INTRODUCTION
Neurofibromatosis type 1 (NF1) is a rare autosomal dominant genetic disorder, with a prevalence of 1 in 3000–4000 (National Institute of Neurological Disorders and Stroke, 2011). Diagnostic criteria for the disorder were established by the National Institutes of Health (NIH, 1988). A diagnosis of NF1 is made when two or more of the following criteria are met: a first degree relative with NF1, six or more cafe au lait patches, axillary or inguinal freckling, two or more neurofibromas or one plexiform neurofibroma, optic glioma, two or more Lisch nodules, or distinctive osseous lesions. Nonetheless, learning disabilities are the most frequent complication in children with NF1, varying from 30% to 65% (Cutting, Clements, Lightman, Yerby-Hammack, & Denckla, Reference Cutting, Clements, Lightman, Yerby-Hammack and Denckla2004).
Cognitive impairment has often been inconsistent, and a specific cognitive phenotype associated with NF1 remains unclear (Levine, Materek, Abel, O’Donnell, & Cutting, Reference Levine, Materek, Abel, O’Donnell and Cutting2006). Most studies have shown a general intellectual functioning within the average range, although somewhat lower than children in normative samples or comparison groups (Lehtonen, Howie, Trump, & Huson, Reference Lehtonen, Howie, Trump and Huson2013). Cognitive disturbances in children with NF1 are heterogeneous and affect language, reading, spelling, mathematical skills, praxis and visuo-spatial abilities, memory, attention, and executive functions (EFs) (Cutting et al., Reference Cutting, Clements, Lightman, Yerby-Hammack and Denckla2004; Levine et al., Reference Levine, Materek, Abel, O’Donnell and Cutting2006). Executive functioning refers to a superordinate capacity underlying goal-directed behavior, which is particularly important in novel or demanding situations (Shallice & Burgess, Reference Shallice and Burgess1991; Stuss, Reference Stuss1992). It is usually considered to be mediated by the prefrontal cortex and its subcortical connections.
Over the past 10 years, an increasing body of evidence has suggested that executive dysfunction is a core deficit in children with NF1 (Plasschaert et al., Reference *Plasschaert, Van Eylen, Descheemaeker, Noens, Legius and Steyaert2016; Ribeiro, Violante, Bernardino, Edden, & Castelo-Branco, Reference Ribeiro, Violante, Bernardino, Edden and Castelo-Branco2015; Roy et al., Reference *Roy, Roulin, Charbonnier, Allain, Fasotti, Barbarot and Le Gall2010, Reference *Roy, Barbarot, Roulin, Charbonnier, Fasotti, Stalder and Le Gall2012, Reference Roy, Barbarot, Charbonnier, Gayet-Delacroix, Stalder, Roulin and Le Gall2015). Thus, some cognitive impairments, which have been considered as a hallmark deficit of children with NF1, are reinterpreted in the light of executive dysfunction (Van Eylen et al., Reference Van Eylen, Plasschaert, Wagemans, Boets, Legius, Steyaert and Noens2017). Table 1 shows an overview of studies investigating EF in children with NF1 and included in the meta-analysis.
Table 1 Overview of studies included in the meta-analysis
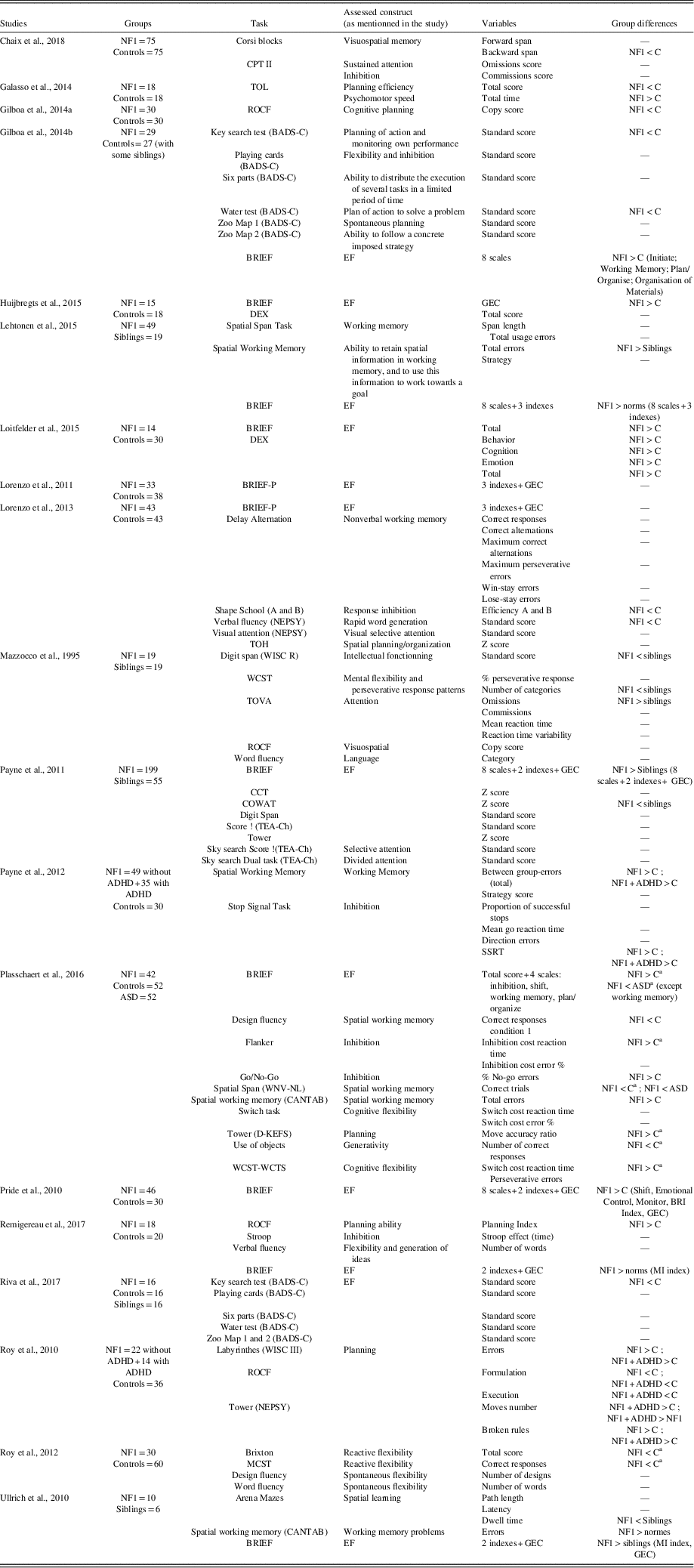
CPT II=Continuous Performance Test; TOL=Tower of London; ROCF=Rey-Osterrieth Complex Figure; BADS-C=Behavioral Assessment of the Dysexecutive Syndrome in Children; BRIEF=Behavior Rating Inventory of Executive Functioning; EF=executive function; DEX=Dysexecutive Questionnaire; GEC=Global Executive Composite; BRIEF-P=Behavior Rating Inventory of Executive Function – Preschool; NEPSY=Developmental Neuropsychological Assessment; WISC=Wechsler Intelligence Scale for Children; TOH=Tower of Hanoi; WCST=Wisconsin Card Sorting Test; TOVA=Test of Variables of Attention; CCT=Children’s Category Test; COWAT=Controlled Oral Word Association Test; TEA-Ch=Test of Everyday Attention for Children; ADHD=attention deficit and hyperactivity disorder; SSRT=Stop Signal Reaction Time; ASD=autism spectrum disorder; WNV-NL=Wechsler Nonverbal Scale of Ability—Dutch version; CANTAB=Cambridge Neuropsychological Test Automated Battery; D-KEFS=Delis–Kaplan Executive Function System; WCST-WCTS=Wisconsin Card Sorting Test with Controlled Task Switching; BRI=Behavioral Regulation Index; MI=Metacognitive Index; MCST=Modified Card-Sorting Test.
a Group differences remains after IQ as covariable.
Several questions emerge from this overview that we aim to study in this meta-analysis. The first objective of this study is to determine whether all aspects of executive functioning are impaired to a similar degree for each aspect of EFs. In fact, papers simultaneously studying all EFs remain scarce. Furthermore, a diversity of measures (tasks or questionnaires) is generally used to investigate EFs (see Table 1) and depending on the study considered, the same measurement may refer to different EFs. Indeed, studies are rarely theoretically guided, and, if so, the theoretical model used varies from one study to another. The first aim of this meta-analysis was thus to collect all published EF-related data and to combine the results with a statistical approach to enable the generalization of findings. This is all the more important as clinical sample sizes in studies are relatively small due to the scarcity of NF1. EF measures were coded separately according to Diamond’s model of EFs (2013).
In this recent model, three core EFs are described: inhibitory control, working memory, and cognitive flexibility. Inhibitory control is the ability to control one’s attention (interference control – selective or focused attention), thoughts (interference control – cognitive inhibition), or behavior (response inhibition – self-control and discipline). Working memory is defined as the ability to keep in mind information and mentally working with or updating it. Two types are distinguished: verbal working memory and visual-spatial working memory. Cognitive flexibility refers to the ability to switch between mental sets or strategies.
Diamond showed the specific development of each of the executive components and their increasingly complex relationships. Working memory and inhibitory control support one another and co-occur. They develop early but show a prolonged developmental progression. Cognitive flexibility builds on the other two and arises much later in development. These three core EFs underpin the establishment of “higher-level executive functions” such as reasoning, problem solving, and planning. In the current meta-analysis, we divided the analysis into four domains. We considered different sets of tasks that address aspects of one of the three domains (working memory, inhibitory control, cognitive flexibility) and the possibility that planning/problem-solving does not overlap with the other three. Indeed, the literature data and our clinical experience seem to indicate that this last domain does not subsume the first three factors.
The second aim was to ascertain whether executive dysfunction may be explained by methodological choices (control group composition, tool types used to assess EFs). First, the composition of the control group (community controls versus siblings) could influence the results of cognitive assessment. For example, a recent study by Lehtonen et al. (Reference *Lehtonen, Garg, Roberts, Trump, Evans, Green and Huson2015) revealed how a healthy control group may differ from a sibling group, although both are matched with the NF1 group by age and socio-economic status. Indeed, calculations of estimated marginal means in the analysis of variance (ANOVA) with IQ as a covariate revealed differences between the community comparisons and each of the other groups (sibling and NF1 groups), leading to the exclusion of the community group for this study. Finally, contrary to performance-based tests, a questionnaire is an ecological assessment based on child’s behavior rating in daily life. Some studies have arrived at different conclusions depending on the tool used to investigate EFs (e.g., Lorenzo, Barton, Arnold, & North, Reference *Lorenzo, Barton, Arnold and North2013). Data collection from performance-based tests on the one hand, and from questionnaires on the other hand, will allow us to determine whether the results from the two tools assessing executive functioning in a complementary manner are similar.
The third question was whether the executive dysfunction may be explained by participants’ selection criteria (age, IQ, neurological history, attention deficit hyperactivity disorder [ADHD]). First, we know that the rudiments of EF emerge before early childhood and continue to be reinforced significantly throughout childhood and adolescence (Best & Miller, Reference Best and Miller2010). Due to this extensive development window, it is reasonable to hypothesize that the developmental changes in children with NF1 do not follow the same curve as healthy children. Thus, differences between patients and children without NF1 may change with advancing age, especially as the structure of EF changes during its development (Lee, Bull, & Ho, Reference Lee, Bull and Ho2013). Thereby, the very broad range of subject ages in some studies (e.g., Ferner, Hughes, & Weinman, Reference Ferner, Hughes and Weinman1996) can limit the accuracy of conclusions.
Second, considering IQ in inclusion criterion may induce a selection bias (Dennis et al., Reference Dennis, Francis, Cirino, Schachar, Barnes and Fletcher2009). Indeed, while IQ monitoring provides a degree of certainty concerning the specificity of neuropsychological disorders in children with NF1, weaknesses in intellectual ability can be the result of cognitive function impairments, which are frequently observed in children with NF1 (Cutting, Koth, & Denckla, Reference Cutting, Koth and Denckla2000). Thus, executive dysfunction could contribute to the weakening of IQ insofar as the relationship between these two components appears to be narrow for some authors (e.g., Diamond, Reference Diamond2013).
Third, neurological history co-occurrence such as prematurity, epilepsy, brain injury, tumors of the central nervous system also contribute to increase the risk of executive dysfunction because these medical contexts are characterized by probable dysexecutive functioning (Roy, Reference Roy2015). Thus, results concerning EF in some studies should be considered with caution because neurological history co-occurrence is not always considered in exclusion criteria or is not specified in the study methodology (e.g., Bluschke, Von der Hagen, Papenhagen, Roessner, & Beste, Reference Bluschke, Von der Hagen, Papenhagen, Roessner and Beste2017a; Casnar & Klein-Tasman, Reference Casnar and Klein-Tasman2016; Rowbotham, Pit-Ten Cate, Sonuga-Barke, & Huijbregts, Reference Rowbotham, Pit-Ten Cate, Sonuga-Barke and Huijbregts2009).
Fourth, the prevalence of ADHD in children with NF1 has been estimated at approximately 30–50% (Pride, Payne, & North, Reference Pride, Payne and North2012). ADHD is more generally associated with executive dysfunction. It is accepted that difficulties in EFs are components of a complex cluster of underlying impairments that result in ADHD (Castellanos, Sonuga-Barke, Milham, & Tannock, Reference Castellanos, Sonuga-Barke, Milham and Tannock2006). Nevertheless, several studies in children with NF1 have shown that executive dysfunction is only partially related to ADHD (Hyman, Shores, & North, Reference Hyman, Shores and North2005; Galasso et al., Reference *Galasso, Lo-Castro, Di Carlo, Pitzianti, D’Agati, Curatolo and Pasini2014; Pride et al., Reference Pride, Payne and North2012; Roy et al., Reference *Roy, Roulin, Charbonnier, Allain, Fasotti, Barbarot and Le Gall2010, Reference *Roy, Barbarot, Roulin, Charbonnier, Fasotti, Stalder and Le Gall2012), although a few studies indicated some poorer EF scores in children with NF1 and ADHD than in children with NF1 only (Pride et al., Reference Pride, Payne and North2012; Roy et al., Reference *Roy, Roulin, Charbonnier, Allain, Fasotti, Barbarot and Le Gall2010).
In summary, in view of the heterogeneity of results between studies and diversity of methodological choices that can explain them, the aim of this meta-analysis, based on the recent Diamond model of EFs (2013), was (1) to specify the degree of impairment for each aspect of EFs namely: working memory, inhibitory control, cognitive flexbility, and planning/problem solving; (2) to investigate the impact of methodological choices: assessment tool format (questionnaire versus performance-based test) and control group composition (percentage of siblings in the control group) on EF performance; and (3) to analyze the impact of participant characteristics (age, intellectual abilities, percentage of ADHD). The theoretical and clinical implications of the results are important. From a theoretical standpoint, these findings will clarify our knowledge of executive dysfunction in children with NF1 and answer the current debate around the independence of EF impairment from other cognitive characteristics. From a clinical standpoint, these findings will serve to guide neuropsychological assessments and rehabilitative interventions.
METHODS
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Shamseer et al., Reference Shamseer, Moher, Clarke, Ghersi, Liberati and Petticrew2015) and Gates and March’s (Reference Gates and March2016) recommendations were followed to conduct this study.
Eligibility Criteria
To be eligible for the current meta-analysis, studies had to fulfill seven criteria: (1) patients included were all diagnosed with NF1 according to the National Institutes of Health Diagnostic Criteria (NIH, 1988), (2) participants were aged between 2 and 18 years, (3) patients with NF1 were compared to a control group matched at least by age and gender, (4) patients had no neurological history other than NF1, (5) at least one neuropsychological task or questionnaire was used to assess EFs, (6) data reported were sufficient to calculate the effect size (ES), and (7) the study was published in English.
Search Strategy and Data Extraction
The literature search was conducted in May 2017 on four computerised databases (Pubmed, Scopus, PsycARTICLES and Cochrane Library). The following combinaison of keywords was used: (“neurofibromatosis type 1”) and (“children” or “preschooler” or “toddler”) and (“executive”, “neuropsychology”, “neurocognitive”, or “cognitive”). The reference sections of publications found through our search were checked to identify missing studies. When data were missing in studies, the authors were contacted to collect them.
Initial searches were carried out by two authors (M.L.B. and A.R.) who screened search results for initial eligibility based on titles and abstracts according to the inclusion criteria. The potentially eligible studies were further assessed using the full text. Nine authors were contacted to obtain further information or missing data. Six responded favorably to this request. All selected studies were conducted in accordance with the Helsinki Declaration and/or approved by an institutional review board.
From each study, the following data were extracted for each group: number of participants (sample size was extracted for each task) and age (mean and standard deviation). When data were available, the full-scale IQ (FSIQ) (mean and standard deviation [SD]), percentage of ADHD in NF1 group and the composition of the control group (percentage of siblings within control group) were also extracted.
We coded EF measures separately when assessing one of the following EF components: working memory, inhibitory control, cognitive flexibility, and planning/problem solving. For each test, we selected one variable as a most appropriate measure of EF. This coding, which was intended to be the least arbitrary possible, was guided by literature data and our clinical experience with children. Table 2 summarizes executive tasks and related variables used in the current meta-analysis.
Table 2 Executive function tasks and outcome measures used in the meta-analysis
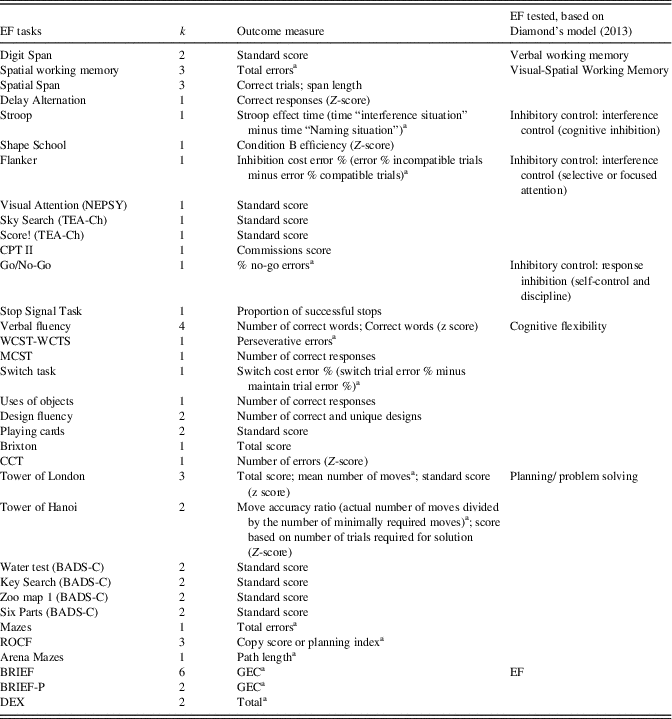
EF=executive functions; k=number of samples; TEA-Ch=Test of Everyday Attention for Children; CPT II=Continuous Performance Test; WCST-WCTS=Wisconsin Card Sorting Test with Controlled Task Switching; MCST=Modified Card-Sorting Test; CCT=Children’s Category Test; BADS-C=Behavioral Assessment of the Dysexecutive Syndrome in Children; ROCF=Rey-Osterrieth Complex Figure; BRIEF=Behavior Rating Inventory of Executive Functioning; BRIEF-P=Behavior Rating Inventory of Executive Function – Preschool; DEX=Dysexecutive Questionnaire.
a The sign of effect size was reversed.
Mean and standard deviation were extracted for each group (NF1 and control). When studies reported results on different NF1 groups compared to the same control group, we averaged means and SDs across subgroups of patients. This was the case for Payne, Arnold, Pride, and North (Reference *Payne, Arnold, Pride and North2012), who examined the impact of ADHD on the cognitive functioning of children with NF1 by comparing two NF1 subgroups (with and without ADHD) to a group of unaffected controls. When many control groups (siblings and community group) were reported in a study (e.g., Lehtonen et al., Reference *Lehtonen, Garg, Roberts, Trump, Evans, Green and Huson2015; Riva et al., Reference *Riva, Vago, Erbetta, Saletti, Esposito, Micheli and Bulgheroni2017), the control groups were combined following established statistical procedures (Higgins, Thompson, Deeks, & Altman, Reference Higgins, Thompson, Deeks and Altman2003).
Data Analysis
Data analysis was carried out with Comprehensive Meta-Analysis software suite version 3 (Biostat, Englewood, NJ). We conducted a random-effects meta-analysis because it does not require the assumption of a common effect size (ES), allowing us to generalize beyond the studies (Borenstein & Higgins, Reference Borenstein and Higgins2013).
From the software, we obtained Hedge’s g for the overall effect, which is less biased for small sample sizes (Borenstein, Hedges, Higgins, & Rothstein, Reference Borenstein, Hedges, Higgins and Rothstein2009). The direction of ES was coded in such a way that a negative score reflects poorer performance for the children with NF1. The sign of ES had to be reversed for some variables (i.e., error and time variables) to avoid obtaining a positive g when children with NF1 made more mistakes or had a longer response time than the control group (see Table 2). When several tasks were used to evaluate the same EF in a sample, the mean of EF scores was used to calculate one ES per sample.
We also obtained an heterogeneity statistic (Cochran’s Q-test) for each ES. The alpha level was set at p=.10. If p-value was less than 0.10, we concluded that the heterogeneity observed between samples could not be exclusively due to within-sample error. We calculated I2, which is a measure of true-effect variance to error variance (Borenstein, Higgins, Hedges, & Rothstein, Reference Borenstein, Higgins, Hedges and Rothstein2017). An I 2 index around 25%, 50%, or 75% would mean, respectively, a low, moderate, or high heterogeneity (Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003). We also calculated tau2, which is an estimate of the variance of true effects (Borenstein et al., Reference Borenstein, Higgins, Hedges and Rothstein2017). A meta-regression was performed, using the mixed-effects model when Q statistic indicated a significant between-sample heterogeneity. The aim of meta-regression analyses was to examine the influence of study characteristics (variable moderators) on ES. The R2 index, which quantifies the proportion of variance explained by the covariate, was also reported.
To detect publication bias, the Fail-Safe Number (FSN) was calculated (Rosenberg, Reference Rosenberg2005). The FSN estimates the number of missing studies with null results we would need to include in the analysis before the p-value became non-significant (p >.05). The results of a meta-analysis can be considered robust if the FSN is greater than 5k+10, where k is the number of samples included in the analysis (Rosenthal, Reference Rosenthal1991). For the overall ES of EF, we used a funnel plot and Egger’s test was used to test for funnel plot asymmetry because there were more than 10 studies (Sterne et al., Reference Sterne, Sutton, Ioannidis, Terrin, Jones, Lau and Higgins2011).
RESULTS
Study Selection
The literature search resulted in 19 studies that satisfied the selection criteria with data from a total of 805 NF1 and 667 controls. See Figure 1 for a flow diagram of studies’ selection process. Sample characteristics are summarized in Table 3.
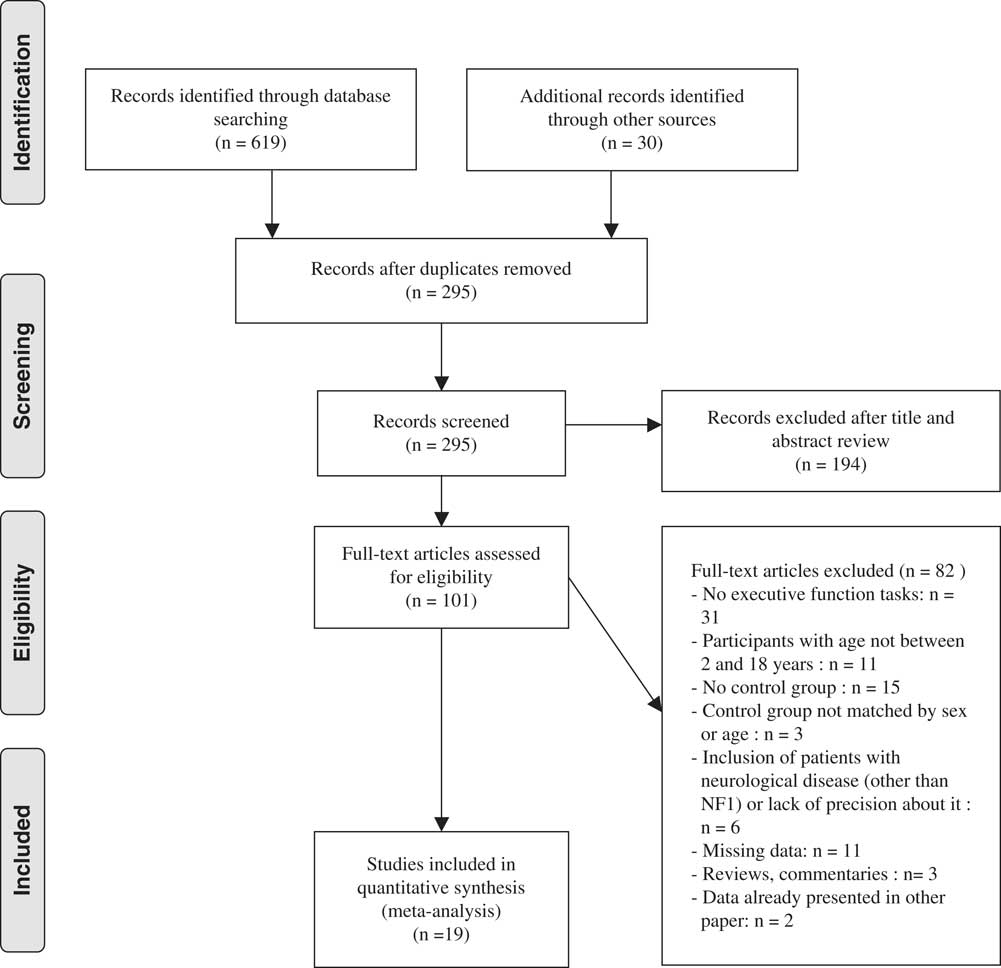
Fig. 1 Flow diagram of the study selection process.
Table 3 Summary of samples characteristics selected in the meta-analysis.
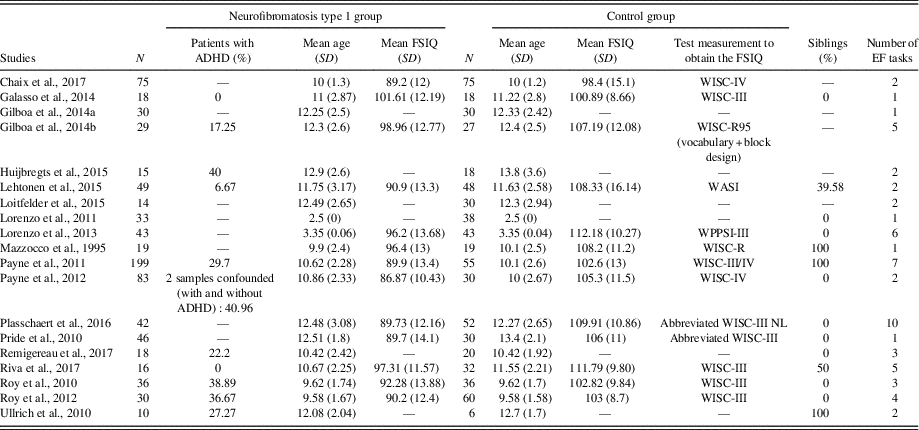
N=sample size; ADHD=attention deficit hyperactivity disorder; SD=standard deviation; FSIQ=Full Scale Intellectual Quotient; EF=executive function; WISC=Wechsler Intelligence Scale for Children; WASI = Wechsler Abbreviated Scale of Intelligence; WPPSI=Wechsler Preschool and Primary Scale of Intelligence.
Overall Effect of EF
The overall effect of EF was moderate and statistically significant (k=19; g=−0.74; 95% confidence interval [CI] [−0.95; −0.53]; Z=−6.82; p<.0001), suggesting an overall executive deficit in children with NF1 relative to controls. The Q-test, however, was significant (Q(18)=59.38; p<.0001) and heterogeneity across studies was moderate (I2=69.99%; tau2=0.14). The funnel plot revealed asymmetry (Figure 2); thus, an Egger’s test of publication bias was conducted and was significant (Egger’s intercept=−3.75; p<.01). There are several possible explanations for funnel plot asymmetry such as the heterogeneity between studies (Sterne et al., Reference Sterne, Sutton, Ioannidis, Terrin, Jones, Lau and Higgins2011).
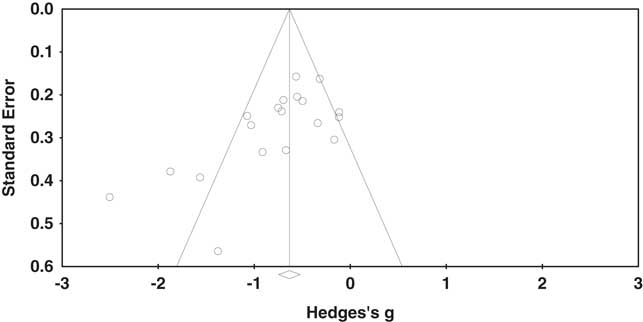
Fig. 2 Funnel plot of standard error by Hedges’s g.
A statistical comparison of “Assessment Tool Type” failed to reveal any significant differences (p=.21) between the performance-based test (k=15; g=−0.65; 95% CI [−0.86; −0.45]; Z=−6.18; p<.0001) and the questionnaire format (k=8; g=−0.98, 95% CI [−1.45; −0.51]; Z=−4.10; p<.0001). Heterogeneity was moderate for the performance-based test (Q(14)=35.47; p<.0001; I2=60.53%; tau2=0.09) and high for the questionnaire format (Q(7)=42.95; p<.0001; I2=83.70%; tau2=0.36).
The subgroup analysis between EF domains was significant (p=.08), demonstrating differential effects across EFs.
EF Domain-Specific Effects
Small to moderate ESs were observed for each of EF (see Figure 3).
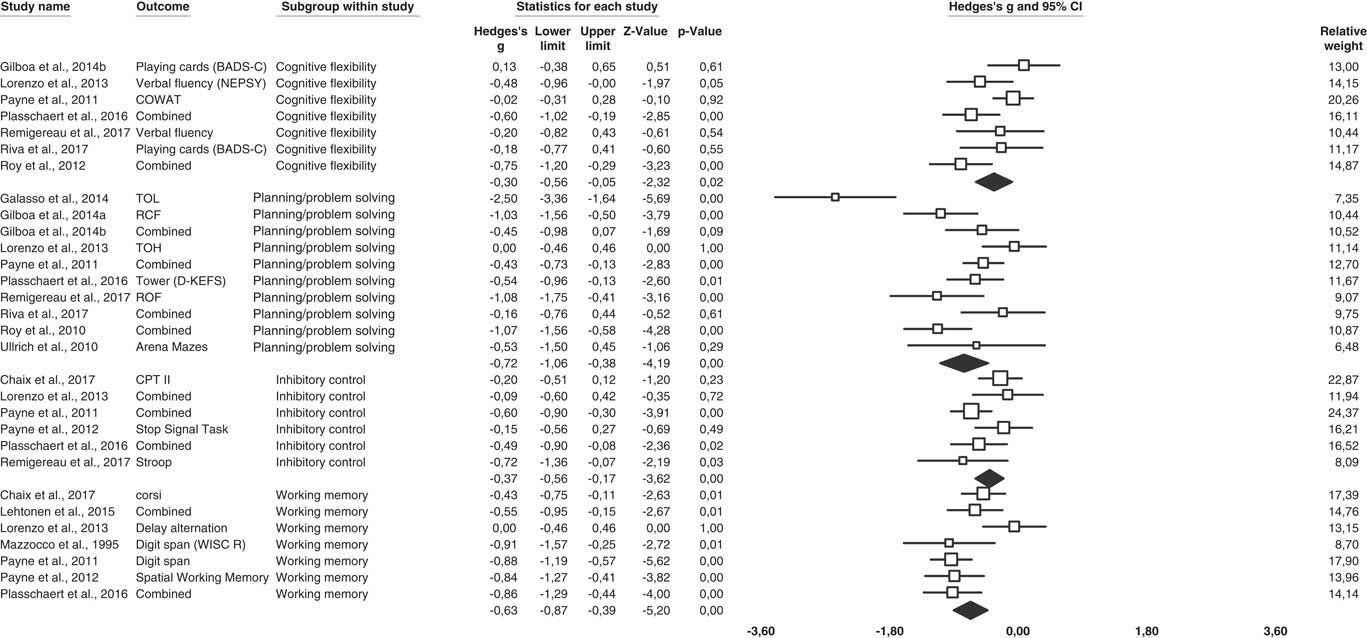
Fig. 3 Forest plot of studies group by EF process.
Working Memory
The overall ES for working memory tasks was significant and moderate (k=7; g=−0.63; 95% CI [−0.87; −0.39]; Z=−5.20; p<.0001). The Q-test was significant (Q(6) =14.24; p<.03) and the I2 test indicated (I2=57,85%; tau2=0.06) moderate heterogeneity between samples. The FSN was 113, indicating results are robust. Nine tasks were used to assess working memory: two to assess verbal working memory (k=2; g=−0,89; 95% CI [−1.17; −0.61]) and seven to assess visual-spatial working memory (results for two samples were pooled) (k=5; g=−0.54; 95% CI [−0.82; −0.26]). A significant difference was observed between these two modalities (p=.08) at the expense of the verbal working memory.
Inhibitory Control
The ES for inhibitory control was significant but small (k=6; g=−0.37; 95% CI [−0.56; −0.17]; Z=−3.62; p<.0001). Results were homogeneous (Q(5)=9.27; p=.21; I2=30.01%; tau2=0.02). The FSN was 24, below the estimations of Rosenthal’s formula (40). Therefore, results were not robust. ES did not differ according to the subcomponents of inhibitory control (p=.96): interference control (cognitive inhibition) (k=2; g=−0.41;, 95% CI [−0.93; 0.11]), interference control (selective or focused attention) (k=4; g=−0.33; 95% CI [−0.58; −0.08]) and response inhibition (self-control and discipline) (k=2; g=−0.37; 95% CI [−0.80; 0.07]).
Cognitive Flexibility
The ES for flexibility was significant but small (k=7; g=−0.30; 95% CI [−0.56; −0.05]; Z=−2.32; p=.02). The Q-test revealed moderate heterogeneity between studies (Q(6)=12.74; p=.05; I2=52.91%; tau2=0.06). The results are not considered to be robust (FSN=14).
Planning/Problem Solving
The overall ES for planning/problem solving tasks was significant and moderate (k=10; g=−0.72; 95% CI [−1.06; −0.38]; Z=−4.19; p<.0001). There was a significant between-sample heterogeneity (Q(9)=36.81; p<.0001; I2=75.55%; tau2=0.21). A publication bias was unlikely (FSN=161).
Moderator Analysis
A significant heterogeneity across overall EF studies and within working memory, cognitive flexibility and planning/problem solving studies was observed, so effects of different variable moderators were assessed (age, IQ, ADHD, percentage of siblings within control group) to determine whether there was a relationship between these variables and the NF1 executive performance (Table 4). No meta-regression was performed for inhibitory control because Q statistics indicated homogeneity of the results.
Table 4 Meta-regression results
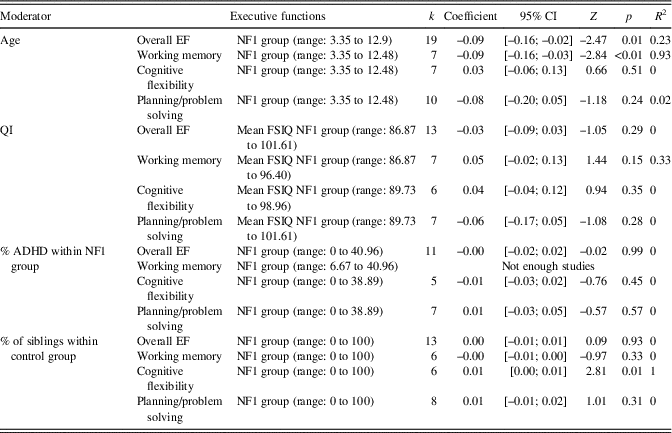
k=number of samples; CI=confidence interval; NF1=neurofibromatosis type 1; FSIQ=Full-Scale Intellectual Quotient; EF=executive functions.
Age
The overall EF meta-regression was significant (regression coefficient=−0.09, 95% CI [−0.16; −0.02]; Z=−2.48; p=.01), suggesting that age has an effect on ES (Figure 4). Furthermore, meta-regressions were performed separately for specific EFs. The working memory meta-regression was significant (regression coefficient=−0.09, 95% CI [−0.15; −0.03]; Z=−2.85; p<.01): age was negatively associated with working memory performances. No significant association was observed for cognitive flexibility (p=.51) and planning/problem solving (p=.24).
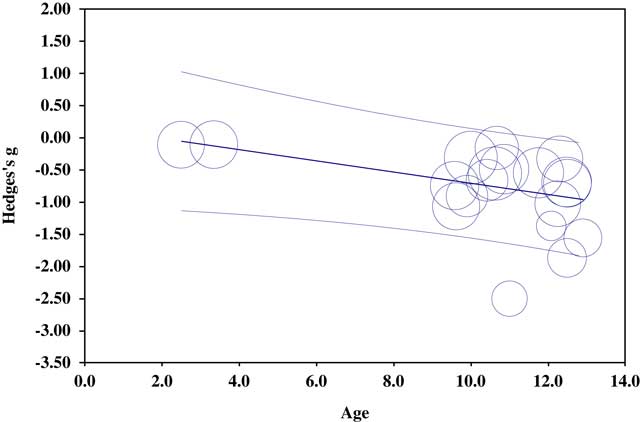
Fig. 4 Regression of Hedges’s g on age for overall EF.
IQ
The effect of FSIQ (NF1 FSIQ) was assessed and showed that NF1 FSIQ has no effect on the ES of overall EF (p=.29), working memory (p=.15), cognitive flexibility (p=.35), and planning/problem solving (p=.28).
ADHD
No association was found between the percentage of ADHD in children with NF1 and ES concerning overall EF (p=.53), cognitive flexibility (p=.38), and planning/problem solving (p=.57). A meta-regression was not possible for working memory because not enough studies with the ADHD variable were available.
Percentage of Siblings Within the Control Group
No association was observed between the overall EFs and the percentage of siblings within the control group (p=.93). Control group composition did not seem to influence working memory (p=.33) or planning/problem solving ES (p=.31). Nevertheless, an association between the percentage of siblings within the control group and cognitive flexibility was observed (regression coefficient=0.01; 95% CI [0.00; 0.01]; Z=2.81; p=.01): the percentage of siblings within the control group was positively associated with cognitive flexibility performances.
DISCUSSION
The aim of this meta-analysis was to determine the magnitude of impairment for each EF, namely working memory, inhibitory control, cognitive flexbility, and planning/problem solving and to clarify the impact of assessment tool format, control group composition, and participant characteristics (age, intellectual abilities, percentage of ADHD) on EFs.
Executive Function Impairments in Children With Neurofibromatosis Type 1
The first question was whether all aspects of executive functioning are similarly impaired. The results demonstrated EF deficits in children with NF1 with moderate ES. These findings are consistent with past research that showed executive dysfunction in children with NF1 (Plasschaert et al., Reference *Plasschaert, Van Eylen, Descheemaeker, Noens, Legius and Steyaert2016; Ribeiro et al., Reference Ribeiro, Violante, Bernardino, Edden and Castelo-Branco2015; Roy et al., Reference *Roy, Roulin, Charbonnier, Allain, Fasotti, Barbarot and Le Gall2010, Reference *Roy, Barbarot, Roulin, Charbonnier, Fasotti, Stalder and Le Gall2012, Reference Roy, Barbarot, Charbonnier, Gayet-Delacroix, Stalder, Roulin and Le Gall2015). However, a heterogeneity was observed across studies. Subgroup analyses showed that ES varied with the specific component of executive functioning. Individual analyses by function showed that working memory and planning/problem solving are more affected than inhibitory control and cognitive flexibility. ES for working memory and planning/problem solving are moderate, whereas the ES for inhibitory control and cognitive flexibility are small. Nevertheless, all ESs are significant. Results for inhibitory control and cognitive flexibility should be considered with caution because the results are not assumed to be robust.
Moreover, working memory deficits vary according to the type of working memory. While the working memory deficit appears to be global, the magnitude of the ES for verbal working memory is larger than that of visual-spatial working memory, which are, respectively, large and moderate. Differences between the two modalities could be due to the psychometric properties of EF tasks. Indeed, in the current meta-analysis, the verbal working memory was assessed solely with the digit span task. This measure is perhaps more sensitive than other working memory tasks, irrespective of the type of working memory assessed. Inhibitory control did not vary according to its component aspects. Indeed, the ES is homogeneous for “cognitive inhibition” and “selective or focused attention.” Nevertheless, heterogeneity between studies for overall EF, working memory, cognitive flexibility, and planning/problem solving studies was observed, thus prompting us to question the influence of moderator variables.
Moderator Variables of Executive Functions
The second question was whether the degree of executive dysfunction is governed by methodological choices (tool type used to assess EFs, composition of control group). There was no significant difference between the assessment tool with a medium ES for performance-based test and a large ES for questionnaire format, contrary to some studies that noted an inconsistency between cognitive test scores and questionnaires (e.g., Payne, Hyman, Shores, & North, Reference *Payne, Hyman, Shores and North2011). On the meta-analysis level, conclusions concerning executive dysfunction from the two assessment tools appear to be similar. It must be kept in mind, however, that these are two different assessment tools used to assess executive functioning in a complementary manner, one with a behavioral approach and the other with a performance-based test.
Control group composition and especially the percentage of siblings within healthy controls seems not to be linked to the ES of EF. To our knowledge, no study to date has investigated this question. Nevertheless, our findings are contrary to some studies that observed a difference between a sibling group and a healthy control group (e.g., Lehtonen et al., Reference *Lehtonen, Garg, Roberts, Trump, Evans, Green and Huson2015; Riva et al., Reference *Riva, Vago, Erbetta, Saletti, Esposito, Micheli and Bulgheroni2017). In the meta-analysis, only one significant relationship between this moderator variable and cognitive flexibility performances was observed: the higher the proportion of siblings in the control group is high, the smaller the ES. This could be explained by the fact that family environments (Bernier, Carlson, & Whipple, Reference Bernier, Carlson and Whipple2010; Rhoades, Greenberg, Lanza, & Blair, Reference Rhoades, Greenberg, Lanza and Blair2011) could also influence EFs, which are expected to be similar in NF1 patients and their siblings. In a complementary way, the education level of parents and parental socioeconomic status may also have an impact on EF development (Er Rafiqi, Roukoz, Le Gall, & Roy, Reference Er-Rafiqi, Roukoz, Le Gall and Roy2017; Noble, Norman, & Farah, Reference Noble, Norman and Farah2005). Nevertheless, this result should be considered with caution because this association is only observed for one domain of EF and the number of samples included in this analysis is relatively small.
The third question was whether the executive dysfunction may be explained by participant selection criteria (age, IQ, neurological history, ADHD). To our knowledge, the influence of age on EF from early childhood to adolescence has never been studied. Only one publication conducted a longitudinal study of a cohort of children with NF1 with some EF tasks (Hyman et al., Reference Hyman, Gill, Shores, Steinberg, Joy, Gibikote and North2003). Concerning cognitive flexibility, the results fail to show a decrease in the gap between children with NF1 and controls with advancing age. In this meta-analysis, an age effect was observed on the ES of overall EF and working memory: executive dysfunction and more particularly working memory seems to be more important when age increases. Thus, executive dysfunction seems to be long-lasting, worsening with advancing age, at least up to adolescence.
These findings can be justified insofar as EFs are increasingly necessary in everyday life, with growing autonomy requirements. Thus, while an EF deficit can pass unnoticed or be of low intensity in younger children, this deficit may become increasingly noticeable during childhood and adolescence, as and when educational and social requirements increase. Data from functional questionnaires tend to reinforce these results. Indeed, while parents did not notice any difference in behavior and executive functioning in younger children (30 and 40 months) (Lorenzo, Barton, Acosta, & North, Reference *Lorenzo, Barton, Acosta and North2011; Lorenzo et al., Reference *Lorenzo, Barton, Arnold and North2013), an emerging executive dysfunction was reported between 3 and 5 years (Casnar & Klein-Tasman, Reference Casnar and Klein-Tasman2016; Sangster, Shores, Watt, & North, Reference Sangster, Shores, Watt and North2011). In school-aged children, EF impairment reported by parents is very significant (Champion, Rose, Payne, Burns, & North, Reference Champion, Rose, Payne, Burns and North2014; Lehtonen et al., Reference *Lehtonen, Garg, Roberts, Trump, Evans, Green and Huson2015; Loitfelder et al., Reference *Loitfelder, Huijbregts, Veer, Swaab, Van Buchem, Schmidt and Rombouts2015; Payne et al., Reference *Payne, Hyman, Shores and North2011; Plasschaert et al., Reference *Plasschaert, Van Eylen, Descheemaeker, Noens, Legius and Steyaert2016; Pride et al., Reference *Pride, Payne, Webster, Shores, Rae and North2010; Roy et al., Reference Roy, Barbarot, Charbonnier, Gayet-Delacroix, Stalder, Roulin and Le Gall2015; Ullrich, Ayr, Leaffer, Irons, & Rey-Casserly, Reference Ullrich, Ayr, Leaffer, Irons and Rey-Casserly2010).
Additionally, these findings consolidate the hypothesis of lasting executive dysfunction in NF1, which is probably due to an atypical development of fronto-sub-cortical circuits. These data are contrary to the temporary developmental shift of EFs assumed by some authors (Itoh et al., Reference Itoh, Magnaldi, White, Denckla, Hofman, Naidu and Bryan1994), especially since, despite heterogeneous results, some studies have shown an executive deficit in adults with NF1 (Descheemaeker, Plasschaert, Frijns, & Legius, Reference Descheemaeker, Plasschaert, Frijns and Legius2013; De Souza Costa et al., Reference De Souza Costa, De Paula, De Rezende, Carneiro Rodrigues, Malloy-Diniz, Romano-Silva and De Miranda2014; Ferner et al., Reference Ferner, Hughes and Weinman1996; Pavol et al., Reference Pavol, Hiscock, Massman, Moore, Foorman and Meyers2006).
No relationship between FSIQ in children with NF1 and EFs was noted. These results recall some previous studies which concluded that EF deficits do not appear to be a side effect of a lower IQ (Plasschaert et al., Reference *Plasschaert, Van Eylen, Descheemaeker, Noens, Legius and Steyaert2016). These findings strengthen the idea that executive dysfunction is specific and not due to the slightly decreased general intellectual functioning. More generally, these results raise questions concerning the link between EF and intelligence in a neurodevelopmental disorder such as NF1, despite the supposed close ties between EF and fluid intelligence in healthy subjects such as proposed by Diamond (Reference Diamond2013).
The meta-analysis shows no association between the percentage of ADHD in the NF1 group and the ES of EF. These results are consistent with previous studies suggesting that executive dysfunction is only partially related to ADHD (Galasso et al., Reference *Galasso, Lo-Castro, Di Carlo, Pitzianti, D’Agati, Curatolo and Pasini2014; Hyman et al., Reference Hyman, Shores and North2005; Roy et al., Reference *Roy, Roulin, Charbonnier, Allain, Fasotti, Barbarot and Le Gall2010, Reference *Roy, Barbarot, Roulin, Charbonnier, Fasotti, Stalder and Le Gall2012). ADHD comorbidity is frequently reported in children with NF1, with an estimated prevalence of 30–50% (Pride et al, Reference Pride, Payne and North2012). The high co-occurrence of ADHD and NF1 testify to semiological proximity. Nevertheless, these findings question the semiological proximity between ADHD and NF1, because executive dysfunction is observed in children with NF1, with or without ADHD. They confirm the presence of executive impairment independently of ADHD symptoms and the specific nature of dysexecutive syndrome in children with NF1. In addition to this observation, these results show a limited repercussion of ADHD in other cognitive domains such as EF. This brings into question the important role attributed to ADHD in the cognitive phenotype of children with NF1. In this context, the results underline the need to study EF in children with NF1, even when ADHD is excluded. Monitoring in schooling and care should not be limited to patients with ADHD symptoms.
Finally, the children included in this meta-analysis did not have a neurological history co-occurrence that could explain or accentuate the executive dysfunction observed among children with NF1. This finding is important because the inconsistency of methodologies used between studies in the scientific literature (children with or without neurological history co-occurrence) could contribute to the heterogeneity of conclusions concerning EF in children with NF1.
Other characteristics specific to NF1, such as T2 hyperintensities (T2H), could not be studied in this meta-analysis, whereas some studies have examined the relationship between neuropsychological impairment and T2H in NF1 (Levine et al., Reference Levine, Materek, Abel, O’Donnell and Cutting2006). T2H are often present in individuals with NF1, at approximately 43% to 79% (Cutting et al., Reference Cutting, Clements, Lightman, Yerby-Hammack and Denckla2004; Levine et al., Reference Levine, Materek, Abel, O’Donnell and Cutting2006). Only a small number have examined the impact of T2H on EFs (Roy et al., Reference Roy, Barbarot, Charbonnier, Gayet-Delacroix, Stalder, Roulin and Le Gall2015). Roy et al. (Reference Roy, Barbarot, Charbonnier, Gayet-Delacroix, Stalder, Roulin and Le Gall2015) compared EF performance in children with and without T2H in 36 school-age children with NF1 (7–12 years). The presence, number, size, and the main cerebral locations of hyperintensities were considered. Results showed that the presence, number, and size of the T2H, as they are currently measured, would be independent of executive functioning. It may also be of interest to study other topics, such as the link between EF and optic pathway gliomas, considering the high prevalence in children with NF1 (approximately 15–20%) (Gutmann et al., Reference Gutmann, Ferner, Listernick, Korf, Wolters and Johnson2017). Neuropsychological profile and, more specifically, EF in children with NF1 and optic pathway gliomas are rarely studied, probably because optic pathway gliomas are often treated and the type of treatment itself (chemotherapy, radiotherapy, surgery) also interferes with cognitive functions (Lacaze et al., Reference Lacaze, Kieffer, Streri, Lorenzi, Gentaz, Habrand and Grill2003).
Executive Functions at the Interface With Higher Functions in Children With Neurofibromatosis Type 1
The EF deficit in children with NF1 may have a negative impact on their daily life, particularly on higher functions. Thus, Remigereau et al. (Reference *Remigereau, Roy, Costini, Barbarot, Bru and Le Gall2017) showed that praxis difficulties disappeared when executive dysfunctions (planning and inhibitory control) were controlled. Gilboa, Josman, Fattal-Valeski, Toledano-Alhadef, and Rosenblum (Reference *Gilboa, Josman, Fattal-Valevski, Toledano-Alhadef and Rosenblum2014) observed that planning underlies poor writing quality in children with NF1.
Similarly, Van Eylen et al. (Reference Van Eylen, Plasschaert, Wagemans, Boets, Legius, Steyaert and Noens2017) found no differences between children with NF1 and controls for any of the visuoperceptual measures when statistically checking for EF confounders (inhibitory control, cognitive flexibility, working memory). These results suggest that reduced visuoperceptual performances in children with NF1 arise from confounding EF impairments and not from visuoperceptual impairments per se. In a complementary manner, other studies show that executive dysfunction is observed even when basic skills such as visuospatial ability is taken into consideration (Roy et al., Reference *Roy, Roulin, Charbonnier, Allain, Fasotti, Barbarot and Le Gall2010, Reference *Roy, Barbarot, Roulin, Charbonnier, Fasotti, Stalder and Le Gall2012). Roy et al. (Reference *Roy, Roulin, Charbonnier, Allain, Fasotti, Barbarot and Le Gall2010) demonstrated that lower Rey-Osterrieth Complex Figure scores are due to a weakness in planning in children with NF1 and cannot be attributed to deficits in either IQ or visuospatial skills. Moreover, these findings emphasize the need for caution with analyses of scores because one neuropsychological task usually assesses several cognitive functions. It is thus essential to propose several tasks to reach a conclusion concerning the nature of impairment.
Another study suggested that the social functioning impairment suffered by children with NF1 is likely to be a result of deficits in higher-order functions rather than of basic face recognition (Lehtonen et al., Reference *Lehtonen, Garg, Roberts, Trump, Evans, Green and Huson2015). Concerning theory of mind, a deficit is observed in children with NF1 but appears to be independent of other cognitive abilities such EF impairments (Payne, Pride, & North, Reference Payne, Pride and North2016). This conclusion, however, was derived from only one study, EF measurement was indirect and not all theory of mind aspects were evaluated. These results should be considered with all the more caution as a link between theory of mind and EF has been demonstrated in many medical contexts (for a review, see Moreau & Champagne-Lavau, Reference Moreau and Champagne-Lavau2014).
Finally, executive dysfunction in children with NF1 partially accounts for their academic achievement difficulties (Gilboa, Rosenblum, Fattal-Valevski, Toledano-Alhadef, & Josman, Reference *Gilboa, Rosenblum, Fattal-Valevski, Toledano-Alhadef and Josman2014).
These data provide an additional argument for considering that EF disorders are the source of several learning disabilities in children with NF1. Contrasting these data with other clinical contexts (e.g., Krivitsky, Walsh, Fisher, & Berl, Reference Krivitzky, Walsh, Fisher and Berl2016), for which executive impairment seems to be a core feature of cognitive phenotype, seems to be important to improve our knowledge of executive dysfunction in pediatric medical conditions.
Limitations and Future Directions
The current meta-analysis had several limitations. From a methodological standpoint, the number of studies included in this meta-analysis was relatively small, particularly for preschool-aged children. For example, only two studies involving preschool children were included in the current meta-analysis, both of these were carried out by the same authors and toddlers come from one group (Lorenzo et al., Reference *Lorenzo, Barton, Acosta and North2011, Reference *Lorenzo, Barton, Arnold and North2013). Thus, the effect of age on the magnitude of the EF deficit needs to be explored with further studies in preschool children, especially since there is an age gap from 3 to 9 in this meta-analysis. Even if an effect of age should be observed on cognitive flexibility and planning/problem solving if the age effect on the overall EF and working memory was solely due to this gap, we cannot fully rule out an effect of age on the other EF domains. These are very important to improve our knowledge of the development of EF in children with NF1, especially since neuropsychological tests are now available. It would also be interesting to confirm this finding with longitudinal studies to observe EF development at the group level as well at the individual level.
Moreover, the small number of studies did not allow us to eliminate those conducted by the same research team. We cannot, therefore, eliminate the fact that school-age children may have been included in several studies at different ages.
Concerning the relationship between intellectual performance and EFs, we have considered the possibility that the FSIQ and working memory may be confounded because, some of FSIQ provided by the meta-analysis was obtained with the Wechsler Intelligence Scale for Children, with Digit Span among subtests. Nevertheless, no effect of FSIQ on the ES of working memory nor of overall EF, cognitive flexibility or planning/problem solving could be found. Thus, we can consider reasonably that executive dysfunction is not due to the slightly decreased general intellectual functioning.
Furthermore, due to the lack of data, some variables such socioeconomic status, specific and preferential location of T2H, or gender influence upon ES in the meta-analysis, could not be analyzed, whereas these variables could have an impact on the development of EF (Roy et al., Reference *Roy, Barbarot, Roulin, Charbonnier, Fasotti, Stalder and Le Gall2012, Reference Roy, Barbarot, Charbonnier, Gayet-Delacroix, Stalder, Roulin and Le Gall2015).
We also decided to exclude studies in languages other than English. The number of non-English studies we came across in our searches, however, was small.
Additionally, we used the FSN to detect publication bias. The FSN estimates the number of missing studies with null results that we would need to incorporate in the analysis before the p-value became non-significant. Nonetheless, it is quite unlikely that all unpublished studies would have a null effect.
On a clinical level, these findings emphasize the need for patients with NFl to undergo a full early neuropsychological assessment, as recommended by Ferner et al. (Reference Ferner, Hughes and Weinman1996) to detect early signs of executive dysfunction and to alert the child’s entourage. In this way, learning disabilities and behavioral problems in children with NF1 could be anticipated. Moreover, while the meta-analysis did not reveal significant differences between performance-based tests and ratings of EF measurement, it would seem essential to study EF with the two assessment tools because they are complementary and probably assess different aspects of cognitive and behavioral functioning (Toplak, West, & Stanovich, Reference Toplak, West and Stanovich2013).
On a theoretical level, the results of this meta-analysis consolidate the biological hypothesis of an atypical development of fronto-sub-cortical circuits in children with NF1. Indeed, executive functioning is associated with a large cerebral network with a preponderant role of the prefrontal cortex and its subcortical connections. These data echo recent studies that observed some differences in neurophysiological processes between adolescents with NF1 and controls in a Go/No-Go task (Bluschke, Von der Hagen, Papenhagen, Roessner, & Beste, Reference Bluschke, Von der Hagen, Papenhagen, Roessner and Beste2017b). These findings should encourage future neurophysiological studies to focus on EF studies which will enrich our knowledge of this topic. These data are reminiscent of the preponderance of T2H in fronto- sub-cortical networks, which could represent cumulative risk factors for executive dysfunction (Roy et al., Reference Roy, Barbarot, Charbonnier, Gayet-Delacroix, Stalder, Roulin and Le Gall2015)
CONCLUSION
To conclude, the current meta-analysis demonstrated EF deficit in children with NF1 compared with controls matched by age and gender. Subgroup analyses showed that impairment varies by specific component of executive functioning. Working memory and planning/problem solving appear to be more affected than inhibitory control and cognitive flexibility. Executive dysfunction and more specifically working memory seems to be more important with increasing age, probably due to the growing autonomy requirements of everyday life. EF performances do not seem to be affected by intellectual performance, ADHD, tool type used to assess EFs, or the percentage of siblings within control groups. Early identification of executive dysfunctions to reduce long-term impact is essential. In fact, these disorders may be a complication affecting the quality of life in NF1, with repercussions on other cognitive abilities (praxis, visuospatial skills), social functioning, and academic achievements.
ACKNOWLEDGMENTS
The authors thank Recklinghaüsen and the Neurofibromatosis Association (France) for its support and financial contribution to this project. No potential conflict of interest was reported by the authors.










