Aside from water, tea is the most popular beverage in the world. Green tea (Camellia sinensis) originates from China and is primarily consumed in Asia but is becoming increasingly common in Europe, in part due to its perceived health benefits. Green tea differs from black tea by the minimal fermentation of the tea leaves during production. The tea leaves are subjected to a steam or dry heat treatment before processing, inactivating the polyphenol oxidases which oxidise and consequently condense the tea leaf polyphenols, a reaction which is promoted in black tea production to form polyphenol dimers and polymers.
Endothelial function has been increasingly recognised as a biomarker of cardiovascular health, and dysfunction of the endothelial layer is recognised as an early aetiological factor in atherogenesis. There is an accumulating body of scientific literature from both human epidemiological and intervention studies which has demonstrated the positive impact of green tea catechins on vascular function. However, there is considerable intra- and inter-study variability which is likely to be attributable to study design, population characteristics including genetics and environmental factors. Furthermore, animal and cell-culture studies have provided some insight into physiological and molecular mechanisms of action. The aim of the present paper is to review current scientific evidence for the effects of green tea and green tea catechins, specifically epigallocatechin-3-gallate (EGCG), on vascular health and function.
Green tea catechins
Green tea is rich in polyphenols, about 70 % of which are catechins, and is the main dietary source of catechin gallates and gallocatechins. Table 1 gives the concentrations of the different classes of phenolics in green tea and the major flavan-3-ols, which is the collective term for the catechin group of compounds. The chemical structures of the main green tea catechins are shown in Fig. 1. Other dietary sources of catechins include red wine, grapes, apples and chocolate. EGCG is one of the most abundant green tea catechins, with a cup of green tea containing 20–200 mg EGCG. It has also been suggested to be one of the most active known compounds in green tea, mediating most of the biological effects(Reference Higdon and Frei1) and accounting for 32 % of the antioxidant potential(Reference Pharn-Huy, He and Phamhuy2). The remaining catechins include: ( − )-epicatechin (( − )-EC), ( − )-epigallocatechin (EGC) and epicatechin-3-gallate (ECG), which provide 5–7, 9–12 and 9–12 % of the antioxidant potential of green tea, respectively.
Table 1 Phenolics in a green tea (Camellia sinensis) infusion*
(Mean values and standard deviations)

* Tea preparation: 3 g leaves in 300 ml boiling water for 3 min(Reference Del Rio, Stewart and Mullen100).
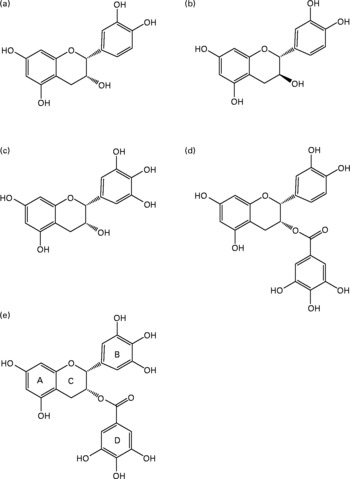
Fig. 1 Major flavan-3-ols found in green tea (Camellia sinensis) infusions: (a) ( − )-epicatechin; (b) (+)-catechin; (c) ( − )-epigallocatechin; (d) ( − )-epicatechin gallate; (e) ( − )-epigallocatechin gallate.
Catechins are thought to be primarily responsible for the beneficial effects of green tea. In comparison, black tea contains about 30 % of the catechins found in green tea and is mainly composed of complex condensation products such as thearubigins and theaflavins.
Bioavailability and metabolism of epigallocatechin-3-gallate and its metabolites
After oral administration green tea catechins are absorbed intestinally, predominately in the jejunum and ileum, with peak absorption occurring at 1.5–2.5 h after consumption. EGCG and ECG are thought to be mainly transported across the intestinal epithelial cell layer via paracellular diffusion. However, bioavailability is low due to instability under digestive conditions, poor absorption, and rapid metabolism and excretion, leading to only about 5 % of consumed catechins appearing in the plasma on average(Reference Miyazawa3). EGCG is the only known polyphenol to be mainly in the free form (77–90 %) in the plasma(Reference Manach, Williamson and Morand4) whereas EGC and EC are mostly in the conjugated form (31 and 21 % in the free form, respectively)(Reference Lee, Maliakal and Chen5). The extensive metabolism in the intestine and liver leads to the formation of glucoronides, sulfides and methylated metabolites(Reference Lee, Maliakal and Chen5, Reference Warden, Smith and Beecher6). This biotransformation alters the chemico-physical properties of the catechins, such as the mass, charge and polarity, aiding the deactivation and rapid excretion of the biologically active compounds. Green tea catechins are methylated by the enzyme catechol-O-methyltransferase, glucuronidated by UDP-glucuronosyltransferases (UGT) such as UGT1A1, 1A8 and 1A9 and sulfadated by sulfotransferases (SULT) such as SULT1A1 and 1A3. Glucuronidation and sulfation can occur to previously methylated EGCG and vice versa, forming mixed metabolites. Green tea catechins have also been shown to undergo microbial degradation in the colon, forming compounds such as valerolactones, and phenolic and benzoic acids which may be absorbed or passed out in the faeces. In comparison with the methylated, glucuronidated and sulfadated metabolites mentioned previously, studies have shown the valerolactones to appear in the blood and urine considerably later, with a lag period of 3 h and a peak at 7.5–13.5 h(Reference Li, Lee and Sheng7).
The major products of EGCG metabolism are 4″-O-methyl-( − )-EGCG and 4′,4″-O-dimethyl-( − )-EGCG (at high or low concentrations of EGCG, respectively)(Reference Lu, Meng and Yang8), ( − )-5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone and ( − )-5-(3′,4′-dihydroxyphenyl)-γ-valerolactone and the respective sulfates and glucuronides. EGC is metabolised to the major products 4′-O-methyl-EGC(Reference Lu, Meng and Yang8), 4′-O-methyl-EGC-glucuronide, 4′-O-methyl-EGC-sulfate, 5-(3′,4′-dihydroxyphenyl)-γ-valerolactone, 5-(3′,5′-dihydroxyphenyl)-γ-valerolactone and ( − )-5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone. EC metabolites mainly include 5-(3′,4′-dihydroxyphenyl)-valerolactone and conjugates of EC, 3′ and 4′-O-methyl-( − )-EC(Reference Kuhnle, Spencer and Schroeter9), whilst ECG metabolites have not been found in human urine or plasma(Reference Feng10).
The biological activity of green tea catechin metabolites have been shown in most cases to be limited compared with the pure form; however, a small selection of metabolites have been shown to have similar or in some cases higher activity than the parent compound. For example, Lu et al. found EGCG-3′-glucuronide and EGCG-3″-glucuronide to have similar radical-scavenging ability compared with free EGCG, as measured by the 1,1-diphenyl-2-picrylhydryl (DPPH) radical assay(Reference Lu, Meng and Li11, Reference Lambert, Rice and Hong12). The major metabolites of EC and (+)-catechin in rats, ( − )-epicatechin-5-O-β-glucuronide and (+)-catechin-5-O-β-glucuronide, were also found to have comparable superoxide-scavenging abilities as assessed by electron spin resonance spectrometry(Reference Nanjo, Mori and Goto13). A limited number of cell-culture studies have also indicated that some catechin metabolites may have a different biological activity to the parent compound. Exposure of human aortic endothelial cells to catechin metabolites extracted from the plasma of (+)-catechin-administered rats was found to inhibit monocyte adhesion when cells were pretreated with the cytokine IL-1β. The unconjugated form, (+)-catechin, had no such effect(Reference Koga and Meydani14). Further study into this area is required to provide insight into the relative potency of the major metabolites observed in human plasma and tissues.
Endothelial function and reactivity
A ‘healthy’ vascular endothelium is crucial for the maintenance of vascular health. This active organ plays an essential role in the intrinsic regulation of vascular tone via the secretion of vasorelaxants, such as NO, and vasoconstrictors, such as thromboxane and endothelin-1, the production of which is regulated by various hormonal, physical and motor signals received from the surrounding vascular wall and vessel lumen. The endothelium is also involved in the inhibition of smooth muscle cell proliferation, inflammation, vascular permeability, leucocyte adhesion and platelet aggregation and maintenance of the balance between prothrombotic and profibrinolytic activity, many of which are mediated by NO. It has been proposed that the initial events leading to atherosclerosis involves the inflammatory process; monocytes adhere to the injured endothelial cells, then enter into the vascular wall where they differentiate into macrophages. Cell adhesion molecules, such as E-selectin, P-selectin, vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1, play an important role in initiating endothelial monocyte recruitment and adhesion. Indeed, expression of VCAM-1 has been shown to be elevated in atherosclerotic lesions together with an increase in the soluble form in the sera of atherosclerotic patients(Reference Cybulsky and Gimbrone15, Reference Rohde, Lee and Rivero16). In addition, mice deficient in P-selectin, E-selectin or ICAM-1 have shown increased protection against atherosclerosis(Reference Dong, Chapman and Brown17, Reference Collins, Velji and Guevara18).
Damage or impairment of the endothelial layer can consequently have a major impact on the regulation and functioning of the vessels. Furthermore, many studies have shown endothelial dysfunction to be highly related to atherosclerosis and CVD(Reference Schachinger, Britten and Zeiher19–Reference Halcox, Schenke and Zalos22) to the extent that endothelial dysfunction has been deemed to be pivotal in the initiation and progression of the aforementioned disease. Vascular reactivity, which refers to changes in the blood vessels' tone and diameter in response to a neural, endocrine or physical (passive distension) stimulus, has thus been defined as a relatively sensitive prognostic indicator of vascular health and CVD risk(Reference Landmesser, Hornig and Drexler23). The classical risk factors for endothelial dysfunction such as diabetes, dyslipidaemia, smoking and hypertension appear to account for less than 20 % of the variation in endothelial function(Reference Chan, Colhoun and Vallance24), leading to the recognition of novel risk factors. Substances that can prevent damage or restore endothelial function may have important clinical applications.
Green tea catechins have been shown in the majority of epidemiological studies to be significantly inversely related to CVD and have been postulated as providing protection against endothelial dysfunction. Research into the emerging area of green tea and endothelial function will be reviewed in the following sections.
Epidemiological studies of green tea and CVD risk
A number of epidemiological studies have investigated the relationship between green tea consumption and CVD risk. For example, Imai et al. (Reference Imai and Nakachi25) conducted a cross-sectional study among 1371 men in Japan and found increased green tea consumption to be negatively associated with total cholesterol and TAG and a decreased atherogenic index (LDL-cholesterol:HDL-cholesterol ratio). In a study of 203 Japanese patients who underwent elective coronary angiography, green tea was found to be an independent predictor of coronary artery disease (defined as having at least one significant coronary stenosis), with an OR of 0·84 (95 % CI 0·76, 0·91) for one or more cups of green tea per d(Reference Sano, Inami and Seimiya26). A protective effect of green tea was also indicated in another cross-sectional study carried out by Sasazuki et al. (Reference Sasazuki, Kodama and Yoshimasu27) in which 512 coronary arteriography patients were assessed on the basis of green tea consumption. Green tea was found to be inversely associated with coronary atherosclerosis in men but not women; in a subgroup of men, excluding those undergoing treatment for diabetes mellitus, the OR associated with green tea consumption of four or more cups per d was found to be 0·4 (95 % CI 0·2, 0·9). However, a similar study by Hirano et al. (Reference Hirano, Momiyama and Takahashi28) found no association between green tea intake and coronary artery disease in 393 Japanese patients who underwent coronary angiography. They did, nevertheless, find a significant association between green tea intake and risk of myocardial infarction, with an OR of 0·58 (95 % CI 0·34, 0·98) for one or more cups per d.
Prospective cohort studies strengthen this association. For example, a recent study following 40 530 Japanese adults aged over 11 years found green tea to be inversely associated with CVD mortality, an association which was stronger than that for all-cause mortality(Reference Kuriyama, Shimazu and Ohmori29). The multivariate hazard ratio of CVD mortality with adjustment for socio-economic status was calculated at 0·74 (95 % CI 0·62, 0·89) for five cups or more per d(Reference Kuriyama, Shimazu and Ohmori29). Similar results were found by Nakachi et al. (Reference Nakachi, Matsuyama and Miyake30), with a relative risk of cardiovascular mortality of 0·72 (95 % CI 0·60, 1·04) for those consuming over ten cups per d in a Japanese study population of 8552, followed up for over 11 years. None of the observational studies conducted to date has reported vascular function as a study outcome.
Chronic human intervention trials of green tea and vascular function
There are a limited number of chronic human intervention studies in the area of green tea and vascular function. The study conducted by Widlansky et al. (Reference Widlansky, Hamburg and Anter31) is currently the only study to specifically investigate the effects of isolated EGCG. In this study, forty-two clinically stable CVD patients (medical history of myocardial infarction or at least one angiographically determined coronary stenosis) were given 300 mg EGCG and vascular function measurements were taken acutely, after 2 h, and chronically, after 14 d of repeated supplementation, using flow-mediated dilatation (FMD). Vascular function was significantly increased after acute (P = 0·01) but not chronic (P = 0·12) supplementation (measurement approximately 14 h after the final dose). Ryu et al. (Reference Ryu, Lee and Lee32) also failed to find a significant improvement in vascular function, measured using pulse wave velocity in fifty-five patients with type 2 diabetes, after chronic consumption of 9 g green tea (900 ml water) for 28 d. Conversely, Kim et al. (Reference Kim, Jeong and Cho33) did find a significant improvement in FMD-assessed vascular reactivity (P < 0·001) after 8 g green tea (1 litre water) per d for 14 d using a study population of twenty smokers. Similar findings were also found in a more recent study by Tinahones et al. (Reference Tinahones Madueño, Rubio and Garrido Sánchez34) in which fourteen healthy women had a FMD of 12 % after 5 weeks of green tea extract (GTE) (daily dose of 375 mg catechols) compared with a 5·7 % vasodilation after placebo (P = 0·02). A significant reduction in oxidised LDL (P = 0·017), which has been previously shown to impair endothelium-dependent dilatation, and anti-oxidised LDL IgM antibodies (P = 0·002) was also found. With regards to other atherosclerotic markers, Lee et al. (Reference Lee, Min and Chun35) conducted a study using 600 ml green tea for 28 d in twenty smokers and found a significant (P < 0·001 and P < 0·05) reduction in soluble P-selectin and oxidised LDL, respectively. However, no change was seen for ICAM, VCAM, total antioxidant capacity, C-reactive protein and lipid profiles.
Acute human intervention trials of green tea and vascular function
In addition to the aforementioned study by Widlansky et al. (Reference Widlansky, Hamburg and Anter31), a number of other acute intervention trials have also been conducted (Table 2). Jochmann et al. (Reference Jochmann, Lorenz and Krosigk36) found an improvement in FMDmax (maximum dilation after hyperaemia as a percentage change from diameter before hyperaemia) from 5·4 % to 10·2 % between baseline and 2 h after consumption of 500 ml green tea in twenty-one healthy participants; no FMD effect was found with hot water. A similar placebo-controlled study by Nagaya et al. (Reference Nagaya, Yamamoto and Uematsu37) reported a 26 % increase in maximum forearm blood flow after reactive hyperaemia measured 2 h after 400 ml green tea compared with baseline. A more recent study conducted by Alexopoulos et al. (Reference Alexopoulos, Vlachopoulos and Aznaouridis38) found that FMD-assessed endothelial function increased from 4·4 % to 8·1 % after 6 g green tea, with no significant effects shown with the caffeine or water controls. However, more intervention studies are needed to confirm these findings and to examine underlying physiological mechanisms.
Table 2 Acute studies of green tea (Camellia sinensis) or epigallocatechin gallate (EGCG) and endothelial function in humans

FBF, forearm blood flow; FMD, flow-mediated dilatation.
Animal studies examining the impact of green tea catechins and vascular function
Animal models, in particular rodents, have proved particularly useful in investigating the relative bioefficacy of different green tea catechins and also elucidate possible molecular mechanisms underlying the physiological responses.
In vivo animal green tea supplementations studies
Similar beneficial effects of green tea catechins have been found using animal models as to those observed in human studies. Antonello et al. (Reference Antonello, Montemurro and Bolognesi39) assigned rats to drinking water with and without GTE at 6 mg/ml together with a high or low angiotensin II dose. The response of the phenylephrine-contracted arteries to the vasodilator acetylcholine was blunted by the high-dose angiotensin II, indicating a diminished endothelial-dependent relaxation, an effect which was improved with the rodents that drank the GTE. Potenza et al. (Reference Potenza, Marasciulo and Tarquinio40) also found that chronic supplementation of 200 mg EGCG/kg per d to spontaneously hypertensive rats for 3 weeks significantly improved the vascular tone of mesenteric vascular beds and reduced the systolic blood pressure. Spontaneously hypertensive rats treated with both EGCG and N ω-nitro-l-arginine methyl ester (l-NAME; NO synthase antagonist) did not show any effect.
The assessment of evolving and established atherosclerotic lesions in apoE-null mice given daily intraperitoneal injections of EGCG (10 mg/kg) has also been investigated by Chyu et al. (Reference Chyu, Babbidge and Zhao41). After 12 and 42 d, atherosclerotic plaque size was reduced by 55 and 73 %, respectively, compared with the saline-injected control mice. However, this was only apparent in evolving plaques and was not found in established lesions. A similar study which supplied GTE at 0·8 mg/ml in the drinking water of the mice for 14 weeks also found a significant reduction (23 %) in atheromatous areas and aortic weight compared with the controls(Reference Miura, Chiba and Tomita42).
Ex vivo green tea catechin exposure to rat aortic rings
A number of studies have utilised rat thoracic aortas with exposure to various concentrations of green tea and green tea catechins and measurement of the vascular tone response(Reference Potenza, Marasciulo and Tarquinio40, Reference Song, Koo and Lau43–Reference Fitzpatrick, Hirschfield and Ricci46) (Table 3). For example, Potenza et al. (Reference Potenza, Marasciulo and Tarquinio40) found a dose-dependent vascular relaxation after acute exposure of mesenteric vascular beds to EGCG in the range 1–100 μm, a response which was nullified by l-NAME (a NO synthase agonist) or the phosphatidylinositol-3-kinase (PI3K) inhibitor, wortmannin. Another study performed by Ajay et al. (Reference Ajay, Gilani and Mustafa45) found similar results using an exposure range of 100–300 μm, with the highest concentration of the range causing a 70·9 % vasorelaxant effect against phenylephrine-induced contractions. This has also been replicated by Lim et al. (Reference Lim, Lee and Park47) with GTE at concentrations of 0·3, 0·6 and 1·2 mg/ml (about 250, 500 and 1000 μm). Conversely in this study, when EGCG at 4 and 12 μg/ml (8·7 and 26·2 μm) was applied, the contractile response evoked by phenylephrine was unchanged. These differing effects were also confirmed when GTE and EGCG were administered intravenously and noradrenaline-evoked arterial blood pressure responses measured. GTE (10 mg/kg per 30 min) resulted in a significant decrease in pressor response whilst EGCG (1 mg/kg per 30 min) had no effect.
Table 3 Animal studies of vascular effect of green tea (GT; Camellia sinensis), green tea extract (GTE) or epigallocatechin gallate (EGCG) exposure

EC50, 50 % effective concentration.
Surprisingly, aortic ring studies using lower concentrations of EGCG ( < 10 μm), comparable with what may be observed in human plasma, have shown a vasoconstrictive effect(Reference Sanae, Miyaichi and Kizu44). It does appear that the mechanisms of action of EGCG on endothelial function in aortic ring studies is dose-dependent with the postulation that lower doses ( < 25 μm) have an impact on the vascular endothelium(Reference Lorenz, Wessler and Follmann48) whilst higher doses work in an endothelium-independent manner such as by inhibition of Ca2+ influx(Reference Fitzpatrick, Hirschfield and Ricci46, Reference Shen, Zheng and Wei49) or inhibition of contractile proteins, mechanisms which may not necessarily occur at concentrations ordinarily consumed and absorbed. This apparent anomaly between human and animal in vivo studies and aortic ring responses in these lower doses is not clearly understood and yet to be fully investigated.
Mechanisms underlying the impact of green tea catechins on vascular function
Cell signalling
The central role of NO in the regulation and maintenance of vascular function has led to this molecule being the focus of attention in cell-culture mechanistic studies. For example, Lorenz et al. (Reference Lorenz, Wessler and Follmann48), whilst investigating the pathway involved in EGCG and endothelial-dependent vasorelaxation in rat aortic rings, found that EGCG activates endothelial NO synthase (eNOS), a protein which catalyses the production of NO from its precursor l-arginine (Fig. 2). Treatment of bovine endothelial cells with 100 μm-EGCG caused activation of protein kinase B (Akt) and cAMP-dependent protein kinase A, inhibition of which prevented the increase in eNOS activity. It was demonstrated that Akt was involved in the phosphorylation of eNOS at Ser1179, causing sustained activation whilst protein kinase A was implicated in the rapid activation of eNOS, most probably by phosphorylation. Further experimentation also identified a common upstream phosphorylator in this pathway, phosphatidylinositol-3-OH-kinase, which activates these two kinases. Bovine aortic endothelial cells pretreated with a phosphatidylinositol-3-OH-kinase inhibitor prevented EGCG-induced eNOS activation(Reference Lorenz, Wessler and Follmann48).

Fig. 2 NO pathway and possible sites of epigallocatechin gallate (EGCG) control in vascular relaxation. eNOS, endothelial NO synthase; p, phosphorylated; GC, guanylate cyclase; GTP, guanosine triphosphate; cGMP, cyclic guanosine monophosphate; SMC, smooth muscle cell; ONOO, peroxynitrite.
Conversely, Alvarez et al. (Reference Alvarez, Campos-Toimil and Justiniano-Basaran50) reported EGCG-induced vasorelaxation to be due to its action as a non-selective phosphodiesterase inhibitor, as demonstrated by a failure of EGCG to modify basal generation of cAMP or cGMP but instead instigating a significant reversal of noradrenaline and KCl inhibition of cAMP and cGMP production in rat aortic smooth muscle cells, albeit at concentrations greater than 25 μm.
In addition to the rapid impact of EGCG on signalling pathways via an effect on phosphorylation status, there is also evidence to suggest that EGCG may influence the expression of cell signalling genes. Treatment of human umbilical vein endothelial cells (HUVEC) with EGCG treatment (50 μm) resulted in specific up- or down-regulation (by more than 1·4-fold) of sixty-five of 12 500 genes, many of which are involved in cell signalling(Reference Pfeffer, Ferrari and Dell'Eva51). Furthermore, chronic EGCG exposure may modulate gene expression by epigenetic changes. Fang et al. (Reference Fang, Chen and Yang52) have shown EGCG to inhibit DNA methyltransferases in vitro, which has subsequently been related to the proposed cancer-prevention properties of these compounds. However, information regarding the impact of EGCG on epigenetic regulation in relation to the vascular function is currently lacking.
NADPH oxidase inhibitor
Endothelial NADPH oxidases are involved in pro-inflammatory, proliferative and apoptotic processes through the catalytic production of superoxide free radicals (O2∙−). It has been proposed that catechins may help to regulate NADPH oxidase activity, reducing the production of O2∙− and thus protecting NO from peroxynitrite formation(Reference Schewe, Steffen and Sies53). An increase in NO bioavailability via reduction in O2∙− production is a plausible mechanism for the improvement in endothelial function from green tea catechin consumption. Indeed, HUVEC supplementation with mono-O-methylated flavanols such as 3′-O-methyl-EC causes a reduction in both the oxidation of NADPH and subsequent O2∙− release, an inhibitory property not seemingly evident with parent compounds(Reference Steffen, Gruber and Schewe54). Structural comparison of these mono-methylated flavanols with apocynin, a known NADPH oxidase inhibitor, substantiates the claim of shared function. It is also of interest to note that mono-methylation, demonstrated with ( − )-EC methylation at the 3′ and 4′ position, appears to remove the O2∙−-scavenging ability possessed by the parent compound(Reference Steffen, Gruber and Schewe54).
Prostacyclin production
Mizugaki et al. (Reference Mizugaki, Ishizawa and Yamazaki55) found prostacyclin (PGI2) production in cultured bovine endothelial cells to be dose-dependently increased by the addition of EGCG (25–200 μm). PGI2 is a known potent vasodilator and thus an increase in PGI2 concentration by EGCG could be a possible mode of action. This response was much greater for EGCG as compared with other catechins such as EC, catechin and EGC. The addition of gallic acid was also found to increase PGI2 production, indicating the importance of a pyrogallol structure in this pathway. An antioxidative effect similar to that found with α-tocopherol may be at play, enhancing PGI2 production. Similarly, a reduction in lipid peroxides, which have an inhibitory effect on PGI2 synthase activity, has also been proposed.
Anti-inflammatory effects
An EGCG inhibition of endothelial exocytosis and P-selectin cell-surface expression has also been demonstrated by Yamakuchi et al. (Reference Yamakuchi, Bao and Ferlito56). HUVEC were pretreated with 0–10 μm-EGCG and stimulated with thrombin. EGCG inhibited von Willebrand factor release concentration-dependently and blocked P-selectin translocation. EGCG inhibition of endothelial exocytosis was confirmed by measuring the adherence of labelled leucocytes. The NO inhibitor l-NAME and a PI3K inhibitor reduced the exocytosis-inhibitory effects of EGCG, suggesting a NO-dependent pathway. EC, ECG and EGC had no significant effect(Reference Yamakuchi, Bao and Ferlito56). Ludwig et al. also found a similar EGCG anti-inflammatory function in that the cytokine induction of VCAM-1 in HUVEC was found to be inhibited by EGCG(Reference Ludwig, Lorenz and Grimbo57). The exact pathway has yet to be elucidated but indications point to this activity being the property of gallate group-possessing catechins only. An additional anti-inflammatory effect of (+)-catechin metabolites has also been found by Koga & Meydani(Reference Koga and Meydani14). Pretreatment of human aortic endothelial cells (HAEC) with 7·6 μm-(+)-catechin metabolites extracted from the plasma of (+)-catechin-treated rats significantly inhibited monocyte adhesion, an effect not found with the extract of control plasma or pure (+)-catechin(Reference Koga and Meydani14). The involvement of adhesion molecules in this mechanism was only partly investigated, with the finding of unaltered ICAM, VCAM and E-selectin expression with catechin treatment; further investigation into this inhibition pathway is therefore required.
Asymmetric dimethylarginine reduction
Asymmetric dimethylarginine (ADMA) has been highlighted as a possible factor in endothelial dysfunction and novel marker of coronary artery disease(Reference Boger58). The endogenous compound is thought to act as an eNOS inhibitor, impairing the production of NO and subsequently decreasing the potential for vasodilation. LDL-induced endothelial dysfunction has also been associated with increased levels of ADMA(Reference Jiang, Jiang and Tang59). Interestingly, EGCG has been shown to reduce the level of ADMA along with increasing NO in Sprague–Dawley rats pretreated with LDL. These effects were related to the observed protective effect on endothelial function in aortic rings(Reference Tang, Hu and Chen60). The EGCG mechanism proposed by the authors is a reduction of the inflammatory cytokine TNF-α and inhibition of lipid peroxidation with consequential enhancement of dimethylaminohydrolase activity, the protein which degrades ADMA. Decreased ADMA may lead to increased NO and an improvement in endothelial function.
Angiotensin-converting enzyme inhibition
Angiotensin-converting enzyme (ACE) is an important mediator in blood pressure regulation, converting angiotensin into its activated vasoconstricting form and inactivating the potent vasodilator bradykinin. Green tea and isolated green tea catechins have been found to significantly inhibit the activity of purified and membrane-bound ACE, evaluated by conversion of hippuryl-l–histidyl-l-leucine to hippuric acid(Reference Actis-Goretta, Ottaviani and Fraga61). The green tea polyphenol EGC was found to be one of the best inhibitors, with a 50 % inhibitory concentration (IC50) in the micromolar range. The authors suggested that hydroxyl groups for hydrogen bonding to ACE and a high molecular mass (dimers and hexamers) were important properties for effective inhibition(Reference Actis-Goretta, Ottaviani and Fraga61). This mechanism may ultimately contribute to increased vascular health by increasing vasodilation and decreasing blood pressure. It is, however, debatable whether polyphenol dimers and trimers are effectively absorbed into the bloodstream; further studies into this and the mechanism of catechin ACE inhibition are needed.
Evidence of green tea catechins as an antioxidant or pro-oxidant
Radical-scavenging ability of green tea catechins
There is speculation on whether the effects of the tea catechins are due to their radical-scavenging ability, with oxidative stress and an associated pro-inflammatory response being atherogenic and associated with a loss of vascular function. Protection of LDL from oxidation (reducing the increased accumulation of oxidised LDL in atherosclerotic plaques), metal-chelating abilities or prevention of NO dissolution by free radicals by catechins may help to prevent vascular dysfunction. Nanjo et al. (Reference Nanjo, Mori and Goto13) found EGCG to be the most effective tea catechin for free radical (superoxide, hydroxyl and 1,1-diphenyl-2-picrylhydryl (DPPH)) scavenging. The scavenging abilities of the α-glucosides were found to vary depending on the position. These investigations led to the conclusion that an ortho-di-hydroxyl group in the B ring of the tea catechins was an important requirement for radical scavenging and that modification of this group by glucosylation hindered the catechin-scavenging abilities. Although NO is crucial in maintaining endothelial function, the copious production of NO by inducible NO synthase formed by macrophages in the vascular wall and up-regulated by various cytokines induced by cellular inflammation may be detrimental. NO is able to react with superoxide radicals sequentially forming peroxynitrite radicals (ONOO− ), which, amongst other effects, can proceed to oxidise LDL or up-regulate pro-inflammatory cytokines and adhesion molecules. EGCG inhibition of the excessive NO production and expression of inducible NO synthase has been shown(Reference Paquay, Haenen and Stender62) and together with an ONOO− -scavenging effect could be aiding healthy endothelial maintenance. However, convincing evidence for a reduction in ex vivo LDL oxidation in green tea intervention studies has not yet been demonstrated.
Furthermore, uncertainty exists regarding the likely antioxidant benefits of the concentrations of catechins present in tissues and biological fluids relative to other dietary antioxidants, suggesting that the radical-scavenging abilities may only partly contribute to the beneficial effects of catechins to the cardiovascular system. The average peak plasma concentration of individual green tea catechins after a single dose of GTE has been reported to be less than 1 μmol/l(Reference Chow, Cai and Alberts63, Reference Yang, Chen and Lee64) compared with an average plasma concentration of about 55 μmol/l for the antioxidant vitamin C(Reference Ruston, Hoare and Henderson65). Although controversial(Reference Duffy, Keaney and Holbrook66–Reference Lotito and Frei68), an increase in antioxidative ability has, however, been shown after green tea catechin oral administration in selected studies(Reference Da Silva, Piskula and Terao69–Reference Erba, Riso and Bordoni72). In addition, polyphenol metabolites have been indicated as significant contributors to antioxidant capacity, as the calculated concentration of polyphenols needed for the antioxidant capacity value measured after consumption is often higher than that which can be accounted for by solely the parent compounds present in the plasma(Reference Scalbert and Williamson73).
Pro-oxidant effect of green tea catechins
Many researchers portray the concept that an alternative mechanism may be responsible. For example, rather than an antioxidant effect, EGCG has been proposed to be operating as a pro-oxidant. It is well known that dietary antioxidants can also behave as pro-oxidants depending on surrounding conditions. EGCG has been found by Kim et al. to generate H2O2 in cultured bovine aortic endothelial cells(Reference Kim, Formoso and Li74). This reactive oxygen species has been shown to phosphorylate Fyn, which in turn has been proposed to activate PI3K, Akt and eNOS, consequentially stimulating the production of NO and mediating the vasodilatory actions observed. Indeed, inhibition of this pathway by N-acetylcysteine (reactive oxygen species scavenger), l-NAME, wortmannin (PI3K inhibitor), or pyruvate dehydrogenase kinase-2 (Src family kinase inhibitor) results in an inhibition of EGCG-induced increase in NO production.
The pathway proposed is similar to that employed by insulin, a well-known potent vasodilator, which promotes vasodilation through activation of PI3K, activating pyruvate dehydrogenase kinase isozyme 1 and Akt with sequential eNOS phosphorylation and NO production. Kim et al. (Reference Kim, Formoso and Li74) have suggested a lack of complete pathway overlap with insulin with regards to its EGCG vasodilatory function. EGCG was shown not to be acting through the insulin receptor for its vasodilatory effects, as unlike with insulin, the receptor was not phosphorylated when cells were treated with EGCG.
Albeit NO-independent, a similar pro-oxidant pathway has also been suggested by Wu et al. who found transcriptional up-regulation of haeme oxygenase-1, a cytoprotective enzyme, with the addition of 50 μm-EGCG in cultured bovine aortic endothelial cells(Reference Wu, Hsu and Hsieh75). Activation of both Akt and extracellular signal-regulated kinase (ERK) 1/2 has again been postulated as being involved in this pathway, downstream of PI3K and mitogen-activated protein ERK kinase (MEK) 1/2. Indeed, a protein tyrosine kinase inhibitor, PI3K inhibitors and a MEK1/2 inhibitor were all independently found to attenuate EGCG-induced haeme oxygenase-1 expression(Reference Wu, Hsu and Hsieh75). The authors have suggested that a minor increase in reactive oxygen species by EGCG may stimulate this pathway leading to an up-regulation in haeme oxygenase-1 expression. The observed cytoprotective effect of EGCG could thus viably reduce any oxidative stress and thus help to maintain the normal functioning of the vascular endothelium.
Systemic effects of green tea catechins on vascular health improvement
In addition to endothelial function, green tea catechins have an impact on CVD risk through a number of alternative mechanisms such as cholesterol lowering and increase in insulin sensitivity. Although not directly related to endothelial function, it is recognised that these mechanisms could indirectly improve endothelial function and are therefore included in this section.
Increased insulin activity and sensitivity
In addition to insulin-mimicked vasodilation, green tea has been reported to reduce glucose levels and to possess an ‘anti-diabetic’ effect in animal(Reference Shenoy76, Reference Renno, Abdeen and Alkhalaf77) and cell-culture studies(Reference Waltner-Law, Wang and Law78). An increase in insulin activity has also been shown with green, black and oolong teas in rat epididymal adipocytes(Reference Anderson and Polansky79). As insulin is a key determinant of eNOS phosphorylation and NO production, insulin sensitivity is imperative for vascular health. The primary active component responsible for the insulin-potentiating effect was found to be EGCG, with ECG, tannins and theaflavins showing a lesser response. An improvement in insulin sensitivity was also found in spontaneously hypertensive rats when supplemented with 200 mg EGCG/kg per d for 3 weeks(Reference Potenza, Marasciulo and Tarquinio40) and Sprague–Dawley rats supplemented with 148 mg green tea catechins/d for 12 d(Reference Wu, Juan and Ho80). This has also been replicated more recently in human subjects, in which twelve healthy males given GTE (equivalent to about 3·5 cups green tea) were tested for glucose tolerance. A significant improvement in insulin sensitivity (13 %) and, thus, insulin response to a glucose load was found (15 % reduction)(Reference Venables, Hulston and Cox81). EGCG supplementation in rat hepatoma cell culture has also shown that EGCG has the ability to interact with the insulin receptor, altering gene expression and increasing specific protein activities, albeit with slower kinetics(Reference Waltner-Law, Wang and Law78). Thus, EGCG may be able to increase insulin activity and sensitivity, improve vascular health and have applications in metabolic and CVD risk reduction in insulin-resistant individuals(Reference Kim, Formoso and Li74).
Cholesterol lowering
A hypocholesterolaemic effect has also been shown with green tea catechin administration in animal(Reference Ramesh, Elanchezhian and Sakthivel82, Reference Yang and Koo83) and human studies(Reference Kono, Shinchi and Wakabayashi84, Reference Tokunaga, White and Frost85). The observed decrease in total cholesterol and LDL-cholesterol levels could be in part responsible for the observed epidemiological reduction in CVD with green tea consumption. It has been proposed that catechins may reduce serum cholesterol levels via reduced cholesterol absorption and increased faecal excretion(Reference Koo and Noh86, Reference Yang and Koo87). More recently, green tea catechins have also been shown to increase the gene expression of the bile acid biosynthesis enzyme, cholesterol 7α-hydroxylase, in HepG2 cells(Reference Lee, Park and Freake88). Cholesterol lowering may contribute to the long-term benefits of chronic green tea consumption with regards to vascular health; however, short-term improvements in endothelial function are most likely to be due to an alternative mechanism.
The complete cellular mechanism in which tea catechins exert their beneficial effect is yet to be elucidated. Radical-scavenging activities, anti-inflammatory properties, inhibition or stimulation of specific enzymes and modulation of cell signalling pathways may all contribute to the beneficial endothelial effects from green tea catechin consumption. However, it is worth noting that many of the in vitro and ex vivo studies discussed use non-physiological concentrations of tea catechins, frequently at levels 100-fold greater than plasma levels; therefore results should be interpreted with caution. Further work is needed, specifically on physiological concentrations of green tea catechins and on the metabolites of green tea catechins found in the plasma after digestion and absorption.
Inter- and intra-study variability in epidemiological and human intervention trials
There is a considerable amount of contradictory evidence in the literature regarding the significance of the effect of green tea on vascular function and cardiovascular health in humans and the ‘size’ of the effect.
Inter-study variability
The heterogeneity found in epidemiological studies may be in part attributed to inconsistent or incomplete correction of statistical models for a myriad of physiological and behavioural confounding factors, and inaccurate assessment of tea and catechin intakes. For example, in the UK, smoking is positively associated with tea intake and smoking is a known potent vasoconstrictor, thus any benefits of tea catechins in the prevention of CVD may be negated. In addition, a Japanese study of 8552 individuals found an increasing percentage of male smokers among those with the highest green tea consumption. This observation was speculated to be counteracting the beneficial effects of green tea as an increased cancer incidence was found amongst males in this green tea group whilst a significant protective effect of high green tea consumption was found in females(Reference Nakachi, Matsuyama and Miyake30). It is also viable that glucuronidation of catechins is modified by lifestyle factors such as diet, smoking, oral contraceptives and alcohol use via competitive inhibition of the glucuronidation enzymes. For example, it has been shown that diet can affect the levels of UGT. Green tea administered to rats for 4 weeks enhanced UGT activity by 100 %(Reference Bu-Abbas, Clifford and Ioannides89). Measurement of these confounding factors is often problematical in epidemiological studies as a result of unavoidable respondent bias and changes in lifestyle. Thus, masking effects of residual confounders remains an ongoing issue in such studies, particularly those of lower statistical power.
The basis for the inter-study variability in the responses evident in intervention studies may be attributable to a number of factors. Differences in the type of product given to the volunteers, the dose of catechins administered (which is often dependent on the catechin content of the particular green tea or GTE used in the study(Reference Khokhar and Magnusdottir90)) and its instruction for use will undoubtedly influence the study outcomes. For instance, green tea preparation could conceivably affect the extent of the vascular response by altering the nature and amount of green tea catechins available in the test beverage. Green et al. found in vitro digestive recovery of green tea catechins to be increased when formulated with additives such as milk, citrus juice and ascorbic acid(Reference Green, Murphy and Schulz91). The use of individual catechins in intervention trials as opposed to mixed catechins could also influence the study outcome. Mixed catechins are thought to act as competitive inhibitors of methylation, sulfation and glucuronidation, unlike pure compounds which have no drug–drug interactions. This results in a lowered plasma maximum concentration and area under the concentration curve with pure compounds such as EGCG as compared with green tea, mixed green tea catechins or GTE(Reference Feng10).
Food intake has been shown to affect tea catechin oral bioavailability. For example, Chow et al. found a 3·5-fold increase in plasma free EGCG when Polyphenon E (decaffeinated green tea catechin mixture) was taken in the fasting condition compared with when taken with food(Reference Chow, Hakim and Vining92). A range of factors may be responsible for this reduction in bioavailability. An altered stomach pH as a result of feeding could result in catechin instability. Indeed, catechins are known to be more unstable in neutral and alkaline conditions resulting in degradation, oxidation and/or polymerisation of free catechins(Reference Feng10). A depletion in metabolic reaction precursors during an acute fast, such as those needed for glucuronidation and sulfation reactions, could also occur resulting in a decrease in pre-systemic metabolism and a sequential increase in free catechins in the plasma(Reference Chow, Hakim and Vining92). A decrease in catechin and food complexation or interactions may also account for the increase in catechin bioavailability when fasted. Thus, supplementation of green tea catechins with or after a meal may result in poor bioavailability and a subsequent reduction in vascular response. When consumed with a meal, the type of food consumed may also have an effect. For example, the absorption of cocoa flavanols has been shown to increase when consumed with carbohydrates such as bread(Reference Schramm, Karim and Schrader93). Therefore inter-study differences in the timing of EGCG consumption relative to habitual meal intakes is likely to have a large impact on the bioavailability of catechins and therefore their physiological effects.
Other aspects of study methodology can also affect findings. The range of methods and protocols available for non-invasive vascular function research does not allow for easy study comparison. Furthermore, there are limited published data comparing the methods, in particular the newer non-invasive techniques, with the best-established yet invasive venous-occlusion plethysmography technique. Thus, standardisation of measurement techniques is firstly required, followed by further research into the extent of correlation between techniques before studies utilising different vascular function measurements can be clustered.
Intra-study variability
A large inter-individual variation in bioavailability of green tea catechins can also be seen in the majority of intervention and pharmacokinetic studies. A number of aforementioned factors may also have an intra-study effect. Lifestyle factors, such as habitual diet, smoking status, alcohol intake and individual characteristics such as BMI and age will give rise to individual differences in response as a result of alterations in catechin bioavailability. Indeed, lifestyle and environmental influences are most likely to be responsible for the intra-individual variations found in acute pharmacokinetic studies where repeated experiments were performed. For example, a pharmacokinetic study conducted by Lee et al. found participant EGCG maximum concentration and area under the concentration curve intra-individual CV values to be as high as 81 and 68 %, respectively, when the experiment was repeated on the same group of participants on three separate occasions(Reference Lee, Maliakal and Chen5). This variation indicates the influence of environmental factors.
Individual differences in intestinal, microbial and hepatic metabolism may also in part provide an explanation for intra-study variation (and may also be responsible for a large component of inter-study variability, in particular when comparing studies conducted in groups geographically and ethnically divergent). Genetic polymorphisms in the enzymes governing the metabolic transformation of tea catechins such as catechol-O-methyltransferase, UGT and sulfotransferases may be partly responsible for the observed variability in flavonoid bioavailability. For example, polymorphisms have been found in the catechol-O-methyltransferase gene, resulting in an enzyme with a 3- to 4-fold difference in metabolic ability(Reference Dawling, Roodi and Mernaugh94). The glucuronidation of many drugs and steroid hormones have also been shown to be affected by UGT polymorphisms(Reference Lampe95), with a recent report of a 25-fold inter-individual difference in the rate of UGT1A6 glucuronidation(Reference Nagar, Zalatoris and Blanchard96). The effect of these polymorphisms on catechin bioavailability is yet to be investigated. The genetic variation of receptors, transporters and regulatory proteins could also have an impact on catechin absorption. For example, the multidrug resistance-associated protein 2 (MRP2), an ATP-binding cassette (ABC) intestinal membrane-bound transporter involved in the efflux of catechins back into the lumen, has been found to have several common single nucleotide polymorphisms, some of which have been suggested to have altered expression and function(Reference Sai, Saito and Itoda97).
Differences between individuals with respect to gastrointestinal function may also influence the metabolism and absorption of the catechins. Green tea catechins are relatively poorly absorbed, with bioavailability dependent on many factors within the small intestine such as lipophilicity, solubility, pH of the lumen, gastrointestinal transit time, mucosal mass and membrane permeability. The range of factors involved in this initial stage could greatly influence the rate and extent of catechin absorption. Indeed, when EGCG stability is increased, absorption is enhanced(Reference Gawande, Kale and Kotwal98).
The importance of microbial degradation of tea catechins has been mentioned previously. Microbial populations in the gut are highly variable and are specific to the food components that they metabolise. The metabolites produced after microbial degradation are also subject to variation depending on the resident microbial species. As a consequence, inter-individual differences in microflora profile can have a large impact on bioavailability and biotransformation of tea catechins. For example, the isoflavone daidzein is degraded to equol, a non-steroidal oestrogen, by colonic bacteria in only 30–40 % of individuals. Consequentially, equol producers are thought to derive a greater benefit from isoflavones compared with non-producers especially with regards to cardiovascular, bone and menopausal health(Reference Setchell, Brown and Lydeking-Olsen99). The levels of the microbial ring-fission metabolites ( − )-5-(3′,4′,5′-trihydroxyphenyl)-γ-valerolactone (M4) and ( − )-5-(3′,4′-dihydroxyphenyl)-γ-valerolactone (M6) in plasma and urine after green tea catechin administration have also been shown to differ greatly between individuals(Reference Li, Lee and Sheng7), which is most likely to be directly related to the variation in gut microbial populations.
Although it is recognised that a whole array of behavioural, physiological and genetic factors have an impact on the metabolism and responsiveness to dietary catechins, such information is often unavailable to add as a covariate to statistical models. However, consideration of these factors when designing studies or evaluating results is advisable.
Conclusion
There is accumulating evidence from human, animal and cell-culture models to indicate that green tea catechins have a positive impact on endothelial and overall vascular functions, with a number of plausible molecular mechanisms proposed. However, these findings are presently inconclusive and not entirely consistent, signifying the need for further research. There is also a concern with the physiological relevance of some in vitro findings which use green tea catechin concentrations far higher than what is achievable through the diet. Additional studies are also required to determine the importance of the metabolised compounds as opposed to the pure compounds, as tissues in humans are often exposed to the metabolites rather than the parent compounds present in food. Wide intra- and inter-individual variation in bioavailability has also been recognised. A greater understanding of this variation is needed to allow specific claims to be made regarding likely dose–response relationships in population subgroups.
Acknowledgements
R. J. M. drafted the manuscript, with all authors deciding on the review content and contributing to the final version. A. M. M. and K. G. J. have a number of ongoing collaborative projects with Unilever Discover (Colworth Science Park, Sharnbrook, Bedford, Beds, UK). Unilever Discover sponsor R. J. M.'s PhD studies.







