INTRODUCTION
Hypofractionated radiotherapy (RT) offers several advantages in the treatment of prostate cancer. As prostate cancer seems to have a low α/β ratio, hypofractionated radiotherapy regimes provide a biological therapeutic advantage over conventional fractionationReference Brenner and Hall1,Reference Duchesne and Peters2. In addition to possible biological therapeutic advantage, it also offers a shorter treatment course, increased convenience for patients and decreased costReference Rene, Faria and Cury3,Reference Mohan, Kupelian and Willoughby4. From the radiobiologic standpoint, however, hypofractionated RT increase the incidence of late radiation toxicity because the late response of normal tissues is influenced by the fraction size rather than the total doseReference Akimoto, Muramatsu and Takahashi5.
Because the potential reduction in dose to critical normal tissues with the use of intensity-modulated radiotherapy (IMRT) facilitates the targeted delivery of hypofractionated RT without associated increase in late toxicityReference Zelefsky, Fuks and Hunt6,Reference Amer, Mott and Mackay7, several studiesReference Martin, Rosewall and Bayley8–Reference Coote, Wylie and Cowan11 have reported the outcomes of hypofractionated IMRT for prostate cancer. At our institution, the standard 3-dimensional conformal RT (3D-CRT) fractionation regime for prostate cancer is a total of 75.6 Gy at 1.8 Gy/fraction. However, from January 2008, we have treated a series of prostate cancer cases with definitive helical IMRT (tomotherapy) using a fractionation scheme of a total of 75 Gy at 2.5 Gy/fraction. In this study, we retrospectively reviewed the toxicity of hypofractionated helical tomotherapy in men with intermediate- and high-risk prostate cancer.
METHODS AND MATERIALS
In January 2008, we began hypofractionated helical tomotherapy for cases of intermediate- and high-risk prostate cancer according to the D’Amico risk classificationReference D’Amico, Whittington and Malkowicz12. Patients were excluded from the analysis if they had received neoadjuvant, adjuvant, or concurrent androgen deprivation or if the follow-up period was less than 12 months. Patients who had pelvic lymph node metastasis at diagnosis were also excluded. A total of 22 patients were treated definitively with hypofractionated helical tomotherapy. The initial evaluation for all 22 patients included pelvic magnetic resonance imaging (MRI), determination of clinical stage, pretreatment prostate specific antigen (PSA) levels, and biopsy Gleason score determination. The 2002 American Joint Committee on Cancer (AJCC) staging system was used for determination of clinical stage. Additional workup with bone scans, transrectal ultrasonography and computed tomography (CT) of the pelvis was obtained, according to the physician’s discretion. A review of medical records was performed with the approval of the institutional review board at our institution.
Simulation
All patients underwent CT simulation in the supine position after immobilization with a vacuum bag (BodyFix®, Medical Intelligence Medizintechnik GmbH, Schwabmünchen, Germany). For immobilization, we also inserted a rectal balloon catheter into the rectum and inflated it with 60–80 cc of air, and patients were instructed to drink 400–600 cc of water an hour before simulation and every treatment. All patients tolerated the rectal inflation procedure well throughout the simulation and treatment course; however, two patients did not tolerate bladder filling procedure during treatment course, and these two patients were instructed to drink 200 cc of water an hour before treatment. Intravenous contrast agents were administered and axial CT images (5 mm slice thickness) were obtained from above the iliac crest through the mid-femur.
RT planning and treatment
The CT data were transferred to a Hi·Art v3.1 Planning Station (TomoTherapy Incorporated, Madison, USA) for inverse planning. The clinical target volume (CTV) 1 was the prostate gland. The seminal vesicles were defined as CTV2 if seminal vesicle invasion was found on pelvic MRI or if the proposed risk of seminal vesicle involvement was >15% as calculated by Roach’s formulaReference Roach, Marquez and Yuo13. Pelvic lymph nodes were defined as CTV3, and elective pelvic nodal treatment was administered if the proposed risk of occult nodal involvement was >15% as calculated by Roach’s formulaReference Roach, Marquez and Yuo13 and seminal vesicle invasion was found on pelvic MRI. The planning target volume (PTV) for the prostate gland (PTV1) and seminal vesicles (PTV2) were generated comprising CTV1 and CTV2 with a margin of 1 cm, respectively, except posterior to the prostate–rectum interface, where the margin was 0.5 cm. Elective nodal PTV (PTV3) was defined by a 0.5-cm expansion of CTV3. The rectum was contoured from the sigmoid flexure to the anorectal junction. The outer bladder wall and both femur heads were each contoured in their respective entirety.
As the treatment volume increased significantly with the inclusion of the pelvic lymph nodes in the target volume, the patients were separated into two groups. One group was composed of patients who received treatment at the prostate gland only or prostate gland and seminal vesicles (Group 1), and the other group was composed of patients who received treatment at the prostate gland, seminal vesicles and pelvic lymph nodes (Group 2).
Treatment was prescribed such that PTV1 received 75 Gy in 30 fractions (2.5 Gy/fraction) over 6 weeks, PTV2 simultaneously received 63 Gy in 30 fractions (2.1 Gy/fraction) and PTV3 simultaneously received 54 Gy in 30 fractions (1.8 Gy/fraction). It has been suggested that the α/β ratio for prostate cancer is relatively lower for use in the linear-quadratic (LQ) model predicting tumour effects. Assuming an α/β ratio of 3, the biologic effective dose (BED) in this study was 137.25Reference Jones, Dale, Finst and Khaksar14.
In general, plans were considered acceptable if ≥95% of the PTV received the prescription dose. Constraints were set so that no more than 25% of the bladder and 20% of the rectum received greater than 50 Gy with a maximum dose level of 75 Gy. The dose-volume limits of the normal structures were met in almost all cases without change of the rectal inflation volume, but the maximum limits of the normal structures were not reached and were put in to try to keep maximum doses in the normal structures as low as possible.
Radiation was delivered by a TomoTherapy (TomoTherapy Incorporated, Madison, WI, USA) with a 6-MV photon beam. The specific method of treatment delivery was helical (delivers radiation continuously from all 360? angles around the patient) IMRT, which uses tens of thousands of narrow beamlets. Patients were set up on a couch with a vacuum bag in the supine position. Triangulation marks were used to make sure the patient did not roll and to quickly position the patient in the correct location. Daily localization for treatment was performed using daily megavoltage (MV) CT images, which were acquired helically using a fan-beam with a conventional CT detector mounted on a ring gantry. First, automatic registration process was performed to align the acquired MV CT image and reference planning CT image. Thereafter, if necessary, manual registration was performed to get perfect alignment. Radiation therapists performed the entire localization process. Daily MVCT images were automatically saved and reviewed by the radiation oncologist.
Toxicity assessment
A baseline gastrointestinal (GI) and genitourinary (GU) symptom assessment was made at the start of radiotherapy. Patients were assessed weekly and also based on patient need during treatment. After treatment, patients were typically seen at 2-month intervals for 1 year and 3- to 4-month intervals thereafter. At each visit, GI and GU toxicity was prospectively scored by physician assessment according to the Radiation Therapy Oncology Group (RTOG) grading system. Toxicities identified during treatment or at the initial 3-month follow-up visit were considered acute toxicities, and late toxicities were defined as any increase of acute toxicities lasting more than 3 months after treatment cessation or occurring for the first time later than 3 months after the end of treatment. The worst toxicity grade documented at any time was considered the final grade for late toxicities.
Statistical analysis
Independent t test was used to compare planning dosimetric data. Toxicity-free survival rates were calculated using the Kaplan-Meier method, and the log-rank test and Cox regression analysis were used to identify factors significantly associated with toxicity-free survival. A p value of <0.05 was considered statistically significant. All analyses were performed with SPSS software system (Version 15.0; Chicago, IL, USA).
RESULTS
Patient characteristics
Between January 2008 and October 2009, 28 prostate cancer patients were treated with hypofractionated helical tomotherapy according to our fractionation schedule (total 75 Gy in 30 fractions, 2.5 Gy/fraction). Of these, 2 patients were classified as clinical stage N1 and received concurrent and adjuvant androgen deprivation hormonal therapy, 3 patients had less than 12 months of follow-up and 1 patient stopped radiotherapy after having received 7.5 Gy because of medical problems unrelated to his prostate cancer treatment. The remaining 22 patients completed their full treatment and were included in this analysis.
The mean age at presentation was 70 years. According to the D’Amico risk classificationReference D’Amico, Whittington and Malkowicz12, none of the patients had low-risk disease, 31.9% had intermediate-risk disease, and 63.1% had high-risk disease. The median pretreatment PSA level was 14.09 ng/mL (range 5.06 to 180.56), and the median follow-up period was 24.5 months. Table 1 summarizes the characteristics of the 22 patients.
Table 1. Baseline characteristics of the 22 patients
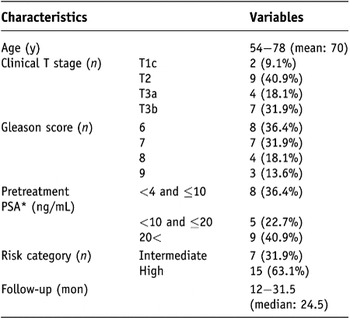
Note: PSA* = prostate-specific antigen.
Planning dosimetric data
Ten patients received treatment to prostate gland only, 5 patients received treatment to prostate gland and seminal vesicles and 7 patients received treatment to prostate gland, seminal vesicles, and pelvic lymph nodes. The dose-volume relationship for PTV and normal tissues was analyzed. The mean doses of prostate gland, seminal vesicles, and pelvic lymph nodes were 76.9 Gy, 65.8 Gy and 56.7 Gy, respectively. The mean volumes of the rectum and bladder that receiving ≥50 Gy were 15.3% and 16.7%, respectively. Group 1 was composed of 15 patients who received treatment at the prostate gland only or prostate gland and seminal vesicles, and Group 2 was composed of 7 patients who received treatment at the prostate gland, seminal vesicles and pelvic lymph nodes. The planning dosimetric data of the two groups were not significantly different (Table 2, p > 0.05).
Table 2. Planning dosimetric data of helical tomotherapy for 22 prostate cancer patients
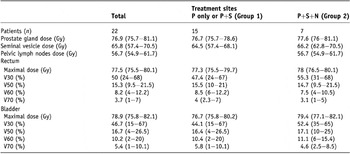
Note: P = prostate gland; S = seminal vesicle; N = pelvic lymph nodes.
Baseline symptoms assessment
A significant number of patients complained of GU symptoms such as urinary frequency, nocturia, and dysuria before treatment. Baseline GU symptoms were measured by RTOG criteria. Fourteen patients presented with Grade 1 GU symptoms, and 3 presented with Grade 2 GU symptoms before the start of radiotherapy. In contrast, only one patient complained of GI symptoms before treatment. Table 3 summarizes the baseline GI and GU symptoms of all 22 patients.
Table 3. Gastrointestinal (GI) and genitourinary (GU) symptoms scored by RTOG criteria before radiotherapy in all 22 patients

Note: RTOG = Radiation Therapy Oncology Group.
Acute toxicity
All patients tolerated treatment well without treatment interruption or major acute side-effects. There was no Grade 3 or more acute toxicity. Acute GI toxicity scores for all 22 patients were as follows: Grade 0 in 7 patients (31.8%), Grade 1 in 12 patients (54.5%) and Grade 2 in 3 patients (13.6%). Acute GU toxicity scores for the 22 patients were as follows: Grade 0 in 5 patients (22.7%), Grade 1 in 12 patients (54.5%) and Grade 2 in 5 patients (22.7%)(Table 4).
Table 4. Incidence of toxicity scored by RTOG criteria in total 22 patients (crude incidence rate)
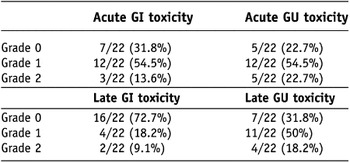
Note: RTOG = Radiation Therapy Oncology Group; GI = gastrointestinal; GU = genitourinary.
The incidence of acute toxicities was compared between Group 1 and Group 2 (Figures 1 and 2). In Group 2, the Grade 2 acute toxicities were presented more frequently.
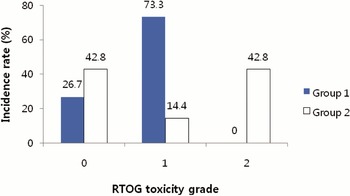
Figure 1. Incidence of acute GI (gastrointestinal) toxicity scored by Radiation Therapy Oncology Group (RTOG) criteria. Group 1 = the patients who were treated prostate gland only, or prostate gland and seminal vesicles; Group 2 = the patients who were treated prostate gland, seminal vesicles and pelvic lymph nodes.
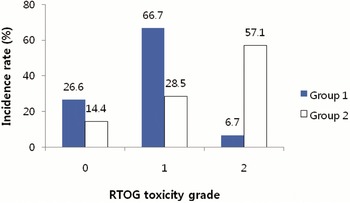
Figure 2. Incidence of acute GU (genitourinary) toxicity scored by Radiation Therapy Oncology Group (RTOG) criteria. Group 1 = the patients who were treated prostate gland only, or prostate gland and seminal vesicles; Group 2 = the patients who were treated prostate gland, seminal vesicles and pelvic lymph nodes.
Late toxicity
As mentioned above, late toxicities were defined as any increase in acute toxicities lasting more than 3 months after treatment cessation or occurring for the first time later than 3 months after the end of treatment. The worst toxicity grade documented at any time was considered to be the final grade of late toxicities.
There was no Grade 3 or more late toxicity. Three patients experienced late rectal bleeding: 1 patient developed Grade 1 late rectal bleeding 11 months after radiotherapy and 2 patients developed Grade 2 late rectal bleeding 17.1 and 21.9 months after radiotherapy, respectively. The late GI toxicity scores for all 22 patients were as follows: Grade 0 in 16 patients (72.7%), Grade 1 in 4 patients (18.2%) and Grade 2 in 2 patients (9.1%). The late GU toxicity scores for the 22 patients were as follows: Grade 0 in 7 patients (31.8%), Grade 1 in 11 patients (50%) and Grade 2 in 4 patients (18.2%)(Table 4).
To find predicting factors for late GU toxicity, we analyzed the effect on late GU toxicity of the following parameters: V30, V50, V60 and V70 of bladder. Using Kaplan-Meier analysis, only the V70 of bladder (<5% vs. ≥5%) was a significant predictor for late GU toxicity(Figure 3). A multivariate analysis of factors affecting late GU toxicity was performed using above mentioned parameter, and the V70 of bladder was a significant predictor for late Grade 2 GU toxicity(Table 5). Analyses were also done to find predicting factors for late GI toxicity. However, there was no predicting factor for late GI toxicity that reach statistical significance.
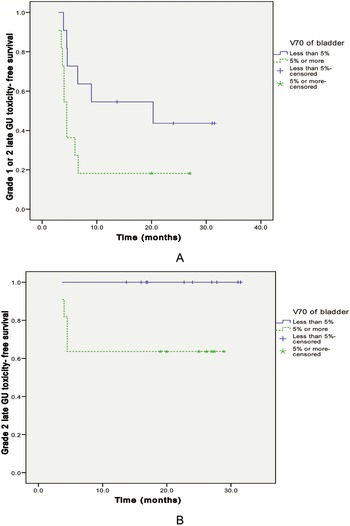
Figure 3. Late GU (genitourinary) toxicity-free survival curve for all 22 cases by bladder volume irradiated ≥70 Gy. (A) V70 of bladder (<5% vs. ≥5%) was a significant predictor for late Grade 1 or 2 GU toxicity (p = 0.049). (B) V70 of bladder (<5% vs. ≥5%) was a significant predictor for late Grade 2 GU toxicity (p = 0.031).
Table 5. Predicting factors for late GU (genitourinary) toxicity

The crude incidence rates of late toxicities in Groups 1 and 2 are depicted in Figures 4 and 5. Grade 2 late toxicities were present more frequently in Group 2. Because of the small sample size, we did not compare the late toxicity-free survival rate between Groups 1 and 2.
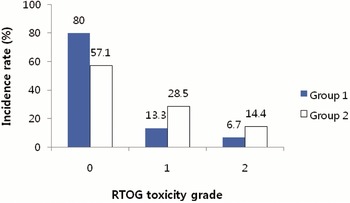
Figure 4. Incidence of late GI (gastrointestinal) toxicity scored by Radiation Therapy Oncology Group (RTOG) criteria. Group 1 = the patients who were treated prostate gland only, or prostate gland and seminal vesicles; Group 2 = the patients who were treated prostate gland, seminal vesicles and pelvic lymph nodes.
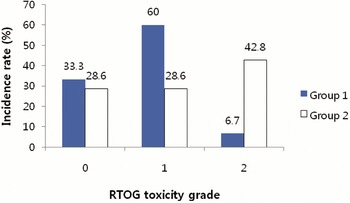
Figure 5. Incidence of late GU (genitourinary) toxicity scored by Radiation Therapy Oncology Group (RTOG) criteria. Group 1 = the patients who were treated prostate gland only, or prostate gland and seminal vesicles; Group 2 = the patients who were treated prostate gland, seminal vesicles and pelvic lymph nodes.
DISCUSSION
There has been interest on the use of hypofractionated RT for prostate cancer because of several advantages—it decreases the inconvenience of lengthy treatments, increases biologic benefit by increasing the daily fraction size and decreases the treatment cost. However, given that late responding normal tissues are considered to have a low α/β ratio, the main concern of hypofractionated RT is late normal tissue toxicity. As reduction in the dose to critical normal tissues is obtainable with IMRT compared with 3D-CRTReference Nutting, Convery and Cosgrove15, there has been recent interest in hypofractionated IMRT with various fractionation schedules for prostate cancer treatment. We report the results of a toxicity assessment after treating 22 prostate cancer patients with hypofractionated helical IMRT (total 75 Gy, 2.5 Gy/fraction). Assuming that the α/β ratio of prostate cancer is 3, the BED of our fractionation schedule was 137.25, and the equivalent total dose for administration of the fractional dose of 2 Gy was 82.2 Gy.
Several studies have reported on the toxicity of hypofractionated IMRT for prostate cancer. Martin et al.Reference Martin, Rosewall and Bayley8 reported on the toxicity of hypofractionated IMRT (total 60 Gy, 3 Gy/fraction) in 92 prostate cancer patients. The treatment sites were the prostate gland and seminal vesicles, and toxicities were graded by RTOG toxicity criteria. Assuming that the α/β ratio of prostate cancer is 3, the BED for the fractionation schedule from that study was 120, lower than the BED of our fractionation schedule. The incidence rates of acute GI and GU toxicities were 66% and 32% for Grade 0, 22% and 43% for Grade 1, 11% and 25% for Grade 2 and 1% and 0% for Grade 3, respectively. In addition, the crude incidence rates of late GI and GU toxicities were 93% and 90% for Grade 0, 2% and 7% for Grade 1, and 4% and 3% for Grade 2, respectively. Compared with the Group 1 in our study, the rate of Grade 0 acute toxicities in that study was higher, but more patients suffered from Grade 2 or more acute toxicities. However, the results of late toxicities were more favourable than our study.
Kupelian et al.Reference Kupelian, Reddy, Carlson, Altsman and Willoughby10 reported on the toxicity of hypofractionated IMRT (total 70 Gy, 2.5 Gy/fraction) for 166 prostate cancer patients. The treatment sites were the prostate gland only or the prostate gland and seminal vesicles. The BED of their fractionation schedule was 128.3, and toxicities were graded by RTOG toxicity criteria. The incidence of acute GI and GU toxicities was 30% and 15% for Grade 0, 55% and 62% for Grade 1, 15% and 22% for Grade 2 and 0% and 1% for Grade 3, respectively. Compared with Group 1 in our study, the incidence rate of Grade 1 acute toxicities was lower, but the incidence rate of Grade 2 or more acute toxicities was higher. For late toxicity, the actuarial combined Grade 1, 2 late GU toxicity rate was very low (1.73%) in that study. The actuarial combined Grade 1, 2 late GI toxicity rate was also low, but Grade 3 late GI toxicity occurred in two patients. In our study, we did not have any cases of Grade 3 late toxicity.
McCammon et al.Reference McCammon, Rusthoven and Kavanagh9 delivered the same fractionation schedule as Kupelian et al. but treated the prostate gland, seminal vesicles and pelvic lymph nodes in 30 patients. Toxicities were graded by the National Cancer Institute Common Terminology Criteria for Adverse Events. The incidence rates of acute GI and GU toxicities were 40% and 10% for Grade 0, 36.7% and 63.3% for Grade 1, 20% and 26.7% for Grade 2 and 3.3% and 0% for Grade 3, respectively. In addition, with a median follow-up of 24 months, the crude incidence rates of late GI and GU toxicities were 66.7% and 46.7% for Grade 0, 20% and 43.3% for Grade 1, 6.7% and 10% for Grade 2 and 6.7% and 0% for Grade 3, respectively. In general, compared with Group 2 in our study, McCammon et al. reported favourable results. However, Grade 3 acute and late GI toxicity was present in 1 (3.3%) and 2 patients (6.7%), respectively, in their study.
Pollack et al.Reference Pollack, Hanlon and Horwitz16 delivered a total of 70.2 Gy with 2.7 Gy fraction size, and the BED was 133.4. A total of 50 prostate cancer patients were treated, and the treatment sites were the prostate gland, seminal vesicles and pelvic lymph nodes. The authors reported on only acute toxicities, and the toxicities were graded by modified RTOG and Late Effects Normal Tissue Task Force (LENT) criteria. The incidence rates of acute GI and GU toxicities were 42% and 8% for Grade 0, 40% and 44% for Grade 1, 18% and 40% for Grade 2 and 0% and 8% for Grade 3, respectively.
To our knowledge, the only study that used a higher BED than our fractionation schedule was implemented in Japan. Akimoto et al.Reference Akimoto, Muramatsu and Takahashi5 reported on late rectal bleeding after hypofractionated RT for 52 prostate cancer patients, but their RT technique was 3D-CRT, not IMRT. The authors delivered a total of 69 Gy in 3 Gy fractions, three fractions weekly. The BED of this fractionation schedule was 138, higher than the BED of our fractionation schedule (137.25). The treatment sites included the prostate gland and seminal vesicles, and the toxicities were graded by RTOG toxicity criteria. The crude incidence rates of late rectal bleeding were 6% for Grade 1, 23% for Grade 2 and 2% for Grade 3. In our study, there was no Grade 3 rectal bleeding, and the crude incidence rates of Grade 1 and 2 late rectal bleeding were 4.5 % and 9.1%, respectively. Meanwhile, Tsuji et al.Reference Tsuji, Yanagi and Ishikawa17 delivered a total of 66 Gy in 3.3 Gy fractions, and the BED was very high (138.6). However, Tsuji et al. used carbon ion for radiotherapy, not photon.
Comparing toxicity results after radiotherapy in prostate cancer patients among several institutions is difficult because of differences in the scoring systems, radiotherapy techniques, the definition of target volumes and the way incidence rates are calculatedReference Faria, Souhami, Joshua, Vuong and Freeman18. Nevertheless, our results seem to be favourable. Although there was a general tendency for higher Grade 2 late toxicities than in other studies, those toxicities were tolerable and there was no Grade 3 or more late toxicity in our study. Considering the high BED of our fractionation schedule, these results are encouraging. In addition, several studies suggest that increasing radiation dose in the treatment of prostate cancer improves local and biochemical controlReference Zelefsky, Fuks and Hunt6,Reference Lyons, Kupelian, Mohan, Reddy and Klein19–Reference Kuban, Tucker and Dong21. As the BED of our fractionation schedule was so high, we anticipate favourable biochemical control after longer term follow-up.
Our study did have several limitations in that we described a relatively small series from a single institution, with somewhat of a limited follow-up period. If longer follow-up periods and larger cohorts confirm the favourable biochemical control rate and toxicity profile of our fractionation schedule, hypofractionated helical IMRT (a total of 75 Gy, 2.5 Gy/fraction) could be implemented in the clinical setting for the treatment of prostate cancer.












