Gestational diabetes mellitus (GDM), a common pregnancy complication, is characterised by hyperglycaemia during pregnancy(1). In recent decades, the prevalence of GDM has become a worldwide growing health concern. In China, the prevalence of GDM has been over 10 % in recent years(Reference Gao, Sun and Lu2–Reference Wei, Yang and Zhu4). GDM has not only been connected to an increased risk of adverse pregnancy outcomes(Reference Farrar, Simmonds and Bryant5) but also an increased risk of several metabolic diseases for both mothers(Reference Li, Cheng and Wang6,Reference Song, Lyu and Li7) and their offspring(Reference Nijs and Benhalima8,Reference Bianco and Josefson9) in the long term. It was estimated that $5·59 billion was spent treating GDM and its complications in China in 2015(Reference Xu, Dainelli and Yu10). Thus, the identification of modifiable risk factors plays a vital role in the prevention of GDM.
Dietary factors, as modifiable risk factors, were associated with GDM risk(Reference Schoenaker, Mishra and Callaway11). Previous studies have shown that the intake of individual macronutrients was associated with GDM risk. The findings have indicated that higher intakes of total fat(Reference Saldana, Siega-Riz and Adair12,Reference Ley, Hanley and Retnakaran13) , animal fat(Reference Bowers, Tobias and Yeung14), total protein(Reference Liang, Gong and Zhang15) and animal protein(Reference Bao, Bowers and Tobias16) and a lower intake of carbohydrate(Reference Ley, Hanley and Retnakaran13,Reference Tajima, Yachi and Tanaka17) were linked to an increased risk of GDM, whereas dietary fat and carbohydrate intakes in early pregnancy were not associated with GDM risk in one study(Reference Radesky, Oken and Rifas-Shiman18). If one macronutrient intake is increased, others will be decreased. Therefore, the balanced intake of the three macronutrients should be simultaneously considered in the overall diets. A comprehensive score, known as the ‘low-carbohydrate diet score’, was created by Halton et al. (Reference Halton, Willett and Liu19) based on the relative levels of carbohydrate, fat and protein intake. A higher low-carbohydrate diet (LCD) score means a lower intake of carbohydrate and higher intakes of fat and protein, and it also represents greater adherence to a low-carbohydrate dietary pattern.
Two studies have examined the association of pre-pregnancy LCD scores with GDM risk(Reference Looman, Schoenaker and Soedamah-Muthu20,Reference Bao, Bowers and Tobias21) . In the Nurses’ Health Study II and the Australian Longitudinal Study on Women’s Health, the LCD score was significantly related to an increased risk of GDM; moreover, the Nurses’ Health Study II observed a positive association of the animal-based LCD score with GDM risk. However, the effect of the LCD during pregnancy on the development of GDM is unclear. Lifestyle modification, including dietary intervention during pregnancy, particularly before the 15th week of gestation, can decrease the incidence of GDM(Reference Song, Li and Leng22). Thus, using data from a prospective cohort study in China, we aimed to examine the association between LCD scores during the first trimester and GDM risk.
Methods
Study population
Participants were drawn from a population-based prospective cohort study conducted in Sichuan Provincial Hospital for Women and Children, Southwest China. From February to July 2017, we recruited 1673 healthy women who met the inclusion criteria: singleton pregnancy, gestational age ranging from 6 to 14 weeks and no chronic metabolic diseases. The study was approved by the Ethics Committee of Sichuan University. All participants provided written informed consent when recruited to the study.
In the analysis, we excluded participants with unfinished dietary surveys (n 8). We also excluded participants with a history of GDM (n 39) because these diagnoses could result in dietary changes in the next pregnancy. Furthermore, we excluded women who had implausible energy intakes (<2092 or >14 644 kJ/d)(Reference Willett23) (n 16) or missing data on the diagnosis of GDM (n 155) (Fig. 1). The final analysis included 1455 women.
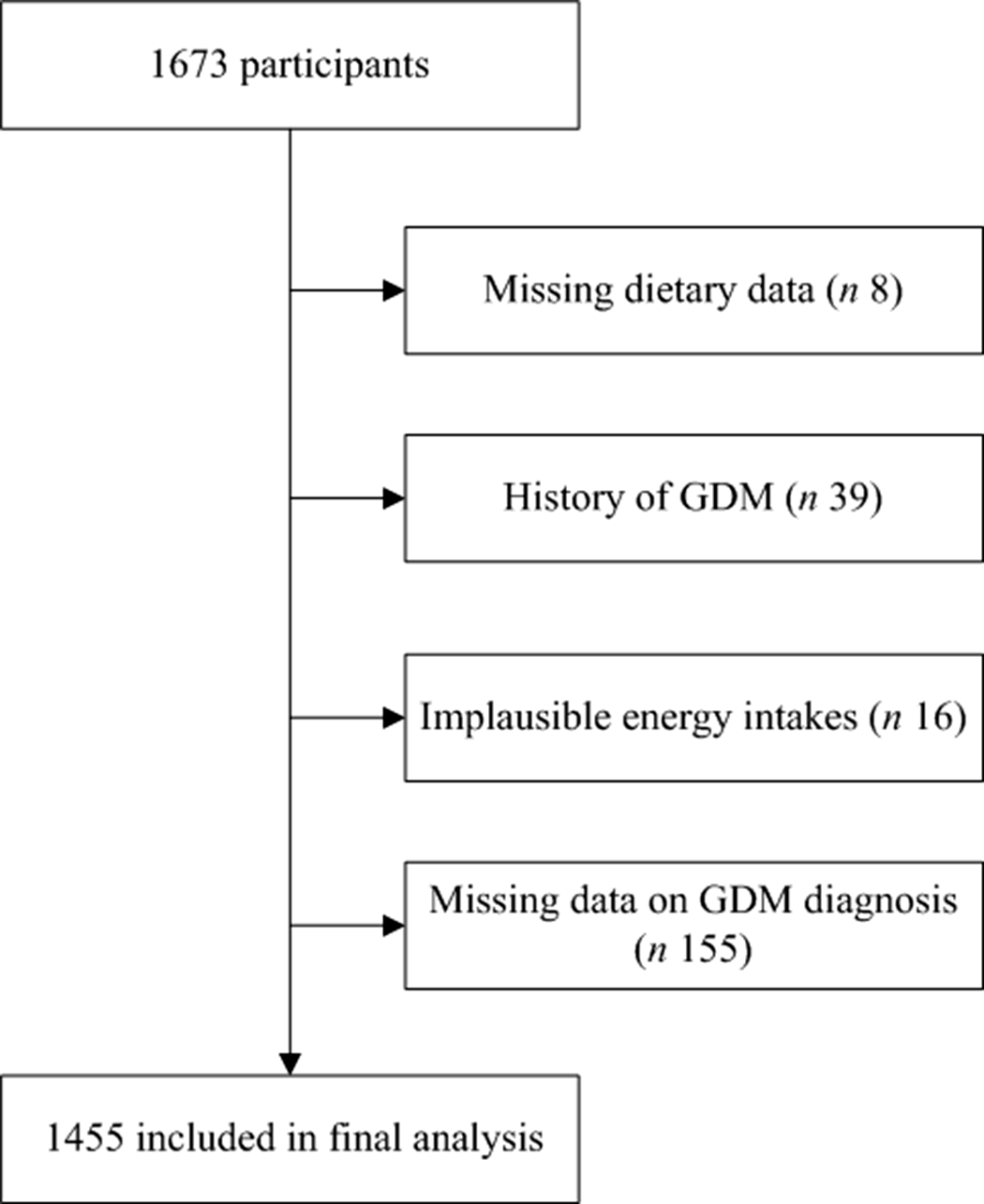
Fig. 1. Flow chart for inclusion and exclusion of the study participants. GDM, gestational diabetes mellitus.
Assessment of dietary intake
Dietary information was assessed by 24-h dietary recalls for three consecutive days including two weekdays and one weekend day. Information on all types and amounts of food consumed by the participants in the past 24 h was collected by specialised investigators via face-to-face interviews at recruitment. To reduce measurement error, standard serving bowls, cups, spoons and illustrative food pictures of various portion sizes(Reference Wang24) were displayed to help the participants estimate intakes of food. The next 2 d of dietary information was collected by specialised investigators through telephone interviews.
To calculate macronutrient intakes, the amount of each food consumed was multiplied by the nutrient content per gram of the food as obtained from the Chinese Food Composition Tables(25). The average daily intakes of energy and nutrients were calculated using a Nutrition Calculator (V2.7.3). Intakes of macronutrients were computed as the percentages of total energy intake by the nutrient-density method, and other nutrient intakes were adjusted for total energy intake by the residual method(Reference Willett23).
Calculation of low-carbohydrate diet scores
Three LCD scores were calculated according to the method of Halton et al. (Reference Halton, Willett and Liu19). The overall LCD score was calculated by dividing the study participants into eleven categories based on the percentages of energy from total fat, total protein and carbohydrate. The total fat and total protein categories were scored 0–10 points from the lowest to the highest intake, respectively. Conversely, carbohydrate categories were scored 10–0 points from the lowest to the highest intake. An overall LCD score ranging from 0 to 30 was created by summing the points for the three macronutrients. A higher score indicated a higher proportion of energy from total fat and total protein and a lower proportion of energy from carbohydrate. Similarly, the animal LCD score was calculated according to the percentages of energy from carbohydrate, animal fat and animal protein. The plant LCD score was calculated according to the percentages of energy from carbohydrate, plant fat and plant protein. Table 1 presents the points of the respective macronutrient categories used for the determination of overall, animal and plant LCD scores.
Table 1. Points for the respective macronutrient categories used to calculate overall, animal and plant low-carbohydrate diet (LCD) scores
(Median and minimum (Min)–maximum (Max))
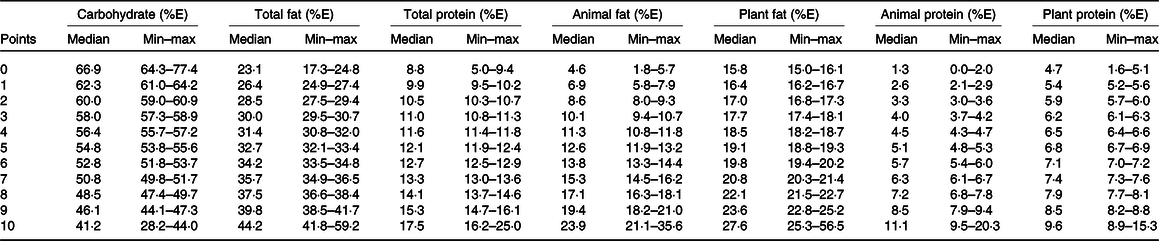
%E, percentage of energy intake.
Ascertainment of gestational diabetes mellitus
Between 24 and 28 weeks of gestation, participants were routinely screened by the 75-g, 2-h oral glucose tolerance test to diagnose GDM. According to the diagnostic criteria of the International Association of Diabetes Pregnancy Study Group guidelines(Reference Metzger, Gabbe and Persson26), GDM was diagnosed if any one or more plasma glucose values met or exceeded the following thresholds: fasting plasma glucose ≥5·1 mmol/l, 1-h plasma glucose ≥10·0 mmol/l or 2-h plasma glucose ≥8·5 mmol/l.
Assessment of covariates
Covariates were chosen based on previous studies(Reference McIntyre, Catalano and Zhang27–Reference Chen, Chen and Wu29). The current review showed that maternal age, pre-pregnancy BMI, ethnicity, parity, family history of diabetes mellitus, cigarette smoking and physical activity were associated with GDM(Reference McIntyre, Catalano and Zhang27). In addition to the above covariates, studies which exploring the relationship between dietary patterns and GDM have additionally selected education level, family income, alcohol consumption and gestational weight gain as covariates that might influence the association between dietary patterns and GDM for analysis(Reference Zhou, Chen and Zhong28,Reference Chen, Chen and Wu29) . Therefore, these covariates were assessed in our study.
Covariates were assessed by a self-designed questionnaire at enrolment via a face-to-face interview. Ethnicity was categorised into Han Chinese and others. Maternal age was divided into four categories (≤24, 25–29, 30–34 and ≥35 years). Self-reported pre-pregnancy weight and measured height at inclusion were collected to calculate pre-pregnancy BMI, which was categorised into three groups (<18·5, 18·5–23·9 and ≥24·0 kg/m2) according to the Chinese obesity criteria(Reference Chen30). Gestational weight gain before GDM diagnosis was calculated by subtracting self-reported pre-pregnancy weight from weight measured at oral glucose tolerance tests. Educational level was divided into three categories (≤12, 13–15, and ≥16 years) based on the number of completed years of education. Family income level was categorised into four groups (≤2999, 3000–4999, 5000–9999 and ≥10 000 Chinese Yuan/month). Parity was divided into two categories (primiparity or multiparity). Physical activity (metabolic equivalent of task-h/week) was measured using the Pregnancy Physical Activity Questionnaire(Reference Chasan-Taber, Schmidt and Roberts31), which has demonstrated good reliability and validity in Chinese pregnant women(Reference Zhang, Zhao and Dong32). The other covariates, including work during early pregnancy, family history of diabetes, and smoking and alcohol drinking status, were regarded as dichotomised variables (yes, no). Smoking was defined as tobacco smoking during the 6 months before conception or during pregnancy, and alcohol consumption was defined as alcohol consumption during the 6 months before conception or during pregnancy.
Statistical analysis
Distributions of categorical variables were described as frequencies and percentages. Means and standard deviations were used to describe continuous variables with a normal distribution, and medians and interquartile ranges (IQR) were used to describe continuous variables with a skewed distribution. Comparisons of categorical variables between groups were performed using χ 2 tests. Continuous variables were compared by one-way ANOVA or non-parametric Kruskal–Wallis tests according to their normal or skewed distributions.
Participants were divided into quartiles according to overall, animal and plant LCD scores. Relative risks (RR) and 95 % CI were estimated through log-binomial models(Reference Wacholder33) with generalised linear regression. In a few cases, the log-binomial models failed to converge when three or more covariates were continuous variables in our study. Then, the Poisson regression with robust standard errors was used(Reference Zou34), which has no difficulty in converging and gives reasonable estimates of the prevalence ratio(Reference Petersen and Deddens35). To test a linear trend, the median values for each quartile of the three LCD scores were assigned and modelled as continuous variables.
In the multivariate analysis, four models were included. Model 1 was the crude model. Model 2 was adjusted for maternal age (≤24, 25–29, 30–34 or ≥35 years), pre-pregnancy BMI (<18·5, 18·5–23·9 or ≥24·0 kg/m2) and total energy intake (kJ/d). Model 3 was adjusted for model 2 plus ethnicity (Han Chinese or others), educational level (≤12, 13–15 or ≥16 years), family income level (≤2999, 3000–4999, 5000–9999 or ≥10 000 Chinese Yuan/month), parity (primiparity or multiparity), family history of diabetes (yes or no), smoking (yes or no), alcohol drinking (yes or no) and physical activity (metabolic equivalent of task-h/week). Model 4 was adjusted for model 3 plus gestational weight gain before GDM diagnosis (kg). The goodness of fit of the models was assessed by the Akaike information criterion.
To examine potential dietary contributors to the association of LCD scores with GDM risk, we additionally and separately adjusted for each macronutrient (e.g. carbohydrate, animal fat, animal protein, plant fat and plant protein), carbohydrate quality (e.g. dietary fibre, glycaemic index and glycaemic load) and each food group (e.g. red meat, poultry, fish, eggs, dairy food, refined grains, whole grains, tubers, fruits, vegetables, nuts and legumes)(Reference Bao, Bowers and Tobias21).
Stratified analyses were performed to assess whether the association of the three LCD scores and GDM risk was modified by maternal age (<35 v. ≥35 years), pre-pregnancy BMI (<24·0 v. ≥24·0 kg/m2), family history of diabetes (yes v. no) and physical activity (<median v. ≥median). Because advanced maternal age, pre-pregnancy overweight or obesity, family history of diabetes and physically inactive lifestyle were risk factors for GDM(Reference McIntyre, Catalano and Zhang27), these variables were chosen as stratified factors. Interaction tests were conducted in multivariable models.
All statistical analyses were performed using Stata version 15.0 (Stata Corp LP). A two-tailed P value <0·05 was considered statistically significant.
Results
Baseline characteristics
Among 1455 participants, 520 cases of GDM were diagnosed. The mean age of participants was 28·5 (sd 4·0) years, and the mean pre-pregnancy BMI was 21·0 (sd 2·7) kg/m2. Women with GDM had higher age and pre-pregnancy BMI (see online supplementary material, Supplemental Table S1). The median of overall, animal and plant LCD scores was 15·0 (IQR 8·0, 22·0), 15·0 (IQR 8·0, 22·0) and 15·0 (IQR 11·0, 19·0), respectively. The median of daily carbohydrate intake for all participants was 245·3 (IQR 202·4, 297·5) g. The median of daily carbohydrate intake from the lowest to the highest quartiles of the overall LCD score was 277·6 (IQR 225·2, 347·8) g, 264·2 (IQR 221·0, 309·5) g, 239·9 (IQR 208·0, 284·6) g and 209·4 (IQR 169·6, 253·8) g, respectively. The median of daily carbohydrate intake from the lowest to the highest quartiles of the animal LCD score was 277·4 (IQR 225·8, 347·5) g, 263·2 (IQR 218·7, 311·8) g, 242·3 (IQR 211·6, 284·7) g and 209·6 (IQR 171·3, 254·0) g, respectively. The median of daily carbohydrate intake from the lowest to the highest quartiles of the plant LCD score was 256·7 (IQR 209·3, 308·7) g, 254·5 (IQR 205·9, 299·0) g, 248·9 (IQR 208·4, 302·5) g and 231·8 (IQR 190·1, 274·3) g, respectively.
Participants with higher overall LCD scores were more often primiparous and more likely to have a family history of diabetes; consumed more vegetables and red meats, eggs, dairy products, legumes and nuts; and consumed less refined grains and fruits (Tables 2 and 3). Participants with higher animal LCD scores were more likely to have a family history of diabetes; consumed more vegetables, red meats, eggs, dairy products and nuts; and consumed less dietary fibre, refined grains and fruits (see online supplementary material, Supplemental Tables S2 and S3). Participants with higher plant LCD scores were more often primiparous and consumed more total energy, dietary fibre, whole grains, vegetables and red meats, eggs, legumes and nuts but consumed less refined grains (see online supplementary material, Supplemental Tables S4 and S5). The correlation coefficients between LCD score distributions were as follows: 0·93 between the overall LCD score and the animal LCD score, 0·51 between the overall LCD score and the plant LCD score and 0·23 between the animal LCD score and the plant LCD score (All P < 0·05).
Table 2. Baseline characteristics of participants according to quartiles of the overall low-carbohydrate diet (LCD) score
(Numbers and percentages; median and interquartile range)
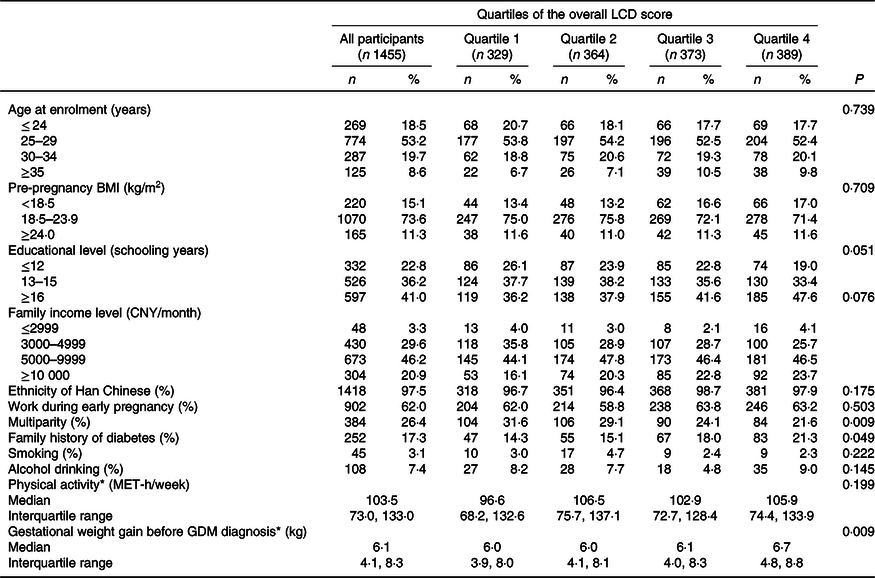
CNY, Chinese Yuan; MET, metabolic equivalent of task; GDM, gestational diabetes mellitus.
* Data of physical activity and gestational weight gain before GDM diagnosis were described by median and interquartile range.
Table 3. Dietary intakes of participants according to quartiles of the overall low-carbohydrate diet (LCD) score
(Median values and interquartile ranges (IQR))
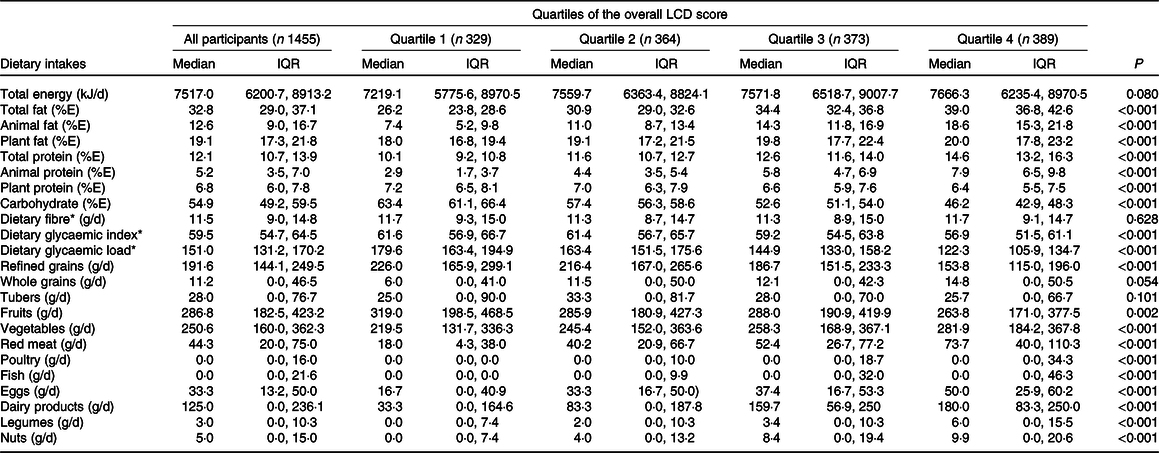
%E, percentage of energy intake.
* Dietary variables were adjusted for total energy intake by the residual method.
Association between low-carbohydrate diet scores and gestational diabetes mellitus risk
The overall and animal LCD scores were significantly and positively associated with GDM risk in crude and multivariable-adjusted models (Table 4). Multivariable-adjusted RR of GDM from the lowest to the highest quartiles of the overall LCD score were 1·00 (reference), 1·15 (95 % CI 0·92, 1·42), 1·30 (95 % CI 1·06, 1·60) and 1·24 (95 % CI 1·01, 1·52) (P = 0·026 for trend) after adjustment for dietary, socio-demographic and lifestyle factors (model 3). The effect size of the overall LCD score on GDM risk was no longer statistically significant after additional adjustment for gestational weight gain before GDM diagnosis (model 4). Multivariable-adjusted RR of GDM from the lowest to the highest quartiles of the animal LCD score were 1·00 (reference), 1·20 (95 % CI 0·96, 1·50), 1·41 (95 % CI 1·14, 1·73) and 1·29 (95 % CI 1·04, 1·59) (P = 0·002 for trend) after adjusting for dietary, socio-demographic and lifestyle factors (model 3). The effect size of the animal LCD score on GDM risk was slightly attenuated but remained significant after additional adjustment for gestational weight gain before GDM diagnosis (model 4). There was no statistically significant association between the plant LCD score and the risk of GDM.
Table 4. Association between low-carbohydrate diet (LCD) scores and risk of gestational diabetes mellitus (GDM)
(relative risk (RR) and 95 % confidence intervals)
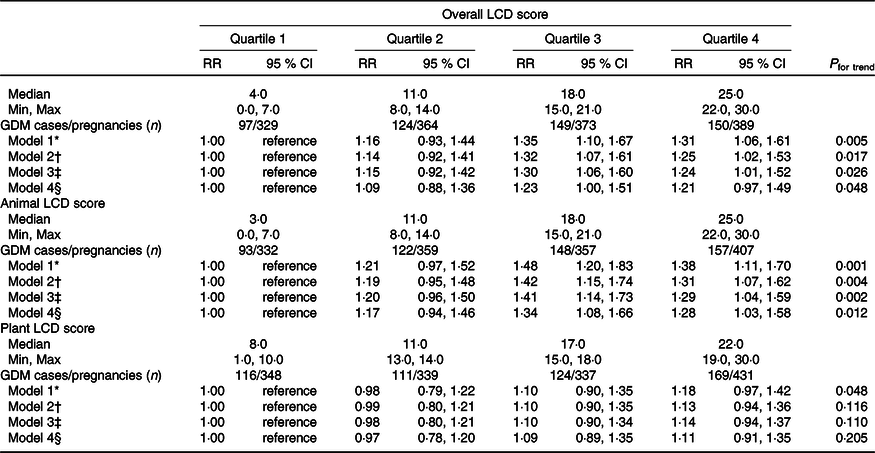
Min, minimum; Max, maximum; CNY, Chinese Yuan; MET, metabolic equivalent of task.
* Crude model.
† Adjusted for maternal age (≤ 24, 25–29, 30–34, or ≥35 years), pre-pregnancy BMI (<18·5, 18·5–23·9, or ≥24 kg/m2) and total energy intake (kJ/d).
‡ Adjusted for model 2 plus ethnicity (Han Chinese or others), educational level (≤ 12, 13–15 or ≥16 years), family income level (≤2999, 3000–4999, 5000–9999 or ≥10 000 CNY/month), parity (primiparity or multiparity), family history of diabetes (yes or no), smoking (yes or no), alcohol drinking (yes or no) and physical activity (MET-hours/week).
§ Adjusted for model 3 plus gestational weight gain before GDM diagnosis (kg). Model 4 was performed by the Poisson regression with robust standard errors.
To examine which dietary variables were main contributors to the association of the animal LCD score with GDM risk, additional adjustments for some nutrients and food groups were modelled. The effect sizes of the animal LCD score on GDM risk in the highest quartile v. the lowest quartile were attenuated after adjustment for carbohydrate (RR: 1·34; 95 % CI 0·91, 1·96), total fat (RR: 1·17; 95 % CI 0·86, 1·59) and animal fat (RR: 1·26; 95 % CI 0·88, 1·80) (see online supplementary material, Supplemental Table S6).
Stratified analyses
The associations between LCD scores and GDM were still consistent in most subgroups after performing stratified analyses (see online supplementary material, Supplemental Table S7). The relationship of overall LCD score and GDM was positive among participants in subgroups of high pre-pregnancy BMI (≥24 kg/m2) and no family history of diabetes, while the interactions for these stratified variables were not significant. The animal LCD score was significantly and positively associated with the risk of GDM among participants in subgroups of low age (<35 years), low pre-pregnancy BMI (<24 kg/m2), no family history of diabetes and low physical activity (<median). However, the interactions for these stratified variables were not significant.
Discussions
In this study, we observed that a higher overall LCD score during the first trimester was significantly associated with a higher incidence of GDM, while the association was no longer statistically significant after additional adjustment for gestational weight gain before GDM diagnosis. The animal LCD score during the first trimester was significantly associated with an increased risk of GDM. In contrast, the plant LCD score during the first trimester was not significantly associated with GDM risk.
Our results were similar to the Nurses’ Health Study II(Reference Bao, Bowers and Tobias21) and the Australian Longitudinal Study on Women’s Health(Reference Looman, Schoenaker and Soedamah-Muthu20), whose results showed that the pre-pregnancy LCD score was positively associated with GDM risk. However, these two studies did not explore the influence of weight gain during pregnancy on the association between LCD scores and GDM. In our study, the positive association of the overall LCD score with GDM was no longer statistically significant after additional adjustment for gestational weight gain before GDM diagnosis, suggesting that this positive association may have been due to the influence of gestational weight gain. The Nurses’ Health Study II also explored the effect of animal and vegetable LCD scores on GDM risk, and the results indicated that a higher animal LCD score was linked to a higher risk of GDM, while the relationship between the plant LCD score and GDM risk was not statistically significant. This was supported by other studies showing that a higher GDM risk was correlated with a higher consumption of animal fat(Reference Bowers, Tobias and Yeung14) and animal protein(Reference Bao, Bowers and Tobias16). In addition, a recent observational study from China indicated that the animal LCD score was positively associated with oral glucose tolerance test 1-h glucose(Reference Chen, Chen and Wu29), suggesting that a low-carbohydrate dietary pattern with high animal fat and protein was associated with an increased risk of abnormal maternal glucose metabolism. However, the positive association between the animal LCD score and GDM risk was observed to be non-linear in our study because the RR was higher in the third quartile than in the highest quartile of animal LCD score. This might be because women in the third quartile of animal LCD score had higher intakes of total energy and lower levels of physical activity than those in the highest quartile group.
To interpret the association of the animal LCD score with the incidence of GDM, the consumption of each macronutrient and its major food source should be taken into consideration. The association between the animal LCD score and GDM risk was attenuated after adjustment for carbohydrate, total fat and animal fat, suggesting that the observed association may have been due to the distribution intakes of carbohydrate, total fat and animal fat across quartiles of the animal LCD score. In our study, 54·9 % of the total energy intake from carbohydrate and 32·8 % of the total energy intake from fat were similar to the average intake of carbohydrate (55·0 %E) and fat (32·9 %E) in Chinese adults of the China Nutrition and Health Surveys in 2010–2012(Reference Ju, Yu and Fang36). However, according to the recommended acceptable macronutrient distribution ranges in the Chinese Dietary Reference Intakes (2013)(37), the median intake of total fat was beyond the standard in our study. In particular, in the highest quartile of overall and animal LCD score groups, participants had high total fat and total protein intakes but low carbohydrate intake. Therefore, an unreasonable macronutrient composition might be the main cause of GDM risk.
Our results were also supported by previous studies of macronutrients. Some studies have found that total fat(Reference Saldana, Siega-Riz and Adair12,Reference Ley, Hanley and Retnakaran13) and animal fat(Reference Bowers, Tobias and Yeung14) intakes were significantly correlated with an increased risk of GDM. Moreover, animal fat is rich in saturated fat, and one study documented that saturated fat has been linked to the risk of developing gestational glucose abnormalities(Reference Bo, Menato and Lezo38). In addition, several studies have suggested that a higher total protein intake, particularly animal protein intake, might increase GDM risk(Reference Liang, Gong and Zhang15,Reference Bao, Bowers and Tobias16) , and one study found a positive association between animal and vegetable protein intake and the risk of GDM in Asian women(Reference Pang, Colega and Cai39). Furthermore, the Tongji Maternal and Child Health Cohort study has shown that a low-carbohydrate and high-protein dietary pattern during pregnancy was linked to an increased risk of developing GDM in Chinese women(Reference Zhou, Chen and Zhong28). These studies might support our finding that a low-carbohydrate dietary pattern was positively associated with GDM risk. Therefore, the total energy intake from carbohydrate, fat and protein should be in an appropriate balance.
Because of the overall balance of macronutrients, low carbohydrate intake means high fat and high protein intake. Our results are biologically plausible from the perspective of the intakes of fat, protein and carbohydrate, although the potential mechanisms need to be further explored. High-fat diets and specific free fatty acids may trigger oxidative stress and apoptosis, resulting in a reduction of β-cell mass and β-cell dysfunction(Reference Cerf40). In addition, high concentrations of free fatty acids are likely one of the reasons for the development of insulin resistance and hyperglycaemia during pregnancy(Reference Sivan, Homko and Whittaker41,Reference Villafan-Bernal, Acevedo-Alba and Reyes-Pavon42) . Regarding dietary protein, a high-protein diet may increase the concentration of plasma amino acids in the short term, which may cause insulin resistance of skeletal muscle and the production of endogenous glucose(Reference Promintzer and Krebs43). In addition, animal protein-rich meals were more likely to lead to high plasma concentrations of branched-chain amino acids than plant protein-rich meals(Reference Brandsch, Shukla and Hirche44), which may be one cause of insulin resistance and type 2 diabetes mellitus(Reference Lynch and Adams45,Reference Newgard, An and Bain46) . In terms of carbohydrate intake, an average of 66·8 % of total carbohydrate was from grains in our study, meaning that complex carbohydrate was main component of total carbohydrate. A randomised crossover study revealed that a higher-complexity carbohydrate diet in GDM achieved glucose targets and lowered postprandial lipids(Reference Hernandez, Van Pelt and Anderson47). Additionally, a higher carbohydrate intake means lower fat and protein intakes. Thus, low-carbohydrate dietary pattern with high fat and protein, particularly animal fat and protein, may lead to insulin resistance and impaired glucose tolerance.
Considering that advanced maternal age, pre-pregnancy overweight or obesity, family history of diabetes and physically inactive lifestyle were risk factors for GDM(Reference McIntyre, Catalano and Zhang27), stratified analyses were performed in our study. Our results indicated that the relationship between the overall LCD score and GDM was positive among participants who were overweight or obese before pregnancy, suggesting that exploring the effects of low-carbohydrate dietary patterns on GDM in overweight or obese women may be potentially important in order to intervene as early as possible and improve pregnancy outcomes. However, the associations between the animal LCD score and GDM were not significant among participants in subgroups of advanced age (≥35 years), high pre-pregnancy BMI (<24 kg/m2), family history of diabetes and high physical activity (≥median), indicating that other factors such as genetic variants(Reference Zhang, Bao and Rong48) and environmental pollutants(Reference Jaacks, Barr and Sundaram49,Reference Zhang, Sundaram and Maisog50) might play roles in the development of GDM.
The strengths of our study include the following. We used the LCD score, a comprehensive indicator, to reflect the overall composition of the three macronutrients. Moreover, this study was the first to explore the association of LCD scores during the first trimester with GDM risk in China. Considering that sources of dietary protein and fat may have different effects on GDM, we also explored the effects of animal and plant LCD scores on the incidence of GDM. Since dietary information was assessed in early pregnancy before the diagnosis of GDM, our findings have important implications for preventing GDM. However, some limitations of our study should be acknowledged. First, measurement error, which is associated with recall bias of 24-h dietary recalls and recognition of portion size, may have resulted in inaccurate collection of food consumption. Second, the associations between the different types of dietary fatty acids may have different effects on the risk of GDM; however, we did not examine these associations, which should be explored in further studies. Third, although we adjusted for a number of socio-demographic and lifestyle factors, residual confounding might still exist. Finally, participants in our study were only from Southwest China, and the incidence of GDM was relatively high. There were some primary reasons to explain this high rate of incidence of GDM in our study. First, in recent years, the incidence of GDM has increased rapidly in China and was up to 24·2 % in urban areas according to the latest Chinese National Nutrition and Health Survey(Reference Wang51). Second, participants in our study underwent oral glucose tolerance test in summer and autumn, while Su WL et al. (Reference Su, Lu and Martini52) showed that summer and fall were associated with a higher risk of GDM diagnosis than winter. Third, participants in our study were only from one hospital with good medical resources in Southwest China, which may cause the sample of our study to not be representative. Thus, the extrapolation of our conclusions may be limited by the population and areas.
In conclusion, a low-carbohydrate dietary pattern with high animal fat and protein during the first trimester is positively associated with the incidence of GDM in Chinese women. However, a low-carbohydrate dietary pattern based on plant-derived food is not significantly associated with GDM risk. The proportions and sources of macronutrients may be important to take into consideration in nutritional intervention for the prevention of GDM.
Acknowledgements
The authors gratefully acknowledge the cooperation of the pregnat women who took part in this study. The authors also thank the all members of this study. This study was supported by the Danone Nutrition Center for Dietary Nutrition Research and Education (grant number DIC2016-06). The funding agency had no role in the study design, data collection, data analysis or writing of this article.
The authors’ responsibilities were as follows: G. Z. designed the research; H. D. analysed the data and wrote the manuscript; H. S. and C. C. edited the manuscript; X. P. and D. B. performed data entry; X. L., Y. Z. and J. Z. performed data collection; G. Z. supervised the study. All authors critically reviewed and approved the final manuscript.
All authors report no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114521000611








