Observational and epidemiological data have shown that a low-Ca diet may be a risk factor for obesity development, and beneficial aspects of milk components on metabolic syndrome are gaining strength(Reference Barba and Russo1, Reference Pfeuffer and Schrezenmeir2), even though intervention trials have yielded inconsistent results to date(Reference Major, Chaput and Ledoux3).
The molecular mechanisms responsible for the impact of dairy products on body weight and fat have been studied in animal models with important contributions from the group of Zemel(Reference Zemel4). Using a transgenic animal model (over-expressing agouti protein under the control of the aP2 promoter), Zemel and co-workers showed that dietary Ca could influence fat deposition by direct modulation on adipocyte metabolism, increasing thermogenesis and lipolysis and decreasing lipogenesis following a high-Ca diet(Reference Zemel, Shi and Greer5, Reference Xue, Greenberg and Kraemer6). However, in normal mice, activation of thermogenesis does not seem to be responsible for the lower rate of weight gain seen with a high-fat diet enriched with dairy Ca(Reference Parra, Bruni and Palou7).
Minerals have been suggested to beneficially modulate cardiovascular risk factors(Reference Scholz-Ahrens and Schrezenmeir8, Reference Vaskonen9). However, there are few data available concerning dietary mineral interactions. The aim of the present work was to assess the effects of a high-fat diet enriched in Ca on body fat and mineral bone retention, particularly on Ca, Mg and Zn, as well as to assess the potential involvement of adaptive thermogenic mechanisms in mice.
Experimental methods
Animals and diets
Twelve male mice (C57BL/6J) (Charles River, Spain) weighing approximately 21 g were housed in groups of three and kept in a single metabolic cage throughout the experiment under controlled conditions. After a 1-week adaptation, mice received ad libitum either a control diet or a high-Ca diet (Research Diets Inc., New Brunswick, NJ, USA) for 56 d. Both diets provided the same digestible energy content (19 kJ/g), 20 % kJ as protein (casein) and 43 % kJ as fat (lard and soyabean oil). In addition, the high-Ca diet supplied 1·2 % (w/w) in Ca (from calcium carbonate and milk powder), three times higher than control group (0·4 % w/w, exclusively from calcium carbonate). High-Ca diet was aimed to supply 42 % Ca from non-fat dry milk which also provided higher levels of Mg (1·09 g/kg) and Zn (0·05 g/kg) than the control diet (Mg, 0·57 g/kg; Zn, 0·03 g/kg). Food and water intake and body weight were recorded weekly.
All experimental procedures were performed according to the national and institutional guidelines for animal care and use at the university.
Sampling
Urine was collected twice a week from each cage, and measured and stored at − 20°C for posterior analysis. Animals were killed by decapitation at the end of the experiment; tissues were dissected, weighed, rinsed with saline containing 0·1 % diethyl pyrocarbonate (Sigma, Madrid, Spain), frozen with nitrogen liquid and stored at − 70°C until analysis.
Analytical procedures
Ashed femur samples and aliquots of urine were measured after appropriate dilution by flame atomic absorption spectrometry (Perkin-Elmer 272). Liver glycogen was extracted(Reference Carroll, Longley and Roe10) and the released glucose was determined by enzymatic assay(Reference Trinder11). Lipids were extracted(Reference Folch, Lees and Sloane Stanley12) and TAG and cholesterol content were determined using commercial kits from Biotécnica 2000. Liver protein content was determined from homogenised samples in PBS(Reference Bradford13).
Muscle and brown adipose tissue (BAT) proteins were extracted using Tripure reagent (Roche, Barcelona, Spain). Protein concentration was determined by the bicinchoninic acid assay (Pierce) using a bovine serum albumin standard. Uncoupling protein 1 (UCP-1) in BAT and UCP-3 in muscle were determined by Western blotting(Reference Parra, Bruni and Palou7). β-Actin was determined in representative gels to confirm equal protein load charge between samples. The immunocomplexes were revealed using an enhanced chemiluminescence detection system (ECL™; Amersham Biosciences, Barcelona, Spain) and visualised by exposure to sensitive films (Hyperfilm™ ECL; Amersham Biosciences). The films were scanned in a ChemiGenius (SynGene) using the software GeneSnap version 6.03, and the bands were quantified using GeneTools version 3.04 (SynGene).
Statistical analysis
The effect of Ca treatment on body weight was assessed by ANOVA followed by post hoc analysis. Comparison between control and Ca animals for the rest of the variables was assessed by Student's t test. The analysis was performed using the SPSS program for Windows version 14 (SPSS, Chicago, IL, USA). Urine mineral data from control and Ca animals were analysed on each day of treatment; no differences were observed throughout the period, and therefore data are presented as the average of all samples collected. The level of significance was set at P < 0·05. Data are presented as means with their standard errors.
Results
Food consumption did not differ between groups during the period studied 52·3 (sem 1·7) kJ/d in the control group and 53·1 (sem 2·1) kJ/d in the Ca group. The amount of food eaten implies an intake of 10·8 (sem 0·3) mg Ca/d, 1·5 (sem 0·04) mg Mg/d and 0·08 (sem 0·002) mg Zn/d in the control group and 46·8 (sem 1·2) mg Ca/d, 4·2 (sem 0·1) mg Mg/d and 0·2 (sem 0·05) mg Zn/d in the Ca group. No difference in water consumption was seen between groups 4·27 (sem 0·08) ml/d in controls and 4·42 (sem 0·05) ml/d in the Ca group.
Body weights were significantly different between groups from week 6 onwards (P = 0·017) and, by the end of the study, Ca-fed animals weighed less than control mice (Fig. 1). During the treatment, the control group gained 7·61 (sem 0·7) g, whereas animals fed the high-Ca diet gained 4·41 (sem 0·2) g (P = 0·003).
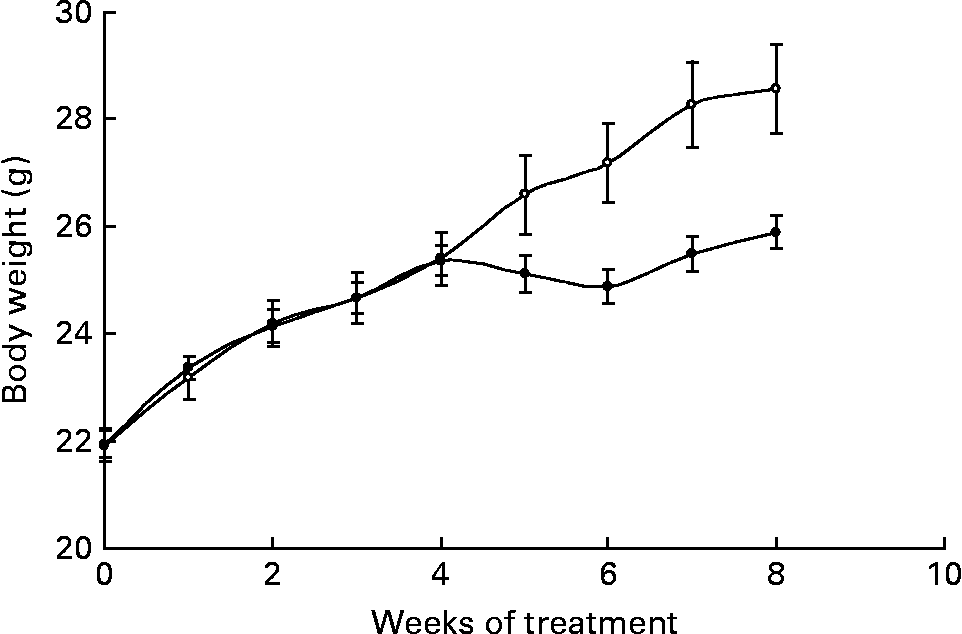
Fig. 1 Body weight evolution of animals fed either the control (○) or the high-calcium (●) diet for 56 d. Values are means with their standard errors depicted by vertical bars (six animals for each group). Body weights were significantly different from week 6 onwards (P = 0·017), determined by repeated-measures ANOVA.
Adipose tissue weights were significantly smaller in mice fed the high-Ca diet than in controls, mesenteric was the least affected (50 %) and the retroperitoneal the highest (69 %) compared with controls. BAT was also 40 % lower in the Ca group than in the control group (Table 1). Saturated lipid concentration and TAG were determined in epididymal adipose tissue and were not different between the control and Ca group (data not shown).
Table 1 Fat depot weights and liver weight and composition in mice fed either the control or the high-calcium diet for 56 d*
(Mean values with their standard errors)
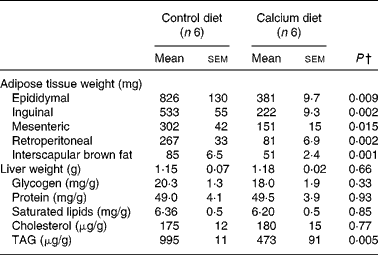
* For details of procedures and diets, see Experimental methods.
† Statistically significant differences between groups were determined by Student's t test.
Liver weight was not affected by dietary treatment and the same was seen for glycogen, proteins, saturated lipid and cholesterol concentrations in liver. However, TAG levels were reduced by half in the Ca group.
UCP-1 levels in BAT were not different between control and Ca-fed animals (100 (sem 11·2) % in control group; 110 (sem 15·4) % in Ca group). The same pattern was seen in muscle UCP-3 levels (100 (sem 10·5) % in control; 89·1 (sem 11·7) % in Ca group).
No significant differences were observed with respect to the volume of urine eliminated daily (control 0·77 (sem 0·05) v. Ca 0·89 (sem 0·07) ml/d, P = 0·19). However, Ca (control 5·18 (sem 1·0) v. Ca 603 (sem 79) μg/d, P = 2·3 × 10− 6), Mg (control 0·50 (sem 0·1) v. Ca 494 (sem 70) μg/d, P = 5·3 × 10− 6) and Zn (control 21·5 (sem 1·7) v. Ca 89·2 (sem 6·0) μg/d, P = 3·6 × 10− 8) urine excretion were significantly higher in the Ca group than in controls. To assess mineral deposition, femur composition was analysed and no statistically significant effects either on femur weight or on Ca, Mg or Zn content at the end of treatment were found (data not shown).
Discussion
The aim of the present work was to assess the effects of high-fat diet enriched in Ca, which has been previously associated with a lower body fat deposition, on Ca, Mg and Zn bone retention, as well as on thermogenic capacity in normal mice.
In accordance with previous results(Reference Parra, Bruni and Palou7), a reduction in body weight gain (by 12·7 %) was observed following the treatment with a high-Ca diet, which was also accompanied by lower fat deposition affecting all the adipose depots. In addition, hepatic lipid profile was not altered in the Ca group and the lower TAG content, associated with diminished hepatic lipogenesis, was in accordance with the lower fat accretion seen in adipose tissues.
No differences in food intake and in the expression of UCP-1 in BAT or in muscle UCP-3 were found, indicating that lower fat accretion in high-Ca-fed animals was not accompanied by lower energy intake either by activation of BAT or muscle thermogenesis, in accordance with results previously found in normal animals(Reference Parra, Bruni and Palou7, Reference Papakonstantinou, Flatt and Huth14) but not in a largely characterised transgenic model(Reference Sun and Zemel15).
Urine mineral excretion (Ca, Mg and Zn) was increased in the Ca group and, to a certain extent, this could contribute to compromised mineral bioavailability. For example, high Ca to Mg dietary ratio has been proposed to pose a risk for Mg deficiency(Reference Seelig16) although, in practice, high-Ca diets have not been demonstrated to affect Mg retention in the long term(Reference Whiting and Wood17). Furthermore, Ca, especially in the presence of phytate, may have an inhibitory effect on Zn absorption(Reference Lonnerdal18) and a reduction in tibia Zn content has been reported in rats fed on high-Ca diet(Reference Forbes19), interestingly this does not seem to happen in the present experimental conditions. The preservation of Ca, Mg and Zn content in femur, despite the higher urine excretion, supports the fact that body levels of these minerals are not compromised in the Ca group, at least during the period studied.
In conclusion, the present results support a lower body weight gain and lower body fat accretion in adult mice fed a high-fat diet enriched with dairy Ca with respect to those fed with standard Ca levels. This could not be attributable to a lower gross energy intake or to activation of thermogenesis in BAT or muscle. Although significant urine mineral loss was found in Ca-fed animals, the presence of dairy Ca was accompanied by preservation of mineral depots in bone. Further characterisation of cellular metabolism of these minerals would be necessary to attain total comprehension of their role in diminishing fat accretion. Nevertheless, the present data support the fact that adding more Ca to the diet, using a combination of milk powder containing among other things higher Zn and Mg, contributes to counteract obesity and improves lipid metabolism in high-fat-fed mice.
Acknowledgements
This work was supported by grants from the Spanish Government (AGL2004-07 496 and AGL2006-04 887) and European Commission (European Nutrigenomics Organisation, NUGO- FP6-506360). CIBER de ‘Fisiopatología de la Obesidad y Nutrición’ is an initiative of ISCIII. P. P. is a recipient of a fellowship from the Spanish Government. M. G. took care of animal handling, sample collections and analytical determinations. J. S. contributed to animal dissection and tissue sample collection. P. P. performed protein extraction and Western determinations. A. P., F. S. and L. P.-G. equally contributed to the conception and design of the study, interpretation of data and drafting of the manuscript. The authors declare no conflict of interest.




