Malnutrition is a global public health issue that encompasses three broad groups of conditions: (a) undernutrition, which includes underweight (low weight-for-age), stunting (low height-for-age) and wasting (low weight-for-height); (b) micronutrient-related malnutrition, which involves deficiencies in vitamins and minerals and (c) overweight and obesity, which is an abnormal or excessive body fat accumulation(1,2) . Globally, the burden of malnutrition remains high, affecting people of all ages, genders and social strata(2). In children, around 20 million babies are born with low birth weight each year, and among those under 5 years old, approximately 45 million are undernourished, 149·1 million are stunted and 37 million are overweight or obese(1,2) .
In Brazil, from 1974 and 2009, there was an increase in adult obesity rates, with a higher annual increment rate for men than women(Reference Conde and Monteiro3). Notably, a substantial decrease in adult undernutrition occurred from 1974 to 1989(Reference Conde and Monteiro3). While the prevalence of overweight in children under 5 years old remained stable, undernutrition decreased notably from 1996 to 2006–2007(Reference Conde and Monteiro3). In addition, there was a disparity in prevalence trends of adult obesity based on household income, with both the poorest and richest adults showing positive incremental rates in the latest period(Reference Conde and Monteiro3). Overall, Brazil faces a shift from undernutrition to excess weight, indicating the success of inclusive social policies in reducing poverty but also emphasising the emerging challenges in obesity control and reduction(Reference Conde and Monteiro3).
In comparison with children with normal height, stunted children are more likely to have learning difficulties in school, earn less as adults and encounter barriers to participating in their communities(1,2) . Otherwise, children with obesity (excessive increase in total body fat) are at risk of the early development of several diseases such as type 2 diabetes mellitus, hypertension, strokes, heart diseases, dyslipidaemia and some types of cancer(Reference Juhola, Magnussen and Viikari4–Reference Costa, Santos and Goldraich6). Studies pointed out that excessive weight during childhood is a risk factor for obesity in adulthood, with high correlations with BMI over time(Reference Ward, Long and Resch7–Reference Tran, Krueger and McCormick9).
Height-for-age and BMI-for-age growth charts are the most used tools to assess the nutritional status of children and adolescents(Reference De Oliveira, Dos Santos Pereira and Melo10,Reference Ferreira11) . The height-for-age growth chart shows the linear growth trajectory, which is essential for detecting stunting, while the BMI-for-age growth chart identifies underweight, overweight and obesity conditions(Reference De Oliveira, Dos Santos Pereira and Melo10,Reference Saari, Sankilampi and Hannila12) . There are international growth references recommended to be applied worldwide, and the most recent published was the MULT growth reference (2023), which was constructed with data from multi-ethnic children and adolescents from ten countries(Reference De Oliveira, Araújo and Ramos13,Reference De Oliveira, Araújo and Severo14) . Other growth references, such as the ones from the World Health Organization (WHO, 2006/2007), the International Task Obesity Force (IOTF/2012) and the Centers for Disease Control and Prevention (CDC, 2000) have been used internationally over the years(15–Reference Kuczmarski, Ogden and Guo19).
The BMI growth charts from these references, although do not directly measure adiposity, they are considered highly sensitive to detect overweight and obesity, showing a strong positive correlation with body fat(Reference Ferreira11,Reference De Onis, Onyango and Borghi16,Reference Cole, Bellizzi and Flegal18,Reference Birch, Perry and Hunt20) . Moreover, they have been essential for assessing nutritional status at the population level, although there is no consensus on which one is the most appropriate for international use, especially in children over five years old(Reference De Oliveira, Dos Santos Pereira and Melo10,Reference Ferreira11) . Therefore, this study aimed to compare the nutritional status classified by the WHO (2007)(Reference De Onis, Onyango and Borghi16), IOTF (2012)(Reference Cole, Bellizzi and Flegal18) and MULT (2023)(Reference De Oliveira, Araújo and Ramos13,Reference De Oliveira, Araújo and Severo14) height and BMI references and verify their diagnostic accuracy in detecting obesity based on body adiposity in Brazilian schoolchildren.
Methods
This study selected data from two Brazilian surveys conducted in the cities of Santos, located on the coast of São Paulo and Porto Alegre, which is the capital of Rio Grande do Sul State(Reference Costa, Santos and Goldraich6,Reference Costa, Cintra and Fisberg21) . Santos, like many Brazilian cities, is ethnically diverse(Reference Careno, Silva and Martins22). The population includes individuals of European, African and Indigenous ancestry, among others(Reference Careno, Silva and Martins22). The city’s historical connection to trade and immigration has contributed to its multicultural makeup(Reference Careno, Silva and Martins22,23) . In addition, it is close to the metropolitan region of the city of São Paulo, composed by approximately 45 % of its adult residents originating from other states or countries(Reference Careno, Silva and Martins22,23) . This demographic makeup underscores the region’s status as a melting pot of cultures and backgrounds, reflecting a dynamic blend of individuals who have migrated to the state of São Paulo(23). On the other hand, even though there are individuals in Porto Alegre with African and Indigenous heritage, the city, situated in the southern part of Brazil, predominantly reflects European influences(Reference Guerreiro-Junior24). The majority of the population has roots tracing back to European countries, notably Portugal, Italy and Germany(Reference Guerreiro-Junior24).
The data from the Santos survey were collected between August and November of 2002 in both public and private schools of the city, through a partnership between the city government, the Federal University of São Paulo (UNIFESP) and the São Marco University(Reference Costa, Cintra and Fisberg21). A total of ninety-nine chools and 10 822 children between the ages of seven and 10 years participated in the data collection(Reference Costa, Cintra and Fisberg21). As for Porto Alegre, the study was conducted by the Federal University of Rio Grande do Sul (UFRGS), and the data were collected during the years 2011 and 2012 in forty-seven schools of the municipal education system, and it included 11 952 children and adolescents aged between five and 16 years(Reference Costa, Santos and Goldraich6).
For this study, demographic data such as sex (determined by the presence or absence of the Y chromosome at birth) and age (in months) were selected from the data pool(Reference Costa, Santos and Goldraich6,Reference Costa, Cintra and Fisberg21) . Additionally, anthropometric measurements of weight and height were included(Reference Costa, Santos and Goldraich6,Reference Costa, Cintra and Fisberg21) . In both surveys, they were taken by trained professionals who followed a standardised data collection protocol to ensure the quality of the measurements(Reference Costa, Santos and Goldraich6,Reference Costa, Cintra and Fisberg21) . Furthermore, the Santos survey provided skinfold thickness data collected in a subsample of participants, so the triceps skinfold (TSF), subscapular skinfold (SSF), suprailiac skinfold and abdominal skinfold (ASF) were selected(Reference Costa, Cintra and Fisberg21). These skinfold measurements were selected to perform the accuracy analysis of the growth references concerning the body composition data that skinfold thicknesses provide. Skinfold measurements are used to estimate the overall percentage of body fat because of their practicality, cost-effectiveness and accessibility(Reference Costa, Santos and Goldraich6).
In the selection process of the data pool, participants with measurement errors (n 4), implausible height-for-age or BMI-for-age values (n 33) or missing data, were excluded (n 3530)(25). For height, values equal to or greater than 250 cm were considered measurement errors, and implausible values were those below –6 standard deviations (sd) or above +6 sd of the WHO (2007) reference height values(Reference De Onis, Onyango and Borghi16,25) . For BMI, implausible values were those below –5 sd or above +5 sd of the WHO (2007) reference BMI values(Reference De Onis, Onyango and Borghi16,25) .
The correlation analysis between adiposity and BMI was conducted using a sub-sample of the Santos survey(Reference Costa, Cintra and Fisberg21). A correlation matrix was calculated, using the Pearson coefficient (r), to measure the degree of linear association between the z-scores of the BMI references and the skinfold measurements(Reference Pearson, Fisher and Inman26). The sum of TSF and SSF, which is one of the commonly used measurements for assessing body adiposity in children and adolescents, was calculated(Reference De Quadros, Gordia and Andaki27,Reference Tang, Bowe and Nguyen28) . The 95th percentile of this sum by age (years) was estimated and defined as the cut-off point to determine high adiposity values. Children were dichotomously classified according to this criterion, indicating whether they had, or not high adiposity values based on their skinfold measurements.
The diagnostic accuracy, which includes sensitivity, specificity, positive likelihood ratio (+LR) and negative likelihood ratio (−LR) of the BMI references, was performed based on the classification of high adiposity values obtained from the sum of skinfold measurements(Reference Van Stralen, Stel and Reitsma29). Additionally, since the MULT BMI reference presented four different cut-off options for obesity, the analyses were performed for all cut-off options, showing the one more adequate to be applied to Brazilian children and adolescents(Reference De Oliveira, Araújo and Severo14).
The prevalence of normal height and stunting was estimated using the WHO and MULT height references(Reference De Oliveira, Araújo and Ramos13,Reference De Onis, Onyango and Borghi16) . Additionally, the prevalence of underweight, normal weight, overweight and obesity was calculated according to the BMI reference values of the WHO, IOTF and MULT(Reference De Oliveira, Araújo and Severo14,Reference De Onis, Onyango and Borghi16–Reference Cole, Bellizzi and Flegal18) . For the MULT BMI reference(Reference De Oliveira, Araújo and Severo14), the following cut-off points were applied for both sexes: (a) underweight for percentile < 3rd; (b) overweight for percentile ≥ 85th and (c) obesity for percentile ≥ 98th.
For the comparison analysis among the growth references, Lin’s concordance correlation coefficient (CCC), Cohen’s Kappa coefficients (Kappa) and the Bland–Altman methods were applied(Reference Lin30–Reference Bland and Altman33). The CCC and the Bland–Altman methods were used to assess the agreement between the estimated z-scores of the BMI references(Reference Lin30,Reference Bland and Altman33) . The CCC evaluates agreement by combining measures of precision and accuracy to determine how much the data deviates from the perfect concordance line(Reference Lin30). On the other hand, the Bland–Altman method calculates the mean difference between the two methods and presents the 95 % limits of agreement as the mean difference (2sd)(Reference Bland and Altman33). Cohen’s Kappa coefficient is applied to assess the reliability between the two methods for categorical outcomes(Reference Cohen31,Reference Cohen32) . In this study, Cohen’s Kappa was used to verify the reliability of the height diagnosis (dichotomous), and weighted Cohen’s Kappa (linear) was used to analyse the reliability of the nutritional status (ordinal categorical) classified by the BMI references(Reference Cohen31,Reference Cohen32) .
The agreement and reliability analyses using CCC, Bland–Altman and Kappa were performed separately by sex and in three age groups(Reference Lin30–Reference Bland and Altman33). The first group included the whole sample of participants aged five to 16 years. The second group consisted of children aged 6–9 years, which is a period that precedes puberty(Reference Loomba-Albrecht and Styne34). The third group included participants aged 10 to 16 years, covering adolescence, a stage characterized by significant physical changes such as the growth spurt and sexual maturation(Reference Loomba-Albrecht and Styne34). All statistical analyses were performed using the R statistical software version 4.2.3 for Windows(35).
Regarding ethical aspects, both surveys included in the data pool were conducted according to the ethical principles for research involving human subjects, laid down in the Declaration of Helsinki and the Brazilian resolution CNS 196/96(Reference Costa, Santos and Goldraich6,Reference Costa, Cintra and Fisberg21) . The Santos survey was approved by the Research Ethics Committee of the UNIFESP under number 0074/02, while the Porto Alegre survey was approved by the Research Ethics Committee of the Hospital de Clínicas de Porto Alegre under number 11–0149(Reference Costa, Santos and Goldraich6,Reference Costa, Cintra and Fisberg21) . The legal guardians of the children and adolescents were informed about the research and gave their consent for participation by signing the Informed Consent Form(Reference Costa, Santos and Goldraich6,Reference Costa, Cintra and Fisberg21) . In addition to that, the participants themselves, if capable of understanding, also signed an assent form(Reference Costa, Santos and Goldraich6,Reference Costa, Cintra and Fisberg21) . The details of these protocol surveys, as well as their approvals from the ethics committees, can be found in previous studies(Reference Costa, Santos and Goldraich6,Reference Costa, Cintra and Fisberg21) .
Results
The selection process is described in Fig. 1, after excluding measurement errors and implausible values, 22 737 subjects (49·4 % males) were included in the BMI reference comparison analysis. The demographic characteristics of the whole sample are shown in Table 1.
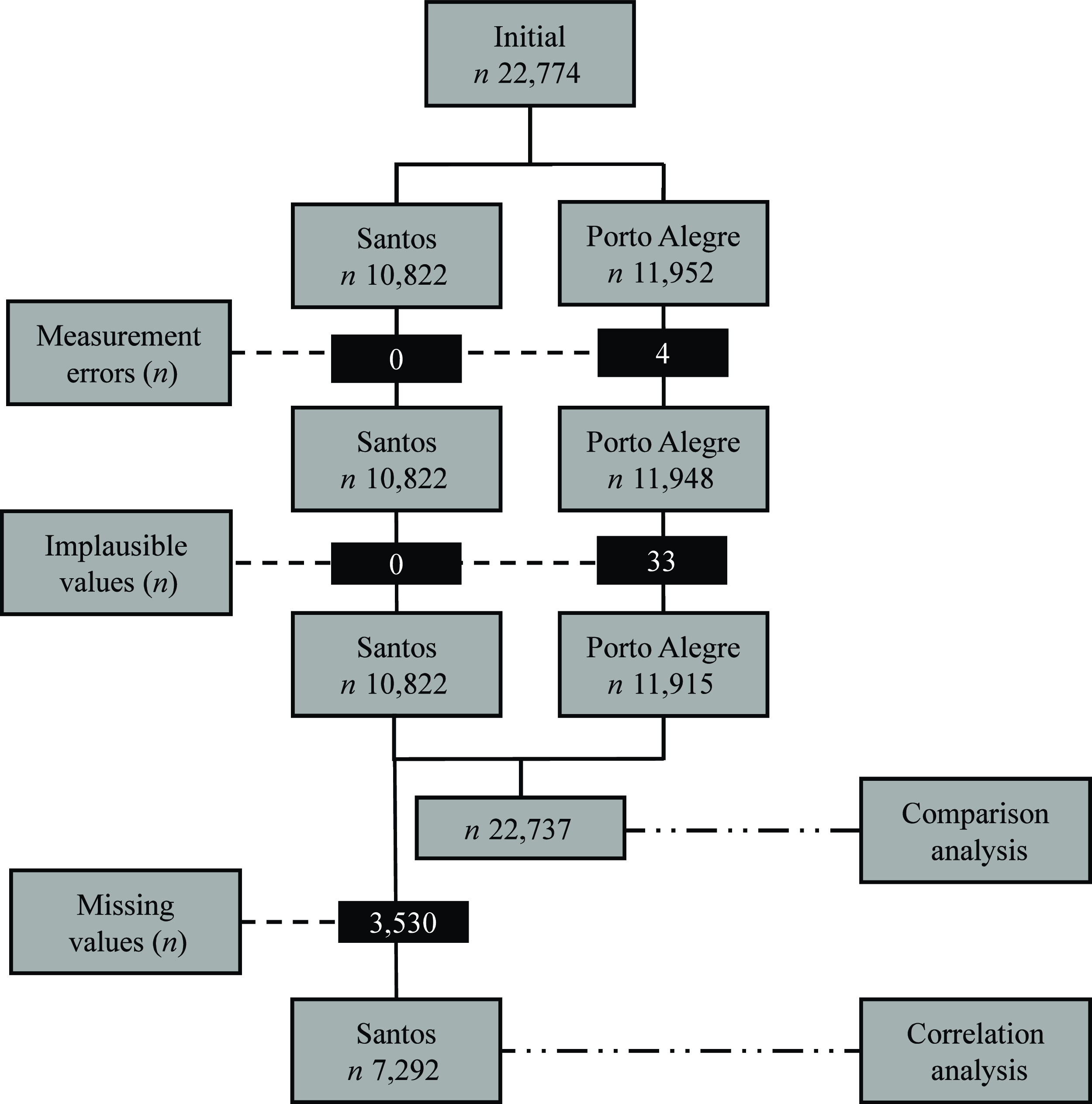
Fig. 1. Flow chart of the subject selection. n, number of participants; measurement errors, height values ≥ 250 cm; implausible values, height-for-age z-score < −6 or > +6 or BMI-for-age z-score < −5 or > +5; missing values, skinfold data.
Table 1. Demographic characteristics of the study population by survey, age and sex
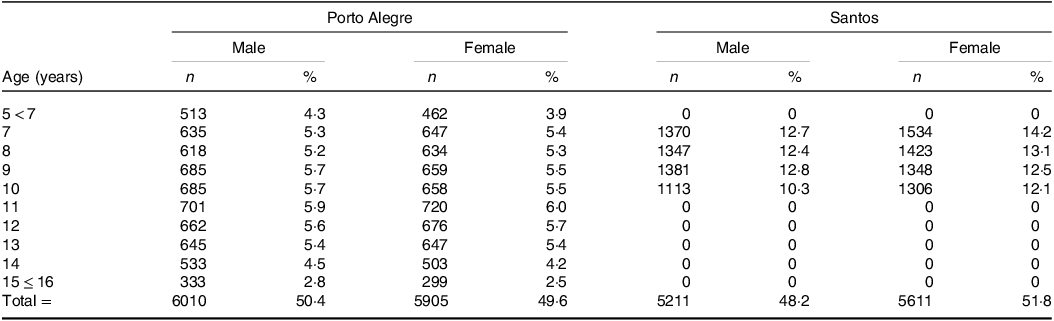
Y, years; n, number of children; %, percentage.
For the correlation and diagnostic accuracy analysis, 7292 participants (48·3 % males) from the Santos survey were included. The correlation matrix (Fig. 2) shows a high positive correlation among the BMI z-scores (r ≥ 0·99) and among the skinfold measurements (r ≥ 0·86). The MULT reference presented higher correlation values than the other references for all skinfold measurements (TSF r = 0·81; SSF r = 0·79; SIF r = 0·79; ASF r = 0·81).
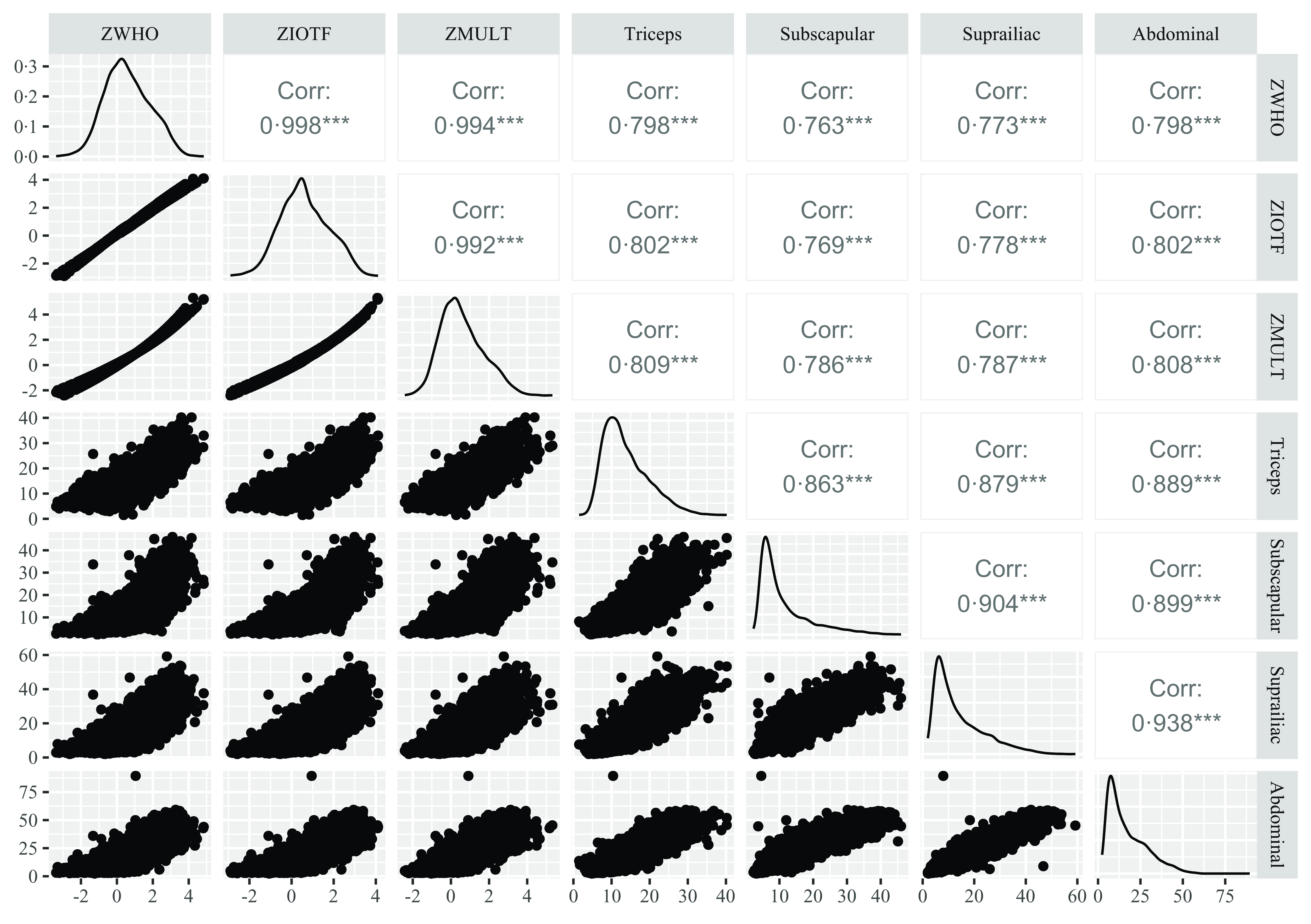
Fig. 2. Pearson’s correlation matrix among the BMI z-scores of WHO (2007), IOTF (2012) and MULT (2023) and four skinfolds ZWHO, BMI z-score of WHO (2007)(Reference De Onis, Onyango and Borghi16); ZIOTF, BMI z-score of IOTF (2012)(Reference Cole and Lobstein17); ZMULT, BMI z-score of MULT (2023)(Reference De Oliveira, Araújo and Severo14).
Regarding diagnostic accuracy, as shown in Table 2, among the BMI references, the IOTF and MULT (17 years) obesity percentiles presented the lowest sensitivity values and the highest specificity, +LR and −LR. For boys, the WHO obesity percentile presented the +LR value of 6·99 for the −LR value of 0·04, while the MULT obesity percentile presented the +LR values of 10·99 (18 years), 8·99 (19 years) and 8·09 (20 years) for the −LR value of 0·04. For girls, the WHO obesity percentile showed better results than for boys; however, MULT 18 years and 19 years still showed higher specificity and +LR values. In the MULT reference, the 98·3th percentile seemed to be the most appropriate for establishing the cut-off point for obesity in boys, while for girls, it was the 98·1th percentile.
Table 2. Sensitivity, specificity, diagnostic accuracy and the positive and negative likelihood of the three BMI references
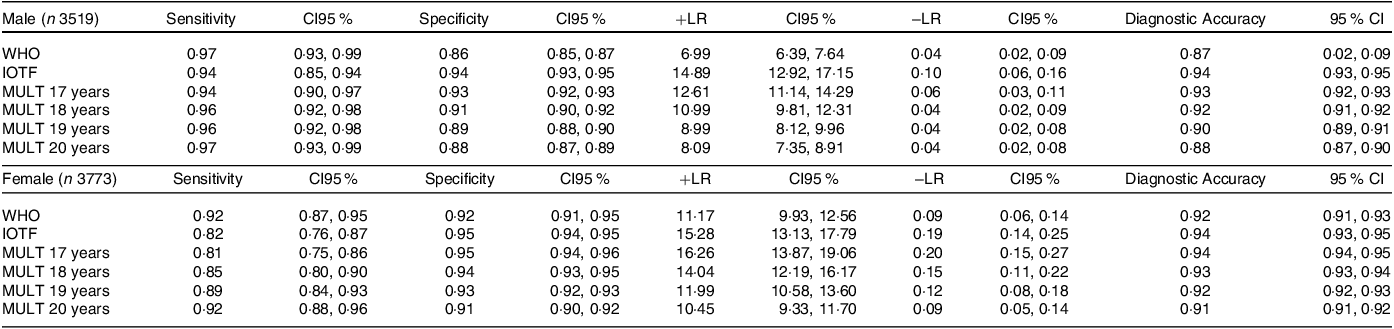
IOTF, International Task Obesity Force; n, number of subjects; n, Number of subjects; +LR, positive likelihood ratio; −LR, negative likelihood ratio; WHO, World Health Organization.
Diagnostic accuracy: Correctly classified proportion.
17 years: The obesity cut-off point was calculated using the BMI value of 30 kg/m2 at 17 years old.
18 years: The obesity cut-off point was calculated using the BMI value of 30 kg/m2 at 18 years old.
19 years: The obesity cut-off point was calculated using the BMI value of 30 kg/m2 at 19 years old.
20 years: The obesity cut-off point was calculated using the BMI value of 30 kg/m2 at 20 years old.
The nutritional status of the participants presented in Table 3 showed that the prevalence of stunting was higher applying the MULT height reference (3·4 %) when compared with the WHO height reference (2·3 %). The highest prevalence of underweight, overweight and obesity was classified using the WHO BMI reference, while the IOTF reference presented the lowest prevalence of overweight and obesity, and the MULT reference showed the lowest prevalence for underweight.
Table 3. The prevalence of stunting, underweight, normal weight, overweight and obesity estimated by three international growth references
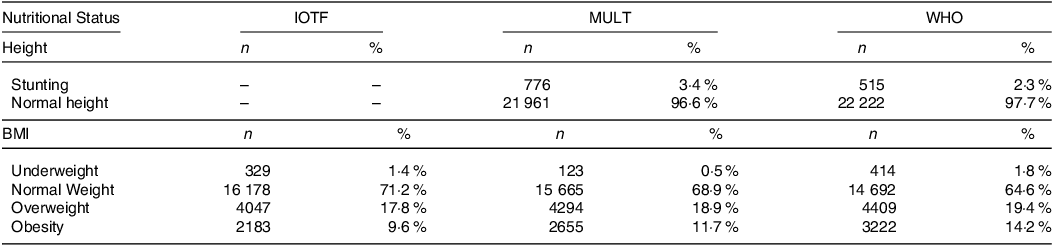
IOTF, International Task Obesity Force; n, number of subjects; WHO, World Health Organization.
In the BMI reference comparison analysis (Fig. 3), the Bland–Altman plots showed the lowest critical difference between the height references of WHO and MULT (critical difference = 0·22) and the highest critical difference between the BMI references of WHO and MULT (critical difference = 0·46). Moreover, the difference between WHO and MULT height references presented positive values, while the difference between WHO and MULT BMI references presented negative values in the extremities.
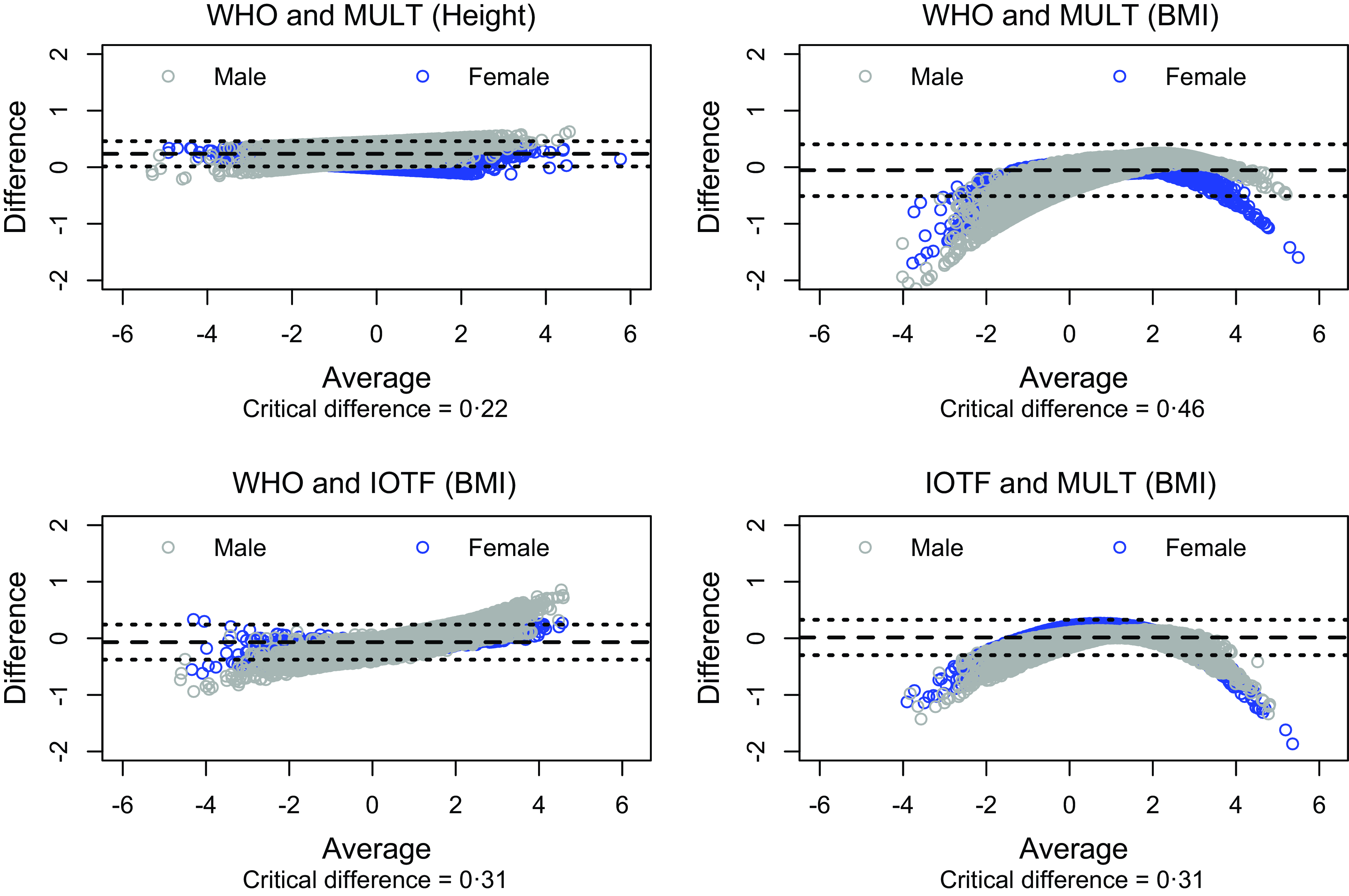
Fig. 3. Bland–Altman plot for comparing the three international growth references.
The concordance analysis (Table 4) showed a substantial agreement (CCC > 0·95 and ≤ 0·99) for the height references in all age groups and both sexes. For the BMI references, in boys there was an almost perfect agreement among the WHO and IOTF references (CCC > 0·99) at the age group 5–9 years and for the IOTF and MULT reference (CCC > 0·99) for the entire sample (5–16 years) for the age group 5–9 years. Among girls, the MULT reference showed an almost perfect agreement (CCC > 0·99) with the WHO for the age group 5–9 years and with IOTF for the age group 10–16 years. Regarding the nutritional status classification, applying the Kappa coefficient (Table 4), there was an almost perfect reliability (Kappa ≥ 0·81 & ≤ 1) between the MULT and WHO BMI references and the MULT and IOTF BMI references for all age groups and both sexes. Additionally, an almost perfect reliability was found between the IOTF and WHO BMI references for females. For the height references, there was an almost perfect reliability (Kappa ≥ 0·81 and ≤ 1) among boys aged five to 9 years and among girls aged 10–16 years.
Table 4. Concordance (CCC) and reliability (Kappa) among WHO, IOTF and MULT growth references, by age group and sex
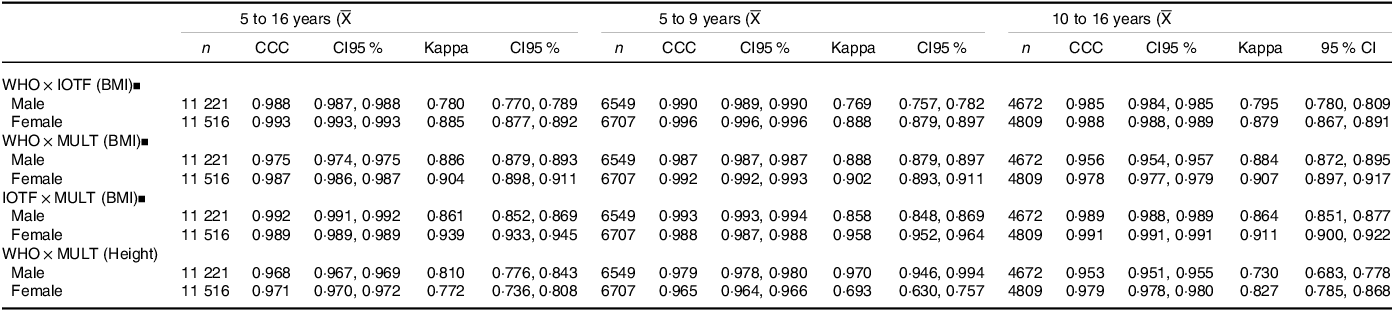
IOTF, International Task Obesity Force; WHO, World Health Organization;
![]() ${\rm{\overline X}}$
, average age in years; y, years; n, number of subjects; CCC, Lin’s concordance correlation coefficient; Kappa, Kappa coefficient; ▪, the weighted Kappa was applied.
${\rm{\overline X}}$
, average age in years; y, years; n, number of subjects; CCC, Lin’s concordance correlation coefficient; Kappa, Kappa coefficient; ▪, the weighted Kappa was applied.
Discussion
This study provided valuable insights into the correlation, diagnostic accuracy and concordance of different growth references based on body adiposity indicators. High positive correlations were identified among BMI z-scores and skinfold measurements, particularly with the MULT reference(Reference De Oliveira, Araújo and Severo14) displaying robust associations. The diagnostic accuracy assessment revealed nuanced patterns, with the IOTF and MULT (17 years) obesity percentiles exhibiting trade-offs between sensitivity and specificity(Reference De Oliveira, Araújo and Severo14,Reference Cole and Lobstein17,Reference Cole, Bellizzi and Flegal18) .
Notably, the MULT reference demonstrated its relevance in establishing obesity cut-offs, with specific percentiles identified for boys and girls(Reference De Oliveira, Araújo and Severo14). MULT growth reference presented a higher stunting prevalence and lower prevalences of underweight, overweight and obesity than the WHO growth reference(Reference De Oliveira, Araújo and Ramos13,Reference De Oliveira, Araújo and Severo14,Reference De Onis, Onyango and Borghi16) . This is because the sample of children included in the MULT height reference presented higher mean height values than the sample included in the WHO height reference(Reference De Oliveira, Araújo and Ramos13,Reference De Onis, Onyango and Borghi16) . Similar findings have been found in studies carried out in European countries and in Australia, which pointed out that children and adolescents in these regions exhibited greater height than those referenced in the WHO height reference(Reference De Onis, Onyango and Borghi16,Reference Rosario, Schienkiewitz and Neuhauser36–Reference Bonthuis, Van Stralen and Verrina38) .
Additionally, a comprehensive investigation encompassing 200 countries and territories, conducted by the NCD Risk Factor Collaboration (NCD-RisC/2020), indicates that although there are variations in average height across different nations, a transformative shift is underway(39). The most substantial increase in height over the past three decades has been seen in emerging economies(40). This positive trend in height in developing nations is attributed to enhancements in healthcare systems, sanitation and socio-economic conditions, all of which have contributed to improved growth for children born in the 21st century(15,Reference De Onis, Onyango and Borghi16,40) . This is the reason why height references that rely on data from children born before the 1990s, such as those from WHO (2007), exhibit lower average heights compared with references based on data from children born in more recent decades(Reference De Oliveira, Dos Santos Pereira and Melo10,Reference De Onis, Onyango and Borghi16,Reference Bonthuis, Van Stralen and Verrina38) . MULT height references have an advantage over WHO height reference since it was developed using height data from multi-ethnic children born more recently who likely overcome the secular trend in height(Reference De Oliveira, Araújo and Ramos13).
Regarding the association with adiposity, the BMI references presented a positive correlation to the adiposity assessed by the skinfolds, which is also observed in children younger than five years old(Reference Aristizabal, Barona and Hoyos41). A study conducted in Medellín (Colombia) with 232 children aged between 2 and 5 years showed a strong positive correlation between BMI and the TSF and SSF sum (r = 0·91)(Reference Aristizabal, Barona and Hoyos41). Another study conducted in Yogyakarta (Indonesia) with 2104 children aged seven to 18 years old pointed out a strong positive correlation between BMI and the TSF, SSF and suprailiac skinfold (r ≥ 0·74)(Reference Hastuti, Rahmawati and Suriyanto42). However, a study conducted in the states of Georgia and Alabama (USA) with 33 896 student-athletes aged from 11 to 19 years old pointed out that applying the BMI percentile from the CDC (2000) there was an overestimation of obesity classification(Reference Kuczmarski, Ogden and Guo19,Reference Etchison, Bloodgood and Minton43) . Other studies also observed this effect in children from South Africa and Saudi Arabia when applying the CDC BMI reference(Reference Kuczmarski, Ogden and Guo19,Reference Moselakgomo and Van Staden44,Reference El Mouzan, Al Herbish and Al Salloum45) .
In this way, even though the international BMI references of WHO (2007), IOTF (2012) and MULT (2023) presented good agreement and reliability, the nutritional status classification differed across them(Reference De Oliveira, Araújo and Severo14,Reference De Onis, Onyango and Borghi16–Reference Cole, Bellizzi and Flegal18) . The WHO BMI reference (2007) classified more children and adolescents as underweight, overweight and obese than the IOTF and the MULT BMI references(Reference De Oliveira, Araújo and Severo14,Reference De Onis, Onyango and Borghi16,Reference Cole and Lobstein17) . These results align with other studies that pointed out that the prevalence of overweight and obesity tends to be higher when applying the WHO BMI reference (2006/2007), which can indicate an overestimation of overweight and obesity conditions(15,Reference De Onis, Onyango and Borghi16,Reference Kêkê, Samouda and Jacobs46) . A study conducted with 1382 schoolchildren from Lille (France) pointed out an overestimation of overweight and obesity by WHO BMI references (2006/2007) in comparison to the IOTF (2012) and the French reference(15–Reference Cole and Lobstein17,Reference Kêkê, Samouda and Jacobs46) .
These findings are important to understand the premises of BMI, as it was primarily developed as an indicator of the relation between weight and height, and even though it does not measure adiposity directly, some of its cut-offs were found associated with obesity-related diseases, which made it to be widely used as a diagnostic tool for obesity screening at a populational level(Reference Bentham, Di Cesare and Bilano5,Reference Eknoyan47) . Additionally, there is a concern about the international growth reference to be used worldwide, especially because the IOTF (2012) BMI reference is just for children from two to 18 years old, and the WHO (2007) references were constructed with older data from a unique country (USA), who probably did not have overcome the secular trend in height that time(Reference De Oliveira, Dos Santos Pereira and Melo10,Reference De Onis, Onyango and Borghi16–Reference Cole, Bellizzi and Flegal18) . MULT references presented an advantage in comparison to these international references since it was constructed with data from multi-ethnic children born more recently (1990s–2000s) presenting growth charts from birth to 20 years old for height and BMI and great accuracy for diagnosing obesity, with a higher correlation to adiposity(Reference De Oliveira, Araújo and Ramos13,Reference De Oliveira, Araújo and Severo14,Reference De Oliveira, Araújo and Severo48) .
The major strengths of this study are being a large sample of children from two Brazilian cities and the use of anthropometric data gathered by trained researchers, which is supposed to reduce the odds of measurement errors and social desirability bias(Reference Costa, Santos and Goldraich6,Reference Costa, Cintra and Fisberg21,25) . Nevertheless, there are certain limitations in our study, such as the lack of a reference to determine obesity in children and adolescents according to their body adiposity, and the absence of skinfold data for children from Porto Alegre, restricting the diagnostic accuracy analysis only for schoolchildren(Reference Costa, Santos and Goldraich6). In addition, another limitation is the time frame for data collection, spanning almost a decade across two surveys. Both data collection points are positioned more than 10 years in the past, introducing the potential for temporal shifts and alterations in the prevalence of overweight and obesity(Reference Costa, Santos and Goldraich6,Reference Costa, Cintra and Fisberg21) . Considering the documented rise in obesity over the years, this temporal gap may affect the study’s ability to accurately capture and reflect the current landscape of overweight and obesity prevalences(Reference Bentham, Di Cesare and Bilano5). Despite not being derived from a representative sample of Brazil, the study’s strength lies in its incorporation of data from two distinct cities with divergent characteristics(Reference Careno, Silva and Martins22–Reference Guerreiro-Junior24). One city, influenced significantly by European heritage, is located in the southern region, while the other, in the south-eastern region, represents the state with the highest influx of immigrants from various parts of the country, resulting in exceptional ethnic diversity(Reference Careno, Silva and Martins22–Reference Guerreiro-Junior24).
In summary, the rise in obesity rates among children and adolescents emphasises the need for efficient monitoring of nutritional status(Reference Conde and Monteiro3,Reference Bentham, Di Cesare and Bilano5) . Improving the diagnosis of obesity in children can mitigate the enduring consequences of obesity on individuals’ overall health and welfare(Reference De Oliveira, Dos Santos Pereira and Melo10). Accordingly, this research assessed the effectiveness of international BMI references in identifying obesity, particularly in relation to body adiposity, and the in light of the extensive analysis conducted in this study, the MULT reference emerges as a compelling option for assessing the nutritional status of children and adolescents, particularly in the context of body adiposity.
The MULT reference exhibited strong positive correlations between BMI z-scores and skinfold measurements, showcasing its robust associations in capturing adiposity indicators. Moreover, the diagnostic accuracy assessment revealed the relevance of the MULT reference in establishing obesity cut-offs, with specific percentiles identified for both sexes(Reference De Oliveira, Araújo and Severo14). The unique strength of the MULT reference lies in its utilisation of data from multi-ethnic children born more recently, overcoming potential limitations associated with older references that may not reflect contemporary growth trends(Reference De Oliveira, Araújo and Ramos13,Reference De Oliveira, Araújo and Severo14) . Notably, the study highlights the limitations of other international references, such as overestimations of overweight and obesity conditions(Reference De Oliveira, Dos Santos Pereira and Melo10,Reference Etchison, Bloodgood and Minton43,Reference Moselakgomo and Van Staden44,Reference Kêkê, Samouda and Jacobs46) . While acknowledging study limitations, including temporal gaps and regional constraints, the MULT reference stands out as a promising tool for accurately diagnosing obesity in Brazilian children and adolescents(Reference De Oliveira, Araújo and Ramos13,Reference De Oliveira, Araújo and Severo14) . This research underscores the pressing need for efficient nutritional status monitoring and suggests that further validation studies could position the MULT reference(Reference De Oliveira, Araújo and Severo14) as a valuable international tool in assessing and addressing the global rise in childhood obesity. In this way, MULT growth reference seems to be a good option to assess the nutritional status of Brazilians, even though there is a need for further studies to validate it to be used internationally.
Acknowledgements
The authors are grateful to the staff and participants of the Santos and Porto Alegre studies and to the UNIFESP and UFRGS for conducting these surveys and making them available to researchers.
All phases of this study were supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) – Finance Code 001 (grant numbers 88887.368190/2019-00, 88887.356471/2019-00) and National Council for Scientific and Technological Development – CNPq, (protocol no. 159754/2010-0).
M. H. De O.: Conceptualisation, methodology, formal analysis, investigation, data curation, writing – original draft, writing – review and editing, visualisation and funding acquisition. R. F. da C.: Conceptualisation, methodology, investigation, writing – review and editing, supervision and funding acquisition. M. F.: Writing – review and editing. L. F. M. K.: Writing – review and editing. W. L. C.: Conceptualization, methodology, formal analysis, writing – review and editing, supervision, project administration.
The authors declare no conflicts of interest in relation to this article.









