Malnutrition and infection are major obstacles to survival, health, growth and reproduction of animals and humans worldwide (Field et al. Reference Field, Johnson and Schley2002; Calder & Yaqoob, Reference Calder, Yaqoob and Cynober2004). This global concern has led to the development of nutritional immunology as a new scientific discipline that integrates nutrition and immunology research methodologies to define a role for nutrients in the metabolism and function of cells of the immune system at molecular, cellular, tissue and whole-body levels. Recent studies indicate that dietary protein deficiency, which reduces concentrations of most amino acids in plasma (Wu et al. Reference Wu, Flynn, Flynn, Jolly and Davis1999) and compromises the immune system, remains a significant nutritional problem in developing countries and also occurs in subpopulations (e.g. the elderly or hospitalised patients) of developed nations (Woodward, Reference Woodward1998; Dasgupta et al. Reference Dasgupta, Sharkey and Wu2005). Thus, there is growing interest in the role of amino acids in the immune function of mammals, birds, fish and other species (Roch, Reference Roch1999; Calder, Reference Calder2006; Grimble, Reference Grimble2006; Kim et al. Reference Kim, Mateo, Yin and Wu2007).
Although dietary supplementation with high-quality protein may be effective in improving protein nutritional status in malnourished subjects (McMurray et al. Reference McMurray, Watson and Reyes1981), this is not feasible for patients who cannot tolerate enteral feeding. Consequently, defining the roles of individual amino acids in immune responses can aid in developing effective strategies to improve health and prevent infectious diseases. However, only in the past 15 years have the underlying cellular and molecular mechanisms begun to unfold (e.g. Wu & Brosnan, Reference Wu and Brosnan1992; Yaqoob & Calder, Reference Yaqoob and Calder1997; Newsholme et al. Reference Newsholme, Procopio, Lima, Pithon-Curi and Curi2003; Field, Reference Field2005; Calder, Reference Calder2006). The major objective of this article is to provide an insight into the specific roles of amino acids in immune function.
An overview of the immune system and assessments of immune function
The immune system
Because leucocytes are important targets for the actions of amino acids, here we briefly review the pertinent literature related to the defence against infectious diseases. The immune system protects the host from various pathogens and consists of the innate (natural, non-specific) and the acquired (adaptive, specific) systems (Calder, Reference Calder1995). These two systems are highly inter-related through cytokines and signalling molecules (Table 1). The innate immune system consists of physical barriers (e.g. skin, the endothelial cell layer in the respiratory tract, and the gastrointestinal tract), mononuclear phagocytes (e.g. monocytes and macrophages), dendritic cells, polymorphonuclear granulocytes (e.g. neutrophils, eosinophils and basophils), mast cells, natural killer (NK) cells, platelets and humoral factors including collectins, complements, lysozymes, C-reactive proteins and interferons. Recently, neutrophil extracellular traps, comprising DNA and proteins as major structural components, were discovered as a mechanism against bacterial infection (Buchanan et al. Reference Buchanan, Simpson, Aziz, Liu, Kristian, Kotb, Feramisco and Nizet2006). The innate immune system can rapidly respond to invading microbes, but its major disadvantages include non-specificity and a lack of memory effect. When infection cannot be fully cleared by the innate immunity over a short period, the adaptive immune system is activated to destroy infectious agents.
Table 1 Innate and adaptive immunity
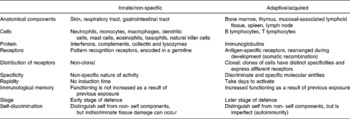
The adaptive (acquired) immune system consists of T lymphocytes, B lymphocytes and humoral factors (Calder, Reference Calder2006). The bone marrow is primarily responsible for haematopoiesis and lymphopoiesis, while the thymus is required for T-cell development. The spleen, lymph nodes and the mucosa-associated lymphoid tissues in the gastrointestinal, respiratory and reproductive tracts, and other organs are secondary lymphoid tissues. Because each lymphocyte carries surface receptors for a single antigen, the acquired immune response is highly specific. This immune system becomes effective over days after initial stimulation and possesses immunological memory. B lymphocytes are unique in their ability to produce and release specific antibodies in the humoral immunity. The antibodies can neutralise microorganisms (including viruses) or toxins by binding to them; activate complement proteins in plasma for the destruction of bacteria by phagocytes; immobolise bacteria; and opsonise various pathogens. When pathogens escape the humoral immunity, they are targeted by the cell-mediated immunity that involves the production of cytokines (e.g. interferon-γ (IFNγ)) and other cytotoxic proteins by T lymphocytes. Antibodies are highly effective against extracellular pathogens. In contrast, host cells infected with intracellular pathogens (e.g. viruses and certain bacteria) are generally cleared by cytotoxic T lymphocytes after the fragments of pathogens and the major histocompatibility complex are presented on the host cell surface and recognised by the T cells.
Innate and acquired immune systems are regulated by a highly interactive network of chemical communications (Fig. 1), which includes the synthesis of the antigen-presenting machinery, immunoglobulins and cytokines (Calder, Reference Calder2006). Both immune systems are highly dependent upon an adequate availability of amino acids for the synthesis of these proteins and polypeptides, as well as other molecules with enormous biological importance (Kim et al. Reference Kim, Mateo, Yin and Wu2007). These substances include nitric oxide (NO), superoxide, hydrogen peroxide, histamine, glutathione and anthranilic acid (Table 2). Individual amino acids affect immune responses either directly or indirectly through their metabolites. While the immune system is vital to health, it can be dysfunctional under certain conditions, resulting in the development of autoimmune and hypersensitivity diseases, such as insulin-dependent diabetes mellitus, rheumatoid arthritis and asthma (Wu, Reference Wu1995; Field, Reference Field2005).

Fig. 1 Interactions among immunocytes through production of regulatory molecules. Abbreviations: αβ T, αβ T cell; Abs, antibodies; Ags, antigens; AbPC, antibody-producing cells; AgPC, antigen-presenting cells; B, B lymphocytes; CD8, cytotoxic T cells carrying CD8 maker; DC, dendritic cell; EC, endothelial cells; ESP, eisinophil; GM-CSF, granulocyte/macrophage colony-stimulating factor; IFNγ, interferon γ; IL, interleukin; LPS, lipopolysaccharide; Mϕ, macrophage; M-CSF, macrophage colony-stimulating factor; Mast, mast cells; MC, monocyte; NETs, neutrophil excellular traps; NK, natural killer cells; NO, nitric oxide; NTP, neutrophil; PMA, phorbol myristate acetate; SCF, stem cell factor; Th0 CD4, T cells carrying CD4 marker; Th1, T helper cell 1; Th2, T helper cell 2; TNFα, tumour necrosis factor α.
Table 2 Roles of amino acids in immune responses
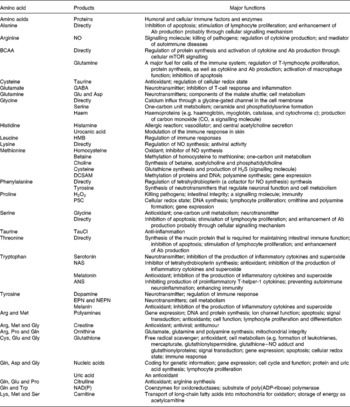
ANS, anthranilic acid; BCAA, branched-chain amino acids (isoleucine, leucine and valine); DCSAM, decarboxylated S-adenosylmethionine; EPN, epinephrine; GABA, γ-aminobutyrate; HMB, β-hydroxy-β-methylbutyrate; mTOR, the mammalian target of rapamycin; NAS, N-acetylserotonin; NEPN, norepinephrine; P5C, pyrroline-5-carboxylate, TauCl, taurine chloramine.
Assessments of immune function
It is likely that nutrients influence several or all aspects of the immune system. Thus, there are multiple, complex methods for assessing immune function in individuals, depending on experimental conditions, the availability of analytical facilities and the investigator's interest (Field, Reference Field1996; Calder & Yaqoob, Reference Calder and Yaqoob1999). The classic functional measurements in vivo include: (1) the delayed-type hypersensitivity response measured by skin testing; (2) serum antibody titres or the humoral immunity in response to primary or secondary (booster) immunisation; (3) blood levels of different lymphocyte subsets as well as serum concentrations of cytokines and other immune mediators; (4) the weights of lymphoid organs; and (5) morbidity and recovery from infectious disease. The in vitro assays of immune function often consist of: (1) the metabolism of immunocytes; (2) lymphocyte blastogenesis (cell proliferation) in response to mitogens; (3) cell morphology and apoptosis; (4) the phagocytosis of particles by monocytes and macrophages; and (5) the production of antibodies, cytokines and low-molecular weight cytotoxic substances.
Roles of amino acids in immune function
Alanine
Alanine is a major substrate for the hepatic synthesis of glucose, a significant energy substrate for leucocytes (Newsholme & Newsholme, Reference Newsholme and Newsholme1989), thereby influencing immune function. There is evidence that supplementation with 2 mm-alanine to the culture medium prevented apoptosis, enhanced cell growth and augmented antibody production in B-lymphocyte hybridoma (Duval et al. Reference Duval, Demangel, Munierjolain, Miossec and Geahel1991; Franek & Sramkova, Reference Franek and Sramkova1996). This supplemental concentration of alanine is approximately 2–4 times that in the plasma of animals and represents 8 % of that in ovine allantoic fluid on day 60 of gestation (Kwon et al. Reference Kwon, Spencer, Bazer and Wu2003). The underlying mechanism is not known, but may involve an alanine-mediated inhibition of protein degradation in immunocytes, as reported for hepatocytes through cellular signalling pathways (Meijer & Dubbelhuis, Reference Meijer and Dubbelhuis2004). At present, little information is available regarding an effect of dietary supplementation with alanine on the immune response in any animal species. However, in patients with total parenteral nutrition (TPN), inclusion of alanine could be highly beneficial for supporting gluconeogensis and leucocyte metabolism (Kudsk, Reference Kudsk2006).
Arginine, citrulline and ornithine
Arginine is synthesised from citrulline as an immediate precursor in virtually all cell types (Wu & Morris, Reference Wu and Morris1998). The small intestine of most mammals, except for cats and ferrets, is capable of synthesising citrulline from glutamine, glutamate and proline (Wu, Reference Wu1998). Plasma concentrations of both arginine and citrulline decrease markedly in subjects with protein malnutrition, fasting, trauma, burn injury, inflammation, sepsis and liver transplantation (Bansal & Ochoa, Reference Bansal and Ochoa2003). Under these conditions, arginine must be provided from the diet to support nitrogen balance and the health of animals and humans (Flynn et al. Reference Flynn, Meininger, Haynes and Wu2002).
Due to membrane depolarisation coupled to transport of the positively charged amino acid, arginine is a potent secretagogue for insulin, growth hormone, prolactin and insulin-like growth factor-I (Newsholme et al. Reference Newsholme, Brennnan, Rubi and Maechler2005). These hormones can mediate an NO-independent effect of arginine on immune function. In particular, insulin and growth hormone regulate the metabolism of glucose and amino acids in major tissues, including skeletal muscle, adipose tissue, liver and heart (Meijer & Dubbelhuis, Reference Meijer and Dubbelhuis2004), thereby influencing the availability of these nutrients for leucocytes. Growth hormone can also increase the production of T-lymphocytes in the thymus, the number of haematopoietic progenitor cells in the bone marrow, the response of T cells to cytokines and the antigen-presenting capability of dendritic cells (Calder & Yaqoob, Reference Calder, Yaqoob and Cynober2004). Interestingly, prolactin enhances the release of cytokines by Th1 lymphocytes and expression of the antigen-presenting major histocompatibility complex class II molecules (Dorshkind & Horseman, Reference Dorshkind and Horseman2000). In addition, insulin-like growth factor-I promotes the maturation of lymphocytes in the bone marrow, ameliorates ageing-related thymic involution and increases lymphocyte number and activity (Dorshkind & Horseman, Reference Dorshkind and Horseman2000).
Early in vitro studies have identified that 0·2 mm-arginine (close to its post-absorptive level in plasma) is required for the maximal proliferation of rodent and human T lymphocytes in response to mitogens and for the killing of tumour cells by activated macrophages (Hibbs et al. Reference Hibbs, Taintor and Vavrin1987). There is also evidence that high concentrations of arginine (e.g. 2 mm) increase the cytotoxicity of monocytes and NK cells in vitro (Abumrad & Barbul, Reference Abumrad, Barbul and Cynober2004). Note that an extracellular concentration of arginine >2 mm occurs in certain physiological fluids. For example, the arginine concentration is as high as 4–6 mm in porcine allantoic fluid during early pregnancy (Wu et al. Reference Wu, Bazer, Wallace and Spencer2006). Intensive research over the past decade has established that NO synthesis by inducible NO synthase (iNOS) in macrophages and neutrophils is an essential mechanism against viruses, bacteria, fungi, malignant cells, intracellular protozoa, and parasites in mammals, birds, terrestrial animals, low vertebrates (e.g. freshwater and marine fishes) and invertebrates (e.g. shrimp) (Bronte & Zanovello, Reference Bronte and Zanovello2005). Expression of iNOS is induced in leucocytes in response to IFNγ and lipopolysaccharide (LPS), and NO is now recognised to play an important role in both innate and acquired immunity (Bogdan et al. Reference Bogdan, Rollinghoff and Diefenbach2000). Therefore, NO production by iNOS is of most relevance to the immune response. Because arginase and iNOS compete for arginine as a common substrate, modulating arginase expression and activity plays a critical role in NO generation by leucocytes (Kepka-Lenhart et al. Reference Kepka-Lenhart, Mistry, Wu and Morris2000). Interestingly, some bacteria, such as Helicobacter pylori, develop a strategy of survival from NO killing through constitutive expression of arginase, which consumes arginine and thus reduces its availability for NO production by iNOS (Gobert et al. Reference Gobert, McGee, Akhtar, Mendz, Newton, Cheng, Mobley and Wilson2001). An exciting new development is that physiological levels of arginine (e.g. 150 μm) modulates expression of the T-cell receptor ζ chain (CD3ζ) that is required for T-cell receptor integrity (Rodriguez et al. Reference Rodriguez, Zea, DeSalvo, Culotta, Zabaleta, Quiceno, Ochoa and Ochoa2003). Further, the addition to culture medium of citrulline at 0·1 mm (close to its plasma level) or 1 mm (10 % of its concentration in ovine allantoic fluid during early pregnancy (Kwon et al. Reference Kwon, Spencer, Bazer and Wu2003)) promoted the synthesis of CD3ξ by prolonging the half-life of its mRNA (Bansal et al. Reference Bansal, Rodriguez, Wu, Eichler, Zabaleta, Taheri and Ochoa2004). This result provides a basis for the use of citrulline to increase arginine availability under immunodeficient conditions associated with elevated arginase activity in blood.
A large body of evidence from animal studies indicates that adequate provision of arginine is required for lymphocyte development and that dietary arginine supplementation en hances immune function in various models of immunological challenges (Field et al. Reference Field, Johnson and Pratt2000; Calder & Yaqoob, Reference Calder, Yaqoob and Cynober2004). In particular, a deficiency of arginine in mice (its plasma concentration < 0·1 mm) resulting from overexpression of arginase in the small intestine impaired the development of progenitor-B to precursor-B lymphocytes in the bone marrow and decreased the number of B lymphocytes in secondary lymphoid organs (De Jonge et al. Reference De Jonge, Kwikkers, Te Velde, Van Deventer, Nolte, Mebius, Ruijter, Lamers and Lamers2002). Importantly, these defects were reversed by subcutaneous administration with arginine (5 mmol/kg body weight twice daily). Also, inadequate intake of dietary arginine (e.g. 0·3 % arginine in the diet) impairs NO synthesis by both constitutive NOS and iNOS in young rats (Wu et al. Reference Wu, Flynn, Flynn, Jolly and Davis1999) and reduces the immune response in growing chickens (Konashi et al. Reference Konashi, Takahashi and Akiba2000). Further, dietary supplementation with 1 or 2 % arginine (~1 or 2 times the arginine content of the regular diet) to healthy rodents and tumour-bearing or septic rats increased thymic weight, the number of thymic lymphocytes, T-lymphocyte proliferation, the cytotoxicity of specific cells (T lymphocytes, macrophages and NK cells), interleukin (IL)-2 production, IL-2 receptor expression on T lymphocytes and the delayed-type hypersensitivity response (Calder & Yaqoob, Reference Calder, Yaqoob and Cynober2004). In addition, supplementing 1 or 2 % arginine to the diet for rats with trauma injury ameliorated thymic involution and body weight loss, while improving wound healing and survival rate (Field et al. Reference Field, Johnson and Schley2002). In animals with burn injury, lethal bacterial peritonitis or intestinal damage, arginine supplementation (1 % of the diet) reduced bacterial translocation, increased the bactericidal activity of host phagocytes and prolonged host survival (Abumrad & Barbul, Reference Abumrad, Barbul and Cynober2004). Further, dietary supplementation with 0·83 % arginine enhanced the immune status of pregnant sows and neonatal pigs, thereby reducing morbidity and mortality in response to infectious pathogens (Kim et al. Reference Kim, Mateo, Yin and Wu2007). Like arginine, ornithine α-ketoglutarate supplementation (1 g/kg body weight per day) modulated immune function in various rat models of catabolic conditions, including burns, sepsis, tumour bearing and glucocorticoid-induced stress (Cynober, Reference Cynober and Cynober2004).
Clinical studies have shown that enteral or parenteral provision of arginine (e.g. 8–20 g/d; corresponding to 1·5–3·6 times the arginine intake by an average adult) improves immune functions and clinical outcomes in patients with burn injury, cancer, HIV infection, major traumas and gastrointestinal surgical operations (Field et al. Reference Field, Johnson and Schley2002; Suchner et al. Reference Suchner, Heyland and Peter2002). The benefits are indicated by enhanced T-cell function, increased antibody production, accelerated wound healing mediated by immune cells or a reduction in infection, ventilator days, intensive care unit stay and hospital stay. The most important outcome of this research is the current availability of arginine-supplemented ‘immunonutrition’ (Kudsk, Reference Kudsk2006). However, these formulas have supplemental l-arginine, n-3 fatty acids and nucleotides, making it unclear whether their benefit derives entirely from arginine. Nevertheless, recent studies with surgical patients have shown that these formulas may save hospital costs by decreasing the length of stay and reducing infection rates (Senkal et al. Reference Senkal, Mumme, Eickhoff, Geier, Spath, Wulfert, Joosten, Frei and Kemen1997; Braga et al. Reference Braga, Gianotti, Radaelli, Vignali, Mari, Gentilini and DiCarlo1999). In contrast, benefits of arginine supplementation to critically ill patients with severe systemic inflammatory response syndrome, sepsis or multiple organ failure are less clear (Suchner et al. Reference Suchner, Heyland and Peter2002). This is probably owing to the complex nature of arginine metabolism and NO production in vivo (Wu & Morris, Reference Wu and Morris1998). Further, it is important to recognise that NO is an oxidant and inhibitor of enzymes that contain an iron–sulphur centre and that high levels of NO rapidly react with H2O2 to form peroxynitrite (ONOO− ) (Fang et al. Reference Fang, Yang and Wu2002). NO and peroxynitrite oxidise biomolecules (e.g. proteins, amino acids, lipids and DNA), which leads to cell injury and death. Thus, large amounts of NO produced by iNOS can exert a deleterious effect on mammalian cells and mediate the pathogenesis of many diseases, including the autoimmune destruction of pancreatic β-cells in type I diabetes mellitus, arthritis, glomerulonephritis, inflammatory bowel disease and neurological disorders (Flynn et al. Reference Flynn, Meininger, Haynes and Wu2002). Under these conditions, arginine supplementation could ‘fuel the fire’ to worsen clinical outcomes (Wu et al. Reference Wu, Meininger, Knabe, Bazer and Rhoads2000).
Asparagine
An early study suggested that asparagine availability could modulate lymphocyte blastogenesis as a possible means to treat childhood acute lymphoblastic leukaemia (Ohno & Hersh, Reference Ohno and Hersh1970). Depletion of asparagine by exogenous asparaginase had been considered to be immunosuppressive until Kafkewitz & Bendich (Reference Kafkewitz and Bendich1983) reported that this effect might also be attributable to glutamine depletion by a glutaminase activity present in asparaginase. However, subsequent research has provided the following lines of evidence that asparagine plays a significant role in immune function. First, the expression of asparagine synthetase was markedly enhanced in lymphocytes and macrophages in response to mitogens and other stimuli (Suzuki et al. Reference Suzuki, Okayasu, Tashiro, Hashimoto, Yokote, Akahane, Hongo and Sakagami2002). Secondly, an increase in intracellular provision of asparagine increased the expression and activity of ornithine decarboxylase for polyamine synthesis in thymocytes (Brand, Reference Brand1987) and of iNOS in activated macrophages (Suzuki et al. Reference Suzuki, Okayasu, Tashiro, Hashimoto, Yokote, Akahane, Hongo and Sakagami2002). Thirdly, asparagine (2 mm) prevented apoptosis and increased cell growth in lymphocytes (Duval et al. Reference Duval, Demangel, Munierjolain, Miossec and Geahel1991). Thus, asparagine is beneficial for mounting a successful immune response in normal subjects but can also contribute to abnormal lymphoblastic growth in patients with leukaemia. At present, little is known about an effect of dietary supplementation with asparagine on immune function in animals or humans.
Aspartate and glutamate
Aspartate and glutamate play versatile roles in the metabolism and function of leucocytes (Newsholme et al. Reference Newsholme, Procopio, Lima, Pithon-Curi and Curi2003). As a substrate for the synthesis of purine and pyrimidine nucleotides, aspartate is crucial for the proliferation of lymphocytes (Newsholme & Calder, Reference Newsholme and Calder1997). Moreover, aspartate is required for the recycling of the citrulline produced by iNOS into arginine in activated macrophages (Wu & Brosnan, Reference Wu and Brosnan1992). This helps maintain an adequate intracellular concentration of arginine for sustaining a high rate of NO production in response to immunological challenges. As noted above, through asparagine synthesis, aspartate contributes to the modulation of immune function. In addition, glutamate regulates iNOS expression in certain tissues (e.g. brain), thereby indirectly modulating immunocompetence of animals (Wu & Meininger, Reference Wu and Meininger2002). Moreover, glutamate is a substrate for the synthesis of γ-aminobutyrate (GABA), which is present in both lymphocytes (Tian et al. Reference Tian, Lu, Zhang, Chau, Dang and Kaufman2004) and macrophages (Stuckey et al. Reference Stuckey, Anthony, Lowe, Miller, Palm, Styles, Perry, Blamire and Sibson2005). Interestingly, T cells express GABA receptors, which mediate an inhibitory effect of GABA on their proliferation (Tian et al. Reference Tian, Lu, Zhang, Chau, Dang and Kaufman2004). Further, as an immediate precursor for glutathione synthesis, glutamate plays an important role in the removal of oxidants and regulation of the immune response (Wu et al. Reference Wu, Bazer, Cudd, Meininger and Spencer2004a). Importantly, dietary aspartate and glutamate, along with glutamine, are the major fuels for enterocytes (Wu, Reference Wu1998). Together, these amino acids help maintain intestinal barrier integrity and prevent the translocation of intestinal microorganisms to the systemic circulation (Van der Hulst et al. Reference Van der Hulst, van Kreel, von Meyenfeldt, Brummer, Arends, Deutz and Soeters1993). Besides their role in leucocyte metabolism as energy substrates, both aspartate and glutamate are excitatory neurotransmitters in central and peripheral nervous systems, acting on ionotropic and metabotropic receptors, which play a role in modulating the immune systems (Newsholme et al. Reference Newsholme, Procopio, Lima, Pithon-Curi and Curi2003). Finally, glutamate and aspartate mediate the transfer of reducing equivalents across the mitochondrial membrane and thus regulate glycolysis and the cellular redox state in cells via the malate/aspartate shuttle (Newsholme et al. Reference Newsholme, Curi, Curi, Murphy, Garcia and de Melo1999).
GABA administration (0·6 mg/d) inhibited the development of the proinflammatory T-cell response and retarded the adoptive transfer of type I diabetes in NOD/SCID mice (Tian et al. Reference Tian, Lu, Zhang, Chau, Dang and Kaufman2004). Interestingly, Lin et al. (Reference Lin, Abcouwer and Souba1999) reported that supplementation with 4 and 8 % glutamate to a glutamine- and glutamate-free diet enhanced the delayed-type hypersensitivity and lymphoproliferation responses in rats recovering from methotrexate treatment. Notably, the beneficial effect of dietary glutamate was dose dependent and more pronounced after a longer period of supplementation (Lin et al. Reference Lin, Abcouwer and Souba1999). These results suggest that dietary glutamate is necessary for maintaining an optimal immune status under conditions of immunosuppression.
Branched-chain amino acids (BCAA)
Lymphocytes express BCAA transaminase and branched-chain 2-oxoacid dehydrogenase for BCAA degradation (Schafer & Schauder, Reference Schafer and Schauder1988). The transport and utilisation of BCAA by lymphocytes are dramatically increased in response to mitogens, with their uptake being highest during the S phase of the cell cycle (Koch et al. Reference Koch, Schroder, Schafer and Schauder1990). Importantly, BCAA provide the α-amino group for the endogenous synthesis of glutamine primarily in skeletal muscle (Fig. 2), which has been considered as part of the immune system (Newsholme and Calder, Reference Newsholme and Calder1997). Also, leucine is an activator of the mTOR signalling pathway that regulates protein synthesis and degradation in cells (Meijer & Dubbelhuis, Reference Meijer and Dubbelhuis2004). This may explain why reducing extracellular concentrations of each BCAA below 0·2 mm (close to the plasma level), as occurs in patients with protein malnutrition, impairs lymphocyte proliferation (Skaper et al. Reference Skaper, Molden and Seegmiller1976).
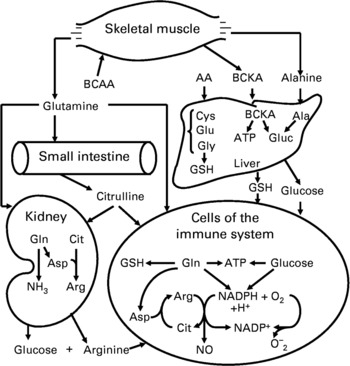
Fig. 2 Inter-organ metabolism of branched-chain amino acids, glutamine and arginine, and their role in immune function. Skeletal muscle takes up BCAA from the arterial blood, synthesises both alanine and glutamine from BCAA and α-ketoglutarate, and releases these two amino acids into the circulation. The small intestine utilises glutamine to synthesise citrulline, which is converted into arginine in kidneys, cells of the immune system and other cell types. The liver is the primary organ for the synthesis of glutathione from glutamate, glycine and cysteine, and of glucose from alanine for use by extrahepatic cells (including leukocytes) and tissues. Abbreviations: Arg, arginine; Asp, aspartate; Cit, citrulline; BCAA, branched-chain amino acids; BCKA, branched-chain α-ketoacids; Gluc, glucose; GSH, glutathione.
There is a paucity of data on an effect of BCAA on the production of cytokines and antibodies by lymphocytes in vitro (Calder, Reference Calder2006). Because the carbon skeletons of BCAA are not synthesised in leucocytes, a lack of leucine, isoleucine or valine in the culture medium results in the complete absence of protein synthesis or proliferation of lymphocytes in response to mitogens (Waithe et al. Reference Waithe, Dauphinais, Hathaway and Hirschhorn1975). However, altering medium concentrations of BCAA between 0·2 and 1 mm (~1 and 5 times plasma levels) had no effect on lymphocyte proliferation (Skaper et al. Reference Skaper, Molden and Seegmiller1976). This finding suggests that normal levels of BCAA in plasma do not limit T-cell responses to mitogens.
A number of animal studies indicate that an inadequate intake of BCAA results in immune impairment. In particular, Jose & Good (Reference Jose and Good1973) reported that dietary restriction of leucine and valine (25 and 50 % of the control dietary intake) caused approximately 80–90 % decreases in lymphocyte-mediated tumour cell lysis. Interestingly, leucine appears to exert a greater effect on immune function than isoleucine and valine (Konashi et al. Reference Konashi, Takahashi and Akiba2000), which may be explained in part by their differential actions on the mTOR signalling (Meijer & Dubbelhuis, Reference Meijer and Dubbelhuis2004). Also, mice fed a BCAA-deficient diet for 3 weeks exhibited enhancement of susceptibility to Sal monella typhimurium, impairment in antibody production, reductions in serum concentrations of transferrin and complement C3, and increased numbers of bacteria in liver and spleen (Petro & Bhattacharjee, Reference Petro and Bhattacharjee1981). In tetrachloride-induced cirrhotic rats fed a 14 % casein diet, supplementing 10 % BCAA increased the number of liver-associated lymphocytes (especially CD8-positive cells) and NK cells, as well as lectin-dependent cellular cytotoxicity, compared with the control rats consuming a 24 % casein diet (Tsukishiro et al. Reference Tsukishiro, Shimizu, Higuchi and Watanabe2000). Consistent with the animal studies, supplementing 35 % BCAA (or 0·7 g/kg body weight per day) to TPN solution increased blood lymphocyte counts in patients recovering from surgery and decreased mortality in septic patients (Freund et al. Reference Freund, Ryan and Fisher1978; Cerra et al. Reference Cerra, Mazuski, Chute, Nuwer, Teasley, Lysne, Shronts and Konstantinides1984). Further, dietary supplementation with a 6 g BCAA mixture (60 % leucine, 20 % isoleucine and 20 % valine) to athletes increased IFNγ production, decreased IL-4 release, prevented an exercise-associated decrease in tumour necrosis factor-α (TNFα) and IL-1 synthesis by mononuclear cells and stimulated lymphocyte proliferation (Bassit et al. Reference Bassit, Sawada, Bacurau, Navarro, Martins, Santos, Caperuto, Rogeri and Costa2002). Because BCAA share the same transporter on the cell membrane, an imbalance in their dietary composition can result in immunity impairment, especially when animals are fed a low-protein diet (Aschken- asy, Reference Aschkenasy1979).
β-Hydroxy-β-methyl-butyrate (HMB), a leucine metabolite, may play a role in immune function. In vitro studies have shown that HMB (0·1–2 mm) increased the proliferation, phagocytosis and expression of Fc receptors in a chicken macrophage cell line (Peterson et al. Reference Peterson, Qureshi, Ferket and Fuller1999). Dietary supplementation with 0·1 % HMB improved immune function and reduced mortality in several animal models of infection, including chickens, fish and pigs (Nissen & Abumrad, Reference Nissen and Abumrad1997).
Glutamine
Glutamine is an abundant amino acid in plasma, skeletal muscle, fetal fluids and milk (Wu & Knabe, Reference Wu and Knabe1994; Newsholme & Calder, Reference Newsholme and Calder1997; Self et al. Reference Self, Spencer, Johnson, Hu, Bazer and Wu2004). As a major energy substrate for cells of the immune system (Wu et al. Reference Wu, Field and Marliss1991; Newsholme et al. Reference Newsholme, Curi, Curi, Murphy, Garcia and de Melo1999), glutamine plays an important role in their function and homeostasis. Interestingly, glutamine is extensively catabolised via the glutaminolysis pathway to yield primarily glutamate and, to a lesser extent, aspartate, alanine, lactate, pyruvate and CO2 in cells of the immune system, including thymocytes, lymph node lymphocytes, blood lymphocytes, intraepithelial lymphocytes, neutrophils and macrophages (Field et al. Reference Field, Wu and Marliss1994; Wu, Reference Wu1996; Newsholme et al. Reference Newsholme, Curi, Curi, Murphy, Garcia and de Melo1999). The action of the NADP+-dependent malate dehydrogenase, which is present in lymphocytes, macrophages, monocytes and neutrophils, converts malate and NADP+ to pyruvate and NADPH (Newsholme, Reference Newsholme2001). Notably, NADPH is required by NOS and NADPH oxidase for the production of NO and superoxide anion, respectively (Fang et al. Reference Fang, Yang and Wu2002). As a major source of glutamate, glutamine regulates the synthesis of glutathione, a tripeptide crucial for defending cells from oxidative stress (Wu et al. Reference Wu, Fang, Yang, Lupton and Turner2004b). As an essential precursor for the synthesis of purine and pyrimidine nucleotides, glutamine is required for proliferation of lymphocytes (Ardawi & Newsholme, Reference Ardawi and Newsholme1983; Wu et al. Reference Wu, Field and Marliss1992). Increasing extracellular concentrations of glutamine from 0·01 to 0·5 mm (a physiological level in plasma) dose-dependently increases lymphocyte proliferation (Wu et al. Reference Wu, Field and Marliss1992). There is also evidence that glutamine is required for NO synthesis in macrophages and monocytes via arginine synthesis. Indeed, arginine derived from glutamine appears to be essential for macrophage activity (Murphy & Newsholme, Reference Murphy and Newsholme1998). Mitogens, changes in cell volume (an early event in the activation of lymphocytes and macrophages in response to immunological stimulation), inflammatory cytokines and an acid–base balance are major regulators of glutamine metabolism in leucocytes (Wu & Flynn, Reference Wu and Flynn1995a, Reference Wu and Flynnb; Newsholme et al. Reference Newsholme, Procopio, Lima, Pithon-Curi and Curi2003).
Several lines of evidence from in vitro studies show that glutamine affects various components of the immune response. First, glutamine is necessary for the proliferation of lymphocytes in response to stimulation by T-cell mitogens and activation of protein kinase C (Parry-Billing et al. Reference Parry-Billings, Evans, Calder and Newsholme1990; Wu et al. Reference Wu, Field and Marliss1992; Wu, Reference Wu1996). Secondly, addition of 2 mm-glutamine to the culture medium prevented apoptosis, stimulated cell growth and promoted antibody production in lymphocytes (Franek & Sramkova, Reference Franek and Sramkova1996). Thirdly, maximal NO production by activated macrophages occurs in the presence of an extracellular concentration of 1 mm-glutamine (Wu & Meininger, Reference Wu and Meininger2002). Fourthly, at or near physiological levels in plasma, glutamine (0·5–2 mm) modulates the production of cytokines by monocytes and macrophages (Spittler et al. 1997; Yaqoob & Calder, Reference Yaqoob and Calder1998). Indeed, a sufficient supply of extracellular glutamine (e.g. 2 mm) is required for the maximal production of IL-1 and TNFα by murine macrophages, and of IL-6 and IL-8 by human monocytes (Field et al. Reference Field, Johnson and Schley2002). Fifthly, the maximum phagocytosis of murine macrophage depends on adequate provision of extracellular glutamine (0·6 mm) (Parry-Billing et al. Reference Parry-Billings, Evans, Calder and Newsholme1990; Newsholme et al. Reference Newsholme, Procopio, Lima, Pithon-Curi and Curi2003). Similarly, glutamine (0·5–2 mm) influences the expression of various genes related to (1) intercellular interactions; (2) the production of cytokines by T lymphocytes; (3) phagocytosis of immunoglobulin G or complement-opsonised particles; (4) antigen presentation; and (5) opsonisation of human monocytes (Spittler et al. Reference Spittler, Holzer, Oehler, Boltz-Nitulescu and Roth1997; Yaqoob & Calder, Reference Yaqoob and Calder1997; Wells et al. Reference Wells, Kew, Yaqoob, Wallace and Calder1999; Newsholme et al. Reference Newsholme, Procopio, Lima, Pithon-Curi and Curi2003). Sixthly, glutamine (0·1–30 mm) in the culture medium enhances the bactericidal function of neutrophils isolated from burn patients in a dose-dependent manner (Ogle et al. Reference Ogle, Ogle, Mao, Simon, Noel, Li and Alexander1994). Note that an extracellular concentration of glutamine >20 mm occurs in certain physiological fluids. For example, glutamine concentration is as high as 25 mm in ovine allantoic fluid during early pregnancy (Kwon et al. Reference Kwon, Spencer, Bazer and Wu2003). Finally, glutamine (2 mm) affects the lytic potential of cultured lymphokine-activated killer cells (Juretic et al. Reference Juretic, Spagnoli, Hörig, Babst, von Bremen, Harder and Heberer1994) and is required for the activation of NK cells that are capable of spontaneous cytolytic activity against a variety of tumour cells (Liang et al. Reference Liang, Cattill and Liang1989). In addition, the activated NK cells participate in other functions through the production of cytokines (Fig. 1).
Animal studies show that enteral or parenteral provision of glutamine enhances the immunity of the host. For example, dietary supplementation with 4 % glutamine maintained intramuscular glutamine concentrations and normalised lymphocyte function in early-weaned pigs infected with endotoxin (Yoo et al. Reference Yoo, Field and McBurney1997). Also, supplementation with 3·5 % glutamine to a casein-based diet resulted in an increased ability of macrophages to produce TNFα, IL-1β and IL-6 (Wells et al. Reference Wells, Kew, Yaqoob, Wallace and Calder1999), and lymphocyte responsiveness to mitogens (Kew et al. Reference Kew, Wells, Yaqoob, Wallace, Miles and Calder1999), providing the first evidence that glutamine supplementation can enhance both macrophage and lymphocyte activities in vivo. Note that this supplemental glutamine corresponds to 1·8 times the glutamine content of the control diet. Further, dietary provision of 2 % glutamine was essential for the maintenance of gut-associated lymphoid tissues and for the synthesis of secretary immunoglobulin A by the small intestine, thereby preventing TNFα-induced bacterial translocation from the lumen of the gut into the circulation (Alverdy, Reference Alverdy1990). Moreover, dietary supplementation with 2 or 4 % glutamine increased the survival of mice to bacterial challenges (Adjei et al. Reference Adjei, Matsumoto, Oku, Hiroi and Yamamoto1994), improved tumour-directed NK cell cytotoxic activity in rats (Shewchuk et al. Reference Shewchuk, Baracos and Field1997) and reduced the growth of implanted tumours in rats (Shewchuk et al. Reference Shewchuk, Baracos and Field1997). Likewise, parenteral provision of glutamine (2 g/100 ml) attenuated the adverse effects of TPN on the gut and respiratory tract immunity in rats (Li et al. Reference Li, Kudsk, Janu and Renegar1997), and increased the survival of rats to endotoxin and bacterial challenges (Ardawi, Reference Ardawi1991; Inoue et al. Reference Inoue, Grant and Snyder1993).
Findings from a majority of human clinical trials indicate that glutamine supplementation in the form of free glutamine or alanyl-glutamine dipeptide (8–30 g of oral glutamine per day or 0·3 g of alanyl-glutamine/kg body weight per day in TPN) is beneficial for the immune system in patients with burn injury and gastrointestinal surgical operations, as well as in critically ill patients (Van der Hulst et al. Reference Van der Hulst, van Kreel, von Meyenfeldt, Brummer, Arends, Deutz and Soeters1993; Melis et al. Reference Melis, ter Wengel, Boelens and van Leeuwen2004). These effects are indicated by increases in lymphocyte number and function, as well as reductions in infectious complications, hospital stay, morbidity and mortality. Notably, a randomised, double-blind study with 28 patients undergoing major abdominal surgery demonstrated that pre-operative glutamine supplementation via TPN improved nitrogen balance, increased lymphocyte function, augmented leukotriene production by neutrophils, reduced the incidence of infection and shortened hospital stay (Morlion et al. Reference Morlion, Stehle, Wachtler, Siedhoff, Koller, Konig, Furst and Puchstein1998). Similar results have also been reported for patients receiving bone marrow transplants (McBurney et al. Reference McBurney, Young, Ziegler and Wilmore1994). Consistent with the favourable clinical outcomes, patients receiving glutamine in TPN (5 g/100 ml) exhibited higher total counts of blood lymphocytes (including CD4 and CD8 T lymphocytes) when compared with patients receiving glutamine-free TPN (Ziegler et al. Reference Ziegler, Bye, Persinger, Young, Antin and Wilmore1998). In addition, oral administration of glutamine (27 mg/kg body weight) increased concentrations of plasma growth hormone in humans (Welbourne, Reference Welbourne1995), which in turn beneficially modulate the immune system (Newsholme et al. Reference Newsholme, Brennnan, Rubi and Maechler2005). Thus, a reduced availability of glutamine may impair immune function, thereby increasing the susceptibility of humans to infectious diseases. Because these published clinical studies involved a relatively small number of subjects, the efficacy of glutamine should be verified in a larger, multicentre study.
Glycine
Glycine participates in the synthesis of many physiologically important molecules, including purine nucleotides, glutathione and haem (Kim et al. Reference Kim, Mateo, Yin and Wu2007). In addition, glycine itself is a potent antioxidant, scavenging free radicals (Fang et al. Reference Fang, Yang and Wu2002). Thus, glycine is essential for the proliferation and antioxidative defence of leucocytes. There is also molecular and pharmacological evidence for a glycine-gated chloride channel in leucocytes (Froh et al. Reference Froh, Thurman and Wheeler2002). The activation of this channel suppresses the agonist-induced opening of L-type voltage-dependent calcium channels and, thus, attenuates intracellular Ca2+ concentrations. As a result, glycine plays a role in regulating the production of cytokines by leucocytes and immune function (Zhong et al. Reference Zhong, Wheeler, Li, Froh, Schemmer, Yin, Bunzendaul, Bradford and Lemasters2003). This notion is supported by in vitro studies showing that an increase in extracellular glycine concentration (0·1–1 mm) within the physiological range activated a glycine-gated chloride channel and hyperpolarised the plasma membrane in a variety of cells types, including macrophages, monocytes, lymphocytes and neutrophils (Froh et al. Reference Froh, Thurman and Wheeler2002). In macrophages stimulated by LPS, glycine (0·1–1 mm) reduced the influx of Ca2+ and an increase in its intracellular concentration, thereby blunting the production of superoxide, IL-1 and TNFα (Wheeler & Thurman, Reference Wheeler and Thurman1999). Glycine did not affect IL-2 production in T-cells in response to stimulation by immobilised anti-CD3 antibody, but inhibited cell proliferation in a dose-dependent manner (0·1–1 mm) by attenuating an increase in intracellular Ca2+ levels (Stachlewitz et al. Reference Stachlewitz, Li, Smith, Bunzendahl, Graves and Thurman2000). Further, addition of 2 mm-glycine to the culture medium prevented apoptosis and enhanced antibody production in B lymphocytes (Duval et al. Reference Duval, Demangel, Munierjolain, Miossec and Geahel1991).
There is also in vivo evidence that glycine reduces inflammatory reactions and morbidity in pathogen-infected animals. A deficiency of dietary glycine impaired immune responses in chickens treated with LPS, which was alleviated by its dietary supplementation (Konashi et al. Reference Konashi, Takahashi and Akiba2000). Additionally, dietary supplementation with 5 % glycine to rats infected with a lethal dose of LPS reduced plasma levels of TNFα and improved survival rate (Ikejima et al. Reference Ikejima, Iimuro, Forman and Thurman1996). Similarly, supplementation with 1 % glycine to a liquid milk diet reduced inflammation and attenuated a rise in body temperature of calves infected with a low dose of endotoxin (Simon, Reference Simon1999). Interestingly, glycine protected animals against peptidoglycan polysaccharide-induced arthritis; chemically and stress-induced gastrointestinal mucosal injury; the ischaemia/reperfusion injury of a variety of organs; and shock caused by haemorrhage, endotoxin and sepsis (Zhong et al. Reference Zhong, Wheeler, Li, Froh, Schemmer, Yin, Bunzendaul, Bradford and Lemasters2003). In particular, dietary supplementation with 5 % glycine prevented experimental colitis in rat models induced by an intracolonic injection of 2,4,6-trinitrobenzene sulphonic acid or oral administration of dextran sulphate sodium (Tsune et al. Reference Tsune, Ikejima, Hirose, Yoshikawa, Enomoto, Takei and Sato2003). In both models of gut inflammation, dietary supplementation with 5 % glycine abolished increases in colonic expression of IL-1β and TNFα, cytokine-induced neutrophil chemoattractant and macrophage inflammatory protein, thereby ameliorating diarrhoea and body weight loss (Tsune et al. Reference Tsune, Ikejima, Hirose, Yoshikawa, Enomoto, Takei and Sato2003). Collectively, these findings indicate that glycine is a novel anti-inflammatory, immunomodulatory and cytoprotective nutrient. Clinical trials are warranted to determine the efficacy of glycine supplementation on improving immune function in humans.
Histidine
Plasma contains a high level of a histidine-rich glycoprotein, which has a multidomain structure, interacts with many ligands and regulates a number of biological processes, including cell adhesion and migration, complement activation, immune complex clearance and phagocytosis of apoptotic cells (Jones et al. Reference Jones, Hulett and Parish2005). Notably, an immunologically significant pathway for histidine utilisation is initiated by histidine decarboxylase to produce histamine, a major mediator of inflammatory reactions (Tanaka & Ichikawa, Reference Tanaka and Ichikawa2006). It was previously thought that only mast cells and basophils could release histamine from storage during degranulation in response to various stimuli. However, it is now clear that many tissues and cell types express this enzyme to synthesise histamine. These cells include haematopoietic progenitors, macrophages, platelets, dendritic cells and T lymphocytes (Dy & Schneider, Reference Dy and Schneider2004). Histamine regulates various physiological and immunological functions by activating various histamine receptors on target cells. The overwhelming evidence that many cell types (e.g. medullary and peripheral haematopoietic cells, eosinophils, basophils, mast cells, T lymphocytes and dendritic cells) express a histamine 4 receptor (H4R) suggests a possible role for these leucocytes in inflammation, haematopoiesis and immunity (Tanaka & Ichikawa, Reference Tanaka and Ichikawa2006). In addition, histamine mediates platelet aggregation and promotes Th2 cell activity by both reducing IL-12 and enhancing IL-10 production (Dy & Schneider, Reference Dy and Schneider2004).
In addition to histamine synthesis, histidine can be deaminated by the catalytic enzyme histidine-ammonia lyase to form urocanic acid (UCA). This substance is a unique photoreceptor, and its conversion from cis-UCA to trans-UCS controls the initiation of the immune suppressive action of solar ultraviolet-B (De Fabo & Noonan, Reference De Fabo and Noonan1983). Further, cis-UCA decreases the following capacities of murine spleenocytes: (1) serving as antigen-presenting cells; (2) proliferating in response to mitogens; (3) suppressing the production of IL-2 and IFNγ; or (4) increasing the production of IL-10 (Holan et al. Reference Holán, Kuffova, Zajicova, Krulova, Filipec, Holler and Jancarek1998). These effects resulted in a reduced T-lymphocyte activity and an increased risk of UV carcinogenesis (De Fabo et al. Reference De Fabo, Webber, Ulman and Broemeling1997).
A limited number of in vitro studies show that supplementation of 2 mm-histidine to the culture medium prevented apoptosis, increased cell growth and promoted antibody production in lymphocytes (Duval et al. Reference Duval, Demangel, Munierjolain, Miossec and Geahel1991). Surprisingly, only a few studies have examined a role for dietary histidine in immune function of animals. Nonetheless, a deficiency of dietary histidine can decrease plasma concentrations of proteins, including histidine-rich glycoprotein (Jones et al. Reference Jones, Hulett and Parish2005), which in turn impairs the immune response. Further, an inadequate intake of dietary histidine reduced the immune response in chickens, which could be reversed by its dietary supplementation (Konashi et al. Reference Konashi, Takahashi and Akiba2000). Moreover, feeding either a high (64 g/kg diet) or low (0·4 g/kg diet) level of dietary histidine to rats resulted in an increase of skin trans-UCA levels above those in the control group and attenuated the UV-induced immunosuppression (De Fabo et al. Reference De Fabo, Webber, Ulman and Broemeling1997). These findings suggest that dietary histidine supplementation may provide a potentially novel means to boost immune function, particularly in the skin.
Lysine
Compelling evidence shows that a dietary deficiency of lysine limits the synthesis of proteins (including cytokines) and the proliferation of lymphocytes, and impairs immune responses in chickens, resulting in increases in morbidity and mortality in response to infection (Kidd et al. Reference Kidd, Kerr and Anthony1997; Konashi et al. Reference Konashi, Takahashi and Akiba2000). There are also findings that an inadequate intake of dietary lysine reduced antibody responses and cell-mediated immunity in chickens (Chen et al. Reference Chen, Sander and Dale2003). By sharing the same transport systems with arginine, the availability of dietary or extracellular lysine can modulate the entry of arginine into leucocytes and thus NO synthesis by iNOS (Wu & Meininger, Reference Wu and Meininger2002). Indeed, increasing extracellular lysine concentrations (0·3–2 mm) reduced the intracellular arginine con- centration and NO synthesis in activated macrophages in a dose-dependent manner (Closs et al. Reference Closs, Scheld, Sharafi and Forstermann2000). The knowledge about an antagonism between lysine and arginine has been exploited to treat effectively dermal infections caused by the herpes simplex virus (Griffith et al. Reference Griffith, Norins and Kagan1978). Topical application of lysine (a therapeutic dosage of 0.8–1 g daily during an overt infection) inhibited the replication of the virus, shortened the course and duration of the disease, and improved clinical outcomes (Griffith et al. Reference Griffith, Norins and Kagan1978). The underlying mechanism involves a decrease in the transport of arginine into the virus and an inhibition of arginase activity by lysine, resulting in a depletion of polyamines for the growth of the virus (Griffith et al. Reference Griffith, Delong and Nelson1981). The successful use of lysine to treat infections by the herpes simplex virus illustrates the power of basic research on amino acid metabolism to solve a significant problem in medicine.
Phenylalanine and tyrosine
Emerging evidence shows that an important function of phenylalanine is to upregulate expression and activity of GTP cyclohydrolase I, which is the first and rate-controlling enzyme for the synthesis of tetrahydrobiopterin, an essential cofactor for NOS (Shi et al. Reference Shi, Meininger, Haynes, Hatakeyama and Wu2004). Thus, phenylalanine can regulates NO synthesis by leucocytes. Consequently, an adequate intake of dietary phenylalanine is required to maintain a sufficient provision of tetrahydrobiopterin for the production of NO by iNOS in activated macrophages and other leucocytes (Wu & Meininger, Reference Wu and Meininger2002).
Tyrosine, a product of phenylalanine degradation, is the immediate precursor for the synthesis of catecholamine hormones (epinephrine and norepinephrine), thyroid hormones (triiodothyronine and thyroxine), as well as dopamine and melanin (Kim et al. Reference Kim, Mateo, Yin and Wu2007). Norepinephrine is a key messenger released from the sympathetic nervous system to act on the immune system (Kin & Sanders, Reference Kin and Sanders2006). Interestingly, both Th1 cells and B cells express β2-adrenergic receptors. The binding of epinephrine and norepinephrine to the receptors triggers the generation of cAMP from ATP and the subsequent activation of protein kinase A, which stimulates the differentiation and proliferation of Th1 cells and B cells (Kin & Sanders, Reference Kin and Sanders2006). In addition, thyroid hormones regulate many important physiological processes, including gene expression, and the metabolism and differentiation of leucocytes (Dorshkind & Horseman, Reference Dorshkind and Horseman2000). Moreover, dopamine and melanin reduce the synthesis of proinflammatory cytokines (including TNFα, IL-1β, IL-6 and IL-10) by monocytes and macrophages, induce the production of anti-inflammatory mediators by leucocytes, and regulate lymphocyte proliferation, platelet aggregation and the phagocytic activity of neutrophils (Basu & Dasgupta, Reference Basu and Dasgupta2000; Mohagheghpour et al. Reference Mohagheghpour, Waleh, Garger, Dousman, Grill and Tuse2000). These biochemical bases explain the finding that a deficiency of dietary phenylalanine plus tyrosine impaired the immune response in chickens, which could be reversed by their supplementation to the diet (Konashi et al. Reference Konashi, Takahashi and Akiba2000).
Proline
Proline is catabolised via proline oxidase in a variety of organs, including the small intestine, liver, kidney, lymphoid organs and placenta, to generate pyrroline-5-carboxylate (P5C) and H2O2 (Wu, Reference Wu1997; Wu et al. Reference Wu, Bazer, Hu, Johnson and Spencer2005). Interestingly, P5C can be reduced to proline by the widespread NADPH-dependent P5C reductase. This proline–P5C cycle functions to regulate the cellular redox state and the proliferation of cells, including lymphocytes (Phang, Reference Phang1985). This may provide a cellular mechanism responsible for a role for proline in protecting lymphocytes from apoptosis, stimulating cell growth and promoting antibody production (Duval et al. Reference Duval, Demangel, Munierjolain, Miossec and Geahel1991). Moreover, proline constitutes one-third of amino acids in collagen, and, thus, is crucial for wound healing and injury recovery mediated by cells of the immune system (Abumrad & Barbul, Reference Abumrad, Barbul and Cynober2004).
An intriguing new discovery is that proline oxidase may play an important role in immunity (Ha et al. Reference Ha, Oh, Bae and Lee2005). Notably, a lack of proline catabolism due to a deficiency of intestinal proline oxidase impairs immune function in the gut (Ha et al. Reference Ha, Oh, Bae and Lee2005). H2O2, a major product of proline oxidation, is a signalling molecule (Shi et al. Reference Shi, Meininger, Haynes, Hatakeyama and Wu2004) and a cytotoxic agent for pathogenic bacteria (Kim et al. Reference Kim, Mateo, Yin and Wu2007). Therefore, a high activity of proline oxidase in the porcine placenta (Wu et al. Reference Wu, Bazer, Hu, Johnson and Spencer2005) and the small intestine of piglets (Wu, Reference Wu1997) may play a crucial role in protecting these organs from infections during the critical periods of fetal and neonatal development. Further, proline oxidase is present in milk and may aid in protecting the neonatal intestine from bacterial and viral challenges (Sun et al. Reference Sun, Nonobe, Kobayashi, Kuraishi, Aoki, Yamamoto and Sakai2002). This may explain, in part, why neonates fed a diet of non-mother's milk have a high risk of intestinal dysfunction in comparison with those nursed by their mothers (Wu et al. Reference Wu, Flynn, Flynn, Jolly and Davis1996; Field, Reference Field2005).
Serine
There are multiple pathways for serine utilisation, which include one-carbon unit metabolism; the hepatic and renal synthesis of glucose, particularly in ruminants; and the synthesis of glycine, ceramide and phosphatidylserine as structural components and signalling molecules of cells, including T and B lymphocytes (Jones et al. Reference Jones, Gonzalez-Garcia, Diez, Martinez, Carrera and Merida1999; Kim et al. Reference Kim, Mateo, Yin and Wu2007). Indeed, phosphatidylserine has been implicated in the regulation of IL-2 production and T-lymphocyte activation in response to an immunological challenge (Pelassy et al. Reference Pelassy, Breittmayer, Mary and Aussel1991). Because glucose is a major fuel for lymphocytes and macrophages (Newsholme et al. Reference Newsholme, Curi, Curi, Murphy, Garcia and de Melo1999), an adequate availability of serine is necessary for the function of these cells, particularly during late pregnancy in ruminants (Wu et al. Reference Wu, Bazer, Wallace and Spencer2006). Interestingly, addition to the culture medium of 2 mm-serine (~7 times its plasma levels and 10 % of its concentration in ovine allantoic fluid during late gestation (Kwon et al. Reference Kwon, Spencer, Bazer and Wu2003)) prevented apoptosis, stimulated cell growth and increased antibody production in lymphocytes (Duval et al. Reference Duval, Demangel, Munierjolain, Miossec and Geahel1991; Franek & Sramkova, Reference Franek and Sramkova1996). Further, there is evidence that inadequate intake of dietary serine reduced the immune response in chickens, which could be reversed by its dietary supplementation (Konashi et al. Reference Konashi, Takahashi and Akiba2000).
Sulphur-containing amino acids
A sufficient intake of dietary methionine and cysteine is important for the synthesis of proteins of the immune system (Grimble, Reference Grimble2006). Through the generation of decarboxylated S-adenosylmethionine, methionine is a donor of the methyl group that participates in the methylation of DNA and proteins, the synthesis of spermidine and spermine, and regulation of gene expression (Wu et al. Reference Wu, Bazer, Wallace and Spencer2006). Because polyamines are important for the proliferation and differentiation of lymphocytes (Flynn et al. Reference Flynn, Meininger, Haynes and Wu2002), methionine may play a role beyond a protein constituent. In addition, methionine is a substrate for the synthesis of choline and thus phosphatidylcholine and acetylcholine that are essential for nerve function and leucocyte metabolism (Kim et al. Reference Kim, Mateo, Yin and Wu2007).
Cysteine is the precursor of glutathione (GSH) and H2S (a signalling molecule) in animal cells, and its metabolism is markedly altered in response to infection (Malmezat et al. Reference Malmezat, Breuillé, Pouyet, Buffiere, Denis, Mirand and Obled2000). Glutathione synthesis is influenced by dietary intakes of sulphur amino acids (Wu et al. Reference Wu, Bazer, Cudd, Meininger and Spencer2004b). Thus, there is a positive correlation between the transulphuration pathway activity and glutathione concentrations in the liver, spleen and muscle (Malmezat et al. Reference Malmezat, Breuillé, Pouyet, Buffiere, Denis, Mirand and Obled2000). Glutathione scavenges free radicals and other reactive oxygen species (e.g. hydroxyl radical, lipid peroxyl radical, peroxynitrite and H2O2) and conjugates with various electrophils and xenobiotics for their detoxification (Fang et al. Reference Fang, Yang and Wu2002). There is evidence that the intracellular concentrations of GSH play an important role in regulating cellular signalling pathways (including the nuclear transcription factor κB pathway) in response to immunological challenges (Fratelli et al. Reference Fratelli, Goodwin, Ørom, Lombardi, Tonelli, Mengozzi and Ghezzi2005). In addition, GSH concentrations in antigen-processing cells modulate immune responses, including T-helper cell function and antibody production (Peterson et al. Reference Peterson, Herzenberg, Vasquez and Waltenbaugh1998). Thus, a deficiency of extracellular cysteine or intracellular GSH decreases the number of CD4 cells, reduces the production of IFNγ, impairs the proliferation of lymphocytes in response to mitogens and diminishes cytotoxic T-cell activity (Obled et al. Reference Obled, Papet, Breuille and Cynober2004). Further, GSH depletion is associated with many diseases, such as malaria, tuberculosis, cancer, AIDS and rheumatoid arthritis, and the requirement for sulphur amino acids increases during trauma, sepsis and injury (Obled et al. Reference Obled, Papet, Breuille and Cynober2004; Grimble, Reference Grimble2006).
Dietary supplementation with methionine or cysteine is beneficial for the immune system under various catabolic conditions. For example, increasing total methionine levels from 0·35 to 1·2 % in the diet for chickens infected with the Newcastle disease virus markedly enhanced the following key aspects of the immune responses: T-cell proliferation in response to mitogen stimulation (Tsiagbe et al. Reference Tsiagbe, Cook, Harper and Sunde1987a), plasma levels of immunoglobulin G (Tsiagbe et al. Reference Tsiagbe, Cook, Harper and Sunde1987a), leucocyte migration and antibody titre (Swain & Johri, Reference Swain and Johri2000). Dietary cysteine provided similar effects to methionine with regard to the immune responses of chickens (Tsiagbe et al. Reference Tsiagbe, Cook, Harper and Sunde1987b). However, high supplemental levels of methionine or cysteine (>1·45 % in the diet) were detrimental to the growth and immune responses of chickens (Tsiagbe et al. Reference Tsiagbe, Cook, Harper and Sunde1987a, Reference Tsiagbe, Cook, Harper and Sundeb), probably due to the excess production of highly toxic substances (e.g. homocysteine and sulphuric acid) (Wu & Meininger, Reference Wu and Meininger2002).
Because cysteine is toxic at a high concentration, N-acetyl cysteine (NAC) and l-2-oxothiazolidine-4-carboxylate (OTC, an analogue of 5-oxoproline) are commonly used to deliver cysteine via intravenous infusion or drinking water to increase endogenous glutathione synthesis in cells (Wu et al. Reference Wu, Bazer, Cudd, Meininger and Spencer2004b). Findings from clinical studies show that daily NAC provision to septic patients (a bolus dose of 150 mg/kg body weight, followed by a constant infusion of 50 mg/kg body weight over 4 h) increased blood GSH content, decreased plasma IL-8 levels and soluble TNF receptors, improved respiratory function, and reduced the number of days in the intensive care unit (Spapen et al. Reference Spapen, Zhang, Demanet, Velminckx, Vincent and Huyghens1998). Further, oral administration of NAC (0·6 g/d every second day) was also effective in raising GSH concentrations in blood and CD4 T cells, increasing NK cell activity and lymphocyte proliferation in response to mitogens, and prolonging the survival time of HIV patients (Breitkreutz et al. Reference Breitkreutz, Pittack, Nebe, Schuster, Brust, Beichert, Hack, Daniel, Edler and Dröge2000). Similarly, OTC supplementation to cirrhotic patients (0·2 g/kg body weight per day) increased GSH content in monocytes and reduced the production of inflammatory cytokines, including IL-1, IL-8 and TNFα (Obled et al. Reference Obled, Papet, Breuille and Cynober2004).
Taurine is the most abundant free amino acid in lymphocytes and a potent antioxidant (Fang et al. Reference Fang, Yang and Wu2002). Further, the reaction of taurine with hypochlorous acid, which is a microbicidal agent produced by activated monocytes and neutrophils, yields taurine chloramine (Wright et al. Reference Wright, Tallan and Lin1986). This long-lived oxidant reduces the production of proinflammatory cytokines (e.g. IL-1, IL-6 and TNFα) and prostaglandin E2 (Weiss et al. Reference Weiss, Klein, Slivka and Wein1982; Choręży et al. Reference Choręży, Kontny, Marcinkiewicz and Maśliński2002), and increases histamine release from neutrophils of carrageenin-induced rats (Wojtecka-Lukasik et al. Reference Wojtecka-Lukasik, Marton, Krajewska, Burakowski, Gujski, Maslinska and Maslinska2004). Thus, dietary supplementation with taurine (1 % in drinking water) can reduce lung inflammation induced by bleomycin (Schuller-Levis et al. Reference Schuller-Levis, Gordon, Wang and Park2003).
Threonine
Threonine is a major component of intestinal mucin and plasma γ-globulin in animals (Kim et al. Reference Kim, Mateo, Yin and Wu2007). Through protein synthesis and cellular signalling mechanisms, addition of 2 mm- threonine to the culture medium prevented apoptosis, stimulated cell growth and promoted antibody production in lymphocytes (Duval et al. Reference Duval, Demangel, Munierjolain, Miossec and Geahel1991). Animal feeding studies indicate that changes in components of the immune system are sensitive to dietary threonine intake (Li et al. Reference Li, Xiao, Qiao, Zhang, Johnson and Thacker1999). Indeed, serum antibody titres increased with increasing dietary intake of threonine in chickens infected with the Newcastle disease virus (Bhargava et al. Reference Bhargava, Hanson and Sunde1971). Also, dietary supplementation with threonine increased serum levels of IgG in sows (Cuaron et al. Reference Cuaron, Chapple and Easter1984). Further, increasing dietary threonine intake increased antibody production, serum IgG levels and jejunal mucosal concentrations of IgG and IgA, while decreasing jejunal mucosal concentrations of IL-6 in young pigs challenged with Escherichia coli (X. Wang et al. Reference Wang, Qiao, Liu and Ma2006). These findings provide support for a role of dietary threonine in modulating immune function in livestock and perhaps humans.
Tryptophan
The products of tryptophan catabolism include serotonin, N-acetylserotonin, melatonin and anthranilic acid (Kim et al. Reference Kim, Mateo, Yin and Wu2007). Tryptophan catabolism is increased to generate anthranilic acid through the indoleamine 2,3-dioxygenase (IDO) pathway during inflammation or stimulation by LPS or certain cytokines (Platten et al. Reference Platten, Ho, Youssef and Fontoura2005). Serotonin, melatonin and N-acetylserotonin can enhance host immunity by inhibiting the production of superoxide, scavenging free radicals and attenuating the production of TNFα (Perianayagam et al. Reference Perianayagam, Oxenkrug and Jaber2005). In addition, N-acetylserotonin is an inhibitor of sepiapterin reductase, an enzyme for the synthesis of tetrahydrobiopterin (Shi et al. Reference Shi, Meininger, Haynes, Hatakeyama and Wu2004). By modulating inducible NO synthesis, this tryptophan metabolite can affect both innate and acquired immunity systems. Excitingly, anthranilic acid was recently found to inhibit the production of proinflammatory Th1 cytokines and prevent autoimmune neuroinflammation (Platten et al. Reference Platten, Ho, Youssef and Fontoura2005). Because there is a progressive decline in tryptophan concentrations in plasma of animals with inflammation, its catabolism plays a critical role in the functions of both macrophages and lymphocytes (Melchior et al. Reference Melchior, Seve and Le Floc'h2004).
Early work indicated that tryptophan starvation resulting from IFNγ treatment was associated with the antiproliferative effect of this cytokine on intracellular parasites (Taylor & Feng, Reference Taylor and Feng1991) and tumours (Ozaki et al. Reference Ozaki, Edelstein and Duch1988). Interestingly, progressively increasing concentrations of IFNγ were required for its growth inhibition in the presence of elevated tryptophan concentrations (Pfefferkorn, Reference Pfefferkorn1984). Subsequently, Munn et al. (Reference Munn, Zhou, Attwood, Bondarev, Conway, Marshall, Brown and Mellor1998) demonstrated that a pharmacological inhibition of IDO suppressed T-cell activity and induced fetal allograft rejection in mice. More recently, N-(3,4,-dimethoxycinnamoyl) anthranilic acid, an orally active synthetic derivative of the tryptophan metabolite anthranilic acid, was found to protect paralysed mice from experimental autoimmune encephalomyelitis (Platten et al. Reference Platten, Ho, Youssef and Fontoura2005). Available evidence suggests that tryptophan catabolism plays a role in immune responses by producing a local immunosuppressive environment that is able to control T-cell homeostasis and self-tolerance during inflammation (Platten et al. Reference Platten, Ho, Youssef and Fontoura2005).
A deficiency of dietary tryptophan impaired the immune response in chickens (Konashi et al. Reference Konashi, Takahashi and Akiba2000). Conversely, oral administration of 300 mg of tryptophan to rats enhanced phagocytosis by macrophages and the innate immune response (Esteban et al. Reference Esteban, Nicolaus, Garmundi, Rial, Rodriguez, Ortega and Ibars2004). Dietary supplementation with 0·22 % l-tryptophan also increased resistance to bacterial and parasitic infections in rats fed a 20 % zein diet (Watson & Petro, Reference Watson and Petro1984). At present, a potential use of crystalline tryptophan for animal health management is not fully developed. However, there are reports that dietary supplementation with 0·36 or 0·5 % tryptophan (corresponding to eight or 11 times the tryptophan content (0·044 %, on a dry matter basis) of the commercial feed) reduced cannibalism in fish (Hseu et al. Reference Hseu, Lu, Su, Wang, Tsai and Hwang2003) and cortisol-mediated immune suppression in the rainbow trout (Lepage et al. Reference Lepage, Tottmar and Winberg2003).
Conclusion and perspectives
Amino acids are required for the synthesis of a variety of specific proteins (including cytokines and antibodies) and regulate key metabolic pathways of the immune response to infectious pathogens. Arginine, glutamine and cysteine precursors are currently the best prototypes, with well-defined roles and expanded applications to human nutrition and livestock production. Adequate dietary provision of all amino acids is necessary for sustaining normal immunocompetence and protecting the host from a variety of diseases in all species. Because of a negative impact of amino acid imbalance and antagonism on nutrient intake and utilisation, an excess supply of amino acids in the diet can be deleterious to the immune system. Thus, care should be exercised in developing effective strategies of enteral or parenteral supplementation to achieve maximum health benefits. Such measures should be based on knowledge about the inter-organ metabolism of amino acids (Fig. 2), their roles in the immune response, nutritional and pathological states of individuals, and expected treatment outcomes. Although great advances have been made in the rapidly growing field of nutritional immunology, there is a paucity of information about the molecular mechanisms that regulate the actions of amino acids on the immune system. Discovery of this new knowledge will probably be facilitated through the integrative applications of modern high-throughput and high-efficient technologies, including genomics, transcriptomics, metabolomics, proteomics, bioinformatics, systems biology and epigenetics (Wu et al. Reference Wu, Bazer, Cudd, Meininger and Spencer2004a; Mathers, Reference Mathers2006; J. Wang et al. Reference Wang, Li, Dangott and Wu2006). Given the increasing commercial availability of food- and feed-grade amino acids for dietary supplementation, we enthusiastically predict that amino acids will widely become cost-effective neutraceuticals for improving health and preventing infectious disease in both humans and animals.
Acknowledgements
Research in our laboratories was supported by funds from USDA CSREES National Research Initiative Competitive Program (2001-35 203-11 247 and 2003-35 206-13 694), National Institutes of Health (R21 HD049449), Texas Agricultural Experiment Station (H-8200), Texas Tech University, Outstanding Overseas Chinese Scholar Fund of The Chinese Academy of Sciences (2005-1-4), China National Natural Science Foundation (30528006, 30671517 and 30121004) and National Basic Research Program of China (2004CB117502 and 2004CB117503). We thank Scott C. Jobgen and Frances Mutscher for assistance in manuscript preparation as well as anonymous reviewers for constructive comments.






