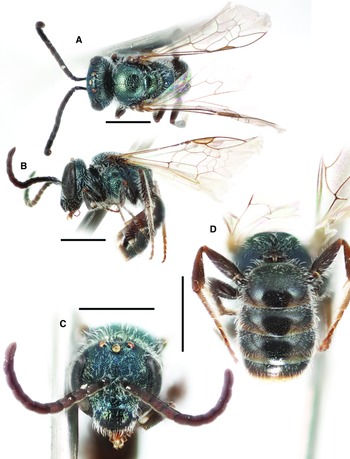
Fig. 3. Lasioglossum immigrans new species (♀): A, lateral habitus (holotype); B, face, mesoscutum, and T1 anterior slope (Manitoba specimen); and C, propodeum and metasoma (Manitoba specimen). Scale bars: 1 mm.
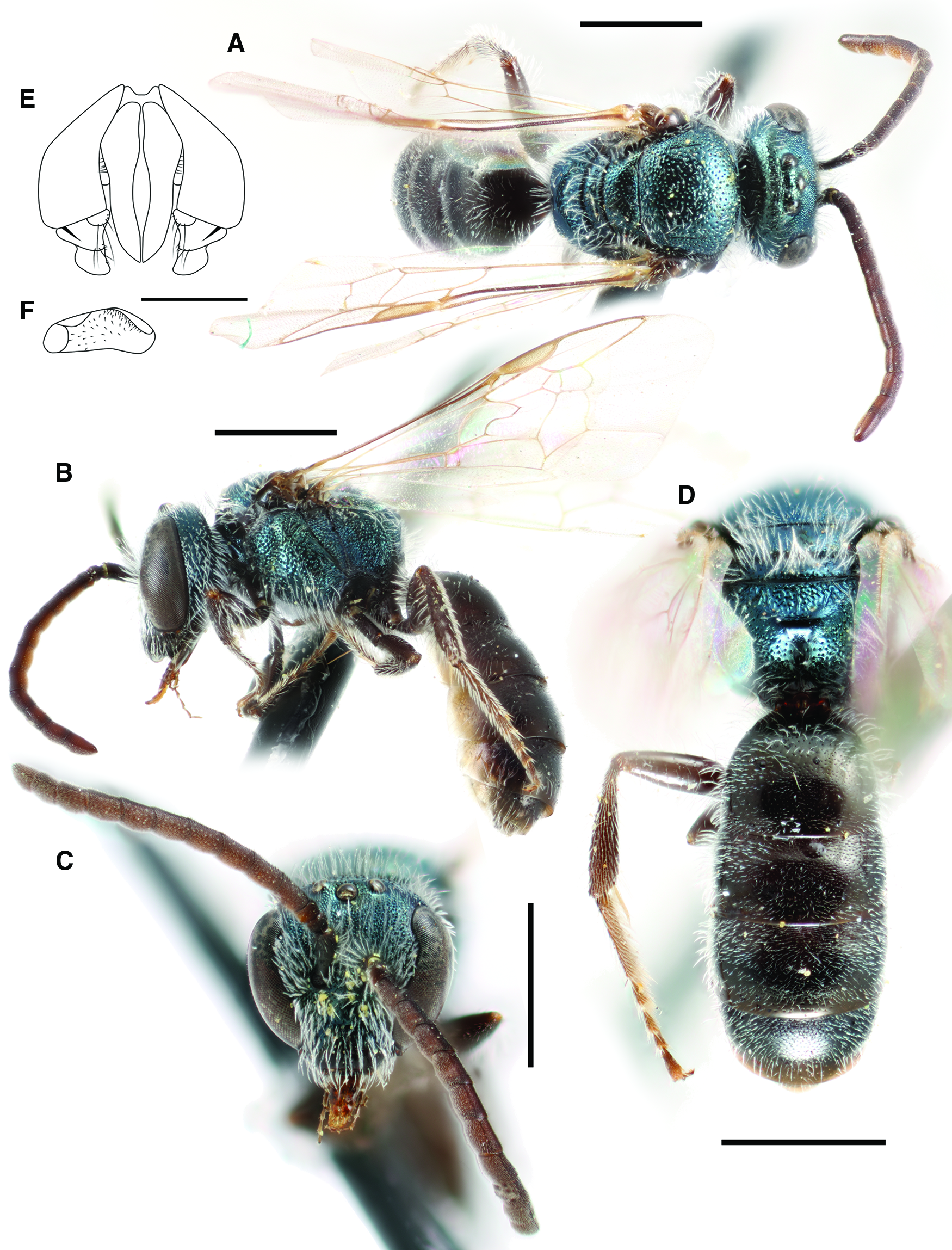
Fig. 4. Lasioglossum immigrans new species (♂): A, dorsal habitus; B, lateral habitus; C, face; D, propodeum and metasoma; E, genital capsule (dorsal view with gonostylus parallel to plane of vision); and F, retrorse lobe (lateral view parallel to plane of vision). Scale bars: A–D, 1 mm; E–F, 0.25 mm.

Fig. 5. T1 anterior slope and acarinarial fan of female: A, Lasioglossum immigrans new species, with fan weakly developed and T1 deeply punctate; and B, Lasioglossum comulum Michener, 1951, with fan well-developed andT1 finely punctate.
Material examined
Holotype. UNITED STATES – Arizona • ♀; Pinal Co., intersection of 347 + 84 S. of Maricopa; 32.875° N, 112.05° W; 8.iii.2018; T.J. Wood leg.; WRME.
[Verbatim label: USA: AZ: Pinal 8.iii.2018 / 347 × 84 intersection S. of Maricopa, 32.875 –112.050, leg. T.J. Wood // BOLD / DLIII097-18 / gard0014-AZ // HOLOTYPE / Lasioglossum (Dialictus) immigrans Gardner and Gibbs // University of Manitoba / Wallis/Roughley Mus. Ent. / Winnipeg Manitoba / Canada R3T2N2 / WRME 0518492]
Paratypes. (See Supplementary material, Table S1 for complete data including coordinates and collection codes.)
CANADA – Manitoba • 1♀; West Interlake, 4.5 km NNW of Ashern; 29.vi.2020; M. Martini leg.; WRME.
MEXICO – Coahuila • 1♂; 41 km NE of San Pedro de las Colonias; 26.iii.1992; R. Brooks leg.; SEMC • 1♀; 5 km W of Ocampo; 30.iii.1992; B. Alexander leg.; SEMC • 3♀; Arteaga, San Antonio de las Alazanas; 21.vii.2013; LRF & CVM leg.; LRF – Guanajuato • 2♀, 2♂; 7 mi. NW of Leon; 9.viii.1954; SEMC – Guerrero • 1♀; Acapulco; 28.vi.1956; R.E. Beer & party leg.; SEMC – Hidalgo • 1♀; 11 mi. E of Pachuca; 26.viii.1962; Naumann & Roberts leg.; SEMC • 1♂; 22 mi. SW of Actopan; 27.viii.1962; Ordway & Marston leg.; SEMC • 1♂; 22.5 mi. SW of Actopan; 27.viii.1962; N. Marston leg.; SEMC • 3♀; 29.4 mi. SE of Zimapán; 9.vii.1961; SEMC • 2♀; 3 mi. W of Pachuca; 24.vi.1953; SEMC • 1♂; ibid.; 24.vi.1954; SEMC • 1♂; 3.5 mi. NE of Tizayuca; 28.viii.1962; SEMC • 8♀; 4 mi. W of Pachuca; 16.vi.1961; SEMC • 3♂; 5 mi. N of Pachuca; 25.viii.1962; Roberts & Naumann leg.; SEMC • 1♂; 5 mi. W of Pachuca; 26.viii.1962; Ellen Ordway leg.; SEMC • 1♀, 1♂; Ixmiquilpan; 23.vi.1953; SEMC • 2♀, 1♂; Lagunilla; 14.vi.1951; H.E. Evans leg.; SEMC • 1♀; Pachuca; 6.vii.1937; Mead & Embury leg.; SEMC • 21♀, 26♂; ibid.; 24.vi.1953; SEMC • 24♀, 14♂; ibid.; 24.vi.1954; SEMC • 1♀, 1♂; ibid.; 28.vii.1954; SEMC • 1♀; Tezontepec, 21 mi. SW of Actopan; 27.viii.1962; Ordway & Marston leg.; SEMC – Jalisco • 1♀, 1♂; Lagos de Moreno; 21.viii.1954; C.D. Michener & party leg.; SEMC – Nuevo Leon • 1♀; 3.9 km NE of Iturbide; 24.iii.1991; R. Brooks, R. Leschen leg.; SEMC • 1♀; 37 km SW of Linares; 21.iii.1991; R. Brooks, R. Leschen leg.; SEMC • 1♀; 37 km SW of Linares, 4.8 km S on Bosque Escuela Road; 22.iii.1991; R. Brooks, R. Leschen leg.; SEMC • 3♂; 4 km S of Linares; 22.iii.1991; R. Brooks, R. Leschen leg.; SEMC • 3♀; 4.2 km S of Iturbide; 23.iii.1991; R. Brooks, R. Leschen leg.; SEMC • 1♀; ANP Corral de Bandidos, Garcia; 11.vii.2009; LRF & CVM leg.; LRF • 2♀; Carretera a Galeana 5 km al O de Puerto Pastores, Galeana; 13.iv.2014; LRF & CVM leg.; LRF • 1♀; Ejido San Juan del Prado, brecha a carretera 57, km 3; 3.iv.2013; LRF & CVM leg.; LRF • 1♀; Galeana, Brecha Cerro El Potosi; 4.iv.2013; LRF & CVM leg.; LRF • 2♀; Pablillo; 13.iv. 2014; LRF & CVM leg.; LRF – Oaxaca • 3♀; 1.4 km SW of Puebla/Oaxaca border, Highway 125 km 58; 14.ix.1996; R. Brooks leg.; SEMC – Puebla • 1♀; 1 km W of San Francisco de Acatepec on Highway 125 km 50.4; 14.ix.1996; R. Brooks leg.; SEMC • 1♀; 4 mi. NW of Petlalcingo; 4.vii.1953; SEMC • 1♀; 5 km E of Cuacnopalan on Highway 1350; 12.ix.1996; R. Brooks leg.; SEMC • 1♀; 8 mi. SE of Tehuitzingo; SEMC • 1♀; Axocopan; 6.vii.2011; J. Gibbs leg.; WRME • 1♀; San Francisco de Acatepec, Highway 125, km 54.5; 13.ix.1996; R. Brooks leg.; SEMC • 8♀, 2♂; Tecamachalco; 2.vii.1953; SEMC • 1♂; Tehuacán; 23.vi.1951; H.E. Evans leg.; SEMC – Querétaro • 9♀, 3♂; 10 mi. E of San Juan del Rio; SEMC – San Luis Potosi • 1♀; 12 mi. NW of Nuevo Morelos; 4.ix.1962; N. Marston leg.; SEMC • 1♀; 20 mi. SW of San Luis Potosi; 25.vii.1962; SEMC • 1♂; 5 mi. E of Ciudad del Maíz; 22–23.viii.1954; SEMC • 7♀; El Huizache; 22.viii.1954; SEMC • 1♀; El Salto Falls; 17.vi.1956; R.E. Beer & party leg.; SEMC – Sonora • 3♀; 30 km E of Agua Prieta; 11.iv.2005; R.L. Minckley leg.; PCYU • 3♀; ibid.; 22.iv.2005; R.L. Minckley leg.; PCYU • 1♀; Rancho San Bernardino; 21.iv.2005; A. Romero leg.; PCYU • 1♀; ibid.; 21.iv.2005; RLM • 1♀; ibid.; 27.iv.2005; R.L. Minckley leg.; PCYU • 1♂; ibid.; 3.v.2005; R.L. Minckley leg.; PCYU • 1♀; ibid.; 19.v.2005; R.L. Minckley leg.; PCYU – State of Mexico • 1♀; 10 mi. N of Atlacomulco; 18.viii.1954; SEMC • 3♀; 10 mi. NW of Chico; 12.viii.1951; SEMC • 1♀; ibid.; 12.viii.1954; SEMC • 1♂; 17 mi. N of Atlacomulco; 18.viii.1954; C. D. Michener & party leg.; SEMC • 1♀; Tepexpan; 12.viii.1954; SEMC.
UNITED STATES – Arizona • 1♀; Cochise Co., 0.7 mi. above (S of) Onion Saddle; 5.ix.2009; J.S. Ascher leg.; AMNH • 1♀; Cochise Co., 1 mi. E of Douglas; 17.viii.2003; J.S. Ascher, J.G. Rozen, M.A. Rozen leg.; AMNH • 1♀; Maricopa Co., 1100 W Mission Drive, Chandler; 27.iii.2017; S.J. Hall et al. leg.; ASUHIC • 1♀; Cochise Co., 13 mi. SW of Apache; 31.viii.1995; J.S. Ascher leg.; AMNH • 1♀; Cochise Co., 14 mi. N of Douglas, road to Rucker Canyon; 7.ix.2009; J.S. Ascher leg.; AMNH • 1♀; Maricopa Co., 1414 E Libra Drive, Tempe; 3.v.2017; S.J. Hall et al. leg.; ASUHIC • 1♀; Maricopa Co., 1926 E Calle De Caballos, Tempe; 4.iv.2017; S.J. Hall et al. leg.; ASUHIC • 1♀; Santa Cruz Co., 2 mi. W of Peña Blanca Canyon; 7.ii.1963; C.E. Mickel leg.; UMSP • 1♀; Cochise Co., 3 mi. NW of Sunizona; 21.viii.1995; J.G. Rozen & S.A. Budick leg.; AMNH • 1♀; Maricopa Co., Apache Trail, 1 mi. E of Tortilla Flat; 12.iii.2018; T.J. Wood leg.; WRME • 1♀; Greenlee Co., Apache-Sitgreaves National Forest, Hannagan Meadow; 3.vii.2017; BBSL • 1♀; ibid.; 7.viii.2017; BBSL • 1♀; Cochise Co., Barfoot Junction; 30.viii.1995; J.S. Ascher leg.; AMNH • 2♂; Cave Creek, 5 mi. W of Portal; 27.vii.1955; Timberlake leg.; UCRC • 1♀; Pinal Co., Copper Hill Wash nr. upper Cañada del Oro, 3 mi. SW Oracle; 11.ix.1962; C.E. Mickel leg.; UMSP • 7♀, 3♂; Cochise Co., Coronado National Forest, Barfoot Park; 12.vi.2017; BBSL • 1♀; ibid.; 1.vii.2017; BBSL • 1♀; Graham Co., Coronado National Forest, Hospital Flat Campground; 13.vi.2017; BBSL • 1♀; Cochise Co., Cottonwood Draw, 26 mi. E of Douglas; 6.ix.2011; J.G. Rozen, E.S. Wyman leg.; AMNH • 1♀; Coconino Co., Highway 67 just N. of Grand Canyon National Park; 22.vi.1999; L. Packer leg.; PCYU • 1♀; Lonesome Valley, 15 miles from Prescott; 3.vii.1937; Timberlake leg.; UCRC • 1♀; Maricopa Co., Phoenix Mountain Preserve; 5.iv.2017; S. J. Hall et al. leg.; ASUHIC • 1♀; Pima Co., Pima Canyon, Santa Catalina Mountains; 9.xi.1962; C.E. Mickel leg.; UMSP • 1♀; Cochise Co., Pinery Canyon, W. side of Chiricahua Mountains; 27.viii.2007; J.S. Ascher, C. Dong leg.; AMNH • 1♂; Prescott; 26.vii.1932; Timberlake leg.; UCRC • 1♀; Cochise Co., Rustler Park; 30.viii.1995; J.S. Ascher leg.; AMNH • 5♂; Cochise Co., Southwestern Research Station; 23.viii.2010; J.S. Ascher leg.; AMNH • 2♀; Cochise Co., Southwestern Research Station, 5 mi. W of Portal; 16.viii.2007; J.S. Ascher leg.; AMNH • 1♀; Pima Co., Tucson, Pima Canyon; 7.iii.2018; T.J. Wood leg.; WRME • 1♀; Maricopa Co., Usery Mountain Regional Park; 11.iii.2018; T.J. Wood leg.; WRME • 1♀; Cochise Co., W. Turkey Creek, Chiricahua Mts.; 2.ix.2003; J.G. Rozen, J.S. Ascher, R.L. Staff, & R.E. Edwards leg.; AMNH – New Mexico • 1♀; Sierra Co., #4, 6 mi. W of Caballo; 30.v.1991; G.W. Byers leg.; SEMC • 1♂; Hidalgo Co., 15 km N of Rodeo; 18.vi.1993; R.L. Minckley leg.; SEMC • 1♀, 1♂; Hidalgo Co., 26 mi. S of Animas; 18.ix.2012; J. Gibbs leg.; WRME • 1♀; Hidalgo Co., 28 mi. S of Animas; 30.viii.2010; J. Gardner leg.; UMSP • 1♂; Carrizozo; 9.vi.1950; L. D. Beamer leg.; SEMC • 2♀; Bernalillo Co., Cibola National Forest, Kiwanis Meadow; 22.vi.2017; BBSL • 1♀; ibid.; 4.viii.2017; BBSL • 1♀; Socorro Co., Cibola National Forest, South Baldy Meadow; 16.vi.2017; BBSL • 1♀; ibid.; 17.vi.2017; BBSL • 4♀; ibid.; 29.vii.2017; BBSL • 1♀; Corona; 8.vi.1950; L.D. Beamer leg.; SEMC • 1♀; Hidalgo Co., near Rodeo; 23.iii.1970; O.R. Taylor & party leg.; SEMC • 1♀; Hidalgo Co., Rodeo; 11.viii.1975; M. Chabot leg.; SEMC • 1♀, 1♂; Lincoln Co., Ruidoso; 10.vii.2007; Gibbs & Sheffield leg.; CMNC • 2♀; ibid.; 10.vii.2007; Gibbs & Sheffield leg.; PCYU • 1♀; San Miguel Co., Santa Fe National Forest, Jack’s Creek Campground; 19.vi.2017; BBSL • 1♀; Socorro Co., Sevilleta NWR-C5S; 15–29.vii.2003; K. Wetherill leg.; PCYU • 1♀; Socorro Co., Sevilleta NWR-S5; 2.vi.2007; K. Wetherill leg.; PCYU • 1♂; Eddy Co., Slaughter Canyon parking lot; 2.vi.2010; A. Druk leg.; BBSL • 1♂; ibid.; 2.vi.2010; J.D. Herndon leg.; BBSL • 1♀; Eddy Co., Slaughter Canyon, 0.4 km NE by N Slaughter Cave; 12.vii.2010; J.D. Herndon, A. Druk leg.; BBSL • 1♀; Sandoval Co., Valles Caldera National Preserve; 9.viii.2017; BBSL • 1♀; Eddy Co.; 13.vii.2010; J.D. Herndon, A. Druk leg.; BBSL • 1♂; ibid.; 23.viii.2010; R. Krauss leg.; BBSL • 1♂; 10.vi.2011; J.D. Herndon leg.; BBSL – Texas • 1♀; Alpine; 28–30 Jun.; Wickham leg.; AMNH • 1♀; Jeff Davis Co., Highway 166 west of Mount Livermore; 24.v.1997; L. Packer leg.; PCYU • 1♀; Jeff Davis Co., Madera Canyon, Davis Mountains; 1.vii.1948; C. & P. Vaurie leg.; AMNH • 3♀; Brewster Co.; 1 May; H. W. Ikerd leg.; BBSL • 2♀; ibid.; 30 Apr.; H.W. Ikerd leg.; BBSL – Utah • 1♀; Washington Co., 0.28 mi. NNE of Spendlove Knoll; 22.vi.2006; B. Hays, F. Nicklen leg.; BBSL • 1♀; Washington Co., 0.4 mi. NE of Spendlove Knoll; 13.vi.2006; B. Hays, F. Nicklen leg.; BBSL • 1♀; Iron Co., 2 mi. E of Cedar City; 20.vi.1999; L. Packer leg.; PCYU • 1♀; Washington Co., Baker Dam; 19.vi.1999; L. Packer leg.; PCYU.
Diagnosis. Females of Lasioglossum immigrans can be recognised by the combination of acarinarial fan present but extremely weakly developed (composed of only a few sparse suberect hairs; Fig. 5A), propodeum with strong oblique carina, mesoscutum and mesepisternum coarsely and densely punctate (IS < 1 PD in large part), frons punctures much smaller and denser than paraocular area punctures, and T1 dorsal surface moderately densely and distinctly punctate (IS = 1–2 PD). They are most similar to L. comulum Michener, 1951, L. dispersum Gibbs, 2018, L. strigosigena Michener, 1954, and a complex of several undescribed species from Mexico. Females of all but one of these species have the acarinarial fan well-developed with dense appressed hairs (Fig. 5B). The only known species with the acarinarial fan weakly developed as in L. immigrans is an undescribed Neotropical species from Veracruz, Mexico; this species has the frons punctures as large and dense as the paraocular area punctures. In addition, females of L. comulum have the T1 dorsal surface more finely and sparsely punctate (IS = 1–3 PD). In Canada, female L. immigrans may be confused with L. rufulipes and L. knereri. Females of L. rufulipes have the mesosoma punctures finer and sparser (IS = 1–2 PD) and no acarinarial fan (T1 anterior surface with evenly distributed erect hairs). Females of L. knereri have the propodeum lacking an oblique carina and have a well-developed acarinarial fan with dense appressed hairs (although often worn).
Males of Lasioglossum immigrans can be recognised by the face with very sparse tomentum not obscuring the surface, T1 anterior surface with no appressed hairs, depressed apical rims of metasomal terga usually punctate basally, mesoscutum and mesepisternum very coarsely and densely punctate (IS = 0 PD in large part, sparser (IS = 1–2 PD) on mesoscutum medially), propodeum lateral surface and often dorsolateral slope shiny and distinctly punctate, and integument blue on head and mesosoma, otherwise black to dark brown including tarsi and ventral surface of antennae. They are most similar to L. dispersum and a complex of several undescribed species from Mexico. They are probably also similar to L. comulum and L. strigosigena, although males of these species have not been definitively associated with females. Males of these similar species have the T1 anterior surface with at least a few appressed hairs (resembling the acarinarial fan of female L. immigrans), mesoscutum more sparsely punctate, and/or depressed apical rims of metasomal terga impunctate. In Canada, male L. immigrans are most similar to L. punctatoventre, which have the propodeum lateral surface and dorsolateral slope rugulose.
Description
Female. Colouration: Head and mesosoma blue–green to olive green; clypeus apex black; labrum black; mandible black with red apex; flagellum black dorsally, dark brown ventrally; pronotal lobe black; metasoma black to dark reddish brown with rims of terga and sterna narrowly translucent yellowish; legs black to dark reddish brown; tegula dark brown; wing membrane hyaline, veins brown to dark brown.
Pubescence: Body hair colour pale yellow on dorsum of head and mesosoma, otherwise white. Tomentum dense on pronotal collar and lobe and space between pronotal lobe and tegula; sparse on T2–T3 basolaterally and T4–T5 throughout. Mesoscutum hair moderately plumose. Wing hairs dark, short, and dense. Acarinarial fan complete but very weak, with only a few sparse, suberect hairs. T2 fringes sparse, T3 fringes sparse.
Surface sculpture: Clypeus shiny, with punctures dense in basal fourth (IS ≤ 1 PD), large and irregularly spaced apically (IS < 3 PD); supraclypeal area shiny, becoming weakly tessellate marginally, with punctures sparse (IS = 1–3 PD); paraocular area shiny, becoming weakly imbricate around antenna socket, with punctures dense (IS < 1 PD), irregularly sparser around antenna socket (IS < 2 PD); frons reticulate, with punctures crowded (IS = 0 PD); vertex shiny, with punctures crowded laterally (IS = 0 PD), sparse and obscure medially (IS = 1–2 PD); gena shiny, with punctures moderately dense (IS = 1–2 PD); postgena shiny; tegula punctures absent; mesoscutum tessellate, becoming shiny posteromedially, with punctures dense (IS < 1 PD), becoming crowded laterally and posteriorly (IS = 0 PD) and moderately sparse anteromedially (IS = 1–2 PD); scutellum shiny, with punctures crowded (IS = 0 PD), becoming sparser submedially (IS ≤ 1 PD), diversopunctate; metanotum finely rugulose; metapostnotum imbricate, with rugae strong, subparallel, nearly reaching posterior margin; preëpisternum areolate–rugose; hypoepimeron imbricate, with punctures crowded (IS = 0 PD); mesepisternum imbricate, with punctures crowded (IS = 0 PD), becoming irregularly sparser below scrobal groove (IS < 2 PD); metepisternum rugulose; propodeum lateral surface rugulose anteriorly, becoming tessellate posteriorly, posterior surface tessellate with weak vertical striae; T1 anterior slope coriarious, disc shiny, with punctures moderately dense (IS = 1–2 PD), impunctate in large apicolateral boss and on rim medially; T2 disc shiny, with punctures dense (IS ≤ 1 PD), apical rim weakly coriarious, with punctures moderately dense (IS = 1–2 PD).
Structure: Face length/width ratio 0.79 (± 0.01 standard deviation). Clypeus projecting ∼67% below suborbital tangent; apicolateral denticles low rounded knobs. Gena/eye width ratio 1 (± 0.05 standard deviation). Pronotal angle obtuse; forewing with three submarginal cells; tegula shape normal. Intertegular distance 1.1 (± 0.07 standard deviation) mm. Propodeum lateral carinae nearly reaching dorsal margin; oblique carina strong, straight. T2 depressed apical rim length about 50% of tergum (n = 10).
Male. Colouration: Head and mesosoma blue; clypeus apex colour black; labrum black; mandible black with red apex; flagellum dark reddish brown dorsally, orange ventrally; pronotal lobe black; metasoma black with rims of terga and sterna and downcurved lateral areas of terga becoming broadly translucent brown; legs black to dark reddish brown; tegula dark brown; wing membrane hyaline, veins with subcosta dark brown, otherwise brown to light brown.
Pubescence: Body hair colour white. Tomentum sparse on face below eye emargination, pronotal angle and lobe, and space between pronotal lobe and tegula. Mesoscutum hair thin to moderately plumose. Sterna hair long (1–3 OD), plumose, dense and erect. Wing hairs dark, short and sparse.
Surface sculpture: Clypeus shiny, with punctures dense (IS < 1 PD); supraclypeal area shiny, with punctures dense (IS ≤ 1 PD); paraocular area shiny, with punctures dense (IS < 1 PD); frons reticulate, with punctures crowded (IS = 0 PD); vertex shiny, with punctures dense laterally (IS < 1 PD), moderately sparse and obscure medially (IS = 1–2 PD); gena shiny, with punctures dense (IS ≤ 1 PD); postgena shiny; tegula punctures absent; mesoscutum shiny, with punctures moderately dense (IS = 1–2 PD), becoming crowded laterad of parapsidal lines and on posterior margin (IS = 0 PD); scutellum shiny, with punctures sparse (IS = 1–3 PD), becoming dense marginally (IS < 1 PD); metanotum shiny and densely punctate (IS < 1 PD); metapostnotum imbricate, with rugae strong, anastomosing, reaching posterior margin; preëpisternum areolate; hypoepimeron shiny, with punctures crowded (IS = 0 PD); mesepisternum shiny, with punctures crowded (IS = 0 PD); metepisternum rugose; propodeum lateral surface tessellate, with punctures crowded anteriorly (IS = 0 PD), becoming irregularly sparser posteriorly (IS < 2 PD), posterior surface shiny and densely punctate (IS ≤ 1 PD); T1 anterior slope shiny, disc shiny, with punctures moderately dense (IS = 1–2 PD), impunctate on rim medially; T2 disc shiny, with punctures dense (IS ≤ 1 PD), apical rim shiny, with punctures moderately dense (IS = 1–2 PD), absent apicomedially.
Structure: Face length/width ratio 0.82 (± 0.01standard deviation). Gena/eye width ratio 0.88 (± 0.04 standard deviation). F1:pedicel length ratio 1.06 (± 0.09 standard deviation); F2:F1 length ratio 1.91 (± 0.11 standard deviation); F2 length/width ratio 1.51 (± 0.14 standard deviation). Pronotal angle obtuse; forewing with three submarginal cells; tegula shape normal. Intertegular distance 0.89 (± 0.04 standard deviation) mm. Propodeum lateral carinae not reaching dorsal margin; oblique carina absent (n = 10).
Genitalia: As in Figure 4E–F. Gonocoxite broad, truncate apically. Gonostylus subrectangular apically, with a raised subcircular area at base dorsally, this area bearing a few long hairs. Retrorse lobe relatively narrow (about 3.5 times as long as broad) with sparse short hair except in subcircular glabrous area apically.
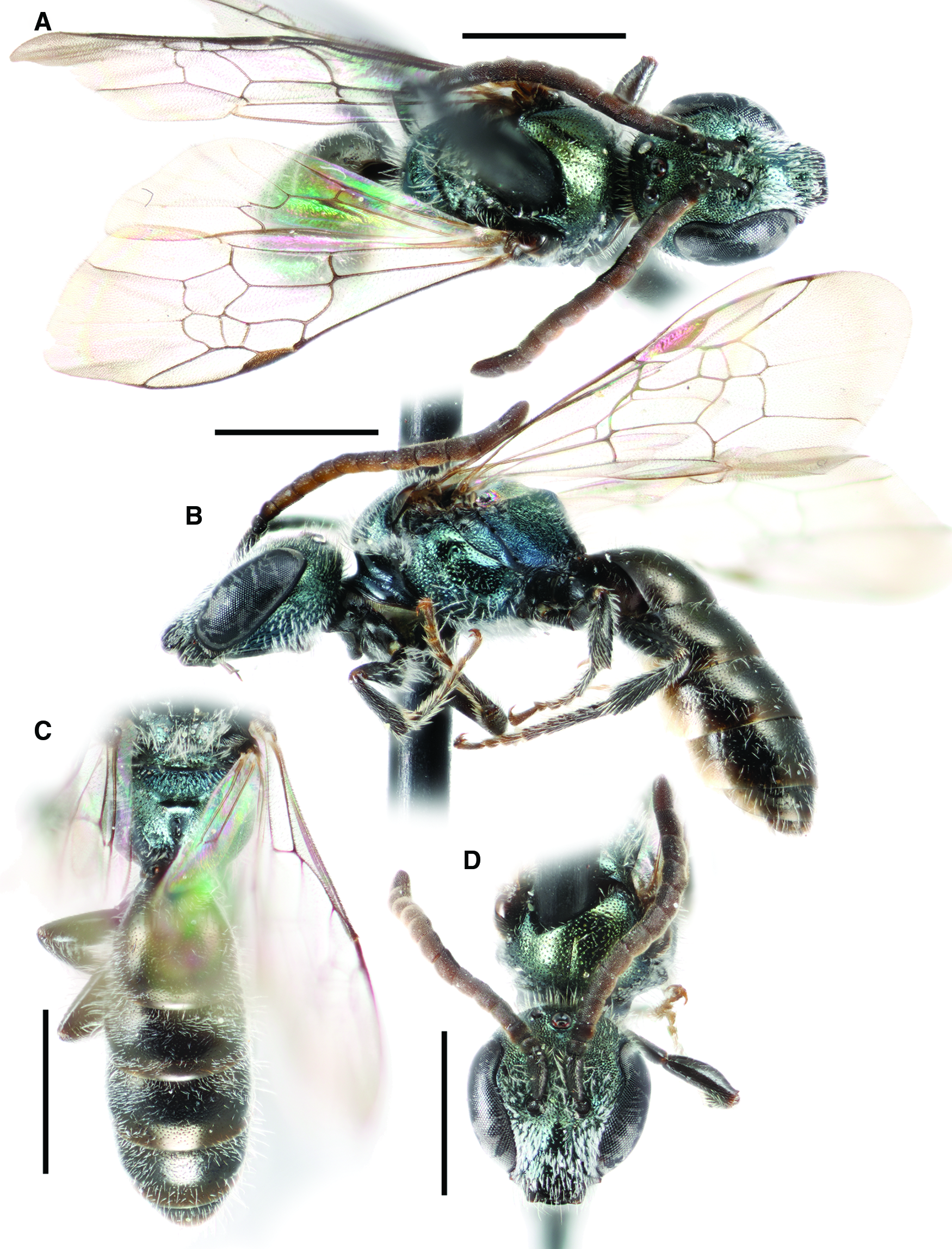
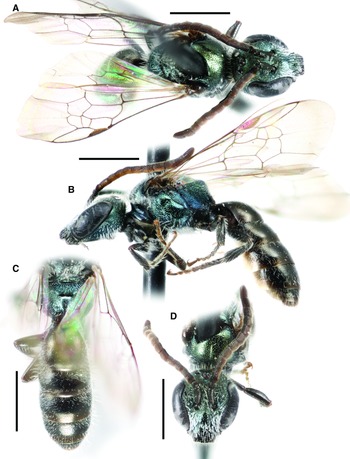
Fig. 6. Lasioglossum obnubilum (Sandhouse, 1924) (♂): A, dorsal habitus; B, lateral habitus; C, propodeum and metasoma; and D, face. Scale bars: 1 mm.
Etymology. The specific epithet immigrans is a Latin present participle meaning “migrating”. It refers to the disjunct population in Manitoba, Canada and its occurrence in three countries. It is meant to honour the diverse human immigrants and refugees who come to Canada and the United States of America from other nations and contribute to a stronger society.
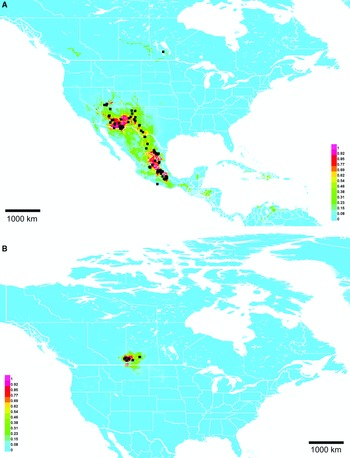
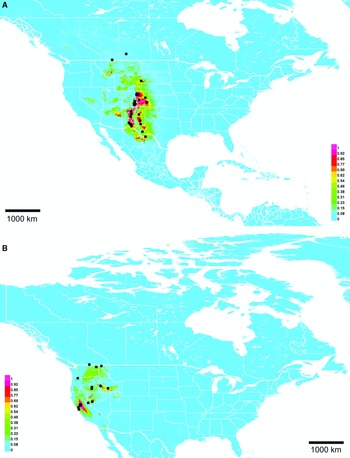
Range. Mountains from Utah, United States of American to Oaxaca, Mexico, with disjunct records in Manitoba, Canada and Acapulco, Mexico (Fig. 10A).
Floral hosts. Broadly polylectic (Supplementary material, Table S2).
DNA barcodes. 17 sequences available (BOLD process IDs: BBHYG462-10, DIAL1164-07, DIAL1165-07, DIAL1167-07, DIAL1168-07, DLII546-07, DLII789-07, DLII791-07, DLII843-07, DLII844-07, DLII845-07, DLII976-07, DLII978-07, DLII1267-08, DLII1332-08, DLIII097-18, HYMBB815-09). A very small amount of divergence occurs among these sequences (maximum P-distance 0.31%). The nearest known neighbour is L. dispersum Gibbs, 2018 from Puerto Rico. The minimum P-distance to L. dispersum is 3.06% and to the morphologically similar L. comulum is 4.69%.
Comments. Common in the southwestern United States of America and Mexico; rare in Canada. Lasioglossum immigrans has been frequently confused with L. comulum in collections. The true L. comulum is comparatively rare in the United States of America but may be more common in Mexico.
Lasioglossum (Dialictus) lionotus (Sandhouse, 1923)
Comments. Lasioglossum lionotus was recorded from Canada in Gibbs (Reference Gibbs2010) as L. asteris. The synonymy of L. asteris with L. lionotus was made by Gibbs (Reference Gibbs2011). The species is a social parasite of L. imitatum (Smith, 1853) (Wcislo Reference Wcislo1997), a common species that forms eusocial colonies (Michener and Wille Reference Michener and Wille1961).
Lasioglossum (Dialictus) obnubilum (Sandhouse, 1924)
Figure 6
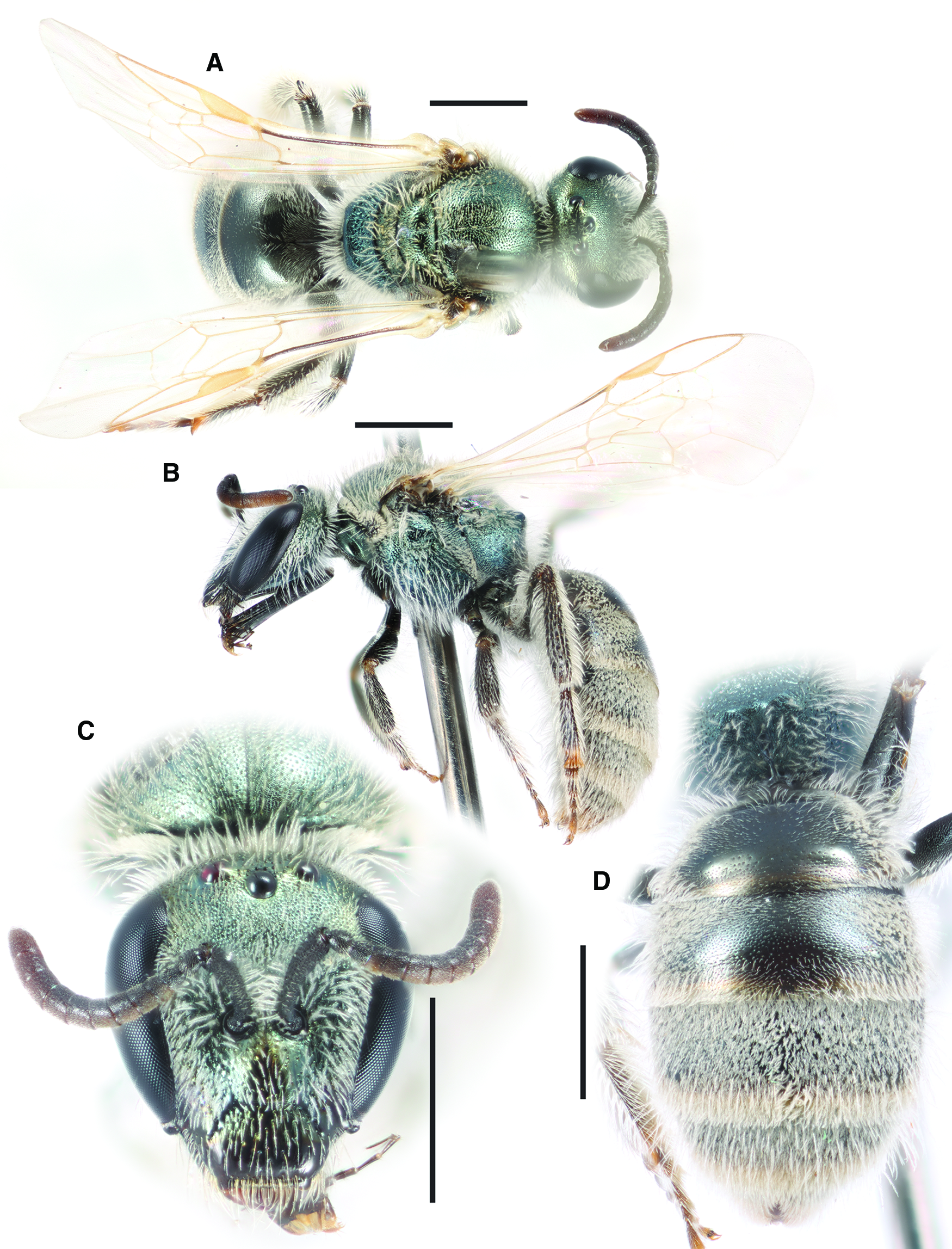
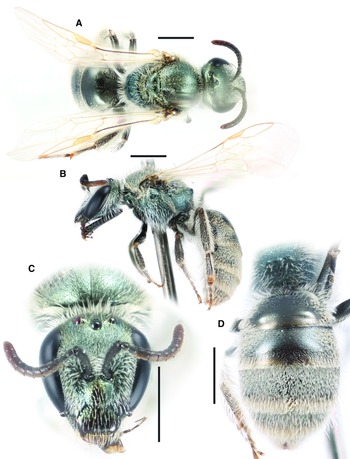
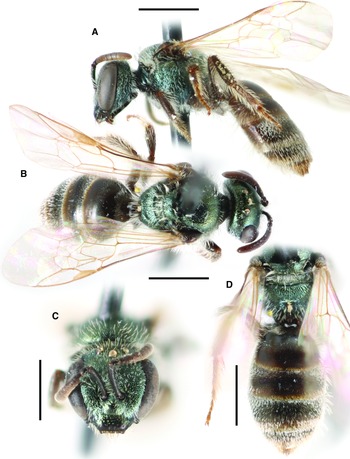
Fig. 7. Lasioglossum onuferkoi new species (holotype ♀): A, dorsal habitus; B, lateral habitus; C, face; and D, propodeum and metasoma. Scale bars: 1 mm.
Halictus (Chloralictus) obnubilus Sandhouse Reference Sandhouse1924: 28 (holotype, ♂, deposited in USNM; examined)
Lasioglossum (Dialictus) lilliputense Gibbs Reference Gibbs2010: 178 (holotype, ♀, deposited in PCYU; examined) new synonymy
Lasioglossum (Chloralictus) obnubilum – Michener Reference Michener1951: 1115 (catalogue)
Dialictus obnubilus – Hurd Reference Hurd1979: 1969 (catalogue) – Moure and Hurd Reference Moure and Hurd1987: 117 (catalogue)
Diagnosis. Males of L. obnubilum can be recognised by the combination of their small size (body length 4–4.5 mm; ITD ∼0.7 mm), face relatively long (length/width ratio ∼0.93), mesepisternum and central mesoscutum finely and moderately densely punctate (IS = 1–2 PD), propodeum relatively smooth with weak rugae, metasomal sterna with very short, sparse, thin hair, face with dense tomentum limited to paraocular area and upper clypeus, clypeus apical margin, flagellum, legs, tegula, and wings all dark brown, and head and mesosoma blue–green to olive green. They are most similar to an undescribed species that occurs at lower elevations in the southern United States of America. This species has the mesepisternum and mesoscutum more coarsely and densely punctate (IS < 1 PD), face covered in dense tomentum below eye emargination, and head and mesosoma blue to blue–green. They are also somewhat similar to L. brunneiventre and L. macroprosopum. Both of these species have very long, dense, plumose hair on the metasomal sterna, the flagellum ventral surface and tarsi orange, and a more western distribution not including the Rocky Mountains.
Comments. Lasioglossum obnubilum was described from the male only (Sandhouse Reference Sandhouse1924). There have been no published records of this species outside of Colorado, United States of America. Lasioglossum lilliputense was described based on morphology and DNA barcode data from female specimens collected in British Columbia, Canada and Idaho and Montana, United States of America (Gibbs Reference Gibbs2010). It was suggested at the time of its description that L. lilliputense could be the female of L. obnubilum. Examination of additional material, including males and females of L. obnubilum from the same locality, confirms this interpretation.
The Lasioglossum perdifficile species complex
Species included. Lasioglossum cyanurus (Cockerell, 1936), L. ebmerellum Gibbs, 2010, L. marinum (Crawford, 1904), L. megastictum (Cockerell, 1937), L. onuferkoi n. sp., L. perdifficile (Cockerell, 1895), L. perpunctatum (Ellis, 1913), L. sheffieldi Gibbs, 2010, and L. yukonae Gibbs, 2010 (possibly L. sombrerense Engel, 2011).
Diagnosis. The Lasioglossum perdifficile complex can be recognised in both sexes by the combination of clypeus strongly projecting below the suborbital tangent, tegula inner posterior margin straight or concave (sometimes weak or obscure, especially when the tegula is folded close to the thorax), metapostnotum relatively long (median length about twice that of metanotum in dorsal view, except in L. cyanurus, L. marinum, and L. megastictum, in which the tegula is punctate) with strong anastomosing rugae reaching the posterior margin (except in L. ebmerellum), and the propodeum with oblique carina absent or very weak (hardly visible). In addition, females have T1 with appressed tomentum laterally joining the acarinarial fan.
Comments. Lasioglossum punctiferellum (Cockerell, 1937) (= Halictus (Chloralictus) punctiferellus Cockerell, 1937) is herein treated as a subjective synonym of L. megastictum (new synonymy). The holotypes of these two species were collected at the same time and place, and they differ only in minor respects. The holotype of L. punctiferellum has less distinct punctures of the clypeus and mesepisternum, shinier supraclypeal area, and slightly sparser tomentum on T2–T4. Cockerell (Reference Cockerell1937) also wrote that L. megastictum has T1 and T4 slightly metallic green, but this character was not evident on re-examination of the type material. However, the holotype of L. punctiferellum has all metasomal terga with a very weak metallic bluish sheen, so it is likely that some specimens can occasionally have the terga weakly metallic. No specimens have been seen that exactly match the type of L. punctiferellum, but numerous intermediates exist. The holotype of L. megastictum is more typical of the species in general, and it is therefore given precedence in the absence of publication priority.
Many species in the L. perdifficile complex are known to be sand-habitat specialists (L. sheffieldi and L. yukonae (Gibbs Reference Gibbs2010); L. marinum (Gibbs Reference Gibbs2011); L. onuferkoi n. sp. (this paper)).
Lasioglossum (Dialictus) onuferkoi new species
ZooBank Registration # urn:lsid:zoobank.org:act:26ADA4CD-655F-4863-9730-7FB785CC888D

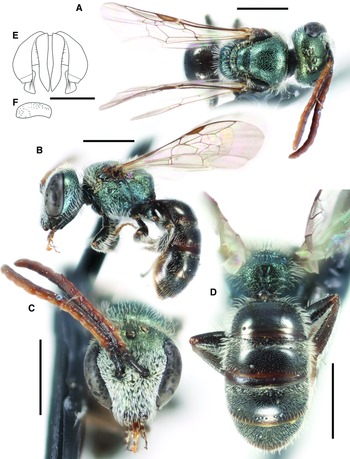
Fig. 8. Lasioglossum onuferkoi new species (♂): A, dorsal habitus; B, lateral habitus; C, face; D, propodeum and metasoma; E, genital capsule (dorsal view with gonostylus parallel to plane of vision); and F, retrorse lobe (lateral view parallel to plane of vision). Scale bars: A–D, 1 mm; E–F, 0.25 mm.

Fig. 9. Diagnostic characters for Lasioglossum onuferkoi new species: A, face of female L. onuferkoi; B, face of female Lasioglossum sheffieldi Gibbs, 2010; C, mesoscutum and tegula of female L. onuferkoi; D, mesoscutum and tegula of female L. sheffieldi; E, metapostnotum of female L. onuferkoi; F, metapostnotum of female Lasioglossum ebmerellum Gibbs, 2010; G, face of male L. onuferkoi; H, face of male L. ebmerellum; I, mesosoma pubescence of male L. onuferkoi; and J, mesosoma pubescence of male Lasioglossum perpunctatum (Ellis, 1913). B, D, F, H, and J reprinted from Gibbs (Reference Gibbs2010).
Material examined
Holotype: CANADA – Saskatchewan • ♀; Great Sand Hills NW (active dune); 50.6834° N, 109.2901° W; 6.viii.2019; T.M. Onuferko leg.; CMNC.
[Verbatim label: Canada: SK: Great Sand Hills NW (active dune) / N 50.6834°, W 109.2901° / 06.viii.2019, ex white pans / T.M. Onuferko, coll. // HOLOTYPE / Lasioglossum (Dialictus) onuferkoi Gardner and Gibbs]
Paratypes. (See Supplementary material, Table S1 for complete data including coordinates and collection codes.)
CANADA – Alberta • 2♀; 10.3 km S of Empress; 24.v.2019; T.M. Onuferko leg.; CMNC • 7♀; ibid.; 10.vii.2019; T.M. Onuferko leg.; CMNC • 4♀; 11.8 km SSW of Empress; 24.v.2019; T.M. Onuferko leg.; WRME • 5♀; ibid.; 10.vii.2019; T.M. Onuferko leg.; CMNC • 1♀; CFB Suffield NWA; 21.vi.1995; A.T. Finnamore, D. Pollock leg.; PMAE • 3♀; ibid.; 29.vi.1995; A.T. Finnamore, D. Pollock leg.; PMAE • 4♀, 1♂; ibid.; 17.vii.1995; A.T. Finnamore, D. Pollock leg.; PMAE • 2♀; ibid.; 31.vii.1995; A.T. Finnamore, D. Pollock leg.; PMAE • 1♀; ibid.; 1–16.vi.1995; A.T. Finnamore, D. Pollock leg.; PMAE • 2♀; ibid.; 1–17.vii.1995; A.T. Finnamore, D. Pollock leg.; PMAE • 1♀, 1♂; ibid.; 17–31.vii.1995; A.T. Finnamore, D. Pollock leg.; PMAE • 1♂; CFB Suffield, Amiens Mounted Rifles Rd. blowout; 28.vii.1994; Finnamore, Pollock leg.; PCYU – Saskatchewan • 1♂; 6.4 km NNW of Webb; 27.vii.2019; T.M. Onuferko leg.; CMNC • 1♀; ibid.; 3.viii.2019; T.M. Onuferko leg.; CMNC • 1♀; 7.6 km NNW of Webb; 27.vii.2019; T.M. Onuferko leg.; CMNC • 1♀; ibid.; 3.viii.2019; T.M. Onuferko leg.; CMNC • 1♀; Douglas Provincial Park, Elbow; 17.vi.2019; T.M. Onuferko leg.; CMNC • 1♂; ibid.; 22.vii.2019; T.M. Onuferko leg.; CMNC • 13♀; Great Sand Hills NW; 11.vii.2019; T.M. Onuferko leg.; CMNC • 2♀; ibid.; 11.vii.2019; T.M. Onuferko leg.; PCYU • 1♂; ibid.; 25.vii.2019; T.M. Onuferko leg.; WRME • 7♀, 5♂; ibid.; 6.viii.2019; T.M. Onuferko leg.; CMNC • 1♀, 1♂; ibid.; 6.viii.2019; T.M. Onuferko leg.; PCYU • 1♀; Great Sand Hills WC; 12.vii.2019; T.M. Onuferko leg.; CMNC • 2♀, 2♂; ibid.; 7.viii.2019; T.M. Onuferko leg.; CMNC • 12♀; N of Bitter Lake, Tunstall; 14.vii.2019; T.M. Onuferko leg.; CMNC • 8♀, 2♂; ibid.; 28.vii.2019; T.M. Onuferko leg.; WRME • 10♀; ibid.; 4.viii.2019; T.M. Onuferko leg.; CMNC.
Diagnosis. Both sexes of L. onuferkoi can be distinguished from other members of the L. perdifficile complex by the combination of face very long (length/width ratio ∼0.91) (Figs. 9A, G) (< 0.85 in all other species except L. ebmerellum) (Figs. 9B, H), mesepisternum shiny and finely and densely punctate (IS < 1 PD) (dull and impunctate in L. yukonae), tegula subovoid with small straight inner posterior margin (Fig. 9C) (more oblong with longer straight or concave inner posterior margin in all other species except L. ebmerellum (Fig. 9D)), metapostnotum with strong anastomosing rugae reaching posterior margin (Fig. 9E) (rugae not reaching margin in L. ebmerellum (Fig. 9F)), and wings hyaline with white hairs (subhyaline with dark hairs in all other species except L. marinum). In addition, the female has T3 completely covered in dense tomentum (tomentum sparse or absent medially in all other species except L. ebmerellum and L. perpunctatum), and the male has the face covered in dense tomentum except for the clypeus apical half (Fig. 9G) (dense tomentum limited to paraocular area in all other species except L. marinum (Fig. 9H)) and the mesoscutum with long, woolly, densely plumose hair (Fig. 9I) (mesoscutum with shorter and thinner hair in all other species (Fig. 9J)). Lasioglossum onuferkoi is also similar to L. pruinosum. Both sexes of L. pruinosum have the mesepisternum rugulose and indistinctly punctate and the metasomal terga metallic blue–green (black in L. onuferkoi), and males have the clypeus apical margin yellow (black in L. onuferkoi). The only one of these similar species known to occur sympatrically with L. onuferkoi is L. pruinosum.
Description
Female. Colouration: Head and mesosoma blue–green; clypeus apex black to reddish brown; labrum black; mandible black in basal half, orange in apical half with red apex; flagellum black to reddish brown dorsally, brown ventrally; pronotal lobe black to reddish brown; metasoma black to dark reddish brown with rims of terga and sterna broadly translucent yellow; legs black to reddish brown with femur–tibia joints brown; tegula brown; wing membrane hyaline, veins with subcosta dark brown, otherwise brown to pale yellow.
Pubescence: Body hair colour white. Tomentum dense on gena, pronotal angle and lobe, metanotum anteriorly, metepisternum, T1–T2 laterally, and T3–T5 throughout; sparse on paraocular area, preëpisternum, mesepisternum, and propodeum lateral face. Mesoscutum hair thin to moderately plumose. Wing hairs light, short, and dense. Acarinarial fan complete, dense. T2 fringes dense, T3 fringes dense.
Surface sculpture: Clypeus shiny, with punctures sparse (IS = 1–3 PD), becoming dense basolaterally (IS < 1 PD); supraclypeal area shiny, with punctures sparse (IS = 1–3 PD); paraocular area shiny, with punctures dense (IS < 1 PD); frons shiny, with punctures fine, crowded (IS = 0 PD); vertex shiny, with punctures dense laterally (IS ≤ 1 PD), sparse medially (IS = 1–3 PD); gena shiny, with punctures moderately sparse (IS = 1–2 PD); postgena shiny; tegula punctures absent; mesoscutum shiny, becoming weakly tessellate anteromedially, with punctures dense (IS < 1 PD), becoming slightly sparser submedially (IS = 1–2 PD) and diversopunctate anteriorly; scutellum shiny, with punctures dense marginally and on median line (IS < 1 PD), sparse submedially (IS = 1–3 PD); metanotum imbricate; metapostnotum shiny to tessellate, with rugae strong, anastomosing, reaching posterior margin; preëpisternum ruguloso-punctate; hypoepimeron weakly ruguloso-punctate, with punctures dense (IS < 1 PD), obscure; mesepisternum shiny, with punctures dense (IS < 1 PD), becoming crowded dorsally (IS = 0 PD); metepisternum lineate dorsally, rugulose medially, imbricate ventrally; propodeum lateral surface tessellate, becoming weakly rugulose on anterior margin, posterior surface tessellate; T1 anterior slope shiny or weakly coriarious, disc shiny, with punctures fine, moderately dense (IS = 1–2 PD), becoming sparse medially and in small apicolateral boss (IS = 2–6 PD); T2 disc shiny, with punctures fine, dense (IS ≤ 1 PD), becoming slightly sparser medially (IS = 1–2 PD), apical rim shiny, with punctures fine, sparse (IS = 1–4 PD).
Structure: Face length/width ratio 0.91 (± 0.01 standard deviation). Clypeus projecting ∼75% below suborbital tangent; apicolateral denticles rounded knobs. Gena/eye width ratio 1.14 (± 0.12 standard deviation). Pronotal angle obtuse; forewing with three submarginal cells; tegula shape round with inner posterior margin straight. Intertegular distance 1.23 (± 0.04 standard deviation) mm. Propodeum lateral carinae not reaching dorsal margin; oblique carina absent. T2 depressed apical rim length less than 50% of tergum (n = 10).
Male. Colouration: Head and mesosoma blue to blue–green; clypeus apex colour black; labrum black; mandible orange with black base and red apex; flagellum black dorsally, orange to light brown ventrally; pronotal lobe black; metasoma black to dark reddish brown with rims of terga and sterna and downcurved lateral areas of terga narrowly translucent yellow; legs black to dark reddish brown with tarsi and sometimes femur–tibia joints yellow–orange; tegula dark reddish brown to light brown; wing membrane hyaline, veins with subcosta black, otherwise brown to pale yellow.
Pubescence: Body hair colour white. Tomentum dense on face below eye emargination and above apical half of clypeus, gena, pronotal angle and lobe, metepisternum, T2–T3 laterally, and T4–T5 throughout; sparse on T1 laterally. Mesoscutum hair densely plumose. Sterna hair moderately long (1–2 OD), densely plumose, dense and erect on S2–S3, thin and suberect on S4–S5. Wing hairs light, short and dense.
Surface sculpture: Clypeus shiny, with punctures dense (IS ≤ 1 PD), becoming sparse apicolaterally (IS = 1–2 PD); supraclypeal area shiny, with punctures dense (IS ≤ 1 PD); paraocular area shiny, with punctures dense (IS < 1 PD); frons shiny, with punctures dense (IS < 1 PD); vertex shiny, with punctures dense laterally (IS < 1 PD), slightly sparser medially (IS ≤ 1 PD); gena shiny, with punctures moderately dense (IS = 1–2 PD); postgena weakly tessellate; tegula punctures absent; mesoscutum shiny, with punctures dense (IS < 1 PD), becoming sparse submedially (IS = 1–3 PD); scutellum shiny, with punctures dense marginally and on median line (IS < 1 PD), sparse submedially (IS = 1–3 PD); metanotum rugulose; metapostnotum shiny, with rugae strong, anastomosing, reaching posterior margin; preëpisternum areolate-rugulose; hypoepimeron shiny, with punctures dense (IS < 1 PD); mesepisternum shiny, with punctures dense (IS < 1 PD); metepisternum rugulose, becoming lineate dorsally; propodeum lateral surface tessellate, with punctures dense (IS < 1 PD), shallow, and obscure, posterior surface rugulose; T1 anterior slope shiny, disc shiny, with punctures fine, moderately dense (IS = 1–2 PD); T2 disc shiny, with punctures fine, moderately dense (IS = 1–2 PD), apical rim shiny, with punctures fine, sparse (IS = 1–4 PD).
Structure: Face length/width ratio 0.91 (± 0.01 standard deviation). Gena/eye width ratio 1.01 (± 0.07 standard deviation). F1:pedicel length ratio 1.23 (± 0.16 standard deviation); F2:F1 length ratio 1.73 (± 0.08 standard deviation); F2 length/width ratio 1.6 (± 0.1 standard deviation). Pronotal angle obtuse; forewing with three submarginal cells; tegula shape round with inner posterior margin straight. Intertegular distance 1.03 (± 0.04 standard deviation) mm. Propodeum lateral carinae not reaching dorsal margin; oblique carina absent (n = 10).
Genitalia: As in Figure 8E–F. Gonocoxite relatively narrow, truncate apically. Gonocoxite boot-shaped, with sparse short hairs. Retrorse lobe broad, ovoid, mostly covered in shallow round dimples and sparse short hairs.

Fig. 10. Georeferenced collection records (black squares) and predicted distribution by maximum entropy ecological niche modelling in Maxent (colour shading). Warmer colours indicate higher cloglog probability of occurrence. A, Lasioglossum immigrans; and B, L. onuferkoi. Duplicate records within 0.1 degree were pruned to reduce bias in A, but only exact duplicates were pruned in B owing to the small number of records.
Etymology. The specific epithet onuferkoi is a dedication to Thomas Onuferko, who collected all the Saskatchewan specimens and contributed to knowledge of this species as a sand dune habitat specialist.
Range. Sand hills and dunes of southwestern Saskatchewan and southeastern Alberta (Fig. 10B).
DNA barcodes. One sequence available (BOLD process ID: DLII737–07). The nearest neighbours are L. sheffieldi and L. yukonae (average P-distance 2.57%).
Lasioglossum (Dialictus) punctatoventre (Crawford, 1907)
Figure 11

Fig. 11. Lasioglossum punctatoventre (Crawford, 1907) (♂): A, dorsal habitus; B, lateral habitus; C, face; and D, metasoma. Scale bars: 1 mm.
Comments. Gibbs (Reference Gibbs2010) neglected to include the male of L. punctatoventre, although Crawford (Reference Crawford1907) described both sexes. Several males have now been associated with females of L. punctatoventre by morphology and geographical co-occurrence, and they are figured here for the first time. See L. reasbeckae (below) for a comparative diagnosis of the male, and Gibbs (Reference Gibbs2010) for a diagnosis and redescription of the female.
Lasioglossum (Dialictus) reasbeckae Gibbs, 2010
Figure 12

Fig. 12. Lasioglossum reasbeckae Gibbs, 2010 (♂): A, lateral habitus; B, dorsal habitus; C, face; and D, propodeum and metasoma. Scale bars: 1 mm.
Diagnosis. Males of L. reasbeckae can be recognised by the combination of mesosoma shiny with large, coarse punctures, basal margins of T2–T3 strongly depressed, clypeus apical half black or brown and becoming brassy in basal half, and metasomal terga with discs sparsely punctate (IS = 1–4 PD) and apical rims entirely impunctate. They are most similar to L. dashwoodi and L. punctatoventre. Males of L. dashwoodi have the metasomal terga metallic blue–green. Males of L. punctatoventre have the metasomal terga densely punctate (IS = 0.5–2 PD) including the apical rims in part. All of these species occur sympatrically in British Columbia, Canada and Washington, United States of America, but only L. punctatoventre and L. reasbeckae are known from Oregon and western California, United States of America, and only L. dashwoodi is known from east of the Cascade and Sierra Nevada ranges, United States of America.
Comments. Gibbs (Reference Gibbs2010) described and diagnosed the female of L. reasbeckae, but the male was unknown. A series of males at UCRC were associated with some females of L. reasbeckae by morphology and geographical co-occurrence. It is now evident that L. reasbeckae is more common at relatively high elevations and ranges as far south as the San Bernardino Mountains in California, United States of America, whereas the similar and closely related L. punctatoventre is more common at lower elevations.
Lasioglossum (Dialictus) rozeni Gibbs, 2011
Comments. Gibbs (Reference Gibbs2010) incorrectly referred to this species as L. cephalotes (Dalla Torre, 1896). The correct usage of L. cephalotes was subsequently determined, and L. rozeni was described as new (Gibbs Reference Gibbs2011). Lasioglossum rozeni has not yet been recorded from Canada, but it is known from adjacent states. It is included in the key because its distribution suggests that it may occur in southern Ontario. It frequently co-occurs with L. versatum (Robertson, 1902). The relatively large size of both species suggests a possible host–parasite association.
Lasioglossum (Dialictus) smilacinae (Robertson, 1897)
Comments. This species was first recorded from Canada by Gibbs (Reference Gibbs2010) as L. zophops (Ellis, 1914). Gibbs (Reference Gibbs2011) resurrected L. smilacinae from synonymy with L. laevissimum (Smith, 1853) and treated L. zophops as a synonym of L. smilacinae.
Lasioglossum (Dialictus) stictaspis (Sandhouse, 1923)

Fig. 13. Lasioglossum stictaspis (Sandhouse, 1923) (♀): A, lateral habitus; B, dorsal habitus; C, face; and D, propodeum and metasoma. Scale bars: 1 mm.

Fig. 14. Lasioglossum stictaspis (Sandhouse, 1923) (♂; A–B, D–F: holotype): A, dorsal habitus; B, lateral habitus; C, face; D, propodeum and metasoma; E, genital capsule (dorsal view with gonostylus parallel to plane of vision); and F, retrorse lobe (lateral view parallel to plane of vision). Scale bars: A–D, 1 mm; E–F, 0.25 mm.

Fig. 15. Lasioglossum tegulariforme (Crawford, 1907) (holotype ♀): A, lateral habitus; B, dorsal habitus; C, face; and D, metasoma. Scale bars: 1 mm.
Dialictus stictaspis Sandhouse Reference Sandhouse1923: 195 (holotype, ♂, deposited in USNM, examined)
Halictus (Chloralictus) albuquerquensis Michener Reference Michener1937: 316 (holotype, ♀, deposited in CAS, examined) new synonymy
Lasioglossum (Dialictus) stictaspis – Michener Reference Michener1951: 1119 (catalogue)
Lasioglossum (Chloralictus) albuquerquense – Michener Reference Michener1951: 1112 (catalogue)
Dialictus stictaspis – Hurd Reference Hurd1979: 1971 (catalogue) – Moure and Hurd Reference Moure and Hurd1987: 131 (catalogue)
Dialictus albuquerquensis – Hurd Reference Hurd1979: 1964 (catalogue) – Moure and Hurd Reference Moure and Hurd1987: 89 (catalogue)
Specimens examined
Holotype: UNITED STATES – New Mexico • ♂; Mesilla; [32.27° N, 106.8° W]; June year and day unknown; Cockerell leg.; USNM
Other material. (See Supplementary material, Table S1 for complete data including coordinates and collection codes.)
CANADA – Alberta • 9♀; PMAE – Saskatchewan • 1♂; CNC.
UNITED STATES – Colorado • 4♀; CUIC • 12♀, 1♂; SEMC • 18♀, 4♂; UCMC – New Mexico • 5♀; BBSL • 1♀; CAS • 1♀; NMSU • 16♀, 9♂; SEMC • 9♀; TAMU • 3♀; UCMC – Texas • 7♀, 1♂; BBSL • 1♂; UCDC – Wyoming • 1♀; UMSP.
Diagnosis. Females of L. stictaspis can be recognised by the combination of tegula enlarged (reaching posterior margin of mesoscutum in dorsal view), with inner posterior margin concave, and densely punctate in centre (IS ≤ 1 PD), metapostnotum dull due to tessellate microsculpture and usually with weak subparallel rugae, mesepisternum shiny (sometimes with weak microsculpture) and densely punctate (IS < 1 PD), mesoscutum coarsely punctate (2–4 punctures between posterior end of parapsidal line and lateral edge of mesoscutum), T3 with dense subapical band of tomentum, and tegula and metasoma black to dark brown. They are most similar to L. ellisiae and several undescribed species from the southwestern United States of America and Mexico. Females of L. ellisiae have the metapostnotum shiny with strong, often anastomosing rugae, mesoscutum usually densely punctate (4–5 punctures between posterior end of parapsidal line and lateral edge of mesoscutum), tegula often more sparsely punctate (IS ≤ 2 PD), T3 usually without subapical tomentum, and tegula and/or metasoma sometimes orange.
Males of L. stictaspis can be recognised by the tegula densely punctate (IS ≤ 1 PD), relatively large (slightly exceeding posterior margin of mesoscutum in dorsal view), and inner posterior margin concave, with a small rounded projection posteriorly, mesoscutum relatively sparsely punctate (IS ≤ 1 PD near parapsidal line), mesepisternum densely punctate (IS < 1 PD), metapostnotum dull due to tessellate microsculpture and usually with weak subparallel rugae, and T1–T3 with dense punctures reaching premarginal line (IS ≤ 1 PD). They are most similar to L. tegulare, L. ellisiae, and L. helianthi. All of these species have the tegula slightly smaller (not exceeding posterior margin of mesoscutum in dorsal view), inner posterior margin more weakly concave, coming to a narrow point or blunt angle posteriorly, and usually more sparsely punctate in part (IS ≥ 1 PD). Males of L. tegulare and L. ellisiae have the metapostnotum shiny with stronger parallel rugae. Males of L. ellisiae and L. helianthi have the mesoscutum slightly more densely punctate (IS < 1 PD and IS < 0.5 PD near parapsidal line, respectively) and tegula often slightly more sparsely punctate (IS ≤ 1 PD). Males of L. ellisiae also have T1–T3 with punctures becoming sparser towards the premarginal line.
Some other species (described and undescribed) from the southwestern United States of America and Mexico are also similar but do not occur sympatrically except in southern New Mexico, United States of America and, in one case, as far north as Colorado, United States of America, and will lack one or more of the diagnostic characters given above. These species will be revised in a forthcoming work.
Range. Western Great Plains and mountain valleys from New Mexico, United States of America to Alberta, Canada (Fig. 17A).
Floral hosts. Polylectic (Supplementary material, Table S2).
DNA barcodes. Three sequences available (BOLD process IDs: DLIII224-20, DLIII225-20, DLIII227-20). These sequences are very similar and poorly distinguished from three other undescribed species (maximum intraspecific P-distance 0.3%; minimum interspecific P-distance 0.64%).
Comments. The name Lasioglossum stictaspis has not been widely applied in the past because the primary diagnostic characters used for this species were the enlarged, punctate tegula and the presence of only two submarginal cells in the forewing (Sandhouse Reference Sandhouse1923). The number of submarginal cells is known to be variable in many bees including several L. (Dialictus) species (Gardner and Gibbs Reference Gardner and Gibbs2020; Scarpulla Reference Scarpulla2018), although this variation is typically rare. Among the specimens reported here, only one additional male from Las Cruces, New Mexico, United States of America was discovered with two submarginal cells. All other specimens have the usual three submarginal cells.
Many other species with an enlarged, punctate tegula can also have only two submarginal cells in rare cases, including L. gaudiale (Sandhouse, 1924) (new combination, see comments on L. tegulariforme below) and several undescribed species. In addition, the holotype of L. stictaspis is missing the head, limiting the number of visible alternative characters. As a result, the few specimens determined as L. stictaspis prior to this work are likely misidentified.
The female of L. stictaspis was associated by a series of both sexes from the same collecting event in Las Cruces, New Mexico, United States of America (very near the type locality), and a series of both sexes from Boulder County, Colorado, United States of America, from which DNA barcodes were obtained. The female was found to match the holotype of L. albuquerquense. This holotype is missing the metasoma, but an additional series of females in good condition from the type locality of Albuquerque, New Mexico, United States of America helped confirm the synonymy.
Lasioglossum (Dialictus) tegulariforme (Crawford, 1907)

Fig. 16. Lasioglossum tegulariforme (Crawford, 1907) (♂): A, dorsal habitus; B, lateral habitus; C, face; D, propodeum and metasoma; E, genital capsule (dorsal view with gonostylus parallel to plane of vision); and F, retrorse lobe (lateral view parallel to plane of vision). Scale bars: A–D, 1 mm; E–F, 0.25 mm.

Fig. 17. Georeferenced collection records (black squares) and predicted distribution by maximum entropy ecological niche modelling in Maxent (colour shading). Warmer colours indicate higher cloglog probability of occurrence. A, Lasioglossum stictaspis; and B, L. tegulariforme. Duplicate records within 0.1 degree were pruned to reduce bias.
Halictus (Chloralictus) tegulariformis Crawford Reference Crawford1907: 194 (holotype, ♀, deposited in USNM, examined)
Lasioglossum (Chloralictus) tegulariforme – Michener Reference Michener1951: 1118 (catalogue)
Dialictus tegulariformis – Hurd Reference Hurd1979: 1972 (catalogue) – Moure and Hurd Reference Moure and Hurd1987: 133 (catalogue)
Specimens examined
Holotype: UNITED STATES – Nevada • ♀; Ormsby Co.; [39.2° N, 119.8° W]; 6.vii; Baker leg.; USNM 12068.
Other material. (See Supplementary material, Table S1 for complete data including coordinates and collection codes.)
CANADA – British Columbia • 1♀; PCYU.
UNITED STATES – California • 2♀; CAS • 25♀; UCDC • 1♀, 2♂; WRME – Idaho • 3♀; CUIC • 6♀; FWSE – Nevada • 1♂; SEMC – Oregon • 23♀; FWSE – Washington • 4♀; FWSE.
Diagnosis. Females of L. tegulariforme can be recognised by the combination of T1 with at least a narrow impunctate median line (often broader) (T1 evenly punctate throughout in all other known species), paraocular area punctures similar in size and density to frons punctures (paraocular area punctures larger and coarser in other Canadian species), T3 densely clothed in short, simple, yellow hairs in addition to basolateral tomentum (T3 with sparser, whiter hairs in other Canadian species, except tomentose in L. helianthi), tegula very large (clearly exceeding posterior margin of mesoscutum in dorsal view) and densely punctate with few or no interspaces up to 1 PD (tegula not exceeding posterior margin of mesoscutum in dorsal view and with many interspaces 1 PD or more in other Canadian species except L. stictaspis), and T1 anterior slope usually with weak microsculpture (T1 shiny in L. gaudiale and strongly coriarious in other Canadian species).
Males of L. tegulariforme can be recognised by the combination of T1 at least slightly more sparsely punctate medially (IS = 2–6 PD) than laterally (IS = 1–3 PD) (T1 evenly punctate throughout in all other known species), metasomal terga with basolateral tomentum very sparse or absent (T2–T3 with distinct basolateral tomentum in L. gaudiale), face with tomentum dense below eye emargination and absent above (face with dense tomentum below median ocellus in L. gaudiale and some other species), tegula very densely punctate with few or no distinct interspaces (surface appearing dull) and inner margin strongly concave with broadly rounded projection directed towards axilla posteriorly (tegula more sparsely punctate with inner posterior margin more weakly concave in other Canadian species except L. stictaspis), and propodeum lateral face with punctures shallow and indistinct (shiny and distinctly punctate in L. gaudiale).
Both sexes of L. tegulariforme are most similar to L. gaudiale and an undescribed species from southern California, United States of America, but the characters of T1 will readily separate them.
Range. California Central Valley, United States of America north to southern British Columbia, Canada and east through Idaho, United States of America (Fig. 17B).
Floral hosts. Broadly polylectic (Supplementary material, Table S2).
DNA barcodes. Three sequences available (BOLD process IDs: BCLRB752-10, DLIII111-18, DLIII112-18). These sequences are highly divergent from L. gaudiale and L. helianthi (maximum intraspecific P-distance 0.31%; minimum interspecific P-distance to L. gaudiale 3.37%; to L. helianthi 2.83%).
Comments. Lasioglossum gaudiale is herein resurrected from synonymy with L. tegulariforme. Lasioglossum gaudiale and L. helianthi were synonymised with L. tegulariforme in Michener (Reference Michener1951). The holotype of L. tegulariforme is a female, that of L. gaudiale a male, so it has seemed plausible that these are different sexes of the same species. Series of associated females and males and DNA barcodes now indicate that the true male of L. tegulariforme is morphologically, genetically, and geographically distinct from L. gaudiale. Lasioglossum gaudiale is primarily a desert species common in the Sonoran and Mojave deserts and the Los Angeles basin, United States of America. The true L. tegulariforme is here considered to have a much more restricted range than previously recorded. It occurs sympatrically with L. helianthi throughout this range.
The holotype of L. helianthi is a female but is in poor condition, making morphological comparison difficult. However, certain diagnostic characters, including the face, tegula, and dorsal surface of T1, are visible and clearly match L. imbrex more closely than L. tegulariforme. Specimens determined by P.H. Timberlake as L. helianthi before he synonymised it in Michener (Reference Michener1951) are also clearly L. imbrex.
The name Lasioglossum tegulariforme has been widely over-applied to specimens from the western Nearctic region. This was first noted by Cockerell (Reference Cockerell1937) and corroborated by Gibbs (Reference Gibbs2010), who suggested that many old records of L. tegulariforme actually pertain to L. imbrex (= L. helianthi). In this work, we suggest that old L. tegulariforme records (especially any from far outside the range in Fig. 17B) may also pertain to L. gaudiale, L. stictaspis, L. paululum (Sandhouse, 1924), L. pseudotegulare (Cockerell, 1896), or an undescribed species. References to L. tegulariforme in Sandhouse (Reference Sandhouse1924) probably pertain to L. stictaspis, and references in Sandhouse and Cockerell (Reference Sandhouse and Cockerell1924) probably pertain to L. gaudiale and/or an undescribed species. Specimen records and floral hosts in Moure and Hurd (Reference Moure and Hurd1987) are likely from several species. Only records from specimens personally examined by J. Gardner or Joe Engler (FWSE) and known to be correctly identified are presented here.
Lasioglossum (Dialictus) tuolumnense Gibbs, 2009
Comments. Lasioglossum tuolumnense is a member of the L. petrellum species complex (Gibbs Reference Gibbs2009). Originally described from the Sierra Nevada Mountains of California, United States of America, a single male was documented from British Columbia, Canada, based on morphology and DNA barcode data (Heron and Sheffield Reference Heron and Sheffield2015).
Lasioglossum (Dialictus) weemsi (Mitchell, 1960)
Figure 18

Fig. 18. Lasioglossum weemsi (Mitchell, 1960) (♂): A, dorsal habitus; B, lateral habitus; C, face; and D, propodeum and metasoma. Scale bars: 1 mm.
Diagnosis. Males of L. weemsi can be distinguished from the similar species in the L. viridatum complex, and especially L. hitchensi, L. paradmirandum, L. admirandum, and L. subviridatum by the combination of mesoscutum entirely dull with tessellate microsculpture (partially shiny in L. subviridatum), mesepisternum rugulose (smooth and imbricate to tessellate in L. paradmirandum), metasomal terga moderately densely punctate (IS = 1–2 PD) (sparsely punctate (i = 2–4 PD) in L. hitchensi) and without tomentum, clypeus apical margin brown (yellow in L. admirandum), and tarsi orange.
Comments. The male of L. weemsi was unknown at the time of the revisions by Gibbs (Reference Gibbs2010, Reference Gibbs2011). A single specimen was associated with some females via DNA barcodes. The DNA barcodes of L. hitchensi, L. leviense (Mitchell, 1960), and L. weemsi are subtly but consistently distinct from other members of the L. viridatum complex, and these three species can almost always be separated from each other by diagnostic nucleotide substitutions (Gibbs Reference Gibbs2018b).
Revised keys to Lasioglossum (Dialictus) of Canada
The identification keys presented in Gibbs (Reference Gibbs2010) were extensively revised to incorporate new species, corrections, and improvements to reliability and ease of use. These keys are available in the online supplemental materials.
Discussion
It has been more than 10 years since the revision of Canadian Lasioglossum (Dialictus) by Gibbs (Reference Gibbs2010), and although that work is still reliable for most specimens, the slow accumulation of new discoveries and taxonomic changes render it more and more difficult to use. The original key also contained a few errors that have caused confusion with users over the years. This paper consolidates all updates and corrections in one place to facilitate the continued use of Gibbs (Reference Gibbs2010) for accurate, up-to-date identifications as of 2021. In the process, two new species are described and two new Canadian distribution records are presented.
Lasioglossum onuferkoi was not discovered during the 2010 revision due to its apparently restricted distribution. It is known only from a small area of sand hills and dunes on the Alberta–Saskatchewan border, Canada despite extensive collecting in other, seemingly suitable sand areas in western Manitoba, Canada. These facts indicate that it is narrowly endemic to this region. The Maxent distribution model (Fig. 10B) supports this hypothesis. Lasioglossum onuferkoi is likely to occur further south in sand hills of Grasslands National Park, Saskatchewan, Canada and north–central Montana, United States of America, but the predicted distribution otherwise fits tightly around known records. Additional collecting should be done to determine the full extent of the range of L. onuferkoi.
A portion of the sand hills on the Alberta side, where some of the paratypes were collected, is strictly protected as part of the Canadian Forces Base Suffield National Wildlife Area (no public access is allowed without prior permission). A portion on the Saskatchewan side (including the type locality) is protected by the Great Sandhills Historical Society (Sceptre, Saskatchewan), a local volunteer organisation, to a lesser extent (certain activities are restricted but the land is still used for grazing and hydrocarbon exploration). The population of Lasioglossum onuferkoi is therefore not likely under immediate threat from human activity, but natural stabilisation of the dunes may be a longer-term threat (Hugenholtz et al. Reference Hugenholtz, Bender and Wolfe2010). Several other insects dependent on dune systems of the southern Canadian prairies were listed as endangered (COSEWIC 2007, 2014, 2016), threatened (COSEWIC 2012), or special concern (COSEWIC 2018) for this reason.
Although the discovery of Lasioglossum immigrans in Canada prompted its description in this paper, it is normally found much further south, from Utah, United States of America to Oaxaca, Mexico. The Canadian specimen is clearly an outlier. A single female was collected near Ashern, Manitoba, about 1800 km away from the nearest other known record (in Santa Fe National Forest, New Mexico, United States of America). The usual northern limit of L. immigrans appears to be about 37–38° latitude: although the Maxent model predicts L. immigrans to occur in Colorado, United States of America with up to 50% probability (Fig. 10A), no confirmed records are known despite examination of about 1000 undetermined Colorado L. (Dialictus) specimens.
Range extensions on similarly large scales have been discovered in Anthophorula micheneri (Timberlake, 1948) (Sellers and McCarthy Reference Sellers and McCarthy2015), Diadasia ochracea (Cockerell, 1903) (Gibbs, unpublished data), Lasioglossum semicaeruleum (Cockerell, 1895) (Scarpulla and Droege, unpublished data), and several introduced Hawaiian bees, including four L. (Dialictus): L. helianthi, L. impavidum (Sandhouse, 1924), L. microlepoides (Ellis, 1914), and L. puteulanum Gibbs, 2009 (Magnacca et al. Reference Magnacca, Gibbs and Droege2013; Tabor and Koch Reference Tabor and Koch2021). It is therefore plausible that the Manitoba specimen is truly conspecific with the American and Mexican specimens and not a separate, cryptic species. It may be that L. (Dialictus) in general have a much greater dispersal ability than might be predicted based on their small body size (Gathmann and Tscharntke Reference Gathmann and Tscharntke2002).
In the United States of America and Mexico, L. immigrans is common at high elevations with cooler temperatures and winter snow cover, so it may survive Manitoba winters and have an established population. The Maxent distribution model (Fig. 10A) predicts several patches of suitable habitat in Canada, primarily associated with orthic greyzem soils. However, these areas are not predicted to be suitable if the Manitoba specimen is excluded from analysis. Whether this population is native or adventive is not known, but a recent introduction seems possible, given the absence of other Canadian and northern United States of America records despite extensive collecting. If adventive, a possible point of introduction is the Ashern landfill about 3 km north of where the L. immigrans specimen was collected; it is conceivable that bees could be brought to Canada by travellers from the United States of America or Mexico in soil or other possible nesting substrates and subsequently discarded. Additional collecting is planned in this area to determine if L. immigrans is established.
Conclusions
There are undoubtedly additional changes and species awaiting discovery in the Canadian L. (Dialictus), but as the rate of change has slowed in the last five years and we pass the 10-year anniversary of Gibbs (Reference Gibbs2010), the time seems ripe for a comprehensive update. Here, we have consolidated all published changes to the Canadian L. (Dialictus) fauna to date and publish all the additional new species, records, and synonymies known to us. It is hoped that this update will be helpful for researchers needing to work with this challenging group of bees.
Acknowledgements
The authors thank Doug Yanega (UCRC) for his thoughts and advice on nomenclature and Timberlake material at UCRC, Joe Engler (FWSE) for independently identifying some L. tegulariforme specimens and sending us his notes and records, Zach Portman (UMSP) for discovering and alerting the authors to the L. lineatulum basitarsus character, and other curators and collectors (listed in Methods) who loaned specimens. They also thank Arash Kheirodin for performing DNA extraction and PCR on the L. weemsi male. Three anonymous reviewers provided valuable comments that improved the final version of the manuscript.
The lead author was supported by the University of Manitoba Graduate Fellowship, Douglas L. Campbell Graduate Fellowship, and USDA National Institute of Food and Agriculture award 2017-68004-26323 (PI: R. Isaacs). The authors acknowledge the financial support of the Natural Sciences and Engineering Research Council of Canada (NSERC), RGPIN-2018–05353 (awarded to J. Gibbs).
Competing interests
The authors declare no competing interests.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.4039/tce.2021.47.