Globally, vitamin D deficiency is common during pregnancy. However, epidemiological studies determining the associations between vitamin D status and adverse pregnancy outcomes such as pre-eclampsia, gestational diabetes, low birth weight, preterm labour and infectious diseases have shown conflicting results( Reference Thorne-Lyman and Fawzi 1 ). Therefore, the benefits of routine vitamin D supplementation for improving pregnancy outcomes remain to be established( 2 ).
Vitamin D plays an important role in defence against infectious diseases by modulating both innate and adaptive immune responses. All major immune cells, including T cells, B cells, neutrophils and antigen-presenting cells (monocytes, macrophages and dendritic cells), express the vitamin D receptor. Macrophages, the front-line responders to microbial infection, sense pathogen-associated molecular patterns by utilising pattern recognition receptors such as Toll-like receptors (TLR). TLR triggering induces Cytochrome p450 27B1 (CYP27B1), a vitamin D-activating enzyme that converts 25-hydroxyvitamin D (25(OH)D) into the active form (1,25-dihydroxyvitamin D; 1,25(OH)2D), and vitamin D receptor expression( Reference Liu, Stenger and Li 3 ). The 1,25(OH)2D–vitamin D receptor complex directly induces cathelicidin antimicrobial peptide (CAMP; gene encoding LL-37) transcription and corresponding increases in the expression of the antimicrobial peptide, LL-37( Reference Wang, Nestel and Bourdeau 4 ).
Antimicrobial peptides function as front-line host defence effector molecules, acting as both endogenous antibiotics and paracrine immunomodulators( Reference Lai and Gallo 5 ). Human cathelicidin (the cleaved active component of which is LL-37) is synthesised by several immune cell types (neutrophils, macrophages, B cells, T cells, natural killer cells and mast cells) as well as by barrier/mucosal epithelial cells( Reference Agerberth, Charo and Werr 6 – Reference Gudmundsson, Agerberth and Odeberg 8 ). In response to some infections (diarrhoeal diseases and gonorrhoea), LL-37 has been found to be down-regulated( Reference Bergman, Johansson and Asp 9 – Reference Shirin, Rahman and Danielsson 12 ), while in some other diseases, it is up-regulated (psoriasis)( Reference Ong, Ohtake and Brandt 13 ). The localisation of LL-37 to skin( Reference Dorschner, Lin and Murakami 14 ), vernix caseosa( Reference Yoshio, Tollin and Gudmundsson 15 ) and breast milk( Reference Murakami, Dorschner and Stern 16 ) suggests a potential role for the antimicrobial peptide in response to infection in young infants. Antimicrobial peptides, including LL-37, exhibited bactericidal activity against organisms responsible for bacterial infections in newborns( Reference Starner, Agerberth and Gudmundsson 17 ). They are up-regulated in tracheal secretions during neonatal lower respiratory tract infections( Reference Schaller-Bals, Schulze and Bals 18 ), suggesting that clinical or nutritional interventions to enhance the production of antimicrobial peptides may reduce the risk of neonatal infections. Little is known regarding the effect of vitamin D supplementation during pregnancy on the synthesis of cathelicidin/LL-37 or other aspects of neonatal innate immune defences.
Therefore, in the present study, we aimed to evaluate the effect of prenatal vitamin D3 (vitD3) supplementation on neonatal innate immune function. In a previous double-blind, placebo-controlled trial of vitD3 supplementation (0·875 mg/week) during the third trimester of pregnancy among Bangladeshi women, vitD3 has been shown to significantly increase the mean 25(OH)D concentration in cord blood by approximately 2·5-fold compared with the placebo group (103 v. 39 nmol/l)( Reference Roth, Al Mahmud and Raqib 19 ). In the present trial cohort, we studied the effect of this substantial rise in vitamin D status on the expression of cord blood LL-37 peptide and mRNA transcript levels in stimulated macrophages and the killing capacity of neonatal macrophages ex vivo.
Methods
Study design and participants
The Antenatal Vitamin D in Dhaka (AViDD) study was a randomised, double-blind, placebo-controlled trial of vitamin D supplementation of pregnant women in the third trimester conducted in Dhaka, Bangladesh. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the Ethics Committees of the International Centre for Diarrheal Disease Research, Bangladesh (ICDDR,B) and Johns Hopkins Bloomberg School of Public Health, Hospital for Sick Children (protocol no. PR-09058). Written informed consent was obtained from all eligible participants. Details of the trial design and primary biochemical outcomes have been published previously( Reference Roth, Al Mahmud and Raqib 19 ). Briefly, 160 pregnant women were enrolled at the Shimantik Urban Primary Health Care Project Maternity Center, a non-governmental facility that provides basic antenatal and obstetric services in a low-income community. Pregnant women were included if they were aged between 18 and < 35 years, at a gestational age of 26–29 weeks, currently residing in Dhaka with plans to stay there throughout pregnancy and for at least 1 month after delivery, and had plans to deliver at the maternity centre. Study participants were allocated to receive a weekly dose of either 0·875 mg vitD3 (VitD group: cholecalciferol, Vigantol oil; Merck KGaA) or placebo oil (placebo group: Miglyol oil; Merck) until delivery. Of the 160 women enrolled in the AViDD trial, there were 129 mother–infant pairs (81 %), sixty-four from the placebo group and sixty-five from the VitD group, with adequate volume of cord blood for immune function assays.
Cord blood collection, plasma and mononuclear cell isolation
Venous cord blood was collected immediately after delivery and transferred to a central laboratory in Dhaka for processing on the same day (within 2–18 h). Maternal blood was also collected during the time of delivery. Cord blood mononuclear cells (CBMC) and cord blood plasma were separated from the whole blood by Ficoll-Paque (Amersham Pharmacia Biotech, Inc.) density gradient centrifugation. The isolated CBMC were resuspended in Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco; Invitrogen) containing autologous plasma, and cultured in two parallel sets of tissue culture plates (NUNC; Thermo Fisher Scientific) for 3 d to develop macrophages. A dose–response assessment was performed to determine the optimum concentration of lipopolysaccharide (LPS; 2·5, 5·0 and 10 μg/ml) and the incubation time needed for monocyte-derived macrophages (MDM) to produce maximum levels of LL-37. We found that stimulation with 5·0 and 10 μg LPS/ml produced similar levels of LL-37 peptide by MDM, and the concentration of the peptide was comparable or slightly higher in 48 h culture as opposed to 24 h incubation. Thus, macrophages derived from CBMC were cultured with or without Toll-like receptor 4 ligand (TLR4L) for a further 48 h. The extracellular fluid was collected from one set of MDM cultures, and the remaining macrophages were treated with 0·1 % saponin in RPMI-1640 medium to release and collect the intracellular fluid (ICF). After removal of the ICF, RNAlater (Qiagen GmbH) was added to the MDM for further extraction of LL-37 transcripts. The LL-37 peptide was measured in the ICF, extracellular fluid and cord plasma. The second set of MDM cultures was used for the assay of MDM-mediated killing capacity. In all assays, LPS (from Escherichia coli serotype 0111:B4, 5 μg/ml; Sigma) was used as TLR4L.
Assessment of vitamin D status
Details of serum 25(OH)D measurements have been described elsewhere( Reference Roth, Al Mahmud and Raqib 19 ). To assess vitamin D status, serum 25(OH)D was measured by HPLC–tandem MS; only 25(OH)D3 was detected in serum, not 25(OH)D2. As no standard classification of 25(OH)D concentrations in cord blood exists, for the purpose of the present study, we followed the classification of vitamin D sufficiency or deficiency based on previous publications( Reference Greer 20 – Reference Walker, Zhang and Rastegar 23 ). All participants were grouped into four categories of 25(OH)D concentrations: (1) ≥ 76 nmol/l (high); (2) 50–75 nmol/l (moderate); (3) 30–49 nmol/l (low); (4) < 30 nmol/l (very low).
Measurement of LL-37 peptide concentration
LL-37 peptide concentration was measured by ELISA (Hycult Biotechnology), according to the manufacturer's recommendations. The lower limit of detection of the kit was 0·14 ng/ml, and the intra- and inter-assay CV were 4·96 and 7·89 %, respectively. The concentration of LL-37 peptide measured in the supernatant was then normalised to per million macrophages.
Quantitative real-time RT-PCR amplification of LL-37 mRNA
RNA was extracted from macrophages using the RNeasy Mini Kit (Qiagen GmbH), according to the manufacturer's instructions, and corresponding complementary DNA was synthesised using the SuperScript III First-Strand Synthesis System (Invitrogen). The CAMP gene encoding LL-37 transcripts relative to the housekeeping 18S rRNA was measured in triplicate from the complementary DNA samples by quantitative real-time RT-PCR using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.) and the 18S rRNA-housekeeping gene kit (Applied Biosystems). The sequences of forward and reverse primers for LL-37 transcripts were 5′-TCACCAGAGGATTGTGACTTCAA-3′ and 5′-TGAGGGTCACTGTCCCCATAC-3′, respectively (Primer Express; Applied Biosystems). Results were analysed using the relative standard method( Reference Bergman, Johansson and Asp 9 ).
Assay of monocyte-derived macrophage-mediated killing capacity
The capacity of MDM to kill ingested bacteria was determined by quantifying the number of viable E. coli K-12 colonies (avirulent) in agar plates after a period of phagocytosis and removal of extracellular bacteria. MDM were infected with E. coli K-12 at a multiplicity of infection of 100 (100 bacteria per one macrophage) and incubated for 2 h. Extracellular bacteria were removed by washing, and infected macrophages were cultured in media with 10 % autologous plasma overnight. The infected macrophages were lysed with 0·3 % saponin to release intracellular bacteria. Cell lysates were cultured on MacConkey agar plates (Becton Dickinson) overnight and viability of the bacteria was determined by counting the colony-forming units (CFU). A ‘relative CFU count’ was calculated for each participant as the ratio of CFU in LPS-stimulated to unstimulated cells, to account for inter-subject variations in the baseline (unstimulated) killing activity of macrophages.
Statistical analyses
Primary outcomes were fold difference in intracellular LL-37 peptide concentration (indicator of the effect of prenatal vitD3 supplementation on LPS-induced LL-37 synthesis in cord blood cells) and the relative CFU count (an indicator of the effect of vitD3 supplementation on LPS-induced neonatal macrophage killing activity). Secondary outcomes were unstimulated and LPS-stimulated LL-37 concentrations in the intracellular and extracellular fluid, LL-37 mRNA transcript copy numbers in MDM, and LL-37 concentration in plasma.
The distributions of LL-37 and bacterial CFU counts are expressed as ranges, means and standard deviations and 95 % CI, or medians and interquartile ranges for non-normally distributed variables. The fold difference in the intracellular concentration of LL-37 peptide was calculated for each participant as the ratio of LL-37 concentration in LPS-stimulated to unstimulated cells. Unstimulated and LPS-stimulated intracellular LL-37 peptide concentrations and fold difference in intracellular LL-37 peptide concentration did not follow normal distributions even after transformation; therefore, they were analysed by non-parametric statistics (Mann–Whitney U test). Bootstrap linear regression was used to verify inferences from non-parametric tests. Factors that have been previously reported in the literature to be associated with cord blood LL-37 concentration including cord serum 25(OH)D concentration, gestational age (categorised by preterm ≤ 37 weeks of gestation or term >37 weeks of gestation), infant sex and delivery mode were assessed for associations with LL-37 concentration using the bootstrap procedure for unadjusted bivariate and adjusted multivariate regression analyses. Cord plasma LL-37 was log-transformed as data were not normally distributed, and between-group comparisons were analysed by Student's t test. For the aforementioned LL-37 outcomes, sensitivity analyses that excluded LL-37 observations below the minimum detectable limit of the ELISA assay ( < 0·14 ng/ml) were also conducted. Participants were categorised into four groups based on 25(OH)D concentrations, as described previously; comparisons between groups with respect to the LL-37 peptide and transcript levels were evaluated by one-way ANOVA.
To calculate the relative CFU count, one CFU was added to all values due to multiple observations with bacterial colony counts of zero. Relative CFU counts did not follow a normal distribution and were therefore log-transformed before the analysis. The number of bacterial colonies that remained after treatment with or without LPS was considered as count data; however, because the Poisson distribution was a poor fit for the over-dispersed count data, comparisons were based on negative binomial regression models, using generalised estimating equations to account for the correlation between colony counts of LPS-stimulated and unstimulated cells within the subjects. The primary parameter of interest in the negative binomial generalised estimating equation model was the interaction between vitamin D supplementation and LPS stimulation on CBMC-derived macrophage killing activity. Statistical analyses were performed using Stata/IC 12.1 for Mac (StataCorp), and P≤ 0·05 was considered significant.
Results
Of the 160 women enrolled in the AViDD trial, there were 129 mother–infant pairs (81 %), sixty-four from the placebo group and sixty-five from the VitD group, with adequate volume of cord blood for immune function assays. The mean age (22·4 (sd 3·5) v. 22·4 (sd 3·5) years) and gestational age (27·73 (sd 1·1) v. 28·0 (sd 1·1) weeks) of the pregnant women in the VitD group were similar to those in the placebo group.
Vitamin D and LL-37 levels in cord blood
25(OH)D concentration ranged from 20 to 192 nmol/l in the study cohort, which was similar to the entire cohort as reported previously( Reference Roth, Al Mahmud and Raqib 19 ). Maternal 25(OH)D concentration in the VitD group (mean 101·52 (sd 30·67) nmol/l) was significantly increased at delivery compared with the placebo group (mean 40·30 (sd 17·83) nmol/l) (P< 0·001) (Table S1, available online). The concentration of LL-37 in cord plasma ranged from 16·6 to 101·7 ng/ml. There was no significant difference in plasma LL-37 concentrations between the two groups (Table 1). Cord serum 25(OH)D was not significantly associated with LL-37 concentration in cord plasma (Table 2).
Table 1 Antimicrobial peptide LL-37 concentration in umbilical cord plasma and cord blood mononuclear cell culture, overall and by treatment group (Medians and interquartile ranges (IQR); geometric means and 95 % confidence intervals)
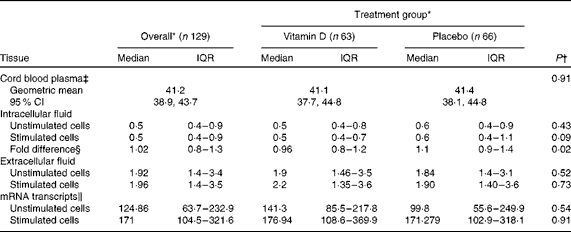
* LL-37 peptide concentrations are expressed as ng/ml and mRNA as copy numbers.
† P values were based on the non-parametric Mann–Whitney U test, unless otherwise indicated.
‡ Vitamin D group n 64 and placebo group n 65; P value was based on the t test.
§ Fold difference was calculated as the ratio of LL-37 concentration in stimulated to unstimulated cells.
∥ Vitamin D group n 42 and placebo group n 37.
Table 2 Unadjusted and adjusted multivariate associations between LL-37 concentration (in cord plasma and intracellular fluid) and cord serum 25-hydroxyvitamin D (25(OH)D), gestational age, infant sex and delivery mode (β-Coefficients and 95 % confidence intervals)

* Cord serum 25(OH) was scaled by 10 nmol/l.
† Gestational age was categorised as preterm ( ≤ 37 weeks of gestation) or term (>37 weeks of gestation).
‡ Infant sex is female v. male.
§ Fold difference was calculated as the ratio of stimulated:unstimulated cells.
Vitamin D3 supplementation suppressed intracellular LL-37 peptide expression in Toll-like receptor 4 ligand-stimulated macrophages
Concentrations of LL-37 ranged from 0·05 to 4·93 ng/ml in the ICF of unstimulated macrophages. No significant differences were observed between the VitD and placebo groups (Table 1). The bootstrap linear regression analysis did not show an effect of vitD3 supplementation on unstimulated intracellular LL-37 concentration (mean between-group difference 0·12 ng/ml, 95 % CI − 0·16, 0·39; P= 0·41).
In TLR4L-stimulated macrophages, LL-37 concentration in the ICF ranged from 0·07 to 7·72 ng/ml. The distribution of TLR4L-induced intracellular LL-37 concentrations was slightly lower in the VitD group than in the placebo group, although the difference did not reach statistical significance as determined by the non-parametric Mann–Whitney U test (P= 0·09; Table 1). Similarly, the results of the bootstrap linear regression analysis revealed that the mean decrease in TLR4L-induced intracellular LL-37 concentrations in the VitD v. placebo group did not reach statistical significance (mean 0·31 ng/ml, bootstrap 95 % CI − 0·64, 0·023; P= 0·068).
Overall, individual fold differences in intracellular LL-37 concentration ranged from 0·12 to 3·64. Fold differences in the VitD group were lower, on average, than those in the placebo group, and the difference between the distributions was significant as determined by the non-parametric Mann–Whitney U test (Table 1). However, when group means were compared using bootstrap regression, the effect of vitD3 supplementation on the fold difference in intracellular LL-37 concentration did not reach statistical significance (P= 0·15). Moreover, there was no significant interaction between vitD3 supplementation and TLR4L stimulation in terms of their effects on mean intracellular LL-37 concentration (data not shown).
Cord serum 25(OH)D was not significantly associated with LL-37 concentration in the unstimulated ICF, or fold difference in intracellular LL-37 concentration (Table 2). However, 25(OH)D in cord serum was inversely related to TLR4L-induced intracellular LL-37 in both unadjusted bivariate and adjusted multivariate analyses (Table 2). For every 10 nmol/l increase in cord serum 25(OH)D concentration, TLR4L-induced intracellular LL-37 concentration decreased by 0·05 ng/ml (P= 0·03). No significant associations were observed between LL-37 concentration and preterm birth, delivery mode or infant sex (Table 2). Excluding low LL-37 values in the sensitivity analysis did not change the inferences from any of the above-mentioned analyses (data not shown). Upon stratifying the participants into four groups based on serum 25(OH)D concentrations, LL-37 peptide concentrations in the ICF of TLR4L-stimulated MDM were significantly reduced in the high ( ≥ 76 nmol/l), moderate (50–75 nmol/l) and low (30–49 nmol/l) groups (P= 0·005, P= 0·035 and P= 0·029, respectively) compared with the very low ( < 30 nmol/l) group (Fig. 1(a)).
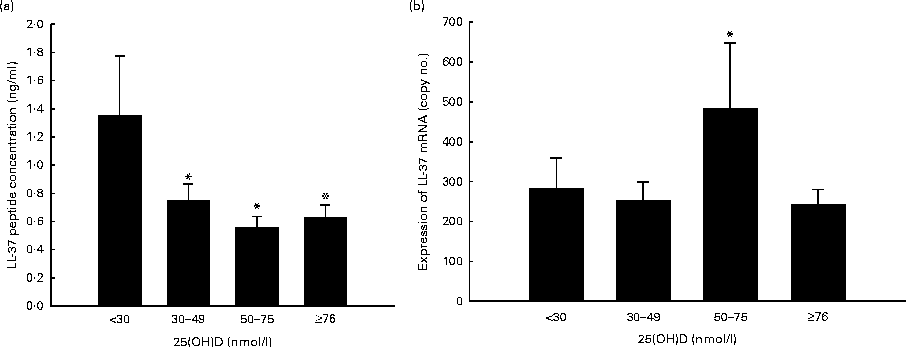
Fig. 1 Participants were stratified into four groups based on serum 25-hydroxyvitamin D (25(OH)D) concentrations: (1) ≥ 76 nmol/l (high, n 57); (2) 50–75 nmol/l (moderate, n 10); (3) 30–49 nmol/l (low, n 37); (4) < 30 nmol/l (very low, n 25). Values are means, with their standard errors represented by vertical bars. (a) * Mean value was significantly different from that of the very low group (P< 0·05). (b) * Mean value was significantly different from that of the high group (P< 0·05).
Effect of prenatal vitamin D3 supplementation on LL-37 mRNA transcription
There was no significant effect of vitamin D supplementation on LL-37 transcript copy number in unstimulated or TLR4L-simulated CBMC (Table 1), nor was there an interaction between vitamin D supplementation and TLR4L stimulation (data not shown). TLR4L stimulation increased LL-37 transcript levels in both groups (Table 1), but no significant differences were observed between the groups.
Among the four groups stratified based on the categories of vitamin D status, we found that TLR4L-induced LL-37 transcript copy number in MDM was higher in the moderate group compared with the low (P= 0·07) and high (P= 0·047) groups (Fig. 1(b)).
Effect of prenatal vitamin D3 supplementation on bacterial killing capacity of activated macrophages
The macrophage killing assay was performed on 112 cord blood cell cultures (fifty-six from the placebo group and fifty-six from the VitD group) since adequate cells were not available from the other seventeen participants. Overall, the range of viable bacterial counts after the MDM-mediated E. coli killing was 0 to 2·50 × 104 CFU for unstimulated cells and 0 to 2·25 × 104 CFU for TLR4L-stimulated cells, and the relative CFU counts ranged from 0 to 1·25 × 103. The regression analysis did not show any significant effect of vitD3 supplementation on the relative CFU count, or on the relative CFU counts in unstimulated and TLR4L-stimulated macrophages (Table 3). However, TLR4L stimulation augmented bacterial killing in both groups. Stratifying the participants based on serum 25(OH)D concentrations did not show any difference among the four groups (data not shown).
Table 3 Effect of prenatal vitamin D supplementation v. placebo on the number of viable Escherichia coli colony-forming units (CFU) after culture with cord blood macrophages, with and without Toll-like receptor 4 ligand stimulation (Medians and interquartile ranges (IQR); ratios of CFU counts and 95 % confidence intervals)
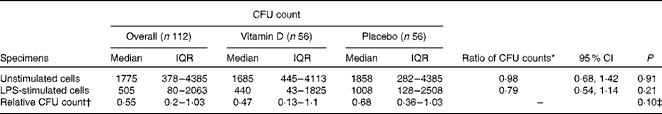
LPS, lipopolysaccharide.
* Based on the negative binomial regression model for comparison between the vitamin D and placebo groups.
† Relative CFU count for each participant was calculated as the ratio of CFU in LPS-stimulated to unstimulated cells.
‡ Based on the t test for comparison between the vitamin D and placebo groups, using log-transformed data.
Discussion
Despite the potent effect of vitD3 supplementation (0·875 mg/week) during the third trimester of pregnancy on maternal–infant vitamin D status( Reference Roth, Al Mahmud and Raqib 19 ), the present study showed that prenatal vitD3 supplementation did not yield significant benefits in terms of indicators of innate immune function. In fact, we observed that vitD3 supplementation may have slightly suppressed intracellular LL-37 peptide synthesis in activated cord blood macrophages, suggesting a potential immunosuppressive effect. Furthermore, we did not find any significant influence of vitD3 supplementation on the bactericidal capacity of macrophages with or without TLR4L activation.
In vitro studies using primary monocytes/macrophages cultured in 25(OH)D-sufficient sera or culture media containing the active form of vitamin D (1,25(OH)2D3) have shown that vitamin D mediates the induction of cathelicidin (LL-37) and β-defensin 4 transcripts in macrophages( Reference Liu, Stenger and Li 3 , Reference Fabri and Modlin 24 , Reference Liu, Stenger and Tang 25 ). In vitro addition of 1,25(OH)2D3 or 25(OH)D3 in cord blood cultures also showed increased TLR-independent or -dependent cathelicidin mRNA expression( Reference Walker, Zhang and Rastegar 23 ). In the present study, in vivo vitD3 supplementation did not affect LL-37 mRNA levels in neonatal macrophages with or without activation. The main difference between the other reports and the present study is that here vitD3 was given orally to pregnant women and autologous plasma was used instead of adding 1,25(OH)2D3 or 25(OH)D3 exogenously in the macrophage culture. However, it is noteworthy that TLR4L activation was associated with increased LL-37 transcript levels in macrophages cultured in the presence of serum 25(OH)D of the moderate group (50–75 nmol/l) compared with those with poorer vitamin D status (30–49 or < 30 nmol/l). This finding was substantiated by Walker et al. ( Reference Walker, Zhang and Rastegar 23 ) who reported that TLR4L-stimulated cord blood monocytes showed higher LL-37 mRNA in the presence of 25(OH)D >50 nmol/l compared with 25(OH)D < 30 nmol/l. They further showed that 25(OH)D concentration < 50 nmol/l was linked with diminished TLR4L-mediated induction of CYP27B1 enzyme, the key enzyme responsible for the conversion of 25(OH)D to the active form in macrophages, and diminished TLR4L-mediated induction of cathelicidin. However, they also showed reduced cathelicidin mRNA levels in the presence of 25(OH)D >75 nmol/l compared with 25(OH)D >50 nmol/l( Reference Walker, Zhang and Rastegar 23 ). Similarly, in the present study, LL-37 transcript levels declined in the high ( ≥ 76 nmol/l) group compared with the moderate (50–75 nmol/l) group. These findings suggest that 25(OH)D concentration >75 nmol/l suppresses the induction of LL-37 transcript levels.
Although most in vitro studies( Reference Liu, Stenger and Li 3 , Reference Walker, Zhang and Rastegar 23 , Reference Liu, Stenger and Tang 25 – Reference Sato, Imafuku and Ishii 29 ) have shown an increase in cathelicidin mRNA expression after exposure to vitD3, none of these studies measured the cathelicidin/LL-37 peptide concentration in the intracellular or extracellular compartment of macrophages. Liu et al. ( Reference Liu, Stenger and Li 3 ) demonstrated the presence of cathelicidin peptide in macrophages by flow cytometry, immunofluorescence staining and the surface-enhanced laser desorption ionisation time-of-flight method, but LL-37 peptide concentration in macrophages was not measured. Only one study in Canada reported the in vivo effects of vitD3 supplementation( Reference Larcombe, Mookherjee and Slater 30 ) that corroborated our findings, showing that 8 months of supplementation with vitD3 significantly decreased serum LL-37 levels compared with pre-supplementation levels in healthy participants. They did not find any significant difference in TLR2/1L-induced LL-37 peptide concentration (assessed by Western blot and densitometry) in MDM between pre- and post-vitD3 supplementation. The decrease in peptide concentration in the ICF of macrophages following TLR4L activation in the VitD group may have been due to a post-transcriptional regulatory block. It is important to acknowledge that this effect was quantitatively small, not consistent across multiple statistical approaches, and not mirrored by a decline in LL-37 mRNA transcript levels. Furthermore, evaluation of the extracellular fluid did not show any increased release of LL-37 from the intracellular compartment of macrophages, which might have been expected in the context of an important increase in intracellular LL-37 peptide synthesis. In our recent report on the oral supplementation of healthy adult volunteers with 0·125 mg vitD3 for 4 d, no significant changes in the concentration of either LL-37 peptide or mRNA were found in macrophages or lymphocytes( Reference Mily, Rekha and Kamal 31 ). The present results thus collectively suggest that in vitro findings related to the effect of vitamin D metabolites on LL-37 expression may not be consistent and may not predict the in vivo effects of vitD3 supplementation.
We did not find any significant effect of oral vitD3 supplementation on the killing activity of macrophages, as determined by the number of viable bacterial colonies remaining after the killing assay in TLR4L-stimulated and unstimulated macrophages. In both groups, TLR4L activation enhanced bacterial killing compared with unstimulated macrophages by reducing the number of viable bacterial colonies by approximately 40 %, which is consistent with previous evidence that the antimicrobial activity of macrophages is dependent upon activation( Reference Liu, Stenger and Li 3 ). Notably, the antibacterial killing capacity of macrophages remained unaffected by 25(OH)D in the context of TLR4L activation, even though intracellular LL-37 peptide expression was slightly reduced by 25(OH)D. In the above-mentioned previous study involving healthy adult volunteers, we found that vitD3 supplementation significantly increased the antibacterial killing activity of macrophages in the absence of any increase in the levels of LL-37 peptide or mRNA expression( Reference Mily, Rekha and Kamal 31 ). However, there are some key differences between our two studies. In our previous study( Reference Mily, Rekha and Kamal 31 ), vitD3 supplementation was administered for only 4 d (even though the daily dose was the same), while in the present study, supplementation was given for about 3 months( Reference Roth, Al Mahmud and Raqib 19 ). The previous study involved healthy adults, while the participants of the present study were healthy pregnant women, and immune function was studied in cord blood, not in maternal specimens. Macrophages have many arsenals; thus, it is possible that other vitamin D-dependent, but cathelicidin-independent, immune responses come into play, contributing to the innate killing by macrophages( Reference Lee, Yang and Shin 32 ). It is likely that vitamin D plays a complex dual role in immunity; on the one hand, it may contribute to direct antimicrobial activities (e.g. by enhancing increased macrophage-mediated antimicrobial activity), on the other hand, it modulates immune cell functions that tend to reduce local inflammation (e.g. by controlling the release of antimicrobial peptides).
One area of particular interest regarding the effect of vitamin D on the immune response is in the treatment of tuberculosis infection, for which several randomised clinical trials of vitamin D supplementation have been reported( Reference Martineau, Timms and Bothamley 33 – Reference Wejse, Gomes and Rabna 36 ) or are ongoing( Reference Wang, Ma and Bygbjerg 37 ) (NCT01130311, NCT00366470, NCT00507000, NCT00677339, NCT00918086 and NCT01580007). The reports published so far have conflicting results. An Indonesian study showed favourable outcome in tuberculosis patients with earlier sputum conversion( Reference Nursyam, Amin and Rumende 34 ). However, studies by Wejse et al. ( Reference Wejse, Gomes and Rabna 36 ) and Martineau et al. ( Reference Martineau, Timms and Bothamley 33 ) did not find any overall effect of vitD3 supplementation on disease outcome; however, the dose and duration of vitD3 supplementation as well as genetic polymorphisms may modify treatment responses( Reference Martineau, Timms and Bothamley 33 ).
There are several studies reporting the effects of vitamin D supplementation on infectious diseases, particularly in lung diseases in children( Reference Charan, Goyal and Saxena 38 – Reference Walker and Modlin 40 ). However, there is little conclusive evidence from controlled trials about the effect of vitamin D supplementation on the risk of infectious diseases in early infancy.
In conclusion, our findings showed that prenatal vitD3 supplementation with a weekly dose of 0·875 mg in the last trimester may have slightly reduced intracellular LL-37 peptide concentration, but did not adversely affect the antibacterial activity of macrophages in neonates. We did not find mechanistic evidence of a promising beneficial effect of prenatal vitD3 supplementation on newborn innate immunity. However, vitamin D has complex immune-regulatory roles, indicating the need for further short- and long-term studies to evaluate the effects of prenatal vitamin D exposures on infant and child immune responses.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114514001512
Acknowledgements
The authors express their gratitude to the pregnant women who participated in the present study and to the staff of Shimantik (non-governmental organisation) for their efforts in the implementation of the AViDD trial. The present study was supported by the Thrasher Research Fund (Salt Lake City, UT), the Swedish Agency for Research Cooperation with Developing Countries (Sida/SAREC Agreement support) and the ICDDR,B. The ICDDR,B acknowledges with gratitude the commitment of all donors to its research efforts.
The contributions of the authors are as follows: R. R. and D. E. R. conceived and designed the study plan; R. R., D. E. R. and A. H. B. obtained funding; A. A.-M. supervised the field activity and collected the data; E. A., A. M. and R. S. R. performed the laboratory experiments: E. A., A. L., N. P. and R. S. R. performed the statistical analysis; R. R. and D. E. R. drafted the manuscript. All the authors revised and approved the final manuscript.
There is no conflict of interest.






