Introduction
The Island Thrush Turdus poliocephalus, a species of the Turdidae Family, is very prone to diverge (Mayr and Diamond Reference Mayr and Diamond2001), with more than 50 described subspecies (Dickinson 2014). Three of these subspecies were found on islands in New Caledonia (Collar Reference Collar, Del Hoyo, Elliot and Christie2005); one, T. p. xanthopus endemic to Grande-Terre (16,890 km2), and at least two surrounding islands, and two on Loyalty islands, T. p. pritzbueri on Lifou (1,207 km2) and T. p. mareensis endemic to Maré (138 km2).
For the latter two subspecies, a few specimens were collected in 1912 but could not be found in 1939 (Macmillan Reference Macmillan1939) and they are now considered to be extinct in New Caledonia (Naurois Reference Naurois1982).
On Grande-Terre, historical data on Island Thrush were summarised by Naurois (Reference Naurois1982) and Baudat-Franceschi (Reference Baudat-Franceschi2013). It was commonly distributed all over the island (Layard and Layard Reference Layard and Layard1882), but listed localities appeared to be in dry forest on the west coast. If we except one sighting in the rainforest (Amieu pass at 500 m) in 1968 (Naurois Reference Naurois1982), the Island Thrush was last seen on Grande-Terre in 1928 and was thought to have been extirpated from New Caledonia. In 1978, the Island Thrush was rediscovered on two small islands north-west of Grande-Terre: Néba (∼3.5 km2) and Yandé (∼13 km2) (Naurois Reference Naurois1982), respectively 8 km and 18 km north-west of Grande-Terre. The T. p. xanthopus population size is currently small: on Yandé an estimate of 100 individuals was obtained at the end of 1970s (Naurois Reference Naurois1982) but only 60 individuals were recorded in 2009 (Baudat-Franceschi Reference Baudat-Franceschi2013). On Néba in December 2015 and January 2016, a census provided a minimum of 75 individuals (Bodin Reference Bodin2016).
Here, we aimed to understand a) how the Island Thrush managed to survive on two islands that are less than 0.1% of the area of Grande-Terre, b) the type of habitat used, c) the diet, d) the breeding success and e) the potential predators, so as to propose conservation plans to ensure the survival of the very rare Island Thrush in New Caledonia.
Study area and Methods
Field work was mainly done on Néba (20°09’S, 163°55’35’’E) from 19 to 26 October and 3 November 2016 to 26 January 2017), followed by a short stay on Yandé (13 to 20 February 2017). Mist-netted birds were measured, colour banded, plumage coloration described and two tertial feathers collected for genetic sexing. DNA was extracted from feathers using the Qiagen extraction kit (Qiagen, Valencia, CA) following the manufacturer’s protocol. Individuals were sexed by PCR using the primer pair 2550F and 2718R under standard PCR-amplification conditions (Fridolfsson and Ellegren Reference Fridolfsson and Ellegren1999).
The Island Thrush is known to occur in the coastal forest. Playback was not reliable for sampling Island Thrush, as they may be present but not responding. Walking through all suitable habitats on the island of Néba, we recorded any encounter with a thrush on a GPS. Using satellite imagery and a GIS (QGIS 2.8), we were able to estimate the area of coastal forest on Néba and Yandé. To understand why this habitat seems to be preferred by thrushes, on Néba, we compared food availability among habitats. To sample invertebrates in the forest litter, we used plastic glasses (internal diameter 64 mm and 98 mm deep). One glass was buried in the soil, with four others at 40 cm of the central one in the design of a cross. Nine stations, each with five glasses, were set 10 m apart, giving 45 glasses by habitat (Illera Reference Illera2001). In total, 180 glasses were set up in four habitat types (wattle tree Acacia spirorbis forest, paperbark tree Melaleuca quinquenervia forest, mimosoid tree Leucaena leucocephala forest and coastal forest) and checked 5–6 times during seven consecutive days. Invertebrates were counted and measured to the nearest millimetre.
To describe nest site preference, we recorded vegetation in a 10-m radius around each nest and measured and identified every shrub and tree (with a diameter > 1 cm and higher than 1 m).
Rats are potential predators of Island Thrush. To update which rodent species occurred in the coastal forest, we set out three lines spaced 20-40 m apart, each with 10 stations, 20 m apart, containing two plastic snap traps spaced 1 m apart, for three nights, representing 180 trap-days per island. We also placed 20 additional traps for one night in the coastal forest in the North of Néba.
Results
Description of the Island Thrush
We confirmed that all individuals caught on Néba and Yandé were phenotypically of the subspecies xanthopus. Adult coloration can be described as: crown dark brown, head light brown, back and wing coverts green-brown, breast and belly reddish-brown, remiges and rectrices dark brown, bill and eye ring yellow-orange, legs brown-yellowish and iris light brown or brown-reddish (Figure S1 in the online supplementary material). All birds have the same colour except for some with a white patch (tiny to small) at the crissum as mentioned by Naurois (Reference Naurois1982). For birds that could be sexed (n = 28, 19 males, nine females), both islands and fledglings-adults combined, 63% of males (n = 16) and 75% of females (n = 8) had a white patch at the crissum. Twenty individuals could not be sexed due to the low amount of DNA. Mass, and wing length of adult birds on Néba were not significantly different between sexes.
Table 1. Morphology measurements of Island Thrush in Néba, New Caledonia

(n): sample size, *: DNA sexing.
The Island Thrush was colour banded only on Néba (31 adults, one fledgling and two nestlings). On Yandé five adults and six fledglings were caught. Fledgling plumage resembles the adult but is less colourful, with a black-yellowish beak, brown-yellowish legs, and reddish-orange underparts with tiny brown spots (Figure S2).
No parasites were found on the Néba Island Thrushes. In contrast, on Yandé, adults were partly bald and carried lice eggs on the feathers. In addition, yellow eggs of chigger mites were found on the skin, close to the top of the legs. Furthermore, four individuals showed evidence of keratitis (Figure S3).
Habitat
The estimated size of suitable forest for the Island Thrush was 153 ha and 42.7 ha for Yandé and Néba, respectively, which is about 12% of the total area of each island. The south-western coast of Néba (Awolo Bay), was our main study site and is the best coastal forest on the island. To estimate its area, we walked the border of the coastal forest and the wattle tree forest included within it and recorded the track on a GPS. Using a GIS we calculated a surface area of 5.65 ha and 0.55 ha respectively for the coastal forest and the wattle tree forest.

Figure 1. Awolo Bay, location of seven nests.
Five types of habitat are available on Néba. Three are native: the paperbark tree forest, the wattle tree forest and the coastal forest, and two are man-made plantations, the mimosoid tree forest introduced to New Caledonia in 1860–1870 (Barrau and Devambez Reference Barrau and Devambez1957) and the coconut Cocos nucifera groves. On Néba, coconut trees were planted around 1880. In 1900, a wharf was built with a railway to carry copra to the ships, and commercial production continued until 1943. Unfortunately for the Island Thrush, those plantations were mainly created in Awolo Bay, following deforestation of the coastal forest.
The Island Thrush forages in four of the five habitats. In the coconut groves, the ground is piled up with dead palms and fallen coconuts; the thickness and size of those materials does not allow the Island Thrush to remove them and search underneath for prey. On the ground, when searching for food, the Island Thrush use its beak to throw aside leaves. Sometimes, it slightly digs into the soil also with its beak, and not with its feet, like the congeneric species, the Blackbird Turdus merula (Snow Reference Snow1958).
The number of invertebrates collected differed markedly among habitats. Ranked by decreasing order, the number of invertebrates collected by habitat (n) was: coastal forest (n = 1,370), followed by wattle tree forest (n = 564), paperbark tree forest (n = 376) and the mimosoid tree forest (n = 173). For each habitat sampled, class sizes 2–12 mm account for the highest number of invertebrates.
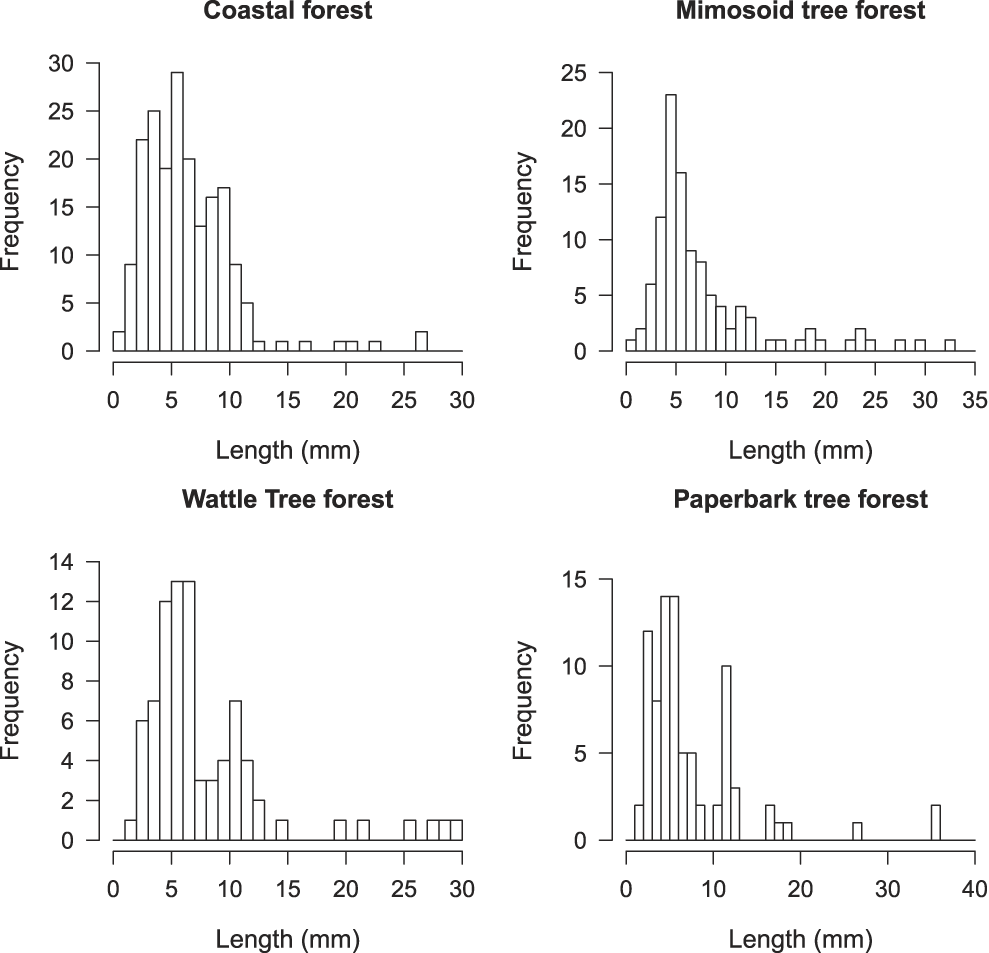
Figure 2. Frequency of the length of invertebrates in four habitats.
Fruits are also a part of the Island Thrush diet. Only fruits of 10 mm (width of the thrush’s gape) or less can be swallowed whole. The subspecies xanthopus was not seen pecking at larger fruits on the ground as documented for the subspecies erebus in New Guinea ( Clarke 2013). If fruit seeds are big, they are regurgitated.
We did not observe see any territorial conflict or hear song duels between neighbouring thrushes so we could not evaluate territorial behaviour. For 103 songs noted, the active vocal months were November (10% of the songs), December (66%) and January (24%). Most of the time the Island Thrush’s song lasted less than two minutes, with the longest of 8 min 30 sec. The song was very soft, rarely been heard further than 50 m away. Inside the 5.65 ha of coastal forest and as far as 60 m away from its edge, 30 adult Island Thrushes were caught, resulting in an estimate of one pair per 0.38 ha but they also move to share fruiting trees, drinking water holes and probably foraging grounds. This territory size could not be applied to other patches of coastal forest on the island, as they are not as optimal. On Néba, we found 13 patches of suitable forest for the Island Thrush and recorded thrushes in eight of them. This allowed us to make a rough estimate of 51 ± 7 individuals in 38 ha.
Breeding
Around nests, most of the plants had a small diameter. Nests were secured in the middle of tangled vegetation including several supports such as trunk, branches, creepers and fallen palms. Support diameters were also small, with a mean for trunks and branches of 34.6 ± 25.5 mm (n = 7 nests). During the nest-building period, the Island Thrush piled up dead twigs, sticks of vines, dead leaves and some leaf compost but did not plaster it with mud as does the congeneric Blackbird. When it has constructed a horizontal platform, it weaves a nice basket-shaped nest. The mean sizes of seven nests were 176 x 149 mm (outside) by 96 x 80 mm (inside) and a depth of 63 mm. The mean height was 2.9 ± 0.8 m (n = 7 measured).
Table 2. Diameter of plants (DBH) around nest support of Island Thrush
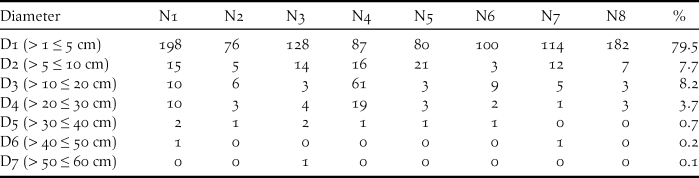
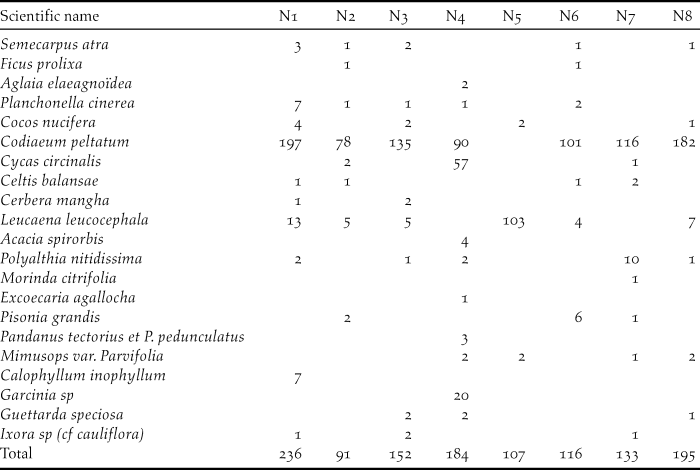
Table 3. Number of trees inside a 10-m radius circle around eight nests of Island Thrush
Clutches were one egg (n = 1), two eggs (n = 3) and three eggs (n = 2). Eggs of four nests were measured: 30.4 ± 1.0 mm x 21.4 ± 0.9 mm (n = 7). Eggs are blue-green with brown blotches (Figure S4). For eight nests discovered, seven had an egg or young and one an incubating female. Only one nest fledged two young, giving a breeding success of 14.3% and for fledglings regarding eggs laid, of 15.4%. In one destroyed nest, broken shell had tracks of small teeth carved which could be from the arboreal skink Epibator nigrofasciolatum seen on Néba.
Twelve days after hatching, young birds left the nest, but were still fed by parents for a month. The day that two fledglings left their nest, one of them was seen on the ground under the nest. To attract attention away from the young on the ground, the male performed a distraction display, with the right leg stretched, toes turned up, and the left wing moving up and down as it slowly hopped away on one leg. For Blackbird, only ruffled body feathers are mentioned (Snow Reference Snow1958). Finally, on Néba, the breeding season runs from November to February, the same as on Maré (Macmillan Reference Macmillan1938a).
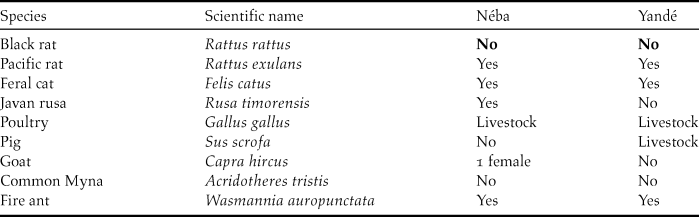
Table 4. Exotic species on both islands
Fruits of burny vine Trophis scandens were found in two nests: between 1 and 50 fruits in a nest with two young and 3 to 40 in a nest with one egg. Fruits were more or less fresh and from red to brown in colour. If these were left over from feeding the young, it is puzzling why there were so many in the case of the egg. In that case, the number of fruits fluctuated between days, indicating that it might be some food provisioning for the incubating female? There seems to be only one annual brood, and no replacement clutch was observed.
Predation and habitat degradation
During 180, 20, and 180 nights trapping on the South of Néba, North of Néba and the East of Yandé, respectively, 29, two and four Pacific rats were caught. Several exotic species on Yandé and Néba could be a threat, being able to kill or compete with the Island Thrush or degrade their habitat. Following our work, we were able to update the list of those exotic animal species. Feral cats are known to predate on Turdus species (Maumary et al. Reference Maumary, Vallotto and Knaus2007), especially newly hatched fledglings. On Néba, five feral cats were caught, but some remain on the island. The stomach content of an adult female contained 15 endemic geckos Bavayia cyclura.
The Java deer Rusa timorensis can swim to Néba, and a small population (potentially more than 10 individuals) is present year-round. Besides hoof prints in soft ground, the main signs on vegetation were trees peeled of their bark when deer rub their antlers in velvet. They are very shy and during three months we only twice encountered one deer.
Discussion
We did not find any sexual dimorphism regarding weight, wing length and coloration. Even the white patch at the crissum is not sex discriminant. The dry forest area, which is the supposed habitat where the Island Thrush lived on Grande-Terre, was estimated to cover 4,500 km2 at the time of human colonisation (Bonvalot et al. Reference Bonvalot, Gay and Habert2012). Following deforestation and fires (for cattle grazing and agriculture), especially after colonisation by France in 1854, the dry forest almost vanished, with less than 100 km2 left (0.6% of the original area) in 1994 (Bouchet et al. Reference Bouchet, Jaffré and Veillon1995) and 66.3 km2 (0.4%) in 2007 (Hequet Reference Hequet2007). Another threat came from the Java deer introduced in 1870 (Barrau and Devambez Reference Barrau and Devambez1957). The species was very successful with around 100,000 animals in the 1990s (Chardonnet and Lartiges Reference Chardonnet and Lartiges1992) and it seems there was an even higher density in the 1920s–1930s (Gargominy et al. Reference Gargominy, Bouchet, Pascal, Jaffré and Tourneur1996) at the time when the Island Thrush population vanished. High numbers of deer result in heavy grazing in the dry forest preventing regeneration and causing the disappearance of understorey (Barrau and Devambez Reference Barrau and Devambez1957).
We do not know the reliability of the last sighting in the rainforest in 1968, 40 years after the species was thought to have been extirpated from Grande-Terre. Dry forest destruction possibly happened too quickly to allow Island Thrush to shift to rainforest habitat as on Norfolk Island, Australia (Higgins et al. Reference Higgins, Peter, Cowling, Higgins, Peter and Cowling2006). On Maré and Lifu, Island Thrush was still common in the early 1900s, but faced a rapid decline over 20 years to the point of extinction. During his stay in 1937–1938, Macmillan tried to explain the reasons by interviewing native villagers. On Lifu, Island Thrush was one of the commonest species, but in 1924 it began to decrease rapidly and in 1931 there were hardly any left. In fact, in 1929, sick Island Thrushes (possibly due to disease or food poisoning) were found in and around the bush and gardens. Black rats could have predated eggs and nestlings/fledglings. The numerous cats were a possible factor to a much greater degree as Island Thrush was not shy around villages where cats hunted. Finally, human persecution by hunting thrushes also played a part (Macmillan Reference Macmillan1938b).
On Maré, native people were responsible for the Island Thrush decline through clearing for agriculture and hunting (snares), but more damaging was predation of Island Thrush nests by the New Caledonia Crow Corvus moneduloides, a species introduced in 1913 for agricultural purposes (Macmillan Reference Macmillan1938a). Macmillan concluded that besides the factors highlighted above for each island, the reason for the extermination of the species on both islands was connected to food (Macmillan Reference Macmillan1938b).
It is puzzling that in the same time period, 1920–1930, Turdus populations of three subspecies, on three islands went extinct. It seems that a combination of several factors contributed to the extinction of Island Thrush. The tameness of the bird did not help, as Turdus on Lifu was not shy, as it was on Maré (Macmillan Reference Macmillan1938b), but still both vanished.
At the end of the 1970s, Naurois estimated the suitable habitat for Island Thrush to be 300 ha on Yandé, compared to 150 ha found in 2009 (Baudat-Franceschi Reference Baudat-Franceschi2013) and in the present study. This means that 40 years later, suitable habitat was reduced by half due to poor forest management.
Here, we recommend that fires are lit only near houses and under careful supervision. For example, in December 2016, uncontrolled burning on a windy day and during drought, destroyed 69 ha of natural habitat on a slope, part of a drinking water catchment.
GPS locations of Island Thrushes recorded were mostly in the coastal forest and they could also be sometimes seen in the mimosoid tree forest but rarely in the paperbark tree or wattle tree forests. The best forest litter, thick, puffy and with leaves from a good number of trees species is found in coastal forest. The wattle tree and paperbark tree forests are almost monospecific and do not have an understorey. Leaves from trees in these two habitats are flat and thick, resulting in a tight litter with a very slow decomposition rate, and low invertebrate abundance, except for ants which account for 83% of those found across the four habitats. The pitfall traps only allowed us to sample part of the invertebrates available in each habitat, those crawling on the ground and inside the forest litter, but not those living on plants, bushes and trees. Yet, even if we sampled only part of the food available, it still gives a good picture of invertebrate richness by habitat, coastal forest being the richest and the mimosoid tree forest the poorest.
Around nests, plants of diameter ≤ 5 cm account for almost 80% of the vegetations. Seven nests were found in the coastal forest and one in the mimosoid tree forest. Regarding the seven nests found in the southern part of Awolo Bay, the surroundings contained a good understorey with frequent patches of creepers. One nest found in the north of Néba differs, where 57 cycas Cycas circinalis a plant with a diameter D3 (10–20 cm) was much numerous. In the southern part of Néba, only three cycas were counted around seven nests and only a few remained on the edge of the coconut grove. In this study, 14.3% of Island Thrush nests resulted in fledglings, matching the 14% found for blackbird nesting in woodland (Snow Reference Snow1958).
Conservation
According to the literature, area size seems to be a key factor in the coexistence of Island Thrush and black rat on islands. On small islands (< 50 km2), black rats are able to extirpate the Island Thrush. On three Australian islands, after black rats became established, the Island Thrush disappeared from two of them: Howe (14.6 km2) and Norfolk (34.6 km2), but the Island Thrush survived on the largest one, Christmas (135 km2) (Garnett et al. Reference Garnett, Szabo and Dutson2011) as well on Erromango (891.9 km2) in Vanuatu (Macmillan Reference Macmillan1938a). Maré, the size of Christmas Island could theoretically have both, the black rat and a population of Island Thrush.
Even with a high density of Pacific rats on Néba, the Island Thrush manages to raise fledglings. If black rats were present, we would very likely have recorded them in our traps.
In New Caledonia, black rat arrived around 1850 (Gargominy et al. Reference Gargominy, Bouchet, Pascal, Jaffré and Tourneur1996) and colonised most of the islands. Some eradication of rats took place only on tiny islands up to 0.07 km2 (Bell Reference Bell1998). Our study shows that Yandé and Néba are still free of black rats. For the survival of the Island Thrush, it is necessary to develop a strong biosecurity programme, with rodent bait boxes set up and maintained to avoid colonisation by black rats.
One trap was left with the warden of Néba to catch any roaming feral cats, another predator. The last primary predator, the New Caledonia Crow is absent from both islands. Another key factor for the conservation of Island Thrush is access to coastal forest which provides shade and humidity, understorey, invertebrates in good leaf litter, fruit and nest support. The area of coastal forest is currently quite limited and under heavy pressure. After securing the islands against rats, the second necessary measure should be to increase the size of the coastal forest. On Néba, since coconut groves were abandoned 70 years ago, the coastal forest is slowly taking over, except in wetter areas. To speed up this process of regrowth of natural habitat, we recommend cutting down most of the coconut trees and planting trees beneficial to the Island Thrush. To avoid any damage during this rehabilitation of natural habitat, fire should not be used at any time to clear the site. In Awolo Bay, the old coconut grove covers about 10 ha. Rehabilitating this area could triple the area of coastal forest, and potentially increase the number of Island Thrushes (26 pairs). We recommend deer removal, especially before planting young trees, to prevent them being browsed by the deer.
Table 5. Tree species to be planted. (Species in bold bear fruit shown to be eaten by the Island Thrush during this study).

On Yandé, we strongly suggest that any future management work (e.g. new roads, new crops) should be planned in areas with coconut trees. The same programme to extend coastal forest should also be tried and later expanded according to the results obtained.
A similar conclusion was also reached by an author familiar with protection of endemic island birds: “The majority of native birds are closely linked to the preserve of native forest, and this is specially true of the endemics. Preservation of native forest is far more important” (Watling Reference Watling2004).
The owner of Néba, the local Nenemas tribe, is willing to save the Island Thrush and want this island to become a nature reserve, to have young people involved in tree planting and follow up work. The local forest service is also eager to produce the forest plants needed and to supervise the rehabilitation of coastal forest. It is not so common to have several partners ready to work together to save a local bird. For the Government of New Caledonia, it is a great opportunity to provide some funding to make this project happen. The Island Thrush could become a New Caledonian success story of management to save island biodiversity, especially as New Caledonia is a hotspot for endemic bird species and already has several birds classified as ‘Critically Endangered’ (BirdLife International 2000).
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270919000091
Acknowledgements
We warmly thank the customary authorities of Yenghebane for their permission to stay on the island of Néba and Waina Dayé; the warden of the island for his constant help and support; Christophe Hatjopoulos, Alice Mathieu and Jurgen Whala-Windi, for numerous boat trips and all the services rendered. Martin Dayé, Arnold Dayé, Shawall Dayé, Jesse Dayé, Waina Dayé and Yvan Watu provided help during field work and Isabelle Brun loaned crucial equipment.
Captures and sampling were realised under North Province authorisation (n°60912-556-2017 JJC of 4th April 2017). This work was supported by the Economic Development and Environment branch, North Province, New Caledonia (grant number 16/0644/04269).









