INTRODUCTION
In developed countries gastroenteritis viruses cause both seasonal acute gastroenteritis (AGE) and occasional outbreaks associated with contaminated food or water. In children, rotaviruses (RVs) are the most common causative agents of seasonal AGE, but are infrequently associated with outbreaks [Reference Lopman1–Reference Svraka3]. RVs cause more severe AGE than other viruses, more commonly requiring rehydration and hospitalization [Reference Mäki4–Reference Giaquinto6]. Human caliciviruses (HuCV) include noroviruses (NoV) and sapoviruses (SaV). NoVs are the second most common causative agent of seasonal AGE in young children in Finland [Reference Pang, Joensuu and Vesikari7] and elsewhere [Reference Pang8, Reference Atmar and Estes9]. In outbreaks of AGE, NoVs are the most common causative agents in both children and adults worldwide [Reference Lopman1, Reference Svraka3, Reference Atmar and Estes9, Reference Pang, Preiksaitis and Lee10]. NoV genotype GII.4 has recently emerged as the most common and virulent type [Reference Lopman11–Reference Johansen13]. SaVs also cause seasonal AGE in children, even though the clinical picture is milder than that of NoVs [Reference Pang, Joensuu and Vesikari7, Reference Pang8, Reference Pang, Preiksaitis and Lee10]. SaVs are rarely reported in outbreaks [Reference Atmar and Estes9, Reference Pang14].
Adenoviruses (AdVs) belonging to group F (types 40 and 41) may also cause AGE in both children and adults in about 1–8% of endemic cases and outbreaks [Reference Svraka3, Reference Vesikari15, Reference Simpson16]. Aichi viruses (AiVs) have been detected with low frequency in children and adults, mainly in AGE outbreaks [Reference Oh17–Reference Kaikkonen19]. Since AiVs have often been found together with other AGE viruses, they might even be considered to be indicators of mixed infections [Reference Ambert-Balay18]. Human bocaviruses (HBoVs) were first recognized in respiratory infections in children, but have recently also been connected with AGE in children [Reference Vicente20–Reference Campe, Hartberger and Sing22].
An outbreak of AGE due to contamination of drinking water with sewage occurred near Tampere in late 2007. We investigated cases of AGE in children from the contaminated area seen in Tampere University Hospital between 28 November and 31 December 2007, and examined the stool specimens for various causative agents.
MATERIALS AND METHODS
Drinking water was contaminated with treated sewage on 28 November 2007 in Nokia, a town of about 30 000 inhabitants near Tampere in southern Finland. It was estimated that thousands of people had symptoms of AGE, and at least 758 patients visited public health centres [Reference Laine23] in the following days and weeks. A total of 115 children requiring rehydration therapy were referred to Tampere University Hospital.
Study subjects
At the time of the incident, we were conducting an epidemiological survey of AGE in children in Tampere University Hospital. The study protocol and consent forms had been approved by the Ethics Committee of the Pirkanmaa Hospital District in 2006. The children treated in the hospital because of the Nokia AGE outbreak were recorded as a subgroup of this epidemiological study.
According to the study protocol, all children aged <15 years presenting with AGE symptoms in Tampere University Hospital were eligible for enrolment. The parents of eligible children (n=115) were informed about the study and asked to sign an informed consent form. We enrolled 65 children and obtained stool samples from 50 cases; of these 28 were hospitalized and 22 were treated as outpatients.
We collected clinical information on each AGE episode, including date of onset of symptoms, frequency of vomiting and diarrhoeal stools, and fever. The information was derived from a questionnaire completed by the parents, and from the hospital records. Some missing information was obtained by telephoning the parents. We also collected information on whether the child had received one or more RV vaccines.
A 20-point scoring system [Reference Ruuska and Vesikari24] was used to assess the severity of AGE episodes.
Laboratory methods
RVs, HuCVs, AdVs, HBoVs, and AiVs were primarily detected with the respective PCR methods. All positive amplicons were sequenced to confirm the result and to determine the virus genotype.
All 50 stool specimens were tested for RV G-types by reverse-transcription (RT)–PCR, as previously described [7], with the modification that the Taq polymerase was replaced by GoTaq® polymerase (Promega, USA). Two different primer sets were used for G-typing (see [Reference Gouvea25, Reference Das26]). For P-typing, an RT–PCR method previously described [Reference Simmonds27] was used with modified primers and conditions. The presence of RV antigen in stools was detected with ELISA, using IDEIA® Rotavirus kit (Oxoid Ltd, UK) according to the manufacturer's protocol. Five stool samples were from nappies only and could not be tested by ELISA.
HuCVs were detected by a modified RT–PCR method [Reference Jiang28, Reference Farkas29]; the primers co-detect NoVs and SaVs. The presence of AiVs was ascertained by a nested PCR after separate RT reaction with random primers [Reference Pang, Preiksaitis and Lee10]. The AiV-detecting PCR method was originally developed by Yamashita et al. [Reference Yamashita30]. AdVs were detected by a previously described nested PCR [Reference Allard, Albinsson and Wadell31] using two primer sets and HBoVs were detected using a PCR-detecting HBoV1 [Reference Sloots32].
Enteropathogenic bacteria were studied in 33/50 stool samples by bacterial culture, which detects Campylobacter, Salmonella, Shigella, Yersinia, Aeromonas, and Plesiomonas. The cultures were performed in the microbiological laboratory of Tampere University Hospital as part of routine examinations and not as part of the study protocol.
Results
Of the eligible 115 children, 35 (30%) were hospitalized and 80 (70%) were treated as outpatients with one or more visits. We enrolled 65 children; 28 were hospitalized and 37 treated as outpatients. Stool specimens were obtained from all 28 hospitalized children and from 22/37 children treated as outpatients. All 50 stool specimens were tested for all viruses (RV, HuCV, AiV, AdV, HBoV).
Of the 50 stool specimens 33 (66%) were RV positive, 31 (62%) were HuCV positive, five (10%) were AdV positive and 25 (50%) were AiV positive. In 20 (40%) cases both RV and HuCV were found; 10 (20%) cases presented with a third virus, and in two (4%) cases four viruses were present. Bacterial culture was performed on 33/50 cases; C. jejuni was found in 20/33 (61%) and Salmonella sp. in 2/33 (6%) of the cases. The complete findings of viruses and bacteria in the 28 hospitalized cases are given in Table 1.
Table 1. Findings of viral and bacterial pathogens and clinical characteristics of the 28 children hospitalized for acute gastroenteritis during the outbreak

RV, Rotavirus; SaV, sapovirus; AiV, Aichi virus; NoV, norovirus; AdV, adenovirus; HBoV, human bocavirus; C.j., Campylobacter jejuni; Salm., Salmonella species; n.a., information not available.
* RV-vaccinated children.
Rotaviruses
RV antigen ELISA in stools was positive in 24/45 (53%) cases tested, and RV RT–PCR in 33/50 (66%) cases. All stool samples which were RV positive by ELISA were also positive by RV RT–PCR. Five stool specimens were negative for RV antigen by ELISA, but positive for RV by RT–PCR. Of the 33 RT–PCR-positive RV cases, 32 were of genotype G1P[Reference Pang8] and one was of genotype G4P[Reference Pang8]. All the RVs of genotype G1 were identical, meaning that >99% of sequenced nucleotides encoding for VP7 were identical (length of amplicon 749 bp).
RV was the only identified viral AGE pathogen in six (12%) stool samples. RV presented with NoV in five (10%), with SaV in three (6%), and with AiV in six (12%) stool samples. Several different combinations of mixed infections with multiple viruses were found (see Table 2).
Table 2. Combinations of viruses detected in the 50 cases of acute gastroenteritis enrolled in the study during a waterborne outbreak
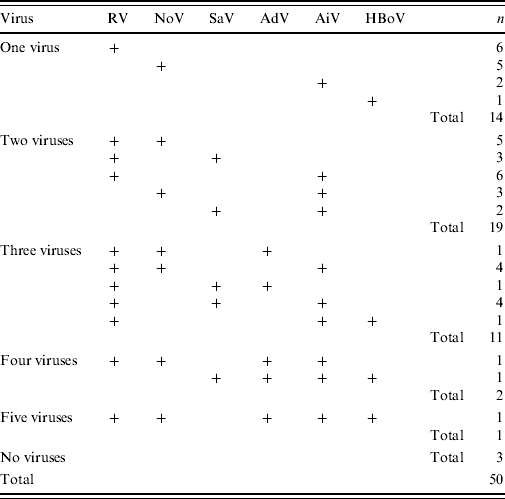
RV, Rotavirus; SaV, sapovirus; AiV, Aichi virus; NoV, norovirus; AdV, adenovirus; HBoV, human bocavirus.
Noroviruses and sapoviruses
RT–PCR for HuCV was positive in 31/50 (62%) cases. Of the 31 HuCVs, 20 (65%) were NoVs and 11 (35%) were SaVs.
Altogether, eight different genotypes of NoV were identified. Of the NoVs, 19/20 belonged to genogroup GII and only one to genogroup GI. Of genogroup GII NoVs, 12 (63%) were genotype GII.4. Of the 11 SaVs, eight different genotypes were found. In five (10%) of all 50 cases NoV was the only viral pathogen found. There were no cases in which SaV was the only viral pathogen. HuCVs presented together with RV in eight cases; in five cases NoV with RV, and in three cases SaV with RV. AiVs, AdVs and HBoVs were also detected in mixed infections with HuCVs (Table 2). There were no cases in which more than one type of HuCV was present.
Adenoviruses
AdV was found in five (10%) of the 50 cases. Two of these were of group A, one of group C, and two of group F. All the AdV-positive cases occurred in mixed infections (Table 1).
Aichi viruses
AiV was present in 25/50 (50%) stool samples. In 24/25 (96%) cases AiV was found in mixed infections; in one case no other AGE viruses were found (Table 1). The median age of the AiV-positive cases was 7 years, i.e. higher than the median of all the enrolled children (see Clinical features below).
Human bocaviruses
HBoV was found in four (8%) stool samples. In one case HBoV was the only pathogen identified in the stool sample. In the other three cases, HBoV presented as a part of a mixed infection.
Bacteria
Bacterial cultures were performed for 33/50 cases. C. jejuni was found in 20/33 (61%) cases and Salmonella sp. in four (12%) cases. In each case these bacteria were found in mixed infections with one or more AGE viruses. C. jejuni was found in 13/33 RV-positive stool samples, in three of which Salmonella sp. was also present. C. jejuni was found in 15/31 HuCV-positive cases, in three of which Salmonella sp. was also present. In five (10%) cases C. jejuni presented with RV, HuCV and AiV, and in one case all the pathogens studied (RV, HuCV, AiV, AdV, HBoV, C. jejuni and Salmonella sp.) were present.
RV vaccination status
There were three children who had been vaccinated against RV. One had been vaccinated with Rotarix® (GlaxoSmithKline, Finland), one with Rotateq® (Sanofi Pasteur MSD, Finland), while in the third case the vaccine was unknown. In all of these cases ELISA for RV antigen was negative, but in one case a weak positive RV-type G1 was detected with RT–PCR. In this case the RV was of wild-type G1 virus similar to the other G1 RVs found in this outbreak, not of the vaccine type. In the remaining two cases RV RT–PCR was clearly negative. In all three cases other pathogens were found: NoV genotype GII.4 in two cases, and SaV, AiV, AdV, and C. jejuni in one case.
Clinical features
The age of enrolled children ranged from 6 months to 13 years (median age 2 years 6 months). There were 19 children aged >7 years (Fig. 1).

Fig. 1. Age distribution of 65 children seen in Tampere University Hospital and enrolled in the study during the waterborne outbreak of acute gastroenteritis.
The median severity score of the hospitalized children (n=28) was 17 (Fig. 1), indicating an unusually severe disease. However, no difference in the severity of AGE was observed between the cases caused by a single virus, different combinations of viruses, or combinations of viruses and bacteria (Fig. 2, Table 1). The mean duration of diarrhoea was 10·3 days (range 2 to >20 days). The mean duration of vomiting was 5 days (range 1–11 days). The mean of maximal fever was 38·7°C (range up to 40·4°C) (Table 1).
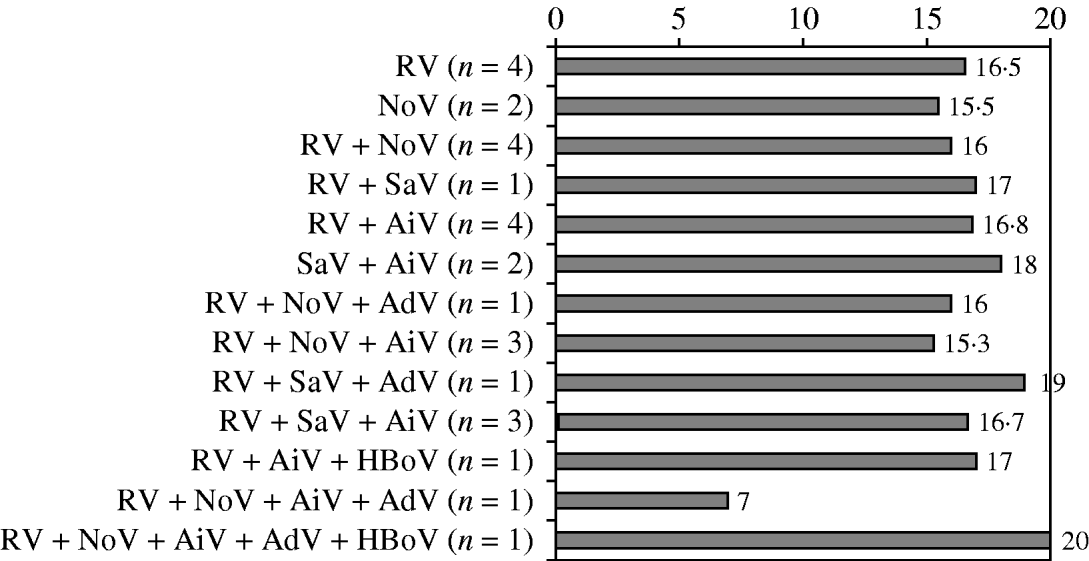
Fig. 2. Mean clinical severity scores in 28 children hospitalized for acute gastroenteritis according to causative viruses. RV, Rotavirus; SaV, sapovirus; AiV, Aichi virus; NoV, norovirus; AdV, adenovirus; HBoV, human bocavirus.
Considering single symptoms, the only significant difference between the various pathogens or combinations of pathogens was that bloody diarrhoea occurred only if C. jejuni was found in the stool samples. Altogether four children had bloody diarrhoea, and in each of these cases a combination of pathogens including C. jejuni and RV was found.
DISCUSSION
We were able to enrol in the study and collect stool samples from 50/115 children (43%) seen in Tampere University Hospital because of this outbreak, including 28/35 hospitalized children (80%). The main reason for failing to enrol more patients was that the emergency room was crowded during the outbreak, and many of the children were discharged before parental consent could be obtained. However, the study material still represents almost half of all the children who were seen in the hospital during this outbreak, and 80% of the hospitalized children. Because our study is hospital-based, the study material includes severe AGE cases rather than being representative for all paediatric AGE cases in the outbreak. Moreover, children were referred to this hospital whenever rehydration was required, effectively selecting the severe cases in children.
This AGE outbreak might be seen as an experimental situation in which many children were simultaneously, and massively, exposed to enteric pathogens. It is impossible to estimate the infectious dose or proportion of each pathogen, but presumably children with severe AGE symptoms were exposed to unusually large doses of several gastroenteritis viruses. As a result, AGE episodes seen in the hospital were unusually severe and enduring. Furthermore, children of all ages suffered from AGE, suggesting that the viral pathogens were able to break through the existing immunity in older children.
The majority of the symptomatic children had multiple AGE pathogens simultaneously. Regardless of the pathogen or combination of pathogens, the AGE symptoms of the hospitalized children were unusually severe, but did not differ for individual pathogens. This is in contrast to the normal endemic or epidemic situation. For example, RV generally causes more severe vomiting, diarrhoea and fever than other viral pathogens [Reference Mäki4, Reference Ehlken5, Reference Pang8], and NoVs typically cause more vomiting than diarrhoea, whereas SaVs cause more diarrhoea then vomiting [Reference Pang, Joensuu and Vesikari7, Reference Pang8]. No such features were seen in this study. Only one child had a severity score <11, usually regarded as a threshold for severe AGE. This male child had mild AGE symptoms, but developed severe post-infectious abdominal pain, which caused a suspicion of intestinal intussusception. NoV type GII.4 and AdV were found in his stool sample, but intussusception was excluded.
Most of the RVs and many NoVs showed genetic similarity. Of the 33 RVs, 32 G1 viruses were genetically 99% identical. While several HuCVs were found, NoV genotype GII.4 detections showed particular genetic similarity. These findings suggest a single common source of G1 RVs and GII.4 NoVs in this outbreak. When the contamination of drinking water occurred, the endemic RV season was just beginning, and a single transmitter of G1 RV might explain all but one of the RV findings. In contrast, some NoV-associated AGE had also already been seen in the hospital throughout autumn 2007, explaining the greater variety of NoVs at the time of the outbreak. Both NoVs and RVs are highly infectious, and even a few viruses are able to cause infection and disease [Reference Moreno-Espinosa, Farkas and Jiang33, Reference Bass, Pappano and Humiston34].
RVs only rarely cause waterborne or foodborne outbreaks. It can be assumed that in countries with a high level of hygiene, the infectious dose of RVs from either person-to-person contact or via airborne infection is probably small. Compared with such a background, the children in this outbreak may have received a much larger amount of RVs from the contaminated drinking water, which would explain the unusually severe clinical picture, as well as the occurrence of RV cases in older children.
As discussed earlier for RVs, the infectious dose of NoVs is probably small in endemic seasonal infections or in usual foodborne or waterborne outbreaks. Usually NoV-associated AGE cases in such epidemics are less severe than those seen in this outbreak [Reference Pang, Joensuu and Vesikari7, Reference Rockx35]. SaVs usually cause milder diarrhoea in children aged <5 years [Reference Pang, Joensuu and Vesikari7, Reference Pang8]. In previous studies on all these viruses, severity scores in prospectively followed-up cases in children are in the range of 8–11, regarded as moderately severe, and in extremely rare cases exceed 17, which was the mean severity score in the present situation. We believe that simultaneous exposure to several pathogens on the one hand and massive exposure on the other, explains the unusually severe clinical picture in these cases.
This is the first AGE outbreak in which AiVs have been found in Finland. In our material, AiV was found in 25/50 (50%) stool samples; 24/25 were in mixed infections. Our finding confirms the earlier notion that AiVs usually present together with other AGE pathogens in mixed infections [Reference Ambert-Balay18].
The role of HBoVs as causative agents in gastroenteritis is unclear. These viruses have been detected in stool samples of AGE cases and also in respiratory infections [Reference Vicente20, Reference Tozer36], but in stool samples they are usually seen in mixed infections. In this outbreak, four cases with HBoV detected in stool samples were seen, of which in one case HBoV was the only pathogen identified. In this case HBoV may have been the causative agent in AGE.
In our study there were three children, who were known to have received RV vaccine. In all three cases the AGE symptoms were severe enough (scores of 14, 15, 19) to lead to hospitalization. In all three cases, HuCVs were presumed to be the main causative agent. NoV type GII.4 was found in two cases and SaV together with AiV, AdV, and C. jejuni in one case. One of the children with NoV GII.4 had a weak positive RV by RT–PCR, but no detectable RV antigen by ELISA in his stool sample, meaning that the amount of RV in the sample was small. This finding suggests that vaccination probably protected this child, and possibly the others, from RV AGE and only minimal replication of wild-type RV occurred in this one case.
ACKNOWLEDGEMENTS
We thank the Pirkanmaa Waterborne Outbreak Study Group (J. Mustonen, J. Antonen, P. Collin, J. Herrala, M. Korpela, E. Kujansuu, A-L. Kuusela, M. Kuusi, J. Laine, J. Lumio, S. Mustajoki, H. Oksa, P. Ruutu, S. Räsänen, T. Uotila, T. Katto) for important work in clarifying the overview of the outbreak. We also thank the clinical and laboratory personnel who worked on the study, and the children and parents that participated in the study.
DECLARATION OF INTEREST
None.






