Cardiovascular diseases (CVD), including coronary heart disease, cerebrovascular diseases, and peripheral circulation diseases are the leading causes of global mortality in adults in both developed and developing countries, and account for almost 16 million deaths annually (Lozano et al., Reference Lozano, Naghavi, Foreman, Lim, Shibuya, Aboyans and Memish2012). Hypertension, hyperlipidemia, hyperglycemia, obesity, and hyperuricemia are important CVD risk factors and independently contribute to the incidence of CVD (Alderman & Aiyer, Reference Alderman and Aiyer2004; Ford et al., Reference Ford, Li, Cook and Choi2007; Hubert et al., Reference Hubert, Feinleib, McNamara and Castelli1983; Kavey et al., Reference Kavey, Allada, Daniels, Hayman, McCrindle, Newburger and Steinberger2007; Yusuf & Bosch, Reference Yusuf and Bosch2002). Atherosclerotic processes of CVD and clustering of CVD risk factors originating in childhood were reported by Srinivasan et al. (Reference Srinivasan, Frerichs, Webber and Berenson1976) and by Steinberger and Daniels (Reference Steinberger and Daniels2003). Furthermore, a number of studies have shown that the clustering of CVD risk factors is evident in childhood and persists into young adulthood (Berenson et al., Reference Berenson, Srinivasan, Bao, Newman, Tracy and Wattigney1998). Lobstein et al. (Reference Lobstein, Baur and Uauy2004) estimated that prevalence of childhood overweight and obesity ranged from 12% to 30% in developed countries and from 2% to 12% in developing countries. In China, the prevalence of overweight and obesity has reached up to 30% among 7- to 18-year-old children and adolescents in some urban areas (Chen et al., Reference Chen, Modin, Ji and Hjern2011), and the prevalence of hypertension up to 14% in the general population age 15 years and older (Yang et al., Reference Yang, Li, Cao, Lu, Wang, Zhan and Li2002). Studies on genetic and environmental variance contributions to these CVD risk factors are important because they can lead to better understanding of their risk factors and thus provide information for developing interventions strategies to prevent development of obesity and other CVD risk factors in youth.
Many twin and family studies have estimated genetic and environmental influences on CVD risk factors and their mutual associations in children and adolescents, primarily based on Caucasian populations (Bodurtha et al., Reference Bodurtha, Chen, Mosteller, Nance, Schieken and Segrest1991; Lajunen et al., Reference Lajunen, Kaprio, Keski-Rahkonen, Rose, Pulkkinen, Rissanen and Silventoinen2009; Schieken et al., Reference Schieken, Eaves, Hewitt, Mosteller, Bodurtha, Moskowitz and Nance1989; Weinberg et al., Reference Weinberg, Webber and Berenson1982). The Georgia Cardiovascular Twin Study also published heritabilities of blood pressure in youth (Snieder et al., Reference Snieder, Harshfield and Treiber2003; Wu et al., Reference Wu, Treiber and Snieder2013), as well as lipids profile in children and adolescents (Iliadou et al., Reference Iliadou, Snieder, Wang, Treiber and Davis2005), based on African and European-American data. However, previous research performed with Asian adolescent twins mostly concentrated on obesity-related phenotypes. For example, the estimated heritabilities of BMI were 0.82 for boys and 0.87 for girls at age 13 to 19 years in South Koreans (Hur, Reference Hur2007). To date, twin studies on the genetic correlations between various CVD metabolic risk factors among children and adolescents in non-Caucasian populations are rare, especially in the Chinese population. In addition, some previous studies on the heritability of blood pressure and cholesterol revealed that there were significant sex-specific genetic contributions in pre-pubertal twins (Bodurtha et al., Reference Bodurtha, Chen, Mosteller, Nance, Schieken and Segrest1991; Schieken et al., Reference Schieken, Eaves, Hewitt, Mosteller, Bodurtha, Moskowitz and Nance1989). The association of anthropometric and maturation variables with CVD risk factors have been well documented in epidemiological studies in childhood as early as in the Bogalusa Heart Study (Foster et al., Reference Foster, Voors, Webber, Frerichs and Berenson1977). Therefore, in addition to estimating genetic and environmental variance contributions to metabolic CVD risk factors, we will explore genetic and environmental associations between them based on twin data from Chinese children (8–12 years) and adolescents (13–17 years). We will also test whether the variance components differ by age or by sex, which would tell us whether there is variation between age groups for boys and girls. The risk factors for CVD in this article included obesity indices, blood pressure, serum lipid profile, and uric acid (UA).
Data and Methods
Participants and Measurements
The Qingdao Twin Registry (QTR) was initiated in 1988 as part of the Chinese National Twin Registry (Li et al., Reference Li, Gao, Yu, Lv, Cao, Zhan and Hu2013). Over 11,000 twin pairs have been recruited into the registry (Pang et al., Reference Pang, Ning, Unger, Johnson, Wang, Guo and Lee2006). Children and adolescents aged 8–17 years (588 twin pairs) of the QTR were examined from May to August, 2006. The procedure of recruitment is described in detail elsewhere (Duan et al., Reference Duan, Ning, Zhang, Wang, Zhang, Tan and Pang2013). Briefly, twins and their parents were invited to attend the survey by telephone or post. The Institutional Review Board of the Qingdao Center for Disease Control and Prevention approved the study protocol. Trained physicians or nurses were responsible for the objectives of the survey and obtained written consent from the parents and verbal consent from their twin children. The survey included a questionnaire, anthropometric measurements, and a 8- to 10-hour fasting blood sample collection.
Eight CVD risk factors, that is, BMI, WC, waist-to-hip ratio (WHR), SBP, DBP, total cholesterol (TC), TG, and UA were included in the analyses. BMI was calculated as weight in kilograms divided by squared height in meters (kg/m2). Weight and height were measured in lightweight clothes and without shoes. Weight was measured using a standing beam scale and rounded to the nearest 0.1 kg, and height using a vertical scale with a horizontal moving headboard and rounded to the nearest centimeter. WC was measured at the midpoint between the rib cage and the iliac crest and rounded to the nearest 0.1 centimeter. Hip circumference was measured over the widest part of the gluteal region and rounded to the nearest centimeter. SBP and DBP were measured on the right arm by a standard procedure using a mercurial table stand model sphygmomanometer. After the appropriate-size cuff for the participant's arm had been applied (covering approximately two-thirds of the upper arm), the cuff was gradually inflated to approximately 20 mm Hg above the point at which the radial pulse disappeared. The pressure within the cuff was then released at a rate of approximately 2 mm Hg/s while auscultating with a stethoscope over the brachial artery. SBP with the subject sitting for at least 10 minutes was measured as Korotkoff phase I (appearance of sound) and DBP as Korotkoff phase V (disappearance of sound). Three measurements were taken and the average of the three was used in the data analysis. Quality control for the blood pressure included calibration protocols of instrument. Serum total cholesterol, serum triglycerides and serum uric acid were measured from the blood sample using the Analyser Medical System (Olympus-AU 640 Automatic analyzers, Olympus Optical, Tokyo, Japan). Sixteen short tandem repeat (STR) markers were used to determine zygosity type of the twins with the same sex and blood type (Becker et al., Reference Becker, Busjahn, Faulhaber, Bähring, Robertson, Schuster and Luft1997). The procedures of DNA tests were conducted in the central laboratory of the Qingdao Blood Station.
Ninety-four subjects with no blood sample, 20 subjects with missing values for the study traits, and 74 twin individuals without information on their co-twin were excluded from the analysis data. As a result, 508 (53% of monozygotic) of children and adolescent twin pairs were included in the data analyses. We conducted the analyses separately for two age groups (aged 8–12 and 13–17 years) based on the median age of onset of spermarche in boys and menarche in girls, according to the Chinese Pubertal Study Group (Ma et al., Reference Ma, Du, Luo, Chen, Liu, Chen and Liu2009, Reference Ma, Chen, Chen, Zhu, Xiong, Li and Du2011). The twins were further classified into five subcategories by sex and zygosity: monozygotic males (MZM), dizygotic males (DZM), monozygotic females (MZF), dizygotic females (DZF), and opposite-sex dizygotic twin pairs (OSDZ).
Statistical Analyses
Twenty-four outliers for TC > 6.5 mmol/L, TG > 2.8 mmol/L, UA > 600 μmol/L and BMI > 30 kg/m2, that is, greater than 4 SD, were excluded from the analyses. TG was log-transformed before analysis to obtain normal distribution, whereas other traits were normally distributed. The SPSS version 18.0 for windows (SPSS, Chicago, IL, USA) and the Stata version 11.0 for windows (StataCorp, TX, USA) were used in descriptive analyses. Mean differences between the age groups and sexes were tested and partial Pearson's correlation coefficients adjusted for age and sex were calculated between the traits. When calculating p values, the effect of within-pair correlations on standard errors was taken into account.
The contributions of genetic and environmental factors were estimated by means of biometrical models for twin data (Posthuma et al., Reference Posthuma, Beem, de Geus, van Baal, von Hjelmborg, Iachine and Boomsma2003). MZ twins share 100% of their genes whereas DZ twins share, on average, 50% of their segregating genes. Both MZ and DZ twins reared together are expected to share the same proportion of environmental variation shared between the co-twins and unique to each individual. These assumptions allow decomposition of the trait variance into components of additive genetic variance (A), common environmental variance (C), dominant genetic variance (D), and unique environmental variance (E). Dominant genetic and common environmental variations are confounded if only information on MZ and DZ twins reared together is available, and thus cannot be estimated simultaneously in our data. Age and sex were treated as covariates in all analyses to control for their main effects. The Mx statistical package (version 1.7.03) with the raw data option was used to estimate the genetic and environmental influences and their 95% confidence intervals (CI). Maximum likelihood method was used in the model fitting.
We started the genetic modeling by fitting univariate models for each trait to test the assumptions of twin models, find the best fitting model used for subsequent multivariate modeling and calculate the proportions of variation explained by genetic and environmental factors. We compared the χ2-goodness-of-fit statistics of saturated models, which do not make any statistical assumptions, to that of univariate twin models. Statistically, non-significant difference in the fit statistics compared to the change of degrees of freedom suggests that the assumptions of twin modeling — that is, equal means and standard deviations for MZ and DZ twins as well as first and second co-twins — are not violated. Saturated models were also used to calculate maximum-likelihood within-pair correlations for each type of twins. Bivariate genetic analysis was conducted using Cholesky decomposition. This method allows partitioning the phenotypic covariance into A, C, and E components and thus estimating genetic and environmental correlations between pairs of traits. Sex limitation models including opposite-sex twins were used for all univariate and bivariate models.
Results
Table 1 presents the descriptive statistics for the CVD metabolic traits by age and sex. Means and standard deviations only for TG were not significantly different at age 8–12 years and 13–17 years. The older (13–17 years) group shows significantly higher BMI, WC, WHR, SBP, DBP, and UA in boys (all p <.001) and lower TC (p < .001) compared with younger (8–12 years) twins. Sex differences were observed for most traits (all p < .05) except age and DBP at both age groups. BMI, WC, WHR, and UA in boys are higher than in girls; however, TC and TG shows opposite results. For SBP, younger (8–12 years) boy twins show slightly smaller levels than that in girls. The effect of age and sex for each trait was adjusted for the further genetic analysis.
TABLE 1 Baseline Characteristic of Study Population in Children (8–12 years) and Adolescents (13–17 years) by Sex
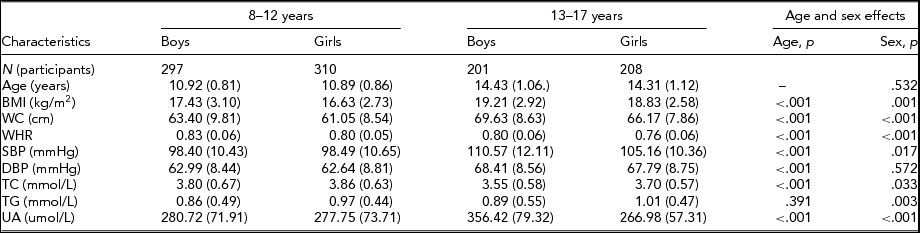
Data denote as mean (SD). BMI = body mass index; DBP = diastolic blood pressure; SBP = systolic blood pressure; TC = total cholesterol; TG = triglyceride; UA = uric acid; WC = waist circumference; WHR = waist-to-hip ratio; p = probability value.
Table 2 presents twin correlations for all traits in the two age groups by zygosity and sex. Twin correlations were higher among MZ than DZ twin pairs in boys and girls, except for BMI in girls aged 8–12 years and TG in girls aged 13–17 years, indicating the presence of genetic effects. Because MZ correlations were less than two times of the DZ correlations except for BMI in boys, TC and UA in girls aged 8–12 years, TC in boys and girls, and DBP in girls aged 13–17 years, we used the additive genetic/common environmental/specific environmental model (ACE model) as the starting point of genetic modeling. We first compared the ACE models to the fully saturated models. The best fit was found for all traits at age 13–17 years. At age 8–12 years, the fit of ACE models for all traits also were not statistically significant compared to saturated models after we had corrected the conventional α-level (p < .05) by Bonferroni correction of 16 tests (p < .003). Thus, the ACE model was performed to estimate the heritability of all traits in univariate modeling.
TABLE 2 Twin Correlations in Children (8–12 years) and Adolescents (13–17 years) by Zygosity and Sex
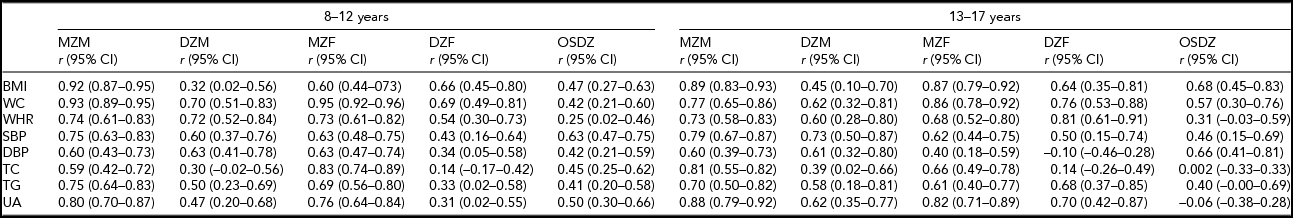
All traits were adjusted for age and sex. BMI = body mass index; CI = confidence interval; DBP = diastolic blood pressure; DZM = dizygotic male twins; DZF = dizygotic female twins; MZM = monozygotic male twins; MZF = monozygotic female twins; OSDZ = opposite-sex dizygotic twins; r = correlation; SBP = systolic blood pressure; TC = total cholesterol; TG = triglyceride; UA = uric acid; WC = waist circumference; WHR = waist-to-hip ratio.
Table 3 presents the standardized variance components of genetic and environmental factors for all traits in the two age groups. In boys aged 8–12 years, the highest heritability was found for BMI 0.81 (95% CI 0.54–0.93) suggesting the importance of genetic effects. Total cholesterol with the heritability estimate of 0.61 (95% CI 0.17–0.72), as well as uric acid 0.49 (95% CI 0.14–0.81) also had evidence of genetic effect. However, for the age 13–17 years, genetic effect clearly decreased and was statistically non-significant for most traits: only BMI and uric acid demonstrated high genetic effect in boys and BMI and total cholesterol in girls. Common environmental effects became important for WC, WHR, triglycerides, and uric acid in girls. The genetic effect for BMI and uric acid were consistently found in the two age groups in boys and for total cholesterol in girls.
TABLE 3 The Relative Variance Component Estimates with 95% Confidence Intervals by Age and Sex
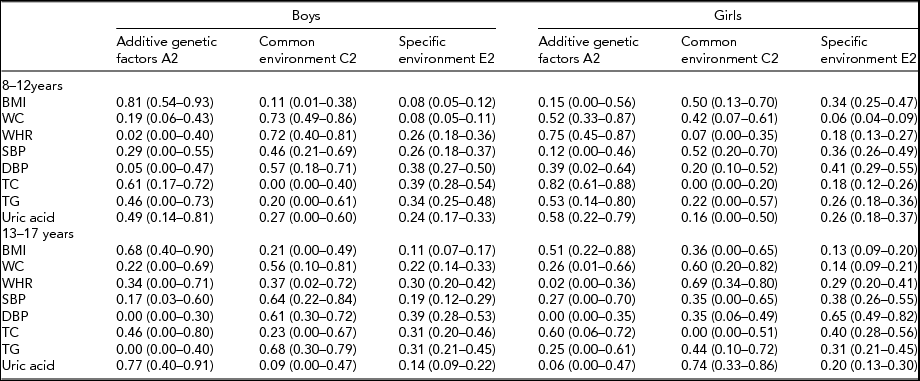
BMI = body mass index; DBP = diastolic blood pressure; SBP = systolic blood pressure; TC = total cholesterol; TG = triglyceride; WC = waist circumference; WHR = waist-to-hip ratio.
The correlations between the traits are presented in Table 4. In the two age groups, moderate to strong correlations in boys and girls were observed between the same groups of traits: BMI-WC, BMI-WHR, SBP-DBP, TC-TG. The highest correlation existed between BMI and WC, in which the corresponding figures were 0.72 for boys and 0.58 for girls in the 8- to 12-year-old age group, and 0.80 for boys and 0.68 for girls in the 13- to 17-year-old age group, respectively. SBP showed constantly moderate correlation with BMI from 8–12 to 13–17 years.
TABLE 4 The Trait Correlations by Age and Sex
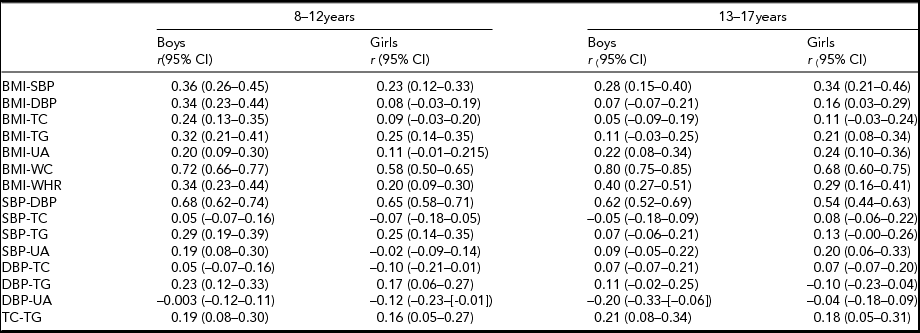
BMI = body mass index; CI = confidence interval; DBP = diastolic blood pressure; r = trait correlation; SBP = systolic blood pressure; TC = total cholesterol; TG = triglyceride; UA = uric acid; WC = waist circumference; WHR = waist-to-hip ratio.
We then decomposed all statistically significant correlations in boys and girls using a bivariate Cholesky decomposition. First, we tested specific environmental correlations and after that common environmental correlations and dropped them from the ACE model if they were not statistically significant. Finally, we decide to retain the r e and r c coefficient unless the coefficient could be eliminated simultaneously from both boys and girls. In the 8- to 12-year-old age group, moderate to strong genetic correlations were found and they showed similar pattern to the trait correlations. However, in the 13- to 17-year-old age group, in both boys and girls, significant genetic correlations were seen only for BMI-SBP, BMI-UA, BMI-WC, BMI-WHR, and TC-TG (Table 5).
TABLE 5 Additive Genetic, Common Environmental and Specific Environmental Correlations in the Best Fitting Cholesky Decomposition by Age and Sex
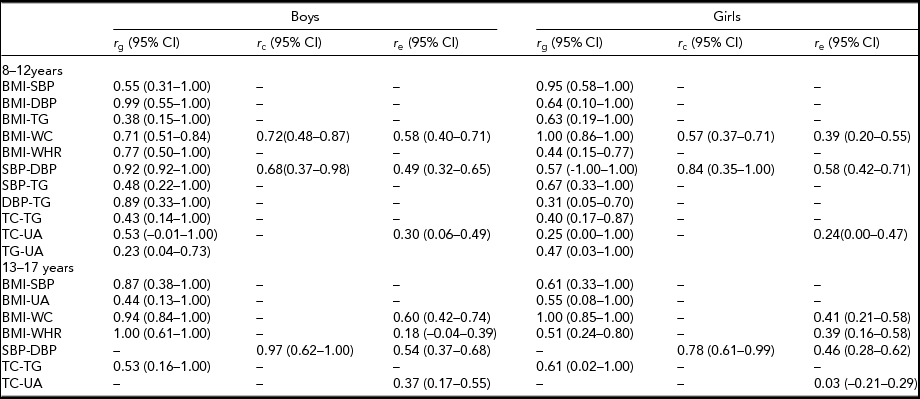
Only trait combination with significant phenotypic correlations were tested in the best-fitting models; — indicates that r e and r c coefficient can be set to zero; BMI = body mass index; CI = confidence interval; DBP = diastolic blood pressure; SBP = systolic blood pressure; TC = total cholesterol; TG = triglyceride; UA = uric acid; WC = waist circumference; WHR = waist-to-hip ratio.
Discussion
A number of studies have identified genetic and environmental contributions to CVD risk factors in adult twins. For example, previous studies based on adult twins reported that heritabilities of SBP were 0.52–0.66 and DBP 0.44–0.66 in six European countries and Australia (Evans et al., Reference Evans, Baal, McCarron, DeLange, Soerensen, Geus and Boomsma2003); and WC 0.59–0.64 and TG 0.46–0.50, respectively, in South Korean twins (Sung et al., Reference Sung, Lee and Song2009) and rural Chinese female twins (Zhang et al., Reference Zhang, Liu, Yu, Hong, Christoffel, Wang and Tang2009). Genetic and trait correlations between BMI-WC and SBP-DBP were high in Danish twins (Benyamin et al., Reference Benyamin, Sørensen, Schousboe, Fenger, Visscher and Kyvik2007). However, few studies have explored whether these factors have shared common genetic and environmental influences in childhood and adolescence. To our knowledge, this is the first study to detect the impact of genetic and environmental factors on several CVD risk factors in Chinese children and adolescents by means of univariate model-fitting methods, and to examine the genetic and environmental associations between traits using a bivariate model-fitting approach.
Our univariate genetic analysis indicated that the heritability estimated for the eight traits associated with CVD risk profile varied from weak to high. The high heritability of BMI in both childhood and adolescence is consistent with prior research results (Ortega-Alonso et al., Reference Ortega-Alonso, Pietiläinen, Silventoinen, Saarni and Kaprio2012; Silventoinen et al., Reference Silventoinen, Rokholm, Kaprio and Sørensen2010); the only exception was somewhat weaker heritability in the 8- to 12-year-old age group of girls. Age and sex difference in statistically significant genetic variances were found in some traits, such as WC, TC, and UA. In contrast to previous findings in Caucasian (Benyamin et al. Reference Benyamin, Sørensen, Schousboe, Fenger, Visscher and Kyvik2007; Elder et al., Reference Elder, Lichtenstein, Pittas, Roberts, Fuss, Greenberg and Neale2009) and Chinese adult populations (Zhang et al., Reference Zhang, Liu, Yu, Hong, Christoffel, Wang and Tang2009), we found moderate to strong effect of common environmental influences specifically on SBP and DBP in boys and girls presented at both age groups. The effect sizes of common environmental factors were smaller in children than in adolescents. Our results support the importance of the environment impact on CVD risk that has started from early life and are modified by factors shared by co-twins, such as childhood family conditions in the Chinese population.
The amount of additive genetic variation shared between CVD risk factors is expressed by genetic correlations. A genetic correlation expresses the extent to which two measurements reflect the same set of genes. Research on genetic correlations can facilitate the search for pleiotropic genetic variant (Povel et al., Reference Povel, Boer and Feskens2011). Findings from our bivariate genetic analyses showed that genetic correlation coefficients between the metabolic traits varied in boys and girls during childhood and adolescence. In childhood, nine significant genetic correlations existed in both boys and girls; the result of high genetic correlation between BMI and blood pressure was similar to those previous twin or family studies of adults across ethnic populations (Benyamin et al., Reference Benyamin, Sørensen, Schousboe, Fenger, Visscher and Kyvik2007; Choh et al., Reference Choh, Gage, McGarvey and Comuzzie2001; Duan et al., Reference Duan, Pang, Zhang, Li, Kruse, Kyvik and Tan2011; Wu et al., Reference Wu, Snieder, Li, Cao, Zhan, Lv and Hu2011). In adolescence, we obtained only five positive genetic correlations in both sexes.
Furthermore, it is noteworthy that first, consistent positively genetic correlation existed between BMI and SBP, BMI and WC, BMI and WHR, and TC and TG in both age groups and sexes. Further research on actual genetic variants responsible for the genetic pleiotropy of these four trait pairs may provide more insight into the etiology of CVD risk factors in China. Generally, unique environmental correlations were smaller in magnitude than genetic correlations. All causal effects between two traits are modeled as part of unique environmental correlations. Thus our results give evidence that causal associations are less important for the trait correlations than genetic pleiotropic effects.
Second, the pattern of correlation between TC and UA is similar in both age groups, with significant unique environmental correlation in boys. It does not appear that these two traits share pleiotropic effects with each other. A direct comparison with other studies is difficult due to lack of information on study of uric acid. Third, Nelson et al. (Reference Nelson, Vogler, Pedersen and Miles1999) reported that WC is more highly genetically correlated to BMI than WHR in both male and female in Swedish older twin pairs. However, in our study, only in girls was WC highly genetically correlated to BMI than WHR at both age groups; for boys, the situation was opposite. More research is needed to find whether genetic associations between central obesity indexes and BMI are different for each gender among the ethnic Chinese population at different ages.
In summary, based on our study, it is clear that the genetic influence on eight metabolic risk factors of CVD decreased with age in both boys and girls. Meanwhile, genetic correlations between the traits also changed with age. In the contemporary Chinese population, environmental factors shared by co-twins play an important role in the etiology of cardiovascular risk profile and correlations.
Acknowledgments
The authors wish to thank the QDCDC twin project staff for assistance in data collection, and the twins and their families for their participation in this research. This study was supported by the EFSD/CDS/Lilly Research Fellowship 2009 and the Natural Science Foundation of Shandong Province (# ZR2009CM111). KS was also supported by the Academy of Finland (#266592).







