Introduction
As part of its effort to eliminate the risks of poliomyelitis disease, the Global Polio Eradication Initiative (GPEI) began a phased process of cessation of oral poliovirus vaccine (OPV) use, starting with the removal of serotype 2 OPV in April–May 2016 [Reference Hampton1]. The GPEI Strategic Plan 2013–2018 [2], which the GPEI extended to 2019 [3], calls for coordinated cessation of all OPV serotypes following the global certification of elimination of the remaining two wild poliovirus (WPV) serotypes (i.e. 1 and 3). By reducing exposure to OPV, the risk of vaccine-associated paralytic poliomyelitis (VAPP) decreases, which occurs in a small fraction of OPV recipients and their close contacts [Reference Duintjer Tebbens4]. Successfully ending OPV use also limits the possibility of creating future circulating vaccine-derived polioviruses (cVDPVs) that can behave like WPVs in settings with low-population immunity to transmission [Reference Duintjer Tebbens4–Reference Thompson and Duintjer Tebbens6]. Finally, stopping OPV prevents new live poliovirus infections in the small number of individuals with some B-cell-related primary immunodeficiency diseases (PIDs) who do not clear poliovirus infections or take significantly longer to clear poliovirus infections compared to individuals with competent immune systems, which we refer to as immunodeficiency-associated long-term vaccine-derived poliovirus (iVDPV) excreters. [Reference Duintjer Tebbens, Pallansch and Thompson7]. While immunocompetent individuals infected with poliovirus excrete on average for around 30 days but no longer than 3 months [Reference Duintjer Tebbens5, Reference Duintjer Tebbens8–Reference Alexander, Gary and Pallansch10], iVDPV excreters can shed virus for variable and substantially longer periods of time [Reference MacCallum11–Reference Kew15]. Consequently, individual iVDPV excreters may increase the risks of re-introducing and re-starting live poliovirus transmission in populations, which becomes more recognisable after other transmission of live polioviruses stop [Reference Duintjer Tebbens4, Reference Duintjer Tebbens, Pallansch and Thompson7, Reference Duintjer Tebbens16, Reference Duintjer Tebbens and Thompson17].
While OPV cessation of any serotype leads to the direct and immediate result of discontinuation of the incidence of recipient VAPP cases of that serotype in immunocompetent individuals, the evolution of OPV-related strains circulating in low-immunity populations can take months until they become apparent as cVDPVs [Reference Thompson and Duintjer Tebbens6]. In addition, individuals in a population can become exposed to OPV excreted by vaccine recipients, and in rare instances develop contact VAPP if not already protected by prior exposure or vaccination [Reference Duintjer Tebbens4]. Individuals infected with polio can present clinically with VAPP at any time during their infections. Thus, during the time that they remain infected, iVDPV excreters can develop VAPP (iVAPP).
Several recent reviews provided information about individual iVDPV excreters [Reference Guo18–Reference Shaghaghi20]. These reviews provide details about the individual cases that they included, however, some inconsistencies exist between the reviews (e.g. inclusion or exclusion of some individuals in some reviews but not others), and in many instances key data gaps remain (e.g. missing information about the nature of the immunodeficiency, which affects all reviews). With the increased use of poliovirus environmental surveillance, the detection of virus likely excreted by iVDPV excreters into catchment areas covered by environmental surveillance also creates a category of potential non-identified iVDPV excreters [Reference Duintjer Tebbens4, Reference Duintjer Tebbens, Pallansch and Thompson7, Reference Burns21–Reference Roivainen27].
A prior stochastic, discrete-event simulation (DES) model provided estimates of the global prevalence of long-term iVDPV excreters based on the best available evidence as of 2014 [Reference Duintjer Tebbens, Hampton and Thompson28]. This model supported a global integrated analysis that suggested iVDPV excreters may present a significant risk in the polio endgame, along with any OPV (un)intentionally remaining in the field or inadequately contained in laboratories and vaccine production facilities [Reference Dowdle29]. In the post-OPV cessation era, inactivated poliovirus vaccine (IPV) remains the only option for immunisation against polio [Reference Thompson and Duintjer Tebbens30]. However, while vaccination with IPV protects vaccine recipients from paralysis if they later become infected by a live poliovirus, IPV does not prevent or stop transmission in most populations (i.e. those characterised by predominantly faecal–oral transmission) [Reference Duintjer Tebbens8, Reference Duintjer Tebbens9]. For example, serotype 1 WPV (WPV1) circulated successfully in Israel for a year despite its very high coverage with IPV in routine immunisation [Reference Anis31, Reference Kalkowska32]. With the cessation of OPV use, population immunity to poliovirus transmission continues to decline as more time passes since OPV cessation, which further exacerbates the inability of IPV to provide sufficient population immunity to prevent or stop potential transmission [Reference Duintjer Tebbens, Pallansch and Thompson7, Reference Duintjer Tebbens, Hampton and Thompson28, Reference Duintjer Tebbens, Hampton and Thompson33, Reference Duintjer Tebbens, Hampton and Thompson34].
The GPEI partners recognised the risk posed by long-term iVDPV excreters and the limited tools available to prevent or to stop future iVDPV-associated outbreaks, which motivated research and development of polio antiviral drugs (PAVDs) [35]. Application of the prior DES model considering different assumptions about patient screening and potential PAVD availability and effectiveness helped to support the economic case for the development of PAVDs and PID screening [Reference Duintjer Tebbens, Pallansch and Thompson7, Reference Duintjer Tebbens16, Reference Duintjer Tebbens and Thompson17]. To date, the first drug (pocapavir) performed well in clinical studies (phases 1 and 2) with respect to safety and reduction of viral excretion [Reference McKinlay36, Reference Collett37]. However, the use of pocapavir also led to the emergence of drug-resistant strains in the study site setting, and a second PAVD that works by a different mechanism is being developed for use in combination with pocapavir. This updated assessment of future iVDPV risks considers the accumulation of new evidence about the prevalence of individuals with specific B-cell related PIDs relevant to prolonged and chronic poliovirus infections, survival rates, and treatment with intravenous immunoglobulin (IVIG) therapy, as well as increased screening of PID patients for long-term iVDPV excretion and delays in the expected availability of PAVDs.
Methods
Synthesis of evidence related to PID patients and iVDPV excretion
The global PID prevalence remains highly uncertain and dynamic due to the large number of PID conditions, differences in case definitions, increased detection and variability between countries in genetic profiles and survival rates of PID patients [Reference Halsey38–Reference Mohammadinejad40]. Some information on PID occurrence comes from surveys among physicians who participate in the Jeffrey Modell Foundation Network (JMFN), a network created and maintained by a private foundation that focuses on identifying and getting treatment for individuals with PIDs (see Table 1) [Reference Modell41–Reference Modell44]. Based on the surveys performed this decade, the estimated number of PID patients increased from ~60 000 PID patients in 2011 to ~94 000 PID patients in 2018 [Reference Modell41–Reference Modell44]. However, the inconsistent and incomplete nature of the data collected from these surveys makes it difficult to estimate the actual rate of increase in the number of PID patients (e.g. the number of countries covered increased, and the numbers of physicians and/or centres in the network and the size of survey response changed over time (see Table 1) [Reference Modell41–Reference Modell44]). Nonetheless, these data provide the only, albeit limited, evidence relevant to the estimation of the number of PIDs globally. Although the JMFN surveys report PID prevalence stratified by different criteria and type of PID, we focus on the subset of PID defects for which known long-term poliovirus excreters exist(ed) (i.e. common variable immunodeficiency disease (CVID) and other PIDs with B-cell involvement relevant to long-term poliovirus excretion (oPIDs), including but not limited to severe combined immunodeficiency disease (SCID), hypogammaglobulinemia (HGG), major histocompatibility complex (MHC) Class II and X-linked agammaglobulinemia (XLA)). Using this subset, we fit the model to produce global CVID and oPID prevalence estimates consistent with the known CVID and oPID prevalence, which we assume provides a reasonable basis for further estimating the prevalence of long-term iVDPV excreters.
Table 1. JMFN surveys and data on patients with PIDs over time [Reference Modell41–Reference Modell44]
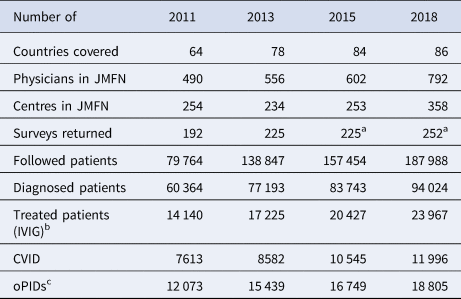
CVID, common variable immune deficiency; JMFN, Jeffrey Modell Foundation Network; IVIG, intravenous immunoglobulin; oPID, other PIDs; PID, primary immune deficiency.
a Number of returned surveys in 2015 and 2018 estimated based on description.
b Treatment includes delivery of IVIG therapy and any other appropriate clinical services, note that treatment does not effectively stop existing excretion.
c oPID defects for which known long-term poliovirus excreters exist(ed), estimated as 20% of all diagnosed patients.
We maintain a database of iVDPV excreters (first discussed in a 2006 paper [Reference Duintjer Tebbens4] and later updated in 2015 [Reference Duintjer Tebbens, Pallansch and Thompson7], but not published in 2015 as an individual line listing). With respect to known individual iVDPV excreters, we updated our database using information obtained from the published literature and personal communications. We added fields to our database to support a cross comparison of the evidence available from different sources. We use prior assessments of iVDPV risks that characterise excreters as chronic if their excretion exceeded 5 years, and prolonged for those excreting at least 6 months but less than 5 years [Reference Duintjer Tebbens4]. The most recent published registry of long-term excreters known to the World Health Organisation (WHO) consists of 101 individuals (94 prolonged and 7 chronic) identified between 1962 and 2016, 21 (21%) diagnosed with CVID and the remainder diagnosed with oPIDs [Reference Macklin19]. In that review, 7 of the 21 (33%) long-term excreters with CVID and none of the long-term excreters with oPIDs met the criterion as chronic excreters [Reference Macklin19]. Since the time of publication of that review [Reference Macklin19], multiple new long-term excreters have been described [Reference Jorba45, Reference Jorba46]. A 2018 systematic review [Reference Shaghaghi20] reported 107 individual iVDPV excreters, 93 of which we matched to entries in the WHO review [Reference Macklin19]. We also found eight iVDPV excreters in the WHO review [Reference Macklin19] missing from the 2018 systematic review [Reference Shaghaghi20], and 14 new iVDPV excreters identified by the 2018 systematic review [Reference Shaghaghi20] not in the WHO review (some that occurred after its publication) [Reference Macklin19]. We included information provided through personal communications with the WHO, which continues to update its registry, and with individuals who reported on specific cases in the literature. We also verified that all of the information that we reported for USA cases in our database contained correct information according to Centers for Disease Control and Prevention (CDC) archives. Our database also includes a list of environmental isolations of ambiguous VDPVs (aVDPVs) that suggest the presence of prolonged and chronic excretion by unidentified iVDPV excreters. The data we present here reflect the information in our database as of 1 May 2019.
Modelling
The previously-developed DES model tracks how CVID and oPID patients move through various clinical and OPV infection stages using a discrete time step of 1 month to estimate long-term poliovirus excreter prevalence over time following each modelled individual for life [Reference Duintjer Tebbens, Pallansch and Thompson7]. The model accounts for different characteristics of transmission and vaccine schedules by stratifying the global population into blocks. We updated the approach that we use [Reference Duintjer Tebbens16] to stratify the world into 72 epidemiological blocks of 107 million people each (corresponding to the global population of 7.2 billion as of 2018). We updated the characterisation of the blocks to use the 2018 World Bank income level (i.e. low-income (LI), lower middle-income (LMI), upper middle-income (UMI), high-income (HI)) [47] and polio vaccine use (i.e. use of OPV, role of IPV). For each block, based on prior modelling experience we assumed a basic reproduction number (R 0) to account for many factors that affect poliovirus transmission and the health system quality [Reference Duintjer Tebbens, Pallansch and Thompson7]. We report the R 0 for WPV1 and then the model applies fixed relative ratios to compute the R 0 for serotype 2 WPV (WPV2) and serotype 3 WPV (WPV3) relative to the R 0 for WPV1 [Reference Duintjer Tebbens, Pallansch and Thompson7]. Table 2 presents the updated inputs for the DES model describing births and attributes assigned at birth, deaths and monthly event probabilities and related relative probabilities (i.e. CVID or oPID onset, diagnosis, OPV infections and iVAPP). With increasing adoption of medical technology globally [Reference Nandakumar48], we updated our assumptions about the delay in PID diagnosis probabilities by the income level for both CVIDs and oPIDs. Similarly, in view of improvements in medicine and changing standards of care over time, we updated our assumptions about patient survival and access to treatment. Specifically, considering a new study that suggested a higher rate of CVID patient survival [Reference Gathmann49] compared to the earlier estimates [Reference Chapel50], we assumed a higher proportion of surviving CVID and oPID patients relative to their year of birth (i.e. improvement over time). Figure 1 shows the updated assumed baseline survival curves for effectively-treated (with IVIG) CVID and oPID patients over time. As in the prior model [Reference Duintjer Tebbens, Pallansch and Thompson7], we assume lower treatment fractions in lower income levels, but we assume an increase in treatment fractions over time, with higher projected values compared to our previous estimates. Figure 2 shows the updated assumed treatment fraction as a function of time for each income level.
Table 2. Inputs for the updated DES model of long-term poliovirus excreter prevalence [Reference Duintjer Tebbens, Pallansch and Thompson7]
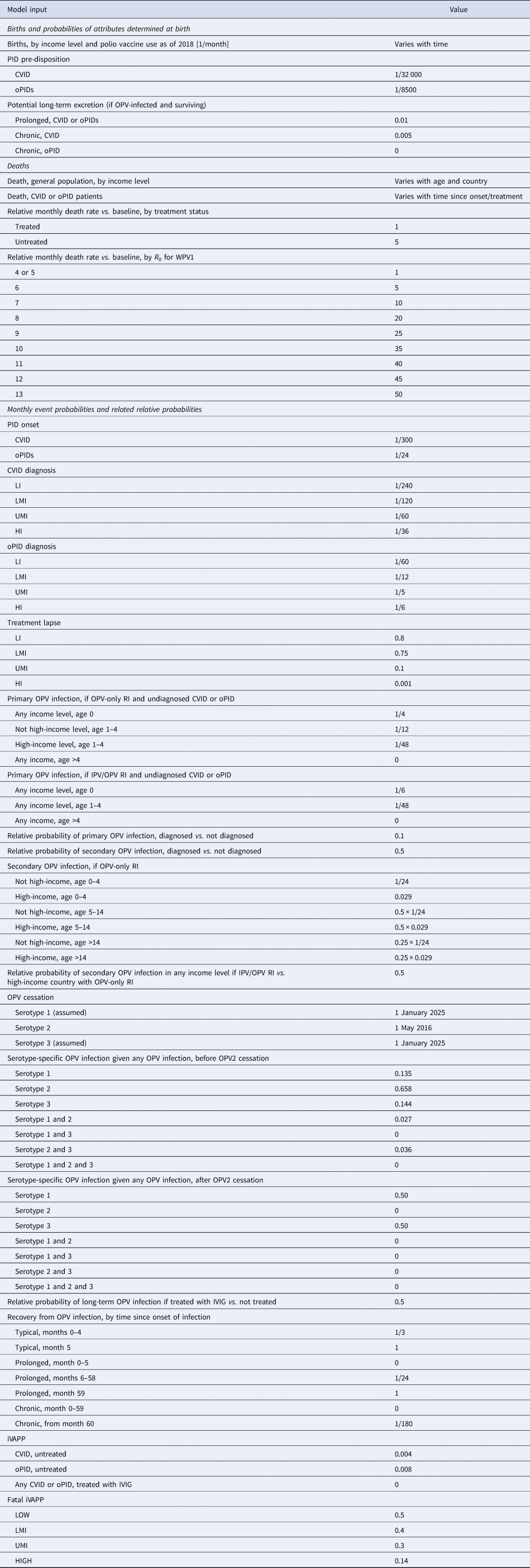
CVID, common variable immune deficiency; HI, high-income; IPV, inactivated poliovirus vaccine; iVDPV, immunodeficiency-related vaccine-derived poliovirus; LI, low-income countries; LMI, lower middle-income; OPV, oral poliovirus vaccine; OPV2, serotype-2-containing OPV; oPID, other PID with B-cell involvement relevant to long-term poliovirus excretion; PID, primary immune deficiency; R 0, average annual basic reproduction number; RI, routine immunisation; UMI, upper middle-income; iVAPP, vaccine-associated paralytic polio in immunodeficient individuals.
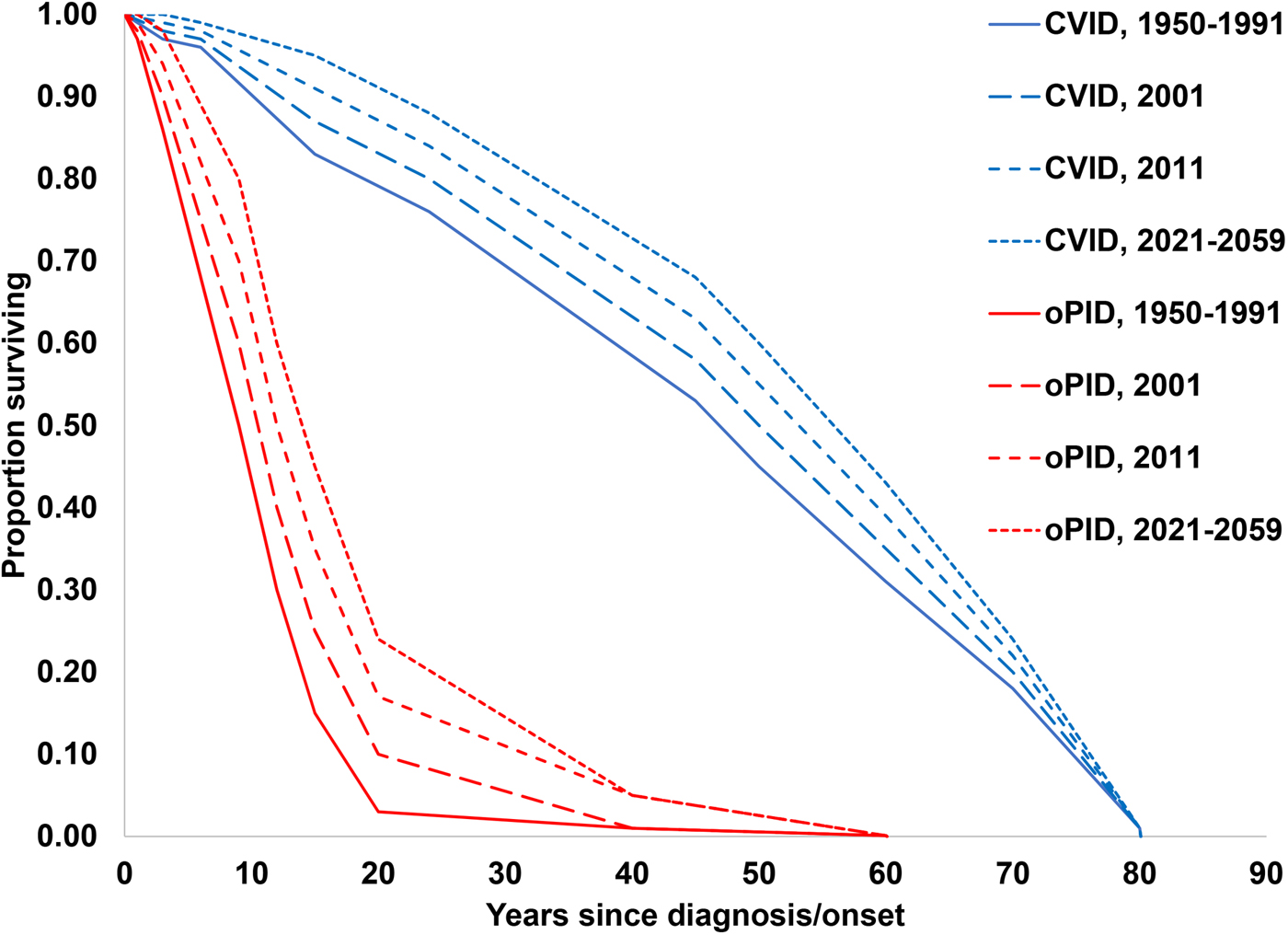
Fig. 1. Assumed baseline survival curves for CVID and oPID patients effectively-treated (with IVIG) in a population with R 0 values for WPV1 of 4 or 5.
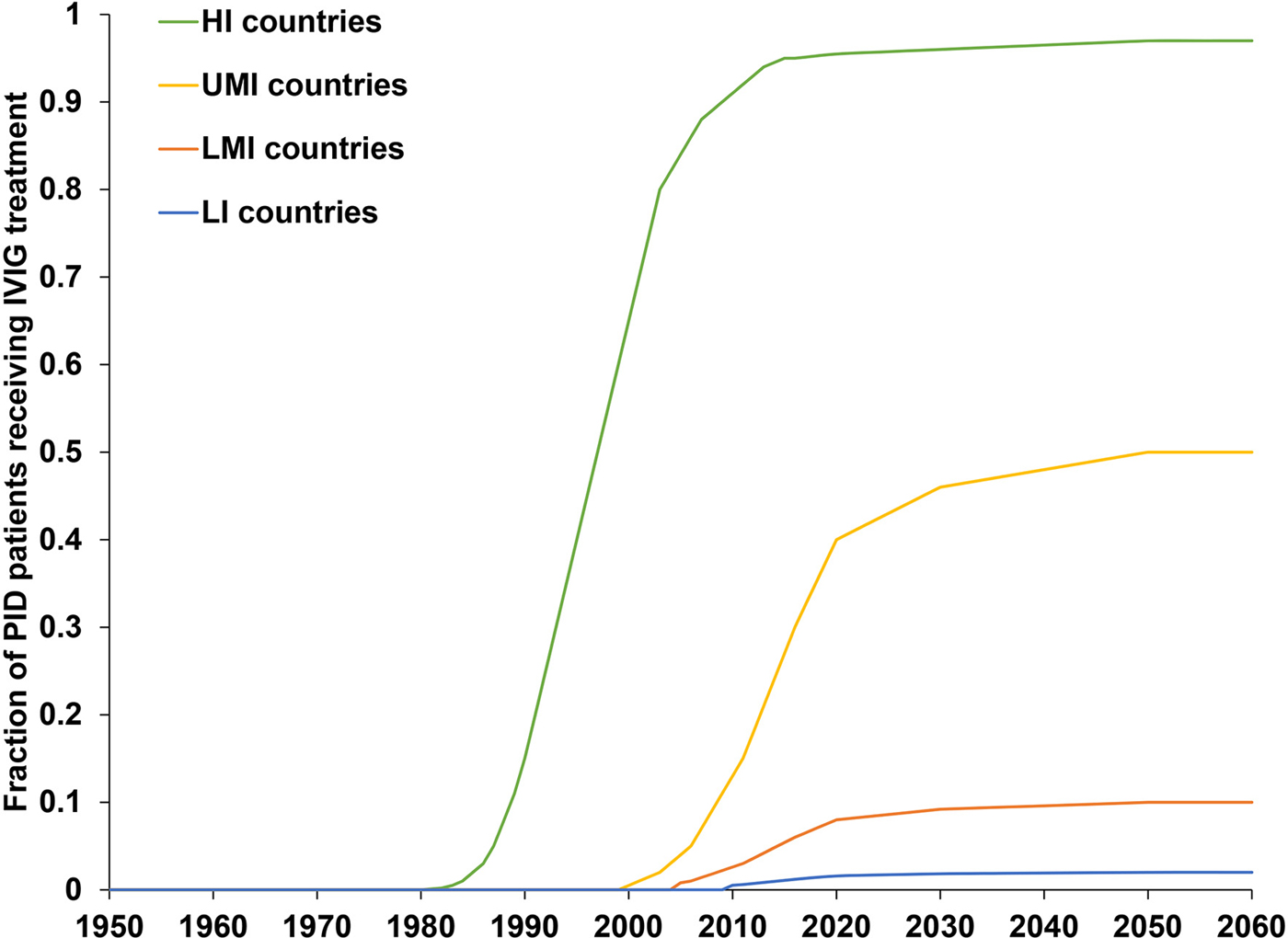
Fig. 2. Assumed fractions of CVID and oPID patients treated with IVIG as a function of time, by income level.
To run the model, we generate the number of births over time on a monthly basis for each block, based on demographic data [Reference Duintjer Tebbens, Pallansch and Thompson7]. We generate the expected number of newborns with a genetic PID predisposition relevant to polio long-term excretion (i.e. a future CVID or oPID) in each month using a random draw from a Poisson distribution with a rate equal to the number of birth times the fraction of births with PIDs. We implicitly assume that the inputs for pre-disposition for CVID and oPID average over any variability that exists in the rate of B-cell immunodeficiencies that result from consanguineous marriage and other risk factors and that these risk factors do not change with time. For each generated CVID or oPID pre-disposed individual at birth, we randomly determine whether he or she will become a long-term excreter after the onset of clinical symptoms if infected with a live poliovirus. On a monthly basis, for each such individual we check whether death occurs prior to clinical PID onset according to age-specific general population death rates for each income level [Reference Duintjer Tebbens, Pallansch and Thompson7]. Once clinical CVID or oPID onset occurs, we assume different monthly probabilities of death according to the CVID or oPID survival curves shown in Figure 1, IVIG treatment status (with the possibility of treatment lapse) and block-specific R 0 values for WPV1. For all surviving CVID or oPID patients, we perform a monthly check to see whether clinical onset of the PID occurs in the model, and if it occurs, we introduce treatment (according to the probabilities of diagnosis and treatment) and apply a monthly probability of OPV infection depending on poliovirus vaccine use, age, diagnosis status, IVIG treatment status and serotype. We randomly and independently sample the serotypes (with a small possibility of two concurrent serotypes) of primary OPV infection, whereas for secondary OPV infection we randomly sample only one serotype. Finally, we determine the monthly progression of OPV infection depending on the long-term poliovirus excreter status determined at birth, and we apply a monthly probability of developing iVAPP while OPV infected, with an income-level-dependent probability that the iVAPP will lead to death. To characterise the updated global iVDPV prevalence behaviour we run 1000 stochastic iterations of the DES model and then aggregate the results.
Results
Table 3 lists our updated database of global iVDPV excreters and isolations of suspected iVDPVs (or aVDPVs given their unknown source) suggesting prolonged excretion detected to date and cross-referenced between multiple sources and studies [Reference Macklin19, Reference Shaghaghi20, Reference Jorba45, Reference Jorba46]. Table 3 includes 143 individuals (120 prolonged, 7 chronic, 15 <6 months and 1 unknown) identified between 1962 and 2018, of which 22 (15%) presented with CVID, while 123 (85%) presented with oPIDs. In the current data, 7 of the 22 individuals (32%) with CVID and none of the 123 individuals with oPIDs met the criteria for chronic excretion. In the column showing the estimated VP1 divergence, we note the individuals for which the estimated duration of iVDPV excretion calculated based on the maximum VP1 divergence exceeds the age of the individual. These cases highlight some of the challenges arising from the use of imperfect information about dose history, inference about the duration of infection from genetic data and the reality that individuals can become infected by viruses in the community. Although we attempted to verify all of the US cases, we could not locate some of the epidemiological and/or virology historical records, and consequently, we most likely missed a small number of iVDPV excreters prior to 2000 (i.e. before recognition of VDPVs). Notably, the definitions of what qualified as a VDPV changed over time for VDPV2, and this impacted the recognition of iVDPV cases.
Table 3. Documented iVDPV excreters and isolations of aVDPVs suggesting prolonged excretion for 1962–2018 (as reported by mid-2019)
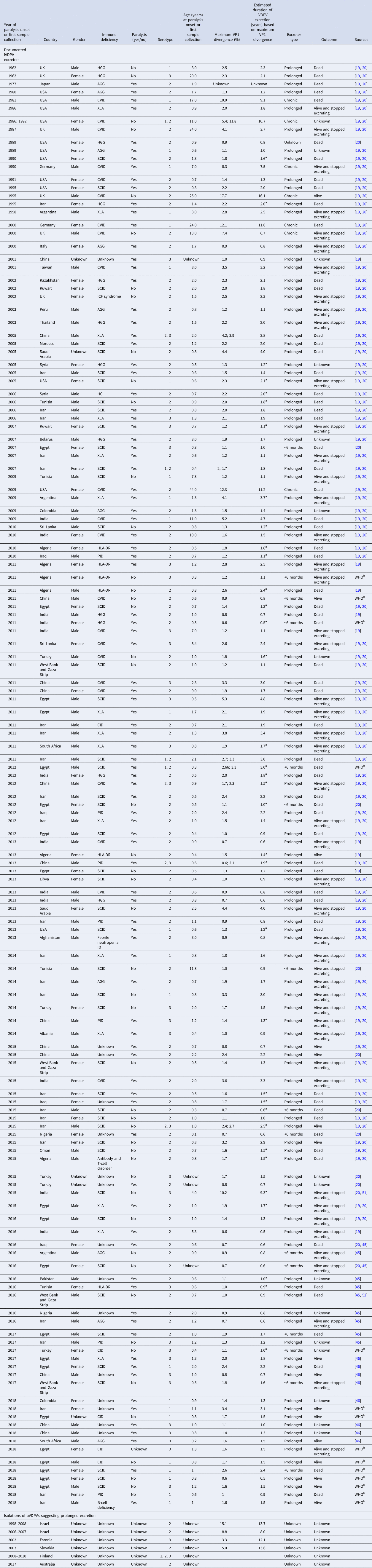
AGG, agammaglobulinemia; iVDPV, immunodeficiency-related vaccine-derived poliovirus; CID, combined immune deficiency; CVID, common variable immunodeficiency disease; ID, immunodeficiency disease; HGG, hypogammaglobulinemia; HLA, human leukocyte antigen; ICF, immunodeficiency, centromeric region instability, facial anomalies; PID, primary immunodeficiency diseases; SCID, severe combined immunodeficiency disease; XLA, X-linked agammaglobulinemia; VP1, viral protein 1; WHO, World health Organisation.
a Estimated duration of iVDPV excretion based on maximum VP1 divergence exceeds the age of patient.
b WHO, personal communication.
Table 4 presents model estimates of global diagnosed CVID and oPID prevalence in January 2011, 2013, 2015 and 2018, which increased from 8326 and 13 660 to 10 494 and 18 868, respectively. These results fall within ~1600 patients compared to the number of patients suggested by the JMFN surveys (which increased from 7613 and 12 073 to 11 996 and 18 805, respectively, as shown in Table 1). For 2011–2018, the model estimates the incidence of 63 iVAPP cases and six chronic excreters compared to 56 known iVAPP cases, one chronic excreter and multiple environmental aVDPV isolates that suggest at least three possible chronic excreters in this period of time (Table 3). We note that delays in reporting of iVDPV cases may lead to higher incidence for the 2011–2018 period at a later point in time, particularly for the more recent years (e.g. our previous study [Reference Duintjer Tebbens, Pallansch and Thompson7] reported 26 iVAPP cases for 2009–2013 known at the time of its publication, but this update reports 34 iVAPP cases for that same period). Overall, we observe an increase in the estimated prevalence over time (compared to our previous study [Reference Duintjer Tebbens, Pallansch and Thompson7]), which we largely attribute to updates in our assumptions about survival and later timing for when some of the 72 blocks stopped or will stop using OPV.
Table 4. Diagnosed CVID and oPID prevalence in the model
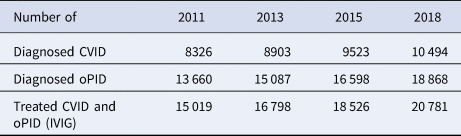
CVID, common variable immune deficiency; IVIG, intravenous immunoglobulin; oPID, other PID with B-cell involvement relevant to long-term poliovirus excretion; PID, primary immune deficiency.
Figure 3 shows the baseline prevalence (i.e. without PAVDs) of long-term iVDPV excreters. Figure 3a shows the prevalence by the income level, which suggests that middle-income countries account for most of the current long-term excreters, while the prevalence in high-income countries continues to decline steadily since these countries stopped all OPV use. Figure 3b shows the impact of stopping serotype 2 containing OPV use (i.e. OPV2 cessation) and assumptions about serotype-specific infections (i.e. a shift to first-infections of individuals with serotypes 1 and 3). Notably, Figure 3b suggests a sharp drop in long-term serotype 2 excreters, but a simultaneous rise in serotype 1 and serotype 3 excreters, caused by the new assumed distribution between serotypes 1 and 3 for the probability of the first OPV infection. Chronic excreters amount to 4–16% of all long-term excreters, most of them residing in HI countries. After the expected global cessation of all OPV use, the prevalence of prolonged excreters drops quickly, while a few chronic excreters continue to exist for over a decade (see Fig. 3c). Only up to 11% of long-term excreters present with iVAPP, while the remainder either recover or die before the paralysis occurs (see Fig. 3d).
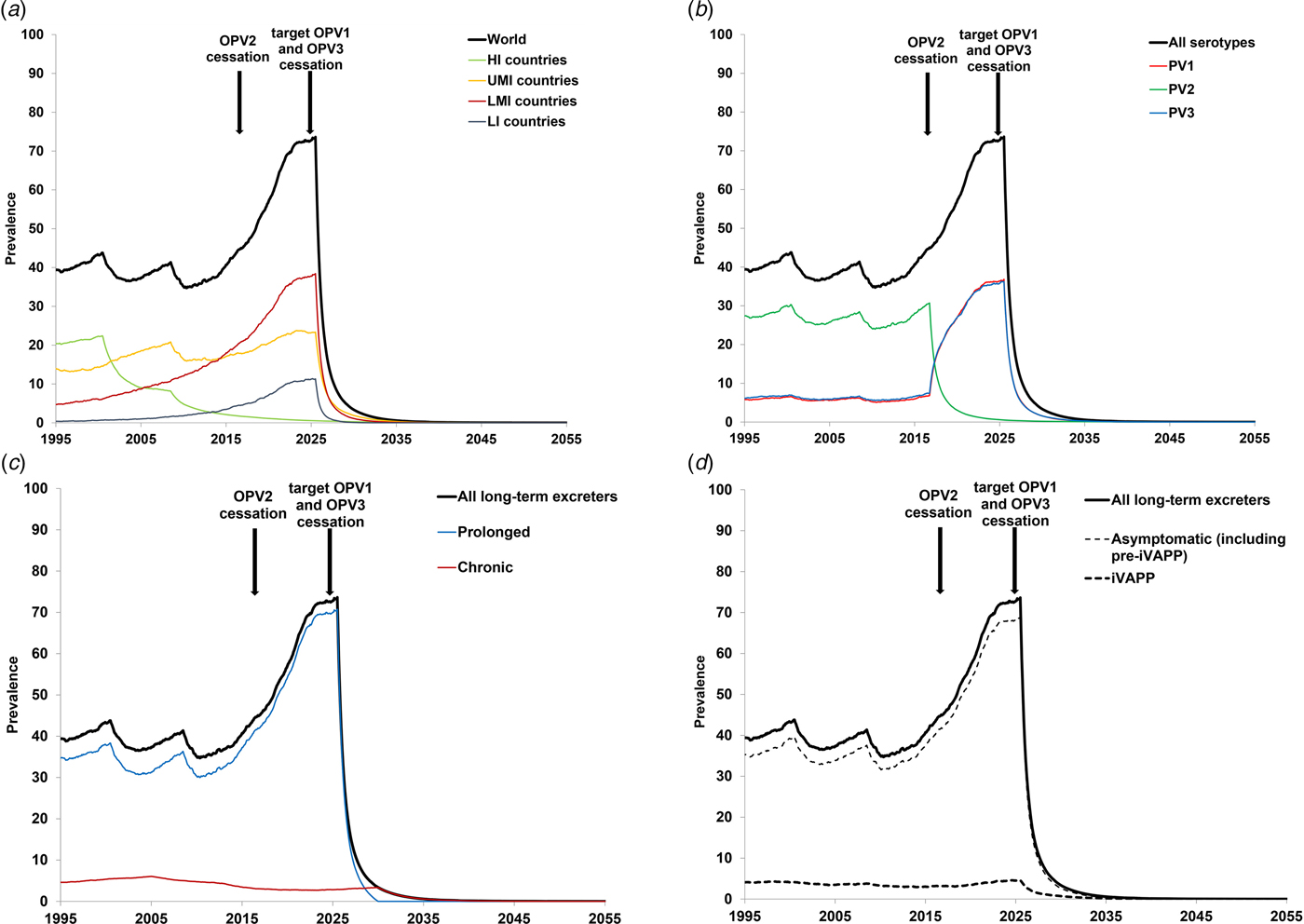
Fig. 3. Prevalence of long-term iVDPV excreters in the absence of PAVD use, based on the monthly averages of 1000 iterations of the DES model.
Discussion
With the GPEI already beginning its transition of activities to countries and other decentralised components of its partner organisations, developing plans for long-term risk management requires attention. Despite the continued circulation of WPV1 in Pakistan and Afghanistan[53], the GPEI aspiration to stop all OPV use and successfully maintain a polio-free world continues to require a longer time horizon and additional resources. This updated analysis of iVDPV risk, despite considerable uncertainty, confirms prior findings [Reference Duintjer Tebbens4, Reference Duintjer Tebbens, Pallansch and Thompson7] that iVDPVs may pose risks that require management after global OPV cessation. Although PID patient screening generally improves with time [Reference Modell41–Reference Modell44], the actual prevalence and the proportion of PID patients who may develop prolonged or chronic excretion (i.e. CVID and oPIDs) and the extent to which health systems will identify and treat these patients remains highly uncertain [Reference Duintjer Tebbens, Pallansch and Thompson7, Reference Duintjer Tebbens and Thompson17]. Our estimates of current long-term excreter prevalence remain higher than reported, as expected given the lack of a comprehensive surveillance system, although the gap should continue to decrease as health systems increasingly expand their capacity to find individuals with PIDs.
Our model remains limited to the assumptions we make based on the insufficient data and high uncertainty around characteristics of PID patients and their relation to iVDPV excretion. Uncertainty about the transmissibility and neurovirulence of viruses re-introduced into populations from iVDPV excreters leads to significant uncertainty about the potential value of developing PAVDs [Reference Duintjer Tebbens and Thompson17]. We combine all non-CVID defects into one homogeneous oPID category despite the differences that exist between them, but as research reveals more evidence about PIDs, this may prove overly simplistic. Insufficient information about their survival and probability of long-term infection (given OPV exposure) led us to a conservative approach of assuming equal probability of becoming prolonged excreter for all oPID patients, which may overestimate prolonged excretion in the model.
The results of our model strongly depend on the assumption and timing of successful cessation of the remaining serotypes. Cessation of all OPV will eventually stop the creation of new long-term and chronic excreters, however it will take time for the existing chronic excreters to clear their infections or die. In the context of discussions related to potentially stopping serotype 3 OPV use (i.e. OPV3 cessation) before stopping serotype 1 OPV (OPV1), the results in Figure 3 would change with the die-out of serotype 3, the shift of all iVDPV risk to serotype 1, and the timing of OPV1 cessation. In Figures 3a, c and d, changing the time of the last OPV cessation will change the timing of the dramatic drop to shortly after the end of the last OPV use (i.e. OPV1 cessation), with the totals expected to continue to increase with time until cessation due to increases in population, PID diagnosis, treatment and survival. In Figure 3b, the blue curve would drop shortly after OPV3 cessation and the red curve would jump up to the level of the black curve, because then all first-infections with OPV would occur with OPV1 (based on our explicit assumption of no inherently lower likelihood of iVDPV from OPV1). In our model, the total (‘all serotypes’) would continue to increase until the last OPV cessation. In addition to these possibilities, OPV2 cessation did not go as smoothly as hoped, and the use of monovalent OPV2 continues to date (as of August 2019) in response to cVDPV2 outbreaks[Reference Duintjer Tebbens and Thompson54]. This means that the model should include the possibility of creating some new iVDPV2 excreters in the areas of serotype 2 outbreaks. In the event of needing to restart OPV2 use globally[Reference Duintjer Tebbens and Thompson54], [Reference Thompson and Kalkowska55], the risks of iVDPV excreters will change due to the exposure of CVID and oPID individuals again potentially becoming first-infected with OPV2. We emphasise that the risks from iVDPV excreters only become recognisable once OPV cessation occurs, and while countries use OPV, the risks from VAPP and cVDPV outbreaks dominate.
In the absence of tools for treating iVPDV excreters, performing surveillance to identify them becomes challenging. However, once PAVDs become available, developing strategies to cost-effectively identify and treat any long-term iVDPV excreters remaining after OPV cessation will help to prevent iVAPP in these individuals and to reduce or eliminate them as a potential source for re-introduction of poliovirus in a world with significantly lowered and decreasing levels of population immunity to poliovirus transmission.
Acknowledgements
The first and last authors thank the Bill and Melinda Gates Foundation for supporting the completion of this work [OPP1129391]. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services. We thank Grace Macklin, Ondrej Mach, Madhu Mohanty, Mohammadreza Shaghaghi and Roland Sutter for help in completing our iVDPV database, and John Modlin for helpful comments.
Conflict of interest
None.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.









