Introduction
Scleractinian corals, which frequently dominate reefs and play a crucial role in building reef frameworks, first appeared in the early Mesozoic. Shallow-water coral reefs are typically distributed within the global tropical latitudinal belt, thriving in areas where mean sea-surface temperatures exceed 18°C (Spalding et al., Reference Spalding, Ravilious and Green2001). However, within this zone, they are generally absent in areas subjected to strong coastal upwelling, which inhibits coral growth and dispersal (Spalding et al., Reference Spalding, Ravilious and Green2001). Indeed, most coral reefs are concentrated in the Indo-Pacific region. Only 8% of the total known coral reefs are situated in the Caribbean and Atlantic. Notably, these reefs are largely absent from the Central Atlantic and the west coast of Africa.
Mature coral communities in the Atlantic, particularly around the Cabo Verde Archipelago, are scarce for various reasons (Spalding et al., Reference Spalding, Ravilious and Green2001; Ramalho, Reference Ramalho2011). Despite being a top priority for reef conservation, well-developed reefs are absent due to factors like upwelling, seasonal currents, Sahara dust, and sediment discharge (Grousset et al., Reference Grousset, Buat-Ménard, Boust, Tian, Baudel, Pujol and Vergnaud-Grazzini1989; Monteiro et al., Reference Monteiro, Almeida, Freitas, Delgado, Porteiro and Santos2008; Carpenter et al., Reference Carpenter, Fleming, Read, Lee, Moller, Hopkins and Purvis2010; Ramalho, Reference Ramalho2011; Gama et al., Reference Gama, Tchepel, Baldasano, Basart, Ferreira, Pio, Cardoso and Borrego2015). Volcanic activity and seawater acidification may also have hindered coral growth locally (Spalding et al., Reference Spalding, Ravilious and Green2001; Ramalho, Reference Ramalho2011).
In addition to extant coral communities, corals are also present in the fossil record of the Cabo Verde Islands, as reported in a number of studies, including those of Charles Darwin (1844), Bebiano (Reference Bebiano1932), Torres and Soares (Reference Torres and Soares1946), Serralheiro (Reference Serralheiro1970, Reference Serralheiro1976), Zazo et al. (Reference Zazo, Goy, Dabrio, Soler, Hillaire-Marcel, Ghaleb, González-Delgado, Bardají and Cabero2007, Reference Zazo, Goy, Hillaire-Marcel, Dabrio, González-Delgado, Cabero, Bardají, Ghaleb and Soler2010), Madeira et al. (Reference Madeira, Mata, Mourão, Brum da Silveira, Martins, Ramalho and Hoffmann2010, Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020), Ramalho et al. (Reference Ramalho, Helffrich, Cosca, Vance, Hoffmann and D. Schmidt2010a, Reference Ramalho, Helffrich, Schmidt and Vance2010b, Reference Ramalho, Helffrich, Cosca, Vance, Hoffmann and Schmidt2010c, Reference Ramalho, Winckler, Madeira, Helffrich, Hipólito, Quartau, Adena and Schaefer2015), Paris et al. (Reference Paris, Giachetti, Chevalier, Guillou and Frank2011, Reference Paris, Ramalho, Madeira, Ávila, May, Rixhon and Engel2018), Ramalho (Reference Ramalho2011), Johnson et al. (Reference Johnson, Baarli, Cachão, da Silva, Ledesma-Vázquez, Mayoral, Ramalho and Santos2012, Reference Johnson, Ramalho, Baarli, Cachão, da Silva, Mayoral and Santos2014, Reference Johnson, Baarli, Marques da Silva, Cachão, Ramalho, Santos and A. Mayoral2016, Reference Johnson, Baarli, Cachão, Mayoral, Ramalho, Santos and A. da Silva2018), Baarli et al. (Reference Baarli, Santos, Mayoral, Ledesma-Vazquez, Johnson, Da Silva and Cachão2013), Mayoral et al. (Reference Mayoral, Ledesma-Vazquez, Baarli, Santos, Ramalho, Cachão, Da Silva and Johnson2013, Reference Mayoral, Santos, Vintaned, Ledesma-Vazquez, Baarli, Cachão, da Silva and Johnson2018), Johnson and Baarli (Reference Johnson and Baarli2015), and Costa et al. (Reference Costa, Dawson, Ramalho, Engel, Dourado, Bosnic and Andrade2021). The islands of the archipelago, shaped by uplift and impacted by volcanic tsunamis, host a diverse marine fossil record primarily embedded in raised marine terrace deposits (generally formed during sea-level high stands; i.e., interglacial periods) and chaotic conglomeratic deposits (Ramalho, Reference Ramalho2011; Ramalho et al., Reference Ramalho, Winckler, Madeira, Helffrich, Hipólito, Quartau, Adena and Schaefer2015). Despite the rich fossil record, with most fossils dating to the Pleistocene (Torres and Soares, Reference Torres and Soares1946; Serralheiro, Reference Serralheiro1970, Reference Serralheiro1976; Serralheiro et al., Reference Serralheiro, Matos Alves, Macedo and J.R. Silva1974; Ramalho et al., Reference Ramalho, Helffrich, Cosca, Vance, Hoffmann and D. Schmidt2010a; Ramalho, Reference Ramalho2011), knowledge about fossil corals and their taxonomy remains limited.
This study provides the first systematic analysis of fossil corals from the Cabo Verde Archipelago, collected over several campaigns to study the Quaternary tsunami deposits and marine terraces of these islands. Following a taxonomic description, we delve into the temporal and spatial distribution of fossil corals, explore their origins and discuss the notable absence of extensive reef frameworks in the archipelago.
Study sites and geologic setting
The Cabo Verde Archipelago consists of a group of 10 main islands located in the north Atlantic about 600 to 900 km to the west of Senegal, Africa, between 14°40′N, 21°30′W and 17°30′N, 25°30′W (Fig. 1). The major islands are traditionally separated into two groups: the windward islands, including Santo Antão, São Vicente, Santa Luzia, São Nicolau, Sal, and Boavista, and the leeward islands, consisting of Maio, Santiago, Fogo, and Brava.
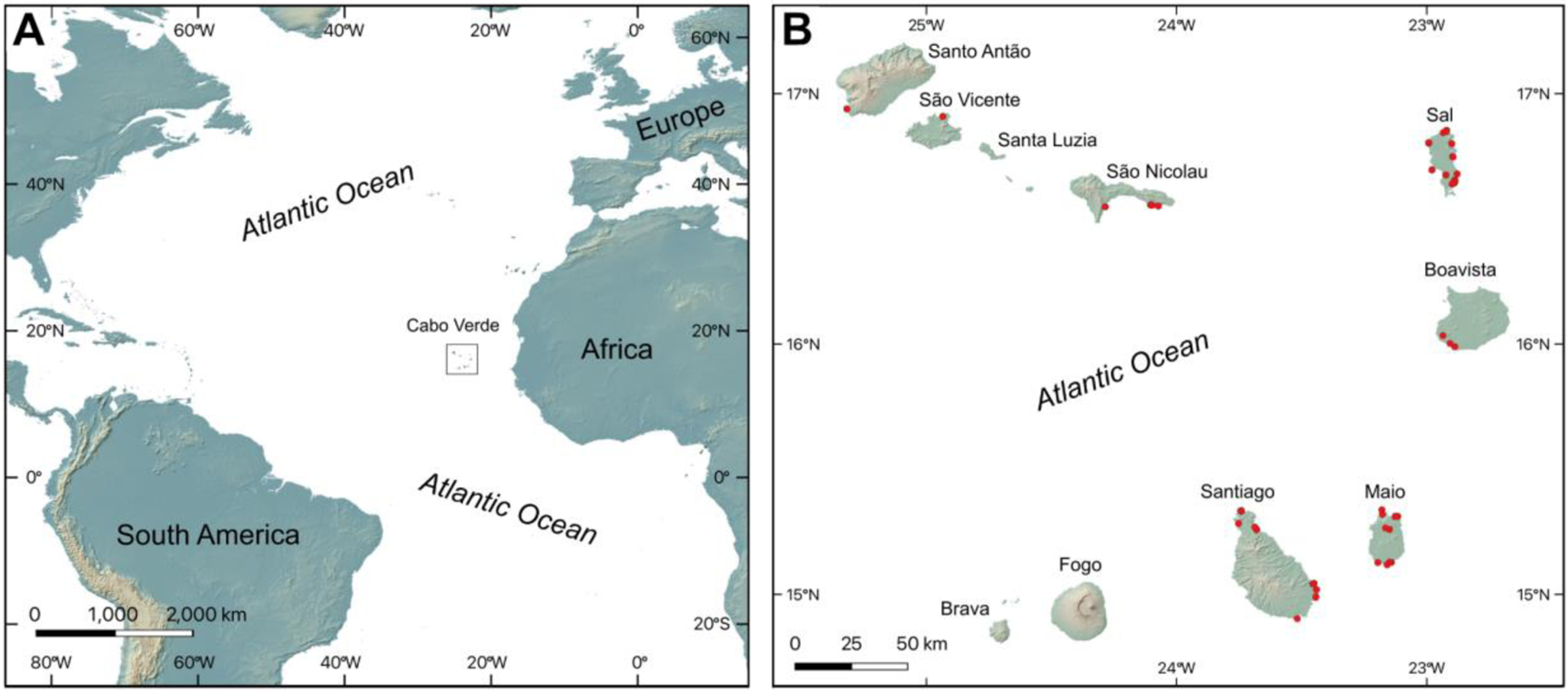
Figure 1. Location of the Cabo Verde Archipelago. (A) Cabo Verde Archipelago located off the western coast of Africa. (B) Location of sampling sites in the Cabo Verde Archipelago marked by red dots. Topography from NASA Shuttle Radar Topography Mission (2013) Global 1-arc second.
The Cabo Verde Islands are the result of long-term but intermittent midplate volcanism over an oceanic hot spot (Ramalho, Reference Ramalho2011). The formation of the archipelago started in the late Oligocene/early Miocene, with the eastern and northern islands emerging during the mid- to late Miocene, and into the Plio-Quaternary in the case of the younger edifices of Fogo and Brava (Ramalho, Reference Ramalho2011; Samrock et al., Reference Samrock, Wartho and Hansteen2019 and references therein).
The islands have an arid to semiarid climate with microclimatic variations and a two-season weather cycle, alternating between a prolonged drought and a temporary monsoon (with significant sediment discharge to the coast by torrential streams) caused by oscillations of the Intertropical Convergence Zone (Duarte et al., Reference Duarte, Romeiras, Gillespie, Clague, Gillespie and Clague2009; Ramalho, Reference Ramalho2011; Lobban, Reference Lobban2019). This climatic regime has contributed to the partial destruction of the fossil record and calcretization of the carbonates. However, marine fossiliferous deposits are still preserved on almost all islands except Fogo, with the older islands possessing more abundant and extensive marine terraces and tsunami deposits.
Materials and methods
Sampling
Coral samples were collected from seven islands of the Cabo Verde Archipelago, as illustrated in Figure 1B (Pleistocene fossil corals are also reported for Brava, but these were not included in this study; Madeira et al., Reference Madeira, Mata, Mourão, Brum da Silveira, Martins, Ramalho and Hoffmann2010). Samples were collected for the purpose of geochronology and do not represent an exhaustive and comprehensive effort to collect a statistically representative population of corals from Cabo Verde, and as such should be considered a preliminary, possibly incomplete approach to the study of fossil corals from this archipelago. Exact coordinates and localities of all samples are provided in Supplementary Tables 1a and 1b. Where outcrops comprised friable sediments, corals were directly collected from the outcrop. In outcrops consisting of consolidated/lithified sediments, coral samples were chiseled out. Each sample was appropriately labeled and briefly described in the field, with its precise location georeferenced using a multiband handheld GPS. At the same time, the elevation was further confirmed using a calibrated altimeter and by plotting sampling locations (in a geographic information system environment) over a 1:5000 digital cartographic database with 1-m-spaced contour lines, allowing for a precision of ±1–2 m.
At the University of Lisbon, Portugal, the samples were washed in distilled water and cut with a diamond-bladed rock saw to expose the interior of the coral or to obtain a manageable size for future studies on their geochronology. Samples were subsequently sent to the Staatliches Museum für Naturkunde, Stuttgart, Germany, for identification purposes and further studies.
All samples are stored at the Staatliches Museum für Naturkunde Stuttgart under the registration number SMNS-P-108.002-#. Supplementary Tables 1a and 1b list all sample and registration numbers.
Stratigraphy and ages
The studied fossil corals were sampled from raised beaches and tsunami deposits. Marine sediments are found both intercalated in the volcanic sequence (largely Pliocene and Pleistocene in age) and on top of the Quaternary volcanic sequences forming a staircase of raised beaches (Zazo et al., Reference Zazo, Goy, Dabrio, Soler, Hillaire-Marcel, Ghaleb, González-Delgado, Bardají and Cabero2007, Reference Zazo, Goy, Hillaire-Marcel, Dabrio, González-Delgado, Cabero, Bardají, Ghaleb and Soler2010; Ramalho, Reference Ramalho2011). Critically, well-exposed, in situ (i.e., attached to the substrate) incipient coral reefs are only known in two raised marine terraces (Fig. 2): one at Ponta das Bicudas in southern Santiago, where it can be found attached to pillow basalts (see Johnson et al., Reference Johnson, Baarli, Cachão, da Silva, Ledesma-Vázquez, Mayoral, Ramalho and Santos2012, Reference Johnson, Baarli, Cachão, Mayoral, Ramalho, Santos and A. da Silva2018; Baarli et al., Reference Baarli, Santos, Mayoral, Ledesma-Vazquez, Johnson, Da Silva and Cachão2013) and at the base of a well-consolidated calcarenite that, in turn, is covered by a subsequent lava delta sequence; and the second at approximately 15 m in elevation, exposed along the north shore of Sal, at Ponta Manuel Lopes, and further eastward along the coast, at the top of the sequence. The outcrop at Ponta Manuel Lopes (16°51′N, 22°56′W; mid-Pleistocene) is noteworthy for the size (up to 60 cm high) and area covered by the coral reef and should constitute a reference for the study of fossil corals in the archipelago.
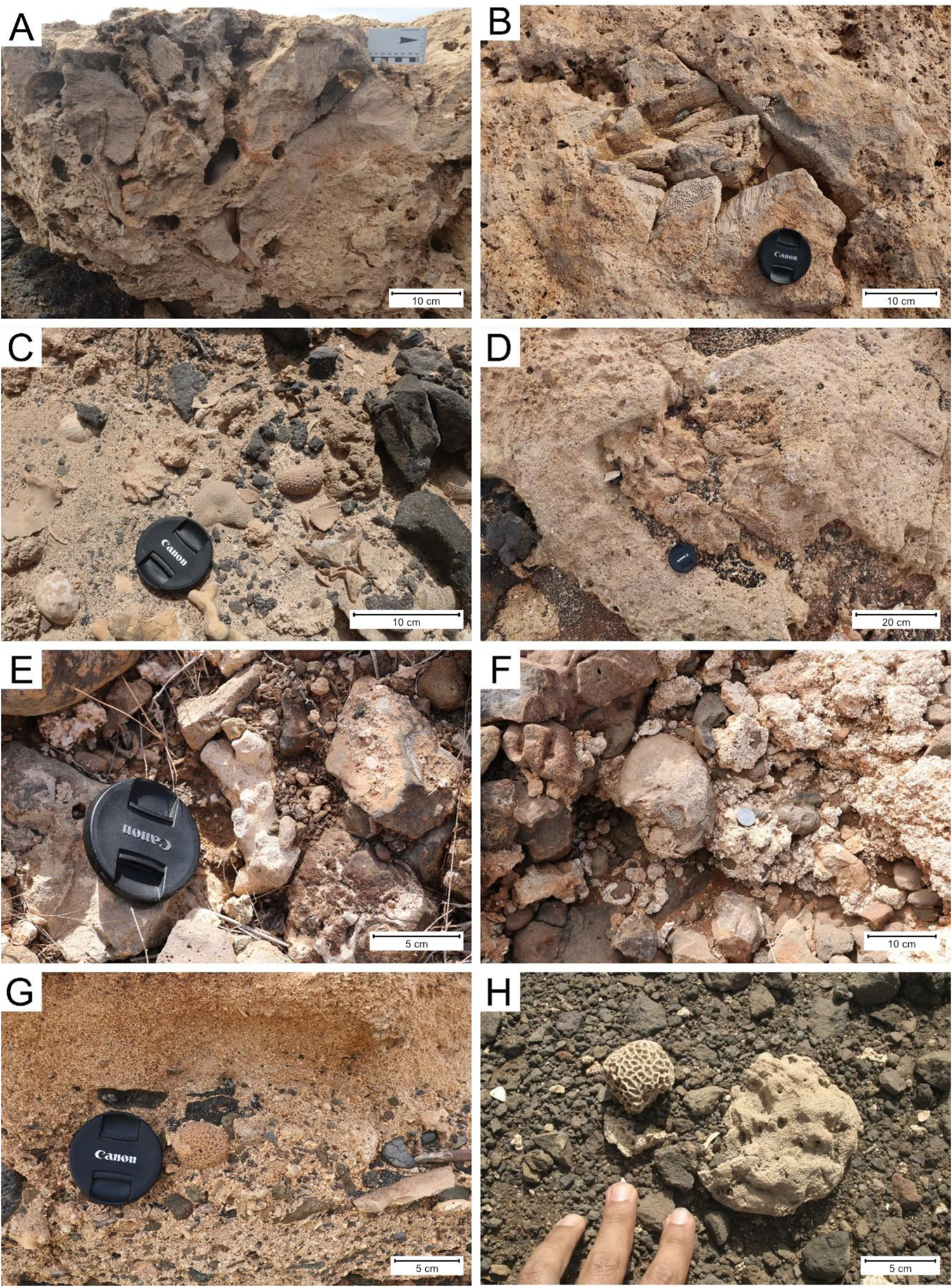
Figure 2. Examples of coral-bearing deposits. (A) In situ coral reef from a raised marine terrace at approximately 15 m in elevation at Ponta do Manuel Lopes, northern Sal Island. (B) In situ coral along the same marine terrace, 100 m farther east. (C) Single cerioid and dendroid corals from raised beach terrace at 5–6 m in elevation, along the coast east of Serra Negra, on Sal Island. (D) In situ coral at Ponta das Bicudas, Santiago Island. (E) Dendroid coral from tsunami deposit at Chão do Porto, northern Santiago Island. (F) Cerioid coral from tsunami deposit at Chão do Porto, northern Santiago Island. (G) Single cerioid coral from a tsunami deposit at Praia dos Barreiros, southeastern Maio Island. (H) Cerioid corals from marine terrace at Ponta do Atum, Santo Antão Island.
The sampled tsunami deposits lie on top of the volcano-stratigraphic sequence and are only locally covered by recent alluvial fans, sabkha deposits, or aeolian sands. They correspond to coarse to very coarse chaotic fossiliferous conglomerates with a bioclastic sand matrix and to bioclastic massive to thinly layered sandstones containing suspended large boulders. The tsunamiites usually overlie terrestrial units (fossil dunes, soils, alluvial fan deposits), blanket irregular subaerial topography, and contain abundant marine fossils demonstrating a marine origin and terrestrial deposition (e.g., see Paris et al., Reference Paris, Giachetti, Chevalier, Guillou and Frank2011, Reference Paris, Ramalho, Madeira, Ávila, May, Rixhon and Engel2018; Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020). The base of the tsunamiites is always erosional, and the deposits include clasts ripped up from both hard and soft substrates. Tsunami deposits usually present indicators of transport by bidirectional currents (landward and seaward). In these deposits, corals are usually found in isolation, entrained in the sandy/conglomeratic matrix, and among the matrix-supported lithic/volcanic pebbles and boulders. For a full characterization of the criteria used to identify tsunamiites and their different facies, as well as the occurrences of tsunamiites used in this study, we refer the reader to the works by Pérez-Torrado et al. (Reference Pérez-Torrado, Paris, Cabrera, Schneider, Wassmer, Carracedo, Rodríguez-Santana and Santana2006), Paris et al. (Reference Paris, Giachetti, Chevalier, Guillou and Frank2011, Reference Paris, Ramalho, Madeira, Ávila, May, Rixhon and Engel2018), Ramalho et al. (Reference Ramalho, Winckler, Madeira, Helffrich, Hipólito, Quartau, Adena and Schaefer2015), Madeira et al. (Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020), and Costa et al. (Reference Costa, Dawson, Ramalho, Engel, Dourado, Bosnic and Andrade2021). Raised beach deposits, in contrast, generally correspond to more horizontal and constant elevation deposits, which are laterally continuous (alongshore) and rest on low-angle surfaces cut in hard substrates, often abutting against a clear shore angle (Zazo et al., Reference Zazo, Goy, Dabrio, Soler, Hillaire-Marcel, Ghaleb, González-Delgado, Bardají and Cabero2007, Reference Zazo, Goy, Hillaire-Marcel, Dabrio, González-Delgado, Cabero, Bardají, Ghaleb and Soler2010; Ramalho et al., Reference Ramalho, Quartau, Trenhaile, Mitchell, Woodroffe and Ávila2013; Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020). Raised beaches typically exhibit normal grading, with well-stratified beds of well-rounded boulders/pebbles at the base (transgressive lag) or filling paleochannels; sedimentary structures are those typical of wave-beaten sandy beaches, displaying even and cross-bedding lamination, or swaley and/or hummocky cross-lamination, as well as frequent bioturbation identified by ichnoassociations (Zazo et al., Reference Zazo, Goy, Dabrio, Soler, Hillaire-Marcel, Ghaleb, González-Delgado, Bardají and Cabero2007; Ramalho, Reference Ramalho2011; Mayoral et al., Reference Mayoral, Ledesma-Vazquez, Baarli, Santos, Ramalho, Cachão, Da Silva and Johnson2013; Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020). In these deposits, corals may be found in situ (attached to the substrate), floating in the sandy matrix, often in coarser layers as “pebbles,” or in coral and rhodolith–rich beds interpreted as beach berm deposits.
These Quaternary sedimentary units (terraces and tsunami deposits) are only locally covered by Holocene deposits such as dunes and alluvial fans (Paris et al., Reference Paris, Giachetti, Chevalier, Guillou and Frank2011, Reference Paris, Ramalho, Madeira, Ávila, May, Rixhon and Engel2018; Ramalho et al., Reference Ramalho, Winckler, Madeira, Helffrich, Hipólito, Quartau, Adena and Schaefer2015; Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020), and their surfaces are frequently calcretized. The age of the coral-bearing units has mostly been determined based on the known volcano-stratigraphy of each island and on detailed field stratigraphic/geomorphological relationships between the fossiliferous outcrops and dated volcanic sequences and/or other sedimentary bodies (such as marine terraces). Coral samples collected in tsunami deposits were attributed the age of the tsunami (Ramalho et al., Reference Ramalho, Winckler, Madeira, Helffrich, Hipólito, Quartau, Adena and Schaefer2015; Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020), although the precise age of some tsunami deposits and marine terraces remains unknown. Moreover, tsunami deposits are known to entrain/rework fossil corals from previous marine terraces or older tsunami deposits (Paris et al., Reference Paris, Ramalho, Madeira, Ávila, May, Rixhon and Engel2018; Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020), and therefore the ages reported for corals collected on tsunami deposits should be interpreted as minimum ages.
All isotopic ages here reported correspond to those published in the literature, namely those reported by Ramalho et al. (Reference Ramalho, Helffrich, Cosca, Vance, Hoffmann and Schmidt2010c), Ramalho (Reference Ramalho2011), and Madeira et al. (Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020). For details on the dating methods and U-Th disequilibrium spectra, we refer to these original works.
The age and stratigraphic assessment of each coral sample are presented in Supplementary Table 1a. All ages in this study should be interpreted as indicative only, unless supported by higher-confidence isotopic U-Th disequilibrium geochronology (bold ages in Supplementary Table 1a).
Sample preparation
At the Staatliches Museum für Naturkunde, Stuttgart, Germany, a total of 168 fossil coral samples were analyzed. The samples were organized based on similarities using a binocular microscope. A concise summary of the key characters used to describe the macromorphology of the corals is provided in Supplementary Table 1b. The samples underwent a 3-minute ultrasonic bath, followed by rinsing with clean water. Any remaining dirt inside the corallites was meticulously removed using a toothbrush and/or a needle to enhance the visibility of corallites and skeletal structures. Once cleaned, the samples were left to dry for a minimum of 24 hours. To facilitate additional measurements and provide a clearer view of the calices, cross sections of coral samples were prepared. The coral samples were subsequently analyzed and photographed using a Keyence Digital Microscope VHX-S550 and a Leica S9i Digital Stereo Microscope for further identification purposes. In addition, scanning electron microscopy was used on some coral samples for detailed examination of skeletal components. The Image J measuring program (Schindelin et al., Reference Schindelin, Arganda-Carreras, Frise, Kaynig, Longair, Pietzsch and Preibisch2012) was used to assess coral structural dimensions, including corallite diameter, distance between corallites, septal thickness, septal margins, and thecal thickness.
Identification of fossil corals
All specimens examined in this study were identified to the genus or species level. The literature used for identification is summarized in Supplementary Table 2. Key characteristics for identification are based on coral morphology as proposed by Budd et al. (Reference Budd, Foster, Dawson and Johnson2001, Reference Budd, Fukami, Smith and Knowlton2012). Corals were primarily identified through a macromorphological approach using a stereoscopic microscope, occasionally supplemented by micromorphology (scanning electron microscopy) when a more detailed observation of the structure of walls, teeth, and granules was necessary.
Results
In the following sections, the systematic description of the studied specimens as well as the stratigraphy of the coral-bearing deposits are presented. An identification key to the described taxa is available as Supplementary Material.
Systematic description
Phylum Cnidaria Hatschek, 1888
Class Anthozoa Ehrenberg, 1834
Subclass Hexacorallia Haeckel, 1896
Order Scleractinia Bourne, 1900
Family Dendrophylliidae Gray, 1847
Genus Balanophyllia Wood, 1844
Balanophyllia sp.
Description: Corallum solitary. Costae hispid and well developed. Corallite diameter measures 11.7 mm on average. Columella spongy and elongated, medium width. Septa arranged in five cycles according to Pourtalès plan. In this study, the term “cycle” describes the arrangement of septa of different sizes as used by the Neogene Marine Biota of Tropical America (Budd et al. Reference Budd, Foster, Dawson and Johnson2001). Septal thickness equal. Synapticulotheca costate. See Figure 3A–C.
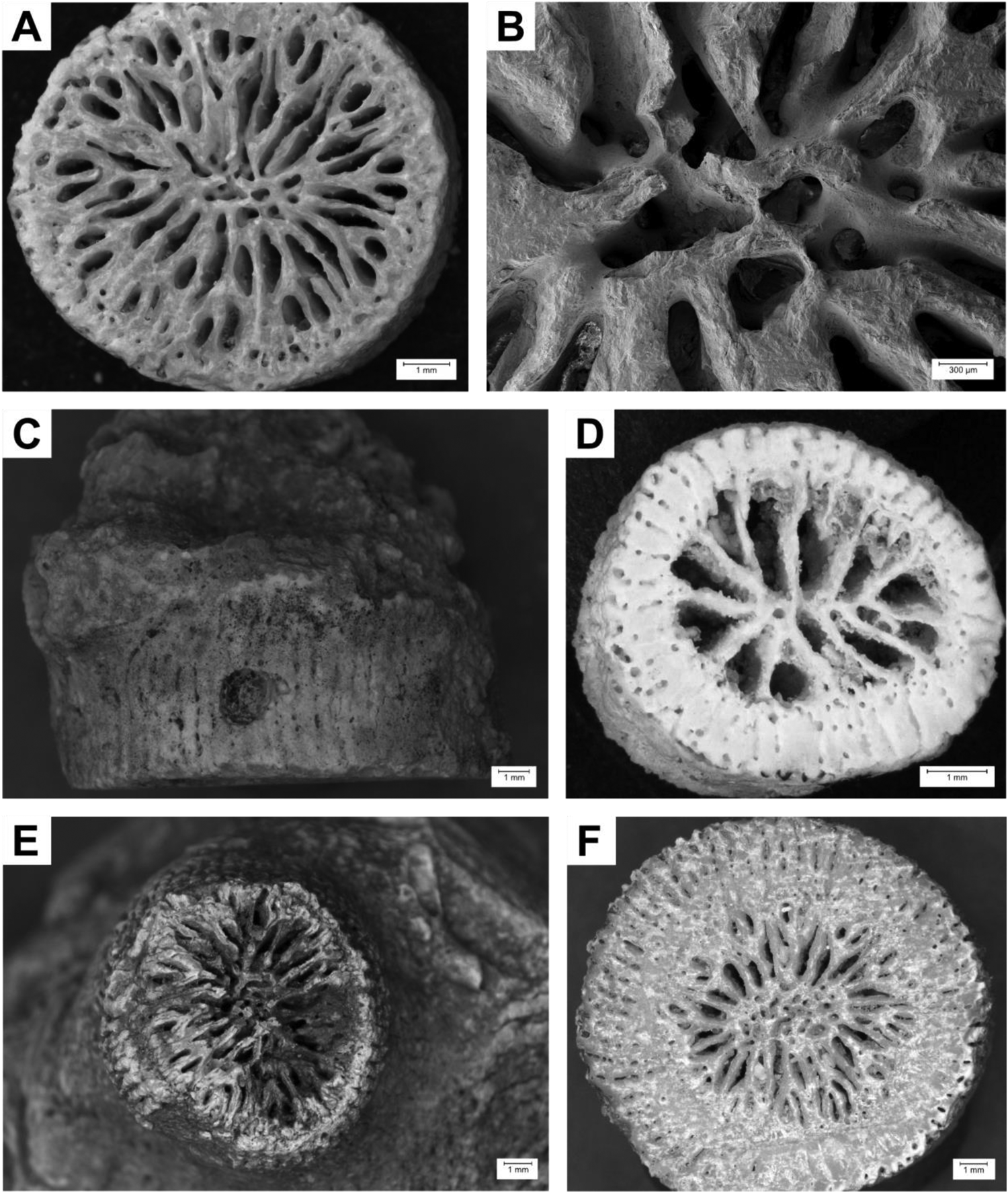
Figure 3. Photos of selected samples. (A–C) Balanophyllia sp. (STG38 – 1; SMNS-P-108.002-165), Santiago, Nossa Senhora da Luz (marine terrace 0–10 m), Marine Isotope Stage (MIS) 5e. (A and B) Transversal section; (C) lateral view. (D) ?Enallopsammia sp. (STG23 C; SMNS-P-108.002-162), Santiago, Praia do Tarrafal (marine terrace 15 m), no information on age, transversal section. (E and F) Dendrophyllia sp. (ST 71–2; SMNS-P-108.002-135), Santiago, Ribeira da Chã do Porto (tsunami deposit), MIS 4 to 5. (E) Corallite surface eroded or broken off; (F) transversal section.
Remarks: Within the Dendrophylliidae family, there are currently 364 recognized valid species, of which 198 are only known as fossils. The family exhibits considerable variability, including differences in growth form, septal arrangement, and absence or presence of zooxanthellae (Cairns, Reference Cairns2002).
The genus comprises two subgenera: Balanophyllia (Balanophyllia) and Balanophyllia (Eupsammia). Species of the first subgenus show a polycyclic development of the juvenile corallum and are firmly attached corals, while species of the second present a monocyclic development and usually remain unattached. Eupsammia seems to occur earlier in the fossil record, during the Late Cretaceous, whereas the polycyclic Balanophyllia probably evolved during the early Paleocene (Cairns, Reference Cairns2002).
Only sample ST7 2-2 presents the base of the corallum, due to how the sample blocks were prepared and the coral samples extracted. The relatively wide and polycyclic base of the corallum suggests that the coral is part of the subgenus Balanophyllia (Balanophyllia). Corallites may differ from their hexameral basis, making it difficult to infer cycles from septal sizes.
Occurrence: Santiago Island. The tsunami deposit has been dated to 84–65 ka (Ramalho et al., Reference Ramalho, Winckler, Madeira, Helffrich, Hipólito, Quartau, Adena and Schaefer2015), and the marine terrace is estimated as mid-Pleistocene. Further details can be found in Supplementary Tables 1a and 1b.
Genus Dendrophyllia De Blainville, 1830
Dendrophyllia sp.
Description: Corallum colonial and branching. Each branch formed by one round corallite with an average diameter of 16.2 mm. Columella fascicular and twisted, medium sized. Septal arrangement follows Pourtalès plan. Septa arranged in five cycles. Relative thickness of major and minor septa unequal. Small teeth along septal margins. Wall composed of coenosteum and porous. See Figure 3E and F.
Remarks: A total of 29 species have been documented and reported worldwide (Choi and Song, Reference Choi and Song2016). Dendrophyllia is the type genus of the family Dendrophylliidae (Cairns, Reference Cairns2002).
Coral samples found in a tsunami deposit of the upper Pleistocene. Samples similar to Dendrophyllia digitalis Michelin, 1842, a species identical to Dendrophyllia rutteni Gerth, 1921 (Umbgrove, Reference Umbgrove1950). Dendrophyllia rutteni is considered fossil only (Horton et al., Reference Horton, Kroh, Ahyong, Bailly, Boyko, Brandão and Costello2021).
Occurrence: Santiago Island. The tsunami deposit has been dated to 84–65 ka (Ramalho et al., Reference Ramalho, Winckler, Madeira, Helffrich, Hipólito, Quartau, Adena and Schaefer2015). Further details can be found in Supplementary Tables 1a and 1b.
Genus Enallopsammia Michelotti, 1871
?Enallopsammia sp.
Description: Sample probably part of a branch of a dendroid colony. Costae hispid. Corallite round with a diameter of 7.1 mm. Endotheca appears to be absent. Columella small and spongy. Septa arranged in three cycles, no apparent Pourtalès plan. Septal margins dentate. Corallite wall prominent with a diameter of about 1.3 mm, synapticulothecal. See Figure 3D.
Remarks: Similar to the genus Tubastraea, but without endotheca and dendroid instead of plocoid. The genus is most similar to Dendrophyllia but presents regularly arranged septa without Pourtalès plan (Cairns, Reference Cairns2002).
Coral sample mostly similar to corals of the genus Enallopsammia due to its prominent and distinctive wall structure.
Occurrence: Santiago Island. Mid- to late Pleistocene. Further details can be found in Supplementary Tables 1a and 1b.
Family Faviidae Milne Edwards & Haime, 1857
Genus Favia De Blainville, 1820
Favia fragum Esper, 1795
Synonyms: Madrepora fragum Esper, 1793; Astraea coarctata (Duchassaing & Michelotti, 1860); Astraea fragum (Esper, 1793); Astraea incerta (Duchassaing & Michelotti, 1860); Astrea ananas (Linnaeus, 1758); Favia ananas (Linnaeus, 1758); Favia coarctata Duchassaing & Michelotti, 1860; Favia incerta Duchassaing & Michelotti, 1860; Favia whitfieldi Verrill, 1901; Madrepora ananas Linnaeus, 1758.
Description: Corallum colonial and massive with pseudo-ceroid to subplocoid corallite arrangement. Calicular platform U-shaped. Calice shape round or angular, often altered by intratentacular budding, with an average diameter of 3.8 mm (ranging between 2.4 and 7.9 mm). Calice relief of corallites low to high. Columella trabecular and spongy, monocentric, and medium to thick. Septa arranged in three to four cycles. Number of septa per corallite ranging between 20 and 50. Due to taphonomic processes, the exact septal number and calice relief of each sample is hard to establish. Relative thickness of major and minor septa unequal. Small teeth positioned irregularly along septal margins. Wall structure septothecal. Synapticular rings absent. Costae mostly discontinuous. Coenosteum appears mostly highly reduced. See Figure 4A and B.
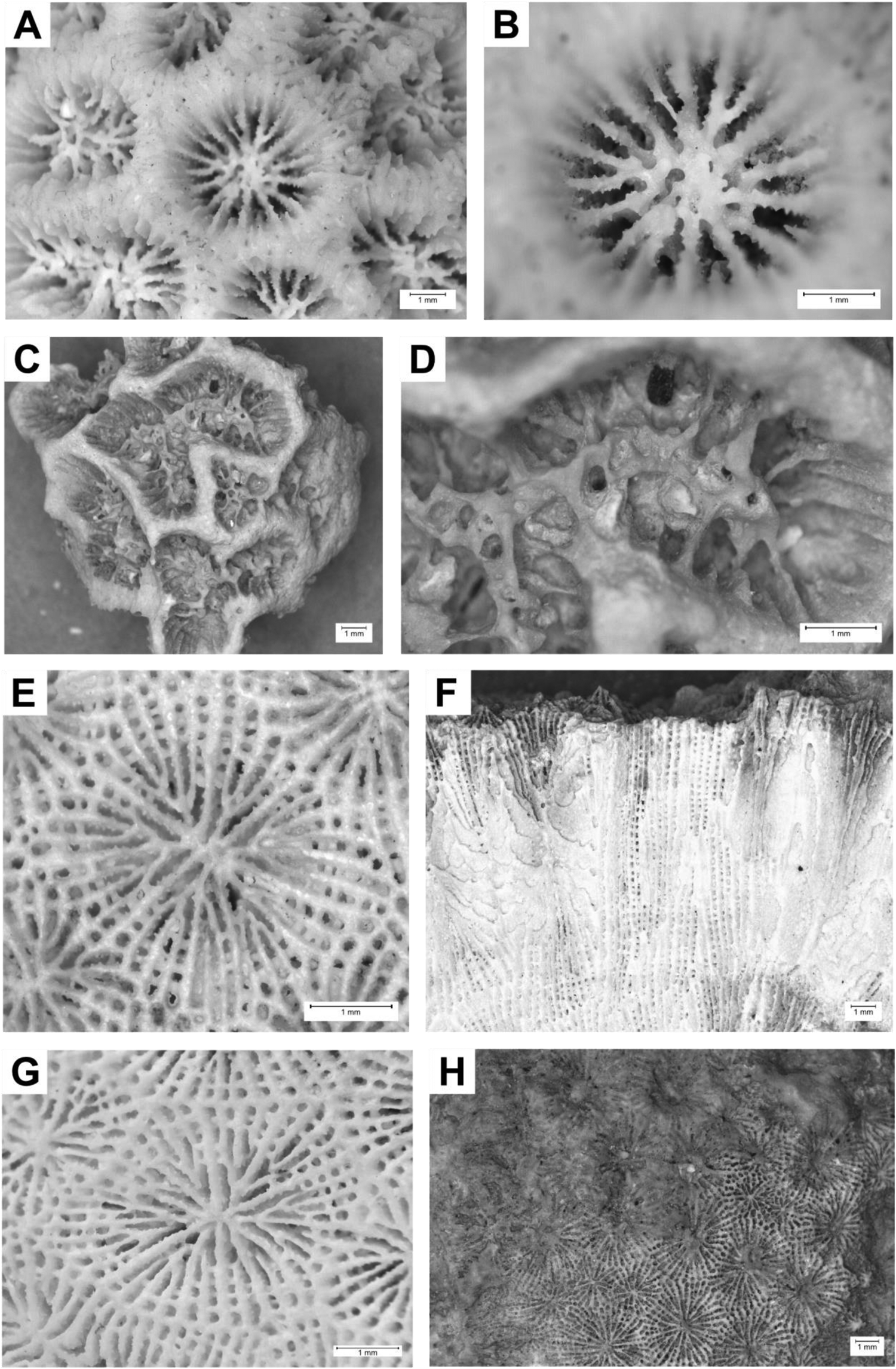
Figure 4. Photos of selected samples. (A and B) Favia fragum (ST 83–1; SMNS-P-108.002-146), Santiago, Nossa Senhora da Luz (marine terrace 0–10 m), Marine Isotope Stage (MIS) 5e, colony surface. (C and D) Favia cf. gravida (SN 60–4; SMNS-P-108.002-113), São Nicolau, Covoada de Chacina (marine terrace 28 m), Pleistocene, colony surface. (E and F) Siderastrea radians (ST 58–4), Santiago, Moia-Moia (marine terrace or tsunami deposit), Pleistocene. (E) Transversal section; (F) longitudinal section. (G and H) Siderastrea cf. siderea (STG 34–3; SMNS-P-108.002-163), Santiago, Nossa Senhora da Luz (marine terrace 0–10 m), MIS 5e. (G) Transversal section; (H) longitudinal section.
Remarks: After taxonomic revision, the genus is now restricted to the Atlantic (Budd et al., Reference Budd, Fukami, Smith and Knowlton2012) and exhibits significant differentiation both among its species (fragum and gravida) and across various biogeographic regions (Teschima et al., Reference Teschima, Zilberberg and Nunes2022). Favia most likely colonized the Cabo Verde Archipelago through at least two separate founder events from the provinces of Brazil and the Caribbean (Teschima et al. Reference Teschima, Zilberberg and Nunes2022). Species can vary in corallite arrangement, ranging from asteroid to meandroid (Zibrowius et al., Reference Zibrowius, Wirtz, Nunes, Hoeksema and Benzoni2014).
Dominant species of coral samples on Cabo Verde Islands, both fossil and extant (Monteiro et al., Reference Monteiro, Almeida, Freitas, Delgado, Porteiro and Santos2008). Favia fragum is amphi-Atlantic (Roos and Cadée, Reference Roos and Cadée2012). Zlatarski and Martínez-Estalella (Reference Zlatarski and Martínez-Estalella2018) point out the high variability of corallites attributed to ecological factors. Despite this acknowledgment, the complete explanation for the morphological divergence remains unclear. Corallites of F. fragum are generally well separated, yet there is a tendency for fusion, giving rise to a pseudo-ceroid appearance, similar to Favites (Riegl, Reference Riegl1996). Additionally, acute walls and higher calice relief might appear due to gradual erosion.
Occurrence: Boavista, Maio, Santo Antão, Sal, São Nicolau, Santiago, and São Vicente Islands. Ages estimated or dated (Ramalho et al., Reference Ramalho, Winckler, Madeira, Helffrich, Hipólito, Quartau, Adena and Schaefer2015; Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020) to the mid- to late Pleistocene. Further details can be found in Supplementary Tables 1a and 1b.
Favia cf. gravida Verrill, 1868
Description: Corallum colonial and massive with ceroid to plocoid corallite arrangement. Calicular platform U-shaped. Calice angular to elongated with an average diameter of 5.8 mm (ranging between 4.2 and 8.2 mm). Columella trabecular and spongy, polycentric, and thick. Septa arranged in three to four cycles. Number of septa per corallite ranging between 20 and 65. Due to taphonomic processes, the exact septal number and calice relief of each sample are hard to establish. Relative thickness of major and minor septa unequal. Small teeth along septal margins. Septothecal wall structure. Synapticular rings absent. Costae discontinuous. Coenosteum highly reduced to narrow. See Figure 4C and D.
Remarks: The identification of this species is controversial, primarily due to its resemblance to F. fragum. Notably, when eroded and fossilized, F. fragum “appears more meandroid, because of the gradual dissolution of thin septa and thin newly formed thecae”; however, it is known to be monocentric, as noted by Boekschoten and Borel Best (1988, p. 105). Species include fossil as well as recent specimens (Horton et al., Reference Horton, Kroh, Ahyong, Bailly, Boyko, Brandão and Costello2021).
Occurrence: Boavista, Maio, Sal, São Nicolau, and Santiago Islands. Ages estimated or dated (Ramalho et al., Reference Ramalho, Winckler, Madeira, Helffrich, Hipólito, Quartau, Adena and Schaefer2015; Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020) to the mid- to late Pleistocene. Further details can be found in Supplementary Tables 1a and 1b.
Family Pocilloporidae Gray, 1840
Genus Madracis Milne Edwards & Haime, 1849
Madracis cf. pharensis (Heller, 1868)
Description: Corallum colonial, growing into thick encruster with ceroid to subplocoid corallite arrangement. Calicular platform U-shaped. Calice round with an average diameter of 1.8 mm (ranging between 1.4 and 2.4 mm). Columella styliform, thin to medium sized. Septa arranged in two cycles. Primary septa thicker than secondary septa. Secondary septa often extremely reduced or weakly developed. Septal number per corallite ranging between 11 and 22. Small teeth along septal margins. Septo- to parathecal wall structure. Coenosteum reduced. See Figure 5E and F.
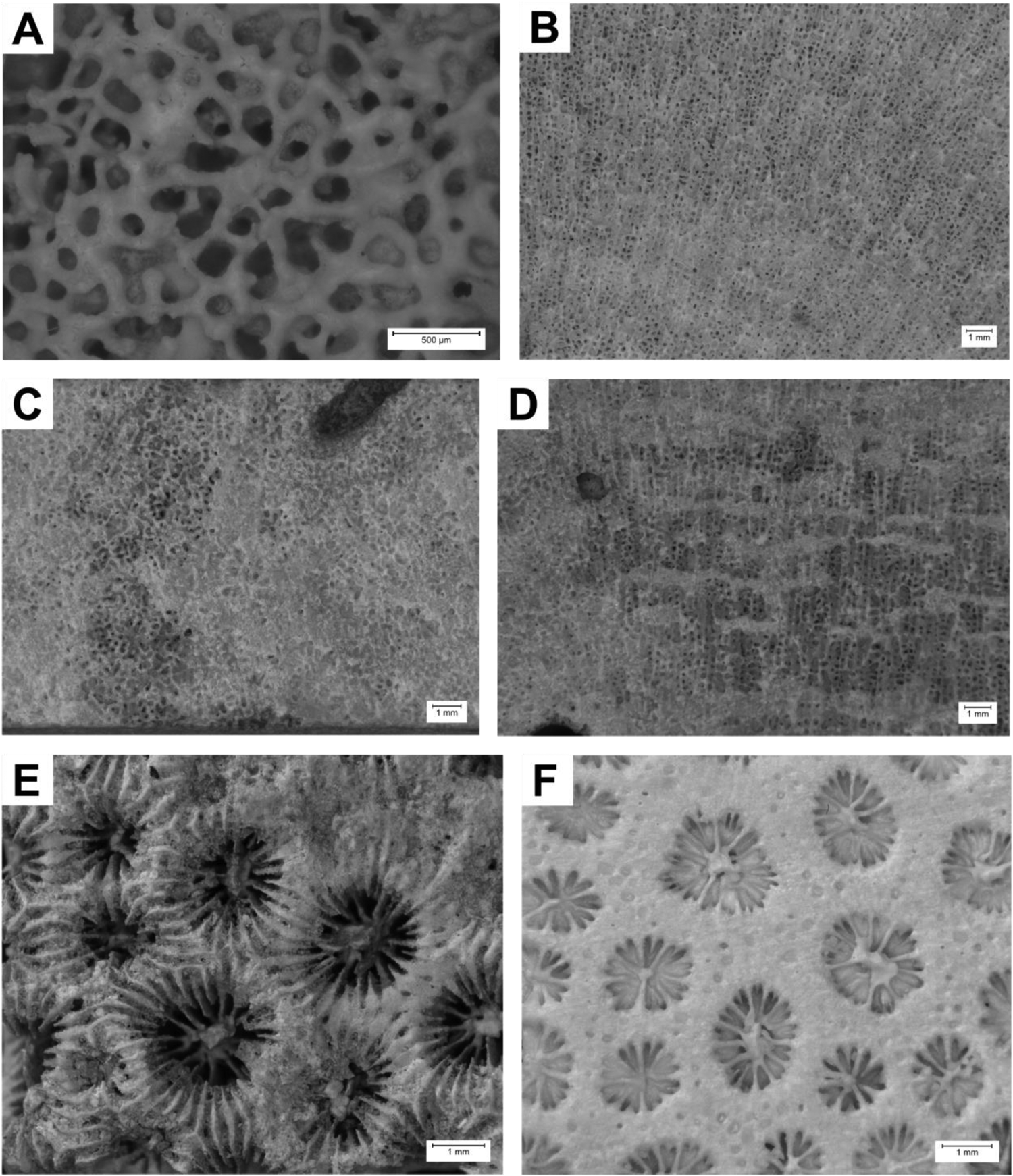
Figure 5. Photos of selected samples. (A and B) Porites porites (MO 24–2; SMNS-P-108.002-022), Maio, Barreiros Lagoon (marine terrace 2–5 m), Pleistocene. (A) Transversal section; (B) longitudinal section. (C and D) Porites sp. (ST 86–1; SMNS-P-108.002-152), Santiago, Nossa Senhora da Luz (marine terrace 0–10 m), Marine Isotope Stage (MIS) 5e. (C) Transversal section; (D) longitudinal section. (E and F) Madracis cf. pharensis (MO 28–1; SMNS-P-108.002-029), Maio, Ribeira do Lugar (tsunami deposit), MIS 11 to 12. (E) Colony surface partially eroded; (F) transversal section.
Remarks: Primarily azooxanthellate coral species (Neves and Johnsson, Reference Neves and Johnsson2009). High variety of color morphs and colony morphologies within the species. Fossils as well as recent species are present (Diekmann, Reference Diekmann2003). Common reef-building species. Occasional parathecal appearance of wall structure, such as shown in Figure 5E, might be a result of erosion. Nevertheless, in addition to a slightly elevated number of septa, this could be a differentiation from M. pharensis. Species fossil as well as recent (Diekmann, Reference Diekmann2003).
Occurrence: Boavista, Maio, Sal, São Nicolau, and Santiago Islands. Ages estimated or dated (Ramalho et al., Reference Ramalho, Winckler, Madeira, Helffrich, Hipólito, Quartau, Adena and Schaefer2015; Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020) between Early and late Pleistocene. Further details can be found in Supplementary Tables 1a and 1b.
Family Poritidae Gray, 1840
Genus Porites Link, 1807
Porites porites (Pallas, 1766)
Synonyms: Madrepora porites Pallas, 1766; Porites flabelliformis Le Sueur, 1820; Porites plumieri Duchassaing & Michelotti, 1864; Porites polymorpha Link, 1807; Porites polymorphus Link, 1807; Porites porites f. clavaria Lamarck, 1816; Porites porites f. typica Vaughan, 1901; Porites porites var. clavaria Lamarck, 1816; Porites recta Le Sueur, 1820; Porites solanderi Duchassaing & Michelotti, 1860.
Description: Corallum colonial, thick branches. Budding extracalicular. Corallite arrangement ceroid, even. Calice round to polygonal with an average diameter of 1.4 mm. Septa arranged in two complete cycles with 12 septa per corallite: one directive septum, four pairs of fused lateral septa, and a triplet of fused septa. Number of pali undetermined. Palar ring intermediate (30–40%). Columella present. Wall reticulum single to double. Inner skeletal structure of vertical trabecular rods and horizontal dissepiments. See Figure 5A and B.
Remarks: Due to its high skeletal plasticity and variability of corallite morphology, Porites is particularly taxonomically challenging (Tisthammer and Richmond, Reference Tisthammer and Richmond2018). While it is the most distributed genus of all reef-building corals worldwide (Garthwaite et al., Reference Garthwaite, Potts, Veron and Done1994), Atlantic and West Indian species show thicker skeletal structures compared with Indo-Pacific forms (Bernard, Reference Bernard1906). Porites presents an excellent fossil record (Forsman et al., Reference Forsman, Barshis, Hunter and Toonen2009), including the earliest record of Eocene age (Tisthammer and Richmond, Reference Tisthammer and Richmond2018).
Both fossil and recent species were identified. Living P. porites at the Cabo Verde Islands was first mentioned by Chevalier (Reference Chevalier1966).
Occurrence: Samples were found on three different islands within marine terraces and date back to the late Pleistocene: Maio, Sal, and Santiago Islands. The estimated ages place it in the mid- to late Pleistocene. Further details can be found in Supplementary Tables 1a and 1b.
Porites sp. 1
Description: Corallum colonial, small to large encruster. Budding extracalicular. Corallite arrangement ceroid, uneven. Calice round with an average diameter of 1.6 mm. Septa arranged in two complete cycles with 12 septa per corallite: one directive septum, four pairs of fused lateral septa, and a triplet of fused septa. Number of pali undetermined. Palar ring intermediate (30–40%). Columella present. Wall reticulum double. Inner skeletal structure of vertical trabecular rods and horizontal dissepiments. See Figure 5C and D.
Remarks: Coral samples share morphological characteristics with P. porites (septal arrangement) as well as Porites astreoides (colony form).
Occurrence: Santiago and São Nicolau Islands. SN 16-4 dated 283 ± 15.1 ka (Ramalho et al., Reference Ramalho, Helffrich, Schmidt and Vance2010b). The other two samples are estimated to date to the mid- to late Pleistocene. Further details can be found in Supplementary Tables 1a and 1b.
?Porites sp. 2
Description: Corallum colonial, encruster. Budding extracalicular. Epitheca reduced. Corallite arrangement ceroid, even. Calice round to polygonal with an average diameter of 1.2 mm. Columella present. Inner skeletal structure of vertical trabecular rods and horizontal dissepiments.
Remarks: The colony exhibits morphological characteristics consistent with Porites, including the inner skeletal structure. The original skeleton has been largely replaced by silica, in combination with extensive erosion, making identification nearly impossible.
Occurrence: Maio Island. Tsunami deposit dated to 422–443 ka (Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020). Further details can be found in Supplementary Tables 1a and 1b.
Family Rhizangiidae d'Orbigny, 1851
Genus Siderastrea Blainville, 1830
Siderastrea radians (Pallas, 1766)
Synonyms: Madrepora radians Pallas, 1766; Astraea (Siderastraea) galaxea (Ellis & Solander, 1786); Astraea (Siderina) galaxea (Ellis & Solander, 1786); Astraea galaxea (Ellis & Solander, 1786); Astraea punctifera Lamarck, 1816; Astraea senegalensis (Milne Edwards & Haime, 1849); Astraeopora punctifera (Lamarck, 1816); Astrea galaxea (Ellis & Solander, 1786); Astrea radians (Pallas, 1766); Astreopora punctifera (Lamarck, 1816); Explanaria galaxea (Ellis & Solander, 1786); Madrepora galaxea Ellis & Solander, 1786; Siderastraea radians var. pulchella Milne Edwards & Haime, 1848; Siderastrea circumfossata Thiel, Reference Thiel1928; Siderastrea galaxea (Ellis & Solander, 1786); Siderastrea senegalensis Milne Edwards & Haime, 1849; Siderina galaxea (Ellis & Solander, 1786).
Description: Corallum colonial, dome-shaped, and encrusting with ceroid corallite arrangement. Calicular platform V-shaped. Budding extracalicular. Calice subhexagonal with an average diameter of 3.3 mm (ranging from 2.5 to 4.0 mm). Columella trabecular, solid, and thin. Septa arranged in three to four cycles. First, second, and third cycles mostly complete; fourth cycle incomplete; and fifth and sixth cycles absent. Number of septa per corallite ranging between 24 and 48, mostly fewer than 40. Relative thickness of major and minor septa unequal. Small teeth along septal margins. Wall structure synapticulothecal. Two to four synapticular rings. Costae continuous. Coenosteum absent. See Figure 4E and F.
Remarks: Species can be identified by their septal numbers and patterns, wall structures, and corallite diameters (Foster, Reference Foster1980). Siderastrea is one of the major reef-building coral genera and shows great resistance to environmental stress (Menezes et al., Reference Menezes, Neves, Kikuchi and Johnsson2014). While contentious, based on morphological data, Siderastrea can be placed in the family Rhizangiidae (Löser et al., Reference Löser, Angel Fernández-Mendiola, Pérez-Malo, Domínguez Pascual and Cahuzac2021), which is yet to be confirmed by additional molecular data. Therefore, we decided to place Siderastrea in the family Rhizangiidae.
Coral species, both fossil and extant, have been identified in Cabo Verde Archipelago (Boekschoten and Borel Best, Reference Boekschoten and Borel Best1988). Extant S. radians colonies are prevalent in Cabo Verde but rare in cold-water areas of the West African coast (Laborel, Reference Laborel1974). The presence of amphi-Atlantic coral species has also been documented (Zibrowius et al., Reference Zibrowius, Wirtz, Nunes, Hoeksema and Benzoni2014).
Occurrence: Boavista, Maio, Sal, Santiago, and São Vicente Islands. Ages estimated or dated (Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020) to mid- to late Pleistocene. Further details can be found in Supplementary Tables 1a and 1b.
Siderastrea cf. siderea (Ellis & Solander, 1786)
Description: Corallum colonial and massive with ceroid corallite arrangement.
Calicular platform V-shaped. Budding extracalicular. Calice hexagonal and pentagonal with an average diameter of 5.4 mm (ranging from 5.2 to 5.5 mm). Columella trabecular, solid, and thin. Septa arranged in four to five cycles. First, second, and third cycles complete; fourth cycle incomplete; and fifth cycle incomplete or absent. Number of septa per corallite ranging between 40 and 46. Relative thickness of major and minor septa more or less equal. Small teeth along septal margins. Wall structure synapticulothecal. Two to four synapticular rings. Costae continuous. Coenosteum absent. See Figure 4 G and H.
Remarks: Taxonomically, the identification poses challenges due to discrepancies in the descriptions of corallite dimensions in the Atlantic S. siderea. Nevertheless, corallites in S. siderea typically appear larger and display more septa when compared with S. radians (Cairns, Reference Cairns1982). The species encompasses both fossil and extant corals (Horton et al., Reference Horton, Kroh, Ahyong, Bailly, Boyko, Brandão and Costello2021).
Occurrence: Santiago Island. Estimated age is mid- to late Pleistocene. Further details can be found in Supplementary Tables 1a and 1b.
Stratigraphic context and geochronology of the coral associations
The most diverse coral associations were found on Santiago Island with a total of seven different taxa, while Santo Antão Island only hosts one species. Fossil corals (Favia, Madracis, Balanophyllia, Dendrophyllia) from Santiago come from two tsunami deposits and two marine terraces. The younger tsunami deposit was emplaced at 85 to 65 ka, the age interval attributed to the impact of the tsunami triggered from nearby Fogo, based on cosmogenic 3He geochronology of megaclasts included in the deposits (Ramalho et al., Reference Ramalho, Winckler, Madeira, Helffrich, Hipólito, Quartau, Adena and Schaefer2015).
However, given that the high-energy tsunami transport and depositional process may erode and entrain corals from older deposits, some of the specimens embedded in the tsunami conglomerates may be older than the event, and correspond to Pleistocene sea-level high stands. Samples found in marine terraces at 0–10 m are postulated to belong to the mid- to late Pleistocene (Balanophyllia, Favia, Siderastrea, Porites).
All coral samples from Sal were found in marine terraces situated at elevations ranging from 0 to 20 m above sea level. Terraces found at elevations of 10 to 20 m are estimated to have been formed during the mid-Pleistocene, and include Favia, Madracis, Porites, and Siderastrea. Meanwhile, terraces at elevations of 0 to 10 m are presumed to correspond to both the mid- and late Pleistocene, featuring Favia and Siderastrea. Fossil coral samples found on São Nicolau were extracted from marine terraces at 9 to 28 m estimated to be mid-Pleistocene (Favia, Porites, Madracis), and samples found at 20 m (Porites, Favia) were dated 283 ± 15.1 ka by Ramalho et al. (Reference Ramalho, Helffrich, Cosca, Vance, Hoffmann and Schmidt2010c). Coral samples found in sediments between lava delta flows are postulated to correspond to the lower to mid Pleistocene (Madracis).
Coral samples from Maio were collected from four tsunami deposits and marine terraces at 2–5 m. Fossils from tsunami deposits have been dated at 78 to 80 ka (Siderastrea), 316 to 348 ka (Favia, Siderastrea), and 422 to 443 ka (Favia, Madracis, Porites) by Madeira et al. (Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020). Coral samples from marine terraces are estimated to be of mid- to late Pleistocene age, including representatives from Favia, Porites, and Siderastrea.
Fossil corals encompassing Favia, Madracis, and Siderastrea from Boa Vista and Favia from Santo Antão were found in marine terraces at 2 to 5 m, likely corresponding to the mid- to late Pleistocene. Additionally, corals from Santo Antão were sampled from a marine terrace that was carved on lava flows dated to 300 ± 70 ka (Ramalho, Reference Ramalho2011).
Samples from São Vicente, found in a potential tsunami deposit, are assumed to be from the mid- to late Pleistocene, with representatives such as Siderastrea and Favia. This assumption is supported by the fact that the deposit overlies lava flows from the youngest volcanic unit, dated at 330 ± 60 ka (Holm et al., Reference Holm, Grandvuinet, Friis, Wilson, Barker and Plesner2008).
Details can be found in Supplementary Tables 1a and 1b and Figure 6.
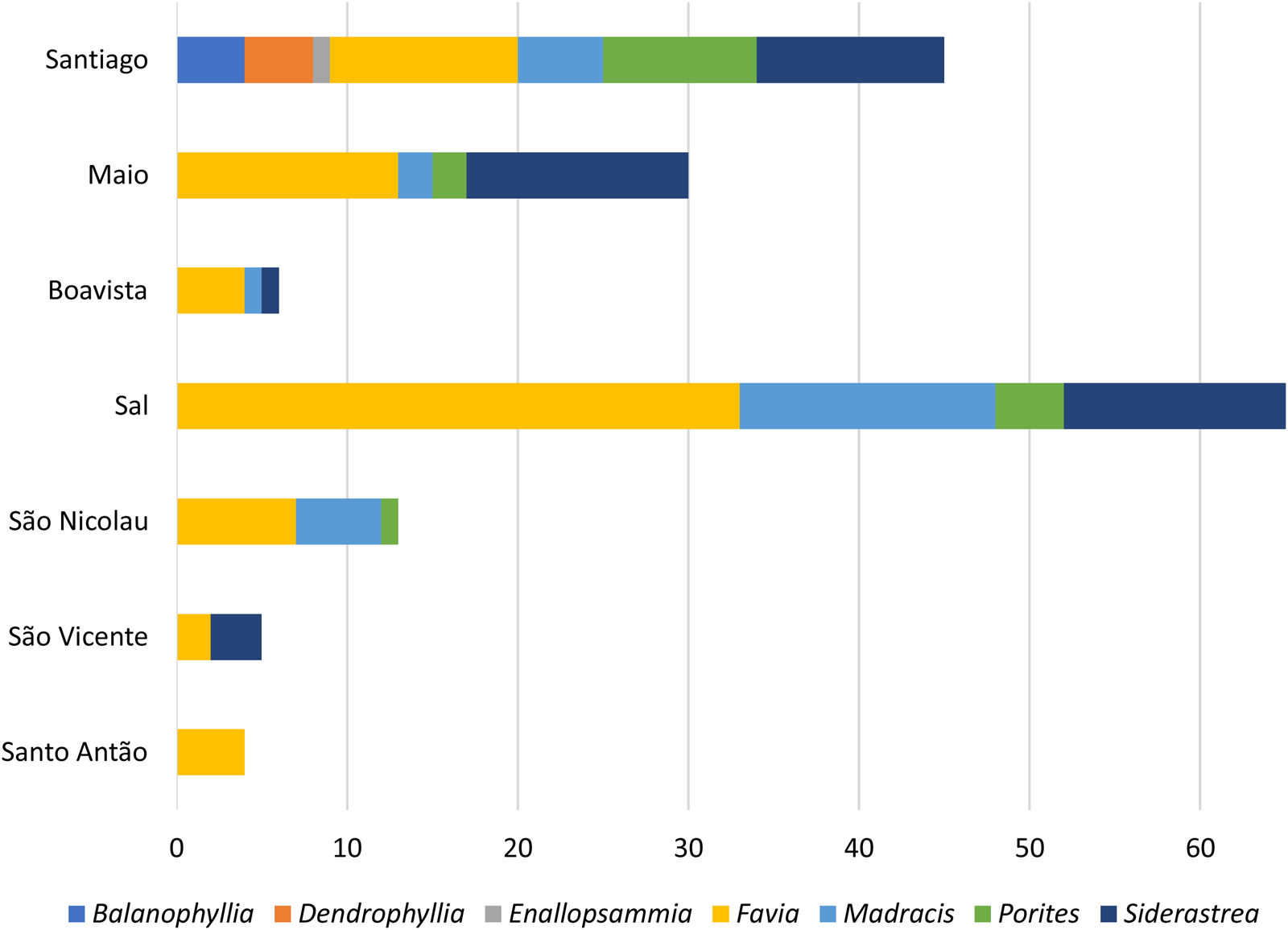
Figure 6. Number of coral specimens studied by genus, per island. Note that this is not a statistical representation of the distribution of coral genera in Cabo Verde, but of the number of specimens available for this study.
Discussion
In this study, a comprehensive analysis was conducted on a total of 168 fossil coral specimens allowing their identification at the genus and/or species level. Thirteen taxa were identified, representing 8 genera and 13 species. These corals were found in 46 different localities on seven islands of the Cabo Verde Archipelago (Santo Antão, São Vicente, São Nicolau, Sal, Boavista, Maio, and Santiago), corresponding to different levels of marine terraces and tsunami deposits (Fig. 2, Supplementary Tables 1a and 1b).
The most diverse coral associations were found on Santiago Island. All coral samples date back to the Quaternary, spanning from the Pleistocene to the Holocene. The most diverse association has been preliminarily attributed to Marine Isotope Stage 5 (MIS 5; Supplementary Tables 1a and 1b).
Taphonomy
Depending on geologic and environmental changes such as water temperature, tectonic events, and timing of burial, the skeletal structure of dead corals can remain intact and relatively unchanged. On oceanic islands, onshore deposits bearing fossil corals are usually preserved either in tsunami deposits or in marine terraces raised above sea-level high stands by the action of island uplift, as is seen in Cabo Verde (Ramalho et al., Reference Ramalho, Helffrich, Cosca, Vance, Hoffmann and D. Schmidt2010a, 2010b, 2010c; Ramalho, Reference Ramalho2011). Interestingly, corals from the high-energetic tsunami deposits are better preserved than those from raised marine terraces. While the former seem to be largely unabraded and exhibit a complete corallite surface, the latter are heavily abraded. This observation may appear contradictory, because tsunamis are high-energy events, but in fact this is a common trait of tsunami deposits, in which the en masse sediment transport by large tsunamis enables the inclusion and preservation of organic remains with fragile or delicate structures within the fine matrix of conglomerates (Paris et al., Reference Paris, Ramalho, Madeira, Ávila, May, Rixhon and Engel2018; Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020), which makes the identification easier. This makes tsunami deposits very particular fossil-lagerstätten (sensu Seilacher, Reference Seilacher1970), serving as an effective proxy for biodiversity within a specific paleoenvironment.
Comparison with extant taxa
Our findings align with the compilation of major extant coral taxa in the area documented in field studies conducted by Boekschoten and Borel Best (Reference Boekschoten and Borel Best1988), Morri et al. (Reference Morri, Cattaneo-Vietti, Sartoni and Bianchi2000), Molodtsova (Reference Molodtsova, Mironov, Gebruk and Southward2006), and Monteiro et al. (Reference Monteiro, Almeida, Freitas, Delgado, Porteiro and Santos2008) around the Cabo Verde Islands, as outlined by Lopes et al. (Reference Lopes, Freitas and Silva2014). The specimens recorded include corals from families such as Oculinidae, Pocilloporidae, Caryophylliidae, Faviidae, Rhizangiidae, Dendrophylliidae, and Poritidae, predominantly observed in shallow waters.
Apart from Oculinidae and Caryophylliidae, our study has identified the presence of the remaining families, including fossil specimens of S. radians. These specimens are notably abundant, existing both in contemporary sandy habitats and as fossils found within marine terraces and tsunami deposits. While living F. fragum only appears occasionally at bedrock reefs/patches and in Siderastrea pavements (Monteiro et al., Reference Monteiro, Almeida, Freitas, Delgado, Porteiro and Santos2008), a considerable number of fossil specimens of this species can be found in Cabo Verde. This disparity may be attributed to the lower resistance of F. fragum to cold water or prolonged exposure to elevated temperatures (Roos and Cadée, Reference Roos and Cadée2012). Today, upwelling along the West African shelf decreases the water temperature, and thus contributes to an unfavorable environment for Favia (Spalding et al., Reference Spalding, Ravilious and Green2001; Ramalho, Reference Ramalho2011). During the Quaternary, particularly during warmer interglacial periods, when most terraces were formed (e.g., MIS 5e and MIS 11), living conditions may have been more suitable for Favia, which would explain the relatively high number of fossil Favia specimens. Siderastrea radians, on the other hand, is known for its efficient response to environmental stresses, such as changes in salinity (Boekschoten and Borel Best, Reference Boekschoten and Borel Best1988). Therefore, both extant and fossil specimens of S. radians are found on the archipelago.
Biogeography
The biogeographic distribution of corals is shaped by a complex interplay of evolutionary, ecological, and geographic factors (Veron, Reference Veron1995; Dawson, Reference Dawson2002; Cairns, Reference Cairns2007; Veron et al., Reference Veron, Stafford-Smith, DeVantier and Turak2015). The existence, continuity, or loss of favorable ecological conditions greatly influences coral distribution. Ongoing changes in environmental factors, such as temperature, water quality, and substrate availability, can impact the ecological suitability of an area for coral growth (Dawson, Reference Dawson2002; Cairns, Reference Cairns2007). This, in turn, affects the presence or absence of coral species. The dispersal and geographic range changes of corals through time are influenced by various geologic and environmental factors. Tectonic activity, eustatic sea-level fluctuations, climate change, oceanographic variations, and geomorphological alterations all contribute to the expansion or reduction of coral ranges.
To the best of our knowledge, Thiel (Reference Thiel1928) was the first to suggest a hypothesis regarding the origin of West African corals. After studying the currents of the Atlantic, Thiel (Reference Thiel1928) proposed that coral larvae most likely migrated from Africa to America. According to this model, Indo-Pacific coral species have crossed the Cape of Good Hope and settled down along the West African coast, carried by the Benguela Current. From there, the south-equatorial current transported the different species to South America and the Caribbean (Chevalier, Reference Chevalier1966). If this is true, the West African coral species of the genera Siderastrea, Porites, and Favia, for instance, would be older than those of the Caribbean. Budd (Reference Budd2000) determined the ages of different Caribbean coral genera and found that Siderastrea and Favia date back to the middle to late Eocene, and Porites from the Oligocene to the earliest Miocene.
The oldest samples studied herein, including the genera Porites, Madracis, and Favia, are from the Pleistocene (443 to 422 ka; Madeira et al., Reference Madeira, Ramalho, Hoffmann, Mata and Moreira2020) (Supplementary Table 1a). Nevertheless, there are fossil corals in sediments from the Pliocene and possibly even from the late Miocene. These are, however, poorly preserved and were not collected, as we are aware that they fall outside the range of U-Th geochronology (which, at most, can date corals up to 600 ka). Chevalier (Reference Chevalier1966) stated that it would have been almost impossible for reef corals with delicate and fragile structures, like those of Favia, to even pass the Cape of Good Hope, and the Benguela Current is possibly too cold for larvae to survive the journey (Rommerskirchen et al., Reference Rommerskirchen, Condon, Mollenhauer, Dupont and Schefuss2011; Woolsey et al., Reference Woolsey, Byrne and M. Baird2013). Consequently, Thiel's hypothesis can most certainly be rejected.
At this point, various authors, including Chevalier (Reference Chevalier1966), suggested that most West African corals originate from the Caribbean, more specifically the Antilles. In this scenario, larvae were transported from the Antilles to West Africa by the Gulf Stream and the Canaries Current. Meanwhile, some corals might have been transported by the Brazil Current and the South Atlantic Current from the Caribbean to South Africa, which would explain the presence of, for example, Siderastrea at the Cape of Good Hope. These migrations probably happened when currents were different and Atlantic Ocean waters were slightly warmer than today, and only resistant genera like Siderastrea and Porites could survive the unfavorable West African environment. Chevalier (Reference Chevalier1966) noted that due to the samples’ relatively young age and minimal diagenesis, the origin of West African corals must be relatively recent (Quaternary) and that migration, therefore, must have started sometime during the Neogene. Laborel (Reference Laborel1974) and other authors expressed skepticism about Chevalier's interpretations, citing sampling bias and uncertainties. The skepticism arose from the fact that the fossil corals in question came exclusively from old museum collections (Laborel, Reference Laborel1974; Boekschoten and Borel Best, Reference Boekschoten and Borel Best1988). Additionally, as mentioned earlier, fossil corals can be found in sediments from the Pliocene and possibly even from the late Miocene, in the Cabo Verde Archipelago, and therefore some coral species must have migrated even before the Quaternary.
Endemism
Chevalier (Reference Chevalier1966) established that 20 (8 zooxanthellate and 12 azooxanthellate) out of 41 coral species found in West Africa originated from there and are an indication of a high degree of endemism in this region. The author mentions that West Africa shares most of its coral species with the Caribbean, with a total of 16 species in common, and that several others show close similarities with the Caribbean ones. As illustrated by Chevalier (Reference Chevalier1966), the African Porites hentscheli, was noted for sharing characteristic traits with the Caribbean P. astreoides. Today, P. hentscheli is acknowledged as a synonym of P. porites (Horton et al., Reference Horton, Kroh, Ahyong, Bailly, Boyko, Brandão and Costello2021). Twelve additional azooxanthellate species are shared with the Atlantic, 12 with the Mediterranean, 11 with the Indo-Pacific, and 9 with the Pacific (Chevalier, Reference Chevalier1966). Later studies by Boekschoten and Borel Best (Reference Boekschoten and Borel Best1988) indicate that meandroid forms of the genus Favia, which can be found along the Brazilian and West African coasts, may have been imported to Cabo Verde in ballast waters of salt vessels. Most probably, however, the only existing species could be F. fragum, which because of the gradual dissolution of thin septa and thin newly formed theca, may appear more meandroid (Boekschoten and Borel Best, Reference Boekschoten and Borel Best1988). Furthermore, it has been shown that fossil and extant coral species of the African genus Schizoculina seem to be limited to the island of Boavista and are therefore an example of erratic colonization of distant islands by an organism that is not a very versatile traveler (Boekschoten and Borel Best, Reference Boekschoten and Borel Best1988).
Origin of West African and Cabo Verde corals
Although we cannot exclude the existence of older fossil corals that have not yet been dated, our results support the paleobiogeographic scenarios of Chevalier (Reference Chevalier1966) and Laborel (Reference Laborel1974). The coral species found on the Cabo Verde Islands match those on other West African islands shown by Laborel (Reference Laborel1974), especially the high abundance of S. radians on almost every island of the archipelago and in the Caribbean. The age of our coral samples from the uplifted marine terraces and their relatively limited modification support Chevalier's theory about a recent migration as well as his theory of most of the species’ origins (Chevalier, Reference Chevalier1966; Ramalho, Reference Ramalho2011). Nevertheless, the existence of older coral samples needs to be considered, and further studies are required to determine the exact age and species of the coral specimens found in sediments from the Pliocene and possibly even from the late Miocene.
Due to their widespread presence in the Indian and Central Pacific Oceans, azooxanthellate corals, such as the Dendrophylliidae, are believed to have originated from the Indo-Pacific (Chevalier, Reference Chevalier1966; Laborel, Reference Laborel1974). Additionally, this group is a cold-water indicator and is therefore only found in deep or cold shallow waters, as is the case around the West African coast (Laborel, Reference Laborel1974). This would explain our findings of Dendrophyllia sp., Balanophyllia sp., and Enallopsammia sp. in the fossil record of Cabo Verde, and their present-day absence.
Favia fragum was first thought to originate from the Indo-Pacific (Chevalier, Reference Chevalier1966). However, there are nomenclatorial as well as taxonomic issues associated with the species, and according to several authors, the type species status for Favia has been erroneously attributed, while this species possibly belongs to Dichocoenia (Veron, Reference Veron2015) and all former Indo-Pacific species of Favia are now placed in Dipsastraea (Budd et al., Reference Budd, Fukami, Smith and Knowlton2012). While the origin of West African F. fragum remains unclear, a study of population genetic parameters of Favia suggests that Cabo Verde “was likely colonized by at least two independent founder events, one from the Caribbean and another from the Brazilian provinces” (Teschima et al., Reference Teschima, Zilberberg and Nunes2022, p. 523).
Where are the reefs?
Extant corals in Cabo Verde are not able to form full reefal frameworks (Spalding et al., Reference Spalding, Ravilious and Green2001; Monteiro et al., Reference Monteiro, Almeida, Freitas, Delgado, Porteiro and Santos2008; Ramalho, Reference Ramalho2011). Several factors contribute to this limitation: (1) One factor is the small size of specimens, which may suggest that they never reached the size necessary for merging and intergrowing to form a reef framework. Factors such as persistent dusty conditions (transported from the Sahara by trade winds), substrate disturbance, fluctuations in sea level, and to a lesser extent, volcanic activity and tsunamis, likely contributed to short-lived and intermittent coral growth. (2) The torrential regime associated with Cabo Verde's (present-day and likely past) arid to semiarid climatic conditions also means that during the rainy season, large sediment plumes may form at the mouth of the canyons due to muddy riverine discharge, greatly reducing water visibility and affecting coral growth, at least in the short term. Presumably, these conditions also prevailed during most of the Quaternary, as reefs are also absent in the fossil sites studied herein. (3) For those corals found in tsunamiites or any other catastrophic deposits, the depositional processes involved would have significantly disrupted any framework that might have developed before the tsunami struck. (4) The coralline algae and microbialite, acknowledged contributors to reef framework and reported for Cabo Verde (Darwin, Reference Darwin1844; Johnson et al., Reference Johnson, Baarli, da Silva, Cachão, Ramalho, Ledesma-Vázquez, Mayoral and Santos2013; Gabriel and Fredericq, Reference Gabriel and Fredericq2019), may face challenges in finding the optimal environmental conditions for binding with corals. (5) Coastal upwelling along the African shelf brings cold and nutrient-rich water, while the Gulf of Guinea river discharge contributes warm, nutrient-rich water with low-salinity. (6) The reef-forming genera commonly found in the Caribbean, including Orcibella, Montastrea, Diploria, Pseudodiploria, and the elkhorn Acropora (Spalding et al., Reference Spalding, Ravilious and Green2001), have not been observed in Cabo Verde. Furthermore, the presence of dendrophylliid corals may be indicative of even cooler water temperatures than today. The majority of our dendrophylliid specimens have been extracted from the main tsunami deposit (84–65 ka), which corresponds to a glacial stage when the sea level was 60 m lower than today and the waters presumably colder (Ramalho, Reference Ramalho2011).
The absence of coral reefs seems to be due to ecological factors rather than the biogeographic distribution and larval recruitment of reef-building species. This is supported by the observation that the taxa documented and discussed herein, such as Siderastrea, Porites, and Favia, are generally capable of forming coral reef frameworks in other locations (Teschima et al., Reference Teschima, Zilberberg and Nunes2022).
Conclusions
Fossil corals from the Pleistocene to Holocene period, collected from seven islands of Cabo Verde, encompass 13 taxa, including a solitary species. Our study brings new insights into the origin of West African corals, supporting the theory that zooxanthellate corals migrated from the Caribbean to the West African coast, while azooxanthellate species likely originated from the Indo-Pacific. The current environmental circumstances make it harder for reef corals to survive in shallow waters around the Cabo Verde Islands, which may explain the lack of true reefs. However, true reefs are also very rare in the fossil record, even though water parameters must have made it somehow possible for the corals to migrate to the East Atlantic. In this instance, the fossil samples themselves could provide additional information about the circumstances surrounding their migration. Further studies on the Cabo Verde fossil samples may uncover more about the past environment and aid our understanding of the influence of different stress factors on coral growth in the face of climate change. The presence of older coral samples should also be taken into consideration, and additional studies are needed to ascertain the exact age and species of the coral specimens found in sediments from the Pliocene and possibly even from the late Miocene.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/qua.2024.20.
Acknowledgments
We express our gratitude to the staff at Staatliches Museum für Naturkunde Stuttgart, particularly Achim Lehmkuhl for sample preparation and Christoph Wimmer-Pfeil for thin-section preparation. Additionally, we extend our thanks to Hannes Löser for his valuable advice and assistance with literature. We also acknowledge the significant contributions by two anonymous reviewers, senior editor Nicholas Lancaster, and associate editor A.E. Mauz, whose insights and feedback greatly enhanced the original version of the article.
Funding
This study was financially supported by projects IF/01641/2015 MEGAWAVE and PTDC/CTA-GEO/28588/2017 - LISBOA-01-0145-FEDER-028588 UNTIeD, funded by FCT–Fundação para a Ciência e a Tecnologia I.P., the latter of which was also cofunded by the European Regional Development Fund–ERDF, through Programa Operacional Regional de Lisboa (POR Lisboa 2020). JM acknowledges funding from projects CVPLUME (PTDC/CTE-GIN/64330/2006) and (PIDDAC)–UIDB/50019/2020 to Instituto Dom Luiz. This work was also financially supported by the German Science Foundation, project DFG Project RA 1597/3-1 “Rhodoliths from the Cape Verde Archipelago: Insights into Climate Change and Megatsunami Sediment Dynamics.”
Competing interests
The authors declare no competing interests.









