Asthma is one of the most common chronic childhood diseases in industrialised countries( Reference Mannino, Homa and Akinbami 1 ). In Denmark, allergic asthma has increased almost twofold over the past 15 years( Reference Thomsen, Ulrik and Larsen 2 ). Most children who develop asthma do so by the age of 7–9 years, suggesting a need to investigate aetiologic factors occurring in early life. The identification of these factors is necessary to best guide future preventive programmes. More than a decade ago, Black & Sharpe( Reference Black and Sharpe 3 ) proposed that the increase in the prevalence of allergic disease may be explained by a shift in the consumption of PUFA away from n-3 PUFA and oily fish towards a higher intake of n-6 PUFA. In support of this hypothesis, animal and in vitro studies have demonstrated the involvement of n-3 PUFA in anti-inflammatory mechanisms by altering membrane phospholipid composition and oxidative balance, influencing cell signalling, cytokine production and T-cell responses (reviewed in Calder et al. ( Reference Calder, Kremmyda and Vlachava 4 ) and Prescott & Dunstan( Reference Prescott and Dunstan 5 )). n-3 PUFA have also been shown to increase the production of anti-inflammatory eicosanoids (EPA) and more recently identified resolvins (both EPA and DHA)( Reference Calder, Kremmyda and Vlachava 4 , Reference Serhan, Arita and Hong 6 ).
Studies in adults and children have provided support for this hypothesis( Reference Hodge, Salome and Peat 7 , Reference Laerum, Wentzel-Larsen and Gulsvik 8 ); yet, evidence on the protective effects of fish intake during pregnancy against child allergic disease has been conflicting. Some observational studies have found lower rates of allergic disease among children whose mothers increased fish consumption or took fish oil supplements during pregnancy( Reference Romieu, Torrent and Garcia-Esteban 9 – Reference Willers, Devereux and Craig 11 ), while others have found no associations( Reference Calvani, Alessandri and Sopo 12 – Reference Miyake, Sasaki and Tanaka 15 ). Maternal fish intake was shown to protect against child eczema in 2- and 5-year-old children( Reference Romieu, Torrent and Garcia-Esteban 9 – Reference Willers, Devereux and Craig 11 ), though this was not a consistent finding( Reference Øien, Storrø and Johnsen 14 , Reference Miyake, Sasaki and Tanaka 15 ). In three studies that specifically examined the risk of child asthma, no associations were found( Reference Salam, Li and Langholz 13 , Reference Øien, Storrø and Johnsen 14 , Reference Willers, Wijga and Brunekreef 16 ). Some of the studies were limited by the retrospective assessment of maternal diet up to 5 years post-pregnancy( Reference Calvani, Alessandri and Sopo 12 – Reference Øien, Storrø and Johnsen 14 ), which may have increased measurement error and reduced the power to detect an association. In randomised clinical trials that supplemented women with fish oil during pregnancy, favourable effects were found on early life sensitisation( Reference Dunstan, Mori and Barden 17 – Reference Krauss-Etschmann, Hartl and Rzehak 20 ). However, these studies were limited by the absence of clinical endpoints and supplementation into the lactation period, making it difficult to draw conclusions about exposure timing.
Fish intake during pregnancy could influence the development of allergy and airway inflammation through fatty acid-driven pathways. The available literature is conflicting and would benefit from additional evidence on clinical outcomes relevant to allergic disease development during childhood. The purpose of the present study was to examine the associations between maternal fish intake during pregnancy, and early and later allergic disease outcomes in children.
Methods
Population and study design
The aim of the Danish National Birth Cohort was to investigate conditions in early life and childhood that may reach into later stages of life. Between 1996 and 2002, we recruited participants at the women's first antenatal visit. Women were eligible to enrol if they planned to carry to term and could speak Danish. Women were interviewed via telephone twice during pregnancy, at 12 and 30 weeks of gestation. Telephone interviews were also administered when the child was 6 and 18 months old. In addition, women completed a 360-item semi-quantitative FFQ during the 25th week of gestation( Reference Olsen, Hansen and Sandstrom 21 ). The FFQ asked about intake in the past 4 weeks and has been validated against 7 d food diaries and blood and urine biomarkers for selected nutrient (protein, retinol, folic acid and n-3 PUFA) and food (fruit and vegetable) intake( Reference Mikkelsen, Osler and Olsen 22 ). The present study showed significant and acceptable to good correlations for all nutrients and food groups in question with correlation coefficients ranging from 0·32 to 0·66. Furthermore, when the children turned 7 years, mothers were asked to complete a mailed questionnaire.
To avoid dependency among correlated observations, out of the 101 045 enrolled pregnancies, we included only the first study pregnancy (n 89 333; Fig. 1). We further limited the present analyses to singletons (n 87 056) and excluded women who took fish oil at any point during pregnancy (n 3546) or who did not have data on fish intake for both pregnancy interviews (n 58 142), generating a total of 28 936 women who were eligible for the analysis. Sample sizes varied somewhat for the individual outcomes due to differential response rates.
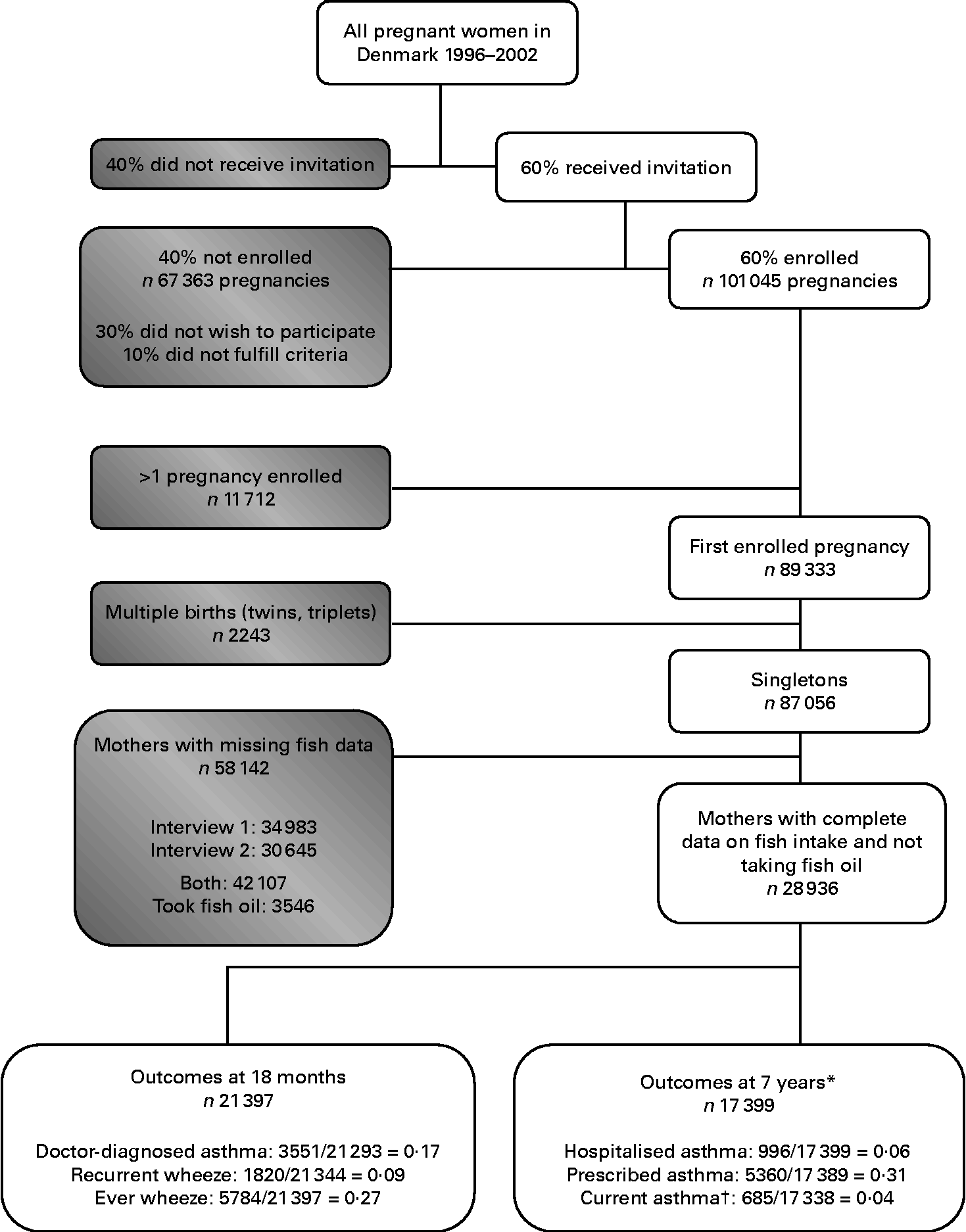
Fig. 1 Flow chart of the participants in the Danish National Birth Cohort. * Study populations limited to children with age 7 data. † Doctor diagnosis of asthma and wheeze in the past 12 months.
Mothers provided written informed consent on behalf of their children. The Regional Scientific Ethics Committee for the municipalities of Copenhagen and Frederiksberg approved all study protocols, and all procedures were in accordance with the Declaration of Helsinki.
Exposure measurements
Fish intake was assessed during both pregnancy interviews. We used a definition of fish intake developed for the Danish National Birth Cohort that best differentiated among the levels of intake. It assessed fish intake with a sandwich or a hot meal, the most common ways of consuming fish in Denmark, with five categories of intake: (1) never eating fish; (2) hot meal and sandwiches both eaten monthly or less than each month, according to both interviews; (3) hot meal monthly and sandwiches weekly, according to both interviews; (4) sandwiches and hot meals both eaten weekly according to both interviews, at a low frequency (hot meals 1 time/week and sandwiches 1–2 times/week, in at least one interview); (5) sandwiches and hot meals both eaten weekly at a high frequency (hot meals >2 times/week or sandwiches >3 times/week, according to both interviews). We also calculated fish intake in g/week using data from the mid-pregnancy FFQ.
Outcome measurements
We asked mothers about the doctor diagnosis of child asthma, wheeze symptoms and the number of wheezing episodes since birth at 6 and 18 months. We defined asthma at 18 months as a doctor diagnosis of asthma, and wheeze at 18 months as reported wheezing or whistling in the chest. We evaluated recurrent wheeze as having >3 episodes in the first 18 months of life compared with having ≤ 3 episodes or no reported wheeze.
We evaluated asthma and allergic rhinitis at 7 years of age using standardised questions based on the International Study of Asthma and Allergies in Childhood questionnaire( Reference Asher, Keil and Anderson 23 ). Current asthma was defined as a positive response to ever doctor-diagnosed asthma and wheezing symptoms in the past 12 months. We defined allergic rhinitis as ever-reported doctor diagnosis of hay fever.
We also followed up on the children through national registries, linked using the Central Person Registry number, a unique identification number provided to all Danish citizens. We had access to two national registries, the Danish National Patient Registry (DNPR) and the Register of Medicinal Product Statistics (RMPS).
The DNPR collects data on all hospital admissions, emergency room and outpatient contacts. The registry has been well validated against asthma diagnosis from hospital records( Reference Moth, Vedsted and Schiøtz 24 ). Data from the DNPR were extracted in August 2010 and linked to our data using the Central Person Registry number. We used the International Classification of Disease 10 for asthma (J45, J45.0, J45.1, J45.8, J45.9, J46 and J46.9) to classify the first registered diagnosis of admitted asthma for every child. Allergic rhinitis diagnosis was not examined due to a low number of hospitalisations (226/47 677 = 0·005).
The RMPS contains detailed individual-level information on prescriptions (with the exclusion of over-the-counter) filled at all pharmacies. We used a definition from a previous validation study to classify ever prescribed asthma cases as individuals with at least two prescriptions of any combination of drugs for obstructive airway disease, except for β-2 agonists as liquid( Reference Moth, Vedsted and Schiøtz 25 ). Classification of ever prescribed allergic rhinitis cases was based on at least two prescriptions of any combinations of anti-allergic drugs.
Covariates
Covariates suspected to be confounders, intermediates in the causal pathway or effect modifiers were considered. Variables that were evaluated for inclusion in the multivariate model included socio-economic status (SES) by parental education level and occupation (SES: high-level proficiency, medium-level proficiency, skilled, unskilled, student or unemployment), maternal age at birth of child ( ≤ 20, 21–39 or ≥ 40 years), parity (nulli- or multiparous), maternal pre-pregnancy BMI (kg/m2) ( ≤ 18·5, 18·6–24·9, 25·0–29·9, 30–34·9 or ≥ 35·0), maternal smoking during pregnancy (non-smoker, occasional smoker, < 15 cigarettes/d or ≥ 15 cigarettes/d), maternal exercise during pregnancy (yes or no), gestational weight gain (g/week), breast-feeding duration (none, 0–1, 2–3, 4–6, 7–9 or ≥ 10), birth weight (g), gestational age (in d since the last menstrual period), child sex and parental history of asthma and allergies. We also evaluated intakes of vitamins A, D, E, Se and Zn from dietary sources and supplements, and intakes of essential fatty acids, fruits, vegetables and alcohol. We energy-adjusted all nutrient intakes from diet and alcohol using the residual method( Reference Willett 26 ).
Statistical analysis
We evaluated the distribution of covariates across the fish intake categories to identify potential confounding variables. There were significant differences in fish intake across the maternal age categories with ‘never’ consumers being younger compared with consistently high fish consumers; we therefore age-standardised the covariate distributions. The final set of covariates was determined by χ2 and partial F-tests with P< 0·10, and prior literature. Covariates suspected to be intermediates (gestational weight gain, birth weight and gestational age) on the causal pathways were excluded from the model to avoid over-adjustment. Fish intake was entered as an indicator variable into the multivariate logistic regression model and individual exposure categories compared with the highest, reference, category. We report here the estimated OR and 95 % CI. We examined models with several levels of confounder adjustment. We adjusted first for sociodemographic variables and then further adjusted for dietary covariates. As further adjustment for dietary factors did not change the results, we report results for the sociodemographic model only. In all adjusted models, breast-feeding accounted for the largest attenuation of OR. Ordinal values for the exposure categories were entered separately into the models as a continuous variable to evaluate the P value for trend. All tests were two-sided and statistical significance was considered at P< 0·05. The analyses were performed using Statistical Analyses System software (release 9.3; SAS Institute).
Results
Study cohort
A total of 28 936 women had information on fish intake based on the two pregnancy interviews. Most women in the study cohort were between the ages of 21 and 39 years (98 %), of higher socio-economic position (high- or medium-level proficiencies 55 %) and multiparous (53 %) (Table 1). Close to a quarter of participants reported any smoking during pregnancy with 12 % daily smokers. The prevalence of maternal history of asthma and allergies was 9 and 31 %, respectively. Paternal asthma and allergies were reported for 8 and 24 % of children, respectively.
Table 1 Age-standardised covariate distribution across the categories of maternal fish intake during pregnancy in the Danish National Birth Cohort (n 28 935)* † (Mean values and standard deviations; number of subjects and percentages)
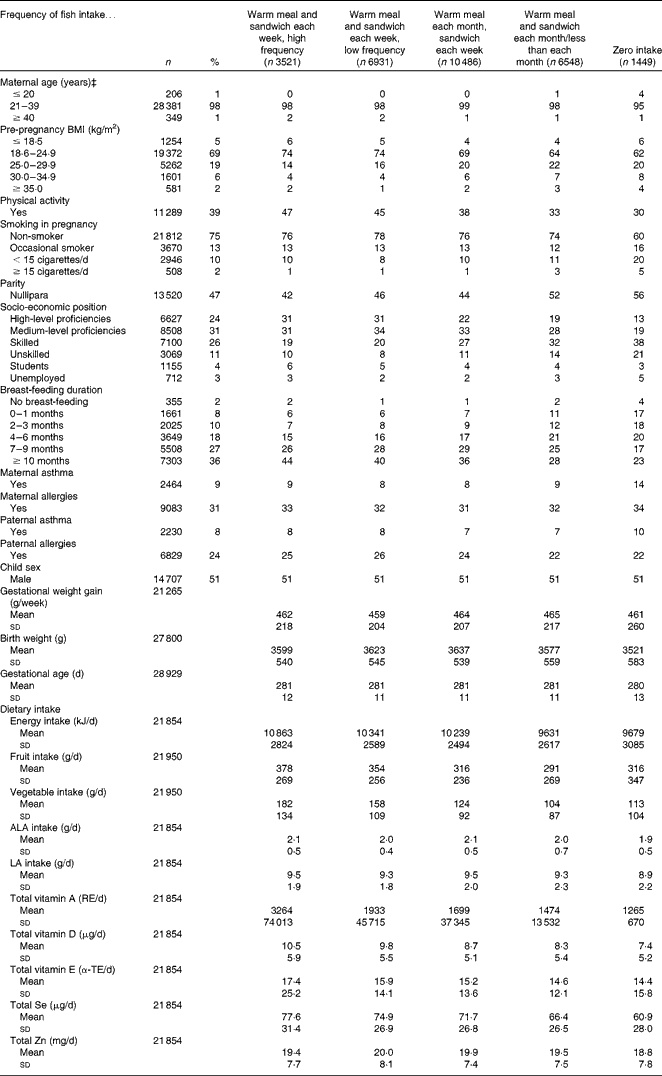
ALA, α-linolenic acid; LA, linoleic acid; RE, retinol equivalents; α-TE, α-tocopherol equivalents.
* One participant excluded because of extreme vitamin D intake (>100 000 μg/d.)
† Values are standardised to the age distribution of the study population.
‡ Values not age-adjusted.
At 18 months, compared with the 7643 eligible participants excluded from the present analysis due to missing outcome data, the 21 293 included participants were more likely to be multiparous (54 v. 51 %) and less likely to smoke during pregnancy (11 v. 13 %). Comparing the 11 598 participants without data on current asthma at 7 years, the 17 338 included mothers were of higher sociodemographic status (high- and medium-level proficiency: 58 v. 53 %), they reported lower pre-pregnancy BMI ( ≥ 30 kg/m2: 7 v. 9 %) and lower pregnancy smoking rates (10 v.14 %); and they were more likely to have breast-fed past 7 months (65 v. 58 %). There were no substantial differences in gestational weight gain, birth weight and intake of fruits, vegetables, micronutrients and essential fatty acids.
A total of 5 % of women reported no fish intake at both interviews, while 12 % of women reported eating fish at a high frequency at both time points. Fish intake estimated by the FFQ increased monotonously across the categories: women in the lowest category reported consuming 21 (sd 49) g fish/week, while intake was 292 (sd 147) g/week among high consumers, confirming successful separation of the exposure categories.
Predictors of fish intake
We examined fish intake across the age-standardised participant characteristics (Table 1). Compared with never consumers, high-frequency fish consumers tended to be of high/medium-level proficiency. They also tended to have a lower pre-pregnancy BMI and more previous pregnancies. They smoked less during pregnancy and breast-fed for ≥ 7 months. They reported an overall healthier diet with a higher intake of fruit, vegetables, vitamins and minerals. Birth weight was generally higher for children whose mothers consumed more fish.
Univariate predictors of the outcomes
We found that younger age, higher parity, higher pre-pregnancy BMI, smoking and lack of physical activity during pregnancy, shorter breast-feeding duration, parental history of either asthma or allergy, and male sex were directly associated with doctor-diagnosed asthma at 18 months (Table 2). Similar results were presented for ever admitted and prescribed asthma, and current asthma at 7 years.
Table 2 Univariate predictors of asthma at 18 months, current asthma at 7 years and ever asthma by hospitalisation or prescription medication in the Danish National Birth Cohort (Odds ratios and 95 % confidence intervals)
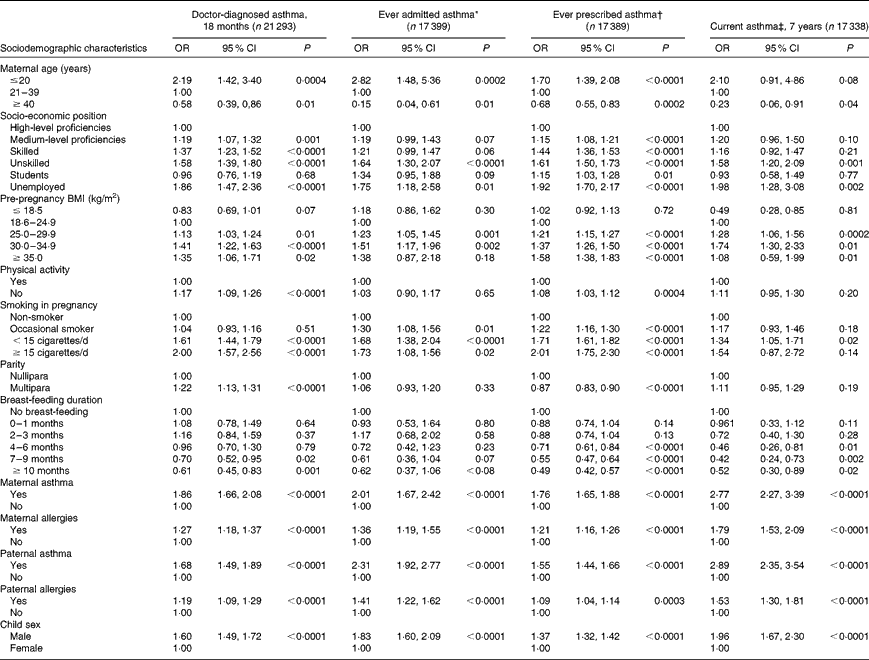
* Ever admitted asthma: first registered asthma diagnosis in the Danish National Patient Registry.
† Ever prescribed asthma: ≥ 2 asthma prescription in the Register of Medicinal Product Statistics except for β-2 agonists as liquid, inhaled β-2 agonists only once or inhaled steroid only once.
‡ Current asthma: self-reported doctor diagnosis of asthma plus wheeze in the past 12 months.
Multivariate analysis
Child asthma, wheeze and recurrent wheeze at 18 months
A total of 17 % (3551/21 293) of children were classified with doctor-diagnosed asthma, 27 % (5784/21 397) with wheeze symptoms and 9 % (1820/21 344) with recurrent wheeze in the first 18 months of life. After adjusting for potential confounders, we found a direct association for asthma (zero v. high frequency intake: OR 1·30, 95 % CI 1·05, 1·63, P= 0·02; P for trend = 0·001) and a suggestive association for recurrent wheeze (zero v. high frequency intake: OR 1·24, 95 % CI 0·94, 1·64, P= 0·13; P for trend = 0·28) (Table 3).
Table 3 Association between maternal fish intake during pregnancy and child asthma diagnosis and wheeze symptoms at 18 months in the Danish National Birth Cohort (Odds ratios and 95 % confidence intervals)
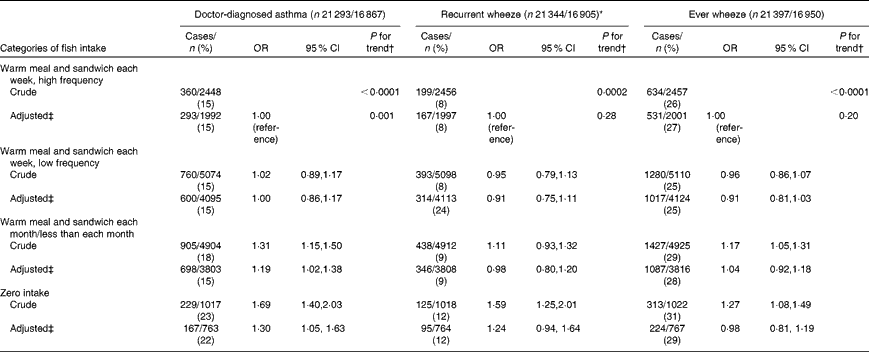
* Recurrent wheeze defined as >3 episodes in the past 18 months v. ≤ 3 episodes or no reported wheeze in the past 18 months.
† P value for trend estimated by modelling exposure as an ordinal variable.
‡ Model adjusted for maternal age, smoking, parity, pre-pregnancy BMI, physical activity, breast-feeding, socio-economic status, maternal history of asthma and allergies, paternal history of asthma and allergies, and child sex.
Ever admitted, prescribed and current child asthma at 7 years
The ever asthma prevalence was 6 % (996/17 399) and 31 % (5360/17 389) by admission and prescription, respectively. About 4 % (685/17 338) of children were classified with current asthma at 7 years of age. Children whose mothers never consumed fish during pregnancy were more likely to have an ever admitted asthma diagnosis compared with mothers in the highest intake category (OR 1·46, 95 % CI 0·99, 2·13, P= 0·05; P for trend = 0·46; Table 4). Similarly, we found a direct association for ever prescribed asthma (OR 1·37, 95 % CI 1·10, 1·71, P= 0·01; P for trend = 0·06). The relationship for current asthma was in the same direction but did not reach statistical significance.
Table 4 Association between maternal fish intake and child asthma ascertained with different data sources (patient registry, medication prescription registry and self-report) in the Danish National Birth Cohort (Odds ratios and 95 % confidence intervals)
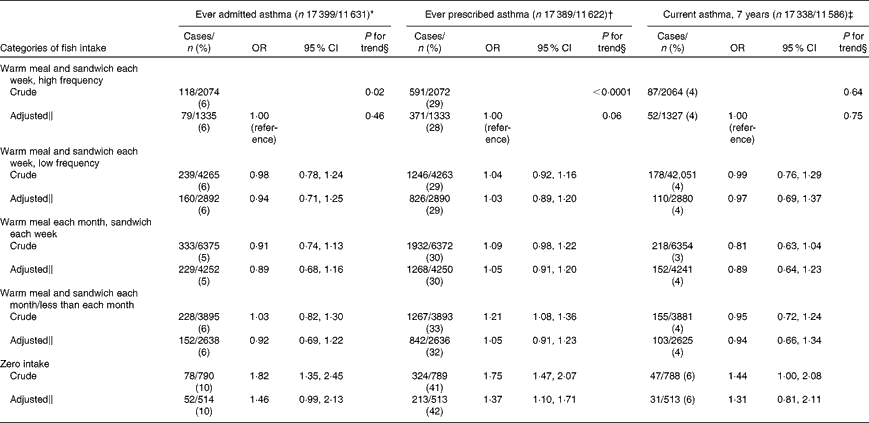
* Ever admitted asthma: first registered asthma diagnosis in the Danish National Patient Registry.
† Ever prescribed asthma: ≥ 2 asthma prescriptions in the Register of Medicinal Product Statistics except for β-2 agonists as liquid.
‡ Current asthma: self-reported doctor diagnosis of asthma plus wheeze in the past 12 months.
§ P value for trend estimated by modelling exposure as an ordinal variable.
∥ Model adjusted for maternal age, smoking, parity, pre-pregnancy BMI, physical activity, breast-feeding, socio-economic status, maternal history of asthma and allergies, paternal history of asthma and allergies, and child sex.
Ever prescribed and reported child allergic rhinitis at 7 years
The prevalence of ever prescribed child allergic rhinitis was 8 % (1332/17 389) and 5 % (821/17 269) based on the 7-year questionnaire. Maternal fish intake was not associated with either allergic rhinitis definition in the zero intake category; however, we found that low fish consumers (weekly and monthly) were less likely to report a child allergic rhinitis diagnosis compared with high fish consumers (Table S1, available online).
Sensitivity analyses
Given that n-3 fatty acids have been implicated as active agents in the association between fish intake and allergic disease, we examined associations with energy-adjusted n-3 fatty acids assessed by the mid-pregnancy FFQ. We found associations that were inconsistent with the fish results. Higher EPA and α-linolenic acid intake were protective of wheeze and recurrent wheeze at 18 months, respectively, but both became insignificant after further adjustment for dietary confounders. No associations were found for asthma or allergic rhinitis (data not shown).
For the early childhood outcomes, we further examined the risk of wheeze and asthma at 6 months but found no associations with fish intake (data not shown). Similarly, reporting of any wheeze episodes in the first 18 months of life ( ≥ 1 episode) was not related to higher fish intake (data not shown). To provide a clearer separation for the recurrent wheeze outcome, we excluded children reporting 1–3 wheeze episodes. This did not alter the results (data not shown).
We excluded the first 3 years of life from the analysis using registry-based outcomes in order to remove potential transient child wheezing. The number of registry cases more than halved and the number of medication-related cases were reduced by close to 80 %. The associations with fish weakened slightly and the CI widened for both ever admitted asthma and ever prescribed asthma (data not shown).
Discussion
In the present large prospective study, we examined the association between maternal fish intake during pregnancy and child allergic disease. The present results show that fish intake may influence child asthma risk as assessed by parent report of doctor diagnosis in early childhood as well as clinically established asthma using hospital admission and prescription registry data. The increase in risk may be especially relevant for non-consumers of fish. We did not find high fish intake to be protective for allergic rhinitis.
Asthma is a heterogeneous group of conditions and its diagnosis is dependent on the timing of assessment. Wheeze in response to respiratory infections in early life is often transient and resolves as the child grows, while allergic asthma persists and peaks at 7–9 years of age( Reference Sporik, Holgate and Cogswell 27 ). The pathological pathways involved in early and later manifestations of asthmatic symptoms may therefore differ, and the influence of fish on these pathways could vary. The present results suggest that fish intake during pregnancy could influence asthma doctor diagnosis, but not wheeze symptoms, in the first 2 years of life. This may be because diagnosis of asthma is a more homogeneous outcome compared with wheeze (which might include other causes of wheezing such as respiratory infections), or because of more accurate maternal reporting of doctor diagnosis compared with more subjective interpretation of wheeze symptoms. We furthermore found a protective association for ever admitted asthma and ever prescribed asthma, but not self-reported asthma diagnosis. The former outcomes may be better at capturing true asthma compared with the questionnaire. The difference may also indicate a difference in the severity of disease in registry v. self-reported diagnoses.
In our adjusted analyses, breast-feeding accounted for the largest attenuation in effect estimates. The role of breast-feeding in atopic disease is unclear. While some studies showed inverse associations with longer duration/exclusive breast-feeding( Reference Guilbert, Stern and Morgan 28 , Reference Oddy, de Klerk and Sly 29 ), others found no( Reference Burgess, Dakin and O'Callaghan 30 , Reference Nagel, Buchele and Weinmayr 31 ) or direct( Reference Sears, Greene and Willan 32 , Reference Fredriksson, Jaakkola and Jaakkola 33 ) relationships. In the present study, women who tended to breast-feed for a longer period of time were less likely to report a doctor diagnosis of early child asthma and to have a registry-based child diagnosis. Similar results were found for exclusive breast-feeding for ≥ 4 and ≥ 6 months. Women in the higher fish intake categories tended to breast-feed for a longer period of time, yet the correlations were modest for both breast-feeding duration and exclusivity (r 0·04–0·16). We found no statistically significant interaction by either breast-feeding duration or exclusivity.
To date, numerous studies have examined the relationship between fish intake during pregnancy and allergic outcomes, with inconsistent findings( Reference Romieu, Torrent and Garcia-Esteban 9 – Reference Øien, Storrø and Johnsen 14 ). Studies examining intake( Reference Miyake, Sasaki and Tanaka 15 ) or blood levels of fatty acids( Reference Newson, Shaheen and Henderson 34 , Reference Notenboom, Mommers and Jansen 35 ) have found protective associations for n-3 fatty acids with early allergic outcomes. These studies have included different allergic disease outcomes and did not have the advantage of the present study to separately examine both early and later manifestations of allergic disease. Furthermore, the protective associations were primarily found for eczema( Reference Romieu, Torrent and Garcia-Esteban 9 – Reference Willers, Devereux and Craig 11 , Reference Miyake, Sasaki and Tanaka 15 ), wheeze( Reference Romieu, Torrent and Garcia-Esteban 9 , Reference Miyake, Sasaki and Tanaka 15 , Reference Chatzi, Torrent and Romieu 36 ) and atopy( Reference Romieu, Torrent and Garcia-Esteban 9 ), rather than asthma( Reference Salam, Li and Langholz 13 ). Randomised clinical trials with fish oil supplementation during pregnancy have found favourable effects on immunological responses and sensitisation in the first year of life rather than on clinical outcomes( Reference Øien, Storrø and Johnsen 14 , Reference Dunstan, Mori and Barden 17 , Reference Furuhjelm, Warstedt and Larsson 18 , Reference Krauss-Etschmann, Hartl and Rzehak 20 ). These results are in some accordance with our fatty acid findings for outcomes at 18 months. The present results do not agree with a highly protective effect of EPA+DHA supplementation during pregnancy and asthma registry diagnosis in a Danish trial( Reference Olsen, Østerdal and Salvig 19 ). Differences in dose and a large variation in intake (2·7 g/d in the trial v. a mean of 0·38 (sd 0·29) g/d in the present study), and the timing of intake (late v. mid-pregnancy) may account for the discrepancies in the results. We are only aware of one previous study looking specifically at fish intake during pregnancy in relation to allergic rhinitis; results from this study( Reference Willers, Devereux and Craig 11 ) were consistent with the present results.
The present findings provide limited support for the lipid hypothesis proposed by Black & Sharpe( Reference Black and Sharpe 3 ) that a lower intake of n-3 fatty acids found in fish may increase the risk of allergic disease development. However, while the results for asthma are in line with this hypothesis, the present findings did not extend to allergic rhinitis; rather we observed lower risks of self-reported allergic rhinitis in the two lower fish intake groups (weekly and monthly), but not in the zero intake group. The differing results for these two outcomes may be explained by the failure to capture true allergic rhinitis since the self-reported definition was based on only one question and did not include questions on symptoms, while ever prescribed allergic rhinitis excluded over-the-counter medications such as histamines which may have decreased the sensitivity of the outcome measure. Asthma and allergic rhinitis share a genetic predisposition for atopy and a common inflammatory aetiology; however, in allergic rhinitis, there is no smooth muscle constriction in the airways( Reference Calder, Albers and Antoine 37 ). The present results could suggest that fish is more strongly related to the tissue, rather than to the immune component, of allergic disease, but these cannot be teased apart using the present data.
Furthermore, we did not find any associations with n-3 PUFA as reported by the FFQ; this may imply that other mechanistic pathways apart from those governed by essential fatty acids and their metabolites are involved. Fish is also an important source of vitamin D, a nutrient that has been implicated in immune function and allergy development( Reference Litonjua 38 ). Yet, we cannot exclude that poor measurement and low variability of fatty acid intake obscured any potential associations. This may be especially relevant if the association lies in the extreme intake categories.
The present study adds to the current literature on prenatal dietary exposure and child allergic disease development. The present prospective study design allowed us to follow a large cohort for the first 7 years of life. We collected detailed information on maternal fish intake during pregnancy and studied the relationship to outcomes at two time points in childhood, allowing for the change in risk across time and by disease manifestation. By adjustment for numerous confounders, including a wide range of other maternal dietary factors, we reduced the potential for residual confounding. We employed both self-reported data and national registries. The DNPR has complete follow-up, but includes only hospital cases, and the International Classification of Disease could be limited by miscoding. However, a recent validation study in Danish male conscripts against medical examination found that any misclassification in the DNPR was too small to nullify observed associations( Reference Østergaard Jensen, Lauge Nielsen and Ehrenstein 39 ). Self-report is more useful for outcomes such as allergic rhinitis. Allergic rhinitis is less likely to be captured by the registries as it rarely results in hospitalisations and use of prescription medication because of moderate symptoms and the access to over-the-counter drugs.
The main limitations of the present study were self-reporting of exposure and outcomes. We expect any misclassification of fish intake to have been non-differential, underestimating our associations. We cannot exclude misclassification of outcomes assessed by the questionnaire; yet, in a recent study( Reference Hansen, Strom and Maslova 40 ), we showed that our definition of current asthma has high agreement among non-cases (>90 %) when compared with the DNPR, suggesting that false positives and biased results were largely avoided. Though associations with all asthma outcomes showed the same directionality, small sample size in the zero intake category may have precluded us from finding an association for current asthma at 7 years of age. Although we did not have full data on child diet, we did have information on early child fish intake, which was only modestly correlated with maternal fish intake. When we included the information on child diet in the present analyses, there was a slight attenuation of the effect estimates, but this did not alter our conclusions. We find this reassuring for our interpretation of the observed associations as indicative of a beneficial effect of maternal fish intake against child asthma. Moreover, while some studies have found a beneficial association between child fish intake and allergic outcomes( Reference Alm, Aberg and Erdes 41 , Reference Kull, Bergstrom and Lilja 42 ), the largest randomised clinical trial did not detect an effect on asthma( Reference Almqvist, Garden and Xuan 43 ). However, we cannot exclude the possibility that child dietary factors other than early fish intake were responsible for the associations observed in the present study. Also, since the associations we observed seemed to be found particularly among the zero fish consumers, who had a lower SES status and a more overall unhealthy lifestyle than the high fish consumers, we cannot exclude the possibility that unmeasured confounding may have accounted for part of the association.
Lastly, loss to follow-up needs always to be considered in longitudinal studies due to the potential for selection bias. A detailed examination of population characteristics comparing participants and non-participants at 18 months and 7 years of the present analysis showed few differences, though participants tended to display healthier lifestyle habits and a higher SES. We also found similar characteristic distributions comparing the full singleton population to populations with outcome data at 18 months (67 % of the population) and 7 years (55 %). However, we cannot exclude residual bias by mismeasured or unmeasured selection variables.
The present results indicate a beneficial relationship between high v. low maternal fish intake in pregnancy and child asthma during the first 7 years of life. We found no associations for fatty acid intake. While several randomised clinical trials have assessed fish oil supplementation in relation to allergic outcomes, we were able to examine the intake of a whole food across a wide range of intakes and account for potential synergistic interactions between nutrients. The present results may also be more pertinent in communicating dietary recommendations. Further examination of which nutrients in fish may exert a protection against allergic disease is warranted. Likewise, studies are also needed to confirm and investigate potential mechanistic differences between asthma and allergic rhinitis, while also taking into account and further exploring the issue of potential residual confounding by lifestyle factors and SES inherent in observational studies.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S000711451300038X
Acknowledgements
The present study was supported by the Danish Council for Strategic Research (09-067124), the Danish Council for Independent Research – Medical Sciences, Danish Agency for Science, Technology and Innovation (09-063410), the Lundbeck Foundation (R13-A907) and the European Union Integrated Research Project EARNEST (FOOD-CT-2005-007036). The European Union project EARNEST (http://www.metabolic-programming.org) received financial support from the Commission of the European Communities under the FP 6 priority 5: food quality and safety. The Danish National Birth Cohort was financed by the March of Dimes Birth Defects Foundation, the Danish Heart Association, the Danish Medical Research Council, the Sygekassernes Helsefond, the Danish National Research Foundation, the Danish Pharmaceutical Association, the Ministry of Health, the National Board of Health and the Statens Serum Institut. We would also like to acknowledge support from the NIH/NICHD (K24 HD069408). The authors' responsibilities were as follows: E. M. and S. F. O. contributed to the study concept and design; M. S. was involved in the data preparation; E. M. and M. S. conducted the statistical analyses; E. M. drafted the manuscript; E. M., M. S., E. O., H. C., C. L., D. G. and S. F. O. contributed to the critical advice and revisions of the manuscript; S. F. O. obtained funding; E. M. and S. F. O. had a role in the acquisition of the data and took responsibility for the entire contents of the manuscript. All authors had full access to the study data. None of the authors had a conflict of interest.







