Introduction
Modern Holarctic distributions of plants and animals were formed in part by the existence of intercontinental dispersal corridors in the Late Cretaceous through early Paleogene, together with a change in climate relative to the tolerances of organisms that might expand their ranges across them. Land connections between North America and Europe via Greenland were present in the Maastrichtian through late Danian by the northern de Geer route to Fennoscandia, and in the Thanetian and early Ypresian by a southern Thulean route through Greenland, the Faroes, and the United Kingdom (Brikiatis Reference Brikiatis2014). North America and East Asia were episodically connected across Beringia in the Danian, Thanetian, and possibly the Eocene. This was a time of “Greenhouse World” climate, with high global mean annual temperatures, low pole-to-equator mean annual temperature gradients, poles free of ice sheets, and low extra-tropical temperature seasonality resulting in mild winters in cooler higher latitudes and elevations (Zachos et al. Reference Zachos, Dickens and Zeebe2008, Reference Zachos, McCarren, Murphy, Röhl and Westerhold2010; Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2010; National Research Council 2011).
In contrast, our post-Eocene “Icehouse World” climatic regime is characterised by cooler mean annual temperatures, a steeper equator-to-pole mean annual temperature gradient, and greater temperature seasonality outside of the tropics (Zachos et al. Reference Zachos, Dickens and Zeebe2008, Reference Zachos, McCarren, Murphy, Röhl and Westerhold2010; Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2010; National Research Council 2011). Thermophilic organisms have become restricted to lower latitudes because they require the high mean annual temperature values found there. Others now restricted to lower latitudes are cryophobic: they do not require high mean annual temperatures but are intolerant of cold extra-tropical winters associated with increased temperature seasonality (Archibald and Farrell Reference Archibald and Farrell2003; Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2010). In the Greenhouse World, however, these two groups partially separated, with cryophobic organisms ranging into cooler higher latitudes and elevations with mild winters – that is, regions with lowered mean annual temperatures that would exclude thermophilic organisms. This allowed the existence of communities without modern analogues – for example, forests with palms, alders, maples, and spruce in upper microthermal localities of the Ypresian Okanagan Highlands. These high-paleoelevation localities in far-western mid-latitude North America (e.g., Archibald and Farrell Reference Archibald and Farrell2003; Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2010, Reference Archibald, Morse, Greenwood and Mathewes2014) reveal clear examples of such mixed climatic–preference plant and insect communities.
This climatic regime and community response has implications for Greenhouse World trans-Arctic dispersal. Analysis by the TEX86 proxy thermometer method gives megathermal background sea surface mean annual temperature values for the Ypresian Arctic, but this method may produce misleadingly high temperature values (Kim et al. Reference Kim, van der Meer, Schouten, Helmke, Willmott and Sangiorgi2010; Sluijs et al. Reference Sluijs, Frieling, Inglis, Nierop, Peterse, Sangiorgi and Schouten2020). Lower estimates of upper microthermal terrestrial mean annual temperatures are given for the Ypresian Arctic using paleobotanical proxies, like the mean annual temperatures determined for most localities of the montane Okanagan Highlands by the same methods (Greenwood et al. Reference Greenwood, Archibald, Mathewes and Moss2005; Sunderlin et al. Reference Sunderlin, Loope, Parker and Williams2011; Mathewes et al. Reference Mathewes, Greenwood and Archibald2016; West et al. Reference West, Greenwood, Reichgelt, Lowe, Vachon and Basinger2020). In the Eocene Arctic, as in the Okanagan Highlands, a suite of frost-intolerant plants and animals indicates mild winters with few, if any, frost days (e.g., Wing and Greenwood Reference Wing, Greenwood, Allen, Hoskins, Sellwood and Spicer1993; Archibald and Farrell Reference Archibald and Farrell2003; Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2010, Reference Archibald, Greenwood, Smith, Mathewes and Basinger2011a, Reference Archibald, Morse, Greenwood and Mathewes2014; Eberle and Greenwood Reference Eberle and Greenwood2012). A microthermal Arctic intercontinental dispersal corridor with mild winters would then have acted as a filter, allowing passage of cryophobic taxa while excluding thermophilic taxa.
Throughout the Ypresian (47.8–56.0 Ma), this temperate and equable Arctic climate was, however, punctuated by brief hyperthermal events of global warming associated with large quantities of carbon injected into the atmosphere–ocean system. The most notable hyperthermal was the Paleocene–Eocene Thermal Maximum, with a release of more than 2000 gigatonnes of carbon commencing at the Paleocene–Eocene boundary and lasting less than 20 ky, decreasing to background values in about 200 ky, with a peak increase in global temperature of 5–8 °C (Wing et al. Reference Wing, Harrington, Smith, Bloch, Boyer and Freeman2005; Zachos et al. Reference Zachos, Dickens and Zeebe2008; McInerney and Wing Reference McInerney and Wing2011; Westerhold et al. Reference Westerhold, Röhl, Frederichs, Agnini, Raffi, Zachos and Wilkens2017; Stokke et al. Reference Stokke, Jones, Tierney, Svensen and Whiteside2020; Vickers et al. Reference Vickers, Lengger, Bernasconi, Thibault, Schultz and Fernandez2020; Reinhardt et al. Reference Reinhardt, von Gosen, Lückge, Blumenberg, Galloway and West2022). Such hyperthermals would have acted as climatic “gates” for thermophilic organisms, briefly opening periodically to allow high-latitude passage between continents.
Archibald et al. (Reference Archibald, Johnson, Mathewes and Greenwood2011b) examined this “bridges with gates” dispersal model regarding size and habitat climate in ants, comparing modern species that have queens of at least 3 cm in length with those of the giant Eocene ants of the extinct subfamily Formiciinae (Hymenoptera: Formicidae) (species of Titanomyrma Archibald et al. and the parataxon Formicium Westwood, those known only from wings). They found that modern ants with the largest queens inhabit only low latitudes, almost exclusively in the tropics, and so great size of queens is associated with being either thermophilic or cryophobic.
The queens of giant species of Formiciine are by far the largest of any extant or fossil Hymenoptera: T. gigantea (Lutz) and T. simillima (Lutz) (Ypresian Messel and Lutetian Eckfeld Maar, Germany), T. lubei Archibald et al. (Ypresian Green River Formation, Wyoming, United States of America), and F. mirabile (Cockerell) (Lutetian Bournemouth Group, United Kingdom). Titanomyrma gigantea queens reached 7 cm in length and possessed a wingspan of about 16 cm (Lutz Reference Lutz1990). Archibald et al. (Reference Archibald, Johnson, Mathewes and Greenwood2011b) found that these are known only at localities of high mesothermal to megathermal mean annual temperatures and never at localities of cooler mean annual temperatures such as the Okanagan Highlands, which is consistent with their being thermophilic, not cryophobic. Smaller Formiciinae are also known from megathermal localities (F. brodiei Westwood, Ypresian–Lutetian Bournemouth Group, United Kingdom) or of undetermined mean annual temperatures (F. berryi Carpenter, middle Eocene Claiborne Formation, Tennessee, United States of America; Archibald et al. Reference Archibald, Johnson, Mathewes and Greenwood2011b). Modern ant species with smaller queens are known from all latitudes where ants are found. The discovery of giant formiciine ants in the Okanagan Highlands would imply that they were cryophobic, not thermophilic. Smaller formiciine there would be consistent with species with giant queens being thermophilic and constrained to higher mean annual temperature regions, and those with smaller queens being distributed across a variety of climates, including regions with lower mean annual temperatures.
Both giant and smaller Formiciinae appear in the second half of the Ypresian in Europe and North America, implying a cross-Arctic dispersal of both size groups in either direction at least once sometime in the Late Cretaceous through early Ypresian under Greenhouse World conditions with episodic hyperthermals. Dispersal of one size group with separate origins of the other on both sides of the Atlantic seems less likely.
Here, we examine the implications for trans-Arctic dispersal of a new Titanomyrma from the microthermal Okanagan Highlands Allenby Formation of British Columbia, Canada.
Material and methods
We examined a fossil from the Allenby Formation near Princeton, British Columbia, Canada, in the collections of the Beaty Biodiversity Museum (Vancouver, British Columbia, Canada) and compared it with other Formiciinae, including two new specimens from the Green River Formation of Wyoming, United States of America in the collections of Fossil Butte National Monument (Kemmerer, Wyoming, United States of America).
Photography of the Allenby ant was done at the Parks Canada laboratory, Vancouver, British Columbia, Canada, using a Zeiss microphotography system (Oberkochen, Germany) and of the Green River specimens at the University of Colorado, Boulder, Colorado, United States of America and the Denver Museum of Nature & Science, Denver, Colorado.
We follow the morphological terminology of Lutz (Reference Lutz1986, Reference Lutz1990) and Wappler (Reference Wappler2003), except that we refer to abdominal, not gaster segment numbers (e.g., abdominal segments A3–7 are gaster segments I–V). Contrary character states in compared taxa are provided in brackets.
We refer to the general mean annual temperature categories of Wolfe (Reference Wolfe1975), rather than estimated values that vary by a few degrees in differing analyses (various equations for leaf margin analyses, the Climate Leaf Analysis Multivariate Program, and nearest living relatives of plants analysis; e.g., Greenwood et al. Reference Greenwood, Archibald, Mathewes and Moss2005), as this level of precision is relevant here. These mean annual temperature categories are as follows: microthermal: ≤ 13 °C; mesothermal: > 13 °C, < 20 °C; and megathermal: ≥ 20 °C.
Localities
The Allenby Formation
This formation is part of the Okanagan Highland series of fossiliferous lacustrine basins scattered across about 1000 km from west–central British Columbia into northern Washington, United States of America (Read Reference Read2000; Archibald et al. Reference Archibald, Greenwood, Smith, Mathewes and Basinger2011a). The ant was collected in an exposure of Vermilion Bluffs Shale Unit about 4 km southwest of the village of Princeton, British Columbia. A nearby Vermilion Bluffs Unit outcrop (the Billy’s Family Restaurant locality) is estimated by U–Pb decay to be Ypresian, 51.85 ± 0.85 Ma (Rubino et al. Reference Rubino, Leier, Cassell, Archibald, Foster-Baril and Barbeau2021). The Allenby Formation forest had an estimated upper microthermal mean annual temperature (Greenwood et al. Reference Greenwood, Archibald, Mathewes and Moss2005; West et al. Reference West, Greenwood, Reichgelt, Lowe, Vachon and Basinger2020), with few or no frost days in its coldest month, as indicated by a suite of frost-intolerant plants such as the coryphoid palm Uhlia allenbyensis Erwin and Stockey (Arecaceae) (Erwin and Stockey Reference Erwin and Stockey1991; Archibald et al. Reference Archibald, Morse, Greenwood and Mathewes2014).
The Green River Formation
This formation includes lacustrine sediments deposited in Wyoming, Colorado, and Utah, United States of America (Smith and Carroll Reference Smith, Carroll, Smith and Carroll2015) in three Ypresian lakes. The ants were found in the laminated limestones of the White Marker Unit near the base of the Angelo Member of the Fossil Lake deposits in Fossil Basin, Wyoming (Buchheim et al. Reference Buchheim, Cushman and Biaggi2011). This unit, approximately 15 cm thick, lies about 5.5 m above the K-spar tuff, which has a radiometric age of 51.98 ± 0.35 Ma (Smith and Carroll Reference Smith, Carroll, Smith and Carroll2015). Green River shales were deposited in a lowland intermontane basin with high mesothermal to mostly megathermal mean annual temperature values, as determined by paleobotanical and isotopic analyses (Wilf Reference Wilf2000; Archibald et al. Reference Archibald, Johnson, Mathewes and Greenwood2011b).
Specimens
The Allenby ant, new Green River Formation ants, and the only specimen of T. lubei vary in preservation and cannot be confidently considered separate species (unless the Allenby ant is small; see below). We, therefore, treat the new fossils as Titanomyrma sp. pending more complete and undistorted specimens. The Allenby ant is a formiciine by the “crowding” of the forewing pterostigma and cells 1–2R, 1M, and 1Rs – that is, these are small and grouped in the anterior middle wing – and this Allenby specimen and the new Green River specimens are Titanomyrma species by their diagnostic slit-like, prominent spiracles (Lutz Reference Lutz1986).
The Allenby Formation ant
Beaty Biodiversity Museum collection BBM-PAL-2022-00001 (part only), queen
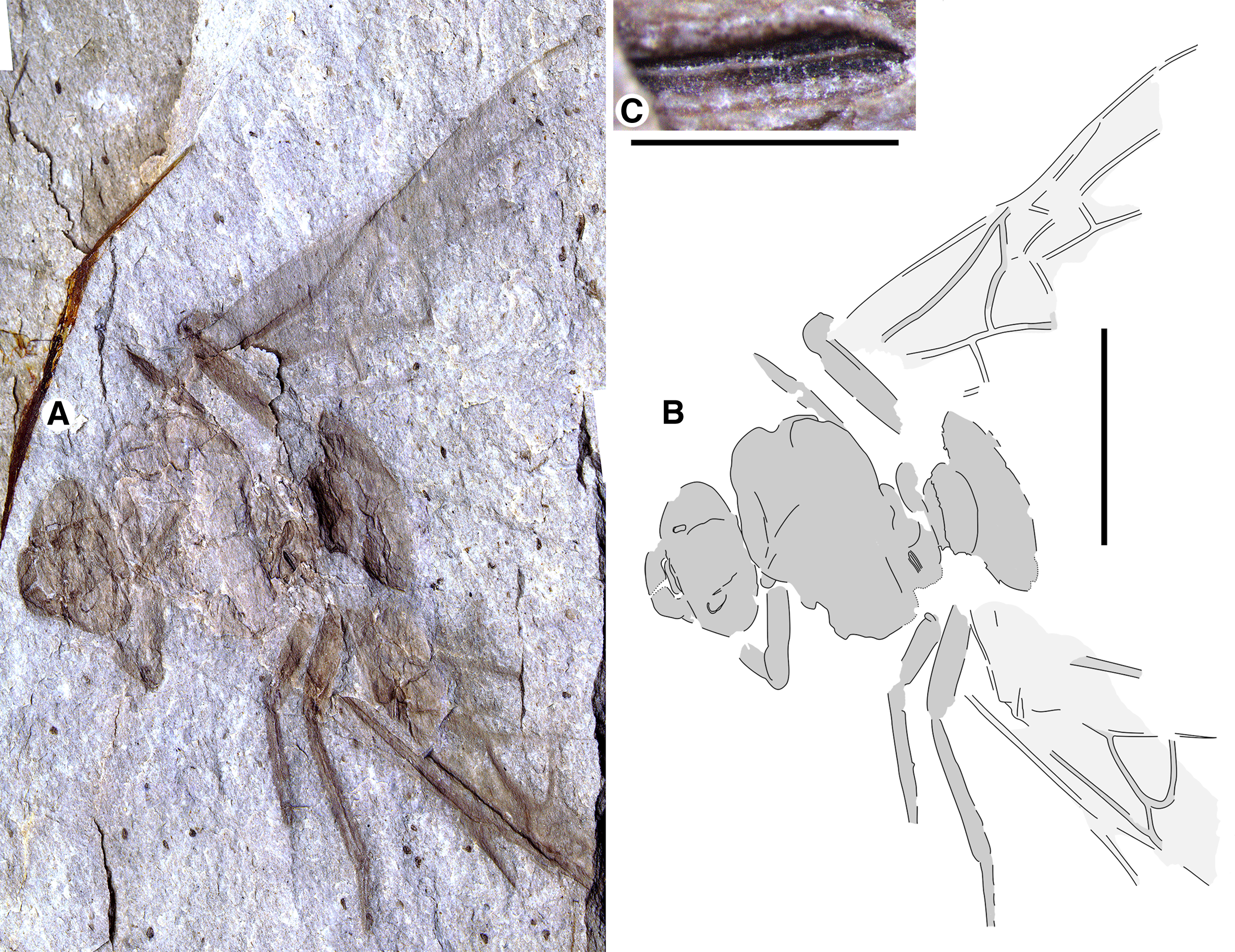
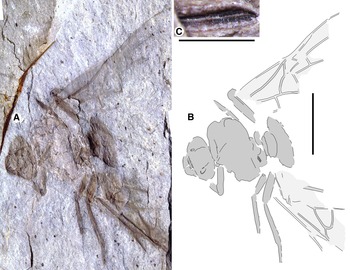
Fig. 1. Allenby Formation ant, Titanomyrma sp. UBC-BBM-PAL-2022-00001. A, photograph; B, drawing; and C, partially preserved spiracle. A, B to scale = 1 cm; C to scale = 1 mm.

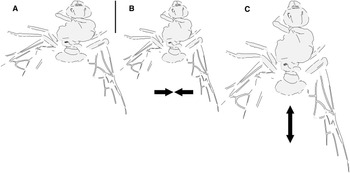
Fig. 2. Allenby Formation ant Titanomyrma sp. BBM-PAL-2022-00001: A, as preserved; B, compressed laterally; and C, extended lengthwise (see text).
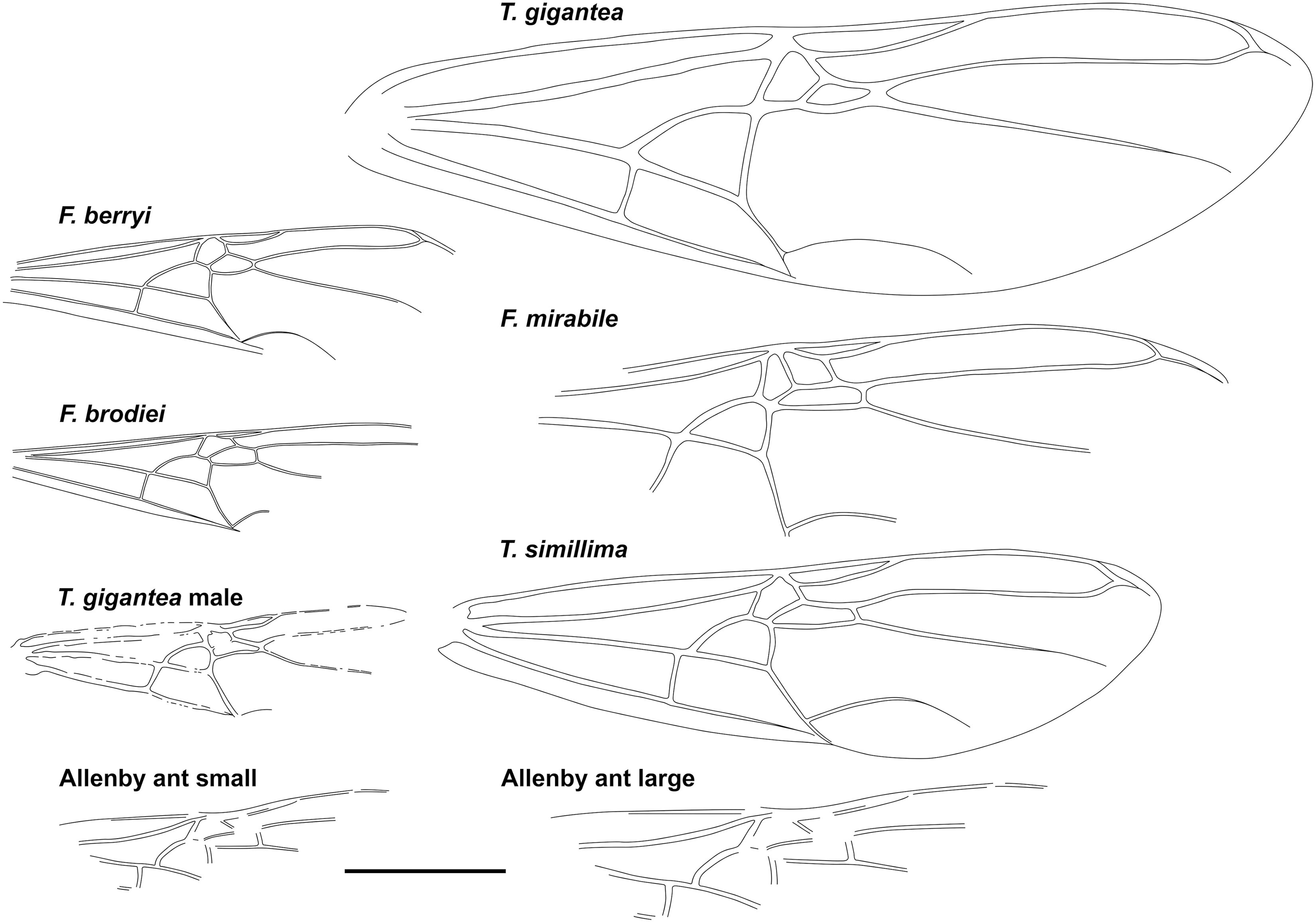
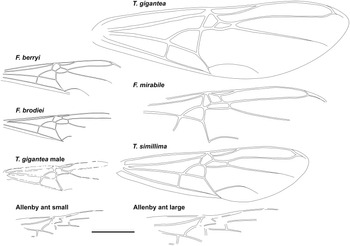
Fig. 3. Comparative forewings of species indicated with the Allenby ant compressed and lengthened. The wings of F. berryi, F. brodiei, T. gigantea (queen), and T. simillima were redrawn from Lutz (Reference Lutz1986), figs. 2C, 1C, 5, and 10, respectively, and the wing of the T. gigantea male was redrawn from Wappler (Reference Wappler2003), fig. 94. Scale = 1 cm.

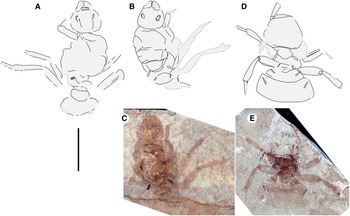
Fig. 4. A, Allenby Formation ant, as lengthened (compare with Fig. 2C), and new Green River Formation ants – Titanomyrma sp.: B, FOBU 9488B drawing; C, FOBU 9488B photograph; D, FOBU 6495 drawing; and E, FOBU 6495 photograph. All to scale, 1 cm.
Measurements are not given by uncertainty due to distortion; shapes are described after graphic adjustment (see Fig. 2 and size discussion, below). Head: with rounded posterior margins, not lobed, missing antennae, capsule about 2/5 alitrunk length; eyes oval, maximum length oriented anterodorsally, located at cephalic mid-length; large, triangular mandibles, about half head length, 8–10 coarse teeth. Alitrunk: poorly preserved. Forewing (basal and distal-posterior portions not known), with venation of preserved portions most like that of T. simillima but differs by: 3-Rs joins M approximately middle between 1-M, m-cu [T. simillima: joins close to m-cu], cell 1Rs evenly widens distally (to rs-m) [narrows distad 2r], cell 1M about as high (Cu to Rs+M) as wide (Cu) [about half as high as wide], cu-a joins M+Cu at approximately half its length [joins very close to 1-M, Cu]. Hind wings missing. Legs: portions preserved short, stout. Waist: single segmented, petiole lacking anterior peduncle, otherwise indistinctly preserved. Partial propodeal spiracle preserved, slit-like, long. Gaster: only the first segment (A3) preserved. Beverly Burlingame (collector), 12.vi.2021.
The Green River Formation ants
Fossil Butte National Monument collection FOBU-9488A, B (part and counterpart), queen
(Fig. 4).
Head: missing antennae; head length, ∼ 5.1 mm; head width, ∼ 6.5 mm; eye length, ∼ 1.1 mm, head capsule about 2/5 alitrunk length (alitrunk indistinctly preserved), with rounded posterior margins, not lobed; eyes large, oval, maximum length oriented anterodorsally, located at cephalic mid-length; large, triangular mandibles, about half head length, with approximately 8–10 coarse teeth. Alitrunk: somewhat distorted, indistinctly preserved; wings not preserved; right legs partially preserved, short, stout, left legs absent. Spiracles slit-like, long; propodeal spiracles robust, widest; petiole spiracles smaller, thinner; one gaster spiracle (A3) present, poorly preserved. Waist: single segmented, petiole lacking anterior peduncle, apparently about 5 mm wide, otherwise indistinct. Gaster: small, indistinct portion preserved. Paul Buchheim (collector), 7.viii.2002.
Fossil Butte National Monument collection FOBU-6495 (part only), queen
(Fig. 4).
Head missing. Alitrunk: well preserved basally, faintly in anterior two thirds; legs short, stout; femur, tibia of the left mesothoracic and metathoracic legs, the right metathoracic leg, the femur of right mesothoracic leg, and tibia of the left prothoracic present. Wings not preserved. Spiracles slit-like, long; propodeal spiracle robust, widest; petiole spiracle shortest; petiole, A3, A4 spiracles narrower; A3, A4 spiracles long. Waist: single segmented, petiole about 4.5 mm wide, length indeterminate, apparently lacking anterior peduncle. Gaster: A3, about 9 mm wide, length indeterminate; A4, 14 mm wide, about 4.5–5.0 mm long, missing posterior to this. Paul Buchheim (collector), 25.vii.2000.
Discussion
Ant size, climate, and trans-Arctic dispersal
The new Allenby specimen is the first occurrence of Formiciinae in a known microthermal climate. Its implications for the relationships between queen size, climate, and trans-Arctic dispersal are complicated, however, by uncertainty of its size in life, as its sole fossil has been distorted by geologic shear forces during diagenesis, a common occurrence in the exposure where it was found (see Archibald and Cannings Reference Archibald and Cannings2021, fig. 3, a similarly distorted fossil from the same locality). It was either compressed lengthwise to shorten it or stretched at a right angle from this to widen it (Fig. 2). These possibilities cannot be distinguished.
The Allenby ant as big
Graphically stretching the fossil a few degrees from lengthwise to be bilaterally symmetrical (Fig. 2C) results in a shape and size indistinguishable from the undistorted and more clearly preserved but incomplete new Green River specimens (Fig. 4) and closely resembles the sizes of T. lubei and T. simillima queens, both about 5 cm long (wings, Fig. 3).
That modern ant species with the largest queens inhabit low latitudes (Archibald et al. Reference Archibald, Johnson, Mathewes and Greenwood2011b) seems to contradict the finding that the average body mass of worker ants decreases along with increasing mean annual temperatures towards the equator (Kaspari Reference Kaspari2009). This might be accounted for by increasing ant species richness towards the equator, lengthening the upper tail of size distribution of workers; that is, although average worker size decreases in low latitudes, individual species with the largest workers are found there – for example, those of Dinoponera Roger, Paraponera Smith, and some Pachycondyla Smith. If this relationship holds true for queens, a giant Allenby queen could result from the high, tropical levels of species richness of insects in the Okanagan Highlands, associated with seasonal temperature stability (Archibald et al. Reference Archibald, Bossert, Greenwood and Farrell2010). Whatever explains gigantism in Titanomyrma queens, the Allenby species being giant would indicate that huge size is decoupled from mean annual temperatures, which would support the hypothesis that the genus was cyrophobic. This would allow giant species to ignore hyperthermal gates and migrate across high latitudes at any time when the land bridges existed, including times of cooler mean annual temperature values. Other truly thermophilic organisms would still require hyperthermals to cross the Arctic, even if the giant formiciines did not.
The Allenby ant as small
Graphically compressing the fossil laterally (Fig. 2B) produces an identical but smaller shape, estimated to be about 3.3 cm in length presumed complete, approximately 65% the size of the stretched version and of the T. simillima and T. lubei queens. Its forewings are then comparable in size to those of the smaller formiciines F. brodiei and F. berryi or to males of T. simillima and T. gigantea (Fig. 3). It has been suggested that specimens of F. brodiei and F. berryi, known only by forewings, might have been males (Fig. 3; Lutz Reference Lutz1986; Katzke et al. Reference Katzke, Barden, Dehon, Michez and Wappler2017). The Allenby ant is not a male, because these are most easily distinguished from queens in T. simillima and T. gigantea by their notably smaller relative head size. Such a small Allenby formiciine queen, although bigger than the 3-cm size set as large for modern ants in Archibald et al.’s (Reference Archibald, Johnson, Mathewes and Greenwood2011b) analysis, would support the hypothesis that species of Formiciine with the largest queens required high mean annual temperatures, whereas smaller species inhabited a wide variety of climates, including cooler microthermal ones that excluded species with large queens. This would be consistent with the hypothesis that giant Formiciinae crossed the Arctic only during hyperthermals and smaller species crossed at any time the corridors were open.
Acknowledgements
The authors thank Beverly Burlingame, collector of the Allenby ant, and Kathy Simpkins, collections manager of the Princeton & District Museum and Archives (Princeton, British Columbia, Canada) for access to the Allenby fossil; Marjorie Holland, president of the Board of Directors, and Todd Davidson, Director, Princeton & District Museum, for facilitating access to the Allenby ant and donating it to the Beaty Biodiversity Museum; Chief Bonnie Jacobsen of the Upper Similkameen Indian Band, and Robin Irwin, Natural Resources Director of the Upper Similkameen Indian Band, for helpful arrangements; David Zelagin for photography of the Green River ants at the Denver Museum of Nature & Science (Denver, Colorado, United States of America); and Marlow Pellatt of Parks Canada (Vancouver, British Columbia, Canada) for access to his laboratory and photography equipment. Funding was provided by a Natural Sciences and Engineering Research Council Discovery Grant 2020-05026 to R.M. Contributions to this paper, including portions of text, are by Arvid Aase, a Government of the United States employee; they were completed as part of his official duties and as such are considered to be a U.S. Government publication. Under provisions of the U.S. Copyright Act (17 USC Section 8), no copyright is claimed on those portions of this work.