Introduction
Reconciling the conservation and human livelihoods aspects of hunting wildlife is crucial (Milner-Gulland et al., Reference Milner-Gulland and Bennett2003; Barrett et al., Reference Barrett, Travis and Dasgupta2011). Hunting provides substantial nutritional and economic benefits to rural people, yet when practised at unsustainable levels it threatens wildlife and the integrity of local ecosystems (Barrett et al., Reference Barrett, Travis and Dasgupta2011; Brashares et al., Reference Brashares, Golden, Weinbaum, Barrett and Okello2011; Golden et al., Reference Golden, Fernald, Brashares, Rasolofoniaina and Kremen2011, Reference Golden, Wrangham and Brashares2013; Myers et al., Reference Myers, Gaffikin, Golden, Ostfeld, Redford and Ricketts2013). Given the scale of hunting globally (Milner-Gulland et al., Reference Milner-Gulland and Bennett2003; Fa et al., Reference Fa, Seymour, Dupain, Amin, Albrechtsen and Macdonald2006), implementing effective regulations that balance the needs of people and wildlife is difficult.
Understanding seasonal patterns in behaviour can increase the efficacy of conservation action by providing information on the timing of and the incentives that drive within-year variation in behaviour (Packer et al., Reference Packer, Ikanda, Kissui and Kushnir2005; Irvine et al., Reference Irvine, Mate, Winsor, Palacios, Bograd, Costa and Bailey2014). To change unsustainable hunting practices and improve the welfare of rural people, conservation strategies must address specifically when and why people hunt threatened species (Milner-Gulland, Reference Milner-Gulland2012). The resources and funds available to implement conservation strategies are limited (Wilson et al., Reference Wilson, McBride, Bode and Possingham2006), and focusing effort on the most critical periods of the year would make the best use of the time and money available. Thus it makes sense to develop conservation strategies that are sensitive to seasonal patterns or cycles. However, seasonal hunting patterns are still poorly understood.
Madagascar is a biodiversity hotspot (Myers et al., Reference Myers, Mittermeier, Mittermeier, da Fonseca and Kent2000). More than 90% of Madagascar's endemic lemurs and 50% of endemic euplerid carnivorans are threatened with extinction (Schwitzer et al., Reference Schwitzer, Mittermeier, Davies, Johnson, Ratsimbazafy and Razafindramanana2013; IUCN, 2014), yet these and other wildlife provide direct nutritional and economic benefits to the people of Madagascar (Golden et al., Reference Golden, Fernald, Brashares, Rasolofoniaina and Kremen2011, Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014). Madagascar's protected areas are home to many mammals; outside protected areas four categories of protection are defined by law, from no restrictions to complete prohibition on hunting (Decree No. 2006-400). However, public awareness of the law is not guaranteed (Keane et al., Reference Keane, Ramarolahy, Jones and Milner-Gulland2011), and knowledge of the law does not ensure compliance. Local people often resent legal intervention, which they perceive as forcing them into poverty and depriving them of a secure food supply (Keller, Reference Keller2008; Sodikoff, Reference Sodikoff2009), and despite the existence of regulations, hunting of threatened species for food is still widespread (Garcia & Goodman, Reference Garcia and Goodman2003; Goodman, Reference Goodman2003; Jenkins & Racey, Reference Jenkins and Racey2008; Barrett & Ratsimbazafy, Reference Barrett and Ratsimbazafy2009; Golden, Reference Golden2009; Randrianandrianina et al., Reference Randrianandrianina, Racey and Jenkins2010; Golden et al., Reference Golden, Fernald, Brashares, Rasolofoniaina and Kremen2011, Reference Golden, Wrangham and Brashares2013, Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014; Jenkins et al., Reference Jenkins, Keane, Rakotoarivelo, Rakotomboavonjy, Randrianandrianina and Razafimanahaka2011; Razafimanahaka et al., Reference Razafimanahaka, Jenkins, Andriafidison, Randrianandrianina, Rakotomboavonjy, Keane and Jones2012; Gardner & Davies, Reference Gardner and Davies2014).
Variation in the characteristics of local communities may affect within-year hunting patterns as a result of seasonal changes in economics (Golden et al., Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014), nutritional availability and value (Goodman, Reference Goodman2006; Golden et al., Reference Golden, Fernald, Brashares, Rasolofoniaina and Kremen2011; Gardner & Davies, Reference Gardner and Davies2014), cultural norms (Jones et al., Reference Jones, Andriamarovololona and Hockley2008), travel, and laws that define legal hunting seasons (Decree No. 2006-400). Furthermore, variation in the characteristics of wildlife may affect within-year hunting patterns as a result of seasonal changes in feeding and ranging behaviour (Andrianjakarivelo, Reference Andrianjakarivelo2000; Randrianandrianina et al., Reference Randrianandrianina, Racey and Jenkins2010), body fat content (Gardner & Davies, Reference Gardner and Davies2014), and flavour (which may be affected by diet). Hunting may also have a greater effect on prey species if it coincides with seasons of mating, pregnancy and lactation in prey species (Jenkins & Racey, Reference Jenkins and Racey2008). Alternatively, hunting may have a greater effect on human health and welfare if it coincides with the season of greatest food and economic insecurity (Cripps, Reference Cripps2009; Harris, Reference Harris2011; Golden et al., Reference Golden, Fernald, Brashares, Rasolofoniaina and Kremen2011, Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014; Tsujimoto et al., Reference Tsujimoto, Homma, Matsuyama and Shiraiwa2012).
The biodiversity-rich Masoala Peninsula is a conservation priority in Madagascar (Kremen et al., Reference Kremen, Raymond and Lance1998, Reference Kremen, Razafimahatratra, Guillery, Rakotomalala, Weiss and Ratsisompatrarivo1999). In a focal village on the Peninsula I established the number and biomass of forest mammals hunted, the seasonal variation in the hunting of forest mammals and the consumption of domestic and wild-caught meat, and the reasons for seasonal variation in hunting. Using these data I investigated (1) what drives seasonal hunting patterns, and (2) how seasonal variation in hunting and meat consumption affects native species and people.
Study area
The Masoala National Park, a UNESCO World Heritage Site on the Masoala Peninsula, in north-east Madagascar (Fig. 1), is a wet, mountainous area far from roads, and contains some of the last remaining lowland coastal rainforest in Madagascar (Kremen et al., Reference Kremen, Raymond and Lance1998). There are two seasons on the Masoala Peninsula: the wet, cool austral winter (March–August) and the hot, dry austral summer (September–February). The Masoala National Park is home to 10 species of lemurs, six euplerids, one introduced viverrid, at least four tenrecs, at least three bat species, the introduced bushpig Potamochoerus larvatus, and numerous native and introduced rodents (Table 1; Sterling & Rakotoarison, Reference Sterling and Rakotoarison1998; Garbutt, Reference Garbutt2007; Farris et al., Reference Farris, Kelly, Karpanty, Ratelolahy, Andrianjakarivelo and Holmes2012; Goodman, Reference Goodman2012). Forty-eight percent of these species are threatened with extinction (Schwitzer et al., Reference Schwitzer, Mittermeier, Davies, Johnson, Ratsimbazafy and Razafindramanana2013; IUCN, 2014).

Fig. 1 The location of the Masoala Peninsula and Masoala National Park in Madagascar.
Table 1 Forest mammals on the Masoala Peninsula of Madagascar (Fig. 1), with their IUCN Red List status, national protected status, regulated hunting season, hunting season in practice, number of forest mammals caught by focal hunter and in focal village, method of hunting used, % of hunters who report the species is hunted seasonally, and incentives for seasonal hunting in a village, based on data collected from household interviews, 24-hour recall surveys, and shadowing a focal hunter during 2011–2012.

1 IUCN (2014), Schwitzer et al. (Reference Schwitzer, Mittermeier, Davies, Johnson, Ratsimbazafy and Razafindramanana2013); EN, Endangered; CR, Critically Endangered; VU, Vulnerable; LC, Least Concern; NT, Near Threatened
2 Designated by Malagasy law (Decree No. 2006-400)
3 The season each species is hunted by local people (data from household interviews and by shadowing a focal hunter for 1 year)
4 The percentage of active hunters who mentioned this as an incentive for hunting the animal seasonally
5 Change in ranging behaviour increases the predictability of the species’ location at key seasonal resources
6 Change in ranging behaviour increases the species' overlap with humans, crops or domesticated animals
There are 250 permanent villages around Masoala National Park, with a total population of > 85,000 (Holmes, Reference Holmes2007). Local people are predominantly Betsimisaraka; 80% make their living as agriculturalists, primarily for subsistence. The staple crop is rice; lowland irrigated rice fields are harvested during the austral summer (in December) and hillside rain-fed rice fields are harvested during the austral winter (in May). Rice production is supplemented by multiple species of tubers, primarily harvested in June, July and November (AGEVAREN, 2009). In addition to agricultural activities, the local people fish, raise domesticated livestock (cows, pigs, chickens, geese and ducks), and supplement their diet with forest animals (Golden et al., Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014). This study was conducted during 12 consecutive months in a focal village (population 118) on the coast, < 2 km from Masoala National Park. There is no formal market within the village.
Methods
I used both direct (focal hunter shadowing) and indirect (household interviews and 24-hour recall surveys) methods to provide a holistic understanding of seasonal hunting and meat consumption. This approach allowed me to study the behaviour of hunters and consumers at the individual, household and village levels. By comparing the results of multiple methods I can evaluate their strengths and weaknesses and control for the inevitable biases in how individuals recall and report behaviour.
Focal hunter shadowing
Firstly, I identified all known trappers of bushpigs in the village. Fourteen people trapped forest mammals but only 5 trapped bushpigs. Bushpigs are considered a pest and can be hunted legally outside the Park (Decree No. 2006-400), and hunters discuss their trapping freely in the presence of other community members. I then selected at random one bushpig trapper as the focal hunter. I discussed the aims, objectives and possible implications of the project with the hunter, and he consented to being shadowed. I followed the hunter for 2 weeks per month over 12 months (July 2011–June 2012) and the hunter reported what he caught at the end of every day he was not followed. During each follow I collected data on the number of animals captured (recoverable and unrecoverable), and estimated the number of escaped individuals (evident from missing limbs, fur, feathers and damage left behind at snare traps). For each animal caught I recorded species, age class and sex. When possible I also weighed and measured carnivorans, insectivores and lemurs, using a spring scale and measuring tape. Bushpigs were too large to weigh, and therefore I weighed cut portions of bushpig meat and used these to estimate total weight. I also interviewed the hunter during all trapping activities about his rationale for seasonal trapping strategies.
Household interviews
During July 2011–June 2012, spaced over the entire year, I conducted 37 semi-structured interviews in the study village, interviewing at least one member of every household. I defined a household as a group of people who share the use of a kitchen. I speak the local dialect of Betsimisaraka, and conducted the interviews without a Malagasy assistant. I asked members of each household if they trapped, caught, and/or ate any of 27 forest mammals (Table 1) in the previous year. For each species eaten I also asked how it was captured, why it was captured (e.g. for food or because of human–wildlife conflict), if the species was available or eaten seasonally, the season it was available, the season it was eaten, and any reasons for variation over time in any of these factors. Interviewees rarely referred to seasons by months but rather referred to seasonal indicators, including crops, fruiting and flowering trees, thunder and the names of moons (for each period between new moons, the moon has a different name).
24-hour recall surveys
A local assistant native to the study village conducted 24-hour recall surveys of diet during 2 weeks per month over 11 months (August 2011–June 2012). The assistant will remain anonymous to protect the identity of the focal hunter and the village. We asked one member of each household about the household's activities, income, expenditure, collection of forest and marine products, and the food and drink consumed (type, amount, source and cost) for meals and snacks in the previous 24 hours. Households were not identified by name or number, to preserve anonymity. One household was excluded from data collection during the second half of the study because of interpersonal conflict (unrelated to this research) with the field assistant collecting the data.
Data analysis
Using the household interview and focal hunter data I calculated the number of individuals and the biomass of each forest mammal species consumed in the village and caught by the hunter each month. I estimated the total biomass based on data collected when following the hunter, and on weights in Goodman (Reference Goodman2012) and Garbutt (Reference Garbutt2007). From these data I calculated the number and biomass of forest mammals that were hunted and eaten each month, and divided these totals into the number and biomass of euplerids, viverrids, Megachiroptera, Microchiroptera, tenrecs, bushpigs and lemurs eaten each month. To determine which species are hunted in each season, I used a hierarchical cluster analysis with Ward's metric. I determined the seasonal variation in the consumption of all animals, using data from the 24-hour recall surveys, and excluded data from the first month, as a sensitization period. From the remaining 10 months of data I calculated the mean number of meals each month that included any animal-based food, and then subdivided this into meals containing marine or freshwater animals (hereafter summarized as fish, although turtles and invertebrates were also eaten), domestic animals and forest animals (including both mammal and avian species). To examine the potential reasons for seasonal hunting I used linear and multiple regression to test if the consumption of domestic animals or fish (calculated from 24-hour recall surveys) could predict either the consumption of forest mammals (calculated from household interview data) or hunter effort (focal hunter shadowing). I also used bivariate linear regressions to test if hunter effort was correlated with the total, recoverable or lost biomass. I analysed seasonal hunting behaviour (from interviews and focal hunter shadowing) in the context of laws that designate hunting seasons and the protected status of wildlife species (Decree No. 2006-400). Using interview data I calculated the percentage of active hunters that cited seasonal variation in human factors (financial or food insecurity, cultural norms, travel, laws) and prey characteristics (ranging behaviour and body fat content) as an incentive for seasonal hunting of each species. To examine the impact of hunting patterns on wildlife and people I compared seasonal hunting patterns (based on interviews and focal hunter follows) with seasonal periods of mating and offspring dependency in each forest mammal species, and with seasonal periods of food insecurity for crop (AGEVAREN, 2009) and animal-based foods in the village (calculated from 24-hour recall surveys).
Results
The extent of hunting and consumption of forest mammals
The focal hunter and other hunters in the village used snare traps to catch forest mammals or caught them opportunistically using sticks, machetes, dogs or (less frequently) slingshots. The focal hunter built snare traps that targeted one or two species, with a broad range of bycatch within (and occasionally outside) the same taxon. Bycatch was incidental (non-targeted animals that were of equal or lower value) but not accidental (non-targeted animals of no value). The focal hunter and other hunters in the village caught forest mammals for subsistence or in response to human–wildlife conflict (e.g. as a result of predation on poultry by Cryptoprocta ferox or crop-raiding of farmed tubers by P. larvatus). Animals that were hunted because of human–wildlife conflict were also consumed.
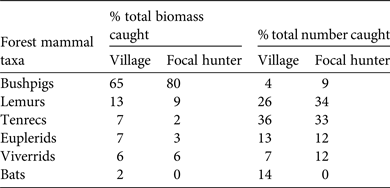
Inhabitants of the study village consumed 1,148.73 kg (before processing) of forest mammal biomass from 292 individuals over 1 year (Table 1). The focal hunter caught 58 of these animals (390.54 kg; Table 1). The two most consumed taxa in terms of numbers of individuals were tenrecs (36%) and lemurs (26%), whereas the two most consumed taxa in terms of biomass were bushpigs (65%) and lemurs (13%; Table 2).
Table 2 Forest mammal taxa hunted on the Masoala Peninsula in Madagascar (Fig. 1), with their percentage of the total biomass and total number of forest mammals caught by the study village and by the focal hunter during 2011–2012, based on data collected from household interviews and by shadowing the focal hunter.
Seasonal variation in hunting and consumption of animal-based foods
Village inhabitants reported eating a mean of 1.29 ± SD 0.19 meals containing meat (wild or domestic) per day. More animal-based meals were consumed during the austral summer (Fig. 2), with 89% of meals from fish, 9% from domestic animals and 2% from forest animals. The number of meals containing fish and domestic animals was greatest during the summer, and of forest animals during the winter.
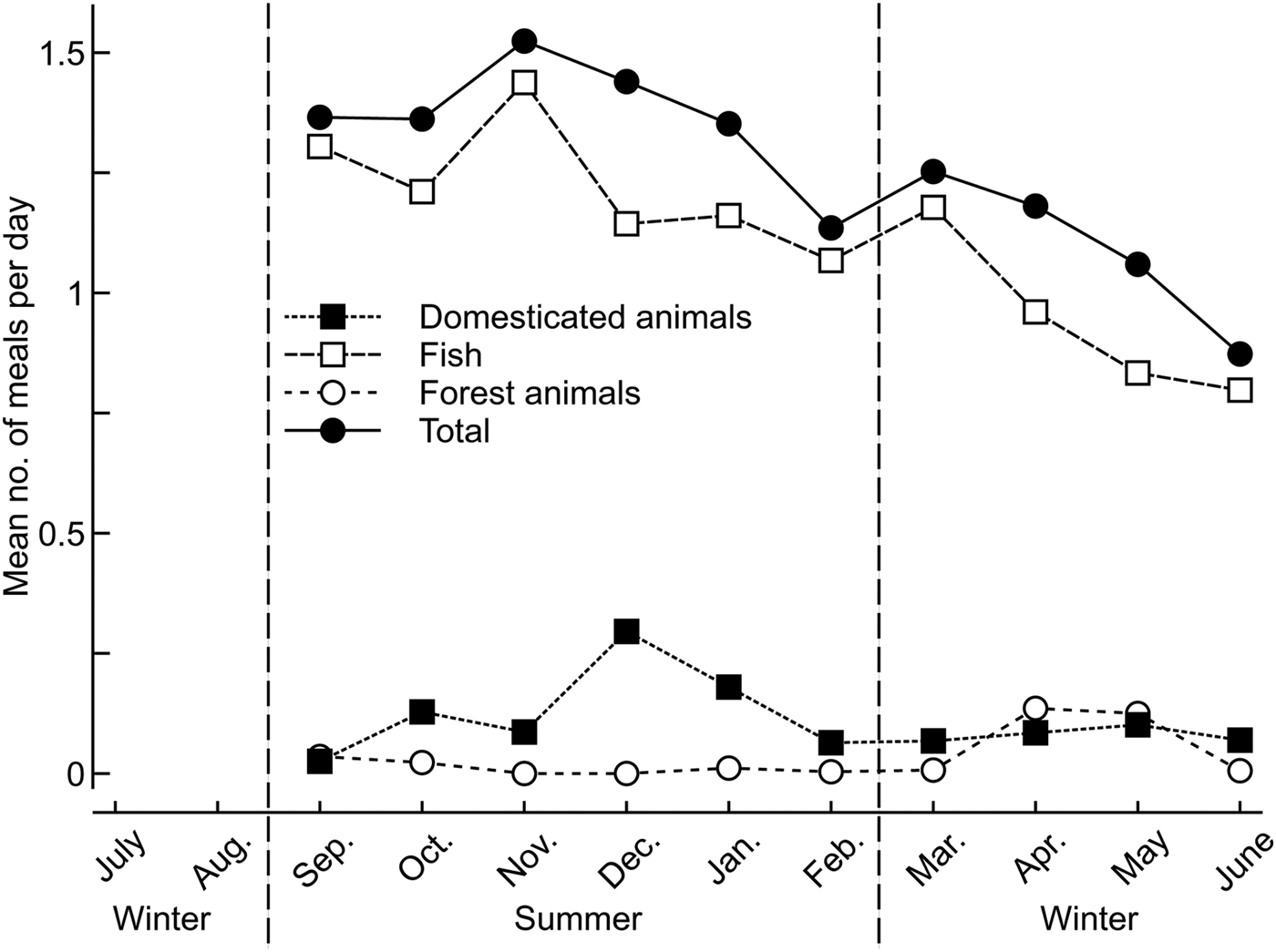
Fig. 2 Mean number of meals per day that contained animals, based on 24-hour recall surveys during September 2011–June 2012 (the August data were not included, to allow for sensitization to the survey), consumed in a village on the Masoala Peninsula (Fig. 1).
There was seasonal variation in the hunting of species (Table 1). In the village people caught a greater biomass of forest mammals during the cool, wet austral winter (Fig. 3). They caught more bushpigs, tenrecs and lemurs during the austral winter and more native euplerid carnivorans and introduced viverrid carnivorans during the warm austral summer (Fig. 4). The focal hunter trapped lemurs most intensively during the austral winter, and small euplerids and viverrids during the austral summer; he set traps for bushpigs throughout the year. He caught more forest mammals early in the austral winter (Fig. 3), primarily opportunistically caught tenrecs (Tenrec ecaudatus) and targeted lemurs (primarily Eulemur albifrons; Fig. 4). Although the hunter caught the most euplerids (Fossa fossana) and viverrids (Viverricula indica) in snare traps early in the austral summer, he also caught euplerids (Galidia elegans) opportunistically in early winter when they predated poultry in the village (Fig. 4). A hierarchical cluster analysis (using Ward metrics) of the number of individuals of each forest mammal species caught by the hunter each month also confirmed the seasonal nature of wildlife hunting, with species clustered into three categories: those targeted primarily during winter (1) and summer (2), and those caught opportunistically throughout the year as bycatch or as a response to human–wildlife conflict (3; Fig. 5).

Fig. 3 Seasonal variation in the total number and biomass of forest mammals caught by inhabitants of a village on the Masoala Peninsula (Fig. 1), based on shadowing a focal hunter during July 2011–June 2012 (a, b) and on responses to annual recall questions during household interviews conducted during July 2011–June 2012 (c, d).

Fig. 4 Seasonal variation (by taxon) in the percentages of total number and of total biomass caught by inhabitants of a village on the Masoala Peninsula (Fig. 1) based on shadowing a focal hunter during July 2011–June 2012 (a, b) and on responses to annual recall questions during household interviews conducted between July 2011 and June 2012 (c, d).
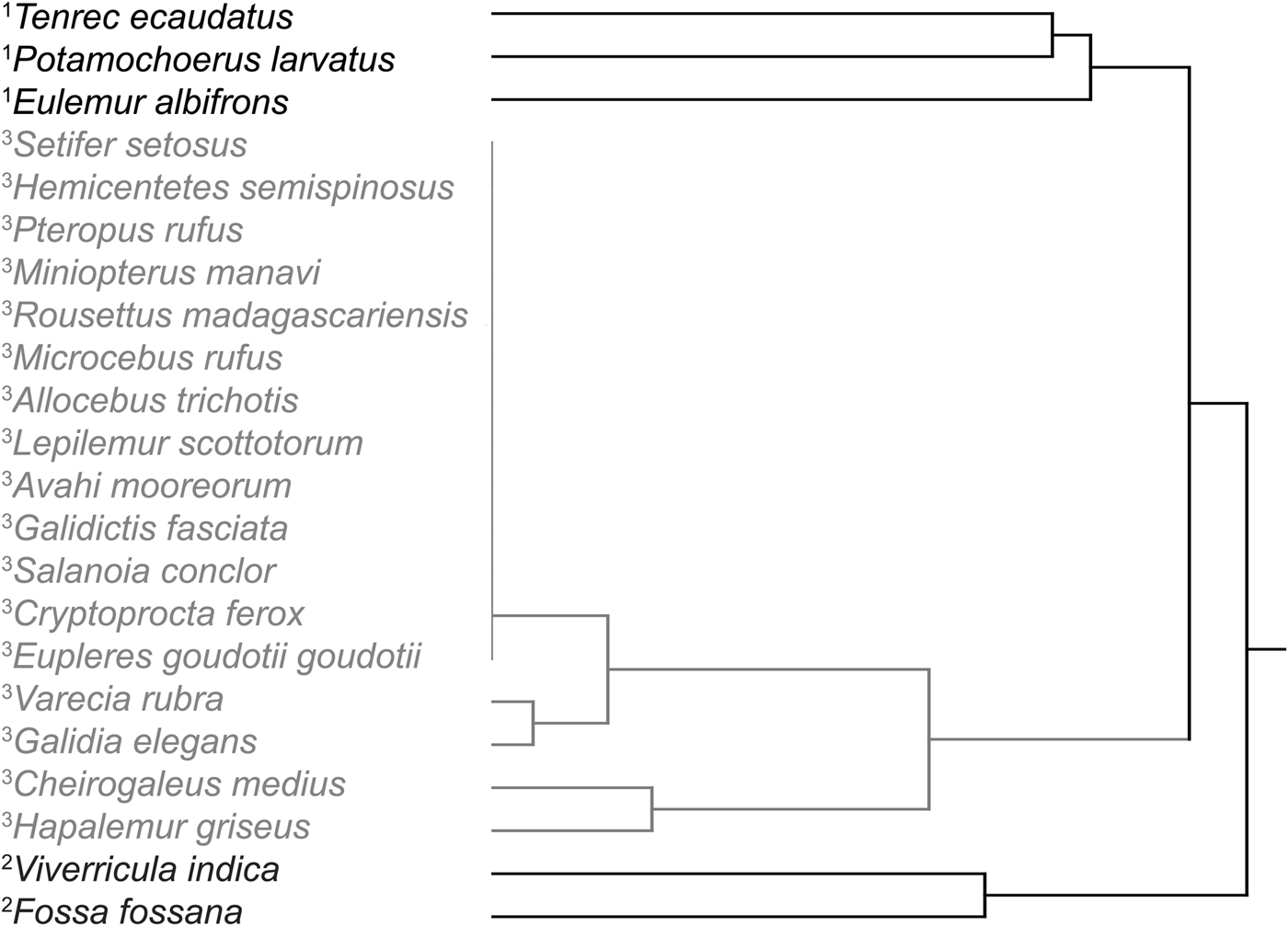
Fig. 5 Hierarchical cluster analysis, using the Ward metric, of the number of each species caught by the focal hunter on the Masoala Peninsula (Fig. 1) each month during July 2011–June 2012. The seasonal patterns of hunting are reflected in three clusters: (1) targeted species that were trapped during the winter, (2) targeted species that were trapped during the summer, and (3) untargeted species that were caught opportunistically or incidentally throughout the year or because of human–wildlife conflict.
Hunter effort did not vary significantly between seasons (t = 0.44, df = 9.99, P = 0.34; Fig. 6). Of the total biomass caught by the focal hunter over 1 year, 20% was unrecoverable because it had rotted in traps or was scavenged before he could collect it, 56% was consumed by members of his household, and 24% was sold (Fig. 6). The hunter sold only the meat of bushpigs, which were too large for his family alone to consume. Bushpigs and lemurs accounted for the greatest amount of recoverable biomass per day spent trapping during March–June and in October (Fig. 6). Unrecoverable biomass was highest during September–December (Fig. 6), when animals rotted in traps because of high ambient temperatures.
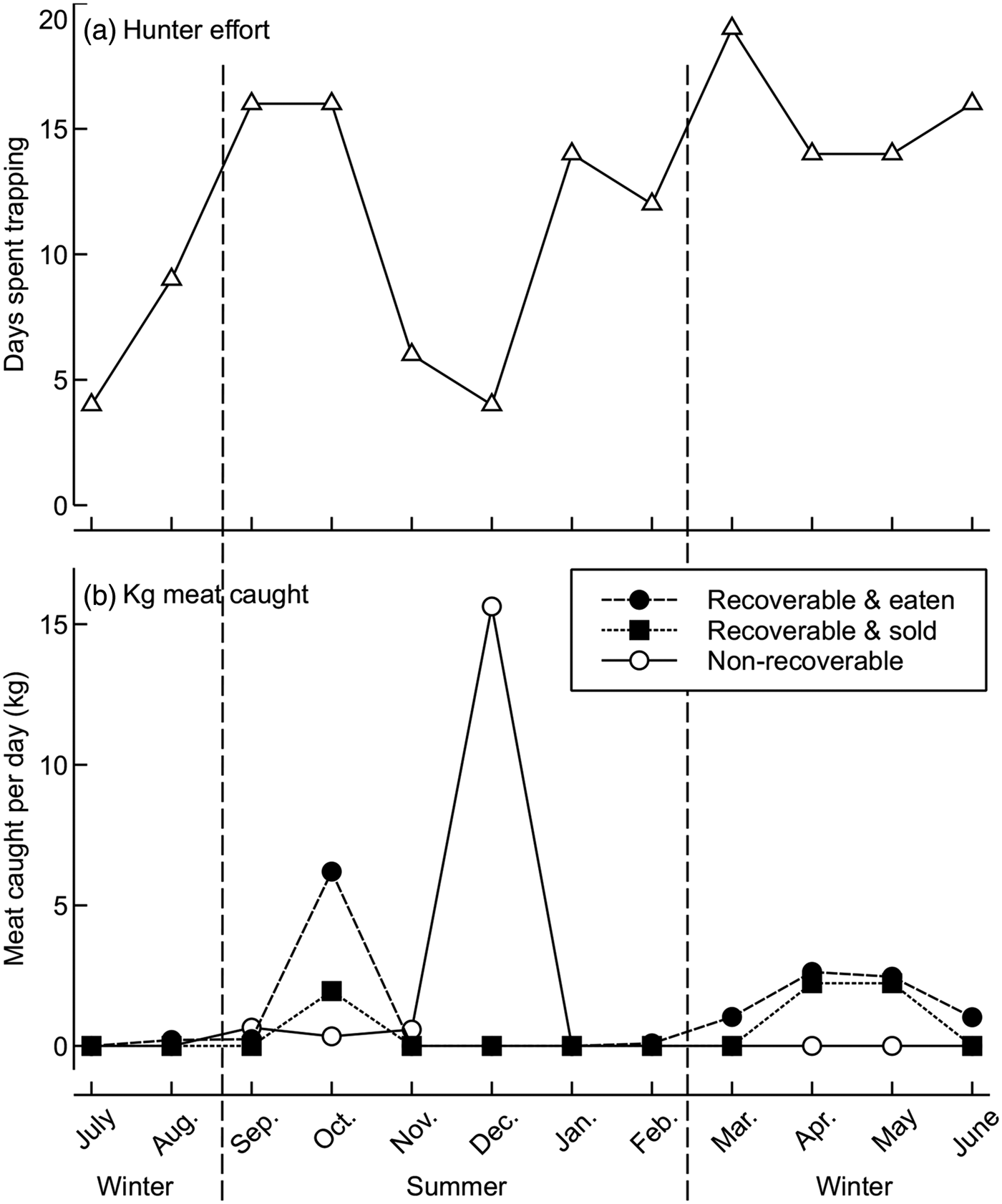
Fig. 6 Seasonal variation in the effort (a) and productivity (b) of a focal hunter on the Masoala Peninsula (Fig. 1) during July 2011–June 2012. The hunter spent 4–19 days per month trapping (mean = 12.00 ± SD 5.55 days) and caught a mean of 0.38 ± SD 0.30 forest mammals or 3.75 ± SD 4.74 kg of forest mammal meat per day spent trapping. He was unable to recover (or eat) a mean of 1.43 ± SD 4.48 kg of forest mammal meat per day spent trapping (these animals decomposed or were predated before the trapper returned to check the trap). This non-recoverable or wasted catch was highest in the austral summer.
Reasons for seasonal hunting
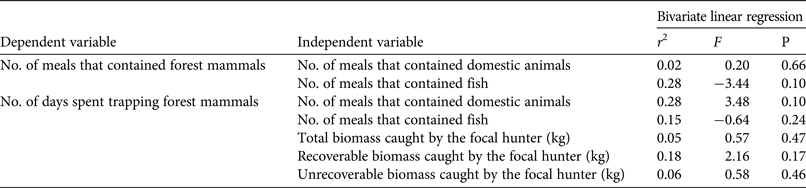
Seasonal variation in hunting patterns on the Masoala Peninsula were not driven by seasonal scarcity of animal-based foods or by cultural norms or hunting laws (Table 1). Although peak consumption of wildlife coincided with seasonal food insecurity, it could not be predicted by consumption of either domestic animals or fish (Table 3). The number of days the hunter spent trapping could not be predicted by the consumption of alternative animal-based foods or by the total, recoverable or unrecoverable biomass he caught per day (Table 3).
Table 3 Results of bivariate linear regression show the lack of effect of total consumption of forest mammals, fish and domestic animals, as well as trapper productivity, on hunting and consumption of forest mammals in a village on the Masoala Peninsula, Madagascar (Fig. 1), based on data collected from 24-hour recall surveys and by shadowing a focal hunter during 2011–2012.
Laws that designate the protected status of wildlife species or specify hunting seasons had little effect on seasonal patterns of wildlife hunting (Table 1). Almost three quarters (73%) of the forest mammals caught by hunters from the village and half (50%) of those caught by the focal hunter were harvested illegally (either out of season or prohibited). Illegal hunting peaked in the village during January–March, when tenrecs were hunted out of season; illegal hunting by the focal hunter peaked in March and June when he trapped lemurs.
Seasonal hunting was driven by characteristics of the prey that changed throughout the year (Table 1), the most important being seasonal variations in prey behaviour and body fat content. Seasonal variation in prey behaviour increased the predictability of prey location (which increased catch per day), or increased the species’ overlap with people, crops or domesticated animals (which increased both catch per day and human–wildlife conflicts; Table 1).
The predictable location of euplerids and lemurs was exploited by trappers. Fossa fossana was trapped on hot, dry days, along travel paths to, from and over rivers when water tables were low. Eulemur albifrons and Varecia rubra were trapped during the winter, along travel paths to seasonally fruiting trees, when these animals had their highest quantity of body fat. The focal hunter spent 30–58% more days trapping in March and June than during the rest of the year, trapping lemurs at seasonally fruiting trees (Fig. 6). He spent fewer days trapping when he was ill, celebrating holidays or working for a salary (opportunistic small jobs).
Human–wildlife conflict resulted in increased hunting of euplerids and bushpigs. Hunting of C. ferox, G. elegans and Galidictis fasciata increased in the austral summer, when they killed poultry, and more bushpigs were caught in the austral winter, when they consumed tuber crops. Whereas the majority of carnivorans (with the exception of most C. ferox) caught during the austral summer were hunted for food, all the carnivorans caught during February–July (C. ferox, G. elegans and G. fasciata) were hunted because of conflict over poultry.
The impact of seasonal hunting patterns on wildlife and people
The consequences of seasonal hunting for the hunted species vary by taxon. Hunting of euplerids coincided with their mating, pregnancy and lactation; F. fossana and Eupleres goudotii were hunted during pregnancy and lactation; C. ferox was hunted during its mating season (October–December, when males increase the size of their range) and again after females had given birth and were nursing (March); and hunting of T. ecaudatus coincided with its reproduction, with entire litters of dependent offspring (14 or more) often caught at one time. In contrast, hunting of lemurs occurred when they had weaned their offspring but had not yet begun mating.
Although seasonal hunting of forest mammals was not driven by the seasonal lack of alternative animal-based foods, more forest mammals were consumed during periods when consumption of fish and domestic animals was lowest. Because people targeted bushpigs, tenrecs and lemurs during this time, these species may provide more nutritional and economic benefits to local people.
Differences between methods
The three methods used to collect data (focal hunter shadowing, household interviews and 24-hour recall surveys) yielded slightly different results. It is likely the 24-hour recall surveys underestimated the consumption of forest animals. During these surveys local people rarely reported eating forest mammals, except bushpigs, even if they had been observed eating forest mammals that day. In contrast, all meals I observed containing forest mammals were reported during annual recall interviews. When questioned about this discrepancy participants revealed that they did not report their consumption of forest mammals during the 24-hour recall surveys because: (1) the surveys were conducted by a Malagasy assistant whom they perceived was likely to share this information if pressured physically by the military in my absence; and (2) because the illegal behaviour had happened more recently (i.e. within the previous 24 hours) and there was likely to be incriminating evidence (e.g. leftovers, hair or bones) available.
Discussion
Although there is a wealth of literature on hunting and the consumption of wild meat (Milner-Gulland et al., Reference Milner-Gulland and Bennett2003; Fa et al., Reference Fa, Seymour, Dupain, Amin, Albrechtsen and Macdonald2006; Brashares et al., Reference Brashares, Golden, Weinbaum, Barrett and Okello2011; Golden et al., Reference Golden, Fernald, Brashares, Rasolofoniaina and Kremen2011, Reference Golden, Bonds, Brashares, Rasolofoniaina and Kremen2014; Milner-Gulland, Reference Milner-Gulland2012), there is a lack of information on seasonality of hunting. By ignoring the seasonality of hunting we risk missing information that could be vital to developing sustainable conservation policies and action plans (Golden et al., Reference Golden, Wrangham and Brashares2013). The results of direct observations of hunting, interviews with local people, and 24-hour diet recalls suggest there is seasonal variation in the hunting of forest mammals and the consumption of fish and domesticated livestock on the Masoala Peninsula. The effects of this timing on native euplerid carnivorans may warrant a revision of the conservation status of some species, as they may be more threatened than previously recognized. Additionally, the extent to which seasonal variation in human factors and prey characteristics drive seasonal hunting patterns suggests that people decide whether or not to hunt independently of their decision on when to hunt particular species. The results also suggest that conservation efforts on the Masoala Peninsula should be focused on various times of year, depending on the species involved, to optimize their effects.
Unlike studies that rely solely on secondary accounts of hunting and meat consumption, collected through interviews and recall surveys, this study also incorporated direct observation of the activities of a focal hunter throughout the year. Although caution is warranted in inferring hunting patterns from observations of a single individual, such observations can be valuable. The behaviour of the focal hunter was consistent with that of other bushpig hunters, as inferred from their reports during interviews. However, the focal hunter differed from opportunistic hunters, who caught fewer animals, consumed less meat from bushpigs and lemurs and more from bats, and used slingshots. When trust has been established other hunters may be willing to collect accurate daily self-report data and record direct observations of hunting activities. Future efforts to understand how hunting varies across the Peninsula will be essential for designing and applying effective action plans for regional conservation and public health.
The reasons for seasonal hunting patterns
Hunters targeted lemurs and bushpigs during the cool, wet austral winter (March–August), and consumed more native euplerid carnivorans (e.g. C. ferox, F. fossana), introduced viverrids (V. indica), fish, and domesticated livestock during the warm austral summer (September–February). Seasonal increases in hunting coincided with food insecurity within the community. However, seasonal changes in hunting patterns were not driven by seasonal scarcity of animal-based foods, or by cultural norms or hunting laws, but by seasonal variation in the ranging behaviour, diet and body fat content of prey species. These findings highlight an important aspect of hunting on the Masoala Peninsula: the reasons people hunt are not, in general, the same as the reasons for seasonal patterns in hunting. Hunters maximize their energetic efficiency, hunting when they can expend the least amount of energy for the greatest gain, be it direct (from eating their catch) or indirect (by saving crops or poultry). If these results are validated elsewhere, conservationists may be able to apply their knowledge of seasonal changes in the ranging behaviour, diet and fat storage of threatened species to predict when they are most likely to be hunted.
The impact of seasonal hunting patterns on native species
Hunting of euplerids and tenrecs coincided with pregnancy and lactation in these animals, which is of particular concern for C. ferox, F. fossana and E. goudotii. Cryptoprocta ferox was hunted during the mating season (October–December, when males increase the size of their range) and again after females had given birth and were nursing their young (March). Thus, hunting of the species increased at the two most sensitive times for its reproduction. Fossa fossana and E. goudotii were also hunted when they were pregnant or lactating. Furthermore, F. fossana and V. indica are caught in the same style of trap, and therefore frequent catch of the introduced V. indica can encourage continued trapping effort and continued incidental catch of native F. fossana. This may place further stress on populations of F. fossana already in decline as a result of habitat alteration. Native carnivorans are also more likely to decompose in traps than are other native mammals because they are targeted during the hot austral summer.
Three native euplerid carnivorans (C. ferox, G. elegans and G. fasciata) are hunted seasonally because of human–wildlife conflict over poultry. This is in line with findings regarding hunting of carnivorans in central south-eastern Madagascar (Kotschwar Logan et al., Reference Kotschwar Logan, Gerber, Karpanty, Justin and Rabenahy2015), with a few important distinctions. On the Masoala Peninsula, as in central south-eastern Madagascar, C. ferox and G. elegans are hunted primarily because of human–wildlife conflict over poultry but, in contrast to central south-eastern Madagascar, G. fasciata was also hunted (albeit less often) in response to predation on chicks. Other distinctions include the infrequent hunting of C. ferox on the Peninsula (but not in central south-eastern Madagascar), and the hunting of V. indica solely for food. Viverricula indica, F. fossana and E. goudotii did not predate village poultry. In contrast to Kotschwar Logan et al. (Reference Kotschwar Logan, Gerber, Karpanty, Justin and Rabenahy2015) I found that taboos provided little protection to native euplerid carnivorans in this region. Taboos deterred the consumption of animals but not their killing in response to predation (e.g. C. ferox).
The conservation community in Madagascar has tended to focus on the impacts of habitat loss and hunting on endemic lemurs (Borgerson, Reference Borgerson2015). We are only beginning to understand the threats to euplerids from hunting and habitat alteration (Farris et al., Reference Farris, Karpanty, Ratelolahy and Kelly2014; Gerber et al., Reference Gerber, Karpanty and Randrianantenaina2012), and the direct (i.e. competition for resources) and indirect (disease transmission) threats from introduced carnivorans (Dollar, Reference Dollar2006; Vanak & Gompper, Reference Vanak and Gompper2009). The conservation status of many euplerids may need to be re-evaluated. Fossa fossana and E. goudotii are currently categorized as Near Threatened, and C. ferox as Vulnerable (IUCN, 2014). The Masoala–Makira landscape is a key conservation area for C. ferox and is one of only two forest regions where the C. ferox population exceeds 300 (Gerber et al., Reference Gerber, Karpanty and Randrianantenaina2012). The C. ferox population within the Masoala–Makira landscape is estimated to be c. 762 individuals (Farris et al., Reference Farris, Karpanty, Ratelolahy and Kelly2014) but, at one of c. 370 villages in the vicinity of the Masoala and Makira protected areas, four individuals were trapped within a single year. The number of C. ferox trapped within this study year is not atypical (C. Borgerson, unpubl. data).
The results show that lemurs were hunted after they had weaned their offspring but prior to their mating season. Thus the impact of hunting on dependent offspring was limited. Conservation efforts that aim to reduce the hunting of threatened lemurs on the Peninsula by increasing poultry production (Andrianjara et al., Reference Andrianjara, Andrianarimisa, Ratelolahy, Schwitzer, Mittermeier, Davies, Johnson, Ratsimbazafy and Razafindramanana2013) will face challenges because people eat more fish and domesticated animals during the austral summer. Poultry is consumed most often during the austral summer, when animal-based foods are most abundant, and poultry and lemur consumption peak during different seasons. Unless efforts to increase the production of poultry also reduce human–carnivoran conflict over poultry, they may unintentionally increase the hunting of native euplerids.
The impact of seasonal variation in hunting and meat consumption on people
Although seasonal hunting patterns are not driven by seasonal scarcity of resources on the Masoala Peninsula, they nonetheless affect the health and livelihoods of hunters. People consume more lemurs, bushpigs and tenrecs when they consume fewer domestic animals and fish, and therefore lemurs, bushpigs and tenrecs may make a greater contribution to human health and nutrition than animals hunted during the austral summer.
If collected sustainably, wild species could provide valuable renewable sources of fat, protein and micronutrients for local people. Tenrecs and bushpigs constituted most of the wild meat consumed, in terms of number of individuals and biomass, respectively. In a single year 105 tenrecs were caught. Tenrecs are not threatened and are designated as a game species in Madagascar, with a legal hunting season. The majority of tenrecs caught during this study, however, were hunted outside the legal hunting season for T. ecaudatus, when females are pregnant and lactating. The species breeds prolifically (Racey & Stephenson, Reference Racey, Stephenson and Lourenco1996; Nicoll, Reference Nicoll, Goodman and Benstead2003) but because it is hunted during critical stages in the reproductive cycle it is unlikely to reach its maximum potential productivity. Local people reported that T. ecaudatus populations had declined significantly in the decade prior to this study, and attributed the decline to their own overhunting. If hunting was confined to the legal season, and policy efforts were designed to assist population rebound, this species could provide significant health and economic benefits to local people.
Seasonal timing of conservation and public health action
Conservation action plans may be more effective on the Masoala Peninsula if actions are timed to coincide with either the hunting of euplerids during the austral summer or the hunting of tenrecs and lemurs during the austral winter. Similarly, programmes to improve the health and welfare of local people could be more effective if conducted during the austral winter, when people eat fewer alternative animal-based foods. Incorporating an understanding of seasonal patterns of hunting and meat consumption could help optimize programmes to improve the health of local people and native species, strengthening both the design and application of conservation policy.
Acknowledgements
I extend my deepest gratitude to the hunter, field assistant, and village community without whom this project would have been impossible. I thank Laurie R. Godfrey, Christopher D. Golden, Margaret A. McKean, Steve King, Mike Sutherland, Tom Leatherman, Timothy Randhir, Joel Borgerson, Martin Fisher, several anonymous reviewers, Madagascar National Parks and MICET for their support. This research was funded by grants from the Margot Marsh Biodiversity Foundation, Conservation International Primate Action Fund, Oregon Zoo Future for Wildlife Fund, and the International Primatological Society. The University of Massachusetts, Amherst Institutional Animal Care and Use Committee (2010-0010 and 2012-0028) and Human Subjects Institutional Review Board (2010-0595) approved this research.
Biographical sketch
Cortni Borgerson has worked in Madagascar since 2007 studying why people choose to hunt threatened species illegally, and the impact this has on humans, wildlife and the environment.











