Introduction
Turmeric (Curcuma longa L.) is one of the important spice and medicinal crop, valued for its diverse uses. It is a rhizomatous herbaceous perennial native to South Asia (Ravindran et al., Reference Ravindran, Nirmal Babu, Shiva, Ravindran, Nirmal Babu and Sivaraman2007) but cultivated and used since time immemorial as a flavouring and colouring agent in Asian households. The crop was grown under area of 0.349 million hectares in India with a production and export of 1.33 and 0.15 million tonnes, respectively (Spices Board, 2023). The powder of turmeric rhizome contains 2–8% curcumin, which is the main biologically active phytochemical compound (Prasath et al., Reference Prasath, Kandiannan, Leela, Aarthi and Sasikumar2019).
The colour of food products has been employed as an indirect measure to assess pigment concentration, as it is simpler, faster, and correlates well with physicochemical metrics (Pathare et al., Reference Pathare, Opara and Al-Said2013). Artificial food colours are used frequently because they are highly stable and inexpensive. Artificial colours have been linked to a wide range of adverse effects, some of which have been shown to be unsafe to humans (Dey and Nagababu, Reference Dey and Nagababu2022). Due to growing health awareness, natural colours are currently more in demand than ever before. According to Bijauliya et al., (Reference Bijauliya, Alok, Kumar, Chanchal and Yadav2017), natural colours are derived from plants, insects, animals, minerals, etc. Applications for natural colours in the food, cosmetics, confectionery, and textile sectors are numerous and varied (Bijauliya et al., Reference Bijauliya, Alok, Kumar, Chanchal and Yadav2017; Dey and Nagababu, Reference Dey and Nagababu2022; Kaur and Chopra, Reference Kaur and Chopra2023). Turmeric is increasingly in demand due to its numerous health benefits, as well as the rise in the market for natural colours and organic supplements. This opens an approach for identifying turmeric genotypes with a range of colour shades and consistent yields.
The colour of a food products often reflects direct correlation to pigment content, serving as an indicator of its quality (Francis, Reference Francis1995). Colour had played a vital role for visual appearance, nutritional composition, judge maturation, preservation or storage (Stewart and Wheaton, Reference Stewart and Wheaton1971; Little, Reference Little1975; Campbell et al., Reference Campbell, Huber and Koch1989; Carreno et al., Reference Carreno, Martinez, Almela and Fernández-López1995). Several colour coordinate systems were validated for the describing colour of an object (Francis, Reference Francis1980; Hunter and Harold, Reference Hunter and Harold1987; Minolta Reference Minolta1994). Among colour spaces RGB (red, green and blue) being the widely used and accepted among the popular systems. The food industry often uses colour scales like Hunter Lab's L*, A*, B* and the CIELAB system to measure colour. In CIELAB, you read the L*, A*, and B* values directly. L* is defined as the lightness index, with higher values being lighter and lower values being darker. The A* value indicates redness if positive and greenness if negative, while the B* value shows yellowness if positive and blueness if negative. Product-specific colour indices were used by several researchers in crops viz., apple, tomato, citrus, carambola using these L*, A* and B* in order to establish a mechanism for understanding the association of the colour traits with maturation and preservation of fruits by Carreno et al., (Reference Carreno, Martinez, Almela and Fernández-López1995). A*/B* ratio was also used as colour index along with A* value in mango and banana (Medlicott et al., Reference Medlicott, Semple, Thompson, Blackbourne and Thompson1992). This ratio was used for the correlation of the maturation of fruits with peel colour.
The quality of turmeric is measured based on colour and curcuminoids content. However, the quality is influenced by temperature, humidity, light intensity and duration of the light and altitude (Sandeep et al., Reference Sandeep, Sahoo, Singh, Sahoo, Nayak and Kar2021). In addition to yield and quality, the genotype of turmeric with rich colour and higher levels of curcuminoids is a desirable characteristic for developing a variety. However, there are only a few publications on the genetic diversity in colour traits and the correlation between the colour of turmeric powder and curcuminoids, and its interactions with the environment make this study significant. Mostly multi-environment trials are conducted to study GEI effects. Since genotypes respond differently in various environments, stability of colour and curcuminoids are major concerns for turmeric growers worldwide. The interaction of environment on the essential oil content and curcuminoids of turmeric was studied by Aarthi et al., (Reference Aarthi, Suresh, Leela and Prasath2020). To address this problem, selecting superior genotypes with optimal redness index and curcuminoid content under different production environments is crucial. Therefore, understanding the influence of genotype, environment, and GEI will be highly beneficial for turmeric breeders in selecting genotypes with a stable redness index and curcuminoids. Additionally, we can rank the environments and genotypes for the redness index using the GGE biplot model. In the present study, our main emphasis is to identify the best and stable genotypes for redness index across environments and to understand the association between colour value and curcuminoids. The genotype main effects plus the GEI of colour traits of 21 turmeric genotypes across the three contrasting production environments, namely vertical farming, greenhouse, and field conditions, were studied.
Materials and methods
Plant material
In the present investigation, 21 turmeric genotypes, collected from the National Active Germplasm Site (NAGS), the Indian Council of Agricultural Research-Indian Institute of Spices Research (ICAR-IISR), Kozhikode, Kerala, India, representing different turmeric growing locations in India, were evaluated under three different environments. The experiment was conducted for two seasons during 2021–2022, and 2022–2023. A randomised complete block design was adopted with two replications for each genotype across three production systems. The list of the genotypes and their characteristic features is given in Table S1 and graphical representation of genotypes origin as Figure S1.
Environments and inter-cultural practices
The present investigation was carried out in three different environments, namely vertical structures (L1), greenhouse (L2), and open field (L3) production for two seasons during 2021–2022, and 2022–2023, with 21 genotypes. The details of the experimental conditions are given in Table 1.
Table 1. The details of experimental conditions for three contrasting production systems

Colour value of the turmeric powder
The fresh rhizome was processed to obtain the dried turmeric by boiling with water, followed by sun drying. One kilogram of fresh rhizome (containing 20% mother rhizomes, 60% primary rhizomes and 20% secondary rhizomes) of each genotype was boiled in a clean and fresh water as part of the curing process. The rhizomes were boiled for 45–60 min, or until they became mushy and emitted a characteristic odour (Prasath et al., Reference Prasath, Krishnamurthy, Praveena, Jayashree, Leela, Sellaperumal and Aarthi2022). The boiled rhizomes were then sun-dried for 10 days (6 −8 h each day) to achieve a constant moisture level of 10% (Prasath et al., Reference Prasath, Kandiannan, Leela, Aarthi and Sasikumar2019). The dried rhizomes were ground into a powder and sieved with a consistent mesh size of 325-micron (ASTA, Reference ASTA2004). Thereafter the colour value (L*, A* and B*) of turmeric powder was measured using HunterLab ColorFlex EZ spectrophotometer (Schmitzer et al., Reference Schmitzer, Veberic, Osterc and Stampar2010; Braga et al., Reference Braga, Vieira and de Oliveira2018). L* lightness index (on a scale from 0 to 100 (0 = black and 100 = white), A* redness index (−60 to + 60) -(negative) values stand for green and + (positive) values stand for red, B* yellowness index (−60 to + 60) – (negative) values stand for blue and + (positive) values stand for yellow were recorded for all 21 genotypes in all three environments. The hue angle (hue°) is expressed in degrees from 0 to 360, where 0 = red, 90 = yellow, 180 = green and 270 = blue. The hue° was calculated from L*, A* and B* values by using the formulae: hue° = tan−1 (B*/A*). The C* (saturation index or chroma) values were determined using A* and B* values by the formula, C* = √ (A*2 + B*2) (Pal et al., Reference Pal, Chowdhury, Dutta, Chakraborty, Chakraborty, Pandit, Dutta, Paul, Choudhury, Majumder, Sahana and Mandal2020). Turmeric Redness Index (A*/B*) was measured using A* and B* values (Pathare et al., Reference Pathare, Opara and Al-Said2013).
Estimation of curcuminoids
To extract curcuminoids, the dried rhizomes were ground into a powder using the Cyclotec 1093 sample mill and sieved with a consistent mesh size of 325 microns. The absorption maxima of curcuminoids was measured in a Shimadzu UV-160 I spectrophotometer at 425 nm and percentage was computed based on the concentration of pure crystalline curcumin (98%) (ASTA, Reference ASTA2004).
where DS-Dilution volume (100 ml); WS-weight of sample in grams (one gram); AS-Absorbance maxima
Statistical analysis
All the analysis was carried out using R studio (a simplified version of R statistical software) (R Core Team, 2022). The Agricolae package was used to conduct an analysis of variance (ANOVA) to measure the variation among genotypes and environments. The treatment means were subjected to Duncan's multiple range test (DMRT) at a 5% level of significance for comparison (de Mendiburu, Reference de Mendiburu2021). Metan (Multi-Environment Trial Analysis) package (Olivoto and Lúcio, Reference Olivoto and Lúcio2020) was used to measure the correlation (Pearson's) among the variables and to analyse the GEI, multivariate stability analysis was conducted graphically based on GGE biplot model.
Results
Estimation of the colour composition
All the colour parameters were statistically significant across the three environments for 21 genotypes (Table S2, S3). In the three different productions systems, the L* value ranged from 41.80 to 54.76 (P < 0.001) (Table 2). Mydukur Local recorded the highest L* value under vertical structures (54.76), greenhouse conditions (53.36) and field conditions (51.35), followed by Acc. 179 (53.20), IISR Alleppey Supreme (53.12), and Acc. 1545 (52.16), respectively. Among the genotypes, the A* value (redness index) was observed in the range of 13.92 to 24.83 (P < 0.001) across three environments. In terms of A* value, IISR Pragati (22.52), Rajendra Sonali (21.66), and CIM Pitambar (21.65) were found to be the best under greenhouse conditions and field conditions, respectively. IISR Alleppey Supreme (24.83), Waigon Turmeric (24.74), and Acc. 1545 (24.60) were found to be the best under vertical structures. B* value, ranged from 31.72 to 47.67 (P < 0.001). Mydukur Local (47.09) recorded the highest B* value under vertical structures, followed by IISR Prathiba (46.35) and IISR Alleppey Supreme (45.46). Under field conditions, IISR Prathiba (47.67) recorded the highest score, followed by Rajendra Sonali (46.12).
Table 2. Pooled mean performances of 21 genotypes for colour characteristics and curcuminoids (%) under three contrasting production systems
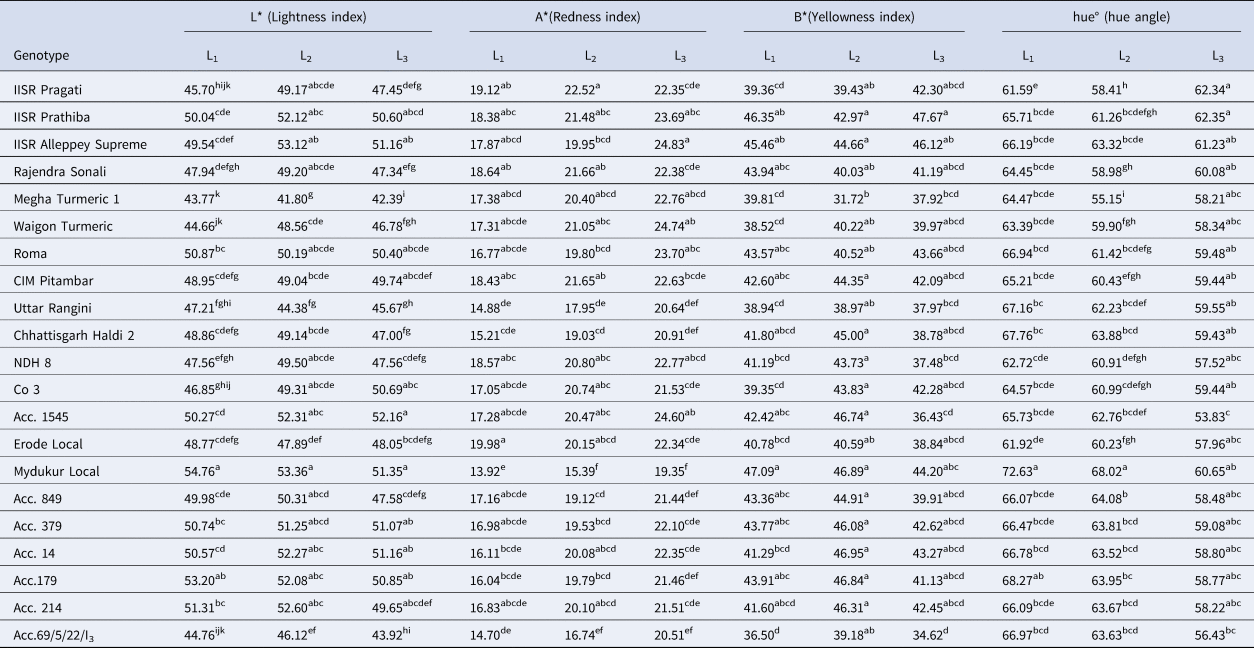
The hue° which shows the redness of the colour ranged from 53.83 to 72.63 (P < 0.001) and was statistically significant for three environments. Mydukur Local recorded the highest hue°, which indicates the lowest redness. The light-yellow genotypes had higher hue° values that were close to the perpendicular angle and outperformed the darker genotypes, which clearly shows that a higher hue° is directly proportional to the lightness of the yellow colour. The C* value varied between 38.02 and 54.73 across the three production systems (Table 3). While IISR Prathiba recorded the highest C* value, under field conditions, IISR Alleppey Supreme for vertical structures and Acc. 14 for greenhouse conditions. In this present study, L*, A*, B*, and hue° showed positive values, indicating no blue or green colours was observed in turmeric powders of 21 genotypes across three production systems.
Table 3. Pooled mean performances of 21 genotypes for colour characteristics and curcuminoids (%) under three contrasting production systems

Estimation of curcuminoids
Among the genotypes, the curcuminoids content ranged from 0.18 to 6.18% across three environments (Table 3). The maximum curcuminoids content under vertical structures (L1) was found in Waigon Turmeric (2.13%), followed by Megha Turmeric 1 (2.09%) and IISR Pragati (2.06%). For greenhouse cultivation (L2), IISR Pragati (3.24%) followed by IISR Prathiba (2.90%) recorded the maximum curcuminoids. In the case of field conditions (L3), IISR Prathiba (6.18%) recorded the maximum, followed by Waigon turmeric (5.94%), Roma (5.63%), and IISR Alleppey Supreme (5.60), which were statistically on par.
Relationship of colour parameters with curcuminoids
In the present study, a positive correlation was observed for curcuminoids with A* values (r = 0.608, P < 0.01 in L1, r = 0.699, P < 0.001 in L2, and r = 0.735, P < 0.01 in L3) (Figs 1a–1c). Significant positive correlation of the A* value with curcuminoids for three environments indicated an association of the redness index with curcuminoids. The A*/B* value also showed a positive correlation with curcuminoids. The correlation between L* and curcuminoids was negative and non-significant under vertical structures. Whereas, under greenhouse conditions and field conditions, a poor and insignificant correlation was found which implies the lighter the colour value of turmeric powder chance of curcuminoids presence is minimum and vice versa darker red coloured ones possess more curcuminoids.
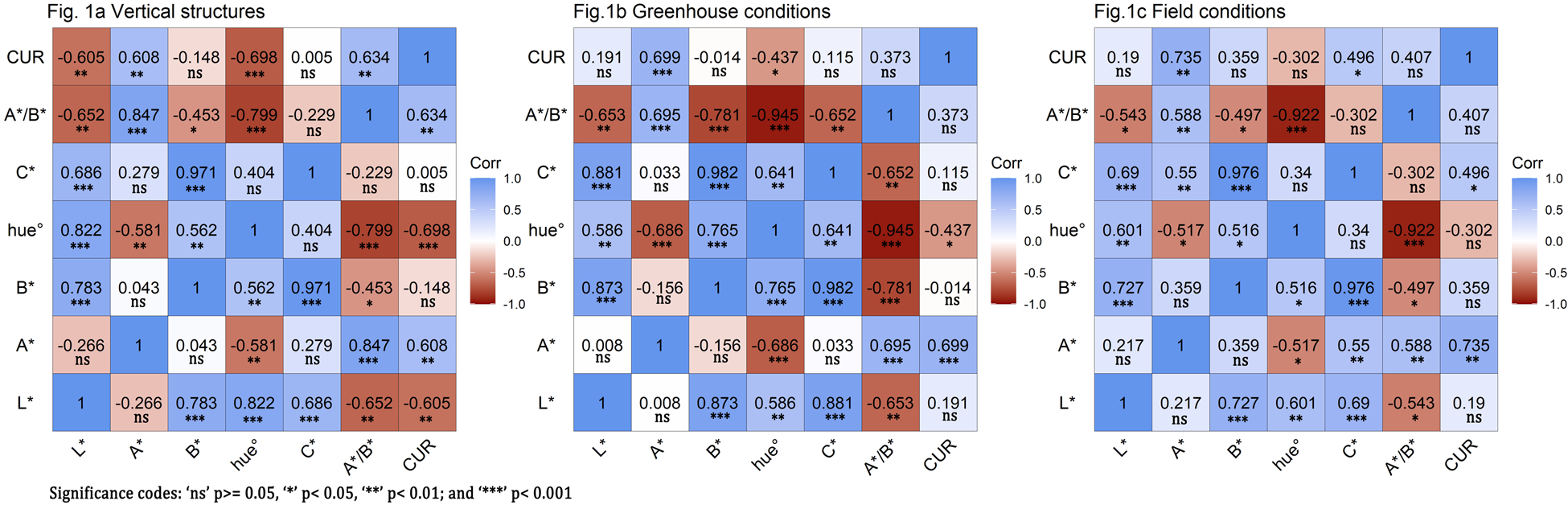
Figure 1. Correlation matrix for colour traits and curcuminoids (a) Vertical structures (L1), (b) Greenhouse conditions (L2) and (c) Field conditions (L3): Where, L = L* (lightness index), A = a* (redness index), B = b* (yellowness index), hue° (hue angle), C = c* (saturation index or chroma) and A*/B* = (Turmeric Redness index).
Biplot pattern for elucidation of multivariate analysis
Further, stability analysis for the colour characteristic, A* value, was conducted as only A* value showed significant positive correlation with curcuminoids. GGE biplot pattern for the A* value is depicted in Figs2a–2e. Variation of about 70.26% and 19.29% difference in GEI for A* value was recorded from the first two principal components of GGE biplot.
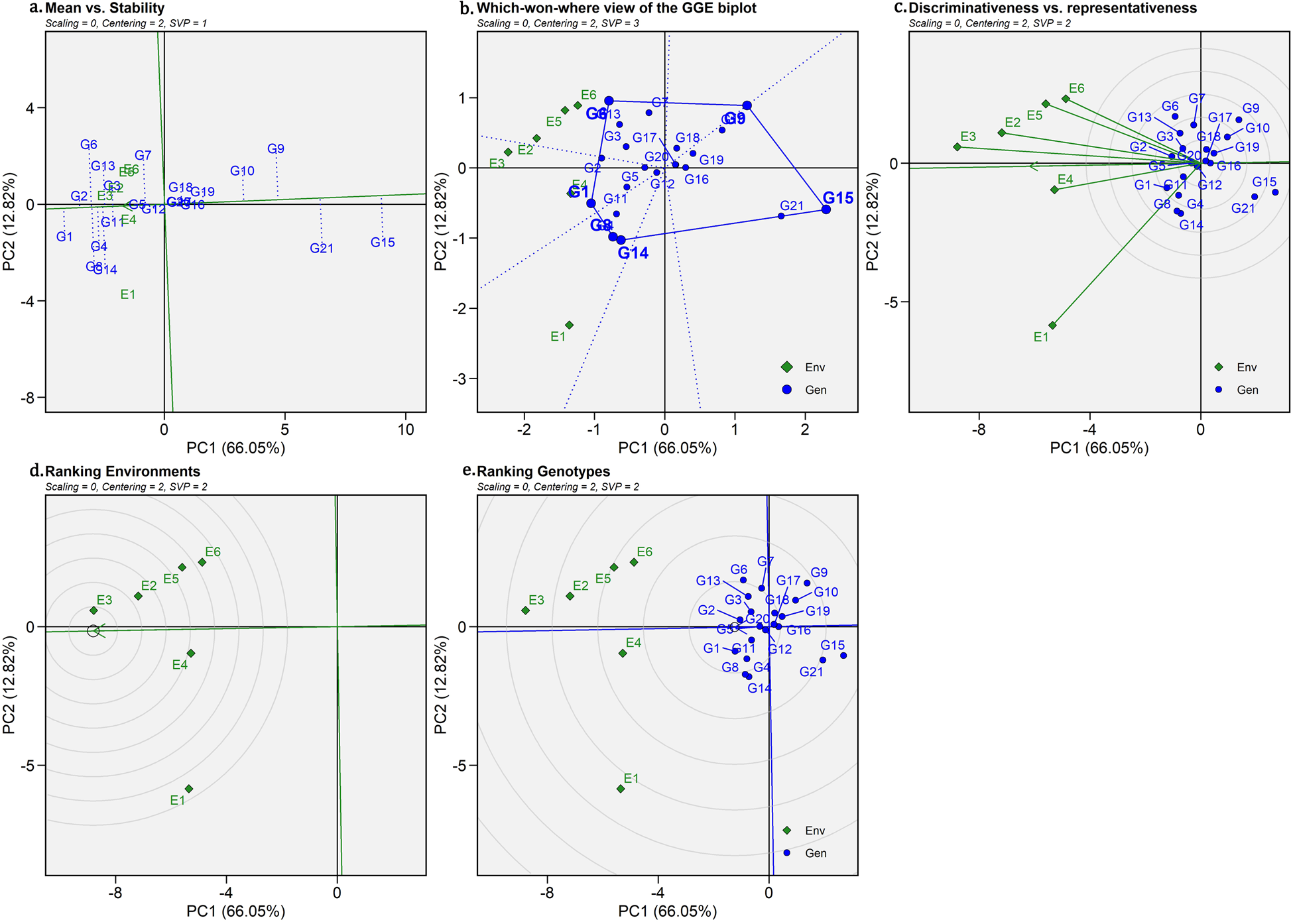
Figure 2. GGE Biplots views displaying the genotype main effect plus G × E interaction effect of 21 turmeric genotypes in two seasons three production systems for redness index (A*).
(a) Mean vs Stability, (b) ‘What-Won-Where’ phenomenon of genotypes, (c) Distinctiveness and representativeness, (d) Ranking of environments and (e) Ranking of genotypes.
Where G1-IISR Pragati, G2-IISR Prathiba, G3-IISR Alleppey Supreme, G4-Rajendra Sonali, G5-Megha Turmeric 1, G6-Waigon Turmeric, G7- Roma, G8-CIM Pitambar, G9- Uttar Rangini, G10- Chhattisgarh Haldi 2, G11- NDH 8, G12-CO 3, G13- Acc. 1545, G14-Erode local, G15-Mydukur Local, G16-Acc. 849, G17- Acc. 379, G18- Acc. 14, G19-Acc. 179, G20-Acc. 214 and G21- Acc.69/5/22/I3.
GGE biplot pattern of ‘mean vs stability’ for A* value
The GGE biplot view of mean vs stability is given in Fig.2a. IISR Pragati was observed as the best genotype, followed by IISR Prathiba, CIM Pitambar, Waigon Turmeric, Rajendra Sonali, and Acc. 1545 for the A* value based on the mean performance across all three production environments. IISR Prathiba found the most stable genotype across all the environments, with high adaptability and a considerable redness index. While Megha Turmeric 1 and Co 3 were identified as stable with a moderate redness index across all environments, their projection of vectors is less and on the positive side of the direction of the Average Environment Coordinate (AEC) abscissa. Genotypes Mydukur Local, Acc.69/5/22/I3, Uttar Rangini, and Chhattisgarh Haldi 2 were the low redness index cultivars as they fell away from the origin and opposite to the direction of the AEC ordinate, indicating the genotypes were less affected by the GEI effects. IISR Pragati, IISR Prathiba, CIM Pitambar, and Waigon Turmeric were identified as superior having redness indexes and better adaptability across three production systems.
GGE biplot pattern of ‘which-won-where’ pattern for A* value
The G (genotype main effect) + GEI variation was recorded as 78.87% for the A* value. The environmental indicators were positioned into three segments of a biplot for the A* value, with different genotypes winning (Fig. 2b). The polygon is formed by connecting the cultivars that are farthest from the biplot origin, so that the polygon contains all other cultivars. The polygon is known as a convex hull, and the cultivars at the polygon's corners are known as vertex cultivars. This result confirmed the presence of distinct genotype and environmental interaction for the A* value. Among the genotypes and three environments, the GGE biplot was divided into six sections. The Erode Local along with CIM Pitambar were the clear winners for vertical structures (E1) as it was the best performer along with high stability. Greenhouse conditions (E3 and E4) along with (E2) all fell in one sector where IISR Pragati was the clear winner as it was placed in the corner of polygon. Whereas for field conditions, Waigon turmeric emerged as the clear winner for both seasons (E5 and E6). However, IISR Pragati and Waigon turmeric were found to be best across three environments and form a mega environment.
Distinctiveness and representativeness view of GGE Biplot for A* value
The E3 (greenhouse condition) and E1 (vertical structure), were the most discriminating and responsive environments, which produced the longest vector across all environments (Fig. 2c). On the other hand, E4 (greenhouse condition) had a short vector and was found to be the least discriminative environment, treated as non-informative or less informative among the tested environments. E3 and E4 (greenhouse conditions) were lying almost on the average environment axis or having a small angle, as they represented the most representative environments. Among all the tested environments, only E6 (field condition) and E1 (vertical structure) had an almost perpendicular angle between them, representing a negative or no relationship for A* value. Among the tested environments, greenhouse condition (E3) was the most discriminative and representative for A* value, which will help choose superior genotypes adapted to most conditions for future breeding programs.
Ranking of environments view of GGE Biplot for A* value
The biplot under consideration uses the ‘ideal’ environment as the center of a set of concentric lines, which function as rulers to measure the distance between an environment and the ideal environment (Yan and Tinker, Reference Yan and Tinker2006). The focus of this biplot is to rank the environments based on their desirability for the A* value. Based on the biplot (Fig. 2d), it can be observed that greenhouse condition (E3) was the closest to the ideal environment and, therefore, the most desirable among the six environments being evaluated. The greenhouse conditions (E3) are ranked higher than the vertical structures (E2), followed by the greenhouse conditions (E4), which are the most desirable environments for the A* value in turmeric genotypes (Yan and Tinker, Reference Yan and Tinker2006). Contrary, vertical structures (E1) was ranked as the least desirable test environment for A* value. From this, it is clearly evident that greenhouse will be an ideally suitable production environment for a superior redness index.
Ranking of genotypes for redness index of GGE Biplot for A* value
According to Yan and Tinker (Reference Yan and Tinker2006), the biplot compares all genotypes to the ‘ideal’ genotype, which is shown as a small circle with an arrow pointing in its direction. The genotype with the highest redness index across environments is defined as the ideal. IISR Pragati was the highest-ranked genotype in this category, followed by IISR Prathiba, Waigon Turmeric, CIM Pitambar, Rajendra Sonali, Erode Local, and Acc. 1545, IISR Alleppey Supreme, and NDH 8, with the best mean yield and absolute stability due to their placement on the optimum genotype concentric circle (Fig. 2e), outperformed all other genotypes in all environments. On the other hand, genotypes Mydukur Local (G15) and Acc. 69/5/22/I3 recorded less redness index among the genotypes. IISR Pragati, IISR Prathiba, and Waigon Turmeric were ranked stable with a higher redness index among 21 genotypes across three production environments.
Discussion
Our findings suggest that colour values of the 21 genotypes varied significantly across production systems, which gives us the opportunity to choose a suitable gradient of colours as per specific requirements. In this regard, studies on influence of GEI plays a key role in identifying the superior colour value and stable genotypes for desirable colour traits and best production systems for cultivation for turmeric for colour value. Recently, Aarthi et al., (Reference Aarthi, Suresh and Prasath2018) reported significant variation in the rhizome colour of turmeric genotypes based on colour chart values. Madhusankha et al., (Reference Madhusankha, Thilakarathna, Liyanage and Navaratne2018) reported that the presence of brilliant yellow curcuminoid pigment was the reason for the observed colour changes, which ranged from bright yellow to orange yellow. Our findings also indicate the influence of genetic and environmental factors on curcuminoids. The variation of curcuminoids in different environments and geographical locations was reported earlier by Anandaraj et al., (Reference Anandaraj, Prasath, Kandiannan, Zachariah, Srinivasan, Jha, Singh, Singh, Pandey, Singh, Shoba, Jana, Kumar and Maheswari2014), Sandeep et al., (Reference Sandeep, Kuanar, Akbar, Kar, Das, Mishra, Sial, Naik, Nayak and Mohanty2016), and Aarthi et al., (Reference Aarthi, Suresh, Leela and Prasath2020). Whereas, the association of colour and curcuminoids will facilitate the choice of superior genotypes based on the colour value for higher curcuminoids. In addition to this, the colour value of turmeric is also a key factor, as it is often used as a colouring agent. The brighter and vibrant colour of turmeric, the more visually appealing to its consumers. Overall, the hue of turmeric is extremely important to both its therapeutic properties and consumer preferences. Given the lack of information on these aspects, a thorough investigation of the relationship between curcuminoids and colour parameters is necessary to establish a simple model for the quantification of curcuminoids based on photometric observations. Our results are in agreement with the earlier studies by Magar and Chowdhury, (Reference Magar and Chowdhury2021); Pal et al., (Reference Pal, Chowdhury, Dutta, Chakraborty, Chakraborty, Pandit, Dutta, Paul, Choudhury, Majumder, Sahana and Mandal2020); Supannarach and Thanapatay, (Reference Supannarach and Thanapatay2008).
Khan et al., (Reference Khan, Rafii, Ramlee, Jusoh and Al Mamun2021) and Oladosu et al., (Reference Oladosu, Rafii, Abdullah, Magaji, Miah, Hussin and Ramli2017) reported the influence of genotype and GEI on yield of bambara groundnut and mutant lines of rice for selecting superior genotypes for further breeding programmes. Without sufficient knowledge of GEI and the stability pattern of genotypes for colour value, breeding programmes become more complicated in order to select a superior genotype across production systems. A similar result was reported by Sincik et al., (Reference Sincik, Goksoy, Senyigit, Ulusoy, Acar, Gizlenci, Atagun and Suzer2021) while studying on yield stability of canola (Brassica napus L.) genotypes to multi-environments. similar interpretations for the GGE biplots were given by Yan and Kang (Reference Yan and Kang2002); Yan and Tinker, Reference Yan and Tinker2006; Abua et al., (Reference Abua, Iwo, Ittah, Obok and Edugbo2020); Oladosu et al., (Reference Oladosu, Rafii, Abdullah, Magaji, Miah, Hussin and Ramli2017). In Nigeria, Abua et al., (Reference Abua, Iwo, Ittah, Obok and Edugbo2020) conducted a multi-location trial of ginger and reported adaptation of few genotypes to the specific soil and agro-climatic conditions. Moreover, studies on stability of colour value of turmeric are very limited. The results of our present study outline the influence of genotype and genotype- environment interaction on colour value along with curcuminoids which gives additional information for further improvement of turmeric genotypes for colour traits adapted to different production systems. Tavares et al., (Reference Tavares, Kirk, Motomura-Wages, Calpito, Bingham, Ahmad, Flanagan, Uyeda, Kantar and Radovich2022) reported the influence of genotypes and GEI on fresh rhizome yield in turmeric. The influence of genotype was more on rhizome yield, and production aspects like nutrient and water management were crucial. Reports by Aarthi et al., (Reference Aarthi, Suresh, Leela and Prasath2020) and Sandeep et al., (Reference Sandeep, Sahoo, Singh, Sahoo, Nayak and Kar2021) suggested a significant environmental influence on quality. From the pooled analysis, it is clearly evident that IISR Pragati, Rajendra Sonali and CIM Pitambar were found to be superior under greenhouse and field conditions; IISR Alleppey Supreme and Waigon turmeric for vertical structures. Whereas from the GGE biplot model, IISR Pragati, IISR Prathiba and Waigon Turmeric were ranked higher in redness index and stability. Whereas, IISR Alleppey Supreme and Rajendra Sonali were unstable and most influenced by particular environments along with GEI. These studies provide information on how quality traits and yield are influenced by genotypes and GEI which is in agreement with our study. Overall factors like production systems, management practices, media, geographical factors and stress influence the quality of turmeric.
Conclusion
In conclusion, our study fills the gap in the current research, as there are no specific turmeric genotypes identified that are particularly adapted to different production systems for colour value and for superior redness index. While numerous genotypes have shown superior performance in terms of yield and quality under field conditions, the adaptation of these genotypes for colour traits across various cultivation systems remains unexplored. There is little information available on the selection criteria for determining the genotypes of turmeric based on colour value. The colour of the turmeric genotypes varied widely across the tested environments. The promising genotypes for A* value across all three environments is IISR Pragati, followed by IISR Prathiba, CIM Pitambar, Waigon Turmeric, Rajendra Sonali, and Acc. 1545 (Fig. 3). Our findings suggested considerable variation and environmental influence for colour traits and curcuminoids among the genotypes. IISR Pragati, IISR Prathiba and Waigon Turmeric were identified as stable genotypes with a higher redness index and curcuminoids. Among three different production environments, greenhouse was found to be the best for producing a superior redness index across the production environments. Further, these findings also shed light on the development of tailored turmeric genotypes for colour based on genotype by environment studies since the preferred colour of turmeric powder on the global market varies, which leads to blending.
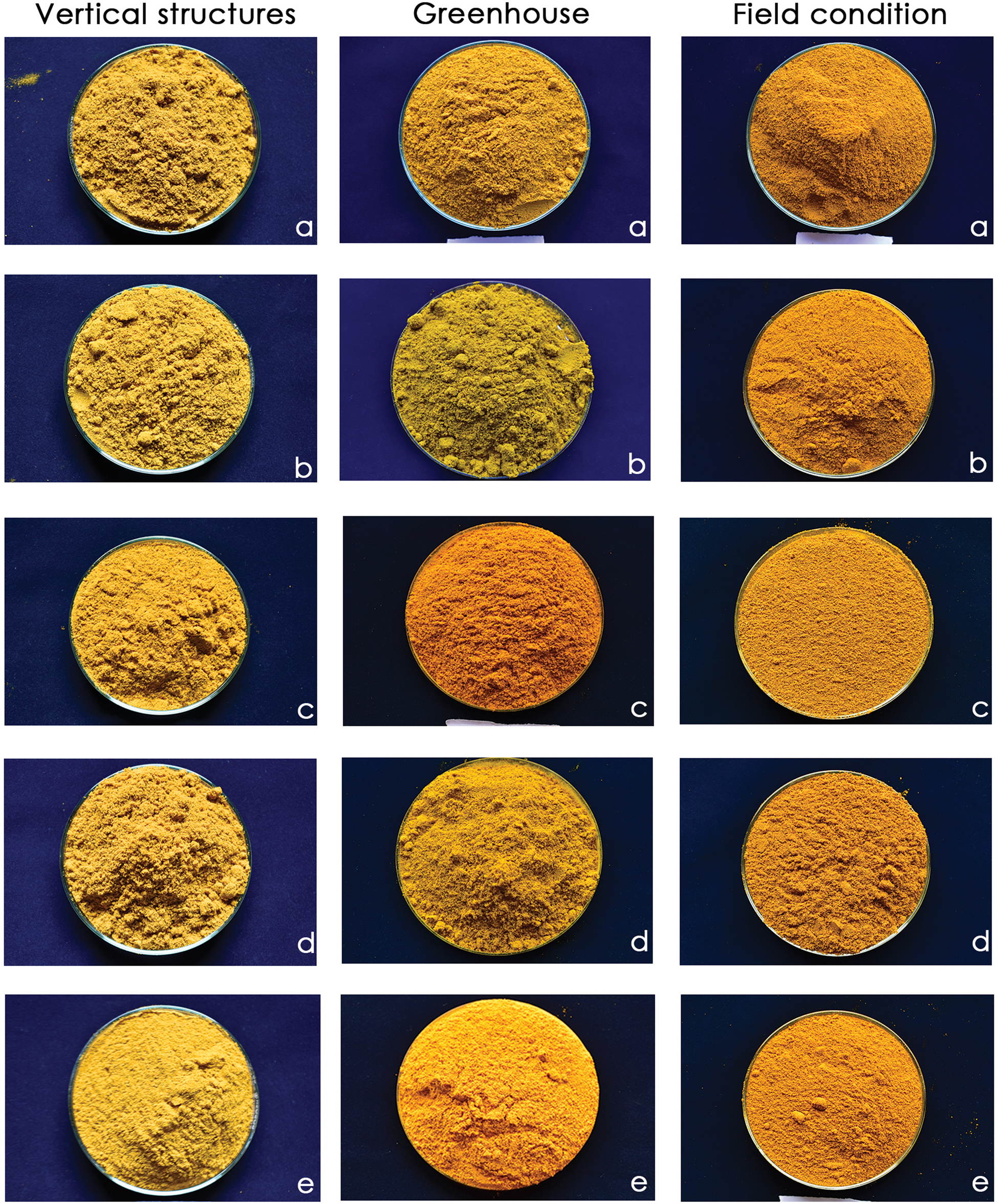
Figure 3. Genotypes for superior A* value across three production systems; (a) IISR Pragati, (b) IISR Prathiba, (c) Rajendra Sonali (d) Waigon Turmeric and (e) Acc. 1545.
CRediT authorship contribution statement
All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
Experiments, analysis, writing-original draft preparation was done by Raghuveer S. Prasath D has done conceptualization, resources, supervision, reviewing and editing. Yuvaraj KM has done contribution to the conception of the study and Aarthi S has done conceptualization, reviewing and editing.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1479262124000339.
Competing interests
All authors read and approved the final manuscript. The authors declare no conflict of interest. This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue. The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript.








