Introduction
The development of a nomenclature to identify and classify minerals is a fundamental step to understand the processes that govern mineral diversity and has direct implications on petrogenetic and provenance information. For example, ambiguity in the naming of a mineral species hinders direct comparison of minerals originating from different types of deposits.
A fundamental criterion for mineral nomenclatures (e.g. Hawthorne et al., Reference Hawthorne, Ungaretti and Oberti1995; Bayliss et al., Reference Bayliss, Kaesz and Nickel2005; Mills et al., Reference Mills, Hatert, Nickel and Ferraris2009) is based on the dominance of a chemical constituent (i.e. ions, vacancies, molecular groups, or atom groups with the same valency state; Nickel and Grice, Reference Nickel and Grice1998; Hatert and Burke, Reference Hatert and Burke2008) of the dominant valency state at a given crystallographic site. This criterion stems from the application of the dominant-valency rule, which is an extension of the dominant-constituent rule (Hatert and Burke, Reference Hatert and Burke2008). Along with valency-imposed double site-occupancy, these two rules may identify a mineral species and its charge-balanced end-member formula as defined by Hawthorne (Reference Hawthorne2002).
However, important aspects need to be clarified when this criterion is applied to minerals of complex mixed compositions. Firstly, a mineral should be identified for the most abundant constituent of the dominant valency state, and not for the most abundant end-member. Indeed, mineral species identified by the dominant valence may not correspond to the dominant end-member component. Secondly, complex coupled substitution mechanisms may lead to compositions which are not directly identified (i.e. an end-member formula is not identified) with the simple application of the dominant-valency rule; in those cases, an extension of the rule may be used (Bosi, Reference Bosi2018).
This study aims at explicitly addressing these issues and at suggesting a coherent procedure to identify mineral species based exclusively on the dominant-valency and dominant-constituent rules, which are currently recommended by the Commission on New Minerals, Nomenclature and Classification (CNMNC) of the International Mineralogical Association (IMA). The following nomenclature proposal was approved by the IMA−CNMNC in May 2019 (Miyawaki et al., Reference Miyawaki, Hatert, Pasero and Mills2019).
Dominant-valency rule versus dominant end-member approach
Usually, two approaches could be used to distinguish mineral species: (1) the dominant-valency approach, which identifies mineral species by determining the dominant root-charge arrangement (e.g. Hawthorne, Reference Hawthorne2002); (2) the dominant-end-member approach, which identifies species by determining the most abundant end-member component (e.g. Bulakh, Reference Bulakh2010; Dolivo-Dobrovol'sky, Reference Dolivo-Dobrovol'sky2010).
These two approaches may converge to the same mineral name in species with chemical substitutions at a single site or at two sites, when the dominant ion of the site-dominant valence state is also the site-dominant ion. Consider the following two examples: (1) the hypothetical composition [8](Ca0.60Na0.25K0.15)Σ1.00[4](Al1.60Si2.40)Σ4.00O8 is dominated by the root-charge arrangement (R 2+)(R 3+2R 4+2)O8 (60%) corresponding to anorthite CaAl2Si2O8, which is also the most abundant end-member (60%) with respect to albite, NaAlSi3O8 (25%) and ‘K-feldspar’, KAlSi3O8 (15%); (2) the composition [8](Ca0.35Na0.40K0.25)[4](Al1.35Si2.65)O8 is dominated by the root-charge arrangement (R +)(R 3+R 4+3)O8 (65%) corresponding to albite (Na > K), which is also the most abundant end-member (40%) with respect to anorthite (35%) and ‘K-feldspar’ (25%).
The dominant-valency approach and dominant-end-member approach do not converge to the same mineral name in the case of coupled heterovalent substitutions coupled at two sites, associated with homovalent substitutions at one site, leading to a situation where one site-dominant ion is not the dominant ion of the dominant valence state. Consider the composition [8](Ca0.40Na0.35K0.25)[4](Al1.40Si2.60)O8: on the basis of the dominant-valency rule, the dominant root-charge arrangement is (R +)(R 3+R 4+3)O8 (60%) as the monovalent cations, Na and K, are dominant at the [8]-fold coordinated site. Thus, the mineral species is identified as albite because the Na content is larger than the K content (dominant-constituent rule). On the other hand, on the basis of the dominant-end-member approach, the mineral would be anorthite as the proportion of this end-member (40%) is dominant over albite (35%) and ‘K-feldspar’ (25%). These relations can be graphically illustrated by ternary diagrams (Fig. 1). Plotting the chemical compositions of the [8]-fold site in a diagram with Ca2+, Na+ and K+ placed at each corner, the typical boundary limits crossing at the centre of the diagram (33% of each component) will represent the dominant-end-member approach (Fig. 1a), whereas the boundary limits are displaced for the dominant-valency approach as follows: 25% for the component Ca2+, 37.5% for both Na+ and K+ (Fig. 1b). Note that the dominant-end-member approach and the dominant-valency rule diverge when mineral compositions occur in the grey triangular area of Fig. 1b. This divergence area is even smaller as the miscibility gap of the feldspar diagram covers most of the grey triangular surface (Fig. 1c).

Fig. 1. Diagram for discriminating anorthite, albite and ‘K-feldspar’ according to the dominant-end-member approach (a) and the dominant-valency rule (b). In (c) the ternary miscibility gap calculated for P = 10 kbar and T = 1200°C (dashed black line) using the method of Kroll et al. (Reference Kroll, Evangelakakis and Voll1993), is shown. Solid/dashed red lines are consistent with the dominant-end-member approach, whereas the solid blue lines are consistent with the dominant-valency rule. The grey colour highlights the area where the dominant-end-member approach and the dominant-valency rule diverge.
This simple example demonstrates that a mineral species identified by the dominant-valency approach may not correspond to that identified by the dominant-end-member approach. It is likely that the dominant-valency rule and the end-member approach would give the same identification in many (simple) cases, but there are other cases in which they do not. In these cases the dominant-valency rule should always prevail and mixed mineral nomenclatures, relying on both the dominant-valency rule and the dominant-end-member approach, should be avoided.
An important reason to avoid the identification of a mineral species on the basis of end-member components is that this approach may be ambiguous, not only when new species belonging to poorly characterised groups are discovered, but even when the end-member formulae of all species in the group are known. Indeed, as demonstrated by Rickwood (Reference Rickwood1968), it is impossible to find a unique algebraic solution for a formula identification, when the number of end-members is higher than the number of independent chemical components. In fact, the results will depend strongly on a specific sequence of calculating end-member proportions, as demonstrated for reciprocal solutions in the garnet or spinel supergroups (e.g. Rickwood, Reference Rickwood1968; Grew et al., Reference Grew, Locock, Mills, Galuskina, Galuskin and Hålenius2013). An informative example of definition of a meaningless mineral phase is found in Putirka (Reference Putirka2016), who identifies a hypothetical new amphibole (‘aluminous kaersutite’) inconsistent with existing nomenclature rules (Hawthorne et al., Reference Hawthorne, Oberti, Harlow, Maresch, Martin, Schumacher and Welch2012). Consequently, the end-member approach is ambiguous in mineral nomenclature, although the calculation of end-member proportions is currently an important step for application of thermodynamics to mineral systems and for petrologic studies.
Application of the dominant-valency rule to complex mineral compositions: the site-total-charge approach
The site-total-charge approach
As the sequential use of the dominant-valency and the dominant-constituent rules are recommended by the IMA−CNMNC, it is worthwhile to analyse their basic aspects, here identified as follows: (1) the principle of the formula electroneutrality; and (2) the definition of an end-member formula (Hawthorne, Reference Hawthorne2002) that includes at most one site with double occupancy (i.e. heterovalent pair of ions or ion–vacancy pair). In addition, the concepts of total charge and charge constraint at the crystallographic sites should be considered. The charge constraint can be defined as an integer number close (or next) to the observed site total charge (STC). It provides information on possible root-charge and atomic arrangements; if such arrangements satisfy all the criteria of an end-member formula, the mineral may be identified.
Again, considering the composition [8](Ca0.4Na0.35K0.25)[4](Al1.4Si2.6)O8, the key aspect is to explore the STC at the [8]- and [4]-fold coordinated sites: [8]STC = +1.4 and [4]STC = +14.6. As the former is close to +1 and the latter is close to +15, the charge constraints (+1 and +15) indicate that the mineral species may be consistent with the end-member root-charge arrangement (R +)Σ1+(R 3+R 4+3)Σ15+O8, rather than (R 2+)Σ2+(R 3+2R 4+2)Σ14+O8. In terms of atoms per formula unit (apfu), the atomic arrangement 0.60[(Na,K)(AlSi3)] (= 3.00 apfu, limited by Na + K content) is larger than 0.40[(Ca)(Al2Si2)] (= 2.00 apfu, limited by Ca content). Thus, in accord with the dominant-constituent rule, Na0.35 > K0.25, the species corresponds to albite.
Note that the site total charge and charge constraint are another important way to manifest the dominant-valency rule in which the STC is used to identify the dominant root-charge arrangement consistent with an end-member formula.
Extension of the dominant-valency rule
For complex mineral compositions, it may appear that the simple application of the dominant-valency rule does not lead to a charge-balanced end-member formula. This is due to the occurrence of heterovalent substitution mechanisms at more than one crystallographic site (or group of sites). Starting from the albite composition [8](Na0.60Ca0.40)[4](Al1.40Si2.60)O8, we could assume a hypothetical heterovalent substitution mechanism affecting the larger cation site: 0.12 [8](Pb2+ + □) → 0.12 [8](Na+ + Na+). This substitution will lead to the composition [8](Na0.36Pb2+0.12□0.12Ca2+0.40)[4](Al1.40Si2.60)O8, which has 0.52 divalent (R 2+) apfu; the simple application of the dominant-valency rule thus leads to the unbalanced charge formula (CaAlSi3O8)Σ1+. Application of the site-total-charge approach to this composition indicates +1.4 charges at the [8]-fold coordinated site, close to +1, thus confirming that the root-charge arrangements with total charge +1 are dominant at that site: in fact, [0.36(R +) + 0.24(R 2+0.5□0.5)Σ1+] = 0.60 apfu is larger than the largest amount of charge arrangement entirely composed by R 2+ cations = 0.52 apfu. In other words, the aggregate-charge arrangement [(R 1+) + (R 2+0.5□0.5)Σ1+] is more abundant than the largest amount of (R 2+) we can calculate from the mineral composition. As the arrangement (R +0.36) (limited by the Na content) is larger than (R 2+0.12□0.12)Σ0.24 (limited by the number of vacancies), the relative dominant cation arrangement is given by [8](Na+), which leads to the end-member formula NaAlSi3O8. Figure 2 shows the boundaries between generalised mineral species in such a complex system involving a heterovalent substitution at two sites (Fig. 2a), followed by a heterovalent substitution at a single site (Fig. 2b).

Fig. 2. Schematic representations of coupled heterovalent substitutions at two sites (a), followed by a heterovalent substitution at a single site (b) leading to the site-total-charge approach.
Hatert and Burke (Reference Hatert and Burke2008) have in fact addressed the cases of (1) coupled heterovalent substitutions at a single site; (2) heterovalent substitutions at two sites; and (3) heterovalent substitutions at two sites plus homovalent substitution at a single site, but have not considered the case (4) of coupled heterovalent substitutions at two sites along with the heterovalent substitution at a single site. This more complex case is now clarified (Fig. 2).
The jervisite example
The application of the site-total-charge approach should be used when the simple application of the dominant-valency rule leads to an unbalanced end-member formula. Let us consider the empirical formula of the jervisite pyroxene of Mellini et al. (Reference Mellini, Merlino, Orlandi and Rinaldi1982):
in which divalent cations seem to predominate at the M(2) site with Ca2+ as dominant constituent, and trivalent cation predominate at the M(1) site with Sc3+. As a result, the simple application of the dominant-valency rule yields (M(2)CaM(1)ScSi2O6)Σ1+, which is not charge-balanced. However, the STC at M(2) (= +1.33) and M(1) (= +2.66) indicate the following charge constraints: (i) M(2)Σ1+ compatible with both M(2)(R +) and M(2)(R 2+0.5□0.5)Σ1+; (ii) M(1)Σ3+ compatible with R 3+ (Fig. 3). As a result, the root-charge arrangements consistent with an end-member are M(2)(R +)M(1)(R 3+)Si2O6 and M(2)(R 2+0.5□0.5)M(1)(R 3+)Si2O6, which are related by the heterovalent substitution M(2)(Na+) ↔ M(2)(R 2+0.5 + □0.5). Regarding the dominant charge arrangements, M(2)[(R +) + (R 2+0.5□0.5)Σ1+] prevails over the divalent M(2)(R 2+) in terms of apfu: the sum of the charge arrangements with total charge +1 at the M(2) site, 0.43 apfu of M(2)(R +) (limited by Na contents) and 0.24 apfu of M(2)(R 2+0.5□0.5)Σ1+ (limited by the number of vacancies), yields an aggregate-charge arrangement M(2)[0.43(R +) + 0.24(R 2+0.5□0.5)Σ1+] equal to 0.67 apfu. The latter is even larger than the largest amounts of charge arrangement characterised by the sole occurrence of M(2)(R 2+), 0.45 apfu. Note that M(2)(R 2+0.45) is a hypothetical value that we can calculate to compare with the dominant-charge arrangements: in fact, the occurrence of 0.24(R 2+0.5□0.5) will actually reduce M(2)(R 2+) to 0.33 apfu. Between the two root-charge arrangements characterised by a total charge +1 at M(2), 0.43[M(2)(R +)M(1)(R 3+)R 4+2O6] is more abundant than 0.24[M(2)(R 2+0.5□0.5)M(1)(R 3+)R 4+2O6] and as the atomic arrangement 0.43[M(2)(Na+)M(1)(Sc3+)Si2O6] > 0.24[M(2)(Ca2+0.5□0.5)M(1)(Sc3+)Si2O6], the end-member formula is NaSc3+Si2O6.

Fig. 3. Schematic representations of site total charge and atomic charge arrangements at the M(2) (a) and M(1) (b) sites of jervisite (see text).
The reason why the dominant-valency rule apparently fails may be shown by the substitution mechanisms involving jervisite. In this regard, it is instructive to arrive at the jervisite empirical formula from the diopside composition CaMgSi2O6:
(1) the heterovalent substitution at two sites
 $$\eqalign{&\hskip-15pt ^{M {\left( 2 \right)}}\left( {{\rm N}{\rm a}^ + } \right)_{0.66} \,+\, ^{M{(1)}} \left( {{\rm S}{\rm c}^{3 + }} \right)_{0.66}\;\to\; ^{M{(2)}}\!\! \left( {{\rm C}{\rm a}^{2 + }} \right)_{0.66} \,+\, ^{M{(1)}} \left( {{\rm M}{\rm g}^{2 + }} \right)_{0.66} \cr & {\rm yield}{\rm s}\;^{M{(2)}} \left( {{\rm N}{\rm a}^ + _{0.66} {\rm C}{\rm a}^{2 + }_{0.34} } \right)\;^{M{(1)}} \left( {{\rm S}{\rm c}^{3 + }_{0.66} {\rm M}{\rm g}^{2 + }_{0.34} } \right){\rm S}{\rm i}_2{\rm O}_6;} $$
$$\eqalign{&\hskip-15pt ^{M {\left( 2 \right)}}\left( {{\rm N}{\rm a}^ + } \right)_{0.66} \,+\, ^{M{(1)}} \left( {{\rm S}{\rm c}^{3 + }} \right)_{0.66}\;\to\; ^{M{(2)}}\!\! \left( {{\rm C}{\rm a}^{2 + }} \right)_{0.66} \,+\, ^{M{(1)}} \left( {{\rm M}{\rm g}^{2 + }} \right)_{0.66} \cr & {\rm yield}{\rm s}\;^{M{(2)}} \left( {{\rm N}{\rm a}^ + _{0.66} {\rm C}{\rm a}^{2 + }_{0.34} } \right)\;^{M{(1)}} \left( {{\rm S}{\rm c}^{3 + }_{0.66} {\rm M}{\rm g}^{2 + }_{0.34} } \right){\rm S}{\rm i}_2{\rm O}_6;} $$(2) the homovalent substitution at a single site
 $$\eqalign{& ^{M {\left( 1 \right)}}\left( {{\rm F}{\rm e}^{2 + }} \right)_{0.15}\; \to\; ^{M{(1)}}\!\! \left( {{\rm M}{\rm g}^{2 + }} \right)_{0.15} \cr & {\rm yield} {\rm s}\;^{M{(2)}} \left( {{\rm N}{\rm a}^ + _{0.66} {\rm C}{\rm a}^{2 + }_{0.34} } \right)\;^{M{(1)}} \left( {{\rm S}{\rm c}^{3 + }_{0.66} {\rm M}{\rm g}^{2 + }_{0.19} {\rm F}{\rm e}^{2 + }_{0.15} } \right){\rm S}{\rm i}_2{\rm O}_6;}$$
$$\eqalign{& ^{M {\left( 1 \right)}}\left( {{\rm F}{\rm e}^{2 + }} \right)_{0.15}\; \to\; ^{M{(1)}}\!\! \left( {{\rm M}{\rm g}^{2 + }} \right)_{0.15} \cr & {\rm yield} {\rm s}\;^{M{(2)}} \left( {{\rm N}{\rm a}^ + _{0.66} {\rm C}{\rm a}^{2 + }_{0.34} } \right)\;^{M{(1)}} \left( {{\rm S}{\rm c}^{3 + }_{0.66} {\rm M}{\rm g}^{2 + }_{0.19} {\rm F}{\rm e}^{2 + }_{0.15} } \right){\rm S}{\rm i}_2{\rm O}_6;}$$(3) the heterovalent substitution at a single site
which substantially corresponds to the empirical composition of jervisite. $$\eqalign{& ^{M {\left( 2 \right)}} \left( {{\rm F}{\rm e}^{2 + } + {\squ}} \right)_{0.12} \,\to\; ^{M{(2)}}\!\! \left( {2{\rm N}{\rm a}^ + } \right)_{0.12} \cr & \hskip-15pt {\rm yield}{\rm s}\;^{M{(2)}} \left( {{\rm N}{\rm a}^ {+} _{0.42} {\rm C}{\rm a}^{2 + }_{0.34} {\rm F}{\rm e}^{2 + }_{0.12} {\squ}_{0.12} } \right)^{M{(1)}} \left( {{\rm S}{\rm c}^{3 + }_{0.66} {\rm M}{\rm g}^{2 + }_{0.19} {\rm F}{\rm e}^{2 + }_{0.15} } \right){\rm S}{\rm i}_2{\rm O}_6,} $$
$$\eqalign{& ^{M {\left( 2 \right)}} \left( {{\rm F}{\rm e}^{2 + } + {\squ}} \right)_{0.12} \,\to\; ^{M{(2)}}\!\! \left( {2{\rm N}{\rm a}^ + } \right)_{0.12} \cr & \hskip-15pt {\rm yield}{\rm s}\;^{M{(2)}} \left( {{\rm N}{\rm a}^ {+} _{0.42} {\rm C}{\rm a}^{2 + }_{0.34} {\rm F}{\rm e}^{2 + }_{0.12} {\squ}_{0.12} } \right)^{M{(1)}} \left( {{\rm S}{\rm c}^{3 + }_{0.66} {\rm M}{\rm g}^{2 + }_{0.19} {\rm F}{\rm e}^{2 + }_{0.15} } \right){\rm S}{\rm i}_2{\rm O}_6,} $$
The alluaudite example
The alluaudite supergroup contains phosphates and arsenates with the structural formula A(2)’A(1)M(1)M(2)2(TO4)3; the cations are distributed mainly among four distinct crystallographic sites: the large cavities coordinated by the A(2)’ and A(1) sites in the channels of the structure, and the octahedrally-coordinated M(1) and M(2) sites. A wet chemical analysis of an alluaudite from the Buranga pegmatite, Rwanda, was published by Héreng (Reference Héreng1989) and gives the following cation distribution:
 $\eqalign { {^{A(2)^{\prime}}(\squ)^{A(1)} ({\rm Mn}^{2 + }_{0.34} {\rm Ca}^{2 + }_{0.17} {\rm Na}_{0.29} {\squ}_{0.21})_{\Sigma 1.00}} {^{M(1)}( {\rm Mn}^{2 + })} \cr {^{M(2)} ( {\rm Fe}^{3 + }_{1.70} {\rm Fe}^{2 + }_{0.15} {\rm Mn}^{2 + }_{0.10} {\rm Mg}_{0.04}{\rm Zn}_{0.01} )_{\Sigma 2.00}} {^{T} ( {\rm PO}_4 )_3}.}$
$\eqalign { {^{A(2)^{\prime}}(\squ)^{A(1)} ({\rm Mn}^{2 + }_{0.34} {\rm Ca}^{2 + }_{0.17} {\rm Na}_{0.29} {\squ}_{0.21})_{\Sigma 1.00}} {^{M(1)}( {\rm Mn}^{2 + })} \cr {^{M(2)} ( {\rm Fe}^{3 + }_{1.70} {\rm Fe}^{2 + }_{0.15} {\rm Mn}^{2 + }_{0.10} {\rm Mg}_{0.04}{\rm Zn}_{0.01} )_{\Sigma 2.00}} {^{T} ( {\rm PO}_4 )_3}.}$In this formula, vacancies are dominant at A(2)’, divalent cations with Mn2+ > Ca at A(1), Mn2+ at M(1) and Fe3+ at M(2), thus leading to the unbalanced end-member formula [□Mn2+Mn2+Fe3+2(PO4)3]Σ1+. The dominant-valency rule cannot be simply applied to the A(1) site population, due to the occurrence of the heterovalent substitution A(1)(R 2+ + □) → A(1)(2Na+) which produces a decrease of the Na content and an increase of the R 2+-cations and vacancies at the A(1) site. However, this substitution mechanism preserves the STC, whose value (+1.31) is close to +1. In fact, the alluaudite structure is dominated by the aggregate-charge arrangement A(1)[(R 2+0.5□0.5)Σ1+ + (R +)]: the sum of A(1)(R 2+0.21 + □0.21) (Σ = 0.42 apfu, limited by the number of vacancies) plus A(1)(R +0.29) (= 0.29 apfu, limited by Na contents) is equal to 0.71 apfu; the latter is larger than the largest amount of charge arrangement calculated using only R 2+-cations at A(1): 0.51 apfu. Looking at the dominant aggregate-charge arrangement with total charge +1, A(1)(R 2+0.21□0.21)Σ0.42 > A(1)(R +0.29). In accord with the dominant-constituent rule, A(1)Mn2+ > A(1)Ca2+, the relative dominant atomic arrangement at the A(1) site is hence (Mn2+0.5□0.5)Σ1+ and the end-member formula is □(Mn2+0.5□0.5)Mn2+Fe3+2(PO4)3. It should be noted that the heterovalent-pair A(1)(R 2+0.5□0.5)Σ1+ and the homovalent A(1)(R +) play the same role in the charge balance (overall electroneutrality).
The garnet example
The approved garnet nomenclature is based on the dominant-valency rule, but this rule was unsuccessful in discriminating schorlomite from morimotoite/andradite. Let us consider the schorlomite composition from the type locality Magnet Cove, Arkansas (Grew et al., Reference Grew, Locock, Mills, Galuskina, Galuskin and Hålenius2013): X[(Ca2+2.907Fe2+0.043Mn2+0.035)Na+0.015]Σ3.000Y[(Ti4+1.069Zr4+0.055)Fe3+0.517(Fe2+0.204Mg2+0.155)]Σ2.000Z[Si4+2.250(Fe3+0.588Al3+0.162)]Σ3.000O12 in which the dominant valence at the X site is +2, and at the Y and Z sites is +4. The simple application of the dominant-constituent rule fails as the dominant constituents XCa2+, YTi4+ and TSi4+ lead to unbalanced end-member formula (Ca2+3Ti4+2Si4+3O2–12)Σ2+. This issue was overcome by Grew et al. (Reference Grew, Locock, Mills, Galuskina, Galuskin and Hålenius2013) by using the most abundant end-member composition: schorlomite Ca3Ti2(SiFe3+2)O12 (37.5%) > morimotoite Ca3(TiFe2+)Si3O12 (35.9%) > andradite Ca3(Fe3+2)Si3O12 (25.9%). This is equivalent to stating the schorlomite-group minerals (which include the mineral species hutcheonite, irinarassite, kerimasite, kimzeyite, schorlomite and toturite) are identified by the dominant-end-member approach.
With regard to the substitution types involved in the empirical formula of this garnet, starting from andradite Ca3(Fe3+2)Si3O12, we have a heterovalent substitution at two sites (schorlomite → andradite substitution type):
yielding the formula Ca3Y(Ti4+0.75Fe3+1.25)Σ2.00Z(Si2.25Fe3+0.75)Σ3.00O12, plus a heterovalent substitution at a single site (morimotoite → andradite substitution type):
yielding the formula Ca3Y(Ti4+1.13Fe3+0.49Fe2+0.38)Σ2.00Z(Si2.25Fe3+0.75)Σ3.00O12, which reasonably corresponds to the empirical one, with Ca = X(Ca2++ Fe2++ Mn2+), YTi4+ = Y(Ti4++ Zr4+), YFe2+ = Y(Fe2++ Mg2+) and ZFe3+ = Z(Fe3++ Al3+). Similarly to the jervisite example above, the garnet from Magnet Cove can also be identified by the dominant-valency rule using the concepts of STC and charge constraint to select the possible root-charge and atomic arrangements among the end-members X(Ca3)Σ6+ Y(Ti2)Σ8+ Z(SiFe3+2)Σ10+O12 (schorlomite), X(Ca3)Σ6+ Y(Fe3+2)Σ6+ Z(Si3)Σ12+O12 (andradite) and X(Ca3)Σ6+ Y(TiFe2+)Σ6+ Z(Si3)Σ12+O12 (morimotoite). In detail: the total charge at X (= +5.99) is very close to +6, at Y (= +6.77) is closer to +6 than +8, and at Z (= +11.25) is closer to +12 than +10; note that the charge constraint Y Σ7+ and Z Σ11+ are ruled out as leading to a formula, (Ca3)Y(TiFe3+)Z(Si2Fe3+)O12, inconsistent with the end-member definition (Hawthorne, Reference Hawthorne2002); the charge constraints (X Σ6+, Y Σ6+ and Z Σ12+) are only compatible with the root-charge arrangements X(R 2+3)Σ6+ Y(R 4+R 2+)Σ6+ Z(R 4+3)Σ12+O12 (morimotoite type) and X(R 2+3)Σ6+ Y(R 3+2)Σ6+ Z(R 4+3)Σ12+O12 (andradite type), but not with the arrangement X(R 2+3)Σ6+ Y(R 4+2)Σ8+ Z(R 4+R 3+2)Σ10+O12 (schorlomite type) that can be considered as a minor component; in this regard, note that amount of the aggregate-charge arrangement at Y with total charge +6, Y[0.718(R 4+0.5R 2+0.5) + 0.517(R 3+)] = 1.235 apfu, is larger than the largest amount of charge arrangement characterised solely by the YR 4+-cations (1.124 apfu); as Y(R 4+0.5R 2+0.5)0.718 > Y(R 3+)0.517, the relative dominant charge arrangement is Y(R 4+0.5R 2+0.5); in terms of constituents, it corresponds to Y[Ti4+0.5(Fe2+,Mg2+)0.5], which in accord with the dominant-constituent rule, Y(Fe2+0.204) > Y(Mg2+0.155), leads to Y(Ti4+0.5Fe2+0.5); therefore, the garnet species from Magnet Cove corresponds to morimotoite, even though the most abundant end-member component is schorlomite (Grew et al., Reference Grew, Locock, Mills, Galuskina, Galuskin and Hålenius2013).
It is worth noting that this garnet example showed that the STC at Y (= +6.77) and Z (= +11.25) were very close to +7 and +11, but we selected the integer numbers +6 and +12, respectively, as the choice of charge constraints Y Σ7+ and Z Σ11+ would lead to the formula X(Ca3)Y(TiFe3+)Z(Si2Fe3+)O12 with double occupancy of two sites. As a rule, if the integer number closest to the STC is not consistent with an end-member, another integer number in line with the end-member definition must be selected. The incorrect integer number can be recognised as it results in root-charge and atomic arrangements leading to a formula with double occupancy of two sites or charge imbalance.
The detailed procedure reported above for garnet can be summarised as follows:
(1) The STC at X (+5.99), Y (+6.77) and Z (+11.25) indicate the charge constraints X Σ6+, Y Σ6+ and Z Σ12+.
(2) These constraints lead to the possible root-charge arrangements X(R 2+3)Σ6+ Y(R 4+R 2+)Σ6+ Z(R 4+3)Σ12+O12 and X(R 2+3)Σ6+ Y(R 3+2)Σ6+ Z(R 4+3)Σ12+O12, which are end-members.
(3) As the condition of the charge balance in end-members is satisfied, we can calculate the amounts of root-charge and atomic arrangements in terms of apfu: XR 2+ ≅ Ca, ZR 4+ = Si and as Y(R 4+0.5R 2+0.5)0.718 > Y(R 3+)0.517 leads to Y[Ti4+0.5(Fe2+,Mg2+)0.5] in which Y(Fe2+)0.204 > Y(Mg2+)0.155 leads to the end-member Ca3(TiFe2+)Si3O12.
The dominant-valency rule is able to identify this garnet if the concepts of site total charges and charge constraints, dictated by the mineral composition and the electroneutrality principle, are taken into account. These concepts emphasise the role of the root-charge arrangements compatible with the site populations and the end-member definition. The dominant-constituent rule can hence be applied to the relative dominant root-charge arrangement.
The aforementioned concepts are also reflected in the ternary composition diagrams. Morimotoite/andradite and schorlomite are related by coupled heterovalent substitutions at the Y and Z sites, whereas morimotoite and andradite are related by a coupled heterovalent substitutions at the Y site. Plotting the compositions of the Y site in a triangular diagram with (R 4+)Σ4+, (R 4+0.5+R 2+0.5)Σ3+ and (R 3+)Σ3+ placed at each corner, the boundaries crossing at the centre of the diagram are displaced as follows: 25% for the component (R 4+)Σ4+, 37.5% for both (R 4+0.5+R 2+0.5)Σ3+ and (R 3+)Σ3+ (Fig. 4). This displacement is dictated by the different charge or cation arrangements at the Z site between morimotoite/andradite and schorlomite: Z(Si4+3)Σ12+ and Z(Si4+R 3+2)Σ10+, respectively. As a result, Fig. 4 may be used to discriminate the schorlomite-group minerals from the ‘Ca-garnet-group minerals’ in line with the dominant-valency rule (cf. Fig. 4 of this study with fig. 7 of Grew et al., Reference Grew, Locock, Mills, Galuskina, Galuskin and Hålenius2013) as well as with the current garnet nomenclature (the first criteria for distinguishing the group in garnets are the total charge at the Z site and symmetry). A similar argumentation should be applied to the ternary diagram andradite–morimotoite–menzerite-(Y), where the boundaries between the species are redefined in Fig. 5.
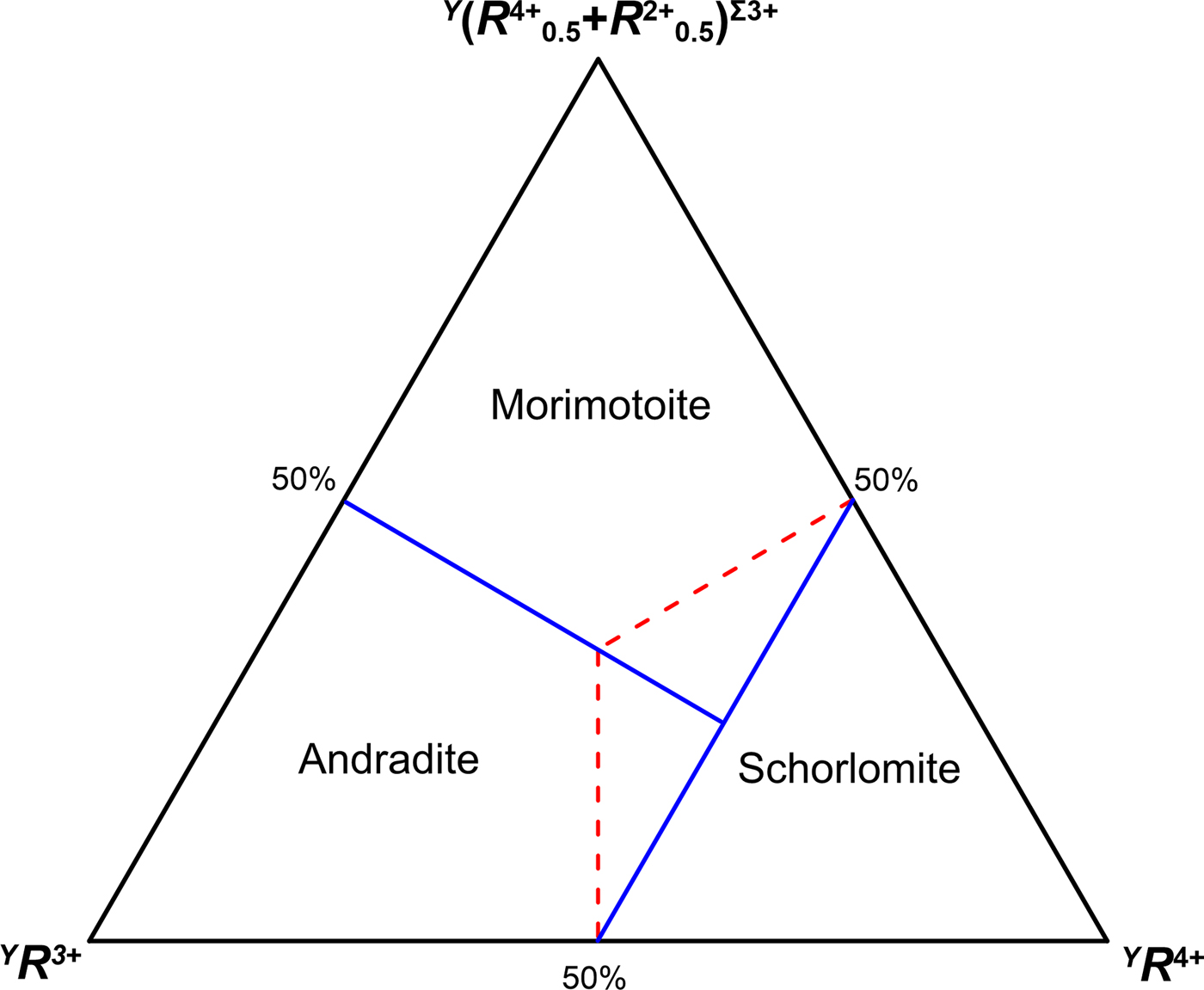
Fig. 4. Diagram for discriminating the species in the schorlomite−andradite−morimotoite system: solid blue lines are consistent with the dominant-valency rule, whereas dashed red lines are consistent with the dominant-end-member approach (cf. with fig. 7 of Grew et al., Reference Grew, Locock, Mills, Galuskina, Galuskin and Hålenius2013).
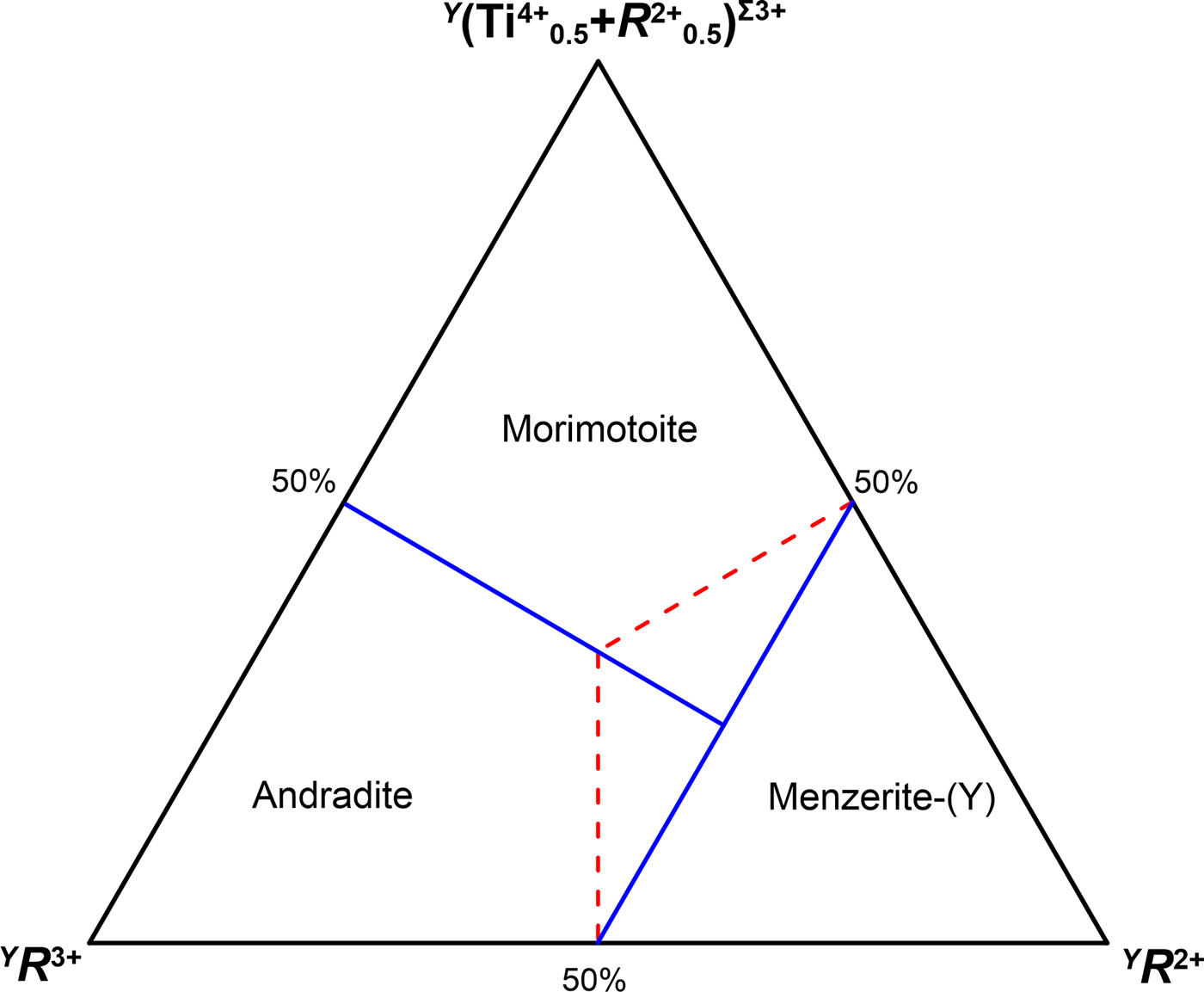
Fig. 5. Diagram for discriminating menzerite-(Y) from andradite and morimotoite: solid blue lines are consistent with the dominant-valency rule, whereas dashed red lines are consistent with the dominant-end-member approach (cf. with fig. 10 of Grew et al., Reference Grew, Locock, Mills, Galuskina, Galuskin and Hålenius2013).
Conclusions
Even if no new nomenclature rules have been introduced in the present paper, the following aspects were discussed with the aim to improve and clarify the application of the dominant-valency rule in mineral nomenclature.
– An end-member formula should always be derived from a mineral composition and it should be consistent with the dominant atomic arrangement of the dominant root-charge arrangement.
– The dominant-valency rule and the dominant-end-member approach may not always converge to the same mineral species.
– The IMA−CNMNC recommends using the dominant-valency rule because it alone may lead to unambiguous mineral identification.
– When the simple application of the dominant-valency rule does not give charge-balanced end-member formulae, it may be useful to use the site-total-charge approach to identify the most appropriated integer number (charge constraint) corresponding to dominant root-charge and atomic arrangements consistent with the end-member formula definition.
The goal of mineral nomenclature should be the identification of the mineral end-member formula (and polymorphic forms). This overriding condition can be achieved by using the dominant-valency rule and/or the site-total-charge approach. Consequently, both procedures should be considered complementary to each other for mineral identification. We cannot exclude the possibility that these procedures will not apply to some mineral compositions, but they may provide the basis to improve our future understanding of mineral systems. The direct consequences of the modifications, here recommended, are a redefinition of the boundaries between garnet species andradite, morimotoite, schorlomite, and menzerite-(Y). As stated by Hatert and Burke (Reference Hatert and Burke2008), these guidelines do not apply automatically to existing mineral species, and any modification of the current nomenclature should be submitted to the IMA−CNMNC for approval.
Acknowledgements
We thank E. Grew, C. Chopin and A. Ertl for their constructive comments on an early version of the manuscript and two anonymous reviewers for their helpful comments. We are sincerely grateful to the IMA−CNMNC members for their suggestions on the proposal and to B. Birch for efficiently handling the whole procedure.










