Introduction
Parrots are one of the most threatened bird orders with 28% of species listed as globally threatened and 56% of species in decline (Olah et al. Reference Olah, Butchart, Symes, Guzmán, Cunningham, Brightsmith and Heinsohn2016). For some highly endangered species, captive breeding programmes might be the only solution for recovery (Earnhardt et al. Reference Earnhardt, Vélez-Velentín, Valentin, Long, Lynch and Schowe2014). Parrots are frequently brought into zoological institutions through confiscations or as rescue birds in the form of unwanted pets from private aviculture. In Europe, a number of such birds are subsequently introduced into breeding programmes of the European Association of Zoos and Aquaria (EAZA), regularly without any or only insufficient previous health screening. Some of these breeding programmes are working towards reintroductions as part of species recovery programmes (Sanz and Grajal Reference Sanz and Grajal1998, Woolaver et al. Reference Woolaver, Jones, Swinnerton, Murray, Lalinde, Birch, de Ravel and Ridgeway2000, Collazo et al. Reference Collazo, White, Vilella and Guerrero2003). In the past there may have been insufficient focus on infectious diseases when considering reintroductions (White et al. Reference White, Collar, Moorhouse, Sanz, Stolen and Brightsmith2012, Collar et al. Reference Collar, Lierz, Stanley Price and Wirth2015).
Case study: disease in a collection of confiscated parrots
Between April 2009 and February 2011, a total of 24 living and one dead parrot, representing 11 species, arrived at one institution from four different locations in Denmark (Table 1). All species involved, apart from Long-billed Corella Cacatua tenuirostris, are considered to have declining in situ populations, one is listed as ‘Endangered’ (IUCN 2016) and all are considered rare in captivity (Zootierliste 2016). The birds were either confiscated on the same day as arrival or had been confiscated earlier and were relocated from other institutions due to space limitations.
Table 1. Parrots confiscated between April 2009 and February 2011, transferred to the same institution and housed in one room.

* the bird has died († cause) or was transferred to Justus-Liebig University in Giessen, Germany (JLU).
Upon arrival, all animals were identified, checked for body condition and endoparasites. The dead Jamaican Amazon Amazona collaria was kept frozen for further investigation. Due to restricted space, all birds were housed in one 100-m2 room in c.4-m2 cages. All parrots were housed in the same pairs or social groups as previously kept. The caretakers only entered the room with exclusive protective clothing. All removed food dishes were disinfected (Vircon S®) and waste was disposed of for incineration. In April 2011 a marten Martes sp. entered one of the cages and killed four amazons (see Table 1 for species). Four weeks later, in May 2011, having shown no prior sign of disease, five birds died within one week: one cockatoo confirmed due to proventricular dilatation disease (PDD) and four Amazons due to herpesvirus infection (Pacheco’s disease). All remaining birds were treated immediately for 14 days orally with acyclovir (100 mg/kg BID) for herpesvirus infection and no further deaths occurred.
The authorities agreed to transfer the remaining 15 parrots to the Justus-Liebig-University (JLU) in Giessen, Germany, in July 2012, because of their importance for managed populations. At JLU they received thorough diagnostics and further transfer to adequate holdings according to the test results. An overview on tests and results is provided in Table 2.
Table 2. Scientific and common name of 15 parrots from the same institution transferred on July 11, 2012 to Giessen University, Germany. Results from serological and PCR examinations for different pathogens.
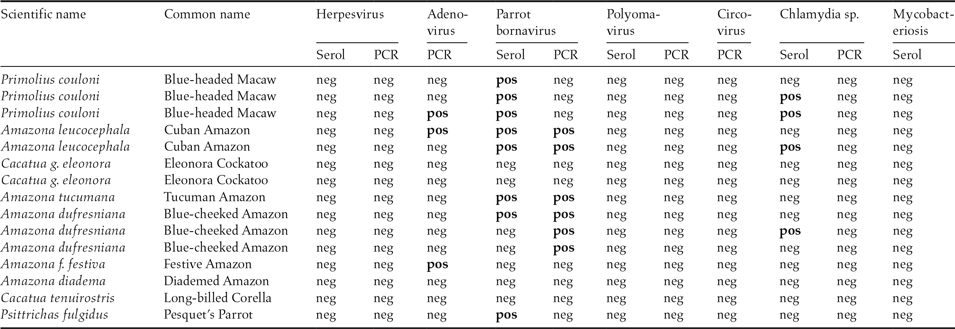
neg = Negative, pos = Positive; Negative serology = no specific antibodies against the pathogen were found; Negative PCR = no DNA/RNA of the pathogen was present in the sample. Positive serology = specific antibodies against the pathogen present; Positive PCR = DNA/RNA of the pathogen was present in the sample.
The major finding was parrot bornavirus (PaBV), with 10 of the 15 birds either demonstrating specific anti-PaBV antibodies and/or shedding PaBV-RNA. Additionally, three birds from three different origins tested positive for adenovirus in the PCR, and four demonstrated specific antibodies against Chlamydia psittaci. Avian tuberculosis was diagnosed microscopically in the Amazon which was dead upon arrival. In contrast, although Pacheco’s disease was diagnosed in the four dead Amazons one year earlier, none of the remaining birds showed antibodies against psittacine herpesvirus 1 or was shedding psittacine herpesvirus DNA. Four of the 15 parrots did not test positive for any of the tests applied.
As a result of these efforts, and with defined health status, all birds were moved to new holders; the four negative birds were included in conservation breeding programmes at zoological institutions, and all others were moved to private holders with birds of same pathogen status in their collection.
Discussion
The considerable risks of introducing captive animals, particularly when having been kept in close proximity to other parrots, have been identified as a concern (Snyder et al. Reference Snyder, Derrickson, Beissinger, Wiley, Smith, Toone and Miller1996), especially since some diseases can remain latent in asymptomatic carriers for long periods (Partington et al. Reference Partington, Gardiner, Fritz, Phillips and Montali1989). Twenty years ago, it was suggested that captive populations should be screened intensively for diseases and only animals that had a long history without exposure to potential disease carriers should be chosen for reintroduction (Snyder et al. Reference Snyder, Derrickson, Beissinger, Wiley, Smith, Toone and Miller1996). A programme to certify disease-free collections was proposed to simplify exchanges between holders (Greenwood Reference Greenwood1992).
Since then, serological and PCR testing methods for most diseases concerning psittacines (Avian polyomavirus, circo-, herpes-, adeno-virus, Chlamydia psittaci) have been established, and are performed by commercial laboratories. Even screening for PaBV, the causative agent of proventricular dilatation disease, is now possible for viral RNA by PCR (Honkavuori et al. Reference Honkavuori, Shivaprasad, Williams, Quan, Hornig, Street, Palacios, Hutchison, Franca, Egholm, Briese and Lipkin2008) and specific serum antibodies by indirect immunofluorescence assay (Herzog et al. Reference Herzog, Enderlein, Heffels-Redmann, Piepenbring, Neumann, Kaleta, Müller, Lierz and Herden2010).
Four of 15 birds in this study tested negative for pathogens despite their close proximity to positive individuals, highlighting the importance of individual susceptibility and indicating that virus-free populations may be achievable. In species where every individual is important to the future of a population, population screening is essential in establishing clinically healthy breeding pairs (Lierz et al. Reference Lierz, Piepenbring, Herden, Oberhäuser, Heffels-Redmann and Enderlein2011). This study illustrates that without appropriate testing, 11 birds carrying infectious pathogens could have transmitted diseases to other collections. Moreover, such unscreened birds could potentially impact wild populations if considered for reintroduction programmes. In-depth screenings were performed in the present case due to the perceived importance of the species for captive breeding; less conservation-relevant species would likely have been euthanized after the exposure to PDD and Pachecos’ disease.
The influence of the marten attack four weeks prior to the disease outbreak is unclear, but it could have potentially stressed one herpesvirus carrier bird enough to shed virus in vast amounts, resulting in disease and eventual death of sensitive animals. No matter what caused the outbreak, the time delay since the birds’ arrival demonstrates clearly the insufficiency of physical quarantine without any specific testing.
Ex situ breeding has played an important role in the recovery of a number of vertebrate species in recent years, contributing to a reduction in their IUCN threat level (Conde et al. Reference Conde, Flesness, Colchero, Jones and Scheuerlein2011). Yet, of 24 EAZA parrot studbooks, only one, Lilacine Amazon Amazona lilacina, has up-to-date Best Practice Guidelines with preventive veterinary recommendations (Pilgrim and Biddle Reference Pilgrim and Biddle2016) which includes testing requirements for all the pathogens mentioned in this case report. Considering that EAZA zoos keep on average 12 different species of Psittacines, routinely catching and screening all parrots in an institution may be beyond available budgets and willingness. Existing concerns regarding individuals that escape detection of disease, despite repeated testing (Collar et al. Reference Collar, Lierz, Stanley Price and Wirth2015), can be minimised through multiple testing of entire collections which helps identify individuals that only sporadically shed pathogens. Additionally, test sensitivities are constantly improving.
Looking at publications regarding releases of captive reared or confiscated parrots, animals have often been released with only selective screening (Brightsmith et al. Reference Brightsmith, Hilburn, Del Campo, Boyd, Frisius, Frisius, Janik and Guillén2005, Saidenberg et al. Reference Saidenberg, Zuniga, Melville, Salaberry and Benites2015). In consequence, the possible threat of introducing pathogens to naïve species has already been demonstrated. PBFD is considered to have emerged in the wild population of the endangered South African Cape Parrot Poicephalus robustus, probably from exposure to infected captive-bred animals (Regnard et al. Reference Regnard, Boyes, Martin, Hitzeroth and Rybicki2015).
In conclusion, in order to ascertain the conservation value of individuals and their reintroduction potential, we recommend the following at all zoological facilities: (1) establish pathogen-testing guidelines for target conservation species; (2) implement regular, mandatory health screening for the pathogens discussed above (see Table 2); and (3) for large collections, perform periodic collection-wide disease-risk assessments and implement strict quarantine and screening protocols. Ideally, untested Psittacines should not be housed at facilities with high-conservation-priority parrots. Specimen transfers should depend upon individual health status and be coordinated by the studbooks accordingly.
Acknowledgements
The authors are grateful to the technicians from Justus-Liebig-University, Giessen for excellent technical support. Two anonymous reviewers are thanked for very helpful comments on earlier drafts of this manuscript and Rebecca Biddle for proofreading.




