Introduction
The family Trypetheliaceae is an almost strictly tropical lineage of nearly exclusively corticolous (very rarely saxicolous), lichenized fungi (Fig. 1). The first members of this family were encountered towards the end of the 18th century on pieces of medicinal bark (mainly Cinchona for quinine) that had been collected in South American forests (Zenker Reference Zenker1827). The conspicuous, often brightly coloured ascomata with complex structures intrigued lichenologists of the time and both Acharius (1817 Reference Achariusa , Reference Achariusb ) and Fée (Reference Fée1831) wrote monographs of what was then known as the genus Trypethelium, treating 10 and 19 species, respectively. More species became known especially from Australia, Brazil and Borneo, which were described in separate papers, mostly by Eschweiler (Reference Eschweiler1824, Reference Eschweiler1833), Krempelhuber (Reference Krempelhuber1875), Montagne (Reference Montagne1851, Reference Montagne1856) and Müller (Reference Müller1884, Reference Müller1885). During this time, a further synopsis of the family was made by Trevisan (Reference Trevisan1861).

Fig. 1 Mosaic of six Astrothelium species (and one Phaeographis) on a tropical rainforest tree at Los Amigos Biological Station in Amazonian Peru. Left, with orange pseudostromata, A. kunzei; centre, with white pseudostromata, A. rufescens; upper right, with green thallus, A. cf. cecidiogenum; right, mottled-green-yellow thallus, A. subscoria; lower right, small thallus, A. nitidiusculum; lower middle and left, A. aeneum.
After this initial burst of activity, which concluded with Malme’s treatment of species gathered during the first Regnellian Expedition in Brazil (Malme Reference Malme1924), relatively few species were added for a long time, and for many species only the type was known. In the meantime, the genus Trypethelium had been split into smaller entities and various classification systems had been proposed and applied, particularly by Müller (Reference Müller1884, Reference Müller1885), Nylander (Reference Nylander1863), Vainio (Reference Vainio1890) and Zahlbruckner (Reference Zahlbruckner1922, Reference Zahlbruckner1924, Reference Zahlbruckner1928). These studies doubled the number of names accepted in the Trypetheliaceae solely through reclassifications. Halfway through the 20th century, this led to the situation where almost as many names existed in the family as there had been specimens collected and illustrated by the only monograph published around that time, by Letrouit-Galinou (Reference Letrouit-Galinou1957, Reference Letrouit-Galinou1958), on the genus Laurera. In part, the genera of Trypetheliaceae were also mixed with those of Pyrenulaceae since the overall ascoma morphology was considered more important than anatomical features such as hamathecium structure and ascospore type (e.g. Dodge Reference Dodge1953). Both these character complexes clearly separate the two families, which are not even closely related; molecular phylogenetic studies placed Pyrenulaceae in Eurotiomycetes and Trypetheliaceae in Dothideomycetes (Del Prado et al. Reference Del Prado, Schmitt, Kautz, Palice, Lücking and Lumbsch2006; Nelsen et al. Reference Nelsen, Lücking, Grube, Mbatchou, Muggia, Rivas Plata and Lumbsch2009; Schoch et al. 2009 Reference Schoch, Sung, López-Giráldez, Townsend, Miadlikowska, Hofstetter, Robbertse, Matheny, Kauff and Wanga , Reference Schoch, Crous, Groenewald, Boehm, Burgess, De Gruyter, de Hoog, Dixon, Grube and Gueidanb ).
Aside from two papers published by Johnson (Reference Johnson1940, Reference Johnson1959) on North American Trypetheliaceae and an anatomical study of the endoperidermal thallus of Trypethelium eluteriae (Lambright & Tucker Reference Lambright and Tucker1980), serious taxonomic and systematic treatments of the Trypetheliaceae were only taken up again by Harris with his studies on Amazonian species (Harris Reference Harris1986) and a revision of Polymeridium (Harris Reference Harris1993). Previously, Eriksson (Reference Eriksson1981) had briefly treated the family in his conspectus of bitunicate Ascomycetes and elaborated a detailed evolutionary scheme for the supposedly closely related Trypetheliaceae and Pyrenulaceae. The work by Harris (Reference Harris1986, Reference Harris1993) redefined the family in a restricted sense and adopted a generic division based on thallus type, ascoma disposition, and ascospore type that has been in use since then. However, Harris (Reference Harris1995) and other workers (Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008) also recognized that this system was at least in part artificial, resulting in cases such as in the Astrothelium variolosum-Trypethelium nitidiusculum complex where a single specimen could be partly assigned to one genus and partly to another (Harris Reference Harris1995). Further systematic treatments were provided by Aptroot (Reference Aptroot1991), introducing the new genus Architrypethelium, and Harris (Reference Harris1998), with a revision of the genus Pseudopyrenula. Harris’s work in particular triggered further inventories and taxonomic studies of the family, particularly in India but also in the Neotropics and Australia, describing many new taxa (Upreti & Singh Reference Upreti and Singh1987; Makhija & Patwardhan Reference Makhija and Patwardhan1988, Reference Makhija and Patwardhan1992, Reference Makhija and Patwardhan1993; McCarthy & Kantvilas Reference McCarthy and Kantvilas1993; McCarthy Reference McCarthy1995; Aptroot & Ferraro Reference Aptroot and Ferraro2000; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Aptroot 2009 Reference Aptroota , Reference Aptrootb ). At the time when Harris (Reference Harris1986) published his Amazonian studies, Hawksworth (1986) provided a revision of the genus Mycomicrothelia, a genus at the time not thought to be related to Trypetheliaceae but recently found to include many species falling in lineages at the base of the family (Nelsen et al. Reference Nelsen, Lücking, Grube, Mbatchou, Muggia, Rivas Plata and Lumbsch2009, Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011, Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ) and now redispositioned in the genera Bogoriella and Novomicrothelia (Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ).
Notably, the Trypetheliaceae were the subject of several detailed studies about ascospore anatomy and ontogeny (Sweetwood et al. Reference Sweetwood, Lücking, Nelsen and Aptroot2012) and pigment chemistry (Mathey & Hoder Reference Mathey and Hoder1978; Mathey Reference Mathey1979; Mathey et al. Reference Mathey, Steffan and Steglich1980), as well as potential pharmaceutical properties of their chemical compounds (Manojlovic et al. Reference Manojlovic, Vasiljevic, Gritsanapan, Supabphol and Manojlovic2010). Ecological and ecogeographical papers with details on Trypetheliaceae were published by Komposch & Hafellner (Reference Komposch and Hafellner2000) on canopy and savanna species in Venezuela, Aptroot et al. (Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008) on pyrenocarpous lichens in Costa Rica, Rivas Plata et al. (Reference Rivas Plata, Lücking and Lumbsch2008) on the correlation between family-level diversity and environmental parameters related to the conservation status of tropical forest ecosystems, and Aptroot (2009 Reference Aptrootb ) on diversity and endemism of Trypetheliaceae and Pyrenulaceae in Malaysia.
The systematics of the family regained interest with the advent of molecular phylogenetic methods. An initial study by Del Prado et al. (Reference Del Prado, Schmitt, Kautz, Palice, Lücking and Lumbsch2006) demonstrated the placement of Trypetheliaceae within Dothideomycetes, and phylogeny of the family was refined through subsequent work by Nelsen et al. (Reference Nelsen, Lücking, Grube, Mbatchou, Muggia, Rivas Plata and Lumbsch2009, Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011, Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014) and Lücking et al. (2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ), showing that lichenized species of Arthopyrenia and Mycomicrothelia also belong here, and laying the groundwork for a much revised genus and species concept (Aptroot et al. 2013 Reference Aptroot, Nelsen and Parnmena ; Hyde et al. Reference Hyde, Jones, Liu, Ariyawansha, Boehm, Boonmee, Braun, Chomnunti, Crous and Dai2013; Aptroot & Cáceres Reference Aptroot and Cáceres2014; Nelsen et al. Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014; Luangsuphabool et al. Reference Luangsuphabool, Lumbsch, Aptroot, Piapukiew and Sangvichien2016; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). This also stimulated further inventory work in several parts of the tropics, with the discovery of many new taxa (Aptroot & Cáceres Reference Aptroot and Cáceres2013, Reference Aptroot and Cáceres2016; Aptroot et al. 2013 Reference Aptroot, Menezes, Lima, Xavier-Leite and Cáceresb , 2016 Reference Aptroot, Ertz, Etayo, Schumm and Weerakoona , Reference Aptroot, Mendonça, Santos, Reis Silva, Martins, Gumboski, Vidigal and Cáceresb ; Lima et al. Reference Lima, Maia, Aptroot and Cáceres2013; Córdova-Chávez et al. Reference Córdova-Chávez, Aptroot, Castillo-Camposa, Cáceres and Pérez-Pérez2014; Weerakoon & Aptroot Reference Weerakoon and Aptroot2014; Flakus et al. Reference Flakus, Kukwa and Aptroot2016; Luangsuphabool et al. Reference Luangsuphabool, Lumbsch, Aptroot, Piapukiew and Sangvichien2016; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Benatti, Binh, Gueidan, Gutiérrez, Jungbluth, Lumbsch and Marcellib ). The order Trypetheliales was eventually established for the family (Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Hyde et al. Reference Hyde, Jones, Liu, Ariyawansha, Boehm, Boonmee, Braun, Chomnunti, Crous and Dai2013), and recently a second family, Polycoccaceae, including lichenicolous fungi, was included in this order (Ertz et al. Reference Ertz, Diederich, Lawrey, Berger, Freebury, Coppins, Gardiennet and Hafellner2015).
In the present work, we provide a concise synopsis of the family Trypetheliaceae to account for the numerous changes that have taken place based on recent phylogenetic studies and a new assessment of the delineation of genera and species within the family. Together with the other papers included within this special issue, this should give a complete overview of the current knowledge of the family. We realize that this synopsis cannot be a definitive monographic treatment; the gaps in knowledge about this megadiverse group of tropical lichens are too large and still many taxa are known only from few or single collections. While available sequence data and revision of phenotypic characters suggest that the concept presented here reflects genus and species boundaries more accurately than previous treatments, we expect that this synopsis will also trigger many further studies. These, we anticipate, would be based on a much more thorough sampling of individual geographical regions, which will unearth novel taxa and also require further revision of Trypetheliaceae species. Furthermore, additional types of old names to be assigned to this family will undoubtely surface.
Material and Methods
Identification and descriptive work was carried out in Soest, using an OLYMPUS SZX7 stereomicroscope and an OLYMPUS BX50 compound microscope with interference contrast, connected to a NIKON Coolpix digital camera, as well as at the Field Museum in Chicago, using a LEICA MS5 dissecting microscope and a ZEISS Axioskop 2 compound microscope, in part connected to JENOPTIK ProgRes C3 and C5 digital microscope cameras. Further digital images were taken with CANON Powershot SX20IS, NIKON F301 and Sony Alpha 33 DSLR digital cameras.
Sections of thalli and ascomata were cut by hand with a razor blade and examined with squash preparations on material mounted in water, KOH and Lugol’s solution. All measurements are given for water mounts. Illustrated thin sections were made by Dr Felix Schumm using a Wild M3 stereomicroscope, an Olympus BX51 compound microscope with interference contrast, a Canon EOS 40D camera with MP-E 65 mm and a Mic HM 560 cryotome. Specimens were measured and illustrated in tap water, unless marked with IKI (mounted in iodine) or LCB (mounted in lactophenol cotton blue).
Chemistry was analyzed by means of a UV lamp and K-tests using 10% KOH solution, both on thallus and ascoma portions including medulla and on sections. Many specimens, including most types (where not previously analyzed), were also investigated using thin-layer chromatography (TLC) in solvents A and/or C (Orange et al. Reference Orange, James and White2010).
Well over 2000 specimens were examined for this study, including most types. Where possible, types were studied through loans or visits to the corresponding major herbaria; in some cases we relied on digitized type specimens available through the Global Plants Initiative (GPI) at JSTOR (https://plants.jstor.org). To complete our own 352 images of type specimens accumulated through studies over the past decades, we added 54 partial images of selected type specimens posted on JSTOR to the plates published here to highlight specific morphological features required for taxon identification, reproduced with permission from the curators at BM (Figs 31E, 34H, 41L, 44F, 49F, 51I, 52K), G (Figs 13F, 15B, 16J & K, 18C & K, 19G, Reference Eriksson23C & E, 26G, 28J, 29B & F, Reference Harris32H, 33B, 35B & I, 37A, G & H, 39G, 44K & L, 52F, 53I, 54F & G, 57G), H (Figs 13D & I, 15H, 18G, 20E, 21A, 27F, 35H, 38I, 43A, B & J, 44B, 48A, 49G), and S (Figs 31D, 39I, 42H, 54A). Quite a number of additional type species not shown here are available through GPI at JSTOR and can be consulted there in case of doubts. Except for types, specimens studied are not cited in full; instead, we summarize the known distribution and give selected new country records. Distributions are given in geographical order following the Flora Neotropica style, from NW to SE, starting with North America and ending with Australia and Oceania.
The numerous collections made by Charles Wright in the 19th century in Cuba, which have been studied (and distributed in exsiccatae) by Tuckerman (Reference Tuckerman1864), Nylander (Reference Nylander1876), and Müller (Reference Müller1885, Reference Müller1894) was an important reference. The citation of these exsiccatae or the corresponding collections has been somewhat ambiguous in the literature, usually giving Wright as editor or with the exsiccate number as his collection number. However, Wright did not perform identification work on his lichen collections; instead they were organized and edited by Tuckerman (as Caroli Wright Lichenes Insulae Cubae or Lich. Ins. Cub.) and Müller (as Graphideae Cubenses (a cl. C. Wright lectae et a cl. W. Nylander determinatae) or Graph. Cub., as Lichenes Cubenses (a cl. Wright lecti et a cl. W. Nylandero pr. p. determinati) or Lich. Cub., and as Verrucariae Cubenses or Verr. Cub.) (http://indexs.botanischestaatssammlung.de)) using a numbering system separate from Wright’s collection numbers. Wright’s original collection numbers are usually not available for his Cuban lichen collections (if he numbered them at all) and they will be cited as Wright s. n.
For the general section, a selection of morphological and anatomical images was provided by Felix Schumm and for the taxonomic section we also included images of five new species of Astrothelium described elsewhere in this issue (Luangsuphabool et al. Reference Luangsuphabool, Lumbsch, Aptroot, Piapukiew and Sangvichien2016), provided by Ek Sangvichien.
Morphology, Anatomy and Chemistry
Morphological, anatomical and chemical characters in Trypetheliaceae are variable and, as in other species-rich families of tropical lichens such as Graphidaceae and Pyrenulaceae, provide a broad array of features for taxonomic and systematic purposes, which in the past have not been fully explored due to the lack of a phylogenetic framework to assess the validity of individual characters, in particular the external morphology of thallus and ascomata. This framework is now available (Nelsen et al. Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ); it shows that morphological variation changes with evolutionary history within the family. The typical and often unique characters of Trypetheliaceae, particularly the astrothelioid ascospores and the thin, net-like hamathecium, often with coarse inspersion, are mostly developed in derived lineages, whereas the more basal lineages are not particularly different from similar, lichenized forms in other, often unrelated orders, such as Monoblastiales, Strigulales and even certain Pyrenulales.
Thallus morphology
Various characters can be assessed with regard to thallus morphology, including whether the thallus is (partially) endoperidermal, the presence of a cortex, the development of surface structures, colour and the associated pigment chemistry (see also below). Basal lineages in the family, such as former species of Arthopyrenia and Mycomicrothelia now dispositioned in the genera Bogoriella, Constrictolumina, and Novomicrothelia, as well as the genera Dictyomeridium, Distothelia, Polymeridium, and Pseudopyrenula, almost invariably have an ecorticate, whitish thallus that is often endoperidermal (Fig. 2A–F), with scattered photobiont cells; further structures except for a black prothallus line (Fig. 2F) are usually absent and the ascomata are usually exposed and black (Fig. 2A–F). Except for Pseudopyrenula, this correlates with the presence of non-astrothelioid ascospores with thin or uniformly thickened septa and rectangular to rounded, but not diamond-shaped, lumina (see below).
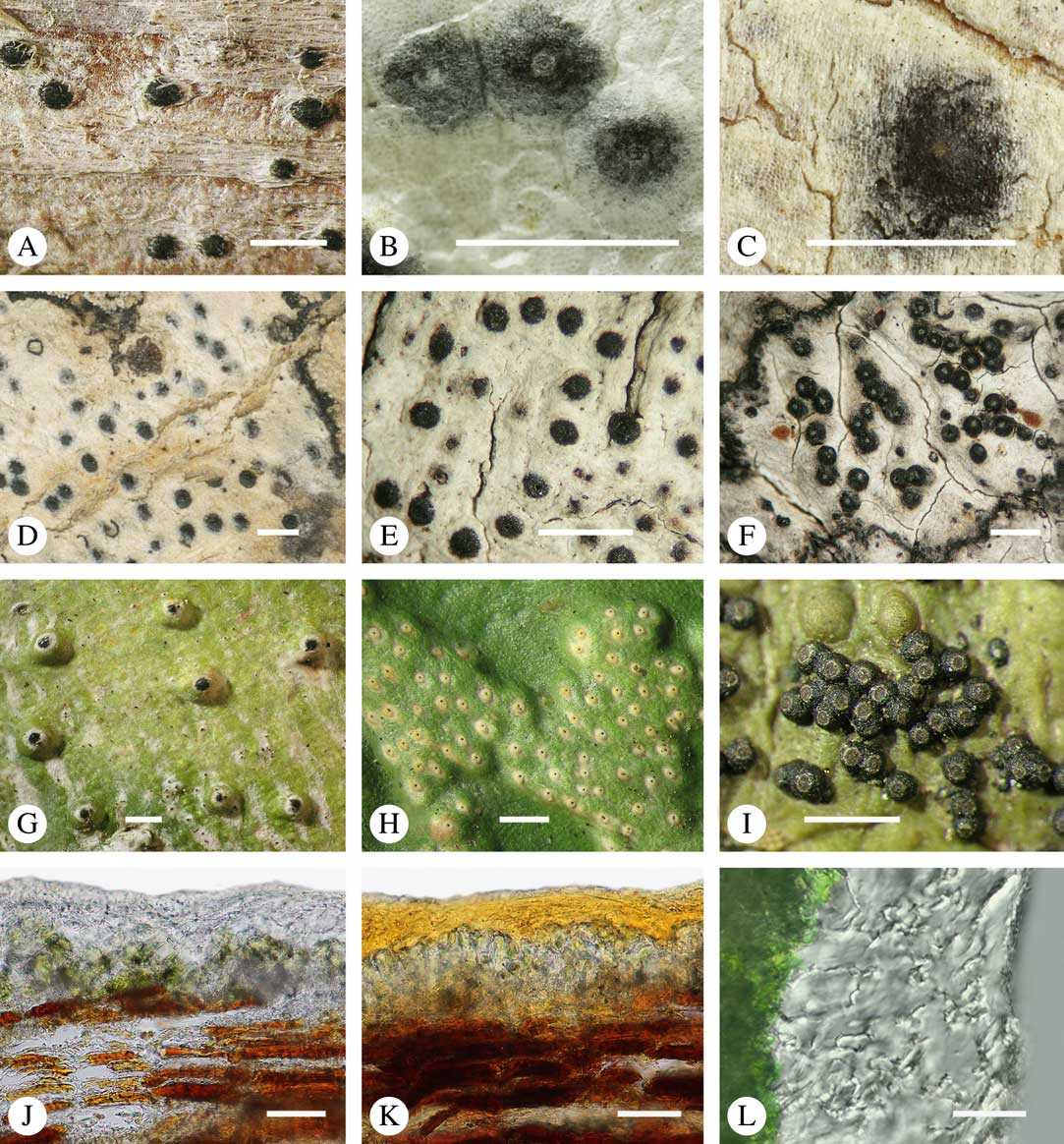
Fig. 2 Thallus morphology and anatomy in Trypetheliaceae. A–C, endoperidermal thallus with outline of periderm cells visible; A, Bogoriella modesta; B, Constrictolumina majuscula; C, Bogoriella megaspora. D–F, ecorticate, whitish thallus with black prothallus line; D, B. miculiformis; E, Polymeridium catapastum; F, P. subcinereum. G–I, corticate, (olive-)green thallus; G, Astrothelium megaspermum; H, A. intermedium; I, Nigrovothelium tropicum. J–L, anatomy of epiperidermal thallus showing gelatinized cortex, photobiont layer, and periderm; J & K, Astrothelium tuberculosum; L, Architrypethelium grande. Scales: A–I=1 mm; J–L=20 µm. (Images B, C, J & K by F. Schumm).
In more derived lineages, the thallus is almost invariably corticate and mostly has an olive-green colour when fresh (Fig. 2G–I). The cortex is epiperidermal and often massive, consisting of a loose network of anastomosing hyphae (similar to the hamathecium, but more irregular; see below) embedded in a gelatinous matrix and becoming more dense and orange-yellow in K; the photobiont layer and medulla (if developed) are either epiperidermal or partly endoperidermal (Fig. 2J–L).
While most species have a smooth to uneven thallus (Fig. 3A–C), a particular feature in Trypetheliaceae rarely found in other families such as Graphidaceae, is the formation of a verrucose-papillose to bullate or folded thallus surface (Fig. 3D–I). In section, these structures either include amorphous white crystal masses or mainly consist of a strongly thickened cortex, with anastomosing hyphae embedded in a gelatinous matrix (Fig. 3J & K). In these cases, in which the thallus might even appear squamulose (Fig. 3I), the photobiont layer is often vertically arranged (Fig. 3J & K), suggesting adaptation to high light intensities, for example in the rainforest canopy where such Trypetheliaceae primarily occur. The available phylogenetic data suggest that such thallus structures are species-specific, although habitat-induced variation has been shown in other groups, such as Graphis pseudocinerea in Graphidaceae (Lücking et al. Reference Lücking, Seavey, Common, Beeching, Breuss, Buck, Crane, Hodges, Hodkinson and Lay2011).

Fig. 3 Thallus surface morphology in Trypetheliaceae. A–C, surface smooth to uneven, following the contours of the bark; A, Astrothelium nitidiusculum; B & C, A. rufescens. D, surface papillose-verrucose, A. papillosum. E, surface bullate, A. tuberculosum. F & G, surface bullate-folded; F, A. megalophthalmum; G, A. versicolor. H & I, surface verrucose-bullate-squamulose; H, Aptrootia elatior; I, Astrothelium puiggarii. J & K, section through bullate ‘squamules’ showing gelatinized cortex and vertically arranged photobiont layer, A. puiggarii. L, true gall formation, A. ceratinum. Scales: A–I & L=1 mm; J & K=20 µm. (Images J & K by F. Schumm).
Independent of the surface structure, many Trypetheliaceae seem to produce thickened bark, which suggests gall formation. This is particularly seen in relation to the ascomata, which often emerge from beneath the upper periderm layers of the bark (see below). However, galls in the strict sense should produce abnormal growth of the periderm, in particular the inner, living layers, viz. the cork cambium (phellogen), which due to its meristematic nature is predestined to produce abnormal growth reactions. In most Trypetheliaceae, the endoperidermal thallus portion is developed only in the upper, dead layers of the periderm (Fig. 3J & K), the cork (phellem), and rather than causing growth of the periderm, the thickening appears to be caused by uplifting of the upper layers, particularly above the ascomata (see below). However, some cases have been observed with true gall formation due to growth of the cork (Fig. 3L). Further studies, particularly on the ontogeny of the thallus and ascomata, are needed to properly understand this phenomenon and its biological implications.
Ascoma morphology
The ascomata in Trypetheliaceae provide some of the richest character sets for taxonomic purposes but at the same time have provided stumbling blocks to properly recognizing and classifying the variation encountered in this family. Several characters can be assessed when analyzing ascoma morphology: 1) arrangement of the ascomata relative to the thallus, 2) orientation of the ostioles, 3) ascoma emergence, 4) degree of thallus cover, 5) configuration of the ostiolar area, and 6) pigmentation (see also below).
Ascomata arrangement can vary from regularly dispersed over the thallus (solitary), to confluent, aggregate, and pseudostromatic (Fig. 4A–L), with the ostioles apical, eccentric (lateral) or fused, in which case the shared portion of the ostiole might be apical or lateral (Fig. 5A–L). Ascoma arrangement can best be assessed when comparing distances between individual ascomata. In dispersed ascomata (Fig. 4A–C), random measurement of paired distances will result in a broad, continuous distribution of distance measures. In contrast, aggregate or pseudostromatic ascomata (Fig. 4E–L) will give a bimodal distribution of randomly measured distances, reflecting small distances between ascomata belonging to the same cluster versus large distances between ascomata belonging to different clusters. In specimens with partially confluent ascomata (Fig. 4D), the distribution will be intermediate between the two extremes. To distinguish between aggregate and pseudostromatic clusters, pseudostromata are defined here as areas that differ from the surrounding thallus in either structure (e.g. being strongly emergent; Fig. 4E), colour (often whitish or dominated by black excipular tissue; Fig. 4F–K), or chemistry (e.g. pigment or lichexanthone limited to the pseudostromata; Fig. 4L), or a combination of these. Thus, aggregate ascomata in which the associated thallus is similar to the surrounding thallus and which lack specific features are not considered pseudostromatic. This applies in particular to fused ascomata with a shared ostiole in many species of Astrothelium, in which the thallus associated with the ascomata is not particularly differentiated.

Fig. 4 Ascoma arrangement in Trypetheliaceae. A, solitary, dispersed, Pseudopyrenula subnudata; B, solitary, dense, Astrothelium floridanum; C, solitary, crowded, Nigrovothelium tropicum; D, solitary to confluent, Astrothelium nitidiusculum; E, pseudostromatic by emergence, A. intermedium; F, pseudostromatic by colour contrast, A. norisianum; G, with fused ostioles, fused ascomata dispersed, A. eustomum; H, with fused ostioles, fused ascomata pseudostromatic, A. interjectum; I, pseudostromatic with exposed ascomata and linear-reticulate pseudostromata, A. neogalbineum; J, pseudostromatic with exposed, prominent to sessile ascomata, Bathelium mastoideum; K, pseudostromatic with erumpent to prominent pseudostromata with white cover, Astrothelium sphaerioides; L, pseudostromatic with prominent to sessile pseudostromata covered by pigment, Trypethelium eluteriae. Scales=1 mm.

Fig. 5 Ascoma disposition and orientation of ostioles in Trypetheliaceae. A–C, ostioles apical, separate, A, Polymeridium subcinereum; B, Astrothelium nitidiusculum; C, Trypethelium subeluteriae. D, ostioles apical, with ostiolar areas forming lobate pattern, T. astroideum. E, ostioles apical, ostiolar area separated from covering thallus by a split, Marcelaria purpurina. F–I, ostioles lateral, eccentric, separate, pointing in various directions, F, Astrothelium gigasporum; G, A. scorizum; H, Polymeridium simulans; I, Dictyomeridium proponens. J–L, ostioles lateral, eccentric, centrally fused to form a shared channel leading to various chambers, J, Astrothelium macrocarpum; K, A. marcidum; L, A. intermedium. Scales=1 mm.
For ascoma emergence, we apply here the same criteria and terminology as used in Graphidaceae and the genus Graphis (Lücking Reference Lücking2009), since this feature has often been imprecisely treated in the literature and the term emergent has been used for morphologies ranging from slightly emergent to sessile. Here we define four states: 1) immersed, if more than 3/4 of the ascoma is beneath the thallus level (Fig. 6A); 2) erumpent, if more than 1/4 to 1/2 of the ascoma is above thallus level (Fig. 6B); 3) prominent, if more than 1/2 of the ascoma is above thallus level and the base is expanded outwards to vertical (Fig. 6C); 4) sessile, if more than 3/4 of the ascoma is above thallus level and the base is constricted (Fig. 6D). This terminology also applies to pseudostromata as a whole. A special feature of some Trypetheliaceae is that the ascomata can be deeply immersed, well below the thallus and often deep into the periderm, up to 2 mm and occasionally up to 5 mm below the thallus surface. This is not restricted to a certain taxonomic group but occurs, for example, in Astrothelium (Fig. 6A), Polymeridium, and Pseudopyrenula. Ascomata that are deeply immersed in the periderm are rare in lichens, otherwise mostly known from Pyrenulaceae.
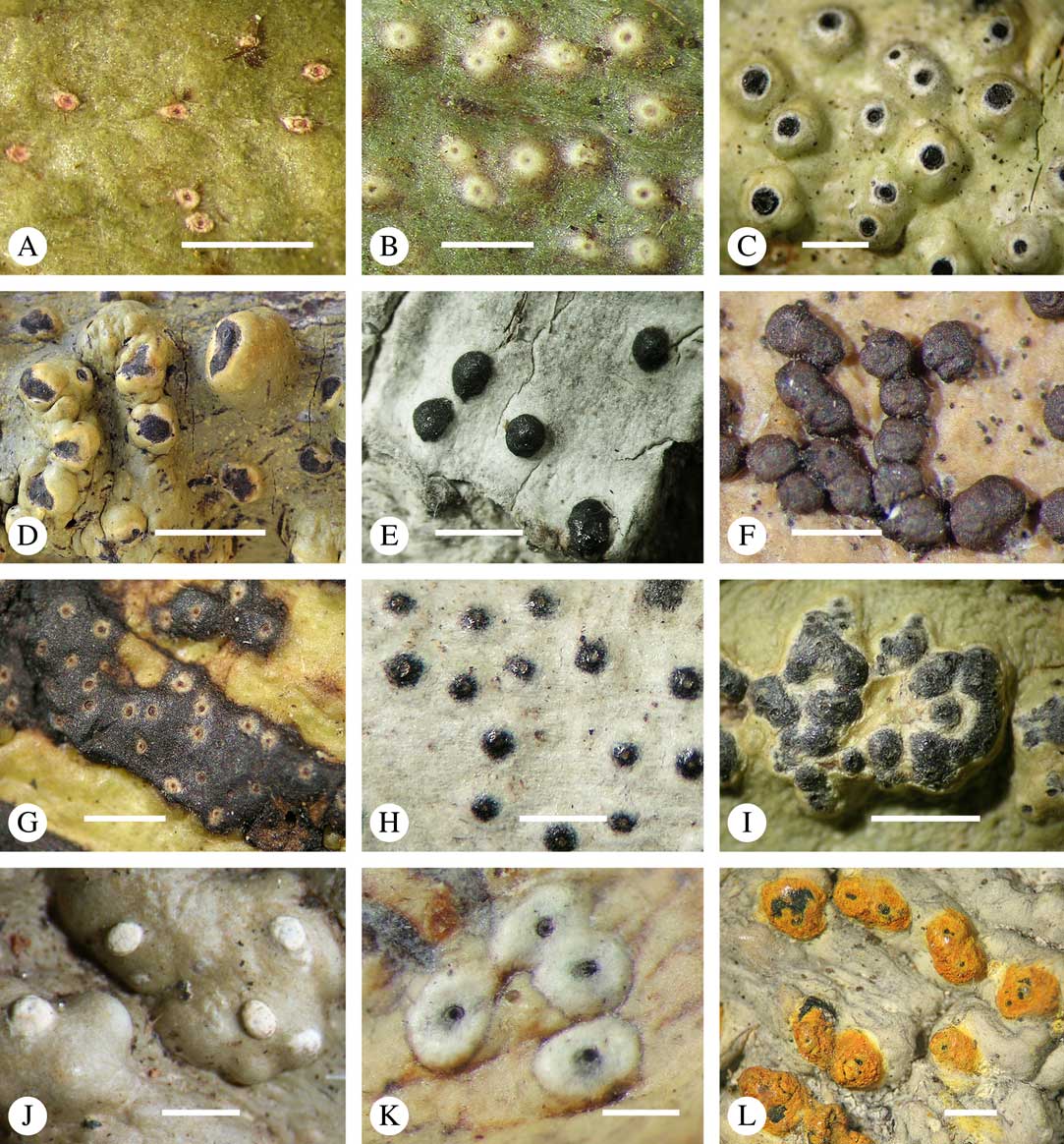
Fig. 6 Ascoma emergence and thallus cover in Trypetheliaceae. A, immersed, Astrothelium calosporum. B, erumpent, A. nitidiusculum. C, prominent, A. megaspermum. D, sessile, A. fallax. E–G, fully exposed, without thallus cover; E, Pseudopyrenula dubia; F, Bathelium porinosporum; G, Astrothelium infuscatulum. H, with basal thalline cover, Polymeridium amyloideum. I, with lateral thalline cover, Astrothelium straminicolor. J, with complete thalline cover, A. megeustomum. K, whitish and exposed ostiolar area, A. fijiense. L, with pigment cover, A. macrocarpum. Scales=1 mm.
With regard to the degree of thallus cover, the following states are distinguished, also based on classifications developed for Graphidaceae and Graphis (Lücking Reference Lücking2009): 1) absent, ascomata are fully exposed from the base (Fig. 6E–G); 2) basal, only the base up to c. 1/4 of the ascoma is covered (Fig. 6H); 3) partial, the ascomata are covered with thallus up to about 3/4 except the area surrounding the ostiole (Fig. 6I); 4) complete, only the ostiole or ostiolar spot remains visible (Fig. 6J). This scheme also applies to pseudostromata or thallus warts with aggregated ascomata (Fig. 6K & L). The configuration of the ostiolar area is also an important taxonomic character, including its visibility as a narrow dot (Fig. 6B) or broad spot (Fig. 6C & D), its colour (mostly black, sometimes white; Fig. 6J), and the presence of a differently coloured, ring-shaped band around the ostiole (usually whitish), which can be sharp and regular (Fig. 6C) or diffuse and often irregular (Fig. 6B & G). In some cases, the entire ascoma is covered with a layer different from the thallus, which is often the case in pseudostromata but can also be found in species with solitary ascomata (Fig. 6K).
Ascoma anatomy
The internal anatomy of the ascomata including the pseudostromata has not been well studied, even though it has the potential to provide useful taxonomic and systematic characters. For instance, Makhija & Patwardhan (Reference Makhija and Patwardhan1993) made a typology of pseudostroma configurations found in Trypethelium, but more data are needed to fully assess the importance of their categories. Letrouit-Galinou (Reference Letrouit-Galinou1957, Reference Letrouit-Galinou1958) also classified the ascomata of the artificial genus Laurera according to wall structures which have subsequently been used, in part, to disperse the species studied over various lineages, including Marcelaria, Bathelium, and Astrothelium. Notably, the species belonging to Marcelaria were placed in different categories by Letrouit-Galinou (Reference Letrouit-Galinou1957, Reference Letrouit-Galinou1958), although the main difference is the nature of the pigments present on and in the pseudostromata (Schumm & Aptroot Reference Schumm and Aptroot2012; Aptroot et al. 2013 Reference Aptroot, Nelsen and Parnmena ).
The ascomata in Trypetheliaceae develop either superficially above the periderm or emerge from beneath the periderm and while this appears to be species-specific, both epiperidermal and endoperidermal ascomata can be found in different species of the same genus. For example, Architrypethelium grande, Astrothelium megaspermum, A. porosum, A. puiggarii, Marcelaria purpurina, Nigrovothelium tropicum and Trypethelium eluteriae all produce epiperidermal ascomata or pseudostromata, with the periderm remaining below the ascomata and not included in the covering layers (Fig. 7A–F, J–L). In contrast, in Astrothelium tuberculosum, Constrictolumina majuscula and Pseudopyrenula diluta the ascomata are endoperidermal (Fig. 7G–I). The ascoma wall is (1–)2–3-layered with a brown to carbonized excipulum (Fig. 7B–K), often with a cortical layer similar to the thallus cortex, which might be thin (Fig. 7B) to thick (Fig. 7E & F, K & L) and in the case of pseudostromata with an amorphous medullary layer (Fig. 7C); in species such as Astrothelium megaspermum, the upper wall also contains a brownish ‘medullary’ layer which might be homologous to the involucrellum in other pyrenocarpous taxa.
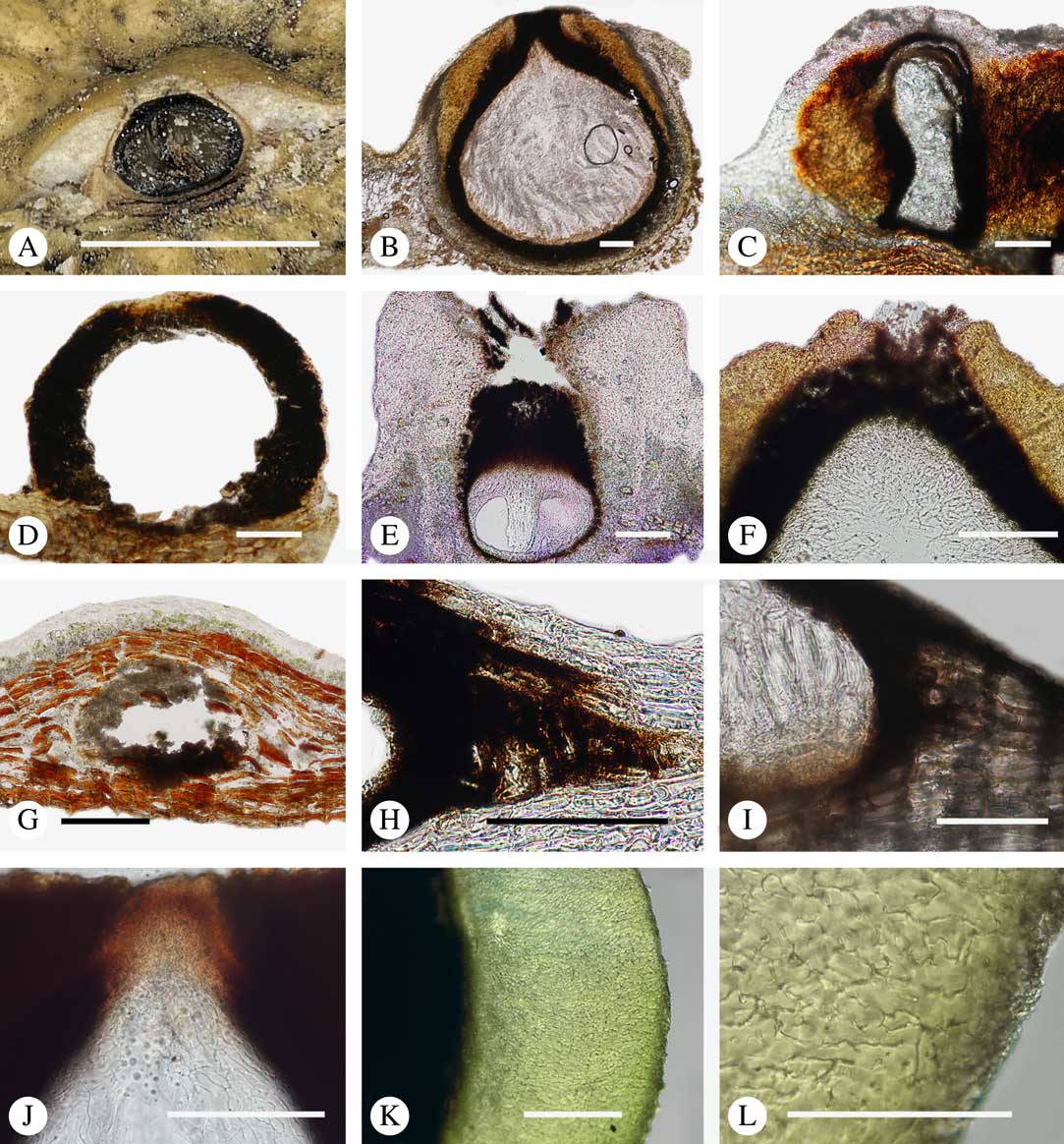
Fig. 7 Ascoma anatomy in Trypetheliaceae. A–C, epiperidermal ascomata with thallus cover; A & B, Astrothelium megaspermum; C, A. porosum. D, epiperidermal ascoma with simple wall, Nigrovothelium tropicum. E–F, epiperidermal ascoma with thick cortical layer, E, Marcelaria purpurina; F, Astrothelium puiggarii. G–I, endoperidermal ascomata, G, A. tuberculosum; H, Pseudopyrenula diluta; I, Constrictolumina majuscula. J, ostiolar area, Trypethelium eluteriae. K–L, gelatinized cortical layer on ascoma, Architrypethelium grande. Scales: A=1 mm; B–K=100 µm; L=50 µm. (Images A-I by F. Schumm).
Ascoma anatomy provides a very rich set of characters but we have not yet fully explored these features here as we await more detailed studies. We expect that ascoma and pseudostroma wall anatomy, explored within a phylogenetic framework, will help to further refine genus and species concepts in Trypetheliaceae.
Hamathecium, asci and ascospores
Most Trypetheliaceae have a rather uniform hamathecium, consisting of thin, straight, much branched and anastomosing paraphyses forming a network embedded in a gelatinous matrix (Fig. 8A). In basal lineages, the paraphyses are usually thicker and less anastomosing and the gelatinous matrix is less obvious. Hymenial inspersion is common throughout the family and usually occurs as large, irregular, colourless oil droplets lining the paraphyses (Fig. 8B) but it can also form a more amorphous, sometimes dirty yellow infusion in basal lineages such as Pseudopyrenula; in some species, the droplets contain anthraquinones and react with K. In most species, inspersion is found along the ostiolar area while the hamathecium is clear. For instance, Harris (Reference Harris1995) suggests muriform-spored species of Bathelium have an inspersed hamathecium, whereas the inspersion in most of these species is developed only around the ostiole. The taxonomic importance of hamathecium inspersion (i.e. of the entire hamathecium) has been neglected, except in a few cases, but phylogenetic studies suggest that it is species-specific (Nelsen et al. Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). Unfortunately most descriptions, especially of historical names, do not mention this character and hence the status of names for which types have not been available is difficult to resolve. On the other hand, inspersion is usually preserved even in old collections as long as hymenium material is present.
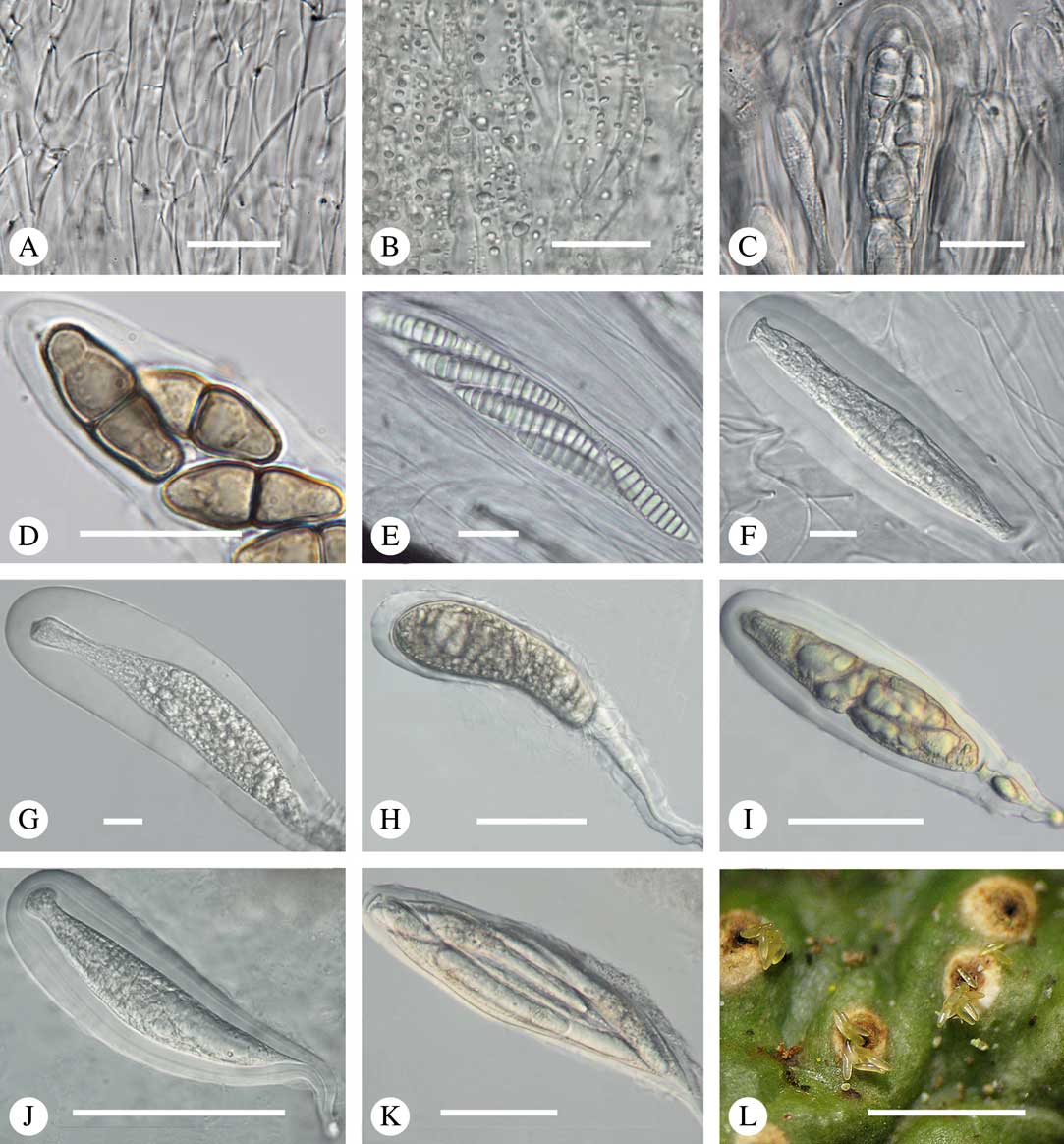
Fig. 8 Hamathecium and asci in Trypetheliaceae. A, anatomosing, net-like filaments, Architrypethelium nitens. B, inspersion with oil droplets lining the filaments, Astrothelium megaspermum. C–E, mature ascus with ascospores; C, Constrictolumina majuscula; D, Novomicrothelia oleosa; E, Trypethelium eluteriae. F–K, young and immature asci showing wall layers and apical apparatus; F, Aptrootia terricola; G & H, A. robusta; I, Architrypethelium nitens; J & K, Astrothelium megaspermum. L, ejection of ascospores through ostiole, A. megaspermum. Scales: A–G=20 µm; H–K=100 µm; L=1 mm. (Images C & D by F. Schumm).
Asci in Trypetheliaceae are typically fissitunicate (Eriksson Reference Eriksson1981; Aptroot Reference Aptroot1991); their internal structure is best observed in species with larger ascospores, as long as the ascospores are young or immature (Fig. 8C–K). Typically the asci have a distinct foot (Fig. 8H & J). The elastic inner wall (endotunica) appears multilayered (Fig. 8J) and the tholus contains a non-staining ring structure. Like ascoma anatomy, ascus structure has not yet been fully studied and explored for taxonomic purposes in this family, but may be helpful to delimit especially the basal lineages.
Besides ascoma morphology, ascospore type is the second most important character complex in Trypetheliaceae (Figs 8L, 9A–L). While in the past ascospore septation was mostly used to separate lineages at the genus level, it is now obvious that the nature of the septa and walls provide the most critical characters at a higher taxonomic level (Fig. 9A–L) whereas septation per se might separate species but varies considerably at genus level. Astrothelioid ascospores are typical of this family , forming secondary wall thickenings that make the lumina diamond-shaped (Fig. 9G). Very similar ascospores occur in some non-lichenized families such as Massariaceae, which Eriksson (Reference Eriksson1981) used to suggest that they should form part of Trypetheliaceae. In fact these families are not closely related to Trypetheliaceae (Schoch et al. 2009 Reference Schoch, Crous, Groenewald, Boehm, Burgess, De Gruyter, de Hoog, Dixon, Grube and Gueidanb ; Hyde et al. Reference Hyde, Jones, Liu, Ariyawansha, Boehm, Boonmee, Braun, Chomnunti, Crous and Dai2013). In species of Trypetheliaceae with muriform ascospores, in which the lumina are small and their morphology is difficult to assess, the young ascospores in lineages with an astrothelioid ascospore type undergo a distinctly astrothelioid stage (Fig. 9J & K) (Sweetwood et al. Reference Sweetwood, Lücking, Nelsen and Aptroot2012). Almost without exception, a given genus has either astrothelioid or non-astrothelioid ascospores. The latter can be assigned to various types, including the multiseptate ascospores with slightly thickened but not astrothelioid septa in Trypethelium (Fig. 9E) and Viridothelium, or the septate to muriform ascospores in Bathelium (Fig. 9F), Polymeridium (Fig. 9D) and Dictyomeridium. In Architrypethelium the ascospores are principally astrothelioid when immature with a reduced endospore when mature, becoming very large with few septa and often including characteristic, needle-shaped crystals (Fig. 9H). Basal lineages formerly placed in Arthopyrenia and Mycomicrothelia now positioned in the genera Bogoriella, Constrictolumina, and Novomicrothelia have almost invariably 1-septate ascospores with thin or thickened walls and septa but are never astrothelioid (Fig. 9A–D); in Constrictolumina these may form secondary invaginations (partial septa), whereas in Bogoriella and Novomicrothelia, ornamented walls may occur. Distothelia has particular ascospores with strong distal thickenings and the lumina near the central septum (Fig. 9C).
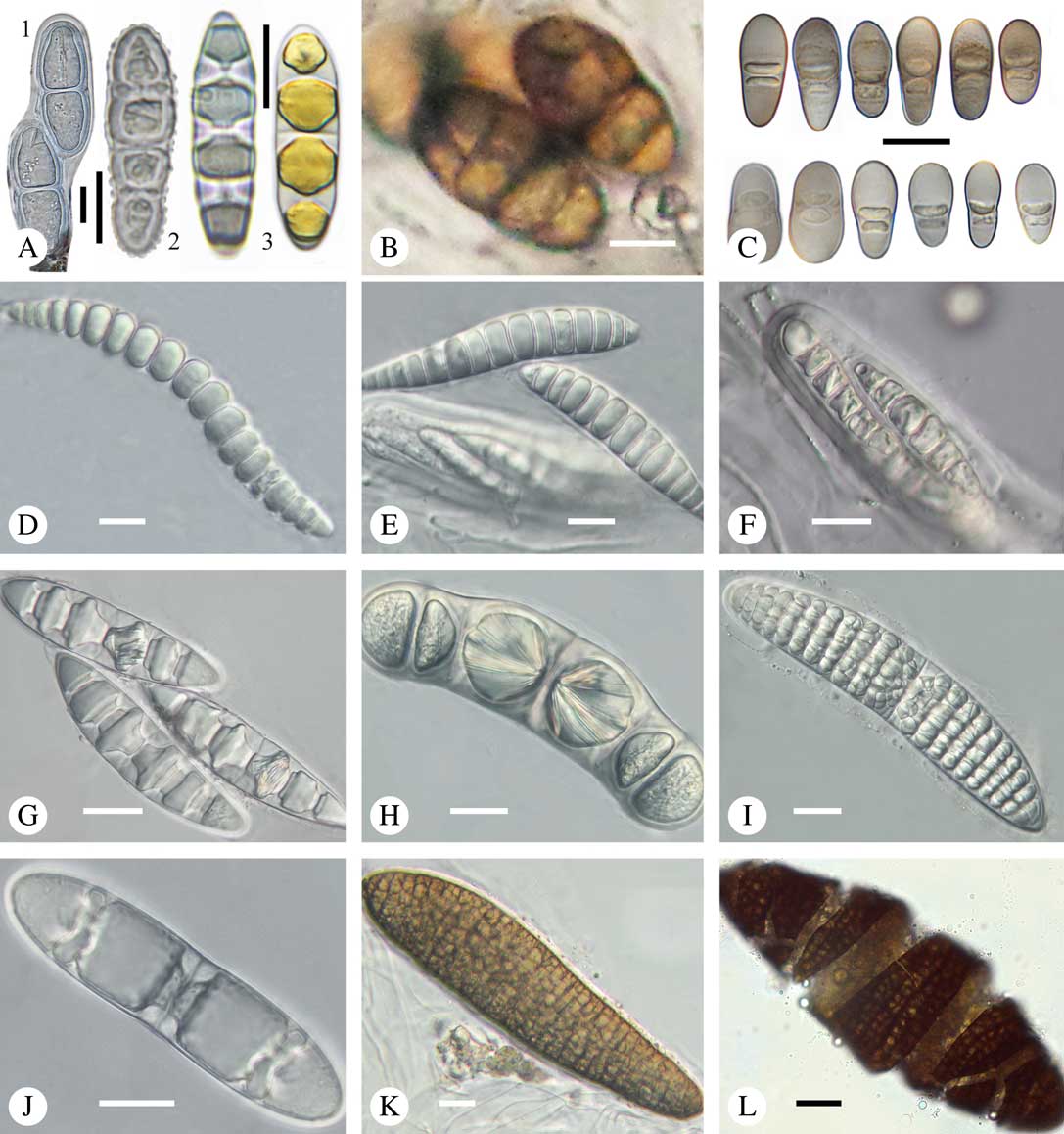
Fig. 9 Ascospores in Trypetheliaceae. A1, Constrictolumina malaccitula; A2, C. majuscula, with ornamented wall; A3, Pseudopyrenula diluta, in part with yellow content in lumina; B, Bogoriella decipiens; C, Distothelia angulata, with thick terminal walls; D, Polymeridium pleurothecium, thin-walled; E, Trypethelium subeluteriae, thin-walled; F, Bathelium nigroporum, thin-walled; G, Astrothelium diplocarpoides, with diamond-shaped lumina; H, Architrypethelium nitens, with crystal-like structures; I, Astrothelium megaspermum, muriform with rounded to diamond-shaped lumina; J & K, Aptrootia terricola, muriform, brown; in J young, still hyaline ascospore showing astrothelioid stage; L, A. robusta, muriform, brown; outer wall breaking apart. Scales: A–F=10 µm; G–L=20 µm. (Images A & C by F. Schumm).
Most species in Trypetheliaceae have colourless ascospores, but brown ascospores are found in the basal genera Bogoriella (Fig. 9B) and Novomicrothelia, in most species of Architrypethelium, and in Aptrootia (Fig. 9K & L), in which the large, muriform ascospores also form a peculiar, easily breakable outer shell (Fig. 9L) (Sweetwood et al. Reference Sweetwood, Lücking, Nelsen and Aptroot2012).
Chemistry
The chemistry of the Trypetheliaceae is rather simple compared to other tropical, crustose families, such as Graphidaceae (Rivas Plata et al. Reference Rivas Plata, Lücking and Lumbsch2012; Lumbsch et al. Reference Lumbsch, Parnmen, Kraichak, Papong and Lücking2014), and secondary substances are restricted to two main groups: xanthones and pigments, mostly anthraquinones.
Lichexanthone is the most commonly found xanthone in the family. It reacts UV+ yellow and can be present on the ascomata, pseudostromata and/or the ostioles, and/or on the thallus (Fig. 10A), rarely in the medulla. The taxonomic value of the presence of lichexanthone has been disputed; species were traditionally separated based on this feature, in others it was considered infraspecific variation (e.g. Harris Reference Harris1995). Based on evidence from phylogenetic data (Nelsen et al. Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014: Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ), we consider the presence and location of lichexanthone species-specific. 1,8-dihydroxy-3,6-dimethoxyxanthone (coronatone) is less often present; it reacts UV+ orange but might be masked by the present of lichexanthone. The presence and proportion of both substances in UV+ taxa needs to be studied further.

Fig. 10 Secondary chemistry and pigments in Trypetheliaceae. A, lichexanthone, Astrothelium phlyctaena. B–G, yellow-orange to red anthraquinones on ascomata and pseudostromata; B, A. croceum; C, A. kunzei; D, Marcelaria cumingii; E, M. purpurina; F, Astrothelium aurantiacocinereum; G, Trypethelium eluteriae. H, pseudostromata with yellow internal and orange and red external anthraquinone pigments, T. astroideum; I, fused ostiole with red quinone isohypocrellin, Astrothelium purpurascens; J, ascomata with pockets of red pigment, A. sierraleonense. K & L, pseudostromata with internal yellow and red pigment; K, A. degenerans; L, A. sanguinarium with isohypocrellin. Scales=1 mm.
Various quinones, mostly anthraquinones, are regularly present on or in the ascomata or pseudostromata, and/or the ostioles and/or on or in the thallus (Fig. 10B–L), sometimes even in the ascoma wall, the hamathecium, or inside the ascospores (Fig. 9A). Many anthraquinones have not yet been identified, especially when quantities are low (e.g. when restricted to the ostiole or ascospore). However, the following substances have been classified (Mathey & Hoder Reference Mathey and Hoder1978; Mathey Reference Mathey1979; Mathey et al. Reference Mathey, Steffan and Steglich1980):
Parietin (=physcione) is the most common yellow-orange pigment, for example in Astrothelium aeneum, A. croceum (Fig. 10B), Marcelaria cumingii (Fig. 10D), and Trypethelium eluteriae (Fig. 10G); it reacts K+ purple.
Teloschistin (=fallacinol) is also yellow and is found in Marcelaria benguelensis; it reacts K+ purple.
Xanthorin (=lauropurpurone) is red and reacts K+ purple; it is found, for example, in Marcelaria purpurina (Fig. 10E).
Secalonic acid derivates are yellow to orange and react K+ yellow; they occur in Marcelaria purpurina.
Emodin and derivates are orange and react K+ purple; these pigments are found, for example, in Marcelaria cumingii (Fig. 10D).
The perylene quinone isohypocrellin is red and reacts with a K+ green efflux: this pigment occurs in Astrothelium purpurascens (Fig. 10I), A. sanguinarium (Fig. 10L), A. sanguineoxanthum, and Dictyomeridium isohypocrellinum.
Skyrin, isopigmentosin A and C, and semivioxanthin are also occasionally found; since these substances are detected in low concentration, they have not yet been assigned taxonomic value, since their absence or presence on chromatographic plates might depend on their concentration in the lichen and on analytical conditions. However, it is expected that detailed chemical analyses with sophisticated methods could reveal characteristic, species-specific patterns including these substances.
With regard to UV testing, it should be stressed that only positive UV-reactions that relate to xanthones and anthaquinones are mentioned in the descriptions. These are yellow or orange for xanthones and usually red for pigments; whitish or greenish reflections of the thallus or ascoma surface are ignored as these correspond to brightening under the UV lamp of ochraceous (often old) specimens; taxa with such UV reflections only are reported as UV−. Similarly, some specimens with a thick, hyaline cortex show a faint, K+ yellow reaction, which is also ignored as it seems to be caused by a structural change from wetting, not by a secondary substance. When carrying out UV testing, it is therefore recommended to make comparisons with previously identified reference specimens of taxa with a set of known chemical compounds, including lichexanthone and the major pigments.
Species Delimitation and Nomenclature
Species delimitation within Trypetheliaceae has been rather inconsistent in the past and, until recently, sometimes used the same characters to either separate species or include specimens within a single taxon (Harris Reference Harris1993, Reference Harris1995). This was also reflected at the genus level, where species with lateral ostioles and transversely septate ascospores were assigned to a single genus, Astrothelium, whereas those with lateral ostioles and muriform ascospores were assigned to two separate genera, Campylothelium (with solitary ascomata) and Cryptothelium (with fused ascomata).
To obtain a more consistent species concept we used two approaches: 1) analysis of morphological, anatomical and chemical variation in phylogenetically defined clades (Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ); and 2) morphological analysis of a larger number of specimens in larger species complexes. As a result, we found that characters such as thallus and ascoma morphology (e.g. surface structure and emergence), hymenial inspersion, ascospore size, and secondary chemistry (including lichexanthone) are diagnostic at the species level, much more so than previously believed; hence, a much larger number of species is recognized here based on a revised species concept alone (exluding the many novel taxa described elsewhere in this issue). For example, the rich material available from Rondônia and Sergipe in Brazil (Aptroot & Cáceres Reference Aptroot and Cáceres2016; Aptroot et al. 2016 Reference Aptroot, Mendonça, Santos, Reis Silva, Martins, Gumboski, Vidigal and Cáceresb ), with several hundred collections representing over 70 taxa, and from Venezuela (Komposch & Hafellner Reference Komposch and Hafellner1999; Komposch et al. Reference Komposch, Aptroot and Hafellner2002), with c. 300 collections belonging to 40 species, allowed us to analyze morphological and anatomical variation in some of the common taxa. As an example, the ascospore width and length of all specimens of the Astrothelium conicum-aggregate were analyzed showing that the species recognized within this complex are separated on a combination of UV-reaction and ascospore dimensions.
To obtain a stable nomenclature that reflects this revised species concept, as many collections as possible were studied including most type specimens of published names. Fifty-seven new lectotypes were designated, including in one case an illustration with an additional epitype. To check possibly erroneous nomenclatural citations, including those given in Index Fungorum and other similar databases, original protologues of all names were studied. As an extreme example of a revised species concept and nomenclatural treatment, Astrothelium variolosum sensu Harris (Reference Harris1993), which in most other treatments corresponds to the four names Astrothelium confusum, A. variolosum (ostioles lateral, fused, without or with lichexanthone), Trypethelium nitidiusculum and T. ochroleucum (ostioles apical, separate, without or with lichexanthone), is now considered to encompass at least 34 different species (Table 1) which are not necessarily closely related (Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). The fact that all of these names were described in the 19th century underlines the importance of critical analysis of historical literature and type specimens to accurately assess the taxonomy and nomenclature of Trypetheliaceae. This situation is similar to that found in the genus Ocellularia where the commonly used names O. papillata, O. perforata and O. terebrata now refer to 69 different taxa, many of them represented by historical names (Lücking Reference Lücking2014).
Table 1 Revised species concept in the Trypethelium nitidiusculum/T. ochroleucum-Astrothelium variolosum complex
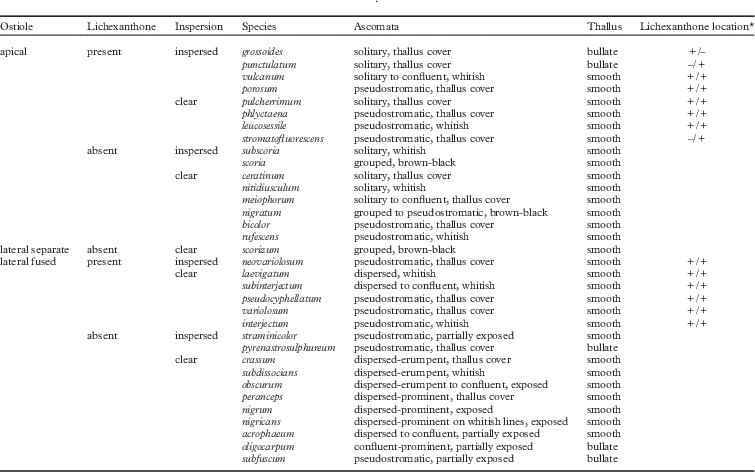
*Lichexanthone location: +/+ indicates lichexanthone present on both thallus and ascomata (pseudostromata); +/– indicates lichexanthone present on the thallus but not on the ascomata (pseudostromata) and –/+ indicates lichexanthone not present on the thallus but is present on the ascomata (pseudostromata).
Keys and short descriptions are presented for the currently accepted species of Trypetheliaceae with the exception of those described elsewhere in this issue as new taxa; these are only cross-referenced in the keys. The delimitation of Astrothelium, Bathelium, and Trypethelium is revised so as to be in concordance with the molecular phylogeny, resulting in mostly monophyletic genera that are still morphologically recognizable. Many taxa, including the genera Campylothelium, Cryptothelium and Laurera, are synonymized for the first time (the latter three with Astrothelium) whereas others, including the genus Bogoriella, have been reinstated.
The revised genus and species concept and the examination of many old type specimens led to the proposal of many new combinations. Numerous species are recorded for the first time from a country or continent. However, there is still a high proportion of species known only from their type material. This also strongly suggests that many species still remain undiscovered, as shown by a statistical prediction exercise (Aptroot et al. 2016 Reference Aptroot, Caceres, Johnston and Lückingc ). This is corroborated by the fact that a few short field trips in small areas in Brazil yielded many undescribed species (Aptroot & Cáceres Reference Aptroot and Cáceres2016; Aptroot et al. 2016 Reference Aptroot, Mendonça, Santos, Reis Silva, Martins, Gumboski, Vidigal and Cáceresb ; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ), and also taxonomic inventories of other understudied areas, such as Panama and Bolivia, unravelled numerous novel taxa (Flakus et al. Reference Flakus, Kukwa and Aptroot2016; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Benatti, Binh, Gueidan, Gutiérrez, Jungbluth, Lumbsch and Marcellib ).
Some commonly used names are synonymized for the first time, such as Trypethelium ochroleucum; many rarely applied names are reinstated, as in the example of the Astrothelium variolosum complex above. Inevitably this resulted in new names for some of the most common taxa. We preferred this solution, over the alternative of proposing well-known names for conservation, for two reasons: 1) Trypetheliaceae are relatively rarely mentioned in the general literature and mostly known to specialists; 2) the new names reflect the revised taxonomy and hence will force future workers to assess morphological, anatomical and chemical characters more critically. For instance, the name Trypethelium ochroleucum has been applied in a pantropical context to a number of species now recognized as different: Astrothelium porosum (pseudostromatic, inspersed), A. pulcherrimum (solitary, clear) and A. phlyctaena (pseudostromatic, clear). The fact that the name ochroleucum has been subsumed in synonymy will hopefully lead to proper revision and identification of herbarium material and newly collected specimens. Similarly, the taxon formerly known as Trypethelium nitidiusculum was split into Astrothelium bicolor (pseudostromatic, clear), A. nitidiusculum (solitary, clear) and A. scoria (grouped, inspersed).
We are very much aware that the present revision is not a thorough monograph of Trypetheliaceae. Rather, we present this revisionary synopsis to stimulate further research in this highly diverse and fascinating family, hoping that future workers will pay more attention to morphological, anatomical and chemical features to identify species and that the molecular phylogenetic data will be much expanded. Undoubtedly, further research will lead to additional changes in the classification of Trypetheliaceae and many additional new species will be discovered (Aptroot et al. 2016 Reference Aptroot, Caceres, Johnston and Lückingc ). Notably, we are more confident with the taxonomic concept of the less common and rare species, which often have a unique combination of diagnostic characters different from similar and related taxa, than with the common species, such as Astrothelium aeneum, A. bicolor, A. porosum, A. phlyctaena, A. scoria and Trypethelium eluteriae which have a degree of variation that needs to be assessed by additional molecular phylogenetic studies.
Distribution and Ecology
Trypetheliaceae is almost exclusively tropical and epiphytic, with very few species (e.g. Viridothelium virens) found in temperate regions and few species growing on other substrata. In general terms, the distribution and ecology of Trypetheliaceae is very similar to that of Graphidaceae although the latter has more taxa in extra-tropical regions and on substrata other than bark (Rivas Plata et al. Reference Rivas Plata, Lücking and Lumbsch2008; Lücking et al. Reference Lücking, Johnston, Aptroot, Kraichak, Lendemer, Boonpragob, Cáceres, Ertz, Ferraro and Jia2014). More commonly than Graphidaceae, Trypetheliaceae are found in (semi-) exposed microhabitats and habitats, such as the forest canopy and open savannahs and dry forest, often forming colourful crustose lichen communities with dominant taxa producing yellow to orange pigments (Komposch & Hafellner Reference Komposch and Hafellner2000, Reference Komposch and Hafellner2002, Reference Komposch and Hafellner2003; Cáceres Reference Cáceres2007; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Cáceres et al. Reference Cáceres, Lücking and Rambold2008; Rivas Plata et al. Reference Rivas Plata, Lücking and Lumbsch2008). It appears that species with green thalli and partially immersed or covered ascomata are more frequently found in the rainforest understorey, but this has not been tested quantitatively. However, the observation that many taxa are typical of the more exposed canopy, a microhabitat that has not been well studied, indicates that this microhabitat might harbour many yet unknown species.
Following this revisionary synopsis, the list of accepted species known from the various countries has changed considerably, especially for countries in which much previous work was carried out, such as India and Brazil. A table with the currently accepted species and their updated, confirmed world distribution is available as supplementary material in Aptroot et al. (2016 Reference Aptroot, Caceres, Johnston and Lückingc ).
Some species are much more widespread and common than others. In order to facilitate identification, lists are given here of the most common species. These are only a fraction of those known. Half of what remains are known only from the type location; the chance of finding any of these elsewhere is probably not much higher than that of finding an undescribed species, as demonstrated by the papers on Bolivian and Rondônian Trypetheliaceae in this issue (Aptroot & Cáceres Reference Aptroot and Cáceres2016; Flakus et al. Reference Flakus, Kukwa and Aptroot2016).
Species with over 100 specimens seen include: Astrothelium macrocarpum, A. phlyctaena, A. scoria, A. scorioides, A. versicolor, Constrictolumina cinchonae, Nigrovothelium tropicum and Trypethelium eluteriae.
Species with between 20 and 100 specimens seen include: Astrothelium aeneum, A. bicolor, A. cinnamomeum, A. degenerans, A. eustomum, A. feei, A. inspersaeneum, A. megaspermum, A. obscurum, A. ochrothelium, A. porosum, A. variolosum, Bathelium madreporiforme, Bogoriella hemisphaerica, B. punctata, B. subfallens, Dictyomeridium proponens, Marcelaria benguelensis, M. purpurina, Polymeridium albocinereum, P. catapastum, P. pleiomerellum, P. subcinereum, Pseudopyrenula diluta, P. subgregaria, P. subnudata, Trypethelium subeluteriae and Viridothelium virens.
Taxonomic Treatment
Trypetheliales Lücking, Aptroot & Sipman
In Aptroot et al., Biblioth. Lichenol. 97: 13 (2008); type: Trypetheliaceae Eschw.
Ascomycetes, usually lichenized with trentepohlioid algae, more rarely bark saprobes or lichenicolous fungi.
Ascomata perithecia, solitary or grouped and fused (with separate or fused ostioles), round, immersed to sessile. Wall dark brown to carbonized. Interascal hyphae thin, richly branched and anastomosing, forming a net-like structure. Asci fissitunicate, I−. Ascospores ellipsoid-fusiform, with variously developed endospore thickenings giving the lumina a diamond-shaped or more rarely a round outline, colourless or rarely (dark) brown.
Discussion. This order encompasses a phylogenetically quite distinct group, now containing two families, viz. the lichenicolous Polycoccaceae and the lichenized Trypetheliaceae (Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Hyde et al. Reference Hyde, Jones, Liu, Ariyawansha, Boehm, Boonmee, Braun, Chomnunti, Crous and Dai2013; Ertz et al. Reference Ertz, Diederich, Lawrey, Berger, Freebury, Coppins, Gardiennet and Hafellner2015).
The order Trypetheliales, based on Trypetheliaceae Eschw., was introduced by Aptroot et al. (Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008) to reflect the phylogenetic distinctiveness of the Trypetheliaceae within Dothideomycetes (Del Prado et al. Reference Del Prado, Schmitt, Kautz, Palice, Lücking and Lumbsch2006; Nelsen et al. Reference Nelsen, Lücking, Grube, Mbatchou, Muggia, Rivas Plata and Lumbsch2009, Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011). The name Trypetheliaceae Eschw. itself (Eschweiler Reference Eschweiler1824) has been considered invalidly published in Index Fungorum (IF 81884) and MycoBank (MB 81884), with the argument that it was associated with the rank term “cohors”; the same applies to the family names Parmeliaceae Eschw., Usneaceae Eschw., and Verrucariaceae Eschw. However, this interpretation appears to be incorrect, since an inappropriate rank term does not invalidate a name. Rather, ICN Art. 17.2 and 18.2 specify that if there is conflict between the intended rank (based on context and/or Latin termination) and the associated rank term, a name is presumed to be validly published at the intended rank, but not invalid. According to Art. 17.2, one might then conclude that Trypetheliaceae Eschw. (Eschweiler Reference Eschweiler1824) is to be accepted as a valid name at the order level (i.e. Trypetheliales Eschw.) because of the associated rank term “cohors”. In that case, because the principles of priority and homonymy do not apply to names above the rank of family, the names Trypetheliales Lücking et al. and Trypetheliales Eschw. would both be legitimate and could be used interchangeably. However, the opposite is the case, since Art. 17.2 states: “Names intended as names of orders, but published with their rank denoted by a term such as ‘cohors’, ‘nixus’, ‘alliance’, or ‘Reihe’ instead of ‘order’, are treated as having been published as names of orders.” This means that a name is to be treated at the order level, if the intention was to designate a name as an order, in spite (not because) of having used a rank term such as “cohors” (i.e. ‘cohors’ is not a rank term that automatically designates the rank of order). There is no further definition of the term ‘cohors’ in the Code, but its best Latin translation would be ‘group’, and there is no provision in the Code that the use of the term ‘cohors’ would preclude the meaning of family (as a group of genera). Correspondingly, the correct interpretation of the analogous Art. 18.2 is that a name is to be treated as family if the intention was to designate a family, in spite of using the rank term ‘ordo’. Eschweiler’s (Reference Eschweiler1824) intention undoubtedly was to establish a family, as he used the term “familia” in the Latin description, defined Trypetheliaceae as a group of genera (the next higher, principal hierarchical level above genus is family), and used the correct Latin termination “-aceae” (Art. 18.1); technically, the termination is not relevant as the Code was not in place at the time, but the termination ‘-aceae’ has been widely accepted since the late 18th century as denoting taxa at the family level in botanical nomenclature. As a consequence, neither Art. 17.2 nor Art. 18.2 apply in this case, since Trypetheliaceae Eschw. was not intended as a name of an order (required for Art. 17.2) and it was not associated with the rank term ‘ordo’ (required for Art. 18.2). On the other hand, by extension of the meaning of both articles, which imply that 1) ‘cohors’ is not a rank term naturally corresponding to order level, 2) a name is to be accepted at the level of family if intended so, and 3) there is no conflict between the use of the rank term ‘cohors’ and the family rank defined in either article or the Code as a whole, the name Trypetheliaceae Eschw. (Eschweiler Reference Eschweiler1824) is valid at the family rank and is not to be treated at the order level or to be considered invalid. Similar considerations would apply to the names Parmeliaceae Eschw., Usneaceae Eschw., and Verrucariaceae Eschw.
Trypetheliaceae Eschw.
Eschweiler, Syst. Lich.: 17 (1824); Fée, Essai Crypt. Écorc.: xxvi (1824; as “Trypetheliées”), nom. inval. [ICN Art. 18.4, 32.1(b)]; Trypetheliaceae Zenker, in Goebel & Kunze, Pharmaceutische Waarenkunde 1(3): 123 (1827; as “Trypethelia”). nom. illeg. [ICN Art. 52.1]; type: Trypethelium Spreng.
Thallus crustose, corticate or not, without pseudocyphellae, sometimes surrounded by a black hypothallus. Algae trentepohlioid.
Ascomata perithecioid, mostly simple, but sometimes with fused ostioles, or the ascomata fused and having shared ostioles, with or without pseudostromatic tissues of a different structure and colour. Ostioles apical or eccentric. Wall usually completely carbonized, rarely only partly carbonized, with or usually without a clypeus, with or without crystals. Hamathecium usually colourless, rarely yellow, IKI−, clear or inspersed with oil droplets. Hamathecial filaments branched and usually anastomosing paraphysoids of 0·5–2·0 μm thick, rarely almost unbranched paraphysoids that are much wider at the base than at the tips; periphyses absent; short periphysoids rarely present. Asci bitunicate, cylindrical, IKI−, usually with a broad ocular chamber that is especially distinct in immature asci. Ascospores 1–8 per ascus, distoseptate, usually IKI−, rarely IKI+ violet, with rounded or angular lumina, without additional eusepta, colourless, rarely becoming brown, rarely constricted at the septa, with or without a gelatinous sheath.
Conidiomata pycnidia, containing microconidia. Conidia colourless, rod-shaped. Conidiogenesis acrogenous.
Chemistry. Lichexanthone (or the closely related 1,8-dihydroxy-3,6-dimethoxyxanthone or coronatone) or quinones, mostly anthraquinones, often present. Non-specific substances such as skyrin, isopigmentosin A and C and semivioxanthin sometimes detected.
Discussion. This family currently comprises 16 genera. Most were traditionally assigned to this family, but some have been erected here to accommodate species aggregates traditionally assigned to other groups (e.g. Arthopyrenia, Mycomicrothelia) that were found to belong here based on phylogenetic studies (Nelsen et al. Reference Nelsen, Lücking, Grube, Mbatchou, Muggia, Rivas Plata and Lumbsch2009, Reference Nelsen, Lücking, Mbatchou, Andrew, Spielmann and Lumbsch2011, Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). The new genus Constrictolumina is used for species formerly placed in Arthopyrenia, which in its strict sense remains a small, non-lichenized, extratropical genus (Hyde et al. Reference Hyde, Jones, Liu, Ariyawansha, Boehm, Boonmee, Braun, Chomnunti, Crous and Dai2013), and Novomicrothelia for several species previously classified in Mycomicrothelia, while Bogoriella is reinstated to accommodate most of the former Mycomicrothelia species. Most genera accepted here are supported by molecular data, whereas the inclusion of the reinstated Distothelia is based on morphological similarities. The circumscription of Astrothelium, Bathelium, and Trypethelium had to be changed so as to be in concordance with the phylogeny (Nelsen et al. Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). Bathelium and Trypethelium are restricted here to small, morphologically recognizable groups of species related to their respective types; some species included in Bathelium sensu Harris (Reference Harris1995), with astrothelioid ascospores, were found to belong in Astrothelium (Nelsen et al. Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). The new genus Viridothelium encompasses a clade with species morphologically similar to Astrothelium but with ascospores reminiscent of those of Trypethelium, whereas Marcelaria was recently established for a small group of species with very conspicuous ascomata and muriform ascospores (Aptroot et al. 2013 Reference Aptroot, Nelsen and Parnmena ).
For the correct nomenclature and authorship of the name Trypetheliaceae, see discussion under Trypetheliales above.

Aptrootia Lücking & Sipman
In Lücking et al., Lichenologist 39: 188 (2007); type: Aptrootia terricola (Aptroot) Lücking, Umaña & Chaves (holotype).
Thallus either corticate and green and bullate or verrucose, or not corticate and grey, ±smooth and cartilaginous.
Ascomata solitary, black, globose to ampulliform, immersed in thalline warts or in the substratum. Ostioles apical. Hamathecium colourless, clear or inspersed with large irregular oil droplets near the ostiole, filaments thin, anastomosing paraphysoids. Ascospores 1(–2) per ascus, IKI+ violet, with scarcely rounded lumina, initially colourless, becoming dark brown but wall internally remaining hyaline (only outer layer brown), ornamented with brown warts or not, fusiform or usually elongate-ellipsoidal to bacilliform with subacute or rounded ends, densely irregularly muriform, not constricted at the septa, surrounded by a gelatinous sheath.
Pycnidia unknown.
Chemistry. No substances detected.
Discussion. This recently established genus (Lücking et al. Reference Lücking, Sipman, Umaña, Chaves and Lumbsch2007) differs from other Trypetheliaceae in the combination of immersed ascomata and dark brown, muriform ascospores, as well as in its peculiar ecology growing often over bryophytes, a feature otherwise unknown in the family. The genus was erected for a terricolous lichen known from mountains in Papua New Guinea and Costa Rica. Two additional Australasian species have been transferred to Aptrootia (Aptroot 2009 Reference Aptroota ).

Aptrootia elatior (Stirt.) Aptroot
Fl. Australia 57: 600 (2009).—Ascidium elatius Stirt., J. Linn. Soc., Bot. 14: 466 (Feb. 1875).—Leptotrema elatius (Stirt.) Müll. Arg., Bull. Herb. Boissier 2 (App. 1): 75 (1894).—Thelotrema elatius (Stirt.) Hellb., K. Svenska Vetensk.-Akad. Handl. 21(3, 13): 79 (1896).—Laurera elatior (Stirt.) D. J. Galloway, New Zealand J. Bot. 21: 193 (1983); type: New Zealand, near Wellington, Buchanan (BM!—lectotype; Galloway, Fl. New Zealand Lichens: 205, 1985).
Ascidium melanosporum C. Knight, Trans. Proc. N. Z. Inst. 7: 363 (Jul. 1875); type: New Zealand, Knight s. n. (NSW!).
Anthracothecium monosporum Müll. Arg., Bull. Herb. Boissier 3: 327 (1895).—Polyblastiopsis monosporum (Müll. Arg.) Upreti & A. Singh, Brunonia 10: 226 (1987).—Julella monospora (Müll. Arg.) D. D. Awasthi, Lichenology in Indian Continent: 15 (2000); type: Australia, Victoria, Knight 214 (G!—holotype).
Verrucaria luteonitens Nyl., Ann. Soc. Sci. Fenn. 26(10): 24 (1900).—Anthracothecium luteonitens (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 464 (1922); type: Sri Lanka, Pedretalagalla, Almquist (H-Nyl 1061!—holotype).
(Fig. 11A)

Fig. 11 Habitus, anatomy and ascospore of Aptrootia (A–C) and Architrypethelium species (D–L). A, Aptrootia elatior (New Zealand, lectotype); B, A. terricola (Papua New Guinea, Aptroot 37658); C, A. robusta (Tasmania, Lumbsch 20012n); D, Architrypethelium grande (Brazil, lectotype); E, A. lauropaluanum (Brazil, holotype); F & G, A. hyalinum (Venezuela, Sipman & van der Werff 10902); H , A. penuriaxanthum (Bolivia, holotype); I & J, A. nitens (I, Venezuela, holotype of Pleurothelium ernstianum; J, Costa Rica, Lücking 15212b); K, A. columbianum (Colombia, lectotype); L, A. uberinum (Costa Rica, Lücking s. n.). Scales: A, B, D–F, H, I, K & L=1 mm; C=100 µm; G & J=50 µm.
Thallus corticolous, corticate, pale yellow-brown to green, verrucose-bullate.
Ascomata trypethelioid, with apical ostioles, ampulliform, oval or subglobose (in section), 0·7–1·7 mm diam., erumpent, covered by thallus except the rather broad, black ostiolar area, with copious hyaline crystals and a conspicuous black ring around the ostiole free of crystals. Wall to 150 µm thick. Hamathecium clear except for large irregular oil droplets near the ostiole. Ascospores 1(–2) per ascus, 200–330×60–90 µm, oblong-ellipsoid, richly muriform, becoming dark brown and ornamented (verruculose), with ascospore wall distinctly bi-layered: outer layer dark brown, inner layer hyaline.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (previously reported from Australia and New Zealand, now also from Sri Lanka and Sabah).
Discussion. This species differs from the other two by the ornamented ascospores and bark as substratum. We suspected that it may represent an undescribed genus, but it is phylogenetically nested between the other two species. The ascospore surface with the irregular brown warts is unique in the family. In a thin hand-section, this outer wall appears to be brittle, and a section through the ascospores reveals the subhyaline internal parts.
New country record. Malaysia: Sabah, Kota Belud, Kinabalu Park, 2800 m, 1989, Sipman & Tan 31305 (B).
Aptrootia robusta (P. M. McCarthy & Kantvilas) Aptroot
Fl. Australia 57: 661 (2009).—Laurera robusta P. M. McCarthy & Kantvilas, Lichenologist 25: 51 (1993); type: Australia, Tasmania, Crater Peak, Kantvilas & James (HO—holotype, not seen; BM!—isotype).
(Fig. 11C)
Thallus muscicolous, with thin, cartilaginous cortex, grey, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, globose (in section), 0·9–1·5 mm diam., immersed, partially covered by thallus except the rather broad, black ostiolar area. Wall to 120 µm thick. Hamathecium clear. Ascospores 1 per ascus, 150–360(–400)×65–140 µm, ellipsoid, richly muriform, becoming dark brown.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Australia.
Discussion. This species differs from Aptrootia terricola principally by the larger ascospores (170–230×40–70 µm in the latter).
Aptrootia terricola (Aptroot) Lücking et al.
In Lücking et al., Lichenologist 39: 188 (2007).—Thelenella terricola Aptroot, Fungal Diversity 2: 45 (1999); type: Papua New Guinea, Simbu Prov., Mount Wilhelm, Pindaunde Valley, near the hut on the S-shore of Lake Piunde, Aptroot 32649 (CBS!—holotype; ABL!—isotype).
(Fig. 11B)
Thallus terricolous, with thin, cartilaginous cortex, grey, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, globose (in section), 0·9–1·5 mm diam., immersed, partially covered by thallus except the rather broad, black ostiolar area. Wall to 120 µm thick. Hamathecium clear. Ascospores 1 per ascus, 170–230×40–70 µm, ellipsoid, richly muriform, becoming dark brown late during maturity, I+ violet when hyaline.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Amphi-Pacific, known from tropical mountains in Costa Rica and Papua New Guinea, now also reported from the Solomon Islands.
Discussion. This species differs from Aptrootia robusta principally by the smaller ascospores (150–360(–400)×65–140 µm in the latter).
New country record. Solomon Islands: Guadalcanal Island: Central part, Mount Popomansiu, summit, c. 2200 m, 1965, Hill 9400 (BM, ABL).
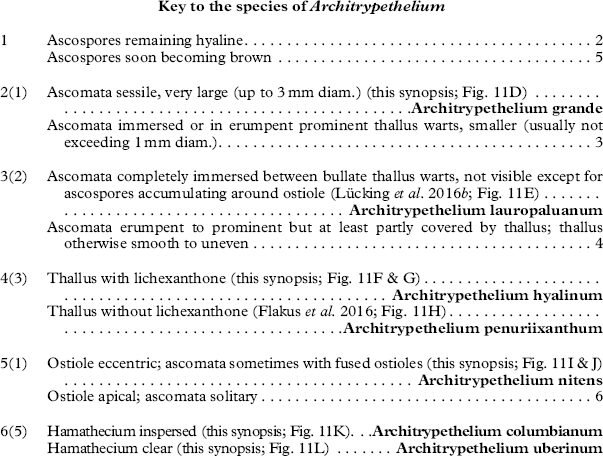
Architrypethelium Aptroot
Biblioth. Lichenol. 44: 120 (1991); type: Architrypethelium uberinum (Fée) Aptroot (holotype).
Thallus corticate.
Ascomata solitary or aggregated. Ostioles apical or eccentric. Wall hyphal (textura intricata), carbonized. Hamathecium clear or inspersed with oil droplets, filaments thin, anastomosing paraphysoids. Ascospores distoseptate, usually also with euseptate walls, mostly brown, 3–5-septate, large, often with longitudinal folds in the wall, rarely colourless.
Pycnidia unknown.
Chemistry. Lichexanthone rarely present.
Discussion. Architrypethelium externally resembles species of Astrothelium, including those previously placed in the genera Laurera, Cryptothelium, and Trypethelium, but differs anatomically by its 3-septate, extremely large ascospores, which are hyaline to dark brown when mature and do not have the diamond-shaped lumina typical of Astrothelium when mature (Aptroot Reference Aptroot1991). Phylogenetically it can be considered the sister clade to Astrothelium. Species of Pyrenula with large, 3-septate ascospores can be confused with Architrypethelium but, in addition to the differences in hamathecium structure, are distinguished as follows: Pyrenula subpraelucida has ascospores with small terminal lumina against the endospore, while in P. laii and P. montocensis, the ascospores have angular lumina with very thick septa and lateral walls. In addition, 3-septate ascospores in Pyrenula are less than 90 µm long while in Architrypethelium they are usually longer than 90 µm (up to 160 µm).
Architrypethelium columbianum (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816650
Trypethelium columbianum Nyl., Ann. Sci. Nat. Bot. sér. 5, 3: 347 (1867); type: Colombia, Rio Negro, Lindig 35 (H-Nyl 321!—lectotype, designated here; BM!, BR—isolectotypes).
(Fig. 11K)
Thallus corticate, light olive-grey, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·7–1·2 mm diam., sessile, covered by a brownish thallus layer. Wall thick, fully carbonized. Hamathecium inspersed. Ascospores 2–4 per ascus, 120–155×35–50 µm, oblong-ellipsoid, 3-septate, outer lumina much smaller than inner lumina, lumina rounded in the corners, often with needle-like crystals, wall 3–5 µm thick, surrounded by a gelatinous sheath 7–10 µm wide, becoming dark brown, I−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Colombia).
Discussion. This species differs from Architrypethelium uberinum mainly in the inspersed hamathecium.
Architrypethelium grande (Kremp.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816651
Ascidium grande Kremp., Flora 59: 249 (1876); Phaeotrema grande (Kremp.) Zahlbr., Catal. Lich. Univ. 2: 607 (1923); type: Brazil, Rio de Janeiro, Glaziou 6271 (M!—lectotype, designated here; BM!, C!—isolectotypes).
(Fig. 11D)
Thallus corticate, olive-green to yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 1–3 mm diam., sessile, covered by a light, orange-brown layer. Wall thick, fully carbonized, with thick, gelatinous cortex composed of anastomosing hyphae. Hamathecium clear. Ascospores 2–4 per ascus, 120–165×40–50 µm, oblong-ellipsoid, 3-septate, lumina angular to almost rounded in the corners, often with needle-like crystals, wall 3–5 µm thick, surrounded by a gelatinous sheath 3–5 µm wide, hyaline (although given as becoming pale olive-brown in the protologue), I−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Brazil).
Discussion. This species differs from the other two species with hyaline ascospores, Architrypethelium hyalinum and A. penuriaxanthum, in the sessile ascomata, and from A. hyalinum also in the absence of lichexanthone. Krempelhuber (Reference Krempelhuber1876) described the ascospores as becoming pale olive-brown, but we observed only hyaline ascospores in the type material. The only species with brown ascospores, an apical ostiole and clear hymenium is A. uberinum, from which A. grande also differs markedly in the sessile, orange-brown perithecial warts.
Architrypethelium hyalinum Aptroot
In Aptroot et al., Biblioth. Lichenol. 98: 37 (2008); type: Costa Rica, Puntarenas, Las Cruces Biological Station, trail to Rio Java, Sipman 53229 (B!—holotype; INB-3993295!—isotype).
Thallus corticate, olive-green, smooth to uneven, sometimes gall-like.
Ascomata trypethelioid, with apical ostioles, solitary, 0·7–1·5 mm diam., prominent, irregularly covered by thallus except for the black ostioles which are sometimes surrounded by an ochraceous zone. Wall 200–400 µm thick, fully carbonized. Hamathecium clear. Ascospores 4–8 per ascus, 100–150×30–50 µm, oblong-ellipsoid, often curved, 3-septate, outer lumina much smaller than inner lumina, lumina rounded in the corners, often with needle-like crystals, constricted at the median septum, wall 3–5 µm thick, surrounded by a gelatinous sheath 7–15 µm wide, hyaline, I−.
Chemistry. Thallus and ascomata UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (previously reported from Costa Rica and Brazil).
Discussion. This Architrypethelium has among the largest 3-septate ascospores in the family. It is also the only species in the genus with lichexanthone. The species could be mistaken for a Trypethelium except for the extremely large ascospores.
New country records. Puerto Rico: Distr. Mayagüez: Reserva Forestal Maricao, 1989, Aptroot & Aptroot 24960, 25322 (ABL).—Venezuela: Falcon: Miranda, 1979, Sipman & van der Werff 10902 (B).—Colombia: Santuario, 1989, Wolf et al. 5992 (ABL, COL).—Guyana: Demerara-Berbice District: Mabura Hill, 1988, Bleij & Biesmeijer s. n. (ABL).
Architrypethelium nitens (Fée) Aptroot
In Aptroot et al., Biblioth. Lichenol. 98: 38 (2008).—Verrucaria nitens Fée, Essai Crypt. Écorc.: 88 (1824); type: South America, on “cinchona” (L!—lectotype; Aptroot et al., Biblioth. Lichenol. 98: 38 (2008)).
Pyrenastrum seminudum Mont., Ann. Sci. Nat. Bot. sér. 2, 19: 64 (1843).—Architrypethelium seminudum (Montagne) Aptroot, Biblioth. Lichenol. 44: 120 (1991); type: French Guiana, Leprieur 588 (PC!—holotype).
Pleurothelium ernstianum Müll. Arg., Flora 60: 475 (1877).—Parathelium ernstianum (Müll. Arg.) Müll. Arg., Hedwigia 34: 36 (1895); type: Venezuela, Caracas, Ernst 130 (G!—holotype).
Parathelium superans Müll. Arg., Bull. Soc. Roy. Bot. Belg. 32(1): 165 (1893).—Splanchnonema superans (Müll. Arg.) O. Erikss., Opera Bot. 60: 133 (1981); type: Costa Rica, Puntarenas, Boruca, Tonduz s. n. (G!—lectotype, designated here; BR!, G!—isolectotypes; Pittier, Pl. Costaric. Exs. 6253).
Thallus corticate, olive-green, smooth to uneven.
Ascomata pleuro- to astrothelioid, solitary with eccentric ostioles or few chambers joined with eccentric, fused ostioles, 1·0–1·5 mm diam., erumpent to prominent, covered by thallus except for the blackish ostiolar area. Wall thick, fully carbonized. Hamathecium clear. Ascospores 8 per ascus, 90–150×25–50 µm, oblong-ellipsoid, 3-septate, outer lumina much smaller than inner lumina, lumina rounded in the corners, often with needle-like crystals, wall 3–5 µm thick, surrounded by a gelatinous sheath 7–10 µm wide, becoming dark brown, I−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from Costa Rica, Venezuela, Guyana, French Guiana and Brazil).
New country records. Puerto Rico: Mayagüez: Reserva Forestal Maricao, 1989, Aptroot & Aptroot 24930, 25420, 25040, 25002 (ABL).—Panamá: Panamá: Alto de Campana, 2001, Etayo 18782 (ABL).
Architrypethelium uberinum (Fée) Aptroot
Biblioth. Lichenol. 44: 122 (1991).—Porina uberina Fée, Essai Crypt. Écorc.: 83 (1824).—Porophora uberina (Fée) Spreng., Syst. Orb. Veget. 4(1): 242 (1827).—Pyrenula uberina (Fée) Fée, Essai Crypt. Écorc. Suppl.: 84 (1837).—Pertusaria uberina (Fée) A. Massal., Ric. Auton. Lich. Crost.: 190 (1852).—Trypethelium uberinum (Fée) Nyl., Mém. Soc. Sci. Nat. Cherbourg 5: 141 (1857).—Verrucaria uberina (Fée) Trevis., Consp. Verruc.: 8 (1860).—Stromatothelium uberinum (Fée) Trevis., Flora 44: 20 (1861).—Pseudopyrenula uberina (Fée) Vain., Ann. Acad. Sci. Fenn., ser. A 15(6): 353 (1921); type: Peru, “cort. cinchonae” (G!—holotype).
Trypethelium uberinoides Nyl., Flora 41: 381 (1858); type: Mexico, Orizaba, Müller (PC—lectotype, designated here).
Verrucaria megalospora Kremp., Flora 59: 525 (1861).—Parathelium megalosporum (Kremp.) Müll. Arg., Hedwigia 32: 134 (1893); type: Brazil, Rio de Janeiro, Glaziou 6276 (M!—holotype; BM!, UPS!—isotypes).
(Fig. 11L)
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, 1·0–1·7 mm diam., erumpent to prominent, covered by thallus except for the blackish ostiolar area surrounded by a whitish rim. Wall thick, fully carbonized. Hamathecium clear. Ascospores 2–4 per ascus, 120–155×35–50 µm, oblong-ellipsoid, 3-septate, outer lumina much smaller than inner lumina, lumina rounded in the corners, often with needle-like crystals, wall 3–5 µm thick, surrounded by a gelatinous sheath 7–10 µm wide, becoming dark brown, I−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Possibly pantropical; previously reported from Costa Rica, Peru, and Brazil. Incorrectly reported from Sri Lanka but now found in Oceania.
New country records. American Samoa: Tutuila: Pago Pago, 1970, Degelius P-279 (UPS).—French Polynesia: Tahiti: Taravao, 1970, Degelius P-371 (UPS).
Astrothelium Eschw.
Syst. Lich.: 18 (1824); type: Astrothelium conicum Eschw. [=A. cinnamomeum (Eschw.) Müll. Arg., lectotype; Massalongo, Atti I. R. Istitut. Veneto, Ser. 3, 5: 335, 1860].
Meissneria Fée, Essai Crypt. Écorc. Suppl.: 66 (1837), nom. illeg., non Meisneria DC. (1828); type: Trypethelium deforme Fée (as Meissneria varia Fée, holotype).
Laurera Reichenb., Deutsch. Bot. Herb.-Buch: 15 (1841), nom. cons., nom. nov. pro Meissneria Fée; type: Trypethelium deforme Fée (as Meissneria varia Fée, holotype).
Heufleria Trevis., Spighe e Paglie: 19 (1853); type: Heufleria conica (Eschw.) Trevis. (= cf. Astrothelium cinnamomeum, holotype).
Meristosporum A. Massal., Atti I. R. Istitut. Veneto, Ser. 3, 5: 327 (1860); type: Meristosporum javanicum A. Massal. (holotype).
Cryptothelium A. Massal., Atti I. R. Istitut. Veneto, Ser. 3, 5: 335 (1860); type: Cryptothelium sepultum (Mont.) A. Massal (holotype).
Leightonia Trevis., Flora 44: 19 (1861), nom. illeg., non. Trevis. (1853); type: Leightonia porosa (Ach.) Trevis. (holotype).
Campylothelium Müll. Arg., Flora 66: 245 (1883); type: Campylothelium puiggarii Müll. Arg. (holotype).
Thallus corticate, mostly olive-green.
Ascomata simple or aggregated or forming pseudostromata, immersed to prominent, with the pseudostromata often of a different structure and colour than the thallus. Ostioles apical or eccentric, simple or fused. Wall hyphal (textura intricata), usually carbonized. Hamathecium clear or inspersed with oil droplets, filaments thin, anastomosing paraphysoids. Ascospores distoseptate, hyaline, transversely septate or muriform.
Pycnidia occasionally present.
Chemistry. Lichexanthone, red crystals and/or anthraquinones regularly present, in or on the thallus, pseudostromata and/or ostioles.
Discussion. The type species, Astrothelium conicum, was not validly published when the genus Astrothelium was published (Eschweiler Reference Eschweiler1824), but only later by Eschweiler (in Martius 1833); however, this does not render the description of the genus invalid. Several other species were described in the genus prior to A. conicum, which makes the lectotypification with the latter slightly unusual, but not invalid either. The synonymy of A. conicum (the type species of the genus) with A. cinnamomeum was confirmed by combining the observations of Müller (Reference Müller1884: 270), who observed the ascospores, and Harris (Reference Harris1986: 59), who observed no ascospores but saw the negative reaction with UV.

Astrothelium acrophaeum Müll. Arg.
Bot. Jahrb. Syst. 6: 383 (1885).—Trypethelium acrophaeum Nyl. (1876), nom. nud.—Pleurotrema acrophaeum (Müll. Arg.) R. C. Harris, Acta Amazon. (Suppl.) 14: 69 (‘1984’) [1986]; type: Cuba, Wright s. n. (G!—holotype; H-Nyl 268!, US!—isotypes; Müller, Verr. Cub. 176).
(Fig. 28B)
Thallus corticate, light olive-green to yellowish, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed to irregularly confluent or diffusely pseudostromatic, 0·7–1·1 mm diam., erumpent to prominent, basally to laterally covered by thallus but upper part and ostiolar area exposed and blackish. Hamathecium clear. Ascospores 8 per ascus, 2(–3)-septate, fusiform, 12–14×4–5 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Cuba).
Astrothelium aeneum (Eschw.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816652
Verrucaria aenea Eschw., in Martius, Icon. Sel. Pl. Crypt. 2: 15, t. 4, fig. 3 (1828).—Pseudopyrenula aenea (Eschw.) Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 207 (1890).—Trypethelium aeneum (Eschw.) Zahlbr., in Engler & Prantl, Nat. Pflanzenfam. 1(1*): 70 (1903); type: Brazil, Bahia, Caetité, Martius s. n. (M!—holotype).
Verrucaria tetracerae var. crocea Eschw., in Martius, Flora Brasil. 1: 134 (1833).—Spermatodium croceum (Eschw.) Trevis., Conspect. Verruc.: 6 (1860); type: Brazil, Martius s. n. (M—holotype, not seen).
Verrucaria myriococca Spreng., Syst. Veget. 4(1): 245 (1827); type: not found in L, probably lost.
Verrucaria heterochroa Mont., Ann. Sci. Nat., Bot., sér. 2, 19: 60 (1843).—Pyrenula heterochroa (Mont.) Trevis., Spighe e Paglie: 17 (1853).—Segestria heterochroa (Mont.) Trevis., Conspect. Verruc.: 6 (1860).—Pseudopyrenula heterochroa (Mont.) Mull. Arg., Flora 66: 248 (1883); type: French Guiana, Leprieur 452 (BM—lectotype, designated here).
Thallus corticate, light olive-green to yellowish but largely covered with orange pigment, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent or diffusely pseudostromatic, 0·3–0·7 mm diam., erumpent, covered by thallus and orange pigment except for dark ostiolar area. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 20–27×7–10 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV+ red, K+ purple, with anthraquinone.
Distribution. Pantropical (previously reported from the USA, Costa Rica, El Salvador, Islas Revillagigedo, Bahamas, Cuba, Jamaica, Colombia, Venezuela, Guyana, Surinam, French Guiana, Galapagos, Bolivia, Brazil, Sri Lanka, Thailand, Malaysia (Sarawak, Sabah), Papua New Guinea, and Australia).
Discussion. Astrothelium aeneum as defined here might still represent a collective taxon, including forms with solitary or almost pseudostromatic, erumpent to sometimes almost prominent ascomata with different size ranges. Due to the lack of sufficient molecular data spanning a wide range of morphodemes, we have not attempted to split this taxon further, after recognizing separate lineages forming flat, linear pseudostromata, such as A. flavostromatum (Aptroot & Cáceres Reference Aptroot and Cáceres2016) and A. kunzei (see below), with an inspersed hamathecium, such as A. inspersaeneum (Lima et al. Reference Lima, Maia, Aptroot and Cáceres2013; see below) and A. neoinspersum (Aptroot et al. 2016 Reference Aptroot, Ertz, Etayo, Schumm and Weerakoona ), or with smaller or larger ascospores, such as A. flavum (Aptroot & Cáceres Reference Aptroot and Cáceres2016) and A. megaeneum (Flakus et al. Reference Flakus, Kukwa and Aptroot2016).
New country record. Ecuador: Zamora-Chinchipe: Cordillera Numbala, Reserva Biologica San Francisco, 2004, Sipman & Mandi 53115 (B).
Astrothelium alboverrucum (Makhija & Patw.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816653
Laurera alboverruca Makhija & Patw., Mycotaxon 31: 571 (1988); type: India, Andaman Islands, South Andaman, Port Mout, 14 ii 1985, Patwardhan, Nagarkar & Sethy AMH 85.36 (ABL!—isotype).
(Fig. 35G)
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·9–1·3 mm diam., prominent, hemispherical, basally to laterally covered by thallus but upper part whitish, rough, with narrow, blackish ostiolar spot. Hamathecium clear, yellow, IKI−. Ascospores 6–8 per ascus, densely muriform, fusiform, (85–)100–170×23–33 µm, with distinctly thickened median septum, yellow, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from India).
Astrothelium amazonum (R. C. Harris) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816654
Cryptothelium amazonum R. C. Harris, Acta Amazon. (Suppl.) 14: 65 (‘1984’) [1986]; type: Brazil, Amazonas, Reserva Biológica de Campina, Dumont 107 (NY!—isotype).
Verrucaria aurantia Eschw. in Martius, Icon. Sel. Pl. Crypt. 2: 15 (1828) nom. illeg., non (Pers.) Wibel (1799); type: Brazil, Martius s. n. (M—holotype, not seen).
(Fig. 37I)
Thallus corticate, light olive-green to yellowish, uneven to somewhat bullate and often pseudogall-inducing.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed, not pseudostromatic, 0·8–1·2 mm diam., erumpent to prominent, wart-shaped to conical, covered by thallus except for papilliform, reddish to brown-black ostiole, internally with yellow-orange, K+ purple, UV+ red pigment. Hamathecium clear. Ascospores 8 per ascus, muriform, oblong-fusiform, 75–95×23–28 µm, median septum thickened, hyaline, IKI−.
Chemistry. Thallus UV−, K−, ascomata internally with yellow-orange, K+ purple, UV+ red anthraquinone.
Distribution. Neotropical (previously reported from Guyana and Brazil).
New country record. Venezuela: Amazonas: Alto Orinoco, 15 km W of Esmeralda, 110 m, 1997, Hafellner & Komposch 178-3-16 (GZU).
Astrothelium ambiguum (Malme) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816655
Laurera ambigua Malme, Ark. Bot. 19(1): 24 (1924).—Meristosporum ambiguum Malme (Reference Malme1924) nom. inval.; type: Brazil, Matto Grosso, Cuyabá, Malme 2009 (S!—lectotype, designated here; BM!—isolectotype).
(Fig. 33F)
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·4–0·5 mm diam., erumpent to prominent, hemispherical, mostly exposed, brown-black. Hamathecium inspersed, IKI−. Ascospores 8 per ascus, muriform, fusiform, 45–60×14–20 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Brazil).
Astrothelium andamanicum (Makhija & Patw.) Aptroot comb. nov.
MycoBank No.: MB 816656
Cryptothelium andamanicum Makhija & Patw., J. Econ. Tax. Bot. 10: 498 (‘1987’) [1988].—Laurera bispora D. D. Awasthi, Lichenology in Indian Subcontinent: 15 (2000) non Laurera andamanica D. D. Awasthi (1991); type: India, Andaman Islands, North Andaman, Tugapur Range, Pathat Tikri, 19 xii 1985, Nagarkar & Sethy AMH 85.2323 (ABL!—isotype).
(Fig. 38J)
Thallus corticate, olive-green, smooth to uneven.
Ascomata pleurothelioid, with eccentric, separate ostioles, solitary, 0·6–1·0 mm diam., erumpent to prominent, partially covered by thallus but becoming exposed and black. Hamathecium clear. Ascospores 2 per ascus, densely muriform, fusiform, 80–130×23–36 µm, hyaline, IKI−, without distinctly thickened median septum.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from India).
Astrothelium annulare (Fée) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816657
Pyrenula annularis Fée, Essai Crypt. Écorc.: 73 (1824).—Verrucaria annularis (Fée) Spreng., Syst. Veget. 4(1): 245 (1827).—Trypethelium annulare (Spreng.) Mont., Ann. Sci. Nat., Bot., sér. 2, 19: 71 (1843).—Pseudopyrenula annularis (Spreng.) Müll. Arg., Flora 68: 331 (1885); type: South America, “corticis annosas Cinchonea lancifoliae” (M!—isotype).
Verrucaria exasperata Zenker, in Goebel & Kunze, Pharmazeut. Waarenkunde 1(3): 183 (1827).—Trypethelium exasperatum (Zenker) Zahlbr., Catal. Lich. Univ. 1: 492 (1922); type: South America, s. col. (lost).
Trypethelium annulare var. detrusum Mont., Ann. Sci. Nat., Bot. sér. 2, 19: 71 (1843); type: French Guiana, Leprieur s. n. (PC-Mont—syntypes, not seen).
Verrucaria myriomma Nyl., Ann. Sci. Nat. Bot. sér. 5, 7: 348 (1867).—Pseudopyrenula myriomma (Nyl.) Müll. Arg., Flora 66: 248 (1883); type: Colombia, Pie de Cuesta, Lindig 98 (H-Nyl 1117!—holotype; BR—isotype).
(Fig. 21A)
Thallus corticate, olive-green to brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to confluent, not pseudostromatic, 0·6–0·8 mm diam., immersed-erumpent, covered by thallus except for the dark ostiole surrounded by a narrow, whitish rim. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 62–80×20–25 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Costa Rica, Colombia, French Guiana).
Discussion. According to Hekking & Sipman (Reference Hekking and Sipman1988), Verrucaria exasperata Zenker is conspecific with Trypethelium annulare var. detrusum Mont. and hence both are included here in the synonymy of Astrothelium annulare.
Astrothelium aurantiacum (Makhija & Patw.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816658
Laurera aurantiaca Makhija & Patw., Mycotaxon 31: 572 (1988); type: India, Karnataka, Agumbe-Shringera road, Patwardhan & Nagarkar s.n. (AMH—holotype, not seen).
(Fig. 33K)
Thallus corticate, olive-green to brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·8–1·3 mm diam., strongly prominent to sessile, hemispherical to barrel-shaped with flattened top, covered by thallus but ostiolar area greyish. Hamathecium inspersed with red, K+ purple droplets. Ascospores 8 per ascus, densely muriform, ellipsoid, 115–135×26–33 µm, with distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected except for red anthraquinone in hamathecium.
Distribution. Eastern palaeotropical (previously reported from India and Papua New Guinea).
New country record. Malaysia: Sarawak: Gunong Mulu National Park, 1978, Coppins 5139 (ABL, E).
Astrothelium auratum (R. C. Harris) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816659
Laurera aurata R. C. Harris, Acta Amazon. (Suppl.) 14: 67 (‘1984’) [1986]; type: Brazil, Acre, km 12 on road to Porto Velho, Lowy 961 (NY!—isotype).
(Fig. 32D)
Thallus corticate, olive-green to brownish, uneven to verrucose.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata 1–2 mm diam., prominent to sessile, each with 5–15 ascomata, with yellow-orange pigment. Hamathecium inspersed, IKI−. Ascospores 8 per ascus, muriform, ellipsoid, 60–75×20–30 µm, without distinctly thickened median septum, hyaline, IKI+ violet.
Chemistry. Thallus UV−, K−; pseudostromata with yellow-orange, K+ purple, UV+ red anthraquinone.
Distribution. Neotropical; reported from Brazil.
New country records. Venezuela: Amazonas: Alto Orinoco, 15 km W of Esmeralda, 110 m, 1997, Hafellner & Komposch 178-5-43 (GZU).—Guyana: Potaro-Siparuni: surroundings of Paramakatoi Village, 800 m, 1996, Sipman 41197 p.p. (B).
Astrothelium aureomaculatum (Vain.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816660
Pseudopyrenula aureomaculata Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 207 (1890).—Trypethelium aureomaculatum (Vain.) Zahlbr., Catal. Lich. Univ. 1: 488 (1922); type: Brazil, Minas Gerais, Caraça, Vainio s.n. (TUR-Vain 30749!—holotype; BM!—isotype; Vainio, Lich. Bras. 1473).
(Fig. 23D)
Thallus ecorticate, light brownish to greenish grey, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata pseudostromatic; pseudostromata 0·5–1·0 mm broad, immersed to erumpent, with several joined ascomata, forming irregular groups or lines, with yellow pigment cover except for blackish ostiolar areas. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 28–35×10–15 µm, hyaline, IKI−.
Chemistry. Thallus UV+ yellow, K−, with lichexanthone; pseudostromata with yellow, K+ purple, UV+ red anthraquinone.
Discussion. This species is removed here from synonymy with Astrothelium versicolor, from which it differs substantially in thallus and ascoma morphology, the latter having a thick, bullate, whitish thallus with the joined ascomata dispersed.
Distribution. Neotropical (reported from Brazil).
Astrothelium basilicum (Kremp.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816661
Verrucaria basilica Kremp., Bol. Acad. Nac. Ci. Republ. Argentina 3: 126 (1879).—Pseudopyrenula basilica (Kremp.) Müll. Arg., Flora 72: 68 (1889).—Porina basilica (Kremp.) Zahlbr., Catal. Lich. Univ. 1: 367 (1922).—Trypethelium basilicum (Kremp.) R. C. Harris, Lichenogr. Thomsoniana: 141 (1998); type: Uruguay, Lorentz & Hieronymus s. n. (M!—lectotype; Harris, Lichenogr. Thomsoniana: 141, 1998; BM!—isolectotype).
(Fig. 21K)
Thallus corticate, olive-green, verrucose-bullate.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·8–1·5 mm diam., erumpent to prominent, covered by thallus except for blackish ostiolar area. Hamathecium clear. Ascospores 8 per ascus, 11–19-septate, fusiform, 140–190×20–25 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Uruguay).
Astrothelium bicolor (Taylor) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816662
Trypethelium bicolor Taylor, in Hooker, London J. Bot. 6: 157 (1847).—Bathelium bicolor (Taylor) C.W. Dodge, Nova Hedwigia, Beih. 12: 20 (1964); type: Africa (BM!—isotype).
?Trypethelium scoria var. convexum Nyl., Mém. Soc. Acad. Maine-et-Loire 4: 74 (1858).—Trypethelium mastoideum var. convexum (Nyl.) Müll. Arg., Mém. Soc. Phys. Hist. Nat. Genève 30(3): 12 (1888): type: “Crotone cascarillae” (PC—not seen).
Trypethelium polychroum Müll. Arg., Bot. Jahrb. Syst. 6: 391 (1885); type: Cuba, Wright s. n. (G!—lectotype, designated here; BM!—isolectotype; Müller, Verr. Cub. 178).
?Trypethelium scoria var. janeirense Zahlbr., Sitzungsber. K. Akad. Wiss., math.-naturw. Classe 111(1): 369 (1902); type: Brazil, Rio de Janeiro, von Höhnel 145 (W—holotype, not seen).
?Trypethelium formosanum Zahlbr., Feddes Repert. 31: 204 (1933); type: Taiwan, Taihoku, Soozan, Asahina 311 (W—holotype, not seen).
?Trypethelium boninense Kurok., Bull. Nat. Sci. Mus. Tokyo 12: 691 (1969); type: Japan, Ogasawara-shoto (Bonin Islands), Inoue 18960 (TNS—not seen).
(Fig. 18J)
Thallus corticate, green to yellowish or brown, smooth to somewhat bullate.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 0·7–1·5 mm broad, erumpent to prominent, rounded to irregular but not becoming confluent or reticulate, covered by thallus but often slightly paler than the surrounding thallus or sometimes whitish. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 15–27×7–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (previously reported from, e.g. Mexico, Guadeloupe, Puerto Rico, Cuba, Dominican Republic, Netherlands Antilles, Costa Rica, Nicaragua, El Salvador, Ecuador, Bolivia, Guyana, Brazil, Argentina, Seychelles, India, China, Hong Kong, Taiwan, Japan, Malaysia, Philippines, Indonesia, Papua New Guinea, Solomon Islands, Australia, New Caledonia, Fiji and Hawai’i).
Discussion. This reinstated taxon belongs in the Astrothelium nitidiusculum complex, in which the epithet bicolor is the oldest for specimens with a clear hamathecium and ascomata arranged in pseudostromata; previously, this and other names were included in a wide species concept of Trypethelium nitidiusculum. Astrothelium bicolor appears to be the most common species in the complex. Due to the fact that the types of some presumed synonyms could not be tested for hymenium inspersion, some of these may eventually turn out to be conspecific with other species in this complex, such as A. nitidiusculum s. str. or the inspersed A. scoria.
New or confirmed country records. Mexico: Chiapas: Ocozocoautla, 1994, Wolf & Sipman 2205 (B).—Guadeloupe: Marie Galante, 1992, Vivant (ABL).—Puerto Rico: Mayagüez: Reserva Forestal Maricao, 1989, Sipman 25757 (B).—Dominican Republic: Distrito Nacional: Sierra Prieta, 1987, Harris 20151 (ABL, NY).—Netherlands Antilles: St. Eustatius: Quill, 2008, Sipman 56749 (B). Saba: The Bottom, 2007, Sipman 54960 (B).—Costa Rica: Puntarenas: Parque Nacional Carara, 2002, Sipman 48382l (ABL, B).— Nicaragua: Rio San Juan: El Castillo, 2001, Breuss 18994 (LI).—El Salvador: Ahuachapán: Parque Nacional El Imposible, 1998, Sipman et al. 44882 (B).—Ecuador: Esmeraldas: San Lorenzo, 1982, Aptroot & Hensen 11022 (ABL). Pichincha: 19 km S of Santo Domingo, 1982, Aptroot & Hensen 11251 (ABL).—Guyana: Upper Takutu: Kusad Mountain, 1992, Sipman 57532 (B).—Brazil: Minas Gerais: Caraça, 1997, Sipman 40779 (B).—Argentina: Misiones: Iguazú, Ferraro et al. 10669 (ABL, CTES).—Seychelles: Mahé: Old Mission, 1973, Norkett 16442 (ABL, B).—India: West Bengal: Sundarbans, Chamta, 2003, Jagadeesh Ram 13547 (ABL).—China: Yunnan: Xishuangbanna, Menglun, 2002, Aptroot 57141 (ABL).—Hong Kong: New Territories: Tai Po Kau, 2000, Aptroot 48748 (ABL).—Malaysia: Pahang: Fraser’s Hill, 1964, Degelius s. n. (UPS).—Philippines: Luzon: Sorsogon, Irosin, 1915, Elmer 14659 (B).—Indonesia: Java: Bogor, Gunung Salak, 1991, Sipman & Tan 30065 (B).—Papua New Guinea: Madang: 10 km W of Brahman Mission, 1995, Sipman 38792 (B).—Solomon Islands: Guadalcanal Island: 1965, Hill 9215 (BM).—Australia: Queensland: 8 km W of Ravenshoe, 1984, Streimann 30176 (B).—New Caledonia: Ouenarou, 1970, Degelius P127 (UPS).—Fiji: Viti Levu: Nadarivatu, 1968, Degener & Degener 31814o (B).—Hawai’i: Hawai’i: Pahoa, 1969, Degener & Degener 32285b (B).
Astrothelium buckii (R. C. Harris) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816663
Trypethelium buckii R. C. Harris, Acta Amazon. (Suppl.) 14: 72 (‘1984’) [1986]; type: Brazil, Amazonas, along Rio Negro, 100 km NW of Manaus, Paraná Concelção, Buck 2157 (NY!—isotype).
(Fig. 14J)
Thallus corticate, olive-green to brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent but not pseudostromatic, 0·6–0·9 mm diam., erumpent, covered with thallus but upper part reddish brown and ostiolar area blackish, internally with hyaline or red, K+ red crystals. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, fusiform, (32–)37–47×14–16 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−; ascomata internally K+ red, with anthraquinone.
Distribution. Neotropical (reported from Costa Rica, Venezuela, Brazil).
Discussion. This species is taken out of the synonymy with Astrothelium cartilagineum as suggested by Aptroot et al. (Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008), differing from the latter by the inspersed hamathecium.
Astrothelium calosporum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816664
Pseudopyrenula calospora Müll. Arg., Bot. Jahrb. Syst. 6: 409 (1885).—Trypethelium calosporum (Müll. Arg.) R. C. Harris, Lichenogr. Thomsoniana: 141 (1998); type: Cuba, Wright s. n. (G!—holotype; BM!—isotype; Müller, Verr. Cub. 234).
Pseudopyrenula calospora var. rhodocheila Vain., Proc. Amer. Acad. Arts Sci. 58: 145 (1923); type: Trinidad, La Selva Valley, Thaxter 63 (S!—isotype).
(Fig. 17I)
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·7–0·9 mm diam., immersed-erumpent, completely covered by thallus except for ostiole furnished with pink pigment. Hamathecium clear. Ascospores 8 per ascus, 14–18-septate, fusiform, 110–150×17–22 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−; ostiole K+ red, with anthraquinone.
Distribution. Neotropical (reported from Cuba and Trinidad).
Astrothelium campylocartilagineum Aptroot & Lücking nom. nov.
MycoBank No.: MB 816665
Campylothelium cartilagineum Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 195 (1890) non Astrothelium cartilagineum (Fée) Aptroot (see below); type: Brazil, Minas Gerais, Sitio, Vainio s. n. (TUR-Vain 30835!—holotype; BM!—isotype; Vainio, Lich. Bras. Exs. 1145).
(Fig. 39C)
Thallus corticate, olive-green, verrucose-bullate.
Ascomata pleurothelioid, with eccentric, separate ostioles, solitary, 0·7–1·0 mm diam., erumpent to prominent, covered by thallus but upper part often irregularly exposed and blackish. Hamathecium clear. Ascospores 2–4 per ascus, densely muriform, fusiform, 120–175×38–50 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Costa Rica and Brazil).
Discussion. This species is not synonymous with Heufleria chlorogastrica, as suggested by Harris (Reference Harris1986), since the type of the latter appears to belong in the genus Phyllobathelium.
Astrothelium cartilagineum (Fée) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816668
Pyrenula cartilaginea Fée, Essai Crypt. Écorc.: 76 (1824).—Trypethelium cartilagineum (Fée) Aptroot, in Aptroot et al., Biblioth. Lichenol. 98: 134 (2008) non Campylothelium cartilagineum Vain. (1890; see above); type: South America, “in America ad corticem Cinchonae lancifoliae et Portlandiae hexonae (quina nova)” (L!—isotype).
Thallus corticate, brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·4–0·5 mm diam., immersed to erumpent, covered by thallus except for dark ostiole surrounded by pale rim, internally with hyaline or red, K+ red crystals. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, (32–)37–47×14–16 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−; ascomata internally K+ red, with anthraquinone.
Distribution. Neotropical; previously reported from Trinidad, Costa Rica, French Guiana, Venezuela, and Brazil.
Discussion. This species is most similar to Astrothelium buckii, but differs by the clear hymenium.
New country record. Guyana: Upper Takutu: Kuyuwini Landing, 1992, Sipman 57073 (B); Karanambo ranch, 1992, Sipman 57280 (B).
Astrothelium cecidiogenum (Aptroot & Lücking) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816669
Cryptothelium cecidiogenum Aptroot & Lücking in Aptroot et al., Biblioth. Lichenol. 98: 57 (2008); type: Costa Rica, Alajuela, Volcán Tenorio National Park, Pilón Biological Station, Sipman 51911 (B!—holotype; INB-4055132!—isotype).
Thallus corticate, olive-green, smooth to uneven, pseudogall-inducing.
Ascomata mostly astrothelioid, several chambers joined with eccentric, fused ostioles, rarely pleurothelioid and with separate ostioles; joined ascomata diffusely pseudostromatic; pseudostromata 1–2 mm broad, prominent, confluent and forming reticulate or brain-like lines, covered with thallus except for the dark ostioles with irregular, whitish rim; pseudostromata 10–30 mm long and 5–10 mm wide, individual ascomata 0·7–1·2 mm diam. Hamathecium clear. Ascospores 8 per ascus, densely muriform, ellipsoid, 125–175×30–40 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution: Neotropical (previously reported only from Costa Rica).
New country record. Brazil: Rio Grande do Sul: Maquiné, 2006, Koch s. n. (ABL, HAS).
Astrothelium ceratinum (Fée) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816670
Pyrenula ceratina Fée, Essai Crypt. Écorc. Suppl.: 77 (1837).—Verrucaria ceratina (Fée) Nyl., Acta Soc. Sci. Fenn. 7(2): 491 (1863).—Pseudopyrenula ceratina (Fée) Müll. Arg., Mém. Soc. Phys. Hist. Nat. Genève 30: 29 (1888).—Trypethelium ceratinum (Fée) R. C. Harris, Lichenogr. Thomsoniana: 141 (1998); type: Peru, “ad corticem cinchonarum” (G!—lectotype; Harris, Lichenogr. Thomsoniana: 141 (1998)).
(Fig. 19E)
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·3–0·4 mm diam., erumpent, covered by thallus but upper portion darker or greyish. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 21–28×9–12 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from Peru).
New country records. Guyana: Upper Mazaruni: N-slope of Mount Roraima, 1985, Sipman & Aptroot 18767 (Lichenotheca Latinoamericana 100, ABL).—Ecuador: Zamora-Chinchipe: Estacion San Francisco, 2004, Sipman 52735 (B).
Astrothelium chapadense (Malme) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816671
Laurera chapadensis Malme, Ark. Bot. 19(1): 23 (1924).—Meristosporum chapadense Malme (Reference Malme1924), nom. inval; type: Brazil, Matto Grosso, Santa Anna da Chapada, Malme 2513 (S!—holotype; S!—isotype).
(Fig. 36A)
Thallus corticate, olive-green to brownish yellow, uneven to verrucose or almost squamulose.
Ascomata trypethelioid, with apical ostioles, irregularly confluent to pseudostromatic; pseudostromata 0·8–2·0 mm broad, prominent to sessile, dark brown, forming irregular groups or lines. Hamathecium clear. Ascospores 8 per ascus, densely muriform, ellipsoid, (60–)70–100(–130)×20–30 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Costa Rica and Brazil).
Discussion. Astrothelium chapadense strongly resembles a species of Bathelium, but is included here in Astrothelium due to the lack of an internal anthraquinone pigment.
Astrothelium chrysoglyphum (Vain.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816672
Thelenella chrysoglypha Vain., Hedwigia 38: 258 (1899).—Laurera chrysoglypha (Vain.) Zahlbr., Catal. Lich. Univ. 1: 503 (1922); type: Guadeloupe, Parnasso, Duss 564 (TUR-Vain 31044!—holotype).
(Fig. 32L)
Thallus corticate, olive to yellowish but covered by orange pigment, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, aggregate to diffusely pseudostromatic, pseudostromata 1–2 mm broad, erumpent, covered by thallus and orange pigment except for blackish ostiolar area. Hamathecium clear. Ascospores 2 per ascus, densely muriform, fusiform, 80–150×25–40 µm, without distinctly thickened median septum, hyaline, IKI− or IKI+ brownish.
Chemistry. Thallus and ascomata UV+ red, K+ purple, with anthraquinone.
Distribution. Neotropical (reported from Guadeloupe).
Astrothelium chrysostomum (Kremp.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816673
Trypethelium chrysostomum Kremp., Nouvo Giorn. Bot. Ital. 7: 57 (1875); type: Malaysia, Sarawak, Beccari 50 (M!—lectotype, designated here following annotation by R. C. Harris).
Trypethelium leucostomum Kremp., Nouvo Giorn. Bot. Ital. 7: 57 (1875) non (Nyl.) C. W. Dodge (Reference Dodge1953); type: Malaysia, Sarawak, Beccari 188 (M!—lectotype, designated here; M!—isolectotype).
Thallus corticate, olive-brown, uneven-rugose.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata solitary to confluent, 0·7–1·0 mm diam., immersed in thick thallus folds, covered except pale ostiolar area. Hamathecium clear. Ascospores 8 per ascus, 3(–5)-septate, fusiform, 32–52×10–17 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV+ yellow, K−. TLC: lichexanthone.
Distribution. Eastern palaeotropical (Malaysia: Sarawak).
Discussion. This taxon was first believed to be conspecific with Astrothelium leucothelium but differs substantially in thallus morphology and also in its distribution.
Astrothelium cinereorosellum (Kremp.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816674
Trypethelium cinereorosellum Kremp., Nuovo Giorn. Bot. Ital. 7: 35 (1875); type: Sarawak, Beccari 48 (M!—lectotype; Makhija & Patw., J. Hattori Bot. Lab. 73: 194, 1993).
Verrucaria antoniae Kremp., Nuovo Giorn. Bot. Ital. 7: 51 (1875).—Pyrenula antoniae (Kremp.) van Overeem-de Haas, Bull. Jard. Bot. Buitenzorg, Ser. 3, 4: 113 (1922).—Pseudopyrenula antoniae (Kremp.) Zahlbr., Catal. Lich. Univ. 1: 354 (1922); type: Sarawak, Beccari (M!—lectotype; Harris, Lichenogr. Thomsoniana: 140, 1998).
Trypethelium microstomum Makhija & Patw., J. Hattori Bot. Lab. 73: 201 (1993); type: India, Andaman Islands, South Andaman, Baratang, Bishmu Nala, 22 ii 1985, Patwardhan & Nagarkar AMH 85.663 (ABL!—isotype).
Trypethelium flavocinereum Makhija & Patw., J. Hattori Bot. Lab. 73: 198 (1993); type: India, Kerala, Cardamom Hills, Munnar-Devicolam road, 25 January 1976, Patwardhan & Prabhu AMH 76.672 (ABL!—isotype).
Thallus corticate, light greenish grey to yellowish or whitish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary or forming irregular groups or lines but not distinctly pseudostromatic, 0·3–0·5 mm diam., erumpent to prominent, covered by thallus except dark ostiole. Hamathecium inspersed. Ascospores 8 per ascus, (5–)7–9-septate, fusiform, (33–)40–50(–73)×8–14 µm, hyaline, IKI−.
Chemistry. Thallus UV+ yellow, K−, with lichexanthone (or 1,8-dihydroxy-3,6-dimethoxyxanthone).
Distribution. Palaeotropical; previously reported from India, Thailand, Borneo, Papua New Guinea, and Australia.
New country record. Indonesia: Java: Cibodas, 1450 m, 1939, Groenhart 1942 (ABL, L).
Astrothelium cinereum (Müll. Arg.) Aptroot & Lücking comb. et stat. nov.
MycoBank No.: MB 816675
Heufleria praetervisa var. cinerea Müll. Arg., Flora 68: 251 (1885).—Cryptothelium praetervisum var. cinereum (Müll. Arg.) Zahlbr., Catal. Lich. Univ. 1: 522 (1922); type: French Guiana, Leprieur 41 (G!—lectotype, designated here).
(Fig. 39G)
Thallus corticate, olive-brown, uneven to coarsely bullate-folded.
Ascomata mostly astrothelioid, several chambers joined with eccentric, fused ostioles, rarely pleurothelioid and with separate ostioles; joined ascomata pseudostromatic, pseudostromata 0·7–1·5 mm diam., erumpent to prominent, forming irregular lines, covered by thallus except for dark ostiole surrounded by whitish rim. Hamathecium clear. Ascospores 8 per ascus, densely muriform, fusiform, 33–52×16–22 µm, without thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from French Guiana).
Notes. This taxon is very similar to Astrothelium praetervisum but appears to lack the thin orange pigment characteristic of the latter.
New country record. Guyana: Upper Mazaruni: Paruima Mission, 1997, Sipman 39819 (B). Upper Takutu: Kuyuwini Landing, 1992, Sipman 57079 (B).
Astrothelium cinnamomeum (Eschw.) Müll. Arg.
Flora 68: 270 (1884).—Pyrenastrum cinnamomeum Eschw., in Martius, Icon. Sel. Pl. Crypt. 2: 18, t. 9, fig. 1 (1828); type: Brazil, Bahia, Caetité, Martius s. n. (M!—holotype).
Astrothelium conicum Eschw., in Martius, Syst. Lich.: 18 (1833); Eschweiler, Syst. Lich.: 26 (1824), nom. nud.—Heufleria conica (Eschw.) Trevis., Spighe e Paglie: 19 (1853); type: Brazil, Martius s. n. (M!—holotype).
Astrothelium minus Müll. Arg., Bot. Jahrb. Syst. 6: 382 (1885); type: Cuba, Wright s. n. (G!—holotype; NY!—isotype; Müller, Verr. Cub. 235).
Thallus corticate, light olive-green to yellowish, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata pseudostromatic, pseudostromata 0·4–0·7 mm diam., erumpent to prominent, conical with flattened top, covered by thallus and upper portion with yellow-orange, K+ purple, UV+ red pigment, area beneath the upper portion often whitish. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 23–30×6–10 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata with yellow to orange, K+ purple, UV+ red anthraquinone.
Distribution. Pantropical (previously reported from the USA, Mexico, Cuba, Trinidad, Tobago, Costa Rica, Guyana, French Guiana, Venezuela, Brazil, Seychelles, Hong Kong, Borneo and Australia).
Discussion. The synonymy of Astrothelium conicum (the type species of the genus) with A. cinnamomeum is confirmed by combining the observations of Müller (Reference Müller1884: 270), who observed the ascospores, and Harris (Reference Harris1986: 59), who observed no ascospores but saw the negative reaction with UV.
New country record. China: Yunnan: Xishuangbanna, Menglun, 2002, Aptroot 57255, 57026, 57267 (all ABL).
Astrothelium coccineum Córdova-Chávez et al.
In Córdova-Chávez et al., Cryptog., Mycol. 35: 159 (2014); type: Mexico, Veracruz, La Cortadure, Coatepec, 2062 m, 2013, Córdova-Chávez 464 (XAL!—holotype; ABL!—isotype).
(Fig. 14K)
Thallus corticate, olive-brown, verrucose-bullate.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata 0·7–1·5 mm broad, erumpent to prominent, rounded to irregular, covered by dark red pigment except for the blackish ostioles, internally with yellow pigment. Hamathecium clear. Ascospores 8 per ascus, 3-septate, oblong-ellipsoid, 25–30×10–13 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV−, outside K+ purple, inside K+ crimson red. TLC: a red anthraquinone at Rf 7 and a yellow anthraquinone at Rf 2.
Distribution. Neotropical (reported from Mexico).
Astrothelium confluens (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816676
Heufleria confluens Müll. Arg., Flora 66: 243 (1883).—Cryptothelium confluens (Müll. Arg.) Zahlbr., Catal. Lich. Univ. 1: 521 (1922); type: Brazil, Rio de Janeiro, Apiahy, Puiggari 143 (G!—lectotype, designated here).
(Fig. 37F)
Thallus corticate, greyish green to pale yellowish, uneven to bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles, rarely pleurotremoid with separate ostioles; joined ascomata 1–3 mm diam., prominent, completely covered by thallus. Hamathecium clear. Ascospores 8 per ascus, densely muriform, fusiform, c. 130×20 µm, with distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ascomata UV+ yellow, K−. TLC: lichexanthone on the ascomata.
Distribution. Neotropical (previously reported only from Brazil).
New country record. Guyana: Potaro-Siparuni: Kaieteur Falls National Park, 1996, Sipman 40482 (B).
Astrothelium consimile (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816677
Heufleria consimilis Müll. Arg., Bot. Jahrb. Syst. 6: 385 (1885).—Cryptothelium consimile (Müll. Arg.) Zahlbr., Catal. Lich. Univ. 1: 521 (1922); type: Cuba, Wright s. n. (G!—holotype).
(Fig. 36L)
Thallus corticate, olive-green yellowish, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles, rarely pleurotremoid with separate ostioles; joined ascomata dispersed to confluent or diffusely pseudostromatic, 0·8–1·2 mm diam., immersed-erumpent, covered by thallus but upper portion whitish with blackish ostiole area. Hamathecium clear. Ascospores 2 per ascus, densely muriform, fusiform, 85–130×23–36 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. No reactions or substances in the type material; however, the specimen from Brazil has the medulla UV+ yellow.
Distribution. Neotropical (previously reported from Cuba).
New country record. Brazil: Rondônia: Porto Velho, Parque Natural Municipal, 2012, Cáceres & Aptroot 15568 (ABL, ISE).
Astrothelium crassum (Fée) Aptroot
In Aptroot et al., Biblioth. Lichenol. 98: 44 (2008).—Trypethelium crassum Fée, Essai Crypt. Écorc.: 66 (1824).—Pyrenodium crassum (Fée) Fée, Essai Crypt. Écorc. Suppl.: 69 (1837); type: South America, “ad ramae Crotonis cascarillae” (G! —lectotype; L!—isolectotype).
Trypethelium clandestinum Fée, Essai Crypt. Écorc.: 68 (1824).—Polyblastia clandestina (Fée) Trevis., Spighe e Pagie: 15 (1853), non Jatta (1900).—Astrothelium clandestinum (Fée) Nyl., Mém. Soc. Sci. Nat. Cherbourg 5: 141(1857).—Pyrenastrum clandestinum (Fée) Müll. Arg., Bot. Jahrb. Syst. 6: 386 (1885); type: South America, “Cinch. jaune” (G—holotype, not seen; L!—isotype).
Astrothelium confusum Müll. Arg., Flora 68: 247 (1885); type: Colombia, Rio Magdalena, Lindig 141 (G!—holotype; BM!, BR—isotypes).
Thallus corticate, olive-green to yellowish brown, rather thick, uneven to bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed to confluent, 0·3–0·4 mm diam., erumpent, covered by thallus but upper portion whitish and ostiolar area blackish. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 21–27×8–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Pantropical; previously reported from Costa Rica, Guyana, Venezuela, Brazil, India, New Caledonia and Papua New Guinea.
New country records. Puerto Rico: Ponce: Caribbean National Forest, 1989, Aptroot & Aptroot 25523 (ABL).—Colombia: Amazonas: Araracuara, opposite Isla Morrocoy, 240 m, 1988, Sipman & Duivenvoorden 28502 (B).—Ecuador: Los Rios: 45 km S of Quevedo, 1982, Aptroot & Hensen 10422 (ABL).—Madagascar: Tamatave: Andasibe (Périnet), 1984, Aptroot & Hensen 13396 (ABL).—Indonesia: Java: South Semeru Lands, Tempur Sewa, 1939, Groenhart 1679, 2595 (ABL, L).
Astrothelium croceum Malme
Ark. Bot. 19(1): 12 (1924); type: Brazil, Matto Grosso, Serra da Chapada, Malme, 26 vii 1984 (S!—holotype).
Thallus corticate, light olive-green to yellowish, yellowish grey, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed to confluent, 0·4–0·7 mm diam., erumpent, covered by thallus and yellow-orange, K+ purple, UV+ red pigment. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 30–35×10–12 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−, without lichexanthone or pigment; ascomata with yellow-orange, K+ purple, UV+ red anthraquinone.
Distribution. Neotropical (reported from Brazil).
Astrothelium deforme (Fée) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816678
Trypethelium deforme Fée, Ann. Sci. Nat. 23: 454 (1831).—Meissneria varia Fée, Essai Crypt. Écorc. Suppl.: 66 (1837).—Bathelium deforme (Fée) Trevis., Spighe e Paglie: 20 (1853).—Trypethelium varium (Fée) Nyl., Mém. Soc. Acad. Maine-et-Loire 4: 78 (1858).—Bathelium varium (Fée) Trevis., Flora 44: 21 (1861).—Laurera varia (Nyl.) Zahlbr., in Engler & Prantl, Natürl. Pflanzenfamil. 1(1*): 71 (1903); type: Malaysia, Sarawak, Beccari (G-G00128943!—lectotype, designated here).
(Fig. 32H)
Thallus corticate, olive-green, uneven-verrucose.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·5–1·0 mm diam., erumpent, covered by thallus but upper portion blackish, internally with orange, K+ purple pigment. Hamathecium clear. Ascospores 8 per ascus, muriform, fusiform, 90–105×26–36 µm, without thickened median septum, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ascomata UV−, externally K−, internally K+ purple.
Distribution. Eastern palaeotropical (reported from Malaysia: Sarawak).
Discussion. Fée later replaced his earlier epithet deforme by varia because he found the species to be variable rather than deformed; however, according to the retroactive nomenclatural rules, his earlier epithet has to be taken up.
Astrothelium defossum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816679
Heufleria defossa Müll. Arg., Flora 68: 250(1885).—Cryptothelium defossum (Müll. Arg.) Zahlbr., Catal. Lich. Univ. 1: 521 (1922) non Campylothelium defossum Müll. Arg. (1891); type: French Guiana, Leprieur 168 (G!—holotype).
Thallus corticate, olive-green to light greyish green, uneven to bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles, rarely pleurotremoid with separate ostioles; joined ascomata dispersed to confluent or diffusely pseudostromatic, 0·8–1·3 mm diam., erumpent, covered by thallus but upper, often papilliform portion whitish with blackish ostiole. Hamathecium clear. Ascospores 8 per ascus, densely muriform, fusiform, 100–150×30–45 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ascomata UV+ yellow, K−. TLC: lichexanthone on the ascomata.
Distribution. Neotropical (previously reported from Venezuela and French Guiana).
New country record. Guyana: Upper Mazaruni: Paruima Mission, 1997, Sipman 39596 (B).
Astrothelium degenerans (Vain.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816680
Pseudopyrenula degenerans Vain., J. Bot. 34: 292 (1896).—Trypethelium degenerans (Vain.) Zahlbr., Catal. Lich. Univ. 1: 490 (1922).—Bathelium degenerans (Vain.) R. C. Harris, More Florida Lichens: 117 (1995); type: Dominica, Laudat, Elliott s. n. (TUR-Vainio 30782! —holotype; BM!—isotype).
Thallus corticate, olive-green to yellowish or brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata 1–2 mm diam., erumpent, forming irregular groups including numerous ascomata, dark brown, internally with yellow-orange, dusty granular pigment. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 15–25×6–9 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV−, externally K−, internally K+ red, with anthraquinone.
Distribution. Pantropical (previously reported from Mexico, Dominica, Jamaica, Puerto Rico, Costa Rica, El Salvador, Venezuela, Galapagos and Thailand).
New country records. Netherlands Antilles: Saba: Maskehorn, 2007, Sipman 54934 (B).—Ecuador: Manabi: Puerto Lopez, 1982, Aptroot & Hensen 10573, 10594 (ABL).
Astrothelium diplocarpoides Müll. Arg.
Bot. Jahrb. Syst. 6: 384 (1885).—Astrothelium diplocarpoides Nyl. (1876) nom. nud; type: Cuba, Wright s. n. (H-Nyl 191!—holotype; BM!—isotype; Müller, Verr. Cub. 143).
(Fig. 25J)
Thallus corticate, light olive-green but with distinct, whitish to pale yellowish pruina (lichexanthone), smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed, 0·8–1·5 mm diam., prominent to almost sessile, covered by thallus and whitish pruina. Hamathecium inspersed. Ascospores 8 per ascus, 5–7-septate, fusiform, 80–85×20 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Cuba).
Astrothelium diplocarpum Nyl.
Flora 47: 618 (1864).—Heufleria diplocarpa (Nyl.) Müll. Arg., Bot. Jahrb. Syst. 6: 385 (1885).—Cryptothelium diplocarpum (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 521 (1922); type: Colombia, Pie de Cuesta, Lindig s. n. (H-Nyl 109!—holotype).
(Figs 26C & D, Reference Hyde, Jones, Liu, Ariyawansha, Boehm, Boonmee, Braun, Chomnunti, Crous and Dai37K)
Thallus corticate, olive-green to yellowish brown, uneven-bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed, 0·7–1·0 mm diam., immersed-erumpent, covered by thallus except for the papilliform, orange to brown, K+ red ostiolar area. Hamathecium clear. Ascospores 8 per ascus, 9-septate to submuriform, fusiform, 90–110×22–28 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ascomata with brown, K+ red anthraquinone.
Distribution. Neotropical (previously reported from the USA, Costa Rica, Colombia and Ecuador).
New country record. Venezuela: Bolivar: Cerro Guaiquinima, 1000 m, 1990, Sipman 26568 (B).
Astrothelium dissimilum (Makhija & Patw.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816681
Trypethelium dissimilum Makhija & Patw., J. Hattori Bot. Lab. 73: 195 (1993); type: India, Meghalaya, Mawsyhram, 30 October 1977, Patwardhan & Nagarkar AMH 77.1169 (ABL!—isotype).
Thallus corticate, olive-green, uneven-verrucose.
Ascomata trypethelioid, with apical ostioles, confluent to pseudostromatic; pseudostromata 0·8–2·0 mm diam., prominent, dark brown, internally with yellow, K+ purple crystals. Hamathecium clear. Ascospores 8 per ascus, (3–)5(–9)-septate, fusiform, 25–40×6–9 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, externally K−; pseudostromata internally K+ red, with anthraquinone.
Distribution. Eastern palaeotropical (India and South Korea).
Discussion. The material from South Korea is tentatively assigned to this species, although the ascomata are smaller and less emergent (Fig. 17D).
Astrothelium effusum (Aptroot & Sipman) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816682
Laurera effusa Aptroot & Sipman in Aptroot et al., Biblioth. Lichenol. 98: 61 (2008); type: Costa Rica, Puntarenas, La Amistad International Park, Altamira Station, 1500–1600 m, Sipman 48034e (B!—holotype; INB-3944626!—isotype).
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·6–1·0 mm diam., immersed-erumpent, covered by thallus except dark ostiole surrounded by pale ring. Hamathecium inspersed. Ascospores 8 per ascus, muriform, ellipsoid, 80–100(–125)×28–48 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Costa Rica).
Astrothelium endochryseum (Vain.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816683
Pseudopyrenula endochrysea Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 206 (1890).—Trypethelium endochryseum (Vain.) Zahlbr., in Engler & Prantl, Natürl. Pflanzenfamil. 1(1*): 70 (1903).—Bathelium endochryseum (Vain.) R. C. Harris, More Florida Lichens: 117 (1995); type: Brazil, Minas Gerais, Caraça, Vainio s. n. (TUR-Vain 30793!—holotype; BM!—isotype; Vainio, Lich. Bras. 1157).
Thallus corticate, green to yellowish or brown, verrucose.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 1–2 mm diam., erumpent to prominent, dark brown, internally with yellow to orange, dusty granular pigment. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 35–50×12–15 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV−, externally K−, internally K+ red, with anthraquinone.
Distribution. Neotropical (Brazil).
Astrothelium eustomum (Mont.) Müll. Arg.
Flora 68: 247 (1885).—Pyrenastrum eustomum Mont., Ann. Sci. Nat. Bot., sér. 2 19: 63 (1843); type: French Guiana, Leprieur 179 (PC!—holotype; G! —isotype).
Trypethelium celatum Stirt., Proc. Roy. Philos. Soc. Glasgow 13: 193 (1881); type: India, Assam, Lezhore, Watt (BM!—isotype).
Astrothelium acroleucum Malme, Ark. Bot. 19(1): 15 (1924); type: Brazil, Matto Grosso, Santa Anna da Chapada, Malme 2358 (S!—lectotype, designated here).
(Fig. 25D)
Thallus corticate, olive-green to greyish green, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed to pseudostromatic, 0·4–0·8 mm diam., erumpent with flattened tops, with whitish to pale yellowish cover contrasting with the thallus. Hamathecium clear. Ascospores 8 per ascus, 3–5-septate, fusiform, 22–30×6–8 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV+ yellow, K−, with lichexanthone (or 1,8-dihydroxy-3,6-dimethoxyxanthone).
Distribution. Pantropical; previously reported from Costa Rica, Cuba, Guyana, French Guiana, Colombia, Venezuela, Brazil, Bolivia, India, Sri Lanka, Thailand and Australia.
Discussion. The three types cited above show the two extremes in terms of ascoma emergence and pseudostroma development, with Pyrenastrum eustomum and Trypethelium celatum showing the ascomata almost fully immersed in the thallus and mostly dispersed, and Astrothelium acroleucum showing erumpent pseudostromata.
New country records. Madagascar: Tamatave: Andasibe (Périnet), 1984, Aptroot & Hensen 13384 (ABL).—Papua New Guinea: Central: Varirata National Park, 1995, Sipman 38662 (B).—Solomon Islands: Guadalcanal Island: 1965, Hill 9119 (BM).
Astrothelium exostemmatis (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB816684
Bathelium exostemmatis Müll. Arg. Mém. Soc. Phys. Hist. Nat. Genève 30(3): 17 (1888); Laurera exostemmatis (Müll. Arg.) Zahlbr. in Engler & Prantl, Natürl. Pflanzenfamil. 1(1*): 71 (1903); type: Jamaica, “cort. Exostemmatis” (G!—holotype; M—isotype, not seen).
(Fig. 35L)
Thallus corticate, olive-brown to pale brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly clustered to diffusely pseudostromatic, 0·8–1·2 mm diam., when confluent pseudostromata up to 5 mm diam., immersed-erumpent, covered by thallus except dark ostiole surrounded by irregular whitish rim. Hamathecium clear. Ascospores 8 per ascus, muriform, ellipsoid, distinctly curved, 70–80×20–25 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances.
Distribution. Neotropical (previously reported from Jamaica).
New country record. Ecuador: Pastaza: Puyo, 1100 m, 1982, Aptroot & Hensen 10406 (ABL).
Astrothelium fallax Müll. Arg.
Bot. Jahrb. Syst. 6: 383 (1885).—Trypethelium pallescens Leight., Trans. Linn. Soc. London 27: 185 (1869) non Fée (Reference Fée1831); type: Sri Lanka, Thwaites 195 (BM!—lectotype, designated here; PC!, S!—isolectotypes).
Thallus corticate, olive-green to yellowish, uneven to strongly bullate, inducing pseudogall formation.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata pseudostromatic, 0·8–1·5(–2·0) mm diam., prominent to sessile, covered by thallus except for the blackish ostiole area surrounded by a whitish ring, forming large warts with uneven to bumpy surface. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 30–39×10–13 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Pantropical; previously reported from Cuba, India and Sri Lanka.
New country record. Guyana: Upper Mazaruni: Mount Latipu, 1985, Sipman & Aptroot 19120 (ABL, B).
Astrothelium feei (C. F. W. Meissn.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816685
Trypethelium feei C. F. W. Meissn., in Fée, Ann. Sci. Nat., Bot. sér. 1 23: 442 (1831).—Trypethelium scoria var. feei (C. F. W. Meissn.) Trevis., Flora 44: 10 (1861).—Bathelium feei (C. F. W. Meissn.) Aptroot, in Aptroot et al., Biblioth. Lichenol. 98: 51 (2008); type: South America, “Cascarill.” (G!—holotype; L!—isotype).
Trypethelium mastoideum var. macerum Müll. Arg., Bot. Jahrb. Syst. 6: 390 (1885); type: Cuba, Müller, Verr. Cub. 153 (G!—lectotype, designated here; BM!—isolectotype).
Thallus corticate, olive-brown to olive-green or yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata 1–2 mm diam., erumpent, dark brown, internally with yellow-orange, dusty granular pigment. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 18–25×5–9 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV−, externally K−, internally K+ yellow, with anthraquinone.
Distribution. Neotropical; previously reported from the USA, Costa Rica, Colombia, French Guiana and Galapagos.
Discussion. The difference in pseudostroma chemistry from the similar Astrothelium degenerans seems to warrant the distinction of the two species, but it is not correlated with distribution or any other character.
New country records. El Salvador: Ahuachapán: Parque Nacional El Imposible, 1999, Sipman & Bohnke 44912 (B).—Venezuela: Acosta, Aqua Salada, 1989, Kalb & Kalb s. n. (hb. Kalb).—Guyana: Marudi Mts, Aishalton, 1982, Stoffers et al. 251e (ABL).
Astrothelium ferrugineum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816686
Trypethelium ferrugineum Müll. Arg., Bot. Jahrb. Syst. 6: 392 (1885); type: Cuba, Wright s. n. (G!—lectotype, designated here; Müller, Lich. Cub. 593).
Trypethelium ferrugineum var. inornatum Müll. Arg., Bot. Jahrb. Syst. 6: 392 (1885); type: Cuba, Wright s. n. (G!—lectotype, designated here; Müller, Verr. Cub. 157).
Thallus corticate, olive-green but sparsely covered by orange pigment, uneven to shallowly verrucose.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata pseudostromatic; pseudostromata 0·8–1·5 mm diam., prominent, covered by thallus and sparse orange pigment except for the blackish ostioles. Hamathecium clear. Ascospores 8 per ascus, 3-septate, 20–27×7–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV+ red, K+ purple, with anthraquinone.
Distribution. Neotropical (Cuba).
Distribution. This species has recently been synonymized with Trypethelium aeneum (Harris Reference Harris1995), but it is kept separate here as no morphological intermediates occur and the world distribution and ecology seems to differ; this appears to be a very rare species.
Astrothelium floridanum Zahlbr. ex M. Choisy
In Choisy, Icon. Lich. Univ.: pl. 5 (1928).—Trypethelium floridanum (Zahlbr. ex M. Choisy) R. C. Harris, Acta Amazon. (Suppl.) 14: 73 (‘1984’) [1986]; type: USA, Florida, Rapp (W—holotype, not seen).
Thallus corticate, olive-green to brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent but not distinctly pseudostromatic, 0·3–0·6 mm diam., erumpent, covered by thallus except for the blackish ostiolar area surrounded by a whitish rim. Hamathecium clear. Ascospores 8 per ascus, 3-septate, 35–45×14–16 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical, extending into temperate regions; previously reported from the USA, Costa Rica and Japan.
Discussion. The synonymization with Astrothelium marcidum by Aptroot et al. (Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008) was based on a specimen in L that was apparently incorrectly thought to be an isotype of that taxon. The correct type has astrothelioid ascomata (see below).
New country records. Puerto Rico: Ponce: Caribbean National Forest, 1150 m, 1989, Aptroot & Aptroot 25608 (ABL).—Venezuela: Bolivar: Cerro Guaiquinima, 1000 m, 1990, Sipman 26683 (B).—Colombia: Amazonas: Araracuara, opposite Isla Mariñame, 240 m, 1988, Sipman & Duivenvoorden 28337 (B).
Astrothelium galligenum (Aptroot) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816687
Trypethelium galligenum Aptroot, Tropical Bryology 14: 29 (1998); type: Papua New Guinea, Central Province, Owen Stanley Range, Kagi Village, along Kokoda Trail towards Gap, Aptroot 39461 (B!—holotype; ABL!—isotype).
(Fig. 21B)
Thallus corticate, pale greenish, uneven to bullate, causing pseudogall formation.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 0·8–1·5 mm diam., erumpent, apically whitish and blackish ostioles surrounded by a brown rim. Hamathecium inspersed with yellow, K+ purple oil. Ascospores 8 per ascus, 7-septate, 50–60×12–17 µm, central septum euseptate and wider, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−; hamathecium internally K+ purple, with anthraquinone.
Distribution. Eastern palaeotropical (Papua New Guinea).
Astrothelium gigantosporum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816688
Bathelium gigantosporum Müll. Arg., Bot. Jahrb. Syst. 6: 394 (1885).—Laurera gigantospora (Müll. Arg.) Zahlbr., in Engler & Prantl, Natürl. Pflanzenfamil. 1(1*): 71 (1903); type: Cuba, Wright s. n. (G!—holotype).
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, diffusely pseudostromatic, pseudostromata 1–3(–5) mm diam., erumpent to prominent, covered by thallus except for dark ostioles surrounded by whitish rim. Hamathecium clear. Ascospores 2 per ascus, densely muriform, fusiform, 200–280×50–65 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from Cuba and Costa Rica).
New country record. Ecuador: Zamora-Chinchipe: Cordillera Numbala, Reserva Biologica San Francisco, 2004, Sipman 52875, 52646 (B).
Astrothelium gigasporum R. C. Harris
Acta Amazon. (Suppl.) 14: 61 (‘1984’) [1986]; type: Brazil, Amazonas, along Rio Curicuriari, Cachoeira Piraiwara, Buck 2532 (ABL!, NY!—isotypes).
Thallus corticate, olive-green, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata diffusely pseudostromatic, pseudostromata 0·8–1·5 mm diam., prominent to sessile, covered by thallus but with strongly protruding, papilliform, brown ostioles. Hamathecium clear. Ascospores 8 per ascus, 19–22-septate, fusiform, 105–115×18–20 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical; previously reported from Guyana, Venezuela and Brazil.
New country records. Colombia: Amazonas: Araracuara, Villazul, 300 m, 1988, Sipman & Duivenvoorden 28498 (B).—French Guiana: Saül, 1988, Sipman 31687 (B).
Astrothelium grossoides Aptroot & Lücking nom. nov.
MycoBank No.: MB 816689
Trypethelium grossum Müll. Arg., Bot. Jahrb. Syst. 5: 139 (1884), non Astrothelium grossum Müll. Arg. (1888); type: Papua New Guinea, Naumann 409 (BM!—isotype).
(Fig. 12I)
Thallus corticate, olive-green to brownish, uneven-bullate, pseudogall-inducing.
Ascomata trypethelioid, with apical ostioles, dense to diffusely pseudostromatic, 0·4–0·5 mm diam., immersed, covered by thallus. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, 15–32×9–12 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV+ yellow, K−. TLC: lichexanthone.
Distribution. Eastern palaeotropical; reported from Papua New Guinea, where it is locally abundant, and New Caledonia.
Astrothelium grossum Müll. Arg.
Flora 71: 141 (1888); type: New Caledonia, Pancher, 1870 (G!—holotype).
(Fig. 23E)
Thallus corticate, greenish to yellowish, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed to confluent but usually not pseudostromatic, 0·4–0·6 mm diam., erumpent to prominent, wart-shaped, covered by thallus and yellow-orange, K+ purple, UV+ red pigment. Hamathecium clear. Ascospores 8 per ascus, 3-septate, 30–35×10–15 µm, hyaline, IKI−.
Chemistry. Thallus UV+ yellow, K−, with lichexanthone; ascomata with yellow to orange, K+ purple, UV+ red anthraquinone.
Distribution. Reported only from New Caledonia, from where we also observed additional specimens.
Discussion. This taxon corresponds to what has traditionally been named Astrothelium versicolor. The type of the latter has a very peculiar thallus morphology and hence we suspect that many collections identified with the name A. versicolor might represent A. grossum.
Astrothelium heterophorum Nyl.
Bull. Soc. Linn. Normand., sér. 2 2: 133 (1868); type: New Caledonia, Lifu, Thiébaut (H-NYL—holotype, not seen).
Trypethelium elmeri (Vain.) R. C. Harris, Lichenogr. Thomsoniana: 143 (1998).—Pseudopyrenula elmeri Vain., Ann. Acad. Sci. Fenn., ser. A 6: 354 (1921); type: Philippines, Luzon, Irosin, Sorsogon, Elmer 14655 (TUR-Vain 30812!—holotype; ABL!, G!—isotypes).
(Fig. 30E)
Thallus corticate, olive-green to yellowish, smooth to uneven.
Ascomata pleurothelioid and with separate ostioles or in part astrothelioid, several chambers joined with eccentric, fused ostioles; single or joined ascomata dispersed, 0·5–1·0 mm diam., immersed-erumpent, covered by thallus except for blackish ostiolar area. Hamathecium clear. Ascospores 8 per ascus, 9–19-septate, fusiform, 60–80×12–17 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (Philippines and New Caledonia).
Astrothelium indicum (Upreti & Ajay Singh) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816690
Laurera indica Upreti & Ajay Singh, Bull. Jard. Bot. Nat. Belg. 57: 372 (1987) non Makhija & Patw. (1988); type: India, Bengal, Kurz 202 (H-Nyl 122!—holotype; BM!—isotype).
(Fig. 33G)
Thallus corticate, olive-green to brownish but whitish pruinose, uneven-rugose.
Ascomata trypethelioid with apical ostioles, more or less grouped but not distinctly pseudostromatic, groups of ascomata 0·8–1·2 mm diam., erumpent, covered by thallus and whitish pruina except for the blackish ostiolar area. Hamathecium inspersed. Ascospores (4–)8 per ascus, muriform, ellipsoid, (33–)50–70×13–17 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (India and Thailand).
Astrothelium infossum (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816691
Verrucaria infossa Nyl., Flora 69: 178 (1886).—Pseudopyrenula infossa (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 357 (1922).—Trypethelium infossum (Nyl.) R. C. Harris, Lichenogr. Thomsoniana: 144 (1998); type: São Tomé, Bom Successo, Moller s. n. (H-Nyl 1034!—holotype; BM!—isotype).
(Fig. 18G)
Thallus corticate, olive-brown to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·3–0·4 mm diam., immersed, covered by thallus except for blackish ostiolar area. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 22–27×7–9 µm, hyaline, IKI+ violet.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Africa (São Tomé).
Discussion. A very inconspicuous species except for the amyloid ascospores, a rare feature in the family. Otherwise, the species falls within the Astrothelium nitidiusculum complex.
Astrothelium infuscatulum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816692
Trypethelium infuscatulum Müll. Arg., Bot. Jahrb. Syst. 6: 389 (1885).—Pseudopyrenula infuscatula (Müll. Arg.) Vain., Ann. Acad. Sci. Fenn., ser. A 6(7): 197 (1915); type: Cuba, Wright s. n. (G!—holotype; L!—isotype; Müller, Verr. Cub. 175).
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata 1–2 mm broad, erumpent, irregular to linear, with flattened surface, blackish brown except for a pale brown rim around the ostioles. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 25–30×7–10 µm, hyaline, IKI+ violet.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical; previously reported from Cuba, Colombia, Venezuela and Guyana.
Discussion. The conspicuous pseudostromata resemble those of Astrothelium degenerans and A. feei, but lack the internal yellow-orange pigment and are conspicuously flattened above. The amyloid ascospores also set A. infuscatulum apart.
New country record. Surinam: Brokopondo: Brownsberg, 1985, Aptroot 14865 (ABL).
Astrothelium inspersaeneum E. L. Lima et al.
In Lima et al. Bryologist 116: 327 (2013); type: Brazil, Pernambuco, Buíque, Catimbau National Park, on bark of tree, 900 m, 3 February 2012, Lima 394 (ISE!—holotype).
(Fig. 14G)
Thallus corticate, light olive-brown to yellowish, covered by orange pigment.
Ascomata trypethelioid, with apical ostioles, usually aggregated in groups or lines but not distinctly pseudostromatic, 0·2–0·4 mm diam., erumpent, covered by thallus and orange pigment except black ostiolar area. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, oblong-ellipsoid, 20–25×8–10 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K+ purple. TLC: an anthraquinone, probably parietin.
Distribution. Neotropical (previously reported only from Brazil).
Discussion. This species is most similar in aspect and internal structures to Astrothelium neogalbineum, but differs markedly by the inspersed hamathecium and the absence of lichexanthone.
New country record. Peru: San Martin: Tarapoto, Santesson & Thor P71:32 (S).
Astrothelium interjectum R. C. Harris
Acta Amazon. (Suppl.) 14: 61 (‘1984’) [1986]; type: Brazil, Amazonas, Serra Aracá, Samuels 838 (NY!—isotype).
Thallus light olive-green to greyish green, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata pseudostromatic; pseudostromata 1–2(–3) mm diam., erumpent to prominent, whitish and darker ostiolar areas. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 25–30×6–11 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV+ yellow, K−, with lichexanthone.
Distribution. Pantropical; previously reported from Costa Rica and Brazil.
New country records. Colombia: Nariño: Tumaco, Estacion Forestal La Espriella, 1986, Sipman & Velosa 33041 (B).—Guyana: Upper Takutu: Kuyuwini Landing, 1992, Sipman 57017 (B).—Papua New Guinea: Madang: Gogol Valley, Tgubi logging site, 1992, Sipman 35965 (ABL, B); 11 km W of Brahman Mission, 1995, Aptroot 38706 (ABL).
Astrothelium intermedium Aptroot & Lücking
In Aptroot et al., Biblioth. Lichenol. 98: 46 (2008); type: Costa Rica, Puntarenas, Las Cruces Biological Station, Lücking s. n. (F!—holotype).
Thallus corticate, olive-green, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata diffusely pseudostromatic; pseudostromata 1–2 cm diam. (individual ascomata 0·3–0·5 mm diam.), erumpent, irregular to linear and in part confluent and anastomosing, covered by thallus except dark ostioles surrounded by a pale rim. Hamathecium clear. Ascospores 8 per ascus, 3-septate, 29–33×12–15 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Costa Rica).
Astrothelium irregulare (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816693
Bathelium irregulare Müll. Arg., Hedwigia 30: 234 (1891).—Laurera irregularis (Müll. Arg.) Zahlbr., Catal. Lich. Univ. 1: 504 (1922); type: Brazil, Theresopolis, Schenck 4643 (G!—holotype).
(Fig. 34F)
Thallus corticate, olive-green to brownish, uneven-verrucose.
Ascomata trypethelioid, with apical ostioles, solitary or rarely irregularly confluent, 0·6–1·0 mm diam., erumpent to prominent, covered by thallus. Hamathecium inspersed. Ascospores 8 per ascus, densely muriform, fusiform, 135–165×36–52 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Brazil).
Astrothelium isabellinum Eschw.
In Martius, Icon. Plant. Cryptog. 2: 20 (1828).—Astrothelium isabellinum Eschw. (1824), nom. nud.—Heufleria isabellina (Eschw.) Trevis., Flora 44: 23 (1861).—Cryptothelium isabellinum (Eschw.) Zahlbr., Catal. Lich. Univ. 1: 522 (1922); type: Brazil, s. col. (M—holotype, not seen).
Thallus corticate, olive-green, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata diffusely pseudostromatic; pseudostromata 1–2 mm diam., erumpent, covered by thallus, internally with red pigment. Hamathecium clear. Ascospores 2 per ascus, densely muriform, fusiform, 150–200×40–50 µm, without distinctly thickened median septum, hyaline, IKI–.
Chemistry. Thallus UV−, K−; pseudostromata UV−, externally K−, internally with K+ purple anthraquinone.
Distribution. Neotropical (Brazil).
Astrothelium keralense (Upreti & Ajay Singh) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816694
Laurera keralensis Upreti & Ajay Singh, Bull. Jard. Bot. Nat. Belg. 57: 374 (1987); type: India, Kerala, Quilon, Kundara, Singh & Ranjan 102951 (LWG—holotype, not seen).
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·6–1·0 mm diam., erumpent to prominent, hemispherical, covered by thallus. Hamathecium clear. Ascospores 8 per ascus, muriform, fusiform, 50–60×15–20 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (India and Thailand).
Astrothelium kunzei (Fée) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816695
Trypethelium kunzei Fée, Ann. Sci. Nat. 23: 445 (1831); type: Surinam, Kunze s. n. (G!—holotype).
Thallus corticate, olive-brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 0·5–1·0 mm broad, immersed, forming irregular, partly reticulate lines, covered by thallus and orange pigment except dark ostioles. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 20–27×7–10 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV+ red, K+ purple, with anthraquinone.
Distribution. Neotropical (Panama and Surinam).
Discussion. The epithet kunzei is taken up here for material in the Astrothelium aeneum aggregate that produce distinct, immersed, linear-reticulate pseudostromata. This distinction is supported by molecular data (Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ).
Astrothelium laevigatum Müll. Arg.
Flora 66: 245 (1883); type: Brazil, Apiahy, Puiggari 1689 (G!—holotype).
Astrothelium simplicatum Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 194 (1890); type: Brazil, Minas Gerais, Sítio, Vainio 1006 (TUR-Vain 30870!—holotype; BM!—isotype).
Thallus corticate, greenish grey (lichexanthone colour), smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed, not pseudostromatic, 0·3–0·5 mm diam., erumpent, covered by thallus but upper part whitish contrasting with the dark ostiolar area. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 20–26×7–9 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV+ yellow, K−, with lichexanthone (or 1,8-dihydroxy-3,6-dimethoxyxanthone).
Distribution. Neotropical (Brazil).
Astrothelium leioplacum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816696
Clathroporina leioplaca Müll. Arg., Revue Mycol. 10: 182 (1888); type: Paraguay, Balansa s. n. (G!—holotype).
Clathroporina irregularis Müll. Arg., Revue Mycol. 10: 182 (1888); type: Paraguay, Balansa 231 (G!—lectotype, designated here).
(Fig. 39H)
Thallus corticate, olive-grey, smooth to uneven.
Ascomata pleurothelioid, with separate ostioles, 0·4–0·6 mm diam., erumpent, completely covered by thallus. Hamathecium clear. Ascospores 8 per ascus, densely muriform, fusiform, 33–52×16–22 µm, without thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Paraguay).
Astrothelium leucoconicum Nyl.
Flora 52: 126 (1869); type: Brazil, Rio de Janeiro, Glaziou s. n. (H-Nyl 118!—holotype).
Thallus corticate, light olive-green to yellowish, uneven to shallowly bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed, not pseudostromatic, 0·4–0·7 mm diam., erumpent, covered by thallus but upper part whitish contrasting with the dark ostiolar area. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 50–72×20–26 µm, hyaline, IKI−.
Chemistry. Thallus UV+ yellow, K−, with lichexanthone (or 1,8-dihydroxy-3,6-dimethoxyxanthone); ascomata UV−, K−.
Distribution. Neotropical (previously reported from Brazil).
New country record. Venezuela: Bolivar: Cerro Guaiquinima, 1500 m, 1990, Sipman 27215 (B).
Astrothelium leucothelium Nyl.
Ann. Sci. Nat., Bot., sér. 5 7: 348 (1867); type: Colombia, Rio Negro, Lindig s. n. (H-Nyl 117!—holotype; S!—isotype).
(Fig. 23G)
Thallus corticate, greenish grey (lichexanthone colour), becoming brownish yellow, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata solitary to confluent or diffusely pseudostromatic, 0·4–0·6 mm diam., erumpent, covered by thallus but upper part whitish, with dark ostiolar area. Hamathecium clear. Ascospores 8 per ascus, 3(–5)-septate, fusiform, 32–52×10–17 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (previously reported from Costa Rica, Colombia and Brazil).
New country records. Venezuela: Merida: Moro Negro, 1980, López Figueras & Rodríguez 22888 (ABL, MERF).—French Guiana: Saül, 1988, Sipman 31769, 31757 (ABL, B).
Astrothelium lugescens (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816697
Verrucaria lugescens Nyl., Flora 69: 177 (1886).—Anthracothecium lugescens (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 463 (1922).—Campylothelium lugescens (Nyl.) Upreti & Ajay Singh, Bull. Soc. Bot. Fr., Let. Bot. 134: 293 (1987); type: São Tomé and Príncipe, Henriques s. n. (H-Nyl 1067!—holotype).
(Fig. 38I)
Thallus corticate, olive-green, smooth to uneven.
Ascomata pleurothelioid, with separate ostioles, 0·6–1·0 mm diam., erumpent, covered by thallus. Hamathecium clear. Ascospores 1 per ascus, densely muriform, fusiform, 210–230×45–70 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. African palaeotropical (São Tomé and Príncipe).
Astrothelium luridum (Zahlbr.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816698
Trypethelium luridum Zahlbr., Ann. Mycol. 14: 47 (1916); type: Japan, Thushima, Faurie 3744 (W—holotype, not seen).
Trypethelium scoria f. endochraceum Nyl, Lich. Jap.: 115 (1890); type: Malaysia, Labuan, Almquist, 1897 (S!—isotype).
Trypethelium endosulphureum Makhija & Patw., J. Hattori Bot. Lab. 73: 197 (1993); type: India, Meghalaya, Mohmtheid-Cherapunji road, 20 October 1977, Patwardhan & Nagarkar AMH 77.905 (ABL!—isotype).
Thallus corticate, olive-brown to yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 0·8–1·5 mm broad, erumpent to prominent, covered by thallus except for dark ostiolar area, internally with yellow pigment. Hamathecium clear. Ascospores 8 per ascus, 7–11-septate, fusiform, 55–88×13–28 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata U−, internally K+ red, with anthraquinone.
Distribution. Asia, extending into temperate regions (India and Japan).
Astrothelium macrocarpum (Fée) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816699
Porina macrocarpa Fée, Essai Crypt. Écorc.: 81 (1824); non Riddle 1920.—Porophora macrocarpa (Fée) Spreng., Syst. Veget. 4(1): 241 (1827).—Pyrenodium macrocarpum (Fée) Fée, Essai Crypt. Écorc. Suppl.: 69 (1837).—Pyrenula macrocarpa (Fée) Massal., Ricerch. Auton. Lich.: 164 (1852).—Astrothelium hypoxylon var varmacrocarpum (Fée) Nyl., Mém. Soc. Sci. Nat. Cherbourg 5: 141 (1857).—Astrothelium sulphureum var. macrocarpum (Fée) Nyl., in Hue, Nouv. Archiv. du Muséum, sér. 3 4: 132 (1892); type: South America, “Cort. cinchonae” (M!—isotype).
Astrothelium galbineum Kremp., Nuovo Giorn. Bot. Ital. 7: 58 (1875); type: Sarawak, Beccari 23 (M!—lectotype; Harris, Acta Amazon. (Suppl.) 14: 60 (‘1984’) [1986]).
Astrothelium ochrothelizum Müll. Arg., Bot. Jahrb. Syst. 6: 382 (1885).—Trypethelium ochrothelizum Nyl., Flora 59: 365 (1876), nom. nud.—Trypethelium ochrothelizum (Müll. Arg.) Nyl., Sert. Lich. Trop. Labuan et Singap.: 26 (1891); type: Cuba, Wright s. n. (G!—holotype; BM!—isotype; Müller, Verr. Cub. 144).
Astrothelium conicum var. pallidum Müll. Arg., Bot. Jahrb. Syst. 6: 382 (1885); type: Cuba, Wright s. n. (G!—lectotype, designated here; BM!—isolectotype; Müller, Verr. Cub. 605).
Astrothelium ochrothelioides Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 194 (1890); type: Brazil, Minar Gerais, Lafayette, Vainio s. n. (TUR-Vain 30868!—lectotype, designated here; Vainio, Lich. Bras. Exs. 310).
Trypethelium discolor Müll. Arg., Hedwigia 34: 35 (1895); type: Brazil, Minas Gerais, Serra do Ouro Preto, Ule 298 (G!—holotype).
Thallus corticate, olive-green to yellowish, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata pseudostromatic; pseudostromata 0·5–1·0 mm diam., erumpent to prominent, conical with flattened top, covered by thallus with lateral part whitish and upper part with yellow-orange pigment and dark ostiole. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 21–28×7–11 µm, hyaline, IKI−.
Chemistry. Thallus UV+ yellow, K−, with lichexanthone (or 1,8-dihydroxy-3,6-dimethoxyxanthone); pseudostromata with yellow-orange K+ purple, UV+ red anthraquinone, probably parietin.
Discussion. The rather well-known name Astrothelium galbineum has to be replaced by the older epithet macrocarpum, which was in use for a century but then surprisingly went out of use. The synonymy of A. ochrothelioides is based on the original description of the ascospores; Harris (Reference Harris1986: 59) reported that no spores remain. Typical A. macrocarpum has conical pseudostromata in which only the upper part is covered by pigment, whereas the types of A. ochrothelioides and Trypethelium discolor have wart-shaped pseudostromata completely covered by pigment; thus, more than one species might be contained here.
Distribution. Pantropical; previously reported from the USA, Cuba, Costa Rica, Panama, Venezuela, Guyana, French Guiana, South Africa, India, Sri Lanka, Singapore, Thailand, Borneo, Papua New Guinea and Australia.
New country records. Colombia: Amazonas: Araracuara, opposite airstrip, 350 m, 1988, Sipman & Duivenvoorden 27863 (B).—Gabon: Nzé, 2006, Ertz 9719 (BR).
Astrothelium macrosporum (Makhija & Patw.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816700
Trypethelium macrosporum Makhija & Patw., J. Hattori Bot. Lab. 73: 200 (1993); type: India, Meghalaya, Cherapunji Road, 27 October 1977, Patwardhan & Nagarkar AMH 77.826 (ABL!—isotype).
Thallus corticate, olive-green to yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·7–1·0 mm diam., erumpent, covered by thallus except dark ostiolar area surrounded by irregular whitish rim. Hamathecium finely inspersed. Ascospores 8 per ascus, 9–13-septate, occasionally with a longitudinal septum, fusiform, 120–195×17–22 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (India).
Astrothelium marcidum (Fée) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816701
Pyrenula marcida Fée, Essai Crypt. Écorc.: 77 (1824).—Verrucaria marcida Spreng. (1827) nom. nud.—Trypethelium marcidum (Fée) Müll. Arg., Mém. Soc. Phys. Hist. Nat. Genève 30(3): 11 (1888); type: South America “ad corticem Cinchonarum”, “b” (G!—holotype).
(Fig. 29F)
Thallus corticate, olive-brown, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata pseudostromatic, pseudostromata 0·7–1·0 mm diam., immersed-erumpent, covered by thallus or yellowish white layer. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 35–45×14–16 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical.
Discussion. The epithet marcidum was suggested to replace the epithet floridanum by Aptroot et al. (Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008), as both agree in thallus morphology, ascospore type, and lack of secondary substances. The type material of Pyrenula marcida is in very bad shape, with almost all ascomata abraded; however, re-examination revealed that the ascoma organization is astrothelioid, not trypethelioid as in Astrothelium floridanum, since several remaining ascoma bases show a pyriform shape with the tip pointing centrally.
Astrothelium megaleium (Kremp.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 8166702
Trypethelium megaleium Kremp., Nuovo Giorn. Botan. Ital. 21(3): 13 (1875).—Bathelium megaleium (Kremp.) Müll. Arg., Linnaea 63: 45 (1880).—Laurera megaleia (Kremp.) Zahlbr., Catal. Lich. Univ. 1: 505 (1922); type: Malaysia, Sarawak, Beccari s. n. (M!—holotype).
?Trypethelium oligosporum Mont. & Bosch, in Mont., Sylloge Gener. Spec. Cryptog.: 374 (1865).—Bathelium oligosporum (Mont. & Bosch) Trevis., Flora 44: 21 (1861).—Laurera oligospora (Mont. & Bosch) Zahlbr., Catal. Lich. Univ. 1: 505 (1922); type: Indonesia, Java, Junghuhn s. n. (L—holotype lost).
(Fig. 33I)
Thallus corticate, olive-brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·8–1·5 mm diam., erumpent, mostly exposed, dark brown. Hamathecium inspersed. Ascospores 2 per ascus, densely muriform, fusiform, 210–225×32–35 µm, with distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (Malaysia: Sarawak, and Indonesia).
Discussion. The synonymy of Trypethelium oligosporum is uncertain, as the type is lost, and therefore this older epithet is not taken up.
Astrothelium megalophthalmum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816703
Trypethelium megalophthalmum Müll. Arg., Hedwigia 34: 35 (1895); type: Brazil, Santa Catarina, Serra Geral, Ule 291 (G!—holotype; NY—isotype).
Thallus corticate, olive-green, verrucose-bullate.
Ascomata trypethelioid, with apical ostioles, diffusely pseudostromatic; pseudostromata 1–3(–5) mm diam., immersed-erumpent, with upper portion of ascomata exposed, blackish brown. Hamathecium clear. Ascospores 8 per ascus, 3–7-septate, fusiform, 85–120×20–32 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Brazil).
Astrothelium megalostomum (Vain.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816704
Heufleria megalostoma Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 193 (1890).—Cryptothelium megalostomum (Vain.) Zahlbr., Catal. Lich. Univ. 1: 522 (1922).—Campylothelium megalostomum (Vain.) Aptroot, Fungal Diversity 9: 29 (2002); type: Brazil, Minas Gerais, Serra do Caraça, Vainio s. n. (TUR-Vain 303838!—holotype; Vainio, Lich. Bras. Exs. 1587).
(Fig. 36E)
Thallus corticate, light olive-green, smooth to rough.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed, 0·8–1·3 mm diam., erumpent, covered by thallus except dark ostiolar area. Hamathecium inspersed. Ascospores 2 per ascus, densely muriform, fusiform, 170–220×50–64 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ascomata UV+ yellow, with lichexanthone, K−.
Distribution. Neotropical (Brazil).
Astrothelium megaspermum (Mont.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816705
Trypethelium megaspermum Mont., Ann. Sci. Nat., Bot., sér. 2 19: 68 (1843).—Bathelium megaspermum (Mont.) Trevis., Spighe e Paglie: 20 (1861).—Laurera megasperma (Mont.) Riddle, Bull. Torrey Bot. Club 44: 323 (1917); type: French Guiana, Leprieur 603 (FH-TAYL 11!—isotype).
Trypethelium ostendatum Kremp., Vidensk. Meddel. Dansk Naturhist. Foren. Kjøbenhavn 5: 398 (1874).—Bathelium ostendatum (Kremp.) Müll. Arg., Linnaea 63: 45 (1880).—Laurera ostendata (Kremp.) Zahlbr., Catal. Lich. Univ. 1: 506 (1922); type: Brazil, Minas Gerais, Serra d’Estrella, Warming 79 (M!—holotype).
Verrucaria euthelia Nyl., Flora 69: 177 (1886); type: São Tomé and Príncipe, Moller s. n. (H-Nyl 1064! —holotype).
Thelenella fulva Vain., Catal. Welwitsch Afric. Plants 2: 451 (1901).—Clathroporina fulva (Vain.) Zahlbr., Catal. Lich. Univ. 1: 418 (1922).—Polyblastiopsis fulva (Vain.) C. W. Dodge, Ann. Missouri Bot. Gard. 40: 275 (1953); type: Angola, Golungo Alto, Mata Quisuculo, Welwitsch 230 (TUR-Vain 31066!—holotype; BM!—isotype).
Julella zenkeriana P. Henn., Bot. Jahrb. Syst. 38: 127 (1905); type: Cameroon, Bipinde, Zenker 1980 (B!—holotype).
Polyblastiopsis pertusarina Zahlbr., Feddes Repert. 31: 199 (1933); type: Taiwan, Mount Arisan, Toroyen, Asahina 299 (W—holotype, not seen).
Campylothelium nitidum Zahlbr., Ann. Mycol. 33: 39 (1935) non Müll. Arg. (1891).—Campylothelium zahlbruckneri R. G. Werner, Bull. Soc. Sci. Nat. Maroc. 24: 130 (1944); type: USA, Florida, Sanford, Rapp 84 (W—holotype, not seen).
Clathroporina diphloea Zahlbr., Ann. Mycol. 33: 37 (1935); type: USA, Florida, Rapp (W—holotype, not seen).
Laurera megasperma f. immersa Letr.-Gal., Rev. Bryol. Lichénol. 26: 234 (1957); type: Sri Lanka, Petsch (BM!—holotype).
Laurera megasperma f. conica Letr.-Gal., Rev. Bryol. Lichénol. 27: 67 (1958); type: Ivory Coast, Mont Orombe Boka, Dimbokro, 20 August 1954, Santesson 10724o (UPS—holotype, not seen).
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·8–1·5 mm diam., prominent, hemispherical with flattened top, covered by thallus except for dark ostiolar area surrounded by whitish rim. Hamathecium inspersed. Ascospores generally 4 per ascus, densely muriform, oblong-fusiform, 140–220×30–75 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical; previously reported from the USA, Costa Rica, Cuba, Jamaica, Puerto Rico, French Guiana, Brazil, Argentina, Ivory Coast, Angola, Cameroon, India, Sri Lanka, Thailand, Vietnam, Taiwan, New Caledonia and Papua New Guinea.
New country records. Colombia: Nariño: Tumaco, Estacion Forestal La Espriella, 1986, Sipman & Velosa 33054 (B).—Guyana: Upper Takutu: Kuyuwini Landing, 1992, Sipman 58222 (B).—Ecuador: Napo: 5 km N of Sta. Rosa, 400 m, 1982, Aptroot & Hensen 10611, 10612 (ABL).—Peru: Maynas: Iquitos, 1980, Schumm & Pudor 11952 (hb. Schumm).—Gabon: Nzé, 2006, Ertz 9725 (BR).—DR Congo: Mongala: Lisala, 2009, Ertz 14181 (BR).—Malaysia: Pahang: Fraser’s Hill, 1989, Sipman et al. 45022 (B).—China: Yunnan: Xishuangbanna, Menglun, 2002, Aptroot 57355 (ABL).—Indonesia: Sulawesi: Tondano, 1000 m, 1988, Hensen s. n. (ABL).
Astrothelium meiophorum (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816732
Trypethelium annulare var. meiophorum Nyl., Bull. Soc. Linn. Normand., sér. 2 2: 132 (1868).—Trypethelium meiophorum (Nyl.) Müll. Arg., Hedwigia 31: 286 (1892); type: New Caledonia, Pancher s. n. (G!—lectotype, designated here; BM!—isolectotype).
Trypethelium flavoalbum Makhija & Patw., J. Hattori Bot. Lab. 73: 197 (1993); type: India, Megahalya, near Sohararim, 20 October 1977, Patwardhan & Nagarkar AMH 86.697 (ABL!—isotype).
Trypethelium parvicarpum Makhija & Patw., J. Hattori Bot. Lab. 73: 205 (1993); type: India, Nicobar Islands, Kamorta, Daring, 18 January 1987, Nagarkar & Patwardhan AMH 87.389 (ABL!—isotype).
Thallus corticate, light olive-green to yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, irregularly grouped to diffusely pseudostromatic; pseudostromata 0·5–0·7 mm broad, erumpent, covered by thallus except for broad, dark ostiolar area. Hamathecium clear. Ascospores (2–)8 per ascus, fusiform-ellipsoid, 3-septate, 20–25×7–8 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (previously reported from India and New Caledonia).
Discussion. Unfortunately the type material of Trypethelium annulare var. meiophorum is extremely small and hence the new synonyms listed here are somewhat tentative, although morphologically they seem to agree well, particularly the type of T. parvicarpum. We were unable to confirm the statement of 2-spored asci given in the protologue of Astrothelium meiophorum, since only two ascomata are left in the type, but we believe that this observation is based on an ascus with most ascospores already expelled. The two synonyms listed regularly have 8-spored asci. Additional material found under this name from New Caledonia is A. scoria. It should also be mentioned that the ascospores in the type specimen of Trypethelium parvicarpum are c. 23×7 µm, larger than given in the protologue of that taxon.
New country record. China: Yunnan: Xishuangbanna, 2002, Aptroot 57350 (ABL).
Astrothelium meristosporoides (P. M. McCarthy & Vongshew.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816706
Laurera meristosporoides P. M. McCarthy & Vongshew. in Vongshewarat et al., Mycotaxon 70: 228 (1999); type: Thailand, Phisanulok Prov., Phu Hin Rong Kla National Park, Vongshewarat 173 (CANB—holotype, not seen).
(Fig. 31E)
Thallus corticate, light greenish yellow, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, prominent, whitish and black ostiolar area, 0·7–1·5 mm diam. Hamathecium inspersed. Ascospores 8 per ascus, muriform, fusiform, 68–120×18–24 µm, with somewhat thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV+ yellow, K−, with lichexanthone or 1,8-dihydroxy-3,6-dimethoxyxanthone.
Distribution. Palaeotropical (previously reported only from Thailand).
New country record. Papua New Guinea: Central: Varirata National Park, 800 m, 1987, Aptroot 19135 (ABL).
Astrothelium meristosporum (Mont. & Bosch) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816707
Trypethelium meristosporum Mont. & Bosch, in Junghuhn, Pl. Jungh. 4: 487 (1855).—Meristosporum javanicum A. Massal., Atti I. R. Istitut. Veneto, Ser. 3 5: 328 (1860).—Bathelium meristosporum (Mont. & Bosch) Trevis., Flora 44: 21 (1861).—Melanotheca javanica (A. Massal.) Zahlbr., in Engler & Prantl, Natürl. Pflanzenfamil. 1(1*): 70 (1903).—Laurera meristospora (Mont. & Bosch) Zahlbr., Catal. Lich. Univ. 1: 505 (1922); type: Indonesia, Java, Junghuhn (L!—isotype).
Bathelium chrysocarpum Müll. Arg., Hedwigia 30: 54 (1891).—Laurera chrysocarpa (Müll. Arg.) Zahlbr., Catal. Lich. Univ. 1: 503 (1922); type: Australia, Queensland, Bellenden Ker, Bailey 540 (G! —holotype; BRI!—isotype).
Verrucaria anoptella Stirt., Proceed. Philosoph. Soc. Glasgow 13: 190 (1881).—Clathroporina anoptella (Stirt.) Zahlbr., Catal. Lich. Univ. 1: 415 (1922).—Polyblastiopsis anoptella (Stirt.) Ajay Singh, Nova Hedwigia 36: 238 (1982); type: India, Assam, Watt s. n. (BM!—holotype).
Thelenella interrupta Vain., Hedwigia 46: 180 (1907).—Clathroporina interrupta (Vain.) Zahlbr., Catal. Lich. Univ. 1: 418 (1922); type: Thailand, Koh Chang Island, Lem Dam, Schmidt 17 (TUR-Vain 31065—holotype, not seen).
Laurera columellata Makhija & Patw., Mycotaxon 31: 574 (1988); type: India, Karnataka, S. Kanara, Hiriyadaka, Udupi-Hebri Road, 22 February 1978, Patwardhan AMH 78.59 (ABL!—isotype).
Laurera andamanica D. D. Awasthi, Biblioth. Lichenol. 40: 3 (1991).—Laurera indica Makhija & Patw., Mycotaxon 31: 578 (1988) non Upreti & Ajay Singh (1987); type: India, Andaman Islands, South Andaman, Baratang Islands, Bishnu Nala, 22 February 1985, Patwardhan & Sethy AMH 85.656 (ABL!—isotype).
Thallus corticate, light olive-green, uneven to verrucose.
Ascomata trypethelioid, with apical ostioles, 1·0–1·5 mm diam., prominent, hemispherical with flattened top, covered by thallus except whitish to greyish ostiolar area, 0·7–2·0 mm diam. Hamathecium hyaline or slightly yellowish, inspersed. Ascospores (4–)8 per ascus, muriform, oblong-fusiform, 120–220×25–40 µm, constricted at the markedly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV+ yellow, K−, with lichexanthone or 1,8-dihydroxy-3,6-dimethoxyxanthone.
Distribution. Palaeotropical (previously reported from India, Sri Lanka, Thailand, Indonesia, Papua New Guinea, Australia); the specimen reported here from French Guiana is atypical.
Discussion. As can be expected for a species with such large ascospores, there is considerable variation in size, even within a single ascoma.
New country records. French Guiana: Montsinery, 20 km W of Cayenne, along forest track called Risque tout, 50 m, 1985, Aptroot 15125 (ABL, B).—Malaysia: Belum, Halong Trail, 1994, Nätebusch s. n. (hb. Kalb).—Solomon Islands: Kolombangara, 1965, Hill 10377 (BM).
Astrothelium neogalbineum (R. C. Harris) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816708
Trypethelium neogalbineum R. C. Harris, Acta Amazon. (Suppl.) 14: 74 (‘1984’) [1986]; type: Brazil, Pará, Santarém, Spruce 259 (BM!—isotype).
Thallus corticate, olive-green to greyish green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 0·5–1·0 mm broad, immersed-erumpent, irregular to elongate or becoming reticulate, dark brown with orange pigment, especially laterally. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 18–26×7–8 µm, hyaline, IKI−.
Chemistry. Thallus UV+ yellow, with lichexanthone; pseudostromata UV+ red, K+ purple, with anthraquinone.
Distribution. Neotropical (previously reported from Costa Rica and Brazil).
New country record. Guyana: East Demarara: along Linden Highway, Soesdyke, Sipman 40277 (B).
Astrothelium nigratum (Müll. Arg.) Aptroot & Lücking comb. et stat. nov.
MycoBank No.: MB 816709
Astrothelium minus var. nigratum Müll. Arg., Bot. Jahrb. Syst. 6: 382 (1885); type: Cuba, Wright s. n. (G!—holotype; FH-TUCK 3982—isotype, not seen; Müller, Verr. Cub. 638).
(Fig. 19B)
Thallus corticate, olive-brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, diffusely pseudostromatic, 0·2–0·3 mm diam., immersed-erumpent, exposed and brown-black. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 20–27×7–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported only from Cuba).
New country records. Puerto Rico: Mayagüez: Reserva Forestal Maricao, 1989, A. & M. Aptroot 24961 (ABL).—Guyana: Rupununi: Kuyuwini Landing, 1991, Jansen-Jacobs et al. 2573 (ABL).
Astrothelium nigricans Malme
Astrothelium nigricans Malme, Ark. Bot. 19(1): 13 (1924); type: Brazil, Matto Grosso, Cuyabá, Malme 2003 (S!—holotype).
(Fig. 28F)
Thallus corticate, olive-green to yellowish, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata diffusely pseudostromatic, formed on irregularly linear to reticulate, whitish thallus portions, 0·6–1·0 mm diam., prominent, wart-shaped, completely exposed and black, covered. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 21–27×8–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Brazil).
Astrothelium nigrorufum (Makhija & Patw.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816710
Trypethelium nigrorufum Makhija & Patw., J. Hattori Bot. Lab. 73: 203 (1993); type: India, Andaman Islands, Baratang, Baludera, 23 February 1985, Patwardhan & Sethy AMH 85.845 (ABL!—isotype).
(Fig. 17A)
Thallus corticate, olive-brown to yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 1–2 mm broad, immersed-erumpent, exposed and brown-black, internally with yellow-orange, K+ purple pigment. Hamathecium clear. Ascospores 8 per ascus, 9–13-septate, fusiform, 29–35(–46)×7–9 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV−, internally K+ purple, with anthraquinone.
Distribution. Eastern palaeotropical (India).
Astrothelium nitidiusculum (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816711
Verrucaria nitidiuscula Nyl., Ann. Sci. Nat. Bot. sér. 4 20: 252 (1863).—Pseudopyrenula nitidiuscula (Nyl.) Müll. Arg., Flora 66: 248 (1883).—Trypethelium nitidiusculum (Nyl.) R. C. Harris, Acta Amazon. (Suppl.) 14: 75 (‘1984’) [1986]; type: Colombia, Villeta, Lindig 2829 (M!, BR—isotypes).
Pseudopyrenula neglecta Müll. Arg., Flora 68: 332 (1885); type: French Guiana, Leprieur 479 (G!—holotype).
Trypethelium hebetum Makhija & Patw., J. Hattori Bot. Lab. 73: 198 (1993); type: India, Andaman Islands, South Andaman, Near Wright Myo, Shol Bay, 20 December 1986, Patwardhan & Nagarkar AMH 77.866 (ABL!—isotype).
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to confluent but not distinctly pseudostromatic, 0·2–0·3 mm diam., erumpent, covered by thallus but upper portion whitish (or a white rim only) with dark ostiolar area. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 15–27×7–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Pantropical, reported from, for example, Colombia, Ecuador, Bolivia, Guyana, French Guiana, Brazil, India, and Papua New Guinea. The species has also been reported from the USA, Mexico, Bermudas, Bahamas, Cuba, Jamaica, Dominican Republic, Trinidad, Costa Rica, El Salvador, Isla del Coco, Guyana, French Guiana, Colombia, Venezuela, Galapagos, Brazil, Bolivia, Argentina, South Africa, Seychelles, India, Sri Lanka, Vietnam, Thailand, Taiwan, Hong Kong, Japan, Papua New Guinea and Australia, but these reports correspond to a much broader species concept including, for example, the now separated, more common taxa A. bicolor and A. scoria. Therefore, below are some additional records from countries from which the species had been reported previously in a wider sense.
Discussion. Astrothelium nitidiusculum is restricted here to include specimens with solitary to confluent, but not pseudostromatic ascomata. Even when dense or confluent, the ascomata can clearly be individually discerned.
Confirmed additional country records. Ecuador: Morona-Santiago: Gualaquiza, 1982, Aptroot & Hensen 10454 (ABL).—Guyana: Upper Takutu: Kuyuwini Landing, 1992, Sipman 58140 (B).—Papua New Guinea: Madang: Balek Wildlife Reserve, 1992, Aptroot 30891 (ABL).
Astrothelium nitidulum Weerakoon & Aptroot
Cryptog., Mycol. 35: 58 (2014); type: Sri Lanka, Central Province, Coolbone tea estate Matale, Cloonan 083/53, 1982 (F!—holotype).
(Fig. 21E)
Thallus corticate, olive-brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to confluent and becoming diffusely pseudostromatic; pseudostromata 0·9–1·9 mm diam. (individual ascomata 0·6–0·9 mm diam.), erumpent to prominent, covered by thallus except for black ostiole with whitish centre. Hamathecium inspersed. Ascospores 8 per ascus, 7-septate, fusiform, 40–49×12–15 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution: Eastern palaeotropical (Sri Lanka).
Astrothelium obscurum Müll. Arg.
Flora 66: 244 (1883); type: Brazil, Apiahy, Puiggari 2255 (G!—holotype).
(Fig. 28J)
Thallus corticate, olive-brown, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata largely confluent but not distinctly pseudostromatic, 0·3–0·4 mm diam., erumpent, applanately conical, exposed and dark brown. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 20–25×7–9 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Brazil).
Astrothelium ocellatum Malme
Ark. Bot. 19(1): 14 (1924); type: Brazil, Matto Grosso, Santa Anna da Chapada, Malme 2411 (S!—holotype).
Thallus corticate, olive-green, smooth to uneven or shallowly bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata solitary to confluent but not pseudostromatic, 1·0–1·8 mm diam., erumpent, covered by thallus but papilliform ostiolar area ochraceous brown, with yellow-orange pigment and surrounded by an irregular whitish rim. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 55–65×15–25 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−, without lichexanthone; pseudostromata with yellow-orange, K+ purple, UV+ red anthraquinone.
Distribution. Pantropical (previously reported from Guyana, Brazil and Papua New Guinea).
New country records. Venezuela: Amazonas: Alto Orinoco, Surumoni, 1998, Hafellner & Komposch 15-13 (GZU).—French Guiana: Saül, 1988, Sipman 31772 (B).
Astrothelium ochrothelium (Nyl.) Müll. Arg.
Flora 68: 255 (1885).—Trypethelium ochrothelium Nyl., Acta Soc. Sci. Fenn 7: 494 (1863); type: Colombia, Villeta, Lindig 2823 (H-Nyl 7574!—lectotype; Harris, Acta Amazon. (Suppl.) 14: 62 (‘1984’) [1986]; S!, BR—isolectotypes).
Thallus corticate, olive-green to yellowish or brownish, uneven to shallowly verrucose.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata pseudostromatic; pseudostromata 0·8–1·5 mm broad, erumpent, irregular, often confluent, covered by thallus and upper portion with yellow-orange pigment. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, (37–)43–45×15–23 µm, hyaline, IKI−.
Chemistry. Thallus UV+ yellow, K−, with lichexanthone; pseudostromata UV−, K+ purple, with yellow-orange anthraquinone.
Distribution. Neotropical (previously reported from Cuba, Guyana, Venezuela, Colombia and Brazil).
New country record. French Guiana: Kourou, 1958, Degelius s. n. (ABL, UPS).
Astrothelium octosporoides Aptroot & Lücking nom. nov.
MycoBank No.: MB 816712
Bathelium octosporum Zahlbr., Sitzungsber. K. Akad. Wiss. Wien, math.-naturw. Klasse 111(1): 372 (1902).—Laurera octospora (Zahlbr.) Zahlbr., Catal. Lich. Univ. 1: 505 (1922) non Astrothelium octosporum (Vain.) Aptroot & Lücking (see below); type: Brazil, Theresiopolis, von Höhnel 150 (W—holotype, not seen).
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·8–1·5 mm diam., erumpent to prominent, hemispherical, covered by thallus. Hamathecium clear. Ascospores 8 per ascus, densely muriform, fusiform, 150–190×40–45 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Brazil).
Astrothelium octosporum (Vain.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816713
Heufleria octospora Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 192 (1890).—Cryptothelium octosporum (Vain.) Zahlbr., Catal. Lich. Univ. 1: 522 (1922); type: Brazil, Minas Gerais, Sitio, Vainio s. n. (TUR-Vain 30839!—holotype; BM—isotype; Vainio, Lich. Bras. Exs. 1031).
(Fig. 37D)
Thallus corticate, olive-green to brownish, uneven to shallowly bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed, 0·8–1·7 mm diam., immersed to erumpent, covered by thallus except dark ostiolar area surrounded by thin whitish rim. Hamathecium clear. Ascospores 8 per ascus, densely muriform, narrowly fusiform, 110–135×19–23 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (Brazil).
Astrothelium oligocarpum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816714
Trypethelium oligocarpum Müll. Arg., Nuovo Giorn. Bot. Ital. 23: 402 (1891); type: Australia, Queensland, Brisbane, Bailey 465 p.p. (G!—lectotype, designated here).
(Fig. 28K)
Thallus corticate, olive-green to yellowish, shallowly bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata grouped but not distinctly pseudostromatic, 0·3–0·4 mm diam., prominent, basally covered by thallus but upper part blackish. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 20–27×8–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (Australia).
Astrothelium olivaceofuscum (Zenker) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816715
Trypethelium olivaceofuscum Zenker, in Goebel & Kunze, Pharmazeut. Waarenkunde 1(3): 190 (1827); type: South America (L!—holotype).
Thallus corticate, brownish to light greyish green or yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to often irregularly confluent but not distinctly pseudostromatic, 0·6–1·2 mm diam., erumpent, covered by thallus but protruding ostiole dark. Hamathecium clear. Ascospores 8 per ascus, 9–17-septate, fusiform, 85–100×8–16 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from Costa Rica).
New country records. Guyana: Potaro-Siparuni Region: surroundings of Paramakatoi Village, 800 m, 1996, Sipman 41275 p.p. (B).—Brazil: Alagoas: Pilar, 2001, Cáceres & Lücking s. n. (ABL, URM).
Astrothelium papillosum (P. M. McCarthy) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816716
Laurera papillosa P. M. McCarthy, Lichenologist 27: 311 (1995); type: Papua New Guinea, Central Prov., 40 km NE of Port Moresby, near Dabamura, on Owers Corner Rd, 580 m, 10 February 1981, Streimann & Naomi 14908 (B!—isotype).
(Fig. 34D)
Thallus corticate, green, distinctly verrucose-papillose.
Ascomata trypethelioid to pleurothelioid, with apical or eccentric but then separate ostioles, 0·6–1·0 mm diam., immersed and covered by thallus except for brownish black ostioles. Hamathecium orange, inspersed. Ascospores 8 per ascus, muriform, fusiform-ellipsoid, 130–195×26–34 µm, constricted at the markedly thickened median septum, hyaline, IKI−.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (Papua New Guinea).
Astrothelium papulosum (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816717
Verrucaria papulosa Nyl., Ann. Sci. Nat. Bot., sér. 5 7: 345 (1867).—Pseudopyrenula papulosa (Nyl.) Müll. Arg., Flora 66: 248 (1883).—Trypethelium papulosum (Nyl.) Makhija & Patw., Int. J. Mycol. Lichenol. 5: 242 (1992); type: Colombia, Pie de Cuesta, Lindig 96 (H-Nyl 1227!—lectotype; BR—isolectotype; Makhija & Patwardhan, Int. J. Mycol. Lichenol. 5: 242, 1992).
(Fig. 20E)
Thallus corticate, yellowish brown, uneven to verrucose-papillose.
Ascomata trypethelioid, with apical ostioles, aggregate in diffuse pseudostromata; pseudostromata 1–3(–5) mm diam. (individual ascomata 0·2–0·4 mm diam.), immersed to erumpent, covered by thallus except for dark ostioles. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 26–38×7–9 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from Costa Rica and Colombia).
New country record. Brazil: Amapá: Floresta Nacional do Amapá, 2015, Cáceres & Aptroot 27267 (ABL, ISE).
Astrothelium peranceps (Kremp.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816718
Trypethelium peranceps Kremp., Nuovo Giorn. Bot. Ital. 7: 55 (1875); type: Malaysia, Sarawak, Beccari 112 (M!—lectotype; Harris, Acta Amazon. (Suppl.) 14: 60 (‘1984’) [1986]).
(Fig. 28E)
Thallus corticate, brownish, thin, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed, 0·3–0·4 mm diam., prominent, conical to wart-shaped, covered by thallus but upper portion often exposed and dark brown. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 21–27×8–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (Malaysia: Sarawak).
Discussion. This name has been reinstated as a separate species in the Astrothelium crassum complex. This complex is still poorly understood, but the considerable morphological differences between specimens that agree in ascoma organization, ascospore type, and lack of secondary substances suggests the existence of several taxa. Within this complex, A. peranceps is distinguished by its thin thallus and widely dispersed, prominent and conical to wart-shaped ascomata.
Astrothelium phaeothelium (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816719
Trypethelium phaeothelium Nyl., Ann. Sci. Nat., Bot., sér. 5 7: 347 (1867); type: Colombia, Rio Magdalena, Lindig 31 (H-Nyl 271!—holotype; BM!, BR, S!—isotypes).
(Fig. 20J)
Thallus corticate, olive-green to brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, irregularly confluent to pseudostromatic; pseudostromata 1–2 mm broad (individual ascomata 0·8–1·2 mm broad), prominent to sessile, blackish brown, with slightly protruding, black ostioles. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 75–90×17–23 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Colombia).
Discussion. This is another species morphologically similar to Bathelium but it has been included in Astrothelium due to the lack of internal pigment in the pseudostromata and the astrothelioid ascospores.
Astrothelium phlyctaena (Fée) Aptroot & Lücking comb. nov.
MycoBank No.: MB816720
Trypethelium phlyctaena Fée, Essai Crypt. Écorc.: 68 (1824).—Trypethelium scoria var. phlyctaena (Fée) Trevis., Flora 44: 19 (1861); type: Saint Lucia, s. col. (G!—lectotype, designated here; BM!—isolectotype).
Verrucaria catervaria Fée, Essai Crypt. Écorc.: 90 (1824).—Pyrenula catervaria (Fée) Massal., Framment. Lich: 25 (1855).—Spermatodium catervarium (Fée) Trevis., Conspect. Verruc.: 10 (1860).—Pseudopyrenula catervaria (Fée) Müll. Arg., Flora 66: 248 (1883).—Trypethelium catervarium (Fée) Tuck., Genera Lich.: 260 (1872); type: South America, “corticem cinchonarum” (G!—lectotype; Harris, Acta Amazon. (Suppl.) 14: 64 (‘1984’) [1986]).
Verrucaria decolorata Fée, Essai Crypt. Écorc.: 91 (1824); type: South America (G!—holotype; L!—isotype).
Verrucaria tessella Pers., in Gaudichaud, Voy. Uranie: 183 (1827).—Pseudopyrenula tessella (Pers.) Graff, Mycologia 9: 16 (1917); type: (L—holotype, lost).
Verrucaria ochroleuca Eschw., in Martius, Icon. Sel. Pl. Crypt. 2: 16, t. 8, figs 3, 4 (1828).—Pseudopyrenula ochroleuca (Eschw.) Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 209 (1890).—Spermatodium ochroleucum (Eschw.) Trevis., Conspect. Verruc.: 11 (1860).—Trypethelium ochroleucum (Eschw.) Nyl., Flora 52: 126 (1869); type: Brazil, Bahia, Caetité, Martius s. n. (M!—lectotype, Harris, Acta Amazon. (Suppl.) 14: 75 (‘1984’) [1986]).
Trypethelium duplex Fée, Ann. Sci. Nat., Bot. 23: 437 (1831).—Chooicia feei Trevis. (1861) nom. illeg.—Trypethelium cascarillae Müll. Arg., Mém. Soc. Phys. Hist. Nat. Genève 30(3): 14 (1888) nom. illeg.—Pseudopyrenula duplex (Fée) Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 208 (1890); Bathelium duplex (Fée) C. W. Dodge, Ann. Missouri Bot. Gard. 40: 290 (1953); type: South America, “corticem Croton cascarillae” (G!—lectotype; Harris, Lichenogr. Thomsoniana: 142, 1998; BM!—isolectotype).
Trypethelium quassiicola Fée, Ann. Sci. Nat., Bot., sér. 1 23: 448 (1831); type: Jamaica, “in cortice Quassiae excelsae” (G!—lectotype, designated here; L—2 isolectotypes).
Trypethelium pallescens Fée, Ann. Sci. Nat., Bot., sér. 1 23: 440 (1831).—Trypethelium ochroleucum var. pallescens (Fée) Müll. Arg., Bot. Jahrb. Syst. 6: 392 (1885).—Pseudopyrenula ochroleuca var. pallescens (Fée) Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 209 (1890); type: Surinam, Weigelt s. n. (G!—lectotype, Müll. Arg., Bot. Jahrb. Syst. 6: 392, 1885; L!—isolectotype).
Trypethelium leprieurii Mont., Ann. Sci. Nat., Bot., sér. 2 19: 70 (1843); type: French Guiana, Leprieur s. n. (L—isotype).
Trypethelium triplex Nyl., Flora 52: 125 (1869); type: Brazil, Rio de Janeiro, Glaziou s. n. (H-Nyl—holotype, not seen).
Trypethelium euporum Kremp., Flora 59: 527 (1876); type: Brazil, Rio de Janeiro, Glaziou 6304 (M!—holotype).
Trypethelium bicolor var. pyrenuloides C. Knight, Trans. Proc. New Zealand Instit. 16: 405 (1884).—Trypethelium phlyctaena var. pyrenuloides (C. Knight) Zahlbr., Catal. Lich. Univ. 1: 498 (1922); type: New Zealand, Knight (WELT—holotype, not seen).
Trypethelium ochroleucum var. depauperatum Müll. Arg., Bot. Jahrb. Syst. 6: 392 (1885); type: Cuba, Müller, Verr. Cub. 571 (G!—lectotype, designated here; US—isolectotype, not seen).
Trypethelium leprosum Müll. Arg., Bot. Jahrb. Syst. 6: 393 (1885); type: Cuba, Wright s. n. (G!—lectotype, designated here; Müller, Verr. Cub. 155 p.p.).
Trypethelium subalbens Nyl., Flora 69: 178 (1886).—Bathelium subalbens (Nyl.) C. W. Dodge, Ann. Missouri Bot. Gard. 40: 290 (1953); type: São Tomé, Henriques s. n. (G!—lectotype, designated here; BM—isolectotype).
Thallus corticate, light greenish to brownish grey or yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, diffusely pseudostromatic; pseudostromata 0·8–1·5(–2·0) mm broad., erumpent to prominent, covered by thallus but usually paler and cream-coloured. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 15–27×7–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV+ yellow, K−, with lichexanthone (or 1,8-dihydroxy-3,6-dimethoxyxanthone).
Distribution. Pantropical; reported from, for example, USA, Mexico, Puerto Rico, Cuba, Netherlands Antilles, Dominican Republic, Jamaica, Costa Rica, Nicaragua, Guyana, Surinam, French Guiana, Brazil, Uruguay, Ascension, São Tomé, Australia and New Zealand.
Discussion. In the complex traditionally labelled Trypethelium ochroleucum, the epithet phlyctaena is the oldest for specimens without hamathecium inspersion and with raised pseudostromata. This complex was even considered to include taxa with eccentric ostioles and hence was temporarily included in the synonymy of Astrothelium variolosum by Harris (Reference Harris1995), which is considered here a separate complex. Specimens previously labelled Trypethelium ochroleucum but with separate ascomata (not pseudostromatic) are assigned here to Astrothelium pulcherrimum.
New or confirmed country records. Nicaragua: Ometepe Island: Madera Volcan, 2001, Breuss 19160 (LI).—Netherlands Antilles: St. Eustatius: Quill, 1980, Sipman 15092 (B). Saba: Mt. Scenery, 1980, Sipman 15355 (B).—Puerto Rico: Ponce: Guanica, 1989, A. & M. Aptroot 25765 (ABL).—Dominican Republic: Monte Cristi: El Morro, 1987, Harris 19604 (ABL, NY).—Guyana: Upper Takutu: Dadanawa ranch, 1992, Sipman 57378 (B).—Uruguay: Rocha, Sierra de San Miguel, 1989, Osorio 9187 (hb. Kalb).—Ascension: Elliot’s Park, 1976, James s. n. (BM, ABL).
Astrothelium pleiostomum Redinger
Hedwigia 73: 58 (1933); type: Brazil, Amazonas, Santarem, Taperinha, Ginzberger (W—holotype, not seen).
Trypethelium violascens R. C. Harris, Acta Amazon. (Suppl.) 14: 78, fig. 11 (‘1984’) [1986]; type: Brazil, Amazonas, along Igarapé Caititu off Rio Uatumá, Buck 2937 (NY!—holotype; ABL!—isotype).
Thallus corticate, olive-green to yellowish, uneven somewhat bullate, often gall-like.
Ascomata pleurothelioid with eccentric, separate ostioles, diffusely pseudostromatic; pseudostromata 0·8–1·3 mm diam., erumpent, covered by thallus but with upper portion whitish, internally with yellowish pigment. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 28–33×8–10 µm, rather thin-walled, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−; pseudostroma internally K+ yellow, with anthraquinone.
Distribution. Neotropical (Brazil).
Discussion. This taxon is tentatively retained in Astrothelium, since the overall morphology and the ascospore type would also suggest placement in Viridothelium. The new synonymy of Trypethelium violascens is based on study of isotypes in ABL, which show that some characters are different from the description. The K-reaction is positive and the IKI-reaction is (at least now) absent; it was probably an artifact of fresh material.
Astrothelium porosum (Ach.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816721
Trypethelium porosum Ach., Synops. Lich: 106 (1814).—Verrucaria porosa (Ach.) Eschw., in Martius, Flora Brasil. 1: 135 (1833).—Leightonia porosa (Ach.) Trevis., Flora 44: 19 (1861).—Trypethelium sprengelii var. porosum (Ach.) Nyl., in Hue, Nouv. Archiv. du Muséum, sér. 3 4: 129 (1892).—Bathelium porosum (Ach.) C. W. Dodge, Ann. Missouri Bot. Gard. 40: 290 (1953); type: West Indies, “corticem Crotonis Cascarillae” (H-ACH 883!—holotype; S—isotype).
Trypethelium erubescens Kunze in Fée, Ann. Sci. Nat., Bot., sér. 1 23: 441 (1831).—Trypethelium ochroleucum var. erubescens (Kunze) Müll. Arg., Flora 68: 254 (1885); type: Surinam, Weigelt s. n. (L!—holotype).
Trypethelium brachysporum Malme, Ark. Bot. 19(1): 30 (1924).—Polymeridium brachysporum (Malme) Aptroot, in Aptroot & Cáceres, Nova Hedwigia 98: 11 (2014); type: Brazil, Mato Grosso, Cuyabá, Malme 2055 (S!—holotype; BM!—isotype).
Thallus corticate, light greenish to brownish grey or yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, diffusely pseudostromatic; pseudostromata 0·8–1·5 mm diam., erumpent to promiment, covered by thallus but usually paler and cream-coloured. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 17–27×7–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV+ yellow, K−, with lichexanthone (or 1,8-dihydroxy-3,6-dimethoxyxanthone).
Distribution. Pantropical (see below).
Discussion. This taxon was until recently not distinguished from Astrothelium phlyctaena and A. pulcherrimum, and therefore its distribution is not well known. Together, these species have for a long time been labelled Trypethelium ochroleucum and under that name have been reported from the USA, Mexico, Bahamas, Jamaica, Cuba, Puerto Rico, Trinidad, Tobago, Costa Rica, El Salvador, Guyana, French Guiana, Surinam, Colombia, Venezuela, Galapagos, Brazil, Paraguay, Cape Verdes, Ascension, South Africa, Mozambique, Seychelles, India, Sri Lanka, China, Singapore, Philippines, Vietnam, Indonesia, Guam, Hawai’i, Northern Mariana Islands, New Caledonia, Papua New Guinea, Australia and New Zealand. Not all specimens on which these reports are based have been studied recently. Astrothelium porosum is the most common species of the three.
New country records. USA: Florida: Sanford, 1939, Johnson 3310 (ABL).—Mexico: Chiapas: Ocozocoautla, 1996, Wolf & Sipman s. n. (B).— Guyana: Upper Takutu: Dadanawa ranch, 1992, Sipman 57358 (B).—French Guiana: Cayenne, 1993, Sipman 31532 (Lichenotheca Latinoamericana 149, ABL).—India: West Bengal: Sundarbans, Jhila, 2003, Jagadeesh Ram 13640 (ABL).—Indonesia: Irian Jaya: 11km W of Bupul, 1988, Hensen s. n. (ABL).—Papua New Guinea: Madang: Karkar, 1987, Aptroot 17623 (ABL).—Australia: Queensland: Cape Tribulation, 1983, Hale 64181 (ABL, US).
Astrothelium praetervisum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816722
Heufleria praetervisa Müll. Arg., Flora 68: 250 (1885).—Cryptothelium praetervisum (Müll. Arg.) Zahlbr., Catal. Lich. Univ. 1: 522 (1922); type: French Guiana, Leprieur 13 (G!—holotype).
(Fig. 37G)
Thallus corticate, brownish to often partly orange-brown and with orange pigment, mottled with whitish areas producing pycnidia, smooth to uneven.
Ascomata pleurothelioid, with eccentric, separate ostioles, pseudostromatic; pseudostromata 0·4–0·6 mm diam., immersed-erumpent, often forming lines, covered by thallus and partly with orange pigment except for blackish ostioles surrounded by whitish rim. Hamathecium clear. Ascospores 8 per ascus, muriform, fusiform, 33–68×12–22 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K+ partly purple, with anthraquinones.
Distribution. Neotropical (French Guiana).
Astrothelium pseudocyphellatum R. C. Harris
Acta Amazon. (Suppl.) 14: 62 (‘1984’) [1986]; type: Brazil, Amazonas, São Gabriel, Spruce 247 (BM!—isotype).
Thallus light greyish green, uneven to rugose, with superficial pockets of calcium oxalate crystals resembling pseudocyphellae.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata pseudostromatic; pseudostromata 0·8–1·7 mm diam., prominent, covered by thallus usually paler or darker cream-coloured. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 23–27×7–8 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV+ yellow, K−, with lichexanthone.
Distribution. Neotropical (previously reported only from Brazil).
New country record. Guyana: Potaro-Siparuni: Kaieteur Falls National Park, 1996, Sipman 40458 (B).
Astrothelium pseudoplatystomum (Makhija & Patw.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816723
Trypethelium pseudoplatystomum Makhija & Patw., Int. J. Mycol. Lichenol. 5: 244 (1992); type: Brazil, Heufler 130 (M—holotype, not seen).
Thallus corticate, green to yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 1–2 mm diam., erumpent to prominent, with flattened top, covered by thallus except upper portion, with individual ascomata appearing dark and arranged in an astroid way. Hamathecium clear. Ascospores 8 per ascus, (5–)10–14-septate, fusiform, 29–52×8–12 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Brazil).
Astrothelium pseudovariatum (Upreti & Ajay Singh) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816724
Laurera pseudovariata Upreti & Ajay Singh, Bull. Jard. Bot. Nat. Belg. 57: 378 (1987); type: India, West Bengal, Howrah, Sibpur, Roychaudhury 675 (CAL—holotype, not seen).
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, diffusely pseudostromatic; pseudostromata 0·8–1·7 mm diam., prominent, hemispherical, covered by thallus. Hamathecium inspersed. Ascospores 8 per ascus, (sub-)muriform, 7–9×2–3-septate, 32–45×11–13 µm, fusiform, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (India).
Astrothelium puiggarii (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816725
Campylothelium puiggarii Müll. Arg., Flora 66: 245 (1883); type: Brazil, Rio de Janeiro, Puiggari 1460 (G!—holotype; NY!—isotype).
Campylothelium puiggarii var. pallescens Müll. Arg., Flora 66: 245 (1883); type: Brazil, Rio de Janeiro, Puiggari 1757 (G!—holotype).
Thallus corticate, olive-green, verrucose-bullate.
Ascomata pleurothelioid, with eccentric, separate ostioles, solitary, 0·8–1·3 mm diam., prominent to almost sessile, covered by thallus except for the protruding, papilliform, brownish grey ostiole, 1·0–1·5 mm diam. Hamathecium inspersed. Ascospores 2 per ascus, densely muriform, fusiform, 70–90×20–25 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from Costa Rica and Brazil).
New country record. French Guiana: Saül, 1986, Montfoort & Ek 222 (B).
Astrothelium pulcherrimum (Fée) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816726
Trypethelium pulcherrimum Fée, Ann. Sci. Nat., Bot., sér. 1 23: 450 (1831).—Pseudopyrenula pulcherrima (Fée) Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 208 (1890); type: South America, “in America ad corticem Crotonis cascarillae” (G!—lectotype, designated here; L!—isolectotype).
Verrucaria diffluens Nyl., Ann. Sci. Nat., Bot., sér. 4 20: 252 (1863).—Pseudopyrenula diffluens (Nyl.) Müll. Arg., Flora 66: 248 (1883); type: Colombia, Bogotá, Lindig 2770 (H-Nyl 2211!—lectotype, designated here; BM!, BR—isolectotypes).
Trypethelium ochroleucum var. effusum Müll. Arg., Bot. Jahrb. Syst. 6: 392 (1885).—Pseudopyrenula ochroleuca var. effusa (Müll. Arg.) Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 209 (1890); type: Cuba, Wright s. n. (G!—lectotype, designated here; Müller, Lich. Cub., Ser. II: 566).
Trypethelium tricolor Müll. Arg., Bull. Soc. Roy. Bot. Belg. 32(1): 166 (1893); type: Costa Rica, Puntarenas, Boruca, Tonduz s. n. (G!—holotype; BR, US—isotypes; Pittier, Pl. Costaric. Exs. 6282).
Pseudopyrenula duplex var. simplicior Vain., Bol. Soc. Broteriana, Ser. 2 6: 177 (1930).—Trypethelium duplex f. simplicius (Vain.) Zahlbr., Catal. Lich. Univ. 10: 101 (1938).—Bathelium duplex f. simplicius (Vain.) C. W. Dodge, Ann. Missouri Bot. Gard. 40: 290 (1953); type: Mozambique, Palma, Pres de Lima 265 (TUR-Vain 34609—holotype, not seen).
Pseudopyrenula portoricensis J. Hedrick, Mycologia 22: 248 (1930); type: Puerto Rico, Mayagüez, Fink 1025 (MICH—holotype, not seen).
Thallus corticate, light olive-brown to brownish greenish grey to yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary but usually dense, 0·2–0·3 mm diam., erumpent, covered by thallus except dark ostioles surrounded by whitish rim. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 15–27×7–10 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV+ yellow, K−, with lichexanthone (or 1,8-dihydroxy-3,6-dimethoxyxanthone).
Distribution. Pantropical, but distribution incompletely known because the species was not previously separated from Astrothelium phlyctaena and A. porosum (all taken together as Trypethelium ochroleucum s. lat.). Previously reported from Cuba, Puerto Rico, Colombia and Mozambique.
New country records. Guyana: Upper Takutu: Karanambo ranch, 1992, Sipman 57281 (B).—Indonesia: Java: Malang, 1939, Groenhart 5095 (ABL, L).
Astrothelium punctulatum Malme
Ark. Bot. 19(1): 14 (1924); type: Brazil, Matto Grosso, Santa Ana da Chapada, Malme, Lich. Regn. 2457 (S!—holotype; S!—isotype).
Thallus corticate, olive-green to greyish, verrucose-bullate, somewhat pseudogall-inducing.
Ascomata trypethelioid, with apical ostioles, solitary to confluent but often dense, 0·8–1·5 mm diam., immersed-erumpent in elevated thallus portions, covered by thallus except for dark ostiole surrounded by white ring. Hamathecium heavily inspersed. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 23–27×8–9 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ostioles UV+ yellow, K−, with lichexanthone.
Distribution. Neotropical (previously reported from Cuba and Brazil).
New country record. Guyana: Demerara-Berbice: Mabura Hill, 1988, Bleij & Biesmeijer s. n. (ABL). Upper Takutu: Kuyuwini Landing, 1992, Sipman 57021 (B).
Astrothelium pupula (Ach.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816727
Pyrenula pupula Ach., Synops. Meth. Lich.: 123 (1814).—Pseudopyrenula pupula (Ach.) Müll. Arg., Flora 68: 331 (1885).—Trypethelium pupula (Ach.) R. C. Harris, Lichenogr. Thomsoniana: 146 (1998); type: West Indies, s. col. (H-ACH 832!—holotype).
Pseudopyrenula porinoides Müll. Arg., Flora 69: 331 (1885).—Verrucaria porinoides Mont., Ann. Sci Nat., Bot. sér. 2 19: 59 (1843) non Ach.—Pseudopyrenula pupuloides M. Choisy, Mém. Soc. Bot. France 1953–1954: 58 (1954); type: French Guiana, Leprieur 731 (G!—lectotype, designated here; BM!—isolectotype).
Thallus corticate, greyish green to yellowish (lichexanthone colour), smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·4–0·6 mm diam., erumpent, covered by thallus except for blackish ostiolar area. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 33–40×10–13 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV+ yellow, K−. TLC: lichexanthone.
Distribution. Pantropical (previously reported from the West Indies, Costa Rica, French Guiana, Colombia, India and Sri Lanka).
New country records. Puerto Rico: Aguadilla: Bosque Estatal de Guajataca, 1989, Aptroot & Aptroot 25744 (ABL).—Guyana: East Demarara: Linden Highway, E of Timehri, 1985, Sipman & Aptroot 19587, 19602, 19558 (all ABL, B).—Venezuela: Bolivar: Cerro Guaiquinima, along Rio Carapo, 800 m, 1990, Sipman 27030 (B).—Ecuador: Morona-Santiago: Cordillera del Condor, 12km E of Los Encuentros, 1982, Aptroot 10442 (ABL).—Brazil: Minas Gerais: Serra do Caraça, Parque Natural do Caraça, 1250 m, 1997, Aptroot 40918 (ABL).—Malaysia: Sarawak: 1866, Beccari 188 p.p. (M, originally filed as syntype of Trypethelium leucostomum Kremp.).—Papua New Guinea: Central: Varirata National Park, 1987, Aptroot 19080 (ABL).
Astrothelium purpurascens (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816728
Heufleria purpurascens Müll. Arg., Bot. Jahrb. Syst. 6: 384 (1885).—Cryptothelium purpurascens (Müll. Arg.) Zahlbr., Catal. Lich. Univ. 1: 522 (1922); type: Cuba, Wright s. n. (G—holotype, not seen; NY!—isotype; Müller, Verr. Cub. 116b).
Cryptothelium rhodotitthon R. C. Harris, Acta Amazon. (Suppl.) 14: 65 (‘1984’) [1986]; type: Brazil, Roraima, along Manaus-Boa Vista Road, Buck s. n. (NY!—isotype).
(Fig. 37L)
Thallus corticate, olive-green to yellowish or reddish, uneven to bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed, 0·7–1·5 mm diam., immersed, completely covered by thallus except for protruding, dark red ostiole. Hamathecium clear. Ascospores 8 per ascus, densely muriform, fusiform, 100–130×25–30 µm, median septum thickened, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ascomata with red, K+ green-reacting isohypocrellin.
Distribution. Neotropical (Brazil and Cuba).
Astrothelium pustulatum (Vain.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816729
Pseudopyrenula pustulata Vain., in Hiern, Cat. Afr. Pl. 2(2): 456 (1901).—Trypethelium pustulatum (Vain.) Zahlbr., Catal. Lich. Univ. 1: 499 (1922); type: Angola, Ambriz, Welwitsch 190 (TUR-Vain 30791!—holotype; BM!—isotype).
(Fig. 21G)
Thallus corticate, olive-green, uneven-verrucose, pseudogall-inducing.
Ascomata trypethelioid, with apical ostioles, diffusely pseudostromatic; pseudostromata 1–2 mm diam., prominent, completely covered by thallus, internally with yellow pigment, chiefly around the ostiolar region. Hamathecium inspersed. Ascospores 8 per ascus, 5–9-septate, 180–220×40–45 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances.
Distribution. African palaeotropical (Angola).
Discussion. In the original description a red pigment was mentioned, which is absent from the type material. The type of Trypethelium lageniferum Ach. also contains this taxon but additionally a species of Pyrenula, on which most of the description was based.
Astrothelium pyrenastrosulphureum Aptroot & Lücking nom. nov.
MycoBank No.: MB 816730
Pyrenastrum sulphureum Eschw., in Martius, Icon. Pl. Cryptog.: 17 (1828).—Astrothelium sulphureum (Eschw.) Müll. Arg., Flora 68: 255 (1885) nom. illeg. non Astrothelium sulphureum Nyl., Ann. Sci. Nat., Bot., sér. 4 20: 260 (1863); type: Brazil, Amazonas, Martius s. n. (M!—holotype).
Pyrenodium hypoxylon Fée, Essai Crypt. Écorc. Suppl.: 69 (1837).—? Astrothelium hypoxylon (Fée) Nyl., Mém. Soc. Sci. Nat. Cherbourg 5: 141 (1857); type not indicated.
Pyrenastrum sulphureum subsp. plicatum Eschw., in Martius, Flora Brasil. 1: 145 (1833); type: Brazil, Amazonas, Martius s. n. (M—holotype, not seen).
Astrothelium sulphureum var. subpallescens Nyl., Bull. Soc. Linn. Normand., sér. 2 2: 133 (1868); type: New Caledonia, Lifu, Deplanche s. n. (H-Nyl—holotype, not seen).
(Fig. 28A)
Thallus corticate, olive-green to yellowish, uneven to bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata irregularly pseudostromatic; pseudostromata 0·8–1·5 mm diam., erumpent to prominent, covered by thallus except for blackish ostiolar area. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 27–30×8–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances.
Distribution. Pantropical (Brazil, India and New Caledonia).
Astrothelium robustum Müll. Arg.
Bull. Soc. Roy. Bot. Belg. 32(1): 164 (1893); type: Costa Rica, Puntarenas, Rio Naranjo, 1893, Tonduz s. n. (G!—holotype; BR—isotype).
Astrothelium conigerum Zahlbr., Bull. Herb. Boissier, sér. 2 8: 459 (1908); type: Brazil, Amazonas, Rio Juruá, Ule 356 (W—holotype, not seen).
Thallus corticate, olive-green to yellowish, uneven to bullate, sometimes pseudogall-inducing.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed to irregularly confluent or diffusely pseudostromatic, 1–3 mm diam., erumpent to prominent, covered by thallus except for blackish ostiolar area. Hamathecium clear. Ascospores 8 per ascus, (3–)5–7(–9)-septate, fusiform, (70–)90–130×20–30 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from Brazil and Costa Rica).
New country records. Puerto Rico: Ponce: Caribbean National Forest, 1989, Aptroot & Aptroot 25603, 25602 (ABL).—Colombia: Nariño: Tumaco, Estacion Forestal La Espriella, 1986, Sipman & Velosa 33054 (B). Amazonas: Araracuara, opposite Isla Mariñame, 240 m, 1988, Sipman & Duivenvoorden 28328 (B).
Astrothelium rufescens (Müll. Arg.) Aptroot & Lücking comb. et stat. nov.
MycoBank No.: MB 816731
Trypethelium catervarium var. rufescens Müll. Arg., Bot. Jahrb. Syst. 6: 391 (1885); type: Cuba, Wright s. n. (G!—lectotype, designated here; FH-TUCK 3970!—isolectotype, designated here; Müller, Verr. Cub. 576).
Thallus corticate, olive-green to brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 0·5–1·5 mm broad, immersed, forming irregular, whitish lines that become fused and reticulate. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 17–27×7–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Cuba).
Distribution. This taxon belongs in the Astrothelium nitidiusculum aggregate, where it is characterized by its linear-reticulate, immersed, whitish pseudostromata.
Astrothelium sanguinarium (Malme) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816733
Laurera sanguinaria Malme, Ark. Bot. 19(1): 21 (1924).—Meristosporum sanguinarium Malme (Reference Malme1924) nom. inval; type: Brazil, Matto Grosso, Serra da Chapada da Buriti, Malme 2253 (S!—lectotype, designated here).
(Fig. 32G)
Thallus corticate, olive-green to yellowish, smooth to uneven to shallowly bullate.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata 1–2 mm diam., erumpent to prominent, dark brown with pale brown ostioles, internally with bright to dark red pigment; pseudostromata up to 5 mm long and 2 mm broad. Hamathecium clear. Ascospores 8 per ascus, muriform, ellipsoid, (75–)90–130×15–25 µm, without strongly thickened median septum, hyaline, IKI+ violet.
Chemistry. Thallus UV−, K−; pseudostromata UV−, internally with red, K+ green-reacting isohypocrellin.
Distribution. Neotropical (Brazil).
Discussion. This taxon resembles a species of Bathelium but molecular data place it in Astrothelium. Specimens with whitish to pale cream-coloured pseudostromata represent an undescribed taxon labelled “Laurera Formosa” by R. C. Harris, the original material collected in Brazil by Malme (2514, S).
Astrothelium santessonii (Letr.-Gal.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816737
Laurera santessonii Letr.-Gal., Rev. Bryol. Lichénol. 37: 68 (1958); type: Ivory Coast, Mont Toukoui, cercle de Man, 14 August 1954, Santesson 10607d (UPS—holotype, not seen).
(Fig. 38B)
Thallus corticate, olive-green to brownish, smooth to uneven.
Ascomata pleurothelioid, with eccentric, separate ostioles, solitary to aggregate but not distinctly pseudostromatic, 0·7–1·5 mm diam., immersed-erumpent, covered by thallus except dark grey ostiolar area surrounded by irregular whitish rim. Hamathecium inspersed with red, K+ green droplets. Ascospores 8 per ascus, muriform, ellipsoid, 70–100×20–32 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−; hamathecium red, K+ green, with isohypocrellin.
Distribution. Pantropical (previously reported only from the Ivory Coast).
New country record. Guyana: Upper Takutu: Kuyuwini Landing, 1992, Sipman 58142 (B).
Astrothelium saxicola (Malme) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816738
Cryptothelium saxicola Malme, Svensk Bot. Tidskr. 30: 246 (1936); type: Brazil, Matto Grosso, Serra da Chapada, Bocca da Serra, Malme 2314 (S!—holotype).
(Fig. 39I)
Thallus pale greenish grey to yellowish, smooth to uneven or rough.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed, 1·0–1·8 mm diam., erumpent, applanately wart-shaped, completely covered by thallus, with upper part yellowish. Hamathecium clear. Ascospores 1 per ascus, densely muriform, ellipsoid, 215–230×60–80 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances.
Distribution. Neotropical (Brazil).
Discussion. This is one of the very few specimens of Trypetheliaceae encountered on rock, and one of the three species that seem restricted to rock; the others are Astrothelium stramineum, from the same locality, and Pseudopyrenula saxicola.
Astrothelium scoria (Fée) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816739
Trypethelium scoria Fée, Essai Crypt. Écorc.: 69 (1824).—Pseudopyrenula scoria (Fée) Vain., Bol. Soc. Broteriana, Ser. 2 6: 178 (1930); type: South America, “corticem Crotonis cascarillae” (G!—lectotype; Harris, Lichenogr. Thomsoniana: 147, 1998; G!, BM!—isolectotypes).
Pyrenula myriocarpa Fée, Essai Crypt. Écorc.: 74 (1824).—Verrucaria myriocarpa (Fée) Spreng., Syst. Veget. 4(1): 245 (1827).—Spermatodium myriocarpum (Fée) Trevis., Conspect. Verruc.: 11 (1860).—Trypethelium myriocarpum (Fée) Müll. Arg., Bot. Jahrb. Syst. 6: 391 (1885); type: South America, s. col. (G!—holotype).
Trypethelium scoria var. sordidius Nyl., Flora 52: 126 (1869); type: Brazil, Rio de Janeiro, Glaziou 1961 (H—holotype, not seen; M!—isotype).
Trypethelium dichroum Makhija & Patw., J. Hattori Bot. Lab. 73: 195 (1993); type: India, Andaman Islands, North Andaman, Diglipue Range, Millangram, 3 January 1986, Nagarkar & Patwardhan AMH 86.250 (ABL!—isotype).
Trypethelium indicum Makhija & Patw., J. Hattori Bot. Lab. 73: 199 (1993); type: India, Meghalaya, Willoe, 30 October 1977, Patwardhan & Nagarkar AMH 77.1130 (ABL!—isotype).
Trypethelium meghalayense Makhija & Patw., J. Hattori Bot. Lab. 73: 201 (1993); type: India, Meghalaya, near Sohararaim, on road to Cherapunji, 28 October 1977, Patwardhan & Nagarkar AMH 86.862 (ABL!—isotype).
Thallus corticate, olive-green to yellowish or brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, diffusely pseudostromatic, 0·3–0·5 mm diam., erumpent, laterally covered by thallus but upper portion blackish brown with whitish rim. Hamathecium inspersed. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 15–27×7–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (e.g. Puerto Rico, El Salvador, Guyana, French Guiana, Ecuador, Brazil, Zaire, India, China, Hong Kong, Papua New Guinea, Solomon Islands, New Caledonia).
Discussion. The epithet scoria is the oldest for the specimens with hamathecium inspersion of the complex previously known as Trypethelium nitidiusculum. As defined here, Astrothelium scoria is still heterogeneous and might comprise several species, but due to the limited material available we were unable to assess the variation of potential lineages represented by the synonyms listed here. The thallus of the newly synonymized Trypethelium indicum is an unspecific UV+ pinkish, not yellow as mentioned in the protologue.
New country records. El Salvador: Ahuachapán: Parque Nacional El Imposible, 1998, Sipman et al. 44570 (B).—Puerto Rico: Aguadilla: Guajataca, 1989, Aptroot & Aptroot 25737 (ABL).—Guyana: Upper Takutu: Dadanawa ranch, 1992, Sipman 57985 (B).—French Guyana: Regina: Camp Aratai, 2003, Sipman 50699 (B).— Ecuador: Napo: Archidona, 1982, Aptroot & Hensen 10328 (ABL).—Zaire: Kivu: Equateur, Kalamba, 450 m, 1991, Müller s. n. (DR).—China: Yunnan: Xishuangbanna, 2003, Aptroot 57283 (ABL).—Hong Kong: Lantau: Ngong Ping, 2000, Aptroot 48638 (ABL).—Papua New Guinea: Madang: 11 km W of Brahman Mission, 1995, Sipman 38912 (B).—Solomon Islands: Santa Isabel: Tatamba, 1965, Hill 11170 (ABL, BM).—New Caledonia: Vieillard s. n. (BM).
Astrothelium scorioides Nyl.
Ann. Sci. Nat., Bot., sér. 5 7: 348 (1867); type: Colombia, Rio Negro, Lindig 132 (H-Nyl 120!—holotype; BM!—isotype).
Thallus corticate, pale greenish to yellowish, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed to confluent, 0·3–0·5 mm diam., erumpent, conical with flattened top, covered by thallus and upper part with yellow-orange pigment. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 33–45×10–17 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ascomata with yellow to orange, K+ purple, UV+ red anthraquinone.
Distribution. Pantropical (previously reported from Venezuela, Colombia, Brazil and Seychelles).
New country records. Guyana: Upper Mazaruni: Mount Latipu, Sipman & Aptroot 19072 (ABL, B).—French Guiana: Saül, 1985, Aptroot 15442 (ABL, B).
Astrothelium scoriothelium Aptroot & Lücking nom. nov.
MycoBank No.: MB 816740
Trypethelium scorioides Leight., Trans. Linn. Soc. London 25: 459 (1866) non Astrothelium scorioides Nyl. (1867); type: Brazil, Amazonas, Spruce s. n. (BM!—holotype).
Pseudopyrenula infuscatula var. tecomae Vain., Ann. Acad. Sci Fenn., ser A 6(7): 197 (1915).—Trypethelium infuscatulum var. tecomae (Vain.) Zahlbr., Catal. Lich. Univ. 8: 128 (1931); type: Guadeloupe, Près du Camp-Jacob, Duss 1501 (TUR-Vain 30780!—holotype).
(Fig. 20C)
Thallus corticate, olive-green, uneven-verrucose.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata irregular, 0·7–1·7 mm broad, immersed to erumpent, exposed and blackish, with ostioles only visible by their pale rings. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 36–39×11–12 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances.
Distribution. Neotropical (reported from Guadeloupe, Venezuela and Colombia).
Astrothelium scorizum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816741
Trypethelium scorizum Müll. Arg., Bot Jahrb. Syst. 6: 389 (1885).—Trypethelium scorizum Nyl. (1876) nom. nud; type: Cuba, Wright s. n. (G!—holotype; BM!—isotype; Müller, Verr. Cub. 166a).
Thallus corticate, light olive-green to pale brownish, smooth to uneven-rugulose.
Ascomata pleurothelioid, with eccentric, separate ostioles, pseudostromatic; pseudostromata 0·7–1·5 mm diam., immersed-erumpent, forming irregular, in part confluent groups or lines, with the individual ascomata exposed and dark brown. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 20–26×8–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances.
Distribution. Neotropical (Cuba and Brazil).
New country record. Brazil: São Paulo: Pratânia, Fazenda Palmeira da Serra, 2009, Lücking 29814 (B, F).
Astrothelium sepultum Mont.
Ann. Sci. Nat., Bot., sér. 2 19: 74 (1843).—Cryptothelium sepultum (Mont.) A. Massal., Atti I. R. Istitut. Veneto, Ser. 3 5: 335 (1860).—Heufleria sepulta (Mont.) Trevis., Flora 44: 23 (1861).—Laurera sepulta (Mont.) O. E. Erikss., Op. Bot. 60: 50 (1981); type: French Guiana, Leprieur s. n. (BR, PC—syntypes).
Trypethelium connivens Nyl., Mém. Soc. Acad. Maine-et-Loire 4: 79 (1858).—Bathelium connivens (Nyl.) Trevis., Flora 44: 21 (1861).—Laurera connivens (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 503 (1922); type: Peru, s. col. (H-Nyl—holotype, not seen; PC!—isotype).
(Fig. 36H)
Thallus corticate, greyish green to pale yellowish brown (lichexanthone colour), smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed to confluent but not distinctly pseudostromatic, 1·0–1·8 mm diam., erumpent, covered by thallus but with upper part whitish and ostiole dark. Hamathecium clear. Ascospores 2 per ascus, densely muriform, fusiform, 150–250×30–65 µm, with distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and usually also ascomata UV+ yellow, with lichexanthone.
Distribution. Neotropical (reported from Costa Rica, Guyana, French Guiana, Surinam, Brazil and Peru).
Astrothelium sierraleonense (C. W. Dodge) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816742
Tremotylium sierraleonense C. W. Dodge, Nova Hedwigia, Beih. 12: 104 (1964); type: Sierra Leone, Kori, Njala, Deighton M5693C (FH-DODGE!—holotype).
(Fig. 32C)
Thallus corticate, olive-green, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, dispersed to confluent but not distinctly pseudostromatic, 0·8–1·5 mm diam., erumpent, laterally covered by thallus but upper part conspicuously black and flattened, surrounded by an irregular whitish rim, internally with pockets of red crystals. Hamathecium clear. Ascospores 4 per ascus, muriform, ellipsoid, c. 80×14 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata externally UV−, K−; ascomata internally with red, K+ purple anthraquinone crystals.
Distribution. African palaeotropical (Sierra Leone).
Astrothelium sikkimense (Makhija & Patw.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816743
Laurera sikkimensis Makhija & Patw., Mycotaxon 31: 580 (1988); type: India, Sikkim, Gangtok, Near Tangshi View Point, 17 September 1977, Patwardhan & Nagarkar AMH 77.1970 (ABL!—isotype).
(Fig. 33L)
Thallus corticate, brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 1–2 mm diam., immersed to erumpent, covered by thallus except black ostiole surrounded by whitish rim. Hamathecium inspersed. Ascospores 6–8 per ascus, muriform, ellipsoid, 120–140×25–30 µm, with somewhat thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (India).
Astrothelium spectabile (Aptroot & Ferraro) Aptroot & Lücking comb. nov.
MycoBank No.: MB 817086
Trypethelium spectabile Aptroot & Ferraro, Kurtziana 31: 59 (2005); type: Argentina, Misiones, Iguazú, 2003, Ferraro & Popoff 6602 (CTES!—holotype; ABL!—isotype).
(Fig. 21L)
Thallus corticate, light green, smooth.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·7–1·2 mm diam., immersed, completely covered by thallus. Hamathecium clear. Ascospores 8 per ascus, 11–19-septate, fusiform, 140–190×20–25 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Argentina).
Astrothelium sphaerioides (Mont.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816744
Trypethelium sphaerioides Mont., Ann. Sci. Nat., Bot., sér. 2 19: 73 (1843).—Bathelium sphaerioides (Mont.) Trevis., Spighe e Paglie: 20 (1861).—Laurera sphaerioides (Mont.) Zahlbr., Catal. Lich. Univ. 1: 506 (1922); type: French Guiana, Leprieur 80 (PC!—lectotype, designated here; H-Nyl!—isolectotype).
(Fig. 35H)
Thallus corticate, olive-green to brownish, smooth to uneven.
Ascomata trypethelioid to pleurothelioid, with apical to eccentric, separate ostioles, distinctly pseudostromatic; pseudostromata irregular, 0·5–1·5 mm broad, erumpent to prominent, whitish and black ostioles. Hamathecium clear. Ascospores 2–4 per ascus, muriform, ellipsoid, 80–175×25–50 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Pantropical; previously reported from Colombia (Isla de Providencia), French Guiana, Brazil and India.
New country records. El Salvador: Ahuachapán: Parque Nacional El Imposible, 1998, Welz s. n. (B).—Costa Rica: Guanacaste: 5 km NNW of Tilarán, 650 m, 2004, Aptroot 60659 (ABL, INB).—Panama: Veraguas: Bahía Honda, 2001, Cabrera & Etayo 18583 (ABL, hb. Etayo).—Puerto Rico: Mayagüez: Reserva Forestal Maricao, 800 m, 1989, Aptroot & Aptroot 24948, 24943 (ABL).—Guyana: Upper Takutu: Rupununi Savannah, Karanambo Ranch, 1992, Sipman 57321 (B).—Ecuador: Zamora-Chinchipe: Estacion Cientifico San Francisco, 40 km S of Loja, 2004, Sipman 52367 (B).
Astrothelium stramineum (Malme) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816745
Thelenella straminea Malme, Ark. Bot. 22A(1): 7 (1928).—Clathroporina straminea (Malme) Zahlbr., Catal. Lich. Univ. 8: 111 (1931).—Laurera straminea (Malme) P. M. McCarthy, Lichenologist 27: 348 (1995); type: Brazil, Matto Grosso, Serra da Chapada, Bocca da Serra, 15 March 1884, Malme 2550 (S!—holotype).
(Fig. 31D)
Thallus saxicolous, corticate, yellowish white, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary, 0·4–0·6 mm diam., hemispherical, covered by thallus. Hamathecium inspersed. Ascospores (2–)4(–6) per ascus, densely muriform, fusiform, 40–80×15–24 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus UV+ yellow, K−, with lichexanthone; ascomata UV−, K−.
Distribution. Neotropical (Brazil).
Discussion. This is one of the very few species of Trypetheliaceae ever encountered on rock, and one of three species that seem restricted to rock; the others are A. saxicola, from the same locality, and Pseudopyrenula saxicola.
Astrothelium straminicolor (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816746
Trypethelium straminicolor Nyl., Lich. Japon: 115 (1890); type: Malaysia, Labuan, Almquist s. n. (H-Nyl 174!—lectotype, designated here; S!—isolectotype).
Trypethelium stramineum Kremp., Nuov. Giorn. Bot. Ital. 7: 56 (1875), non Astrothelium stramineum (Malme) Aptroot & Lücking, see above; type: Sarawak, Beccari 212 (M!—lectotype, Makhija & Patwardhan, Int. J. Mycol. Lichenol. 5: 246, 1992).
Astrothelium subvariolosum Makhija & Patw., Biovigyanam 15: 64 (1989); type: India, Nicobar Islands, Great Nicobar, Campbell Bay to Laful Bay, 2 January 1987, Patwardhan & Nagarkar AMH 87-70 (ABL!—isotype).
Thallus corticate, olive-green to yellowish brown, uneven to shallowly bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata pseudostromatic; pseudostromata 1–2 mm diam., erumpent to prominent, covered by thallus except for blackish upper part surrounded by a thin, whitish rim, with blackish ostiolar areas often arranged in lobate fashion. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 20–25×7–9 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (India and Malaysia, including Sarawak).
Astrothelium subaequans Müll. Arg.
Bot. Jahrb. Syst. 6: 383 (1885); Astrothelium subaequans Nyl. (1876) nom. nud.; type: Cuba, Wright s. n. (G!—holotype; H-Nyl 108!—isotype; Müller, Verr. Cub. 146).
(Fig. 37H)
Thallus corticate, olive-green to yellowish, uneven to shallowly bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed, 1–2 mm diam., erumpent, covered by thallus except dark, protruding and papilliform ostiole, thinly covered with yellow-orange pigment. Hamathecium clear. Ascospores 8 per ascus, submuriform, fusiform, (45–)72–83×15–25 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ascomata with yellow-orange, K+ purple, UV+ red anthraquinone.
Distribution. Neotropical (previously reported from Costa Rica and Cuba).
New country records. Venezuela: Bolivar: Cerro Guaiquinima, 1200 m, 1990, Sipman 26848 (B).—Guyana: Upper Mazaruni: N of Paruima Mission, Aymatoi savannah, 1000 m, 1997, Sipman 39806 (B). Potaro-Siparuni: Kaieteur Falls, around airstrip, 400 m, 1996, Sipman 40408 (B).
Astrothelium subcatervarium (Malme) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816747
Trypethelium subcatervarium Malme, Ark. Bot. 19(1): 29 (1924); type: Brazil, Matto Grosso, Serra da Chapada, Buriti, Malme s. n. (S!—holotype).
(Fig. 15L)
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed to confluent or diffusely pseudostromatic, 0·4–0·6 mm diam., erumpent, whitish and dark brown ostiolar area thinly covered with yellow-orange pigment. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 27–33×10–12 µm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV+ orange, K+ red, with anthraquinone.
Distribution. Neotropical (reported from Cuba and Brazil). Incorrectly reported from Costa Rica: the specimen (Breuss 21057 in LI) proved to have K− pseudostromata.
Astrothelium subclandestinum Leight.
Trans. Linn. Soc. London 25: 460 (1866); type: Brazil, Amazonas, São Gabriel, Spruce 242 (BM!—holotype).
(Fig. 29L)
Thallus corticate, olive-green to yellowish, uneven to shallowly bullate.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata diffusely pseudostromatic; pseudostromata 1·0–1·5 mm diam., erumpent, laterally covered by thallus but apically greyish. Hamathecium inspersed. Ascospores 8 per ascus, 5-septate, fusiform, 50–75×14–22 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (previously reported from Costa Rica, Brazil and Australia).
New country records. Venezuela: Bolivar: National Park Canaima, 2003, Berger 18510 (ABL, hb. Berger).—Guyana: Demerara-Berbice: Mabura Hill, 1985, Cornelissen 486 (ABL).—Papua New Guinea: Madang: Foothills of Finisterre Range, 1992, Aptroot 33209 (ABL).
Astrothelium subdiscretum (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816748
Trypethelium subdiscretum Nyl., Flora 52: 73 (1869).—Bathelium subdiscretum (Nyl.) Müll. Arg., Bot. Jahrb. Syst. 6: 395 (1885).—Laurera subdiscreta (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 506 (1922); type: India, West Bengal, Kurz s. n. (H-Nyl 322!—holotype; M! —isotype).
Bathelium phaeomelodes Müll. Arg., Bot. Jahrb. Syst. 6: 394 (1885).—Trypethelium phaeomelodes Nyl. (1876) nom. nud.— Laurera phaeomelodes (Müll. Arg.) Zahlbr., in Engler & Prantl, Natürl. Pflanzenfamil. 1(1*): 71 (1903); type: Cuba, Wright s. n. (G!—holotype; BM!—isotype; Müller, Verr. Cub. 170).
Laurera nigeriensis C. W. Dodge, Ann. Missouri Bot. Gard. 40: 297 (1953); type: Nigeria, Abadan, 1951, Thorold 165 (FH-Dodge—holotype, not seen).
Laurera subphaeomelodes Upreti & Ajay Singh, Bull. Jard. Bot. Nat. Belg. 57: 382 (1987); type: India, West Bengal, Howrah, Sibpur, Roychaudhury 649 (CAL—holotype, not seen).
Laurera verrucoaggregata Makhija & Patw., Mycotaxon 31: 586 (1988); type: India, Andaman Islands, North Andaman, Lamia Bay, 5 January 1986, Nagarkar & Sethy AMH 86.550 (ABL—isotype).
Laurera fusispora Makhija & Patw., Mycotaxon 31: 576 (1988); type: India, Karnataka, Anamod ghat, Anamod-Goa Road, 10 December 1974, Patwardhan & Prabhu AMH 74.2499 (ABL—isotype).
Thallus corticate, olive-green to brownish, uneven to verrucose-rugulose.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 0·8–1·5(–2·0) mm broad, immersed-erumpent, irregular to linear and often confluent and reticulate, with ascomata exposed and black. Hamathecium inspersed, clear. Ascospores 8 per ascus, small muriform, ellipsoid, 35–50×12–18 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (previously reported from Cuba, Costa Rica, Nigeria, India and Thailand).
New country records. Netherlands Antilles: Bonaire: Ceru Kammia, 1977, van Slageren 8406-b (ABL).—Dominican Republic: La Altagracia, Bayahibe, 1981, Buck 5141 (NY, hb. Kalb).—Colombia: Caqueta, Araracuara, opposite airstrip, 250 m, 1988, Sipman & Duivenvoorden 27909 (B).—Venezuela: Bolivar: Cerro Guaiquinima, along Rio Carapo, 800 m, 1990, Sipman 26942 (B).—Guyana: between Karanambo and Kwaimatta, 1988, Maas et al. 7709d (ABL).—Sri Lanka: Anuradhapura: Wipattu, Santesson 26185 (S).—Singapore: Pulau Ubin Island: 2000, Sipman & Tan 46340 (B).
Astrothelium subdisjunctum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816749
Bottaria subdisjuncta Müll. Arg., Bot. Jahrb. Syst. 6: 395 (1885).—Laurera subdisjuncta (Müll. Arg.) R. C. Harris, Acta Amazon. (Suppl.) 14: 66 (‘1984’) [1986]; type: Cuba, Wright s. n. (G!—holotype; Müller, Verr. Cub. II: 676).
Laurera dodgei Letr.-Gal., Rev. Bryol. Lichénol. 27: 71 (1958); type: Costa Rica, Puntarenas, Rio Sandalo, Galjo Dulce, Peninsula de Osa, 0–10 m, 23 August 1936, Dodge & Goerger 10063 (FH-DODGE!—holotype).
Clathroporina wainiana Zahlbr., Sitzungsber. K. Akad. Wiss. Wien, math.-naturw. Classe 111(1): 364 (1902); type: Brazil, Rio de Janeiro, Höhnel 151 (W—holotype, not seen).
Thallus corticate, olive-green to brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 0·7–1·5 mm broad, immersed-erumpent, irregular to linear and often confluent and reticulate, with ascomata exposed and black. Hamathecium clear. Ascospores 4 per ascus, densely muriform, fusiform, 120–175×30–45 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from the USA, Cuba, Costa Rica, Guyana, Venezuela and Brazil).
New country records. French Guiana: Cayenne: Cayenne, along forest track called Risque tout, 50 m, 1985, Aptroot 15126 (ABL, B). Saint-Laurent-du-Maroni: Saül, 1985, Aptroot 15440 (ABL, B); Saül, 1986, Montfoort & Ek 290 (ABL).
Astrothelium subdissocians (Nyl. ex Vain.) Aptroot & Lücking comb. et stat. nov.
MycoBank No.: MB 816750
Pseudopyrenula ochroleuca var. subdissocians Nyl. ex Vain., Bot. Tidskr. 29: 147 (1909).—Trypethelium ochroleucum var. subdissocians (Vain.) Hue (1892) nom. nud.—Trypethelium pallescens var. subdissocians Nyl. (1864) nom. nud; type: Thailand, Koh Chang, Schmidt 11 (TUR-Vain 30761!—holotype).
(Fig. 28L)
Thallus corticate, light olive-green to yellowish brown, smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed to irregularly confluent, 0·4–0·6 mm diam., erumpent to prominent, with complete whitish cover. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 20–25×7–9 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Palaeotropical (reported from Thailand).
Astrothelium subfuscum Kremp.
Nuovo Giorn. Bot. Ital. 7: 64 (1875); type: Singapore, Beccari 236 (M!—holotype).
Thallus corticate, olive-green to brownish, rather thick, uneven to bullate-folded.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata confluent to diffusely pseudostromatic; pseudostromata 0·7–1·3 mm diam., erumpent, irregular in outline, laterally covered by thallus but upper part of ascomata exposed, blackish and groups of ascomata bordered by thin, whitish line. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 21–27×8–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (previously reported from India, Singapore, Sabah, New Caledonia and Papua New Guinea). Reports from the Neotropics refer probably to Astrothelium crassum.
New country records. China: Hong Kong: New Territories, Tai Po Kau Nature Reserve, 1998, Aptroot 43290 (ABL).—Philippines: Luzon: Sorsogon, Irosin, 1916, Elmer 17111 (B).
Astrothelium superbum (Fr.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816751
Trypethelium superbum Fr., Syst. Orb. Veget. 1: 287 (1825) .— Campylothelium superbum (Fr.) Müll. Arg., Flora 68: 251 (1885); type: Indonesia, s. col. (UPS—holotype, not seen).
Thallus corticate, olive-green, smooth to uneven.
Ascomata pleurothelioid, with eccentric, separate ostioles, solitary to irregularly grouped, 1–2 mm diam., immersed-erumpent, covered by thallus. Hamathecium clear. Ascospores 8 per ascus, densely muriform, fusiform, 130–165×35–45 µm, without distinctly thickened median septum, hyaline or becoming yellowish, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from India and Indonesia).
Astrothelium tenue (Aptroot) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816752
Campylothelium tenue Aptroot in Aptroot et al., Biblioth. Lichenol. 98: 53. (2008); type: Costa Rica, Alajuela, Volcán Tenorio National Park, surroundings of Pilón Biological Station, Sipman 51867 (B!—holotype).
Thallus corticate, olive-green, smooth to uneven.
Ascomata pleurothelioid, with eccentric, separate ostioles, 0·4–0·7 mm diam., erumpent, completely covered by thallus. Hamathecium clear. Ascospores 4–8 per ascus, muriform, fusiform, 95–115×20–28 µm, with distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Possibly pantropical (so far reported only from Costa Rica).
Discussion. This species is characterized by the fusiform distoseptate ascospores, in which the stellate primary locules remain recognizable in the mature ascospores.
New country records. Guyana: Demerara-Berbice: Mabura Hill, 1988, Bleij & Biesmeijer (ABL).—Singapore: Nee Soon, 2000, Sipman et al. 46164 & 46159 (B).
Astrothelium thelotremoides (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816753
Verrucaria thelotremoides Nyl., Ann. Sci. Nat., Bot., sér. 5 7: 346 (1867).—Pseudopyrenula thelotremoides (Nyl.) Müll. Arg., Flora 66: 248 (1883).—Trypethelium thelotremoides (Nyl.) R. C. Harris., Lichenogr. Thomsoniana: 148 (1986); type: Colombia, Rio Negro, Lindig 51 (H-Nyl 1113!—lectotype, designated here; BM!—isolectotype).
Thallus corticate, olive-green to brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·5–0·8 mm diam., erumpent, covered by thallus with ostiolar area dark greyish with thin whitish rim, internally with red pigment. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 50–65×15–21 µm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−; ascomata internally K+ purple, with an anthraquinone.
Distribution. Neotropical (previously reported only from Colombia).
Discussion. The type sheet contains three specimens; two are Ocellularia species and the third specimen is designated here as lectotype.
New country record. Brazil: Bahia: Entre Rios, 1981, Boom & Mori 1010 (hb. Kalb, NY).
Astrothelium trypethelizans (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816754
Verrucaria trypethelizans Nyl., in Crombie, J. Linn.Soc. London, Bot. 20: 60 (1883).—Pseudopyrenula trypethelizans (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 359 (1922).—Trypethelium trypethelizans (Nyl.) R. C. Harris, Lichenogr. Thomsoniana: 148 (1998); type: Malaysia, Malacca, Tanjong, Maingay 149 (BM!—lectotype; Harris, Lichenogr. Thomsoniana: 148, 1998).
(Fig. 21D)
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, dispersed to largely confluent, 0·3–0·5 mm diam., erumpent with flattened top, brownish black. Hamathecium clear. Ascospores 8 per ascus, 7–9-septate, fusiform, 32–38×10–11 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from Malaysia).
Astrothelium tuberculosum (Vain.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816755
Pseudopyrenula annularis var. tuberculosa Vain., Ann. Acad. Sci Fenn, ser. A 6(7): 196 (1915).—Trypethelium crassum var. tuberculosum (Vain.) Zahlbr., Catal. Lich. Univ. 8: 128 (1932).—Trypethelium tuberculosum (Vain.) R. C. Harris, Acta Amazon. (Suppl.) 14: 76 (‘1984’) [1986]; type: Guadeloupe, Safraga, Duss 1415 (TUR-Vain 30778!—holotype).
(Fig. 20L)
Thallus corticate, olive-green to brownish, strongly bullate.
Ascomata trypethelioid, with apical ostioles, diffusely pseudostromatic; pseudostromata 1–2 mm diam., prominent to sessile, irregular, fully covered by thallus except dark ostioles surrounded by whitish rim. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 48–65×17–20 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from Guadeloupe, Trinidad, Tobago, Costa Rica, Guyana, French Guiana, Venezuela, Galapagos and Colombia).
New country record. Brazil: São Paulo: Campos de Jordão, 1800 m, 1997, Sipman 41127 (SP, B).
Astrothelium ubianense (Vain.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816756
Pseudopyrenula ubianensis Vain., Ann. Acad. Sci. Fenn., ser. A 15(6): 353 (1921).—Trypethelium ubianense (Vain.) Zahlbr., Catal. Lich. Univ. 8: 129 (1932); type: Philippines, Ubian, Merrill 5403 p.p. (TUR-Vain 30798!—holotype).
(Fig. 21C)
Thallus corticate, light olive-green to yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata irregular, 0·8–1·5 mm broad, basally covered by thallus but otherwise whitish, with the ostiolar areas of individual ascomata black and strongly contrasting. Hamathecium clear. Ascospores 8 per ascus, 5–11-septate, fusiform, 30–36(–49)×7–10 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances reported.
Distribution. Eastern palaeotropical (reported from India and the Philippines).
Astrothelium variatum (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816757
Trypethelium variatum Nyl., Ann. Sci. Nat., Bot., sér. 5 7: 347 (1867).—Laurera variata (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 507 (1922); type: Colombia, Rio Negro, Lindig s. n. (H-Nyl!—holotype).
Heufleria subvariata Müll. Arg., Bot. Jahrb. Syst. 6: 384 (1885).—Trypethelium subvariatum Nyl. (1876) nom. nud.— Cryptothelium subvariatum (Müll. Arg.) Zahlbr., Catal. Lich. Univ. 1: 523 (1922); type: Cuba, Wright s. n. (G!—holotype; Müller, Verr. Cub. 164).
Thallus corticate, olive-green, smooth to uneven or shallowly rugose.
Ascomata trypethelioid, with apical ostioles, diffusely pseudostromatic; pseudostromata 0·7–1·4 mm diam., prominent, irregular, covered by thallus except dark ostioles. Hamathecium clear. Ascospores 8 per ascus, small muriform, fusiform, 24–35×11–13 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from Cuba and Colombia).
New country record. Venezuela: Amazonas: Alto Orinoco, 15 km W of Esmeralda, 110 m, 1997, Hafellner & Komposch 209-3-7 (GZU).
Astrothelium variolosum (Ach.) Müll. Arg.
Flora 68: 255 (1885).—Trypethelium variolosum Ach., Synops. Lich.: 104 (1814); type: “ad corticem Chinchonae flavae” (H-ACH 877!—holotype; S!—isotype).
Trypethelium papillosum Ach., Synops. Lich.: 104 (1814) non Knight (1886).—Bathelium papillosum (Ach.) C. W. Dodge, Ann. Missouri Bot. Gard. 40: 288 (1953); type: Guinea, s. col. (H-ACH 878A!—holotype; S!—isotype).
Pyrenula epapillata Fée, Essai Crypt. Écorc.: 78 (1824).—Spermatodium epapillatum (Fée) Trevis., Conspect. Verruc.: 11 (1860); type (PC—syntypes, not seen).
Trypethelium papillosum var. fuscum Müll. Arg., Flora 68: 255 (1885); type: French Guiana, Leprieur 467 p.p. (G!—holotype).
Thallus corticate, pale greenish grey to pale yellowish brown (lichexanthone colour), smooth to uneven.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata pseudostromatic; pseudostromata 0·7–1·5 mm broad, erumpent to prominent, irregular in outline, covered by thallus but often paper to whitish, with dark ostioles. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 20–26×7–9 µm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV+ yellow, K−, with lichexanthone (or 1,8-dihydroxy-3,6-dimethoxyxanthone).
Distribution. Pantropical (previously reported from the continental USA, Bahamas, Cuba, Trinidad, Costa Rica, French Guiana, Venezuela, Colombia, Brazil, Bolivia, Sri Lanka, Thailand, Guam, Northern Mariana Islands and Australia).
Discussion. Further synonyms in the genera Verrucaria and Spermatodium are mentioned by Zahlbruckner (Reference Zahlbruckner1922), but not cited here as the species was used in a different sense at that time and the material has not been checked. Astrothelium variolosum is accepted here in a very much more restricted sense than by Harris (Reference Harris1995), who included specimens with or without lichexanthone, with apical or eccentric ostioles, with simple or aggregated ascomata and with or without hamathecium inspersion, each of which are species characters in our concept. Specimens which differ only in an inspersed hamathecium are known from, for example, Guyana (Sipman 58039, 39954, and 39969). A new species is not described for them because it cannot be ruled out that one of the taxa that have been synonymized with A. variolosum has an inspersed hamathecium.
New country records. Puerto Rico: Aguadilla: Bosque Estatal de Guajataca, 1989, Aptroot & Aptroot 25730 (ABL).—Guyana: Upper Demerara-Berbice: Mabura Hill, 1994, DePriest et al. 9086 (B).—Indonesia: Java: Malang, 1932, Groenhart 412 (ABL, L).—Papua New Guinea: Madang: Madang, 1992, Aptroot 30078 (ABL).
Astrothelium versicolor Müll. Arg.
Flora 71: 495 (1888); type: Puerto Rico, Sintenis 6 (G!—holotype).
(Fig. 23C)
Thallus corticate, whitish to pale yellowish, strongly bullate-folded.
Ascomata astrothelioid, several chambers joined with eccentric, fused ostioles; joined ascomata dispersed but dense, 0·2–0·4 mm diam., completely immersed in thallus folds and warts, visible only by their darker ostioles thinly furnished with yellow-orange pigment. Hamathecium clear. Ascospores 8 per ascus, fusiform-ellipsoid, 3-septate, 28–35×10–15 µm, hyaline, IKI−.
Chemistry. Thallus UV+ yellow, K−, with lichexanthone; ascomata apically K+, UV+ red, with anthraquinone.
Distribution. Pantropical (previously reported from the USA, Bahamas, Cuba, Puerto Rico, Trinidad, Costa Rica, Guyana, French Guiana, Venezuela, Brazil, Bolivia, Philippines and New Caledonia).
New country record. Papua New Guinea: Central: Varirata National Park, 1995, Aptroot 39772 (ABL).
Astrothelium vezdae (Makhija & Patw.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816758
Laurera vezdana Makhija & Patw., Mycotaxon 31: 587 (1988) [as ‘vezdae`]; type: India, Maharashtra, Vishalgarh, Amba-Gajapur Road, 6 December 1974, Nagarkar & Prabhu AMH 74.2183 (ABL!—isotype).
(Fig. 32K)
Thallus corticate, olive-green to brownish, uneven-rugulose.
Ascomata trypethelioid to pleurothelioid, with apical to eccentric, separate ostioles, pseudostromatic; pseudostromata 1·0–1·3 mm diam., erumpent to prominent, covered by thallus but thinly covered with orange pigment. Hamathecium clear. Ascospores 2–4 per ascus, muriform, ellipsoid, 80–175×25–50 µm, without distinctly thickened median septum, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV+ reddish, K+ purple, with orange anthraquinone.
Distribution. Possibly pantropical (previously reported only from India).
New country record. Guyana: Upper Takutu: c. 35 km S of Aishalton, 250 m, 1992, Sipman 51722 (B).
Bathelium Ach.
Methodus: 111 (1803); type: Bathelium mastoideum Ach. (holotype).
Riddlea C. W. Dodge, Annals of the Missouri Botanical Garden 40: 287 (1953); type: Riddlea papillosa C. W. Dodge (= Bathelium cf. madreporiforme, holotype).
Thallus corticate, olive-green to brownish or greyish.
Ascomata with apical ostioles, solitary to grouped or mostly pseudostromatic, prominent to sessile, brown-black or rarely whitish pruinose, with a peripheral layer of compressed cells. Hamathecium clear, hyaline, filaments thin, anastomosing paraphysoids. Ascospores 1–8 per ascus, transversely septate to muriform, with thin septa and more or less angular lumina, fusiform with acute or rounded ends, not constricted at the median septum, hyaline, IKI−, surrounded by a gelatinous sheath.
Conidiomata unknown.
Chemistry. Anthraquinones often present internally in pseudostromata, lichexanthone occasionally present.
Discussion. A genus of currently 11 species, all tropical epiphytes, which are phylogenetically separate from Astrothelium. Bathelium is morphologically characterized by the usually prominent pseudostromata not covered by thallus, superficially somewhat similar to those of Trypethelium s. str. and like the latter usually with internal pigment granules, and the apparently thin-walled ascospores with a reduced endospore, best seen in species with transversely septate ascospores. The genus was first properly recognized by Harris (Reference Harris1995), who also included some species now shown to belong in Astrothelium, such as A. degenerans and A. endochryseum (Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). Harris (Reference Harris1995) therefore did not note the lack of an endospore in species of this genus but instead mentioned hamathecium inspersion as a character of taxa with muriform ascospores. However, all Bathelium species have a clear hamathecium except for the ostiolar region, which is inspersed in many Trypetheliaceae.
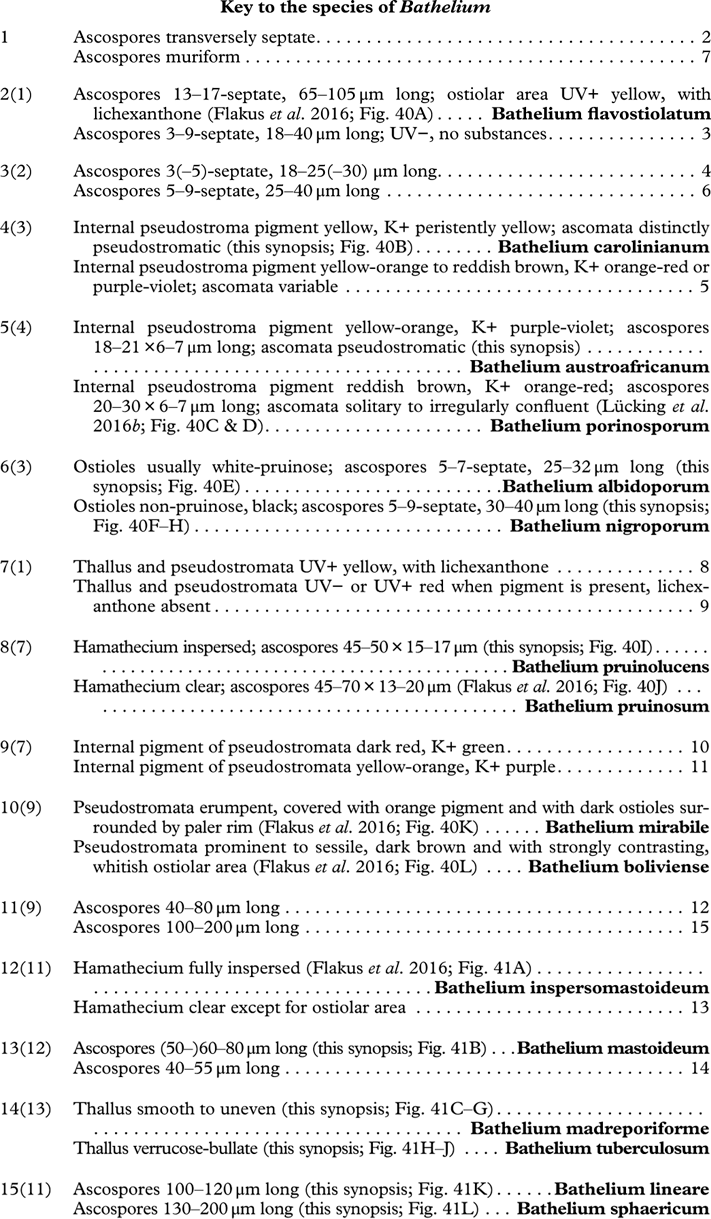
Bathelium albidoporum (Makhija & Patw.) R. C. Harris
More Florida Lichens: 116 (1995).—Trypethelium albidoporum Makhija & Patw., Internat. J. Mycol. Lichenol. 5: 238 (1992); type: Vietnam, Tu-phap, Balansa s. n. (BM!—holotype).
(Fig. 40E)
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·6–1·0 mm diam., prominent to sessile, blackish brown, often with white-pruinose ostioles, internally with orange-brown, dusty granular pigment. Hamathecium clear. Ascospores 8 per ascus, 5–7-septate, fusiform, 25–32×6–9 μm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ascomata UV−, internally K+ red, with anthraquinone.
Distribution. Eastern palaeotropical (previously reported from Thailand, Vietman and Myanmar).
New country records. China: Yunnan: Xishuangbanna, Menglun, 2002, Aptroot 57020 (ABL).—Indonesia: Java: Bay of Serang, S of Bliter, 1937, Groenhart 5813, 5826 (ABL, L).—Papua New Guinea: Madang: S side of Ramu River, Brahman Mission, 1995, Aptroot 38525 (ABL); Gogol Valley, Tgubi logging site, 1992, Aptroot 32903 (ABL).
Bathelium austroafricanum (Zahlbr.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816759
Trypethelium austroafricanum Zahlbr., Ann. Cryptog. Exot. 5: 204 (1932); type: South Africa, Cape Region, Knysna, van der Byl 918 (W—holotype, not seen; BM!—isotype).
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 0·8–1·5 mm diam. (individual ascomata 0·5–0·9 mm diam.), prominent to sessile, blackish brown, internally with yellow-orange, dusty granular pigment. Hamathecium clear. Ascospores 6–8 per ascus, 3-septate, fusiform, 18–21×6–7 μm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV−, internally K+ purple-violet, with anthraquinone.
Distribution. African Palaeotropics (reported from South Africa).
Bathelium carolinianum (Tuck.) R. C. Harris
More Florida Lichens: 116 (1995); Trypethelium carolinianum Tuck., Amer. J. Sci. Arts, ser. 2 25: 429 (1858); type: USA, South Carolina, Ravenel 298 (FH-TUCK—lectotype; Harris, More Florida Lichens: 117, 1995, not seen; S!—isolectotype).
(Fig. 40B)
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 0·8–1·5 mm diam. (individual ascomata 0·5–0·8 mm diam.), prominent to sessile, blackish brown, internally with yellow, dusty granular pigment. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 18–28×6–9 μm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV−, internally K+ yellow, with anthraquinone.
Distribution. South-eastern North America.
Bathelium lineare (C. W. Dodge) R. C. Harris
More Florida Lichens: 117 (1995).—Polyblastiopsis linearis C. W. Dodge, Ann. Missouri Bot. Gard. 40: 276 (1953).—Laurera linearis (C. W. Dodge) Letr.-Gal., Rev. Bryol. Lichénol. 36: 253 (1957); type: Sierra Leone, Njala, Deighton M4634 (BM!—isotype).
(Fig. 41K)
Thallus corticate, olive-green to yellowish brown, uneven to shallowly verrucose.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·9–1·2 mm diam., prominent to sessile, blackish brown, internally with yellow-orange, dusty granular pigment. Hamathecium clear. Ascospores 8 per ascus, muriform, fusiform, 100–120×13–20 μm, central septum not strongly thickened, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ascomata UV−, internally K+ purple, with anthraquinone.
Distribution. Possibly pantropical (reported from Costa Rica, Senegal, Mozambique and Sierra Leone).
Bathelium madreporiforme (Eschw.) Trevis.
Flora 2: 21 (1861).—Trypethelium madreporiforme Eschw., Syst. Lich.: 24 (1824).—Laurera madreporiformis (Eschw.) Riddle, in Howe, Torreya 16: 50 (1916); type: Brazil, Serra dos Montes Altes, Martius s. n. (M-0207125!—lectotype, designated here).
?Riddlea papillosa C. W. Dodge, Annals of the Missouri Botanical Garden 40: 287 (1953); type: Sierra Leone, Njala (Kori), Deighton M4624 (FH!—holotype).
Laurera kundaraensis Upreti & Ajay Singh, Bull. Jard. Bot. Nat. Belg. 57: 375 (1987); type: India, Kerala, Quilon, Kundara, Singh & Ranjan s. n. (LWG—holotype, not seen).
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 1–2 mm broad (individual ascomata 0·7–1·0 mm diam.), prominent to sessile, blackish brown, internally with yellow-orange, dusty granular pigment. Hamathecium clear. Ascospores 8 per ascus, small muriform, fusiform-ellipsoid, 40–55×11–17 μm, central septum not strongly thickened, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV−, internally K+ purple, with anthraquinone. TLC also revealed terpenoid-like spots but these are probably from the bark.
Distribution. Pantropical (previously reported from the USA, Mexico, Costa Rica, French Guiana, Colombia, Venezuela, Brazil, Paraguay, Bolivia, Ivory Coast, India, Thailand, Myanmar and Australia).
Discussion. The type of Riddlea papillosa contains only immature ascospores without septa. The size of these (c. 30×10 μm) agrees with immature ascospores of Bathelium madreporiforme, as does the external morphology of the pseudostromata, and hence R. papillosa is tentatively listed here as a synonym of B. madroporiforme. However, there is a possibility that R. papillosa is conspecific with B. mastoideum, which has larger ascospores but is more common in tropical Africa.
New country record. Guyana: Upper Takutu: Rupununi Savannah, Dadanawa ranch, 1992, Sipman 57387 (B).
Bathelium mastoideum Ach.
Methodus: 111 (1803).—Trypethelium mastoideum (Ach.) Ach., Lichenogr. Univers.: 307 (1810); type: Sierra Leone, Afzelius s. n. (S!—lectotype, Harris, More Florida Lichens: 117, 1995; H-ACH 879E!—isolectotype).
Trypethelium marginatum Fée, Ann. Sci. Nat. Bot. 23: 433 (1831).—Laurera marginata (Fée) C. W. Dodge, Ann. Missouri Bot. Gard. 40: 297 (1953); type: Senegal, Presque-Ile du Cap Vert, Perrottet s. n. (G!—holotype; BM!—isotype).
Thelenella elegans Vain., Bull. Soc. Broteriana, ser. 2 6: 174 (1930).—Laurera elegans (Vain.) Zahlbr., Catal. Lich. Univ. 10: 103 (1938); type: Mozambique, Palma, Pires de Lima 84 (TUR-Vain 34704—holotype, not seen).
Laurera submadreporiformis Abbayes, Bull Inst. Franç. Afr. Noire 15: 52 (1953); type: Equatorial Guinea, des Abbayes s. n. (type probably lost).
Polyblastiopsis pyriformis C. W. Dodge, Ann. Missouri Bot. Gard. 40: 276 (1953).—Laurera pyriformis (C. W. Dodge) Letr.-Gal., Rev. Bryol. Lichénol. 26: 252 (1957); type: Sierra Leone, Njala, Deighton M4404 (BM!—isotype).
(Fig. 41B)
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 1–2(–3) mm broad (individual ascomata 0·8–1·2 mm diam.), prominent to sessile, blackish brown, internally with yellow-orange, dusty granular pigment. Hamathecium clear. Ascospores 8 per ascus, muriform, fusiform, 50–80×13–20 μm, central septum not strongly thickened, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV−, internally K+ purple, with anthraquinone.
Distribution. Pantropical (previously reported from Costa Rica, Colombia, Brazil, Seychelles, Senegal, Mozambique and Sierra Leone). Wrongly reported from many other areas, such as the Galapagos.
New country records. China: Yunnan: Xishuangbanna, Menglun, 2002, Aptroot 57033 (ABL).
Bathelium nigroporum (Makhija & Patw.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816760
Trypethelium nigroporum Makhija & Patw., J. Hattori Bot. Lab. 73: 202 (1993); type: India, Nagaland, Dimapur-Kohima Road, 7 November 1977, Padwardhan & Nagarkar AMH 77.1381 (ABL!—isotype).
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·7–1·0 mm diam., prominent to sessile, blackish brown, internally with orange-brown, dusty granular pigment. Hamathecium clear. Ascospores 8 per ascus, 5–9-septate, fusiform, 30–40×6–9 μm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; ascomata UV−, internally K+ red, with anthraquinone.
Distribution. Palaeotropical (reported from Gambia and India).
Bathelium pruinolucens Aptroot & Lücking nom. et stat. nov.
MycoBank No.: MB 816761
Laurera madreporiformis var. pruinosa Malme, Ark. Bot. 19(1): 23 (1924); type: Brazil, Matto Grosso, Cuyaba, Malme, Regn. Lich. 2560A (S!—lectotype, Harris, More Florida Lichens: 117, 1995).
(Fig. 40I)
Thallus corticate, olive-grey, whitish pruinose, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 1–2 mm broad (individual ascomata 0·7–1·0 mm diam.), prominent to sessile, blackish brown but whitish pruinose, internally with yellow-orange, dusty granular pigment. Hamathecium clear. Ascospores 4 per ascus, small muriform, fusiform, 45–50×15–17 μm, central septum not strongly thickened, hyaline, IKI−.
Chemistry. Thallus UV+ yellow, K−; pseudostromata UV+ yellow, internally K+ purple; with lichexanthone and anthraquinone.
Distribution. Neotropical (reported from Brazil).
Discussion. Rather than elevating the epithet pruinosum to the species level, we introduce the new name pruinolucens to emphasize the presence of lichexanthone, unusual for this genus, thus reserving the epithet pruinosum for a new species described from Bolivia (Flakus et al. Reference Flakus, Kukwa and Aptroot2016).
Bathelium sphaericum (C. W. Dodge) R. C. Harris
More Florida Lichens: 118 (1995).—Polyblastiopsis sphaerica C. W. Dodge, Ann. Missouri Bot. Gard. 40: 277 (1953).—Laurera sphaerica (C. W. Dodge) Letr.-Gal., Rev. Bryol. Lichénol. 26: 254 (1957); type: Sierra Leone, Njala, Deighton M4795 (BM!, F!—isotypes).
(Fig. 41L)
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 1–3 mm broad (individual ascomata 1·0–1·3 mm diam.), prominent to sessile, blackish brown, internally with yellow-orange, dusty granular pigment. Hamathecium clear. Ascospores 8 per ascus, muriform, fusiform, 130–200×13–20 μm, central septum not strongly thickened, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV−, internally K+ purple, with anthraquinone.
Distribution. African palaeotropical (reported from Sierra Leone).
Bathelium tuberculosum (Makhija & Patw.) R. C. Harris
More Florida Lichens: 118 (1995).—Laurera tuberculosa Makhija & Patw., Mycotaxon 31: 584 (1988); type: India, Karnataka, Sirsi-Kumtha Roas, Kulkarni 74.2689 (LWG—holotype, not seen).
Thallus corticate, olive-green to yellowish brown, verrucose-bullate.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 1–2 mm broad (individual ascomata 0·8–1·0 mm diam.), prominent to sessile, blackish brown, internally with yellow-orange, dusty granular pigment. Hamathecium clear. Ascospores 8 per ascus, small muriform, fusiform-ellipsoid, 40–50×12–17 μm, central septum not strongly thickened, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV−, internally K+ purple, with anthraquinone.
Distribution. Eastern palaeotropical (reported from India).
Bogoriella Zahlbr.
Ann. Cryptog. Exotique 1928(1): 111 (1928); type: Bogoriella subpersicina Zahlbr. [=Bogoriella decipiens (Müll. Arg.) Aptroot & Lücking, holotype].
Ornatopyrenis Aptroot, Biblioth. Lichenol. 44: 128 (1991); type: Ornatopyrenis queenslandica (Müll. Arg) Aptroot [=Bogoriella queenslandica (Müll. Arg.) Aptroot & Lücking, holotype].
Thallus ecorticate, endoperidermal, or non-lichenized, usually forming whitish or pinkish patches, sometimes bordered by dark prothallus lines, sometimes indistinct and not discolouring the bark surface.
Ascomata with apical or rarely eccentric ostioles, solitary or irregularly confluent, usually exposed and black. Wall carbonized, dimidiate, often with basal fringe, originating subepidermally but becoming exposed, sometimes with only ostiole exposed, sometimes becoming sessile due to erosion of surrounding bark, carbonized parts in thin section dark (reddish) brown, K+ more or less strongly olivaceous. Hamathecium clear or rarely inspersed, IKI−, with c. 2 µm wide, sparsely septate, branched and anastomosing filaments, hardly branched at ascus level, supposed to be pseudoparaphyses. Asci clavate to subcylindrical, I−, wall gradually thickening towards the tip, with small ocular chamber c. 1/4 of ascus width. Ascospores 8 per ascus or inconstantly in somewhat reduced number, olivaceous grey, becoming brownish with age, with one (sub)central euseptum, where they are constricted and a torus ring can sometimes be seen, rarely 3-septate; internally an endospore layer can be seen against the spore wall in certain stages of the spore development; the endospore may protrude into the locule and form rings or transverse or occasionally longitudinal septa; outer surface covered with a more or less distinct gelatinous sheath (halo), usually at some stage verruculose or spinulose; lower cell usually smaller than upper cell.
Pycnidia absent in some species, common in others, often concentrated along the hypothallus border lines.
Discussion. The genus Bogoriella is reinstated here to accommodate most of the tropical lichen species that have so far been united with some temperate non-lichenized fungi in the genus Mycomicrothelia Keissl. The phylogenetic position of only a small number of species has been confirmed so far; several others that cluster separately in the family Trypetheliaceae are united below in the new genera Distothelia and Novomicrothelia. Many of the almost 40 species placed here in Bogoriella differ from related ones mainly in ascospore shape and size; therefore measurements are very important for the identification, but also comparison with published ascospore drawings in Hawksworth (1986) and Aptroot (Reference Aptroot1991, Reference Aptroot1995). Much information on this group, under the name Mycomicrothelia, was given by Sipman & Aptroot (Reference Sipman and Aptroot2005) and its accompanying internet key (Sipman Reference Sipman2005), but descriptions, type citations and full synonymy, partly taken from Hawksworth (1986), are given here for all species of Bogoriella.
Mycomicrothelia willeyana (Müll. Arg.) D. Hawks. was reported from Florida (Harris Reference Harris1995), based on clearly lichenized specimens (Lücking et al. Reference Lücking, Seavey, Common, Beeching, Breuss, Buck, Crane, Hodges, Hodkinson and Lay2011), although the type appears to be a non-lichenized fungus (no thallus formed) from temperate North America (Massachussetts). After restudy of the (sub-)tropical Florida material, we noticed that the specimens have partially grouped ascomata and are better identified with Bogoriella socialis, a tropical, lichenized taxon known from Puerto Rico and Ecuador. This name is therefore added as a new country record below.

Bogoriella alata (Groenh. ex Aptroot) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816762
Mycomicrothelia alata Groenh. ex Aptroot, Biblioth. Lichenol. 44: 130 (1991); type: Indonesia, Java, East Java, Semaru Lands, ravine of the Glidik River near Tempur Sewu estate, Groenhart 1681 (L!—holotype).
Thallus pinkish grey, with dark prothallus lines.
Ascomata with apical ostioles, solitary, 0·5–0·9 mm wide and 0·1–0·2 mm high, erumpent, with fringe. Wall to 150 μm thick. Asci 60–80×10–15 μm. Ascospores 8 per ascus, 14–17×4·0–6·5 μm, with slightly larger upper locule, with rounded ends, verruculose.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from Indonesia).
Bogoriella annonacea (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816763
Microthelia annonacea Müll. Arg., Hedwigia 34: 145 (1895).—Mycomicrothelia annonacea (Müll. Arg.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 67 (1986) [‘1985’]; type: Venezuela, Caracas, Ernst s. n. (G!—holotype).
Thallus indistinct.
Ascomata with apical ostioles, solitary, 0·4–0·5 mm diam. and 0·10–0·17 mm high, erumpent, with 50–100 μm wide fringe, only ostiole exposed. Wall 30–50 μm thick. Ascospores (15·5–)17·0–19·0(–23·0)×5–7 μm, with equal locules, with rounded ends, verruculose, olivaceous brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Venezuela, Ecuador and Costa Rica) and also from Hawai’i.
Bogoriella apposita (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816764
Verrucaria apposita Nyl., Ann. Sci. Nat. Bot., sér. 4 20: 254 (1863).—Arthopyrenia apposita (Nyl.) H. Oliv., Exp. Lich. Ouest Fr. 2: 258 (1902).—Microthelia apposita (Nyl.) Boistel, Nouv. Fl. Lich. 2: 288 (1903).—Mycomicrothelia apposita (Nyl.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 68 (1986) [‘1985’]; type: Colombia, Choachi, Lindig 815 (H-Nyl 702!—holotype).
(Fig. 43J)
Thallus whitish, without prothallus lines.
Ascomata with apical ostioles, solitary, c. 0·4 mm diam. and 0·15–0·20 mm high, erumpent. Wall 40–80 μm thick. Asci 90–120×14–16 μm. Ascospores (22·0–)24·0–28·0(–29·5)×(8·5–)9·0–11·5(–12·5)μm, with larger upper locule, with attenuated ends, verruculose, reddish brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Colombia).
Discussion. Bogoriella apposita, B. obovata and B. thelena are very similar and possibly conspecific. In that case the earliest name would be B. thelena.
Bogoriella captiosa (Kremp.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816765
Verrucaria captiosa Kremp., Flora 59: 524 (1876).—Microthelia captiosa (Kremp.) Müll. Arg., Bot. Jahrb. Syst. 6: 416 (1885).—Mycomicrothelia captiosa (Kremp.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 73 (1986) [‘1985’]; type: Brazil, Rio de Janeiro, Glaziou 5057a (M—lectotype, Hawksworth, Bull. Br. Mus. Nat. Hist. (Bot.) 14: 73 (1986) [‘1985’], not seen).
Microthelia flavicans Müll. Arg., Bull. Soc. Roy. Bot. Belgique 32: 170 (1894); type: Costa Rica, Tonduz s. n. (G—holotype, not seen; Pittier, Pl. Costaric. Exs. 6307).
(Fig. 43D)
Thallus whitish rose, with black prothallus lines.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam. and 0·15–0·25 mm high, erumpent, conical, without distinct fringe. Wall 50–65 μm thick. Asci (55–)65–73×(12–)13–14 μm. Ascospores (13·5–)17·0–19·0(–21·0)×(5·5–)6·5–7·5(–8·5) μm, with equal locules or larger upper locule, with rounded (-attenuate) ends, verruculose, olivaceous brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Possibly pantropical (reported from Brazil, Costa Rica, Colombia, Guyana and Hawai’i).
Bogoriella collospora (Vain.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816766
Pyrenula collospora Vain., Bot. Mag. Tokyo 32: 162 (1918).—Mycomicrothelia collospora (Vain.) Aptroot, in Kashiwadani et al., Biblioth. Lichenol. 99: 249 (2009); type: Japan, Honshu, Tsunoda s. n. (TUR-Vain 31241!—holotype; TNS!—isotype).
(Fig. 42B)
Thallus dark olive-brown, nearly absent.
Ascomata with lateral, fused ostioles, 2–10 together in dense groups, exposed and black. Ascospores 8 per ascus, brown, ellipsoid to clavate with rounded ends, granularly ornamented, 3-septate, 34–38×11–13 μm, wall not thickened but surrounded by a hyaline gelatinous sheath.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern temperate (reported from Japan).
Bogoriella confluens (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816767
Microthelia confluens Müll. Arg., Flora 68: 333 (1885).—Verrucaria confluens (Müll. Arg.) Stizenb., Ber. Tät. St Gall. Naturw. Ges. 1889–1890: 217 (1891), non (Weber) Hoffm. (1790).—Mycomicrothelia confluens (Müll. Arg.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 75 (1986) [‘1985’]; type: South Africa, Cape of Good Hope, s. col. (G!—holotype).
(Fig. 43H)
Thallus indistinct.
Ascomata in groups of 2–8 with common fringe, 0·8–1·0 mm diam. and 0·12–0·17 mm high, with 200–400 μm wide fringe. Wall 60–100 μm thick. Ascospores (21–)22–25×7·5–9·0(–10·5) μm, with equal locules or larger upper locule, with rounded ends, verruculose, golden brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Costa Rica, South Africa and Fiji Islands).
Bogoriella conothelena (Nyl.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816768
Verrucaria conothelena Nyl., Bull. Soc. Linn. Normandie 2, 7: 180 (1873).—Microthelia conothelena (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 258 (1921).—Mycomicrothelia conothelena (Nyl.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 81 (1986) [‘1985’]; type: India, Andaman Islands, Kurz 54 (H-Nyl 704!—lectotype, Hawksworth, Bull. Br. Mus. Nat. Hist. (Bot.) 14: 81 (1986) [‘1985’]).
Verrucaria conothelena var. errans Nyl., Bull. Soc. Linn. Normandie 2, 7: 180 (1873).—Microthelia conothelena var. errans (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 258 (1921); type: India, Andaman Islands, Kurz 78 (H-Nyl 710!—holotype).
(Fig. 43B)
Thallus pale brown, with dark prothallus lines.
Ascomata with apical ostioles, solitary, c. 0·4 mm diam. and 0·20–0·25 mm high. Wall 30–50(–70 below) μm thick. Asci 80–100×10–20 μm. Ascospores 19–23(–27)×(7·5–)9·0–11·0(–12·0) μm, with larger upper locule, with rounded ends, verruculose, golden brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (previously reported from India).
New country record. Sri Lanka: Eastern Prvince: Ampara, 2013, Weerakoon s. n. (ABL, F).
Bogoriella decipiens (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816769
Anthracothecium decipiens Müll. Arg., Bot. Jahrb. Syst. 6: 415 (1885).—Mycomicrothelia decipiens (Müll. Arg.) R. C. Harris, Mem. New York. Bot. Gard. 49: 78 (1989); type: Cuba, Wright 130 (G!—holotype).
Bogoriella subpersicina Zahlbr., Ann. Cryptog. Exotique 1928(1): 111 (1928); type: Indonesia, Java, Bogor, van Overeem 45 (W!—holotype).
Ornatopyrenis muriformis Aptroot in Aptroot et al., Biblioth. Lichenol. 64: 123 (1997); type: Papua New Guinea, Madang Prov., Gogol Valley, c. 30 km W of Madang, Tgubi logging site, Sipman 35978 (B!—holotype).
Thallus pale brownish to whitish, with dark prothallus lines.
Ascomata with apical ostioles, solitary, 0·7–0·9 mm diam. and c. 0·2 mm high, with wide fringe. Asci c. 100×28 μm. Ascospores 4–8×(1–)2–4 loculate, 25–35×12–15 μm, with rounded ends, smooth, grey.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (previously reported from Cuba, Guyana, Philippines, Indonesia and Papua New Guinea).
New country record. Mexico: Yucatán: Chichén Itzá, 1993, Hensen s. n. (ABL).
Bogoriella exigua (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816770
Microthelia exigua Müll. Arg., Bot. Jahrb. Syst. 6: 416 (1885).—Verrucaria microthelena Nyl., Flora 59: 364 (1876), nom. inval.—Mycomicrothelia exigua (Müll. Arg.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 84 (1986) [‘1985’]; type: Cuba, Müller, Verr. Cub. 14 (G—holotype, not seen).
Microthelia intermedia Müll. Arg., Bot. Jahrb. Syst. 6: 416 (1885); type: Cuba, Wright s. n. (G—holotype, not seen).
(Fig. 42I)
Thallus pale creamy brown, with dark prothallus lines.
Ascomata with apical ostioles, solitary, 0·20–0·25 mm diam. and 0·08–0·11 mm high, without fringe. Wall 25–45 μm thick. Asci 42–60×8·5–12·0 μm. Ascospores 12·5–14·0(–15·5)×4·0–5·5 μm, with equal locules or larger upper locule, with rounded ends, weakly constricted, weakly verruculose, pale olivaceous brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Cuba, Papua New Guinea and Australia).
Bogoriella fumosula (Zahlbr.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816771
Microthelia fumosula Zahlbr., in Handel-Mazzetti, Symbol. Sin. 3: 18 (1930).—Mycomicrothelia fumosula (Zahlbr.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 85 (1986) [‘1985’]; type: China, Kweishou, Dodjie, Handel-Mazzetti 10728 (W—holotype, not seen; S-L1444!—isotype).
(Fig. 42H)
Thallus whitish, with distinct border lines.
Ascomata with apical ostioles, solitary, with purplish basal fringe, 0·2–0·3 mm diam. and 0·07–0·11 mm high. Wall 25–40 μm thick. Asci 55–65×10–14 μm. Paraphyses c. 2·5 μm thick. Ascospores (11–)12–15(–17)×(5·0–)5·5–6·5(–7·5) μm, with larger upper locule, with rounded to weakly attenuated ends, verruculose, pale brown to golden brown.
Pycnidia usually present. Conidia unknown.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Ecuador, Guyana, China, Japan and Australia).
Bogoriella hemisphaerica (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816772
Microthelia hemisphaerica Müll. Arg., Bot. Jahrb. Syst. 6: 47 (1885).—Mycomicrothelia hemisphaerica (Müll. Arg.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 86 (1986) [‘1985’]; type: Cuba, Wright s. n. (G—lectotype, Hawksworth, Bull. Br. Mus. Nat. Hist. (Bot.) 14: 86 (1986) [‘1985’], not seen; Müller, Verr. Cub. 66).
Microthelia intercedens Müll. Arg., Bull. Soc. Roy. Bot. Belgique 32: 171 (1894); type: Costa Rica, Boruca, Tonduz s. n. (G—lectotype; BR—isolectotype, Hawksworth, Bull. Br. Mus. Nat. Hist. (Bot.) 14: 86 (1986) [‘1985’], not seen; Pittier, Pl. Costaric. Exs. 6304).
Didymosphaeria philippina Vain., Ann. Acad. Sci. Fenn., ser. A 15(6): 348 (1921); type: Philippines, Luzon, Nueva Vizcaya, Dupax, McGregor, Bureau of Science 14308 (TUR-Vain 32528!—holotype).
(Fig. 43I)
Thallus whitish, without prothallus lines.
Ascomata with apical ostioles, solitary, 0·4–0·5 mm diam. and 0·15–0·18 mm high, without distinct fringe. Wall 35–60 μm thick. Asci 75–90×12–18 μm. Ascospores (21–)24–28(–30)×5–9(–11) μm, with equal locules or slightly larger upper locule, with rounded ends, verruculose, sometimes with 2 pseudosepta, olivaceous brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (previously USA, Cuba, Costa Rica, Colombia, Guyana, Ecuador, Brazil, Philippines and Hawai’i).
New country record. Malaysia: Sarawak: Gunung Mulu, Coppins 5127 (E, ABL).
Bogoriella lateralis (Sipman) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816773
Mycomicrothelia lateralis Sipman, in Sipman & Aptroot, Lichenologist 37: 309 (2005); type: Norfolk Island, Mt Pitt Reserve, track from Red Road to Mt Bates, Streimann 34411 (B!—holotype).
(Fig. 42E)
Thallus whitish, with dark prothallus lines.
Ascomata with apical ostioles, solitary, usually oval, without fringe, 0·4–0·5 mm diam. and c. 0·15 mm high, with lateral ostiole. Asci c. 130×10 μm. Ascospores 18–22×6–7 μm, with equal locules or slightly larger upper locule, with rounded ends, verruculose, grey.
Pycnidia near the border lines, c. 0·3 mm wide. Conidia bacilliform, simple, hyaline, c. 3·0×0·5 μm.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from Norfolk Island).
Bogoriella leuckertii (D. Hawksw. & J. C. David) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816774
Mycomicrothelia leuckertii D. Hawksw. & J. C. David, Biblioth. Lichenol. 57: 98 (1995); type: Mauritius, Plaines Wilhelms, Vacoas, Hawksworth (IMI 400619!—holotype).
Thallus pinkish white, becoming whitish grey, with dark prothallus lines.
Ascomata with apical ostioles, solitary, with fringe, c. 0·5–0·7 mm diam. and 0·1–0·2 mm high; exciple K+ crimson red, colour dissolving. Asci 45–80×11–14(–16) μm. Ascospores 17–22×(6·0–)7·0–10·5 μm, with upper locule about twice as large as lower locule, with rounded ends, minutely punctate-verruculose, olivaceous brown to red-brown.
Pycnidia mainly near the border lines, c. 50 μm wide. Conidia bacilliform, simple, hyaline, 2·5–3·5×1·0–1·5 μm.
Chemistry. Thallus UV−, K−; excipulum K+ crimson red, with anthraquinone.
Distribution. Possibly pantropical (reported from Costa Rica and Mauritius).
Bogoriella macrocarpa (Komposch, Aptroot & Hafellner) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816775
Mycomicrothelia macrocarpa Komposch et al., Lichenologist 34: 226 (2002); type: Venezuela, Estado Amazonas, Alto Orinoco c. 15 km W of La Esmeralda, plot of tower crane on the W riverbank of Surumoni, Hafellner & Komposch 3095 (GZU!—isotype).
Thallus creamy white, with dark prothallus lines.
Ascomata with apical ostioles, solitary, with wide fringe, c. 0·7–1·1 mm diam. and 0·3–0·4 mm high. Hamathecium with oil droplets, these mainly restricted to the centre and margins. Asci c. 140–190×20–24 μm. Ascospores c. 30–45×10–15 μm, with slightly larger upper locule, with rounded ends, verruculose, olivaceous brown.
Pycnidia 75–125 μm wide; conidia bacilliform with submedian enlargement, simple, hyaline, c. 15–21(–26)×0·4 μm, at enlargement c. 0·8 μm.
Chemistry. Thallus UV+ yellow, K−, with lichexanthone.
Distribution. Neotropical (reported from Venezuela).
Bogoriella megaspora (Aptroot & M. Cáceres) Aptroot & Lücking comb. nov.
MycoBank No.: MB 817004
Mycomicrothelia megaspora Aptroot & M. Cáceres, Lichenologist 45: 768 (2014); type: Brazil, Rondônia, Estação Ecológica de Cuniã, km 760 on road BR 319 NNE of Porto Velho, 2012, Cáceres & Aptroot 15747 (ISE!—holotype; ABL!—isotype).
(Fig. 43K)
Thallus pinkish to whitish grey, smooth to uneven, surrounded by a black prothallus line.
Ascomata with apical ostioles, solitary, 0·5–0·9 mm diam., erumpent to prominent, conical, exposed and black. Hamathecium clear, hyaline. Ascospores 8 per ascus, 1-septate, clavate, often constricted at the septum, 27–40×8–12 μm, brown, often surrounded by a gelatinous layer 6–15 μm thick.
Pycnidia not observed.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Brazil).
Discussion. This species has among the largest 1-septate ascospores of all the species now placed in Bogoriella, Distothelia, and Novomicrothelia. Only two species in Bogoriella have 1-septate ascospores that are in the same size class, viz. B. macrocarpa and B. xanthonica (see above). Both differ by the inspersed hamathecium and the presence of lichexanthone.
Bogoriella miculiformis (Nyl. ex Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816776
Microthelia miculiformis Nyl. ex Müll. Arg., Bot. Jahrb. Syst. 6: 417 (1885).—Verrucaria miculiformis Nyl., Flora 59: 364 (1876) nom. inval.—Mycomicrothelia miculiformis (Nyl. ex Müll. Arg.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 99 (1986) [‘1985’]; type: Cuba, Wright s. n. (H-Nyl 678!—holotype; BM!—isotype; Müller, Verr. Cub. 68).
Microthelia miculiformis var. detincta Müll. Arg., Bot. Jahrb. Syst. 6: 417 (1885).—Verrucaria miculiformis var. detincta Nyl., Flora 59: 364 (1876) nom. inval.—Verrucaria miculiformis var. distincta Nyl. ex Hue, Nouv. Arch. Mus. Paris 3, 4: 126 (1892) nom. inval.—Didymosphaeria detincta (Müll. Arg.) Vain., Ann. Acad. Sci. Fenn., ser. A 6(7): 211 (1915); type: Cuba, Wright s. n. (H-Nyl 683!—holotype; BM!—isotype; Müller, Verr. Cub. 87).
Didymosphaeria coccifera Vain., Ann. Acad. Sci. Fenn, ser. A 15(6): 349 (1921); type: Philippines, Luzon, Laguna, Banajao, Robinson, Bureau of Science 9828 (TUR-Vain 32526!—holotype).
(Fig. 43A)
Thallus whitish to pale brown, with dark prothallus lines.
Ascomata with apical ostioles, solitary, with fringe, 0·4–0·5 mm diam. and (0·08–)0·10–0·1 mm high. Wall 35–45 μm thick. Asci 65–80×12–15 μm. Ascospores 14·5–17·5(–19·0)×6·0–7·5 μm, with upper locule about twice as large as lower locule, with rounded ends, verruculose, brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Cuba, Costa Rica, Brazil, Philippines and Papua New Guinea).
Bogoriella minutula (Zahlbr.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816777
Microthelia minutula Zahlbr., in Handel-Mazzetti, Symbol. Sin. 3: 18 (1930).—Mycomicrothelia minutula (Zahlbr.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 102 (1986) [‘1985’]; type: China, Yunnan, Loping, Handel-Mazzetti 10428 (W—holotype, not seen).
(Fig. 42J)
Thallus indistinct, without prothallus lines.
Ascomata with apical ostioles, solitary, c. 0·3 mm diam. and 0·06–0·11 mm high. Wall 14–35 μm thick. Paraphyses 1·0–1·5 μm thick. Asci 55–65×12–14 μm. Ascospores (12·5–)13·5–15·0(–16·0)×(5·5–)6·0–6·5(–7·0)μm, with larger upper locule, with rounded ends, verruculose, olivaceous brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from China).
Bogoriella modesta (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816778
Microthelia modesta Müll. Arg., Bull. Herb. Boissier 2: 93 (1894).—Mycomicrothelia modesta (Müll. Arg.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 104 (1986) [‘1985’]; type: Mexico, Jalisco, Eckfeldt 182 (G—holotype, not seen).
(Fig. 43E)
Thallus olivaceous, without prothallus lines.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam. and 0·07–0·09(–0·10) mm high. Wall 20–35 μm thick. Asci 63–67×17–20 μm. Ascospores (17·5–)19·0–22·0(–23·0)×(8·0–)8·5–10·0(–10·5) μm, with equal locules or slightly larger upper locule, with rounded ends, verruculose, olive brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Mexico, Costa Rica, South Korea and Japan).
Discussion. Part of the material identified as Bogoriella modesta from Costa Rica has consistently 4-spored asci. It remains to be seen whether this material represents a species.
Bogoriella nonensis (Stirt.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816779
Verrucaria nonensis Stirt., Proc. Phil. Soc. Glasgow 11: 320 (1879).—Microthelia nonensis (Stirt.) Zahlbr., Catal. Lich. Univ. 1: 264 (1921).—Mycomicrothelia nonensis (Stirt.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 105 (1986) [‘1985’]; type: India, Kerala, Nilghiri Hills, Watt (BM!—isotype).
(Fig. 42K)
Thallus whitish, without prothallus lines.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam. and 0·12–0·15 mm high. Wall 30–40 μm thick. Asci 50–60×8–15 μm. Ascospores (14–)16–18×(6·0–)6·5–7·5 μm, with equal locules or somewhat larger upper locule, with rounded ends, smooth to verruculose, olivaceous brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Ecuador and India).
Bogoriella obovata (Stirt.) Aptroot &Lücking comb. nov.
MycoBank No.: MB 816780
Verrucaria obovata Stirt., Trans. Proc. Roy. Soc. Victoria 17: 74 (1881).—Pyrenula obovata (Stirt.) Shirley, Lich. Fl. Queensland 4: 176 (1890).—Microthelia obovata (Stirt.) Müll. Arg., Rep. Australas. Soc. Advancem. Sci. 6: 454 (1895).—Mycomicrothelia obovata (Stirt.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 106 (1986) [‘1985’]; type: Australia, Queensland, Brisbane, Bailey 125 (BM!—holotype).
Microthelia alba Müll. Arg., Rep. Australas. Soc. Advancem. Sci. 6: 455 (1895); type: Australia, Queensland, Brisbane, Knight s. n. (G!—holotype).
Microthelia brisbanensis Müll. Arg., Rep. Australas. Soc. Advancem. Sci. 6: 455 (1895); type: Australia, Queensland, Brisbane, Bailey s. n. (G!—holotype).
Didymosphaeria tetraspora Massee, Kew Bull. 1907: 124 (1907); type: Malaysia, Sarawak, Ridley s. n. (K!—holotype).
Didymosphaeria thelenoides Vain., Ann. Acad. Sci. Fenn, ser. A 6(7): 211 (1915); type: Dominica, Mt. Conliabon, Elliot 1533 (TUR-Vain 32530!—lectotype, Aptroot, Nova Hedwigia 60: 352, 1995).
Thallus pinkish white, border lines.
Ascomata with apical ostioles, solitary, 0·5–0·6 mm diam. and 0·2–0·3 mm high. Wall 45–60 μm thick. Asci (65–)75–90×20–30 μm. Ascospores (21–)22–28(–30)×(8·5–)9·0–11·0(–12·0) μm, with slightly larger upper locule, with rounded ends, often with 2 pseudosepta and seemingly 3-septate, verruculose, brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from Borneo, Philippines, Papua New Guinea and Australia).
Bogoriella pachytheca (Sacc. & Syd.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816781
Didymosphaeria pachytheca Sacc. & Syd. in Sydow & Sydow, Bull. Herb. Boissier 8: 79 (1900).—Mycomicrothelia pachytheca (Sacc. & Syd.) Aptroot, Nova Hedwigia 60: 352 (1995); type: Brazil, Isla S. Francisco, Ule 403 (S!—holotype).
Thallus indistinct.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., conical. Ascospores 21–22×8 μm, with rounded upper and attenuated lower ends, verruculose.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Brazil).
Bogoriella punctata (Aptroot) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816782
Mycomicrothelia punctata Aptroot, Biblioth. Lichenol. 44: 134 (1991); type: Papua New Guinea, Morobe Prov., Huon Peninsula, Honzeukngon Village S of Derim in Timbe Valley, Aptroot 17994 (ABL!—holotype).
(Fig. 42F)
Thallus brownish, with dark prothallus lines.
Ascomata with apical ostioles, solitary or in groups of 1(–3), with fringe, 0·4–0·7 mm diam. and 0·1–0·3 mm high, completely exposed. Wall to 100 μm thick. Asci 80–90×10–15 μm. Ascospores (12–)15–18(–21)×(6–)7–8 μm, with larger, rounded upper locule, and much smaller, often attenuated lower locule, smooth.
Pycnidia mainly near the border lines, c. 0·1 mm wide; conidia not known.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical, mostly at c. 2000–3000 m (previously reported from Costa Rica, Philippines and Papua New Guinea).
New country records. Ecuador. Azuay: Road Cuenca-Girón, 1977, Andersson et al. s. n. (UPS, ABL).—Malaysia: Sarawak: Borneo, Sandakan, Elmer 20019 (L, ABL).—Fiji: Vanua Levi, 1970, Degelius s. n. (UPS).
Bogoriella queenslandica (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816783
Microthelia queenslandica Müll. Arg., Rep. Australas. Assoc. Advancem. Sci. 6: 455 (1895).—Ornatopyrenis queenslandica (Müll. Arg) Aptroot, Biblioth. Lichenol. 44: 128 (1991).—Mycomicrothelia queenslandica (Müll. Arg.) Sipman & Aptroot, Lichenologist 37: 309 (2005); type: Australia, Queensland, Knight 71 (G!—lectotype, Hawksworth, Bull. Br. Mus. Nat. Hist. (Bot.) 14: 161 (1986) [‘1985’]).
(Fig. 43L)
Thallus whitish, with dark prothallus lines.
Ascomata with apical ostioles, solitary, 0·8–1·1 mm diam. and c. 0·2–0·3 mm high. Asci 110–130×30 μm. Ascospores 35–45×13–15 μm, with equal locules or slightly larger upper locule, with rounded ends, often with 2 pseudosepta and seemingly 3-septate, verruculose, grey.
Pycnidia between the ascomata and near the border lines. Conidia not seen.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Costa Rica, Colombia, Indonesia, Papua New Guinea and Australia).
Bogoriella socialis (Zahlbr.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816784
Microthelia socialis Zahlbr., Mycologia 22: 69 (1930).—Mycomicrothelia socialis (Zahlbr.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 110 (1986) [‘1985’]; type: Puerto Rico, Yauco, Fink 1459a (W 1925/9360 —holotype, not seen).
(Fig. 42G)
Thallus whitish grey (to pale pink), without prothallus lines.
Ascomata in groups of (1–)2–6, with wide fringe to 100 μm, c. 0·15–0·20 mm diam. (groups to c. 1 mm diam.) and 0·08–0·12 mm high, only ostiole exposed. Wall 10–15(–29) μm thick. Asci 45–60×15–24 μm. Ascospores 15–20×6–9 μm, with equal locules, with rounded or somewhat attenuated ends, delicately verruculose, brown.
Pycnidia between the ascomata, 50–75 μm wide; conidia bacilliform, simple, hyaline, 12–15×0·5–1·0(–1·5) μm.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported from Puerto Rico and Ecuador).
New country record. USA: material previously reported as Mycomicrothelia willeyana (Lücking et al. Reference Lücking, Seavey, Common, Beeching, Breuss, Buck, Crane, Hodges, Hodkinson and Lay2011).
Bogoriella striguloides (Sérus. & Aptroot) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816785
Mycomicrothelia striguloides Sérus. & Aptroot, Bryologist 101: 145 (1998); type: New Zealand, North Island, near Orewa, 30 km N of Auckland, Bartlett (ABL!—isotype).
(Fig. 42A)
Thallus foliicolous, hypocuticular and lobed.
Ascomata in groups of 1–8, 0·1–0·2 mm diam. and 0·1 mm high. Asci 40–50(–55)×10–12(–15) μm. Ascospores c. 14×4 μm, smooth, dark brown.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from New Zealand).
Bogoriella subfallens (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816786
Microthelia subfallens Müll. Arg., Bot. Jahrb. Syst. 6: 416 (1885).—Verrucaria subfallens Nyl., Flora 59: 364 (1876) nom. inval.—Mycomicrothelia subfallens (Müll. Arg.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 111 (1986) [‘1985’]; type: Cuba, Wright s. n. (G—holotype, not seen; Müller, Verr. Cub. 5).
Didymosphaeria baccharidis Starbäck, Bih. K. Svenska Vetensk.-Akad. Handl. 25, 3(1): 59 (1879) [as ‘bacharidis’]; type: Brazil, Rio Grande do Sul, Santo Angelo near Cachoeira, Regnell 257 [‘251’ in publication] (S—holotype).
Didymosphaeria coccifera var. cinereorubricosa Vain., Ann. Acad. Sci. Fenn., ser. A 15(6): 349 (1921); type: Philippines, Pollilo, Robinson, Bureau of Science 9090 (TUR-Vain 32527—holotype).
(Fig. 42L)
Thallus whitish to pale pink or brownish and nearly absent, with dark prothallus lines.
Ascomata with apical ostioles, solitary, with c. 50 μm wide fringe, 0·4–0·5 mm diam. and 0·06–0·10 mm high. Wall 25–50 μm thick. Asci 50–65×12–14 μm. Ascospores 13·5–16·0×6–7 μm, with upper locule almost twice as large as lower locule, with rounded ends, verruculose, golden brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (previously reported from Cuba, Costa Rica, Venezuela, Guyana, Ecuador, Brazil, Philippines, Singapore, Taiwan, Papua New Guinea, Australia and Hawai’i). Introduced on Dracaena in the Netherlands.
New country record. Solomon Islands: Santa Cristobal Island: 1965, Hill 8936 (BM).
Bogoriella thelena (Ach.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816787
Verrucaria thelena Ach., Syn. Lich.: 92 (1814).—Microthelia thelena (Ach.) Trev., Consp. Verruc.: 10 (1860).—Mycomicrothelia thelena (Ach.) D. Hawksw., Bull. Br. Mus. Nat. Hist. (Bot.) 14: 112 (1986) [‘1985’]; type: South America, s .col. (H-ACH 692B!—holotype).
Microthelia albidella Müll. Arg., Flora 66: 272 (1883); type: Brazil, Apiahy, Puiggari 349 (G—holotype, not seen).
Microthelia thelena var. subtriseptata Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 232 (1890); type: Brazil, Caraça, Vainio s. n. (BM!—isotype; Vainio, Lich. Brasil. 715).
Microthelia leucothallina Vain., Ann. Acad. Sci. Fenn., ser. A 6(7): 210 (1915); type: Danish West Indies, Boergesen s. n. (TUR-Vain 32469—holotype, not seen).
Didymosphaeria palaquii Vain., Ann. Acad. Sci. Fenn., ser. A 15(6): 348 (1921); type: Philippines, Mindanao, Zamboanga, Merrill 5006 (TUR-Vain 32529!—holotype).
(Fig. 43C)
Thallus creamy or whitish or indistinct, with dark prothallus lines.
Ascomata with apical ostioles, solitary, with or without fringe, c. 0·5 mm diam. and 0·10–0·25 mm high. Wall 30–70 μm thick. Asci 80–90(–100)×(23–)26–31 μm. Ascospores (17·5–)21·0–24·0(–26·0)×(7·5–)9·0–11·5(–13·0) μm, with larger upper locule, with rounded ends, verruculose, deep golden brown.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Costa Rica, West Indies, Colombia, Ecuador, Brazil, Philippines and Hawai’i).
Bogoriella triangularis (Aptroot) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816788
Mycomicrothelia triangularis Aptroot in Sipman & Aptroot, Lichenologist 37: 309 (2005); type: Puerto Rico, Mayagüez, Reserva Forestal Maricao, N of Sabana Grande, along road 120, km 16–17, Aptroot & Aptroot 24949 (B!—holotype).
(Fig. 42D)
Thallus whitish, with dark prothallus lines.
Ascomata with apical ostioles, solitary, usually oval to triangular, with conspicuous fringe, 0·4–0·5 mm diam. and c. 0·2 mm high, with lateral ostiole. Asci c. 120×15 μm. Ascospores 25–37×7–10 μm, with equal locules or slightly larger upper locule, with rounded ends, roughly verruculose with warts sometimes in lines, brown.
Pycnidia near the border lines, c. 0·1 mm wide; conidia not observed.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Puerto Rico).
Bogoriella xanthonica (Komposch, Aptroot & Hafellner) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816789
Mycomicrothelia xanthonica Komposch et al., Lichenologist 34: 224 (2002); type: Venezuela, Estado Amazonas, Alto Orinoco c. 15 km WSW of La Esmeralda, plot of tower crane on the W riverbank of Surumoni, Hafellner & Komposch 3399 (GZU!—isotype).
Thallus whitish to creamy, with dark prothallus lines.
Ascomata with apical ostioles, solitary, with wide fringe, c. 0·5–0·7(–0·8) mm diam. and 0·15–0·20 mm high. Hamathecium with oil droplets, these often restricted to less fertile parts. Asci c. 130–160×20–30 μm. Ascospores c. 30–40×10–14 μm, with slightly larger upper locule, with rounded ends, verruculose, olivaceous brown.
Pycnidia 75–125 μm wide; conidia bacilliform with submedian enlargement, simple, hyaline, c. 12–15×0·4 μm, at enlargement 0·8 μm wide.
Chemistry. Thallus UV+ (sometimes weakly), K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Venezuela).
Constrictolumina Lücking et al.
In Lücking et al., Lichenologist 48 (in press); type: Constrictolumina cinchonae (Ach.) Lücking, M. P. Nelsen & Aptroot (holotype).
Thallus not corticate.
Ascomata single, roughly conical, not in pseudostroma, but sometimes fused sideways. Ostiole apical. Hamathecium hyaline, completely clear, filaments thick at the base, much thinner above, but not anastomosing. Asci clavate. Ascospores 1–3-septate, rarely submuriform, with irregular endospore formation, sometimes with pseudosepta, often one or two cells pinched in the middle, smooth or ornamented, hyaline, rarely becoming brown, often becoming granular ornamented.
Pycnidia sometimes present.
Discussion. This group of species has thus far been retained in the genus Arthopyrenia, a collective genus from which successively aberrant groups have been split off. Species details have been given primarily by Harris (Reference Harris1975, Reference Harris1995), either restricting the group to an even smaller number of species or using a wider concept, including the type of the genus Arthopyrenia and all synonym genera, viz. A. analepta (Ach.) Massal. The genus Arthopyrenia s. str. has now been restricted to a very small core group (Hyde et al. Reference Hyde, Jones, Liu, Ariyawansha, Boehm, Boonmee, Braun, Chomnunti, Crous and Dai2013). Regarding the species concept, the present treatment is heavily based on Harris’s work and should be regarded as preliminary, since many taxa are not well sampled and molecular data are scarce. Constrictolumina unites c. 10 essentially tropical members, that is almost all tropical species that had remained in Arthopyrenia after the split-off of Anisomeridium (Harris Reference Harris1975). Interestingly, the new genus is partly characterized by the unique hamathecium structure, a character which differs from almost all remaining Trypetheliaceae.

Constrictolumina cinchonae (Ach.) Lücking et al.
In Lücking et al., Lichenologist 48 (in press); Verrucaria cinchonae Ach., Synops. Lich.: 90 (1814).—Arthopyrenia cinchonae (Ach.) Müll. Arg., Flora 66: 287 (1883); type: “cort. Cinchonae officinalis” (H-ACH 781B!—holotype).
Verrucaria prostans Mont., Ann. Sci. Nat. Bot., ser. 2 19: 53 (1843); type: French Guiana, Leprieur 215 (BM!—isotype).
Verrucaria alboatra var. detergens Nyl., Flora 52: 125 (1869); type: Brazil, Rio de Janeiro, Glaziou 1915 (H-Nyl 1662!—holotype; M—isotype, not seen).
Verrucaria concamerata Stirt., Proc. Phil. Soc. Glasgow 13: 192 (1881).—Porina concamerata (Stirton) Zahlbr., Cat. Lich. Univ. 1: 377 (1922); type: India, Assam, Watt s. n. (BM!—isotype).
Arthopyrenia nieteriana Müll. Arg., Flora 66: 288 (1883); type: Sri Lanka, Nieter 30 (G!—holotype).
Arthopyrenia planipes Müll. Arg., Flora 73: 345 (1890); type: Kenya, Hannington s. n. (G!—holotype; BM!—isotype).
Thallus thin, whitish.
Ascomata with apical ostioles, solitary, 0·4–0·6 mm diam., erumpent to prominent, hemispherical to flattened. Wall lacking below, often surrounded by a grey ring. Asci mostly narrowly obovate, rarely almost cylindrical, (85–)100–125×l7–22 μm. Ascospores 4–8 per ascus, biseriate, sub-biseriate or almost uniseriate, narrowly ovate, 1-septate, lower cell occasionally slightly constricted in the middle; perispore well developed, 20–30×7–11 μm.
Pycnidia often present. Conidia rod-like, 4–5×1 μm.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from the USA, Mexico, Bermuda, Bahamas, Cuba, Puerto Rico, Dominica, French Guiana, Ecuador, Brazil, Argentina, Kenya, Maldives, India, Sri Lanka, China, Indonesia (Java), Philippines, Papua New Guinea and Japan).
Constrictolumina esenbeckiana (Fée) Lücking, M. P. Nelsen & Aptroot comb. nov.
MycoBank No.: MB 817006
Melanotheca esenbeckiana Fée, Essai Crypt. Écorc. Suppl: 71 (1837).—Tomasellia esenbeckiana (Fée) Müll. Arg., Flora 68: 257 (1883); type: Antilles, “cort. Exostemmatis” (G!—lectotype, Harris, More Florida Lichens: 81, 1995).
(Fig. 45E)
Thallus thin, whitish.
Ascomata with apical ostioles, solitary, 0·4–0·6 mm diam., erumpent to prominent, hemispherical to flattened, sideways confluent in dense groups of 5–20. Wall lacking below. Asci mostly narrowly obovate, rarely almost cylindrical, c. 100–125×l7–22 μm. Ascospores 4–8 per ascus, biseriate, subbiseriate or almost uniseriate, narrowly ovate, 1-septate, lower cell occasionally slightly constricted in the middle, perispore well developed, 22–27×7–10 μm.
Pycnidia often present. Conidia rod-like, 4–5×1 μm.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from the Antilles and Brazil; mostly old reports, but also recently found in Sergipe State in Brazil).
Constrictolumina leucostoma (Müll. Arg.) Lücking, M. P. Nelsen & Aptroot comb. nov.
MycoBank No.: MB 817010
Tomasellia leucostoma Müll. Arg., Flora 68: 257 (1883); Arthopyrenia confluens R. C. Harris, More Florida Lichens: 80 (1995), non Arthopyrenia leucostoma (Ach.) A. Massal. (1852); type: “cort. Crotonis Cascarillae, ex hb. Hampe”, 1877 (G!—holotype).
Thallus thin, whitish.
Ascomata with apical ostioles, solitary or grouped, 0·1–0·2 mm in diam., erumpent. Wall poorly developed, lacking below; tip of ascocarp surrounded by a broad, thin, black ring, 0·2–0·3 mm in width, often confluent with the rings of other ascomata forming groups of 2–10. Ostioles often depressed, surrounded by a whitish ring. Asci mostly narrowly ovate, rarely almost cylindrical, 60–105×16–26 μm. Ascospores 8 per ascus, irregularly arranged to biseriate, narrowly ovate to narrowly ellipsoidal, 3-septate, perispore well developed, 17–22×6–8 μm.
Pycnidia often present. Conidia linear, 6–9×1 μm.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from the USA, Bahamas and Antilles).
Constrictolumina lyrata (R. C. Harris) Lücking, M. P. Nelsen & Aptroot comb. nov.
MycoBank No.: MB 817011
Arthopyrenia lyrata R. C. Harris, in Tucker & Harris, Bryologist 83: 6 (1980); type: USA, Florida, Daytona, 1911, Merrill s. n. (MSC!—holotype; BM!, NY!, US!—isotypes; Merrill, Lich. Exs. 2: 38).
Thallus thin, whitish.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., erumpent to prominent, less commonly immersed, hemispherical. Wall usually lacking below. Asci narrowly elliptical to elliptical, usually with a distinct ocular chamber, 75–120(–130)×20–30 μm. Ascospores 4–8 per ascus, irregularly arranged to biseriate, narrowly elliptical or narrowly ovate, 1-septate, with one or both cells weakly constricted near the middle, rarely becoming 3-septate; wall granular ornamented, perispore well developed, 18–30×7–12 μm (excluding perispore).
Pycnidia often present. Conidia linear, 6–9×1 μm.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from the USA, Mexico, Indonesia, Papua New Guinea and Australia).
Constrictolumina majuscula (Nyl.) Lücking, M. P. Nelsen & Aptroot comb. nov.
MycoBank No.: MB 817013
Verrucaria majuscula Nyl., Ann. Sci. Nat. Bot., ser. 4 20: 253 (1864).—Arthopyrenia majuscula (Nyl.) Zahlbr., Cat. Lich. Univ. 1: 332. 1922; type: Japan, Ogasawara-shoto (Bonin Islands), Wright s. n. (H-Nyl 824 p.p.!—lectotype: the left-hand specimens with cream/pink thallus, designated here; BM!—isolectotype).
Thallus thin, whitish.
Ascomata with apical ostioles, solitary, 0·4–0·9 mm diam., erumpent; hymenium flattened hemispherical. Ostioles surrounded by a broad, thin hyphal ring. Wall thin, lacking below. Asci narrowly obovate, (80–)105–125×25–30 μm. Ascospores 4–8 per ascus, irregularly arranged, narrowly elliptical to narrowly ovate, 1-septate, each cell partially subdivided by a flat-topped ring-like thickening of the wall, becoming 3-septate; wall strongly granular ornamented, perispore thick, up to 2 μm, 27–37×10–12 μm (excluding perispore).
Pycnidia often present. Conidia linear, 6–8×1 μm.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from the USA, Bahamas, Cuba, Puerto Rico, Chagos, Seychelles, Philippines and Japan).
Constrictolumina malaccitula (Nyl.) Lücking, M. P. Nelsen & Aptroot comb. nov.
MycoBank No.: MB 817014
Verrucaria malaccitula Nyl., J. Linn. Soc., Bot. 20: 61 (1883).—Arthopyrenia malaccitula (Nyl.) Zahlbr., Cat. Lich. Univ. 1: 284 (1921); type: Singapore, Malacca, Water Islands, Maingay 191 (H-Nyl 1706!—holotype; BM!—isotype).
Arthopyrenia bifera Zahlbr., Ann. Mycol. 33: 34 (1935); type: USA, Florida, Seminole County, Sanford, Rapp 80 [published as 86] (BM!—isotype).
Didymella gigantea Räsänen, Arch. Soc. Zool. Bot. Fenn. “Vanamo” 3: 89 (1949); type: Malaysia, Sarawak, Borneo, Sandakan, Myburgh, Elmer 20019, 1921 (H!—lectotype, designated here based on annotation label by R. C. Harris Reference Harris1986; H!—isolectotype).
Thallus thin, whitish.
Ascomata with mostly eccentric ostioles, solitary, 0·2–0·4 mm diam., mostly immersed, subglobose to hemispherical. Wall thinner or lacking below. Asci narrowly elliptical to elliptical, 75–105(–120)×(20–)25–35 μm. Ascospores 2 per ascus, narrowly elliptical, 1-septate, with a slightly thickened area of the wall outlining a smaller subchamber, ultimately becoming 3-septate; wall strongly granular ornamented, perispore well developed, 37–48×15–16 μm (excluding perispore).
Pycnidia often present. Conidia rod-like to linear, 5–10×1 μm.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from the USA, Singapore, Sarawak and Papua New Guinea).
Constrictolumina planorbis (Ach.) Lücking et al.
In Lücking et al., Lichenologist 48 (in press); Verrucaria planorbis Ach., Synops. Lich.: 92 (1814).—Arthopyrenia planorbis (Ach.) Müll. Arg., Mem. Soc. Phys. Genève 30: 27 (1888); type: “cort. Crotonis Cascarillae” (H-ACH—holotype, not seen).
Arthopyrenia planior Müll. Arg., Bot. Jahrb. Syst. 6: 404 (1885); type: Cuba, Wright s. n. (G-G00290453!—lectotype, designated here; BM!—isolectotype; Müller, Lich. Cub., Ser. II: 627a).
Arthopyrenia planorbiculata Müll. Arg., Bot. Jahrb. Syst. 6: 405 (1885); type: Cuba, Wright s. n. (G-G00290457!, lectotype, designated here; BM!—isolectotype; Müller, Verr. Cub. 64).
(Fig. 45D)
Thallus thin, whitish.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., erumpent, flattened to hemispherical. Wall usually extended outwards above, forming a broad shield up to 1 mm diam., lacking below. Asci narrowly elliptical, 55–100×12–17(–20) μm. Ascospores sub-biseriate to biseriate, narrowly ovate, 1-septate, rarely 3-septate; perispore thin, 17–23×5–7 μm.
Pycnidia often present. Conidia filiform, usually curved, 20–27×1 μm.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from the USA, Bahamas, Cayman Islands, Jamaica, Puerto Rico, Dominican Republic, Cuba, Papua New Guinea and Australia).
Constrictolumina porospora (Vain.) Lücking, M. P. Nelsen & Aptroot comb. nov.
MycoBank No.: MB 817015
Arthopyrenia porospora Vain., J. Bot. 24: 296 (1896); type: Dominica, Morne Trois Pitons, 1892, Elliot s. n. (TUR-Vain—holotype, not seen; BM!—isotype).
(Fig. 44F)
Thallus thin, whitish.
Ascomata with apical ostioles, solitary, 0·8–1·2 mm diam., erumpent to prominent, hemispherical. Wall thin, lacking below. Asci narrowly obovate. Ascospores 8 per ascus, biseriate, narrowly elliptical to narrowly ovate, 1-septate, each cell partially subdivided by a flat-topped ring-like thickening of the wall, becoming 3-septate; wall strongly granular ornamented, perispore thick, up to 2 μm, 42–46×16–24 μm (excluding perispore).
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Dominica).
Dictyomeridium Aptroot et al.
In Lücking et al., Lichenologist 48 (in press); type: Dictyomeridium proponens (Nyl.) Aptroot, M. P. Nelsen & Lücking (holotype).
Thallus not corticate.
Ascomata single or a few aggregate, roughly conical to pyriform, not in pseudostroma. Ostioles eccentric. Hamathecium hyaline, clear. Asci with 2–8 ascospores. Ascospores muriform, hyaline, smooth, often IKI+ violet.
Pycnidia sometimes present.
Discussion. This species aggregate is kept separate here, as a split-off from Polymeridium, uniting seven muriform species that were mostly assigned to the genus Polymeridium before. This is done because these species are phylogenetically distant from the core group of Polymeridium. The type and most common species was for a long time known under different names in the genus Campylothelium. Not much new information is reported here, as compared to Aptroot et al. (2013 Reference Aptroot, Menezes, Lima, Xavier-Leite and Cáceresb ) and Aptroot & Cáceres (Reference Aptroot and Cáceres2014), but descriptions, type citations and full synonymy, partly taken from Harris (Reference Harris1993), are given for all species, as well as additional new synonyms.
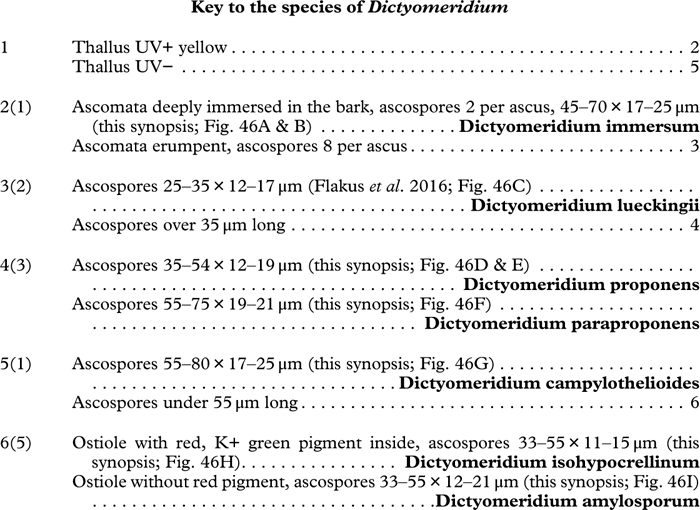
Dictyomeridium amylosporum (Vain.) Aptroot, M. P. Nelsen & Lücking comb. nov.
MycoBank No.: MB 817017
Thelenella amylospora Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 218 (1890).—Polyblastiopsis amylospora (Vain.) Zahlbr., Catal. Lich. Univ. 1: 347 (1922).—Campylothelium amylosporum (Vain.) R. C. Harris, in Tucker & Harris, Bryologist 83: 7 (1980).—Polymeridium amylosporum (Vain.) Aptroot, in Aptroot & Cáceres, Nova Hedwigia 98: 10 (2014); type: Brazil, Rio de Janeiro, Sepitiba, Vainio s. n. (M!, UPS—isotypes; Vainio, Lich. Bras. 419).
Polyblastiopsis dealbens Fink in Hedrick, Mycologia 25: 307 (1933); type: USA, South Carolina, Green (MICH-Fink 11252—lectotype, Harris, Contr. Univ. Michigan Herb. 11: 96, 1975, not seen).
(Fig. 46I)
Thallus ecorticate, white, UV−.
Ascomata pleurothelioid, with lateral ostioles, solitary, 0·4–0·6 mm diam., erumpent to prominent, black. Hamathecium clear, filaments profusely anastomosing. Ascospores 8 per ascus, IKI+ violet, muriform, 33–55×12–21 μm, with partly oblique septa, not ornamented, wall not thickened.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Hawai’i, USA, Mexico, Puerto Rico, Costa Rica, Brazil, South Africa, Seychelles, Hong Kong, India, Fiji, New Caledonia, Papua New Guinea and Australia).
Dictyomeridium campylothelioides (Aptroot & Sipman) Aptroot, M. P. Nelsen & Lücking comb. nov.
MycoBank No.: MB 817018
Polymeridium campylothelioides Aptroot & Sipman, in Aptroot et al., Biblioth. Lichenol. 57: 37 (1995); type: Papua New Guinea, Madang Prov., Laing Island in Hansa Bay near Bogia, Sipman 34767 (B!—holotype).
(Fig. 46G)
Thallus ecorticate, white, UV−.
Ascomata pleurothelioid, with lateral ostioles, solitary or two aggregated with common ostiole, 0·3–0·6 mm diam., erumpent, grey-black. Hamathecium clear, filaments profusely anastomosing. Ascospores 8 per ascus, IKI−, muriform, 55–80×17–25 μm, with partly oblique septa, not ornamented, wall not thickened.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Asian (previously reported from Taiwan and Papua New Guinea). Incorrectly reported from Venezuela (Komposch & Hafellner Reference Komposch and Hafellner1999).
New country record. Indonesia: Java: Bay of Tapen, S of Wlingi, 1939, Groenhart 5796 (ABL, L).
Dictyomeridium immersum (Aptroot, A. A. Menezes & M. Cáceres) Aptroot, M. P. Nelsen & Lücking comb. nov.
MycoBank No.: MB 817019
Polymeridium immersum Aptroot et al., in Aptroot et al., Lichenologist 45: 546 (2013); type: Brazil, Rondônia, Porto Velho, UNIR Federal University campus S of city, on bark of tree, 100 m, 2012, Cáceres & Aptroot 11138 (ISE!—holotype; ABL!—isotype).
Thallus ecorticate, pale pinkish white, surrounded by a brown prothallus line.
Ascomata pleurothelioid, with lateral ostioles, solitary, 0·3–0·5 mm diam., immersed-erumpent, whitish. Hamathecium clear. Ascospores muriform, 2 per ascus, hyaline, IKI−, 9–15(–19)×1–4-septate, ellipsoidal to fusiform, (45–)60–70×17–25 μm, outer wall generally constricted at the median septum.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropics (Brazil).
Discussion. This species differs from all other known Dictyomeridium species by the ascomata that are deeply immersed in the bark below the thallus. The species is easily taken for a sterile crust, because the ascomata are below the thallus in the bark and would escape notice when only a superficial section is made through the thallus at the ostiole. It is also the only species of Dictyomeridium with just two ascospores per ascus; all other species have 8 per ascus.
Dictyomeridium isohypocrellinum (Xavier-Leite et al.) Aptroot, M. P. Nelsen & Lücking comb. nov.
MycoBank No.: MB 817021
Polymeridium isohypocrellinum Xavier-Leite et al., in Aptroot et al., Lichenologist 45: 548 (2013); type: Brazil, Paraíba, Reserve Muralha, on bark of tree, 2012, Xavier-Leite s. n. (ISE 15890!—holotype).
(Fig. 46H)
Thallus ecorticate, pale pinkish to cream-coloured, surrounded by a black prothallus line.
Ascomata pleurothelioid, with lateral ostioles, solitary or occasionally two with fused ostioles, 0·3–0·5 mm diam., erumpent to prominent, black; ostioles with red pigment inside. Hamathecium clear. Ascospores muriform, 8 per ascus, hyaline, IKI−, 9–15×1–3-septate, ellipsoidal, 33–47(–55)×11–15 μm, not constricted.
Chemistry. Thallus UV−, red pigment in ostiole K+ green; isohypocrellin present in ostiole.
Distribution. Neotropical (reported from Brazil).
Discussion. This species differs from all other known Dictyomeridium species by the red, K+ green pigment in the ostiole. It has previously been found many times since its description, always in north-eastern Brazil.
Dictyomeridium paraproponens (Aptroot, et al.) Aptroot, M. P. Nelsen & Lücking comb. nov.
MycoBank No.: MB 817022
Polymeridium paraproponens Aptroot et al., in Aptroot et al., Lichenologist 45: 548 (2013); type: Brazil, Rondônia, Porto Velho, UNIR Federal University campus S of city, on bark of tree, 100 m, 2012, Cáceres & Aptroot 11162 (ISE!—holotype; ABL!—isotype).
(Fig. 46F)
Thallus ecorticate, pale pinkish white, sometimes flaking off, surrounded by a broad brown prothallus line.
Ascomata pleurothelioid, with lateral ostioles, grouped together by two or three, 0·3–0·5 mm diam., erumpent to prominent, mostly covered by thallus, grey-black. Hamathecium clear. Ascospores muriform, 8 per ascus, hyaline, IKI−, 15–25×1–3-septate, long ellipsoidal, 55–75×19–21 μm, not constricted, surrounded by a gelatinous sheath 3 μm wide.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Brazil).
Discussion. This species differs from all other known Dictyomeridium species by the grouped ascomata with lateral, fused ostioles. It has been found already several times since its description, also in north-eastern Brazil.
Dictyomeridium proponens (Nyl.) Aptroot et al.
In Lücking et al., Lichenologist 48 (in press); Verrucaria proponens Nyl., Bull. Soc. Linn. Normandie, sér. 2 2: 130 (1868).— Polyblastia proponens (Nyl.) Müll. Arg., Flora 65: 402 (1882).—Campylothelium proponens (Nyl.) Müll. Arg., Hedwigia 31: 286 (1892).—Polyblastiopsis proponens (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 351 (1922).—Polymeridium proponens (Nyl.) R. C. Harris, Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 637 (‘1991’) [1993]; type: New Caledonia, Lifu, Loyalty Islands, Thiebaut s. n. (H-Nyl—holotype, not seen).
Verrucaria anoista Stirt., Proc. Phil. Soc. Glasgow 13: 191 (1881).—Anthracothecium anoistum (Stirt.) Zahlbr., Catal. Lich. Univ. 1: 458 (1922).—Campylothelium anoistum (Stirt.) Patw. & Makhija, Biovigyanam 7: 44 (1981); type: India, Assam, Watt s. n. (BM!—lectotype, Patwardham & Makhija, Biovigyanam 7: 44, 1981).
Campylothelium album Müll. Arg., Bull. Soc. Roy. Bot. Belg. 30(1): 89 (1891); type: Costa Rica, Puntarenas, Baia de Salinas, 1890, Tonduz s. n. (G!—holotype; BR—isotype; Pittier, Pl. Costaric. Exs. 5173).
Campylothelium nitidum Müll. Arg., Nuovo Giorn. Bot. Ital. 23: 401 (1891); type: Australia, Brisbane, Bailey 460 (G!—holotype).
Thallus ecorticate, white.
Ascomata pleurothelioid, with lateral ostioles, solitary, 0·4–0·6 mm diam., erumpent, grey-black. Hamathecium clear, filaments profusely anastomosing. Ascospores 8 per ascus, IKI+ violet, muriform, 33–54×12–19 μm, with partly oblique septa, not ornamented, wall not thickened.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Pantropical (reported from USA, Mexico, Costa Rica, Puerto Rico, Venezuela, Guyana, Brazil, Canary Isles, Papua New Guinea, Australia and Hawai’i).
Distothelia Aptroot
In Seaward & Aptroot, Bryologist 108: 284 (2005); type: Distothelia isthmospora Aptroot (holotype).
Thallus only causing a bleaching of the bark; algae only loosely associated with the thallus, chlorococcoid, c. 1·5 μm diam.
Ascomata single. Ostioles apical or lateral. Hamathecium hyaline, filaments only little branched or much anastomosing. Ascospores pale brown, essentially 1-septate, asymmetrically sole-shaped with the upper end widest; wall extremely thickened leaving two small reniform to halter-shaped lumina near the central septum.
Pycnidia sometimes present.
Chemistry. No substances detected.
Discussion: Only three species are known in this genus. Little new information is reported here as compared to Schumm & Aptroot (Reference Schumm and Aptroot2013), but it is the first time that this genus is considered to belong to the Trypetheliaceae, following the realization that much or most of the species classified in Mycomicrothelia, to which Distothelia is probably close, belong in this family.

Distothelia angulata Aptroot & Schumm
In Schumm & Aptroot, Flechten Madeiras, der Kanaren und Azoren 2: 200 (2013); type: Portugal, Azores, Terceira, 430 m, 2008, Schumm 14061 (B!—holotype; ABL!—isotype).
(Fig. 9C)
Thallus only causing a bleaching of the bark or pinkish; algae only loosely associated with the thallus, chlorococcoid or Trentepohlia.
Ascomata emergent, 0·25–0·50 mm diam., black with grey covering. Ostioles black, strongly lateral. Hamathecium hyaline, IKI−, filaments only little branched, c. 1·5 μm wide. Asci cylindrical, IKI−, with 5–8 ascospores, ascus tips with long ocular chamber. Ascospores pale brown, IKI−, essentially 1-septate, asymmetrically sole-shaped with the upper end widest, with a submedian constriction, 14–21×5·5–9·5 μm; wall extremely thickened leaving two small reniform lumina near the central septum.
Pycnidia not observed.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Azores.
Distothelia isthmospora Aptroot
In Seaward & Aptroot, Bryologist 108: 284 (2005); type: China, Hong Kong Island, Hillside, Wright s. n. (FH!—holotype).
(Fig. 46L)
Thallus pinkish, UV+ whitish, mosaic-forming, c. 1–3 cm wide, with very thick (up to 1 mm wide) dark brown border lines, seemingly associated with scattered Trentepohlia in the bark cells.
Ascomata regularly scattered, single, low-conical to hemispherical, c. 0·5–0·8 mm diam., and 0·1–0·2 mm high. Ostioles apical, 50–80 μm diam., black. Wall dark brown, in K blackish, c. 100 μm thick. Hamathecium clear, IKI−, filaments up to 1 μm wide, non-septate, anastomosing above the asci. Asci bitunicate, IKI− (but contents dextrinoid), c. 100–130×15–20 μm, tips without ocular chamber. Ascospores 8 per ascus, irregularly biseriate, 15–17×6–7 μm, elongate, reddish brown (in tap water), in K blackish grey, bilocular with unequal locules, upper locule wider and shorter than lower locule, conspicuously constricted at the septum, each locule with a halter-shaped lumen with a tiny isthmus (in tap water; unchanged in IKI or K) with rounded ends, surface smooth.
Pycnidia mostly near the border lines, c. 0·1–0·2 mm diam., wall dark brown, in K blackish, containing copious gel. Conidia fusiform, simple, hyaline, c. 4–5×0·8 μm, with pointed ends.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distothelia rubrostoma (Aptroot) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816790
Mycomicrothelia rubrostoma Aptroot, Biblioth. Lichenol. 44: 135 (1991); type: Guadeloupe, Basse-Terre, Vieux Fort, trail above the town, Culberson & Culberson 14551 (NY!—holotype).
(Fig. 46K)
Thallus pinkish grey, without prothallus lines.
Ascomata single, with fringe, 1·0–1·3 mm wide and 0·2–0·6 mm high, young ostiole with red, K+ purple anthraquinone. Wall completely carbonized, to 80 μm thick. Asci c. 150×20 μm. Ascospores 23–29×11–14 μm, with equal locules, with rounded ends, walls much thickened with a halter-shaped endospore formation, verruculose.
Pycnidia 60–100 μm wide; conidia bacilliform, simple, hyaline, 6–8×0·3–0·5 μm.
Chemistry. Ostiole with anthraquinone.
Distribution. Neotropical (reported from Dominican Republic and Guadeloupe).
Marcelaria Aptroot et al.
Glalia 5(2): 3 (2013); type: Marcelaria purpurina (Nyl.) Aptroot, M. P. Nelsen & Parnmen (holotype).
Thallus crustose, corticolous on tree trunks, in part endoperidermal, with a thin, prosoplectenchymatous, cartilaginous cortex. Photobiont Trentepohlia.
Ascomata abundant, perithecioid, sessile, in unilocular, solitary or aggregated warts but not pseudostromatic, covered by bright (orange-)yellow or red pigment, usually with a split between the inner wall and the surrounding covering tissue; the covering tissue mostly formed by a thick, gelatinous inner cortex that becomes greenish in K. Ostioles apical. Hamathecium composed of thin, straight, branched and anastomosing paraphysoids embedded in a gelatinous matrix, IKI−. Asci bitunicate, with clearly discernible layers and a broad, flat ocular chamber, clavate with short stipe, IKI−. Ascospores hyaline, muriform, oblong-oval to ellipsoid or fusiform, without distinctly thickened median septum, with endospore and more or less rounded lumina.
Pycnidia with punctiform ostiole, internally brain-like lobate. Conidia hyaline, bacillar.
Chemistry. Several red, orange and/or yellow anthraquinones present (sometimes partly restricted to thallus or ascomata) and often also lichexanthone.
Discussion. The type and the other known species were for a long time known under different names in the genus Laurera. Much information has already been reported by Aptroot et al. (2013 Reference Aptroot, Nelsen and Parnmena ).
Marcelaria benguelensis (Müll. Arg.) Aptroot et al.
Glalia 5(2): 4 (2013).—Bathelium benguelense Müll. Arg., Flora 68: 256 (1885).—Laurera benguelensis (Müll. Arg.) Zahlbr., Cat. Lich. Univ. 1: 503 (1922); type: India, Kurz 173 (G!—lectotype, Aptroot et al., Glalia 5(2): 4, 2013).
Laurera subbenguelensis Upreti & Ajay Singh, Bull. Jard. Bot. Nat. Belg. 57: 380 (1987); type: India, Singh & Ranjan 102285 (BM!—isotype).
Thallus corticate, olive-green, often partly to completely (orange-)yellow pruinose (a different pigment compared to that of the ascomata), smooth to uneven.
Ascomata sessile with flattened top, solitary to irregularly grouped and confluent, covered with (orange-)yellow pigment except for blackish ostiole, 0·6–1·0 mm diam., with groups up to 2·5 mm diam. Hamathecium densely and heavily inspersed. Ascospores 8 per ascus, muriform, fusiform-ellipsoid, 50–80×17–23 µm, hyaline, IKI−, surrounded by a gelatinous sheath 3–12 µm thick.
Conidia 5×0·5 µm.
Chemistry. Thallus UV+ yellow, K− or K+ blood red where orange pigment is present as pruina or in the thin medulla; ascomata UV+ yellow, K+ blood red with purplish hue. TLC: two anthraquinones (parietin=physcione, teloschistin=fallacinol) plus lichexanthone.
Distribution. Eastern palaeotropical (India, Cambodia, Myanmar and Thailand).
Marcelaria cumingii (Mont.) Aptroot et al.
Glalia 5(2): 6 (2013).—Trypethelium cumingii Mont. in Hooker, London J. Bot. 4: 5 (1845).—Bathelium cumingii (Mont.) Trevis., Flora 44: 21 (1861).—Trypethelium cumingianum Stirt., J. Linn. Soc. Bot. 14: 473 (1875) [nom. illeg.].—Melanotheca cumingiana (Stirt.) Müll. Arg., Bull. Herb. Boissier 2(App. 1): 96 (1894) [nom. illeg.].—Laurera cumingii (Mont.) Zahlbr., Catal. Lich. Univ. 1: 503 (1922), also ‘cummingii’; type: Philippines, Cuming s. n. (BM!—holotype; H!—isotype).
Thallus corticate, olive-green to yellowish, smooth to uneven.
Ascomata sessile with flattened top, solitary to irregularly grouped and confluent, covered with (orange-)yellow pigment except for blackish ostiole, 0·5–0·8 mm diam., with groups up to 3 mm diam. Hamathecium densely and heavily inspersed. Ascospores 2 per ascus, muriform, fusiform-ellipsoid, 50–70×14–22 µm, hyaline, IKI− or sometimes IKI+ brownish, surrounded by a gelatinous sheath 3–9 µm thick.
Conidia 4–5×0·5 µm.
Chemistry. Thallus UV+ yellow, K+ purple where medullary pigment is present; ascomata warts UV+ yellow, K+ purple. TLC: three anthraquinones (parietin=physcione, emodin, and a trace derivate) plus lichexanthone.
Distribution. Eastern Palaeotropics (India and the Philippines). Incorrectly reported from New Zealand.
Marcelaria purpurina (Nyl.) Aptroot et al.
Glalia 5(2): 9 (2013).—Trypethelium purpurinum Nyl. in Leighton, Trans. Linn. Soc. London 25: 459 (1866).—Bathelium purpurinum (Nyl.) Müll. Arg., Linnaea 63: 45 (1880).—Laurera purpurina (Nyl.) Zahlbr., Denkschr. Kaiserl. Akad. Wiss. Wien, Math.-Naturwiss. Kl. 83: 93 (1909); type: Brazil, Spruce 236 (PC!—holotype; NY!—isotype).
Tremotylium sprucei Müll. Arg., J. Linn. Soc., London 30: 454 (1895); type: Brazil, Spruce 187 (G!—holotype).
Thallus corticate, olive-green but sometimes with patches of bright red pruina, smooth to uneven or shallowly bullate.
Ascomata sessile with flattened top, solitary to irregularly grouped and confluent, covered with red pigment, 0·7–1·2 mm diam., with groups up to 4 mm diam. Hamathecium clear. Ascospores 8 per ascus, muriform, fusiform-ellipsoid, 110–170×14–26 µm, hyaline, IKI+ violet, surrounded by a gelatinous sheath 5–10 µm thick.
Conidia not observed.
Chemistry. Thallus UV−, K+ purple where pigment is present; ascomata UV−, K+ purple. TLC: five anthraquinones (xanthorin=lauropurpurone, parietin=physcione, and three other orange anthraquinones, possibly secalonic acid derivates).
Distribution. Neotropical and Africa (Colombia, Venezuela, Guyana, Brazil, Bolivia, Ivory Coast and DR Congo).
Nigrovothelium Lücking et al.
In Lücking et al., Lichenologist 48 (in press); type: Nigrovothelium tropicum (Ach.), Lücking et al. (holotype).
Thallus corticate, olive-green to brownish.
Ascomata with apical ostioles, solitary but usually densely crowded, prominent to sessile, exposed and black. Hamathecium clear or inspersed, hyaline, filaments thin, anastomosing paraphysoids. Asci clavate. Ascospores transversely 3-septate, astrothelioid, with diamond-shaped lumina, hyaline.
Discussion. The genus was established to accommodate the species that was until then known as Trypethelium tropicum and its relatives (Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). Its ascoma morphology is unique, somewhat similar to that of Bathelium species but with the ascomata black and the ascospores astrothelioid. Nigrovothelium is most closely related to Polymeridium, but differs from that genus in the corticate thallus and astrothelioid ascospores.

Nigrovothelium tropicum (Ach.) Lücking et al.
In Lücking et al., Lichenologist 48 (in press).—Verrucaria tropica Ach., Lichenogr. Univ.: 278 (1810).—Sagedia tropica (Ach.) A. Massal., Ricerch. Auton. Lich.: 161 (1852).—Pyrenula tropica (Ach.) Trevis., Spighe e Paglie: 17 (1853).—Spermatodium tropicum (Ach.) Trevis., Conspect. Verruc.: 11 (1860).—Pseudopyrenula tropica (Ach.) Müll. Arg., Flora 66: 248 (1883).—Trypethelium tropicum (Ach.) Müll. Arg., Bot. Jahrb. Syst. 6: 393 (1885); type: America, Swartz s. n. (H-ACH 707A!—lectotype; BM-ACH—isolectotype).
Verrucaria gaudichaudii Fée, Essai Crypt. Écorc.: 87 (1824).—Pyrenula gaudichaudii (Fée) Pers., in Freycinet, Voyage Uranie, Bot.: 182 (1826); type: (G—holotype, not seen).
Verrucaria tristis Hepp, in Zollinger, Syst. Verzeichn. Ind. Archip. Ges. Pfl.: 8 (1854); type: Indonesia, Java, Tjibodas, Zollinger s. n. (M!—lectotype, designated here).
Trypethelium tropicum var. nigratum Müll. Arg., Rep. Australas. Assoc. Advancem. Sci. 1895: 460 (1895); type: Australia, Queensland, Bellenden Ker, Bailey s. n. (G!—holotype; BRI!—isotype).
Zignoella magnoliae Tracy & Earle, Bull. Torrey Bot. Club 23: 211 (1896); type: USA, Mississippi, Ocean Springs, Tracy s. n. (NY—holotype, not seen).
Zignoella lichenoidea Höhn., Sitzungsber. Kaiserl. Akad. Wiss., math.-naturwiss.Cl. Abt. 1, 118: 331 (1909); type: Indonesia, Java, Buitenzorg, von Höhnel s. n. (BPI!—isotype; Rehm, Ascomyceten 1862).
Pseudopyrenula pyrenuloides Zahlbr., in Rechinger, Denkschr. Kaiserl. Akad. Wiss., math.-naturwiss. Kl. 88: 12 (1911); type: Solomon Islands, Buka, Rechinger 4756 (W—holotype, not seen).
Pseudopyrenula verrucosa Vain., Ann. Acad. Sci. Fenn., ser. A 6(7): 198 (1915).—Trypethelium verrucosum (Vain.) Zahlbr., Catal. Lich. Univ. 8: 129 (1932) nom. illeg. non Fée (1824); type: Guadeloupe, Camp Jacob, Duss 1394 (TUR-Vain 30797—holotype, not seen).
Zignoella nobilis Rehm, Leafl. Philipp. Bot. 8: 2950 (1916); type: Philippines, Luzon, Laguna, Mount Maquiling, Baker s. n. (BPI—isotype; Baker, Fungi Malayana 200).
Pseudopyrenula composita Vain., Bol. Soc. Broteriana ser. 2 6: 177 (1930).—Trypethelium compositum (Vain.) Zahlbr., Catal. Lich. Univ. 10: 101 (1938).—Bathelium compositum (Vain.) C. W. Dodge, Ann. Missouri Bot.Gard. 40: 289 (1953); type: Mozambique, Palma, Pines de Lima 310 (TUR-Vain—holotype, not seen).
Pseudopyrenula bicincta Zahlbr., Repert. Spec. Nov. Regni Veg. 31: 200 (1933); type: Taiwan, Rengechi, Asahina F304 (W—holotype, not seen).
Pseudopyrenula deightonii C. W. Dodge, Ann. Missouri Bot.Gard . 40: 279 (1953); type: Sierra Leone, Njala, Deighton M4340 (BM—isotype).
Massarina salicicola var. minor Batista & Maia, Brotéria Ci. Nat. 29: 134 (1960) [as ‘Massaria salicinicola’, illustrations sub ‘Massarina salicinicola’ and ‘Massariaa salicinicola’]; type: Brazil, Bahia, Serrinha, Batista 11646 (URM 16277—holotype).
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata with apical ostioles, solitary but usually densely crowded, 0·2–0·3 mm diam., prominent to sessile, subglobose to barrel-shaped with flattened top, exposed and black with ostiolar area greyish. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 20–25×7–10 μm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (previously reported from the USA, Mexico, El Salvador, Cuba, Guadeloupe, Colombia, Venezuela, Guyana, French Guiana, Galapagos, Brazil, Argentina, Sierra Leone, Mozambique, Tanzania, Seychelles, India, Sri Lanka, China, Taiwan, Hong Kong, Singapore, Thailand, Vietnam, Cambodia, Philippines, Indonesia, Papua New Guinea, Solomon Islands and Australia).
New country records. Puerto Rico: Mayagüez: Reserva Forestal Maricao, 1990, Sipman 25925 (B).—Madagascar: Tulear: Ifaty, 1984, Aptroot & Hensen 12617 (ABL).—Bangladesh: Dhaka: Bhawal National Park, 2002, Hopman s. n. (ABL).
Novomicrothelia Aptroot et al.
In Lücking et al., Lichenologist 48 (in press); type: Novomicrothelia oleosa (Aptroot) Aptroot, M. P. Nelsen & Lücking (holotype).
Thallus ecorticate, usually whitish.
Ascomata with apical ostioles, solitary, exposed and black. Hamathecium clear or inspersed, hyaline, clear, filaments thin, anastomosing paraphysoids. Asci clavate. Ascospores transversely 1-septate, with irregular endospore formation, becoming ornamented, brown, rather elongated.
Pycnidia sometimes present.
Discussion. The genus thus far accommodates a single tropical lichen species that was previously placed in the genus Mycomicrothelia Keissl. Its morphological distinction from the reinstated genus Bogoriella is not yet very clear, although it occupies a distinct phylogenetic position (Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). Besides hamathecium inspersion, the formation of ascospore wall invaginations similar to those found in Constrictolumina (Fig. 8D) might be diagnostic (Harris Reference Harris1995).
Novomicrothelia oleosa (Aptroot) Aptroot et al.
In Lücking et al., Lichenologist 48 (in press); Mycomicrothelia oleosa Aptroot, Biblioth. Lichenol. 44: 133 (1991); type: Trinidad and Tobago, Trinidad, Caroni, North Bank Road, Britton et al. 869 (NY!—holotype).
Thallus ecorticate, whitish grey, smooth to uneven, without prothallus lines.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., erumpent to prominent, lens-shaped to hemispherical with spreading base (fringe), exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 1-septate, ellipsoid-oval, not constricted at septum, 24–27×8–11 μm, with nearly equal locules, brown, verruculose.
Pycnidia absent.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Trinidad and Tobago, Colombia, Brazil and Guyana).
Polymeridium (Müll. Arg.) R. C. Harris
In Tucker & Harris, Bryologist 83: 12 (1980).—Arthopyrenia sect. Polymeridium Müll. Arg., Flora 46: 317 (1883); type: Polymeridium contendens (Nyl.) R. C. Harris (holotype).
Exiliseptum R. C. Harris, Acta Amazon. (Suppl.) 14: 65 (‘1984’) [1986]; type: Exiliseptum ocellatum (Müll. Arg.) R. C. Harris [=Polymeridium ocellatum (Müll. Arg.) Aptroot].
Thallus ecorticate except in two species, white to yellowish or grey.
Ascomata simple or fused with partly shared walls or only the ostioles fused, black, without pseudostromatic tissues, globose to pyriform, erumpent from the substratum. Ostioles apical to lateral, brown to black or with red anthraquinone. Hamathecium colourless, sometimes inspersed with hyaline or red (K+ green) oil droplets. Ascospores 4–8 per ascus, IKI− or rarely IKI+ violet, irregularly biseriate, colourless, ellipsoid to fusiform with rounded to subacute ends, symmetrically 3–13-septate to muriform, not constricted at the septa, sometimes surrounded by a thin gelatinous sheath to 2–5 μm thick; septa not thickened; lumina rectangular, slightly rounded but not diamond-shaped.
Conidiomata rather rare.
Chemistry. Thallus occasionally with lichexanthone; anthraquinones rarely present in ostiole or hamathecium.
Discussion. Not much new information is reported here, as compared to Aptroot et al. (2013 Reference Aptroot, Menezes, Lima, Xavier-Leite and Cáceresb ) and Aptroot & Cáceres (Reference Aptroot and Cáceres2014), but descriptions, type citations and full synonymy, partly taken from Harris (Reference Harris1993), are given for all species. One species aggregate, now united in the genus Dictyomeridium, is split off from Polymeridium. Both genera have their centre of diversity in north-eastern Brazil, and additional species continue to be reported from there (Cáceres et al. Reference Cáceres, Lima, Aptroot and Lücking2014).

Polymeridium albidoreagens Aptroot et al.
In Aptroot & Cáceres, Nova Hedwigia 98: 7 (2014); type: Brazil, Rondônia, Porto Velho, Parque Natural Municipal, 100 m, 2012, Cáceres & Aptroot 15194 (ISE—holotype; ABL—isotype).
(Fig. 49D)
Thallus ecorticate, whitish grey, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., erumpent, exposed and black. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 20–23×6–8 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Brazil)
Polymeridium albidovarians Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 7 (2014); type: Indonesia, Java, Kawi-complex, Tjemarakandang, 2700 m, 1937, Groenhart 5755 (L—holotype; ABL—isotype).
(Fig. 48I)
Thallus ecorticate, yellowish white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., erumpent, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, fusiform, 20–23×6–8 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Pantropical (reported from Brazil and Indonesia)
Polymeridium albidum (Müll. Arg.) R. C. Harris
Acta Amazon. (Suppl.) 14: 69 (‘1984’) [1986].—Arthopyrenia albida Müll. Arg., Flora 67: 664 (1884); type: Brazil, Bahia, Caetité, Martius s. n. (G!—holotype).
(Fig. 50H)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., prominent, conical to wart-shaped, exposed and black. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 20–23×6–8 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Pantropical (previously reported from USA, Venezuela, Colombia, Brazil, Bolivia, Mozambique, Malaysia, Sarawak, Papua New Guinea and Australia). Incorrectly reported from Thailand.
New country record. Solomon Islands: Santa Isabel Island: 1965, Hill 11123 (BM).
Polymeridium albocinereum (Kremp.) R. C. Harris
Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 625 (‘1991’) [1993].—Verrucaria albocinerea Kremp., Flora 59: 524 (1876).—Arthopyrenia albocinerea (Kremp.) Müll. Arg., Flora 66: 318 (1883); type: Brazil, Rio de Janeiro, Glaziou 5025 (M—lectotype, Harris, Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 625 (‘1991’) [1993], not seen; G!—isolectotype).
Pseudopyrenula pentameria Vain., Ann. Soc.Zool. Bot. Fenn. Vanamo 1(3): 55 (1921); type: Philippines, Robinson 9623 (TUR-Vain 30818!—holotype).
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam., prominent, hemispherical, exposed and grey-black. Hamathecium clear. Ascospores 8 per ascus, 7–11-septate, fusiform, (24–)28–39×6–9 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−; TLC: no substances detected.
Distribution. Pantropical (previously reported from Colombia, Brazil, Tanzania and Thailand).
New country record. Madagascar: Tulear: Betioky, 1984, Aptroot & Hensen 12595 (ABL).
Polymeridium alboflavescens Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 8 (2014); type: Venezuela, Est. Amazonas, Alto Orinoco, 15 km W of Esmeralda, 110 m, 1997, Hafellner & Komposch 991-6-4 (GZU!—holotype)
(Fig. 51F)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam., erumpent to prominent, exposed and black. Hamathecium clear. Ascospores 8 per ascus, 7–11-septate, fusiform, 28–39×6–9 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (Venezuela and Brazil).
Polymeridium albopruinosum (Makhija & Patw.) Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 8 (2014).—Trypethelium albopruinosum Makhija & Patw., J. Hattori Bot. Lab. 73: 193 (1993); type: India, Andaman islands, Middle Andaman, Dhanimali, Patwardhan & Nagarkar AMH 85.2342 (ABL!, BM!—isotypes).
(Fig. 49A)
Thallus ecorticate, yellowish white, smooth to uneven, often in patches around ascomata.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., erumpent, exposed and grey-black. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 13–17×4–7 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Pantropical (reported from Brazil, Tanzania and India).
Polymeridium amyloideum R. C. Harris
Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 626 (‘1991’) [1993]; type: Brazil, Pará, Serra do Cochimbo, Brako 6107 (NY!—isotype).
Thallus ecorticate, white, smooth to uneven.
Ascomata with lateral, fused ostioles, fused ascomata dispersed, 0·3–0·5 mm diam., erumpent, lens-shaped to applanately hemispherical, exposed and black but ostiolar area often pale. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 16–23×5–7 μm, hyaline, IKI+ violet (septa), wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Brazil).
Polymeridium bambusicola Aptroot & L. I. Ferraro
Bonplandia 10: 140 (2000); type: Argentina, Corrientes, Dep. Mercedes, Macrosistema Iberá, Estancia Rincón del Diablo, Arbo et al. 8269a (ABL!—isotype).
Thallus absent, not lichenized.
Ascomata with apical ostioles, solitary, 0·5–0·8 mm diam., erumpent, exposed and grey-black. Hamathecium clear. Ascospores 8 per ascus, 13–17-septate, oblong, 85–150×20–30 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Argentina).
Discussion. The absence of lichenization and the extremely large ascospores compared to other species of Polymeridium suggest that this might not actually be a genuine Polymeridium but instead belong in a non-lichenized genus in Dothideomycetes.
Polymeridium bengoanum (Vain.) Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 11 (2014).—Pseudopyrenula bengoana Vain., in Hiern, Cat. Afr. Pl. 2(2): 457 (1901); type: Angola, Quifandango, Welwitsch 437 (TUR-Vain 30820!—holotype; BM—isotype).
(Fig. 49B)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., erumpent, exposed and black. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 16–19×4·5–6·0 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Pantropical (reported from USA, Venezuela, Angola, Mozambique, South Africa and Australia).
Polymeridium biloculare R. C. Harris
Acta Amazon. (Suppl.) 14: 70 (‘1984’) [1986]; type: Brazil, Roraima, Manaus-Boa Vista Road, Buck s. n. (NY!—isotype).
(Fig. 50A)
Thallus ecorticate, white, smooth to uneven.
Ascomata with lateral, fused ostioles, usually two aggregated, 0·3–0·5 mm diam., erumpent, pear-shaped, upper part exposed and black. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 26–30×8–10 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Brazil).
Polymeridium catapastoides Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 12 (2014); type: Australia, Queensland, Cape Tribulation, N of Daintree, 1983, Hale 64184 (US!—holotype; ABL!—isotype).
(Fig. 50C)
Thallus ecorticate, yellowish white, smooth to uneven.
Ascomata with mostly apical, rarely somewhat eccentric ostioles, solitary, 0·3–0·5 mm diam., erumpent, hemispherical, exposed and black. Hamathecium clear. Ascospores 4–8 per ascus, 3-septate, fusiform, 24–32×6–11 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from Indonesia, Papua New Guinea and Australia).
Polymeridium catapastum (Nyl.) R. C. Harris
Acta Amazon. (Suppl.) 14: 70 (‘1984’) [1986].—Verrucaria catapasta Nyl., Acta Soc. Sci. Fenn. 7: 488 (1863).—Arthopyrenia catapasta (Nyl.) Müll. Arg., Flora 66: 318 (1883); type: Colombia, Bogotá, Lindig 2869 (H-Nyl 7236!—lectotype, designated here; BM, BR—isolectotypes).
Pyrenastrum album var. verrucarioides Eschw., in Martius, Flora Bras. 1: 147 (1833).—Arthopyrenia tumida Müll Arg., Flora 67: 669 (1884); type: Brazil, Bahia, Martius s. n. (G—lectotype, Harris, Acta Amazon. (Suppl.) 14: 70 (‘1984’) [1986], not seen).
(Fig. 49E)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm, erumpent, exposed and black but ostiolar area sometimes whitish. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 25–33×7–10 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Possibly pantropical (previously reported from the USA, Bahamas, Cayman Islands, Cuba, El Salvador, Guyana, Colombia, Venezuela and Brazil).
New country record. Madagascar: Fianarantsoa: Ambositra, Ambalamanakara, 1984, Aptroot & Hensen 12822 (ABL).
Polymeridium chioneum (Mont.) R. C. Harris
Lichenogr. Thomsoniana: 141 (1998).—Verrucaria chionea Mont., Ann. Sci. Nat. Bot., sér. 2 19: 58 (1843).—Pyrenula chionea (Mont.) Trevis., Spighe e Paglie: 17 (1853).—Pseudopyrenula chionea (Mont.) Zahlbr., Catal. Lich. Univ. 1: 355 (1922); type: French Guiana, Leprieur 613 (PC-Mont—lectotype, Harris, Lichenogr. Thomsoniana: 141, 1998, not seen).
Polymeridium pleiomeroides (Müll. Arg.) R. C. Harris, Acta Amazon. (Suppl.) 14: 70 (‘1984’) [1986].—Arthopyrenia pleiomeroides Müll. Arg., Bot. Jahrb. Syst. 6: 407 (1885); type: Cuba, Wright s. n. (G!—holotype; Müller, Verr. Cub. 76).
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., erumpent, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 8–12-septate, oblong, 40–50×9–11 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Cuba, French Guiana, Guyana, Venezuela and Brazil).
Polymeridium cinereonigricans (Vain.) R. C. Harris
Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 631 (‘1991’) [1993].—Thelenella cinereonigricans Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 216 (1890).—Clathroporina cinereonigricans (Vain.) Zahlbr., Catal. Lich. Univ. 1: 416 (1922); type: Brazil, Minas Gerais, Caraça, Vainio s. n. (TUR-Vain 31357—holotype, not seen; BM!—isotype; Vainio, Lich. Bras. 657).
(Fig. 52K)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., erumpent, lens-shaped, with upper portion exposed and grey-black. Hamathecium clear. Ascospores 8 per ascus, 8–11-septate, oblong-fusiform, 30–45×9–11 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Brazil) and Hawai’i.
Polymeridium contendens (Nyl.) R. C. Harris
In Tucker & Harris, Bryologist 83: 12 (1980).—Verrucaria contendens Nyl., Acta Soc. Sci Fenn. 7: 492 (1863).—Arthopyrenia contendens (Nyl.) Müll. Arg., Flora 66: 317 (1883); type: Colombia, San Antonio, Lindig 2877 (H-Nyl 7331!—holotype; BM!, BR—isotypes).
Arthopyrenia atroalba Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 234 (1890); type: Brazil, Minas Gerais, Lafayette, Vainio s. n. (TUR-Vain 32132—holotype, not seen; BM!—isotype; Vainio, Lich. Bras. 334).
(Fig. 49H)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., erumpent, lens-shaped, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, oblong-fusiform, 12–16×4–6 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from USA, Costa Rica, Colombia, Venezuela, Guyana and Brazil). Incorrectly reported from Australia, Papua New Guinea and Tanzania.
Polymeridium corticatum A.A. Menezes et al.
In Aptroot et al., Lichenologist 45: 546 (2013); type: Brazil, Ceará, Chapada do Araripe, 2012, Menezes s. n. (ISE 15888!—holotype).
Thallus corticate, cream-white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., erumpent, conical, exposed and black. Hamathecium clear. Ascospores 8 per ascus, 9–13-septate, oblong, 32–36×6–7 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Brazil).
Polymeridium costaricense Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 14 (2014); type: Costa Rica, Guanacaste, Palo Verde National Park, trail to Rio Tempisque, 2003, Will-Wolf 12512d (INB!—holotype; WIS!—isotype).
(Fig. 52A)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·4–0·6 mm diam., erumpent, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 8–12-septate, oblong-fusiform, 40–50×9–11 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Costa Rica and Brazil).
Polymeridium dithecium R. C. Harris
Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 632 (‘1991’) [1993]; type: Venezuela, Amazonas, Río Casiquiare, Kubitzki s .n. (NY!—holotype).
(Fig. 51A)
Thallus ecorticate, white, smooth to uneven.
Ascomata with lateral, fused ostioles, usually two aggregated, 0·3–0·5 mm diam., erumpent, lens-shaped, upper part exposed and black. Hamathecium clear. Ascospores 8 per ascus, 7–9-septate, fusiform, 28–35×6–8 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Venezuela and Brazil).
Polymeridium endocrocinum R. C. Harris
Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 633 (‘1991’) [1993]; type: Brazil, Amazonas, Serra Aracá plateau, Samuels 521 (NY!—holotype).
(Fig. 49K)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., erumpent, hemispherical, exposed and black but with pale ostiolar area, internally with yellow-orange, K+ purple pigment. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 30–34×9–10 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−; ascomata internally K+ purple, with yellow-orange anthraquinone.
Distribution. Neotropical (reported from Brazil).
Polymeridium flavothecium R. C. Harris
Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 633 (‘1991’) [1993]; type: Dominican Republic, Dist. Nacional, Sierra Prieta, 18 km NW of Villa Melia, Harris 20160 (NY!—holotype).
(Fig. 51G)
Thallus ecorticate, white, smooth to uneven.
Ascomata with lateral, separate ostioles, solitary, 0·3–0·4 mm diam., prominent, wart-shaped to conical, exposed and black. Hamathecium inspersed with yellow, K+ red granules below. Ascospores 8 per ascus, 8–12-septate, oblong-fusiform, 40–55×9–11 μm, hyaline, IKI−, wall smooth, often breaking into part spores.
Chemistry. Thallus UV−, K−; hamathecium K+ red, with yellow anthraquinone.
Distribution. Neotropical (reported from Costa Rica and the Dominican Republic).
Polymeridium glaucoatrum (Vain.) R. C. Harris
Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 634 (‘1991’) [1993].—Arthopyrenia glaucoatra Vain., Ann. Acad. Sci Fenn. Ser. A 19(15): 12 (1923); type: Philippines, Luzon, Benguet, Pauai, McGregor 8553 (TUR-Vain 32107!—holotype; BM!, F!, NY!, US!—isotypes).
(Fig. 49F)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam., erumpent, laterally covered by thallus but apically exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, fusiform, 26–34×6–9 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from Sri Lanka and the Philippines).
Polymeridium inspersum Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 16 (2014); type: Australia, Queensland, Kuranda, Jumrum Creek track, 1988, Aptroot & Aptroot 22755 (CANB!—holotype; ABL!—isotype).
(Fig. 49I)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., erumpent, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, fusiform, 16–20×3–5 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Brazil, Philippines and Australia).
Polymeridium jordanii (C. W. Dodge) Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 18 (2014).—Pseudopyrenula jordanii C. W. Dodge, Nova Hedwigia, Beih. 12: 11 (1964); type: Sierra Leone, Colony, Sugar Loaf Mountain, Deighton M44440 (BM!—isotype).
(Fig. 52E)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., erumpent, exposed and black. Hamathecium clear. Ascospores 8 per ascus, 4–7-septate, fusiform, 21–30×5–9 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from the USA, Puerto Rico, Brazil, Mozambique, Sierra Leone, Yemen, Aldabra, Hong Kong, the Philippines and Indonesia).
Polymeridium julelloides E. L. Lima et al.
In Aptroot et al., Lichenologist 45: 548 (2013); type: Brazil, Pernambuco, Buíque, Vale do Catimbau National Park, 900 m, 2011, Lima 145 (ISE!—holotype).
(Fig. 52L)
Thallus ecorticate, pale pinkish brown, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam., erumpent to prominent, conical, exposed and black, with brownish ostiole. Hamathecium clear. Ascospores 8 per ascus, small muriform, ellipsoid to slightly clavate, 25–29×11–13 μm, hyaline, IKI−, wall smooth but generally constricted at some septa, septa often partly oblique.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Brazil).
Polymeridium microsporum (Makhija & Patw.) Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 18 (2014).—Trypethelium microsporum Makhija & Patw., J. Hattori Bot. Lab. 73: 201 (1993); type: India, Andaman Islands, Middle Andaman, Yeratta, Sethy & Nagarkar AMH 85.2210 (ABL!, BM!—isotypes).
(Fig. 50D)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·3 mm diam., erumpent, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, fusiform, 11–16×3–6 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Brazil, India, Papua New Guinea, Hawai’i and Fiji).
Polymeridium multiforme Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 18 (2014); type: Guyana, Rupununi Distr., trail at base of Mt. Makarapan, 1988, Maas et al. 7586c (B!—holotype; ABL!—isotype).
(Fig. 51C)
Thallus ecorticate, whitish grey, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·3 mm diam., erumpent, exposed and black. Hamathecium clear. Ascospores 8 per ascus, 7-septate, fusiform, 19–26×5–7 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Venezuela, Guyana and Brazil).
Polymeridium multiseptatum Aptroot et al.
In Aptroot & Cáceres, Nova Hedwigia 98: 19 (2014); type: Brazil, Ceará, Chapada do Araraipe, 2012, Menezes 782 (ISE 15943!—holotype).
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., erumpent, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 4–7-septate, fusiform, 18–28×5–7 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Pantropical (reported from Brazil and Angola).
Polymeridium neblinae R. C. Harris
Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 635 (‘1991’) [1993]; type: Venezuela, Amazonas, Cerro de la Neblina, Buck 10788 (NY!—holotype).
(Fig. 52B)
Thallus ecorticate, white-grey, smooth to uneven.
Ascomata with lateral, separate ostioles, solitary, 0·3–0·5 mm diam., erumpent, mostly exposed and black. Hamathecium clear. Ascospores 8 per ascus, 7–9-septate, fusiform, 40–50×8–9 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (previously reported only from Venezuela).
New country record. Brazil: Sergipe: Santa Luzia do Itanhy, Mata do Junco, 2011, Cáceres 8809 (ISE, ABL).
Polymeridium neuwirthii Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 20 (2014); type: Venezuela, Puerto Ajacucho, at Orinoco, 2005, Neuwirth 8409 (B!—holotype; ABL!, hb. Neuwirth!—isotypes).
(Fig. 51H)
Thallus ecorticate, pinkish grey, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., erumpent, exposed and black. Hamathecium inspersed with very large droplets. Ascospores 8 per ascus, 7-septate, fusiform, 30–33×9–10 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Venezuela).
Polymeridium ocellatum (Müll. Arg.) Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 20 (2014).—Polyblastia ocellata Müll. Arg., Flora 65: 402 (1882).—Verrucaria ocellata Leight., Trans. Linn. Soc. London 35: 458 (1866) non Hoffm. (1790).—Polyblastiopsis ocellata (Müll. Arg.) Zahlbr., Cat. Lich. Univ. 1: 351 (1922).—Exiliseptum ocellatum (Müll. Arg.) R. C. Harris, Acta Amazon. (Suppl.) 14: 66 (‘1984’) [1986]; type: Brazil, Amazonas, São Gabriel, Spruce 244 (BM!—isotype).
Thallus ecorticate, greenish grey, smooth to uneven.
Ascomata with lateral, fused ostioles, a few aggregated, 0·2–0·3 mm diam., immersed, only uppermost part exposed and black. Hamathecium clear. Ascospores 8 per ascus, submuriform, fusiform, with constrictions at septa, 15–16×6–8 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Brazil).
Polymeridium pleiomerellum (Müll. Arg.) R. C. Harris
In Egan, Bryologist 90: 164 (1987).—Arthopyrenia pleiomerella Müll. Arg., Bot. Jahrb. Syst. 6: 406 (1885); type: Cuba, Wright s.vn. (G!—holotype; Müller, Verr. Cub. 107a).
(Fig. 51L)
Thallus ecorticate, white-grey, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam., erumpent, exposed and grey-black. Hamathecium inspersed. Ascospores 8 per ascus, 7–11-septate, fusiform, 25–36×5–9 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Probably pantropical (previously reported from the USA, Mexico, Jamaica, Puerto Rico, Cuba, Brazil and Papua New Guinea).
New country record. New Caledonia: Yaté, 1950, Baumann-Bodenheim 6431 (hb. John).
Polymeridium pleurothecium R. C. Harris
Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 636 (‘1991’) [1993]; type: Guyana, Mt Ayanganna, Potaro, Samuels 5085 (NY!—holotype).
(Fig. 52C)
Thallus ecorticate, white-grey, smooth to uneven.
Ascomata with lateral, separate ostioles, solitary, 0·3–0·6 mm diam., prominent, wart-shaped, exposed and grey-black. Hamathecium clear. Ascospores 8 per ascus, 9–13-septate, fusiform, (35–)50–70×12–15 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Costa Rica, Venezuela, Guyana, Papua New Guinea and Australia).
Polymeridium pyrenastroides Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 22 (2014); type: Venezuela, Est. Amazonas, Alto Orinoco, 15 km W of Esmeralda, W bank of Surumoni, 110 m, 1997, Hafellner & Komposch 209-6-41 (GZU!—holotype).
(Fig. 48J)
Thallus ecorticate, whitish grey, smooth to uneven.
Ascomata with lateral, fused ostioles, a few aggregated, 0·4–0·6 mm diam., erumpent, pear-shaped and appearing triangular from above, upper part exposed and black. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 28–32×9–11 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Venezuela).
Polymeridium pyrenuloides (Müll. Arg.) Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 22 (2014).—Arthopyrenia pyrenuloides Müll. Arg., Mém. Soc. Phys. Genève 30(3): 27 (1888).—Arthopyrenia verrucarioides Zahlbr., Catal. Lich. Univ. 1: 312 (1921) nom. superfl; type: South America, “in cortice cinchonarum” (G!—lectotype, Harris, Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 639 (‘1991’) [1993]).
Arthopyrenia paulensis Zahlbr., Akad. Wiss. Wien, math.-naturwiss. Kl. Denkschr. 83: 89 (1909); type: Brazil, São Paulo, S. Cruz, Bella Vista, Wettstein & Schiffner s. n. (W—holotype, not seen).
(Fig. 48H)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·3 mm diam., prominent, hemispherical to wart-shaped, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, fusiform, 17–20×5–7 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Probably pantropical (reported from Brazil and Australia).
Polymeridium quinqueseptatum (Nyl.) R. C. Harris
In Tucker & Harris, Bryologist 83: 12 (1980).—Verrucaria quinqueseptata Nyl., Mém. Soc. Acad. Maine-et-Loire 4: 58 (1858).—Arthopyrenia quinqueseptata (Nyl.) Müll. Arg., Flora 68: 326 (1885); type: USA, South Carolina, Ravenel s. n. (BM!—isotype).
Verrucaria pustulosa Stirt., Proc. Roy. Philos. Soc. Glasgow 13: 191 (1881); type: India, Assam, Tezpore, Watt s. n. (BM!—isotype).
Arthopyrenia comparatula Müll. Arg., Bot. Jahrb. Syst. 6: 406 (1885); type: Cuba, Wright s. n. (G!—lectotype, designated here; Müller, Verr. Cub. 152).
Pseudopyrenula polyphragmia Vain., Bol. Soc. Broteriana sér. 2 6: 178 (1930); type: Mozambique, Palma, Pires de Lima 303 (TUR-Vain 34713—lectotype, Harris, Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 639 (‘1991’) [1993], not seen).
Thallus ecorticate, white-grey, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam., erumpent to prominent, hemispherical, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 4–7-septate, fusiform, 18–28×5–7 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from the USA, Costa Rica, Cuba, Jamaica, Netherlands Antilles, Venezuela, Brazil, Angola, Mozambique, Seychelles, India, Thailand, Philippines and Australia).
Polymeridium refertum (Stirt.) Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 23 (2014).—Trypethelium refertum Stirt., Proc. Roy. Phil. Soc. Glasgow 11: 321 (‘1878’) [1879].—Plagiotrema refertum (Stirt.) Makhija & Patw., J. Hattori Bot. Lab. 73: 209 (1993); type: India, Nilgiris, 1814, Watt s. n. (BM!—isolectotype, Makhija & Patwardhan, J. Hattori Bot. Lab. 73: 209, 1993).
Thallus ecorticate, white, smooth to uneven.
Ascomata with lateral, separate ostioles, solitary, 0·2–0·4 mm diam., erumpent to prominent, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, (3–)4(–6)-septate, fusiform, 22–26×7–9 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from India).
Polymeridium siamense (Vain.) Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 24 (2014).—Arthopyrenia siamensis Vain., Ann. Soc. Zool. Bot. Fenn. Vanamo 1(3): 55 (1921); type: Thailand, Doi Suthep, Hosseus s. n. (TUR-Vain 32114—holotype, not seen).
Arthopyrenia obvelata Vain., Ann. Acad. Sci Fenn., ser. A 19(15): 11 (1923); type: Philippines, Luzon, Benguet Prov., Pauai, McGregor 8606 (TUR-Vain 32108—holotype, not seen).
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., erumpent, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, fusiform, 20–23×6–8 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Guyana, Brazil, Australia, Philippines and Thailand).
Polymeridium simulans R. C. Harris
Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 641 (‘1991’) [1993]; type: Brazil, Amazonas, 5 km E of Borba, Nelson 1310 (NY!—holotype).
(Fig. 50B)
Thallus ecorticate, white, smooth to uneven.
Ascomata with lateral, separate ostioles, solitary, 0·4–0·6 mm diam., erumpent, pear-shaped, exposed and black. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 30–35×12–13 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Venezuela, Guyana and Brazil).
Polymeridium stramineoatrum (Vain.) Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 24 (2014).—Arthopyrenia stramineoatra Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 234 (1890); type: Brazil, Minas Gerais, Caraça, Vainio s. n. (TUR-Vain 32133—holotype, not seen; Vainio, Lich. Bras. 1566).
Thallus ecorticate, white, smooth to uneven.
Ascomata with mostly lateral, separate ostioles, solitary, 0·3–0·4 mm diam., erumpent to prominent, exposed and black. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 24–30×7–10 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Venezuela and Brazil).
Polymeridium subcinereum (Nyl.) R. C. Harris
In Tucker & Harris, Bryologist 83: 12 (1980).—Verrucaria subcinerea Nyl., Mém. Soc. Acad. Maine-et-Loire 4: 37 (1858).—Pyrenula subcinerea (Nyl.) Tuck., Gener. Lich.: 273 (1872).—Porina subcinerea (Nyl) Zahlbr., Catal. Lich. Univ. 1: 405 (1922); type: USA, Texas, Tuckerman 85 (H-Nyl 1529!—lectotype, designated here).
Arthopyrenia subimitans Müll. Arg., Bull. Soc. Roy. Bot. Belg. 32(1): 169 (1893); type: Costa Rica, Puntarenas, Boruca, Tonduz s. n. (G!—lectotype, designated here; BM, BR—isolectotypes; Pittier, Pl. Costaric. Exs. 6287).
Pseudopyrenula follmannii C. W. Dodge, Nova Hedwigia 12: 308 (1966); type: Chile, Aconcagua Prov., Los Molles, Follmann 14852-D (FH-Dodge—holotype, not seen).
Massarina operculicola M. Morelet, Bull. Soc. Sci. Nat. Archéol. Toulon & Var 36: 14 (1980); type: France, Entrecasteaux, Morelet 1276 (hb. Morelet, Champenoux [‘PFN’, ‘CNRF 898’, ‘INRA, Nancy’]—holotype).
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary to sometimes confluent, 0·3–0·5 mm diam., erumpent, exposed and black. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 15–20×5–7 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Pantropical, extending into the subtropics (reported from the USA, Costa Rica, Dominican Republic, Colombia, Venezuela, Guyana, Brazil, Chile, France, Mozambique, Philippines, Papua New Guinea and Australia).
Polymeridium submuriforme Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 35 (2014); type: Philippines, Leyte, Prov. Leyte, between Camp Pandong Bato and Mt. Agipo, 2000, Schumm & Schwarz 774 (B!—holotype).
(Fig. 52J)
Thallus ecorticate, pinkish grey, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·6 mm diam., erumpent, wart-shaped to conical, exposed and black. Hamathecium inspersed. Ascospores 6–8 per ascus, submuriform, with partly oblique septa, fusiform, 18–20×6–8 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from the Philippines).
Polymeridium subvirescens (Leight.) Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 25 (2014).—Verrucaria subvirescens Leight., Trans. Linn. Soc. London 25: 488 (1866).—Porina subvirescens (Leight.) Zahlbr., Cat. Lich. Univ. 1: 406 (1922); type: Brazil, Santarém, Spruce 358 (BM!—lectotype, Harris, Acta Amazon. (Suppl.) 14: 70 (‘1984’) [1986]).
(Fig. 48G)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical and partly lateral, separate ostioles, solitary, 0·3–0·4 mm diam., erumpent to prominent, exposed and black. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, fusiform, 24–33×6–10 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Probably pantropical (reported from Brazil and Australia).
Polymeridium suffusum (Knight) Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 26 (2014).—Verrucaria suffusa Knight, Trans. Proc. New Zealand Inst. 15: 356 (1882).—Arthopyrenia suffusa (Knight) Müll. Arg., Bull. Soc. Roy. Bot. Belgique 31(2): 40 (1892); type: New Zealand, Knight s. n. (BM!—lectotype, designated here; H-Nyl 881!—isolectotype).
(Fig. 49G)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical or partly lateral, separate ostioles, solitary, 0·3–0·4 mm diam., erumpent, mostly covered by thallus but ostiolar area exposed and grey-black. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, fusiform, 24–30×6–9 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Pantropical (reported from Costa Rica, Cuba, Puerto Rico, Brazil, Réunion, Sarawak and New Zealand).
Polymeridium sulphurescens (Müll. Arg.) R. C. Harris
Lichenogr. Thomsoniana: 147 (1998).—Arthopyrenia sulphurescens Müll. Arg., Flora 65: 518 (1882).—Pseudopyrenula sulphurescens (Müll. Arg.) Müll. Arg., Flora 66: 249 (1883); type: Australia, Queensland, Toowoomba, Hartmann s. n. (G!—holotype).
Arthopyrenia oculata Müll. Arg., Rep. Australas. Assoc . Advancem. Sci. 1895: 451 (1895).—Polymeridium oculatum (Müll. Arg.) R. C. Harris, Bol. Mus. Paraense Emílio Goeldi, Ser. Bot. 7: 635 (‘1991’) [1993]; type: Australia, Queensland, Knight 136 (G!—holotype).
Arthopyrenia suboculata Müll. Arg., Bull. Herb. Boissier 3: 325 (1895); type: Australia, Queensland, Knight 199 (G!—holotype).
(Fig. 50G)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical or partly lateral, separate ostioles, solitary, 0·3–0·5 mm diam., erumpent, exposed and black but ostioles usually with a white rim. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 19–27×8–10 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from Papua New Guinea and Australia), rarely also on rock.
Polymeridium tribulationis Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 26 (2014); type: Australia, Queensland, Cape Tribulation, 2 km off main road between Oil Palms and Coopers Creek, 1983, Hale 641717 (CANB!—holotype; ABL!, US!—isotypes).
(Fig. 49J)
Thallus ecorticate, yellowish white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam., prominent to sessile, mostly covered by thallus and grey, except for black upper part, and ostioles usually with a white rim. Hamathecium inspersed. Ascospores 8 per ascus, 3-septate, fusiform, 20–24×7–10 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV−, K−. TLC: no substances detected.
Distribution. Possibly pantropical (previously known only from Australia), below reported from Brazil; the first species of the P. sulphurescens-group, characterized by subglobose, rather sessile ascomata with a ring around the ostiole, found in the Neotropics.
New country record. Brazil: Ceará: Chapada do Araripe, Malhada Bonita, 2012, Alves 1526 (ISE, ABL).
Polymeridium xanthoreagens Aptroot
In Aptroot & Cáceres, Nova Hedwigia 98: 27 (2014); type: Australia, Queensland, E of Mareeba, Davies Creek Road 17 km S of Kennedy Highway; 1983, Hale 64168 (CANB!—holotype; ABL!, US!—isotypes).
(Fig. 49C)
Thallus ecorticate, yellowish white, smooth to uneven.
Ascomata with apical and partly lateral, separate ostioles, solitary, 0·2–0·4 mm diam., erumpent, covered by thallus except grey-black ostiolar area with a white ring around the black ostiole. Hamathecium clear. Ascospores 8 per ascus, 3-septate, fusiform, 17–25×7–11 μm, hyaline, IKI−, wall smooth.
Chemistry. Thallus UV+ yellow, K−. TLC: lichexanthone.
Distribution. Eastern palaeotropical (Australia).
Pseudopyrenula Müll. Arg.
Flora 66: 247 (1883); type: Pseudopyrenula diluta (Fée) Müll. Arg. (lectotype; Clements & Shear, The genera of fungi: 288, 1931).
Plagiotrema Müll. Arg., Bot. Jahrb. Syst. 6: 387 (1885); type: Plagiotrema cubanum Müll. Arg. [=Pseudopyrenula subnudata Müll. Arg., previously cited as synonym of Pseudopyrenula diluta s. str. by Harris Reference Harris1998, holotype].
Thallus ecorticate, more or less endoperidermal, white to pale yellowish or greyish, or absent in presumably non-lichenized species, smooth to uneven.
Ascomata solitary, exposed and black, not pseudostromatic, erumpent to prominent. Ostioles apical or rarely eccentric. Hamathecium colourless or sometimes yellow, usually inspersed with oil droplets. Ascospores 8 per ascus, irregularly biseriate, symmetrically 3–5-septate, fusiform with subacute ends, not constricted at the septa when young, often surrounded by a thin gelatinous sheath, hyaline, IKI−, lumina diamond-shaped.
Pycnidia rather rare.
Chemistry. Thallus with or without lichexanthone; hamathecium and/or ascospore lumina occasionally with yellow anthraquinone.
Discussion. The genus Pseudopyrenula is a mostly tropical genus. So far, almost 100 species have been described within it, but a significant proportion of those are synonyms or have been transferred to other genera. Pseudopyrenula forms a monophyletic group that is characterized by a non-corticate or indistinct thallus (absent in apparently non-lichenized species) and hyaline, transversely septate, astrothelioid ascospores with diamond-shaped lumina, which separate this genus from superficially similar genera such as Bogoriella, Constrictolumina, Dictyomeridium, Novomicrothelia, and Polymeridium, while on the other hand it is the only genus combining an ecorticate thallus with astrothelioid ascospores. The presence of yellow oil in the ascospore lumina and/or the hamathecium is characteristics of many species in Pseudopyrenula. Presently, the genus encompasses less than 20 species.
Pseudopyrenula cerei Vain.
Acta Soc. Fauna Fl. Fenn. 7(2): 212 (1890); type: Brazil, Rio de Janeiro, Cerei, Vainio s. n. (TUR-Vain 30815!—holotype; Vainio, Lich. Bras. 21).
(Fig. 53L)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., prominent to almost sessile, hemispherical to subglobose, exposed and black, with yellow pigment in wall. Hamathecium inspersed, hyaline. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 21–32×7–10 μm, hyaline, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV−, K−; ascomata internally K+ deep yellow, with yellow pigment.
Distribution. Neotropical (reported from Brazil).
Pseudopyrenula cryptotheca Komposch et al.
Lichenologist 34: 229 (2002); type: Venezuela, Estado Amazonas, Alto Orinoco c. 15 km W of La Esmeralda, plot of tower crane on the W riverbank of Surumoni, Hafellner & Komposch 3085 (GZU!—isotype).
(Fig. 53A)
Thallus ecorticate, white to yellowish, apparently not lichenized, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam., immersed-erumpent, covered by thallus. Hamathecium clear, hyaline. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 30–45×8–11 μm, hyaline, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (reported from Venezuela).
Pseudopyrenula cubana (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816791
Plagiotrema cubanum Müll. Arg., Bot. Jahrb. Syst. 6: 387 (1885); type: Cuba, Wright s. n. (G!—lectotype, designated here; L!—isolectotype; Müller, Verr. Cub. 62).
(Fig. 54F)
Thallus ecorticate, whitish to pale brownish, smooth to uneven.
Ascomata with eccentric ostioles, solitary, 0·3–0·5 mm diam., erumpent, pear-shaped, exposed and black. Hamathecium inspersed, hyaline. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 21–25×6–9 μm, hyaline but sometimes lumina filled with yellow oil, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Cuba).
Pseudopyrenula diluta (Fée) Müll. Arg.
Flora 66: 249 (1883).—Verrucaria diluta Fée, Essai Crypt. Écorc. Suppl.: 85 (1837).—Pyrenula diluta (Fée) Tuck., Proceed. Americ. Acad. Arts Sci. 7: 234 (1868); type: South America, s.col. (G!—lectotype, Harris, Lichenogr. Thomsoniana: 137, 1998).
Verrucaria diremta Nyl., Ann. Soc. Sci. Fenn. 7: 492 (1863).—Pseudopyrenula diremta (Nyl.) Müll. Arg., Flora 66: 249 (1883); type: Colombia, Villeta, Lindig 2827 (H-Nyl 1039!—holotype; BM, BR—isotypes).
Pseudopyrenula albonitens Müll. Arg., Flora 66: 271 (1883); type: Brazil, Apiahy, Puiggari 497 (G!—holotype).
Pseudopyrenula atroalba Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 211 (1890); type: Brazil, Minas Gerais, Caraça, Vainio s. n. (TUR-Vain 30816!—holotype; BM!—isotype; Vainio, Lich. Bras. 1402).
Pseudopyrenula sitiana Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 213 (1890); type: Brazil, Sitio, Vainio s. n. (TUR-Vain 30814!—holotype; BM!—isotype; Vainio, Lich. Bras. 1089).
Pseudopyrenula erumpens Müll. Arg., Bull. Soc. Roy. Bot. Belg. 32(1): 170 (1893); type: Costa Rica, Puntarenas, Boruca, Tonduz s. n. (G!—holotype; BR—isotype; Pittier, Pl. Costaric. Exs. 6300).
Pseudopyrenula oahuensis H. Magn., Ark. Bot. ser. 2 3(10): 234 (1955); type: Hawai’i, Oahu, Andersson s. n. (UPS—holotype, not seen).
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., prominent, hemispherical, exposed and black. Hamathecium inspersed, hyaline (reports of yellow hamathecium can be attributed to yellow ascospore lumina). Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 24–32×7–10 μm, hyaline but often partly with yellow oil in the lumina, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical, extending into temperate regions (previously reported from Honduras, Costa Rica, Cuba, Hispaniola, Jamaica, Puerto Rico, Nevis, St Lucia, Guadeloupe, Dominica, Martinique, Tobago, Colombia, Venezuela, Guyana, French Guiana, Galapagos, Brazil, Paraguay, Azores, Cape Verdes, St Helena, Cameroon, Guinea, Ivory Coast, Tanzania, Seychelles, India, Sri Lanka, Thailand, Sarawak, Japan, Marquesas, New Caledonia, Hawai’i and Australia).
New country records. Netherlands Antilles: Saba: Mt. Scenery, 2007, Sipman 54819 (B).—Papua New Guinea: Eastern Highlands: Mount Gahavisuka Provincial Park, 2300–2450 m, 1992, Aptroot 32430 (ABL).—Malaysia: Sabah: Kota Belud, Mount Kinabalu, 2800 m, 1989, Sipman & Tan 31290 (B).
Pseudopyrenula dubia Vain.
Ann. Acad. Sci. Fenn., ser. A 6: 354 (1915); type: Guadeloupe, Savane-aux-Ananas, Duss 1496 (TUR-Vain 30831!—holotype; BM!—isotype).
(Fig. 54B)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam., prominent, hemispherical, exposed and black. Hamathecium inspersed, hyaline. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 33–37×10–12 μm, hyaline, IKI−, not ornamented.
Chemistry. Thallus UV+ yellow, K−, with lichexanthone; ascomata UV− yellow, K−.
Distribution. Neotropical (previously reported from Guadeloupe).
Discussion. Although synonymized with Pseudopyrenula diluta by Harris (Reference Harris1995), the species is reinstated here, because the ascospores are substantially larger than in the latter species.
New country record. Venezuela: Bolivar: Cerro Guaiquinima, 1200 m, 1990, Sipman 26840 (B).
Pseudopyrenula endoxantha Vain.
J. Bot. 34: 292 (1896); type: Dominica, Morne Anglais, Elliott s. n. (TUR-Vain 30821!—holotype; BM!—isotype).
(Fig. 53H)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·4–0·6 mm diam., prominent, hemispherical, exposed and black. Hamathecium inspersed, yellow. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 37–44×10–12 μm, hyaline, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV−, K−; hamathecium, K+ reddish, with yellow anthraquinone.
Distribution. Neotropical (previously reported from Dominica).
New country record. Costa Rica: Guanacaste: Volcán Tenorio National Park, 15 km NNW of Tilarán, 950–1000 m, 2004, Aptroot 60602 (ABL, INB).
Pseudopyrenula endoxanthoides Vain.
Hedwigia 46: 180 (1907); type: Thailand, Koh Chang, Schmidt 16 (TUR-Vain 30829—holotype, not seen).
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., prominent, hemispherical, exposed and black. Hamathecium yellow inspersed. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 18–22×6–8 μm, hyaline, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV−, K−; hamathecium K+ weakly purplish, with yellow pigment.
Distribution. Eastern palaeotropical (reported from Thailand).
Discussion. This taxon is reinstated here from synonymy with Pseudopyrenula subgregaria, due to subtle anatomical differences supported by molecular data (Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). Thus, P. subgregaria has slightly larger ascospores and the hamathecium reacts K+ deep yellow to reddish, not weakly purplish, indicating the presence of different pigments.
Pseudopyrenula papuana Aptroot
In Aptroot et al., Biblioth. Lichenol. 64: 148 (1997); type: Papua New Guinea, Madang, near Bogia, between Bunapas and Bunapas Mission, Aptroot 30326 (B!—holotype).
(Fig. 53E)
Thallus ecorticate, brownish, apparently not lichenized, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·8–1·6 mm diam., erumpent, lens-shaped, exposed and black. Hamathecium clear, hyaline. Ascospores 5–8 per ascus, 3-septate, fusiform-ellipsoid, 25–29×7–9 μm, hyaline, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from Papua New Guinea).
Pseudopyrenula saxicola Malme
Ark. Bot. 22A(6): 8 (1928); type: Brazil, Minas Gerais, São João d’el Rey, Malme 239 (S!—lectotype, Harris, Lichenogr. Thomsoniana: 139, 1998).
(Fig. 54A)
Thallus saxicolous, ecorticate, pale brownish, uneven.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., erumpent to prominent, hemispherical, exposed and black. Hamathecium inspersed (rarely indistinctly so), hyaline. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 35–45×11–13 μm, hyaline, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Brazil).
Pseudopyrenula serusiauxii Aptroot
Tropical Bryology 14: 29 (1998); type: Papua New Guinea, Madang Province, Balek Wildlife Sanctuary, c. 15 km S of Madang along road to Lae, Aptroot 36864 (B!—holotype).
(Fig. 53F)
Thallus ecorticate, brownish (bark colour), not lichenized.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., erumpent to prominent, hemispherical with flattened top, exposed and black. Hamathecium clear, hyaline. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 30–39×6–8 μm, hyaline, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Eastern palaeotropical (reported from Papua New Guinea).
Pseudopyrenula staphyleae (Petr.) Aptroot
Nova Hedwigia 66: 156 (1998).—Massarina staphyleae Petrak, Sydowia 6: 7 (1952); type: USA, Florida, Wekiwa Spa, Shear P787 p.p. (W 06032!—holotype).
Thallus ecorticate, brownish (bark colour), not lichenized.
Ascomata with apical ostioles, solitary, 0·3–0·5 mm diam., erumpent to prominent, hemispherical, exposed and black. Hamathecium clear, hyaline. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 45–55×12–15 μm, hyaline, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. North American (USA).
Pseudopyrenula subgregaria Müll. Arg.
Bot. Jahrb. Syst. 6: 408 (1885); type: Cuba, Wright s. n. (G!—holotype; L!—isotype; Müller, Verr. Cub. 80).
Pseudopyrenula endoxanthoides Vain., Hedwigia 46: 180 (1907); type: Thailand, Koh Chang, Schmidt 16 (TUR-Vain 30829—holotype, not seen).
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam., prominent, hemispherical, exposed and black. Hamathecium yellow inspersed. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 21–25×6–9 μm, hyaline, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV−, K−; hamathecium, K+ deep yellow to reddish, with yellow anthraquinone.
Distribution. Pantropical (previously reported from the USA, Costa Rica, Cuba, Sri Lanka and Australia).
Discussion. Specimens with comparatively small ascospores and yellow hamathecium inspersion (which constitutes a rare, constant character, not to be confused with yellow oil inside the ascospores, which seems to be an inconstant character in this group) are now recognized as a species separate from Pyrenula subnudata, with P. subgregaria being the oldest available name; the yellow-inspersed taxon is less widespread than P. subnudata. Unfortunately, the name P. subgregaria has been used mostly in the sense of P. subnudata s. lat. (including both hyaline and yellow-inspersed specimens), so that most reports in the literature cannot be evaluated; they are tentatively listed under P. subnudata below.
New country records. El Salvador: Ahuachapan, San Francisco Menendez, 1993, Sipman et al. 37961 (B).—Panamá: Veraguas: Bahía Honda, 2001, Cabrera & Etayo 18518 (ABL, hb. Etayo).—French Guiana: Saül, 1986, Montfoort & Ek 8 (B).—Guyana: Upper Takutu: Rupununi Savannah, Karanambo Ranch, 1992, Sipman 57268 (B).—Brazil: Minas Gerais: Serra do Caraça, Parque Natural do Caraça, 1270 m, 1997, Aptroot 41518, 41519, 41425, 41474 (ABL, SP).—China: Yunnan: Xishuangbanna, Menglun, 2002, Aptroot 57111 (ABL).—India: West Bengal: Sundarban Biosphere Reserve, Jagadeesh Ram 749 (ABL, BSA).—Indonesia: Irian Jaya: Padjah Ampat Archipelago, P. Misool, Lilinta, Zaneveld, 1949 (ABL, L).—Java: Serang, 1937, Groenhart 592 (ABL, L).
Pseudopyrenula subnudata Müll. Arg.
Flora 66: 272 (1883); type: Brazil, Apiahy, Puiggari 138b p.p. (G!—holotype).
Pseudopyrenula flavicans Müll. Arg., Bot. Jahrb. Syst. 6: 408 (1885); type: Cuba, Wright s. n. (G!—lectotype, designated here; BM!—isolectotype; Müller, Lich. Cub., Ser. II: 650).
Pseudopyrenula elliptica Müll. Arg., Bot. Jahrb. Syst. 6: 409 (1885); type: Cuba, Wright s. n. (G!—holotype).
Pseudopyrenula araucariae Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 212 (1890); type: Brazil, Minas Gerais, Caraça, Vainio s. n. (TUR-Vain 30775!—holotype; Vainio, Lich. Bras. 1461).
Arthopyrenia minutissima Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 234 (1890); type: Brazil, Minas Gerais, Lafayette, Vainio s. n. (TUR-Vain 32106—holotype, not seen; BM!—isotypes; Vainio, Lich. Bras. 323).
Pseudopyrenula diluta var. degenerans Vain., in Schmidt, Bot. Tidsskr. 29: 148 (1909); type: Thailand, Koh Chang, Schmidt 10 (TUR-Vain 30823!—holotype).
Pseudopyrenula confluens G. Merr. ex Hedrick, Mycologia 22: 247 (1930); type: Puerto Rico, Aibonito, Fink 1856 (MICH!—holotype).
Pyrenula hagmannii Redinger, Hedwigia 73: 56 (1933); type: Brazil, Amazonas, Ginzberger s. n. (W!—holotype).
Pseudopyrenula limitata Szatala, Ann Mus. Nat. Hungar. n.s. 7: 17 (1956); type: Papua New Guinea, Biró s. n. (BP!—holotype).
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·2–0·4 mm diam., prominent, hemispherical, exposed and black. Hamathecium inspersed, hyaline. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 21–25×6–9 μm, hyaline but sometimes lumina with yellow oil, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (previously reported from the USA, Guatemala, Honduras, Costa Rica, Cuba, Cayman Islands, Hispaniola, Jamaica, Puerto Rico, St Eustatius, Nevis, Dominica, Trinidad, Colombia, Venezuela, Guyana, Surinam, French Guiana, Ecuador, Galapagos, Brazil, Bolivia, Cameroon, Sierra Leone, Zaire, Tanzania, Seychelles, India, Sri Lanka, Thailand, Malaysia, Sarawak, Philippines, Hong Kong, Taiwan, Hawai’i, Papua New Guinea and Australia).
Discussion. This species and Pseudopyrenula diluta have recently been reduced to varietal status (Harris Reference Harris1998), but they are maintained as distinct species here based on preliminary phylogenetic data (Nelsen et al. Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ) and because no intermediates occur and their distributions differ, with P. diluta extending further into temperate regions (e.g. Azores and Japan). Furthermore, specimens with yellow hamathecium inspersion are now recognized as an additional, separate species, P. subgregaria. Unfortunately, these names have been used mostly in a broad sense, including specimens with and without hamathecium inspersion. Therefore, the distribution given above is tentative, although there is no doubt that P. subnudata s. str. is the most common species of the genus.
New country records. China: Yunnan: Xishuangbanna, Menglun, 2002, Aptroot 5732 (ABL).—Indonesia: Java: Ngangtang, 1937, Groenhart 4125 (ABL, L); Cibodas, Mt. Gede, 1950, Nurta & Madrodji s. n. (ABL, L).—New Caledonia: Oui Pouen, 1950, Baumann-Bodenheim 7600 (hb. John).
Pseudopyrenula superans Müll. Arg.
Bot. Jahrb. Syst. 6: 408 (1885); type: Cuba., Wright s. n. (G!—holotype; Müller, Verr. Cub. 71).
(Fig. 53D)
Thallus ecorticate, white, smooth to uneven.
Ascomata with apical ostioles, solitary, 0·3–0·4 mm diam., prominent, hemispherical, exposed and black. Hamathecium inspersed, hyaline. Ascospores 8 per ascus, 3-septate, fusiform-ellipsoid, 30–35×9–11 μm, hyaline, IKI−, not ornamented.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (reported from Cuba and Jamaica).
Trypethelium Spreng.
Anleit. Kenntn. Gew. 3: 350 (1804); type: Trypethelium eluteriae Spreng. (holotype).
Holstiella P. Henn., in Engler, Die Pflanzenwelt Ostafrikas C: 33 (1895); type: Holstiella usambarensis P. Henn. [=Trypethelium eluteriae Spreng.]
Thallus green to grey or yellowish to brownish, smooth to uneven or becoming somewhat bullate, corticate. Ascomata black, globose, immersed in prominent to sessile pseudostromata or rarely erumpent from the thallus, often at least the sides covered by the thallus. Hamathecium colourless, clear or inspersed with oil droplets. Ascospores 8 per ascus, IKI−, irregularly biseriate, colourless, fusiform with subacute ends, transversely septate, not constricted at the septa, when young surrounded by a gelatinous sheath, lumina rectangular when mature.
Conidiomata rather rare.
Chemistry. Thallus and/or pseudostromata often with lichexanthone (or 1,8-dihydroxy-3,6-dimethoxyxanthone) or anthraquinones.
Discussion. Trypethelium as defined here is a strictly tropical genus. Well over 300 taxa have been described or recombined under this name, but a significant proportion of these are synonyms of other species or now accepted in other genera, mostly Astrothelium. Phylogenetic studies of the family Trypetheliaceae (Nelsen et al. Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ) have shown that the type species, T. eluteriae Spreng., does not cluster with the majority of the species that were until recently classified under this name, including the common species known as T. nitidiusculum, T. ochroleucum and T. tropicum, which are now included in Astrothelium and Nigrovothelium, respectively. As already suggested by Harris (Reference Harris1995), Trypethelium s. str. forms a small, monophyletic group of taxa; this group is characterized by more or less distinctly pseudostromatic ascomata usually filled or covered with anthraquinone crystals, but the most distinctive feature are the hyaline, multiseptate, transversely septate ascospores with thin septa and more or less rectangular lumina when mature. This ascospore type appears to be rare within the family, otherwise found only in the genera Bathelium, Polymeridium, and Viridothelium, whereas most other derived members of the Trypetheliaceae have astrothelioid ascospores with diamond-shaped lumina. While Polymeridium is distinguished from Trypethelium by the ecorticate, whitish thallus and solitary, exposed ascomata, species of Bathelium have fully exposed, brown-black pseudostromata with usually few ascomata, a morphology not found in the known species of Trypethelium. The distinction between Trypethelium and Viridothelium is less straightforward; where Trypethelium typically has prominent to sessile pseudostromata and in Viridothelium the ascomata are usually immersed-erumpent and solitary, the type species of the latter, V. virens, has pseudostromatic, erumpent ascomata very similar to those of Trypethelium inaequale (see below). Yet, both are placed within their corresponding genera based on molecular data. In its now restricted sense, the genus Trypethelium encompasses 15 species. Its closest relative is the muriform-spored genus Marcelaria (Aptroot et al. 2013 Reference Aptroot, Nelsen and Parnmena ; Nelsen et al. Reference Nelsen, Lücking, Aptroot, Andrew, Cáceres, Rivas Plata, Gueidan, Cañez, Knight and Ludwig2014; Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ).

Trypethelium eluteriae Spreng.
Anleitung zur Kenntnis der Gewächse 3: 351 (1804).—Pseudopyrenula eluteriae (Spreng.) Vain., Ann. Acad. Sci. Fenn., ser. A 6(7): 353 (1921); type: Indonesia, Sprengel s. n. (L!—holotype).
Trypethelium sprengelii Ach., Lichenogr. Univ.: 306 (1810).—Pseudopyrenula eluteriae var. sprengelii (Ach.) Vain., Bol. Soc. Broteriana ser. 2 6: 177 (1930).—Trypethelium eluteriae var. sprengelii (Ach.) Zahlbr., Catal. Lich. Univ. 10: 102 (1938); type: Surinam, Swartz s. n. (H-ACH 875!—holotype; BM!—isotype).
Trypethelium perrotetii Fée, Ann. Sci. Nat., Bot. 23: 432 (1831); type: Senegal, s. col. (G—holotype, not seen).
Trypethelium anacardii Fée, Ann. Sci. Nat., Bot. 23: 430 (1831).—Trypethelium sprengelii var. anacardii (Fée) Nyl., Mém. Soc. Sci. Nat. Cherbourg 5: 141 (1857).—Trypethelium eluteriae var. expallidum Müll. Arg., Mém. Soc. Phys. Hist. Nat. Genève 30(3): 16 (1888).—Trypethelium eluteriae var. anacardii (Fée) Zahlbr., Catal. Lich. Univ. 1: 491 (1922).—Pseudopyrenula eluteriae var. anacardii (Fée) Vain., Bol. Soc. Broteriana ser. 2 6: 177 (1930); type: Guadeloupe, Bertero s. n. (G!—lectotype, Harris, Lichenogr. Thomsoniana: 142, 1998).
Trypethelium sprengelii var. nigricans Fée, Ann. Sci. Nat., Bot. 23: 430 (1831).—Trypethelium eluteriae var. nigricans (Fée) Trevis., Flora 44: 20 (1861): type: s.dat. (G—holotype, not seen).
Astrothelium varium Eschw., in Martius, Flora Bras. 1: 161 (1833); type: Brazil, Martius s. n. (M!—lectotype, Harris, Acta Amazon. (Suppl.) 14: 73 (‘1984’) [1986]).
Astrothelium varium var. citrinum Eschw., in Martius, Flora Bras. 1: 162 (1833).—Trypethelium sprengelii var. citrinum (Eschw.) Müll. Arg., Flora 67: 671 (1884).—Trypethelium eluteriae var. citrinum (Eschw.) Müll. Arg., Bot. Jahrb. Syst. 6: 393 (1885); type: Brazil, Martius s. n. (M!—lectotype, Harris, Acta Amazon. (Suppl.) 14: 73 (‘1984’) [1986]).
Trypethelium areolatum Mont., in Hooker, London J. Bot. 4: 5 (1845); type: Philippines, s. col. (BM—holotype, not seen).
Trypethelium luteum Taylor, Lond. J. Bot. 6: 157 (1847); type: India, Madras, Wight s. n. (BM!—isotype).
Verrucaria trypetheliiformis Mont., Ann. Sci. Nat., Bot., sér. 3 16: 67 (1851).—Trypethelium montagnei Trevis., Flora 44: 20 (1861); type: French Guiana, Leprieur s. n. (PC-Mont—holotype, not seen).
Trypethelium crocosarca Cooke, in Berkeley & Broome, Grevillea 20(95): 83 (1873); type: Sri Lanka, Thwaites 131 (BM—holotype, not seen).
Trypethelium assimile Stirt., Proc. Roy. Philos. Soc. Glasgow 13: 193 (1881); type: India, Assam, Watt s. n. (BM!—isotype).
Trypethelium insigne Müll. Arg., Flora 68: 256 (1885); type: South America, “cinchonicola” (G!—lectotype, designated here).
Trypethelium eluteriae var. truncatum Müll. Arg., Bot. Jahrb. Syst. 6: 393 (1885); type: Cuba, Wright s. n. (G!—lectotype, designated here; Müller, Verr. Cub., Ser. II: 585 p.p.).
Trypethelium eluteriae var. endochlorum Müll. Arg., Flora 68: 255 (1885); type: South America, “quassiaecola et cort. Bonplandiae” (G-G00295257!—lectotype, designated here).
Pseudopyrenula eluteriae ssp. subsulphurea Vain., Acta Soc. Fauna Fl. Fenn. 7(2): 205 (1890).—Trypethelium eluteriae var. subsulphureum (Vain.) Riddle, in Britton & Millspaugh, Bahama Flora: 530 (1920).—Trypethelium subsulphureum (Vain.) Zahlbr., Catal. Lich. Univ. 1: 500 (1922); type: Brazil, Rio de Janeiro, Sepitiba, Vainio 413 (TUR-Vain 30744!—holotype; BM!, M!—isotype).
Trypethelium pringlei Eckfeldt, Bull. Torrey Bot. Club 21: 395 (1894); type: Mexico, San Luis Potosi, Las Palmas, Pringle 226 (NY!, US!—isotype).
Trypethelium scitulens Eckfeldt, Bull. Torrey Bot. Club 21: 395 (1894); type: Mexico, San Luis Potosi, Las Palmas, Pringle 200 (NY!, US!—isotype).
Holstiella usambarensis P. Henn., in Engler, Die Pflanzenwelt Ostafrikas C: 33 (1895); type: Tanzania, Dodo, Holst s. n. (FH-Höhnel!—lectotype, Aptroot, Nova Hedwigia 66: 156, 1998).
Trypethelium medians Harm., Bull. Soc. Sci. Nancy sér. 3 12: 142 (1911); type: New Caledonia [“Australia”], Pionnier s. n. (DUKE!—holotype).
Trypethelium leprosulum Zahlbr., Ann. Mycol. 30: 499 (1932); type: China, Fukien, Kulang, Chung 371 & 376 (W—syntypes; FH—isosyntypes, not seen).
Thallus corticate, olive-green to yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata containing numerous ascomata, 1–2 mm diam., prominent to almost sessile, brownish to dark brown but usually covered by yellow pigment except for dark ostioles; internally usually with yellow pigment. Hamathecium clear. Ascospores 8 per ascus, 9–13-septate, fusiform, 37–52×8–11 μm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV+ red, K+ purple, with yellow anthraquinone.
Distribution. Pantropical (previously reported from the USA, Mexico, Guatemala, El Salvador, Costa Rica, Guadeloupe, Cuba, Colombia, Venezuela, Surinam, French Guiana, Galapagos, Brazil, Paraguay, Gambia, Tanzania, Seychelles, China, Hong Kong, India, Sri Lanka, Thailand, Cambodia, Vietnam, Indonesia, Papua New Guinea, Philippines, Taiwan, Japan, New Caledonia and Australia).
Discussion. Trypethelium eluteriae as circumscribed here most certainly remains a collective taxon. Typically, the pseudostromata are covered by a yellow pigment, but specimens particularly from the Caribbean deviate by their dark brown pseudostromata completely lacking an external pigment but having the pigment inside the pseudostromata instead. Phylogenetic data support their separation as a taxon basal to T. eluteriae s. str. (Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ), and T. sprengelii would then be the oldest available name, as tentatively suggested by Lücking et al. (2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). Other collections, for example from Africa, have light brown pseudostromata lacking an external pigment. The situation is complicated by the fact that the external pigment in old collections is often abraded.
New country records. Nicaragua: Rivas: Playa El Coco, 2001, Breuss 19271 (LI).—Puerto Rico: Mayaguez: Bosque Estatal de Susua, 1989, Aptroot & Aptroot 25894, 25879, 25900, 25905 (ABL).—Dominican Republic: La Romana: 13 km NE of La Romana, Harris 20282 (ABL, NY).—Guyana: Upper Takutu: Rupununi, Dadanawa Ranch, 1992, Sipman 57390 (B).—Madagascar: Tulear: Ifaty, 1984, Aptroot & Hensen 12616 (ABL).—Bangladesh: Dhaka Division: Bhawal National Park. 2001, Hopman s. n. (ABL).—Singapore: University campus, 2000, Sipman 45531 (B).—Vanuatu: Efate: Iririki Island, 1998, Streimann & Ala 61992 (B, CBG).
Trypethelium epileucodes Nyl.
Lich. Japon.: 116 (1890); type: Malaysia, Labuan, Almquist s. n. (H-Nyl 272!—lectotype, Makhija & Patwardhan, Int. J. Mycol. Lichenol. 5: 241, 1992; S!—isolectotype).
Bathelium sundaicum Müll. Arg., Nuovo Giorn. Botan. Ital. 23: 277 (1891).—Laurera sundaica (Müll. Arg.) Zahlbr., Catal. Lich.Univ. 1: 507 (1922); type: Singapore, Beccari s. n. (G!—holotype).
Trypethelium virginicum Müll. Arg., Rep. Australas. Assoc. Advancem. Sci. 1895: 461 (1895); type: Australia, Queensland, Knight 351 (G!—lectotype, designated here).
Trypethelium subnitidiusculum Makhija & Patw., J. Hattori Bot. Lab. 73: 207 (1993); type: India, Karnataka, Coorg, Talcauvery, Patwardhan & Prabha AMH 74.3368 (ABL—isotype).
Trypethelium andamanicum Makhija & Patw., J. Hattori Bot. Lab. 73: 193 (1993); type: India, Andaman Islands, South Andaman, Baratang, 1985, Nagarkar & Sethy AMH 85.471 (ABL!—isotype).
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata containing few to numerous ascomata, 0·5–1·5 mm broad, erumpent, whitish with dark ostioles. Hamathecium clear. Ascospores 8 per ascus, 5–9(–13)-septate, fusiform, 30–60×(5–)8–13 μm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Palaeotropical (previously reported from India, Sri Lanka, Taiwan, Thailand, Hong Kong, Singapore, Indonesia, Papua New Guinea and Australia).
New country records. Malaysia: Johor: Gunung Pulai Forest Reserve, 2000, Sipman et al. 46414 (B).—Philippines: Sorsogon: Luzon, Irosin, 1916, Elmer 15761 (ABL, B).—Solomon Islands: Santa Isabel Island: 1965, Hill 9918 (BM).
Trypethelium foveolatum Müll. Arg.
Flora 68: 255 (1885).—Trypethelium papillosum Kremp., Flora 59: 527 (1876), nom. illeg., non Ach; type: Brazil, Rio de Janeiro, Glaziou 5071 (G!—lectotype, designated here; M!—isolectotype).
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata usually containing few ascomata, 0·7–1·5(–2·0) mm diam., prominent, laterally covered by thallus but upper part cream-coloured, with relatively broad, dark, often confluent ostiolar spots. Hamathecium clear. Ascospores 8 per ascus, 9–15-septate, fusiform, 58–78×10–13 μm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Brazil).
Trypethelium inaequale Fée
Ann. Sci. Nat., Bot. 23: 439 (1831).—Trypethelium sprengelii f. inaequale (Fée) Nyl., Mém. Soc. Acad. Maine-et-Loire 4: 77 (1858).—Trypethelium eluteriae var. inaequale (Fée) Müll. Arg., Bot. Jahrb. Syst. 6: 393 (1885); type: Peru (G!—holotype).
Trypethelium inamoenum Müll. Arg., J. Linn. Soc. London, Bot. 29: 230 (1892); type: India, Manipur, Watt 98 (G!—lectotype, Makhija & Patwardhan, J. Hattori Bot. Lab. 73: 199, 1993, not seen; BM!—isolectotype).
Thallus corticate, olive-green to yellowish or brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, diffusely to distinctly pseudostromatic; pseudostromata with numerous ascomata, 0·5–1·0 mm broad, immersed to erumpent, often linear, with individual ascomata largely exposed and blackish brown. Hamathecium clear. Ascospores 8 per ascus, 5–11-septate, fusiform, 21–45×9–15 μm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Pantropical (previously reported from Peru and India).
Discussion. The collections from the Neotropics and the eastern Palaeotropics agree well with each other in morphology and ascospore type. The species is virtually indistinguishable from Viridothelium virens (see below), both in morphology and ascospore type, but collections of both taxa collected within or close to the area of origin of either type fall phylogenetically within either Trypethelium (specimen from Thailand) or Viridothelium (specimen from the USA).
New country records. Guyana: Upper Mazaruni: Pakaraima mountains, Kamarang, 1985, Sipman & Aptroot 18259 (ABL, B).—Thailand: Lücking 24125 (B, F).—Indonesia: Borneo: Kalimantan, Wolseley 1288 p.p. (BM).
Trypethelium krempelhuberi Makhija & Patw.
Int. J. Mycol. Lichenol. 5: 241 (1992) [as krempelhuberii]; type: Vietnam, Tu-phap, Balansa s. n. (S!—isotype).
Trypethelium platystomum var. denudatum Malme, Ark. Bot. 19(1): 27 (1924); type: Brazil, Matto Grosso, Cuyabá, Malme 2600 (S!—lectotype, designated here).
Thallus corticate, olive-green to brownish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata with few ascomata, 0·5–1·2 mm diam., erumpent to prominent, laterally covered by thallus but upper portion of individual ascomata exposed and blackish brown, conspicuously flattened, disc-shaped and margins of ‘discs’ with numerous small, black papillae arranged in circular fashion, around the ostioles thinly furnished with orange pigment. Hamathecium clear. Ascospores 8 per ascus, 13–15-septate, fusiform, 59–72×13–15 μm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata apically UV+ red, K+ purple, with thin, orange anthraquinone.
Distribution. Brazil and Vietnam.
Trypethelium ornatum Müll. Arg.
Bot. Jahrb. Syst. 6: 393 (1885) non Hellb. (1896); type: Cuba, Wright s. n. (G!—holotype; Müller, Verr. Cub., Ser. II: 557).
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata with few ascomata, 0·7–1·5 mm diam., prominent, grey-black but usually covered by a thin whitish layer, with ostiolar areas forming broad, weakly contrasting, white spots. Hamathecium inspersed. Ascospores 8 per ascus, 11–15-septate, fusiform, 60–70×10–14 μm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Neotropical (Cuba, Venezuela and Brazil).
Trypethelium platystomum Mont.
Ann. Sci. Nat., Bot. Sér. 2 19: 72 (1843); type: French Guiana, Leprieur (BR, L!—isotypes).
Thallus olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata with few ascomata, 0·8–1·5 mm diam., prominent, cream-coloured and usually paler than the thallus, but ostiolar spots dark and usually confluent in lobate pattern, surround by thin layer of orange pigment. Hamathecium clear. Ascospores 8 per ascus, 12–18-septate, fusiform, 55–75×8–15 μm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata apically UV+ red, K+ purple, with orange anthraquinone.
Distribution. Pantropical (previously reported from Costa Rica, Guyana, French Guiana, Venezuela, Brazil, Paraguay, India, Sri Lanka and Thailand).
New country records. Puerto Rico: Aguadilla: Bosque Estatal de Guajataca, 1989, Aptroot & Aptroot 25746 (ABL).—El Salvador: Santa Ana, Metapan, Parque Nacional Montecristo, 1993, Sipman et al. 37370 (B).—Colombia: Risaralda: Puerto Caldas, 1985, Wolf 407 (B).—DR Congo: Mongala Province: Lisala, 2009, Ertz 14181 (BR).—Gabon: 2006, Ertz 9715 (BR).—Sierra Leone: Southern Province: Njala, 1954, Deighton 5843 (BM).—China: Yunnan: Xishuangbanna, Menglun, 2002, Aptroot 57272 (ABL).—Singapore: University campus, 2000, Sipman 45605 (B).—Indonesia: Java: Mt. Parangklakah, 1937, Groenhart 5830 (ABL, L); Malang, 1932, Groenhart 356 (ABL, L); Bogor, Djalan Bamtam, 1941, Groenhart 3142 (ABL, L).—Australia: Queensland: Cairns, near Centenary Lakes, 1988, A. & M. Aptroot 22229 (ABL).
Trypethelium plicatorimosum Makhija & Patw.
J. Hattori Bot. Lab. 73: 205 (1993) [as ‘plicato-rimosum’]; type: India, Maharashtra, Amboli, 1975, Patwardhan & Prabhu AMH 75.528 (ABL!—isotype).
Trypethelium rubrocinctum Makhija & Patw., J. Hattori Bot. Lab. 73: 206 (1993); type: India, Assam, Gauhati-Shilong road, near Burhatti, 1977, Patwardhan & Nagarkar AMH 77.704 (ABL!—isotype).
(Fig. 57F)
Thallus corticate, olive-green to yellowish, verrucose-rugulose.
Ascomata trypethelioid, with apical ostioles, diffusely pseudostromatic; pseudostromata with few ascomata, 0·5–1·0 mm broad, erumpent, basally covered by thallus but upper part exposed, brown-black, flattened and disc-shaped. Hamathecium clear. Ascospores 8 per ascus, 12–17-septate, fusiform, 45–55×6–8 μm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Palaeotropical (previously reported only from India,).
New country record. Zaire: Kivu: Pinga, 1100 m, 1991, Müller s. n. (DR).
Trypethelium regnellii Malme
Ark. Bot. 19(1): 25 (1924); type: Paraguay, Colonia Risso, Malme 1875 (S!—holotype).
Trypethelium globolucidum Aptroot et al., Lichenologist 46: 100 (2014); type: Argentina, Prov. Misiones, Iguazú National Park, along railroad, 200 m, 2013, Ferraro et al. 10630 (CTES!—holotype; ABL!—isotype).
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata with mostly few ascomata, 0·7–1·3 mm diam., erumpent to prominent, covered by thallus except brown ostiolar areas surrounded by cream-coloured to whitish rim; pseudostromata 1–3 mm diam., individual ascomata 0·4–0·8 mm diam. Hamathecium inspersed. Ascospores 8 per ascus, 13–19-septate, fusiform, (65–)83–97×11–15 μm, with slight constriction at the median septum, hyaline, IKI−.
Chemistry. Thallus UV−, K−; whitish parts of pseudostromata surrounding the ostioles UV+ yellow, K−. TLC: lichexanthone.
Distribution. Neotropical (Argentina, Paraguay and Brazil).
Discussion. The type specimens of Trypethelium regnellii and T. globolucidum look superficially different, but the numerous collections from Brazil show all intermediate stages.
Trypethelium sphaerocephalum (Vain.) Zahlbr.
Catal. Lich. Univ. 1: 500 (1922).—Pseudopyrenula sphaerocephala Vain., in Hiern, Cat. Afr. Pl. 2(2): 456 (1901); type: Angola, Luanda, Welwitsch 191 (TUR-Vain 30790!—holotype; BM!—isotype).
Trypethelium platystomum f. leucostomum Nyl., Flora 69: 178 (1886).—Trypethelium anomalum f. leucostomum (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 487 (1922).—Trypethelium leucostomum (Nyl.) C. W. Dodge, Ann. Missouri Bot. Gard. 40: 292 (1953) non Kremp. (1875).—Trypethelium platyleucostomum Makhija & Patw., Int. J. Mycol. Lichenol. 5: 243 (1992); type: São Tomé and Príncipe, Bom Puccesso, Müller s. n. (H-Nyl 254!—holotype).
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata with few ascomata, 0·6–1·3 mm diam., sessile, exposed and laterally grey-black, apically black, with ostiolar areas forming broad, strongly contrasting, white spots. Hamathecium inspersed. Ascospores 8 per ascus, 13–19-septate, fusiform, 80–115×14–18 μm, hyaline, IKI−.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. African palaeotropical (Uganda, São Tomé and Príncipe).
Discussion. This species is rather similar to the neotropical Trypethelium ornatum but differs in the sessile pseudostromata with strongly contrasting ostiolar spots and the larger ascospores.
Trypethelium subeluteriae Makhija & Patw.
Int. J. Mycol. Lichenol. 5: 245 (1992); type: Sri Lanka, Peradenyia, Thwaites 184, “2” (BM—holotype).
Trypethelium eluteriae var. polystomum Malme, Ark. Bot. 19(1): 25 (1924) [incorrectly given as ‘platystomum’ in Zahlbruckner’s Catalogus]; type: Brazil, Matto Grosso, Buriti, 1894, Malme 16 vi 1894 (S!—lectotype, designated here).
Trypethelium ceylonicum Makhija & Patw., Int. J. Mycol. Lichenol. 5: 239 (1992); type: Sri Lanka, Peradenyia, Thwaites 184, “3” (BM!—holotype).
Thallus olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, distinctly pseudostromatic; pseudostromata with numerous ascomata, 1–2 mm diam., prominent to sessile, brownish but covered by orange pigment except for dark ostioles. Hamathecium clear. Ascospores 8 per ascus, 11–19-septate, fusiform, 55–75×11–12 μm, hyaline, IKI−.
Chemistry. Thallus UV−, K−; pseudostromata UV+ red, K+ purple, with orange anthraquinone.
Distribution. Pantropical (USA, Costa Rica, Cuba, Puerto Rico, Venezuela, Guyana, French Guiana, Brazil, Paraguay, Bolivia, Argentina, South Africa, India, Sri Lanka, Malaysia, Philippines, New Caledonia, Papua New Guinea and Australia, often reported as ‘aff. eluteriae’).
Viridothelium Lücking et al.
In Lücking et al., Lichenologist 48 (in press); type: Viridothelium virens (Tuck. ex Michener) Lücking et al. (holotype).
Thallus corticate, often warted.
Ascomata simple or aggregated in pseudostromata, which can be hardly to clearly raised and are usually not of a different structure and colour to the thallus. Ostioles apical or eccentric, simple or fused. Wall hyphal (textura intricata), usually carbonized. Hamathecium clear or inspersed with oil droplets, filaments thin, anastomosing paraphysoids. Ascospores distoseptate, hyaline, transversely septate.
Pycnidia occasionally present.
Chemistry. No secondary substances known except anthraquinones in one species (V. leptoseptatum).
Discussion. The newly established genus Viridothelium is a phylogenetically distinctive clade (Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ), but combines features of the genera Astrothelium (general morphology) and Trypethelium (ascospores). Species can hence be readily assigned to Viridothelium if they have Trypethelium-type ascospores (i.e. with rather thin but numerous septa) but their ascomata are not distinctly pseudostromatic and raised, and the thallus is more or less greenish when fresh. The placement of several taxa, such as V. leptoseptatum, is tentative and requires molecular data. An interesting situation arises with Trypethelium inaequale (including its current synonym, T. inamoenum); this taxon is very similar to Viridothelium virens in most aspects but a sequenced specimen clusters within Trypethelium, whereas a morphologically similar specimen of V. virens from North America defines the Viridothelium clade (Lücking et al. 2016 Reference Lücking, Nelsen, Aptroot, Barillas de Klee, Bawingan, Benatti, Binh, Bungartz, Cáceres and Canêza ). This rather striking example of homoplasy is comparable to what has been found in Graphidaceae, with phenotypically similar taxa belonging to often unrelated clades (Rivas Plata & Lumbsch Reference Rivas Plata and Lumbsch2011).

Viridothelium cinereoglaucescens (Vain.) Lücking, M. P. Nelsen & Aptroot comb. nov.
MycoBank No.: MB 817024
Pseudopyrenula cinereoglaucescens Vain., Bot. Mag. Tokyo 35: 76 (1912).—Trypethelium cinereoglaucescens (Vain.) R. C. Harris, Lichenogr. Thomsoniana: 141 (1998); type: Japan, Kozuke, Yasuda 197 (TUR 30813!—holotype).
(Fig. 59L)
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·4–0·6 mm diam., immersed-erumpent, completely covered by thallus. Hamathecium clear. Ascospores 8 per ascus, (5–)7–11-septate, fusiform, 50–80×8–15 μm, with rather thin septa, hyaline, IKI+ weakly violet.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. Subtropical to temperate Asia (reported from India, Japan, South Korea).
Viridothelium indutum (Stirt.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 817025
Trypethelium indutum Stirt., Proc. Philosph. Soc. Glasgow 13: 193 (1881); type: India, Assam, Tejpore, Watt s. n. (BM!—isolectotype, Makhija & Patwardhan, J. Hattori Bot. Lab. 73: 199, 1993).
Trypethelium deforme Makhija & Patw., Int. J. Mycol. Lichenol. 5: 240 (1992) non Fée [as deformis]; type: Singapore, Maingay 165 (BM!—holotype).
(Fig. 59G)
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, solitary to irregularly confluent, 0·2–0·4 mm diam., erumpent, laterally covered by thallus but upper part exposed, brown-black. Hamathecium clear. Ascospores 8 per ascus, (9–)13–17-septate, fusiform, 90–105×12–16 μm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−. TLC: no substances detected.
Distribution. Palaeotropical (previously reported from India, Singapore and Australia). Reports of Trypethelium elmeri from Australia in Aptroot (2009 Reference Aptroota ) also belong here.
New country records. Indonesia: Sulawesi: Tondano, 1000 m, 1988, Hensen s. n. (ABL).—Philippines: Sorsogon: Bulusan Volcano National Park, 1994, Diederich 13131 (hb. Diederich).—Papua New Guinea: Central: Varirata National Park, 1987, Aptroot 19140, 19147, 1987 (ABL).
Viridothelium megaspermum (Makhija & Patw.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 817027
Pleurotrema megaspermum Makhija & Patw., Biovigyanam 16: 24 (1990), non Trypethelium megaspermum Mont. (1843).— Trypethelium karnatakense R. C. Harris, More Florida Lichens: 147 (1995); type: India, Karnataka, Agumbe, 1974, Kulkarni AMH 74.2991 (ABL!—isotype).
(Fig. 59C)
Thallus corticate, olive-green to yellowish brown, smooth to uneven.
Ascomata trypethelioid, with mostly eccentric ostioles, solitary, 0·4–0·6 mm diam., deeply (c. 2 mm) immersed in the bark, covered by thallus except for black ostioles. Hamathecium clear. Ascospores 4 per ascus, (9–)15–17-septate, fusiform, 99–152×26–33 μm, hyaline, IKI−.
Chemistry. Thallus and ascomata UV−, K−; reported to be K+ orange, but no substances detected.
Distribution. Eastern palaeotropical (India).
Discussion. The hamathecium of this species was erroneously reported as heavily inspersed in the protologue, and the thallus as K+ orange (Makhija & Patwardhan Reference Makhija and Patwardhan1990). The original epithet megaspermum can be used in the genus Viridothelium.
Viridothelium virens (Tuck. ex Michener) Lücking et al.
In Lücking et al., Lichenologist 48 (in press); Trypethelium virens Tuck. ex Michener, W. Dard. Fl. Cest. ed. 3: 453 (1853).—Trypethelium eluteriae var. virens (Tuck. ex Michener) Trevis., Flora 44: 20 (1861); type: USA, Arkansas, Dardanelle, 1853, Michener s. n. (FH—holotype, not seen; M!—isotype).
Trypethelium exocanthum Tuck. in Nyl., Ann. Sci. Nat., Bot., sér. 4 20: 258 (1863); type: USA, Louisiana, Hale s. n. (H-Nyl!—holotype).
Trypethelium scorites Tuck. in Nyl., Ann. Sci. Nat., Bot., sér. 4 20: 259 (1863); type: USA, Mississippi, s.col. (H-Nyl—holotype, not seen).
Verrucaria concatervata Nyl., J. Linn. Soc. London, Bot. 20: 68 (1883).—Pseudopyrenula concatervata (Nyl.) Vain., Bot. Mag. Tokyo 35: 76 (1921).—Trypethelium concatervatum (Nyl.) Zahlbr., Catal. Lich. Univ. 1: 489 (1922); type: Japan, Yokohama, Maingay s. n. (BM!—isotype).
Thallus corticate, olive-green to yellowish, smooth to uneven.
Ascomata trypethelioid, with apical ostioles, pseudostromatic; pseudostromata 0·6–1·5 mm diam., immersed-erumpent, with the upper parts of individual ascomata exposed and dark brown. Hamathecium clear. Ascospores 8 per ascus, 7–11-septate, fusiform, 38–52×7–10 μm, with rather thin septa, hyaline, IKI+ weakly violet.
Chemistry. Thallus and pseudostromata UV−, K−. TLC: no substances detected.
Distribution. North America and temperate East Asia (USA and Japan).
Taxa newly excluded from the Trypetheliaceae
Phyllobathelium chlorogastricum (Müll. Arg.) Aptroot & Lücking comb. nov.
MycoBank No.: MB 816792
Heufleria chlorogastrica Müll. Arg., Flora 66: 243 (1883).—Cryptothelium chlorogastricum (Müll. Arg.) Zahlbr., Cat. Lich. Univ. 1: 521 (1922).—Campylothelium chlorogastricum (Müll. Arg.) Aptroot in Aptroot et al., Biblioth. Lichenol. 98: 52 (2008); type: Brazil, Apiahy, Puiggari 1079 (G!—holotype).
Thelenella thaxteri Vain., Proc. Am. Acad. Arts Sci. 58: 144 (1923).—Phyllobathelium thaxteri (Vain.) Zahlbr., Catalogus Lichenum Universalis 8: 142 (1931); type: Trinidad and Tobago, Trinidad, Port of Spain, La Seiva Valley, Thaxter 46 (TUR! —holotype).
Thelenella thaxteri var. heterogena Vain., Proc. Am. Acad. Arts Sci. 58: 144 (1923); type: Trinidad and Tobago, Trinidad, Port of Spain, La Seiva Valley, Thaxter 66 (TUR!—holotype).
Thelenella megapotamica Malme, Arkiv för Botanik 22A: 7 (1928).—Phyllobathelium megapotamicum (Malme) R. Sant., Symb. Bot. Upsal. 12(1): 290 (1952); type: Brazil, Rio Grande do Sul, Silveira Martins, Malme 1190 (S!—lectotype fide Santesson 1952: 290; UPS!—isolectotype).
Discussion. The type material of Heufleria chlorogastrica is a typical representative of Phyllobathelium and the ascospore measurements (50–65×23–30 μm) fit those of Thelenella thaxteri well; hence this is an older name for the taxon long known as Phyllobathelium thaxteri (Lücking Reference Lücking2008).
Laurera monospora Breuss in Breuss & Brunnbauer, Ann. Naturhist. Mus. Wien, Ser. B 99: 730 (1997); type: Sri Lanka, Sinharaje Forest, 1984, Brunnbauer s. n. (LI—isotype).
Discussion. This is a new synonym of Ocellularia massalongoi (Mont.) Hale, with columella, K+ red thallus, unbranched paraphyses and ascospores c. 300×45 μm.
Laurera subsphaerioides Makhija & Patw., Mycotaxon 31: 582 (1988); type: India, Andaman Islands, South Andaman, Wimberliganj, Patwardhan et al. AMH 85.168 (ABL, BM—isotypes).
Discussion. The isotypes contain a sterile Astrothelium and a fertile Ocellularia from which the ascospore and hamathecium characters in the protologue were derived, so an application of this name to any species within Trypetheliaceae is not possible.
Trypethelium deustum G. Mey., Syst. Veg., Ed. 16, 4(1): 326 (1827); type: Brazil, Serra dos Orgaos (L—isotype).
Discussion. A saprobic fungus, cf. Botryosphaeria sp.: stromatic, ascospores hyaline, simple, c. 15×7 μm.
Trypethelium favulosum Ach., Mém. Soc. Imp. Nat. Moscou 5: 166 (1817).
Discussion. The type is a mixture of Pyrenula anomala (Ach.) Vain. and Glyphis cicatricosa Ach.
Trypethelium lageniferum Ach., Synops. Lich.: 105 (1814).—Plagiotrema lageniferum (Ach.) Müll. Arg., Flora 68: 251 (1885); type: (BM-Ach!—isotype).
Discussion. The type consists of two specimens, one is Pyrenula adacta Fée (P. marginatula Müll. Arg.), for which this would be an older epithet, but the description of the spores probably originates from the second specimen, which is now sterile.
Trypethelium melanothrix Eschw. (1833).—Phyllothelium melanothrix (Eschw.) Trevis. (1861); type: Brazil (type lost).
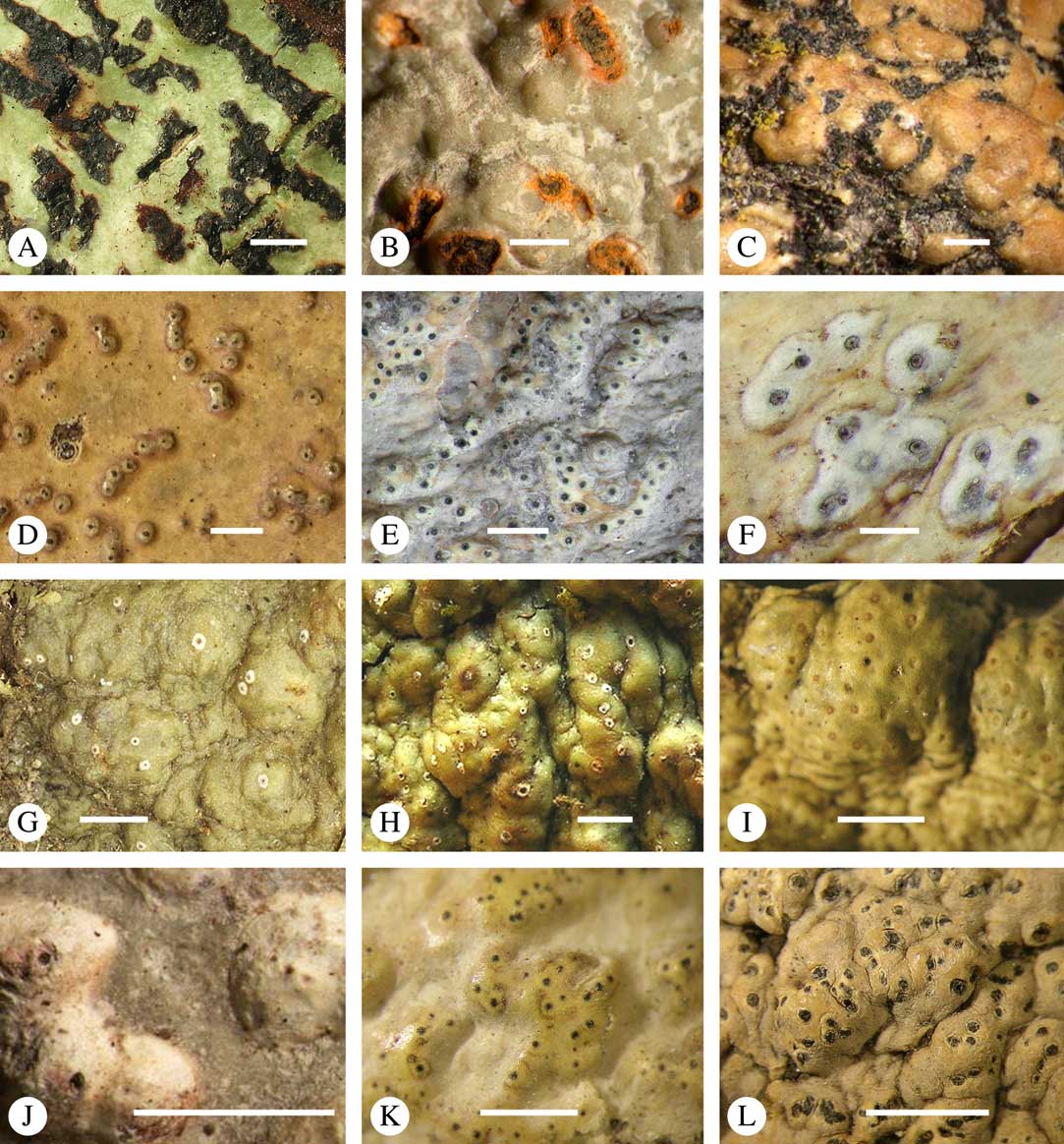
Fig. 12 Habitus of Astrothelium species. A & B, A. neogalbineum (A, Costa Rica, Lücking 16358a; B, Brazil, Cáceres & Aptroot 11436); C, A. pictum (Brazil, holotype); D & E, A. cinereorosellum (D, Indonesia, Borneo, lectotype of Verrucaria antoniae; E, India, isotype of Trypethelium flavocinereum); F, A. fijiense (Fiji, holotype); G & H, A. punctulatum (Brazil, isotype); I, A. grossoides (Papua New Guinea, isotype of Trypethelium grossum); J, A. vulcanum (Guyana, holotype); K & L, A. porosum (K, ‘West Indies’, isotype; L, Brazil, lectotype of Trypethelium brachysporum). Scales=1 mm.

Fig. 13 Habitus of Astrothelium species. A, A. stromatofluorescens (Brazil, holotype); B–D, A. pulcherrimum (B, ‘America’, lectotype; C, Costa Rica, holotype of Trypethelium tricolor; D, Colombia, lectotype of Verrucaria diffluens); E, A. leucosessile (Panama, holotype); F–L, A. phlyctaena (F, St. Lucia, lectotype; G, Brazil, lectotype of Verrucaria ochroleuca; H, Brazil, holotype of Trypethelium euporum; I, French Guiana, lectotype of T. leprieurii; J, Cuba, lectotype of T. leprosum; K, São Tomé and Príncipe, lectotype of T. subalbens; L, Brazil, Cáceres 2028). Scales=1 mm.

Fig. 14 Habitus of Astrothelium species. A & B, A. pupula (A, 'West Indies', holotype: B, French Guiana, lectotype of Pseudopyrenula porinoides); C, A. megochroleucum (El Salvador, holotype); D & E, A. thelotremoides (D, Colombia, lectotype; E, Brazil, van den Boom 1010); F, A. neoinspersum (El Salvador, holotype); G, A. inspersaeneum (Brazil, holotype); H, A. aenascens (Papua New Guinea, isotype); I, A. rubrocrystallinum (Brazil, holotype); J, A. buckii (Brazil, isotype); K, A. coccineum (Mexico, holotype); L, A. aurantiacocinereum (New Caledonia, holotype). Scales=1 mm.

Fig. 15 Habitus of Astrothelium species. A, A. flavum (Brazil, holotype); B–F, A. aeneum (B, French Guiana, isotype of Verrucaria heterochroa; C, Brazil, Cáceres 517; D, USA, Florida, Lücking 26814; E, Colombia, Moncada 3374; F, Costa Rica, Dodge 6486); G, A. flavostromatum (Brazil, holotype); H & I, A. kunzei (H, Surinam, holotype; I, El Salvador, Lücking 28137); J, A. megaeneum (Bolivia, holotype); K, A. pallidoflavum (Bolivia, holotype); L, A. subcatervarium (Brazil, holotype). Scales=1 mm.

Fig. 16 Habitus of Astrothelium species. A & B, A. endochryseum (A, Brazil, holotype; B, El Salvador, Lücking 28121); C & D, A. laevithallinum (Brazil, holotype); E, A. aeneoides (Brazil, holotype); F–I, A. degenerans (F, Dominica, holotype; G, Puerto Rico, Aptroot 25734; H & I, Costa Rica, Lücking 16657); J–L, A. feei (J, ‘South America’, holotype; K, Cuba, lectotype of Trypethelium mastoideum var. lacerum; L, USA, Florida, Nelsen s. n.). Scales=1 mm.
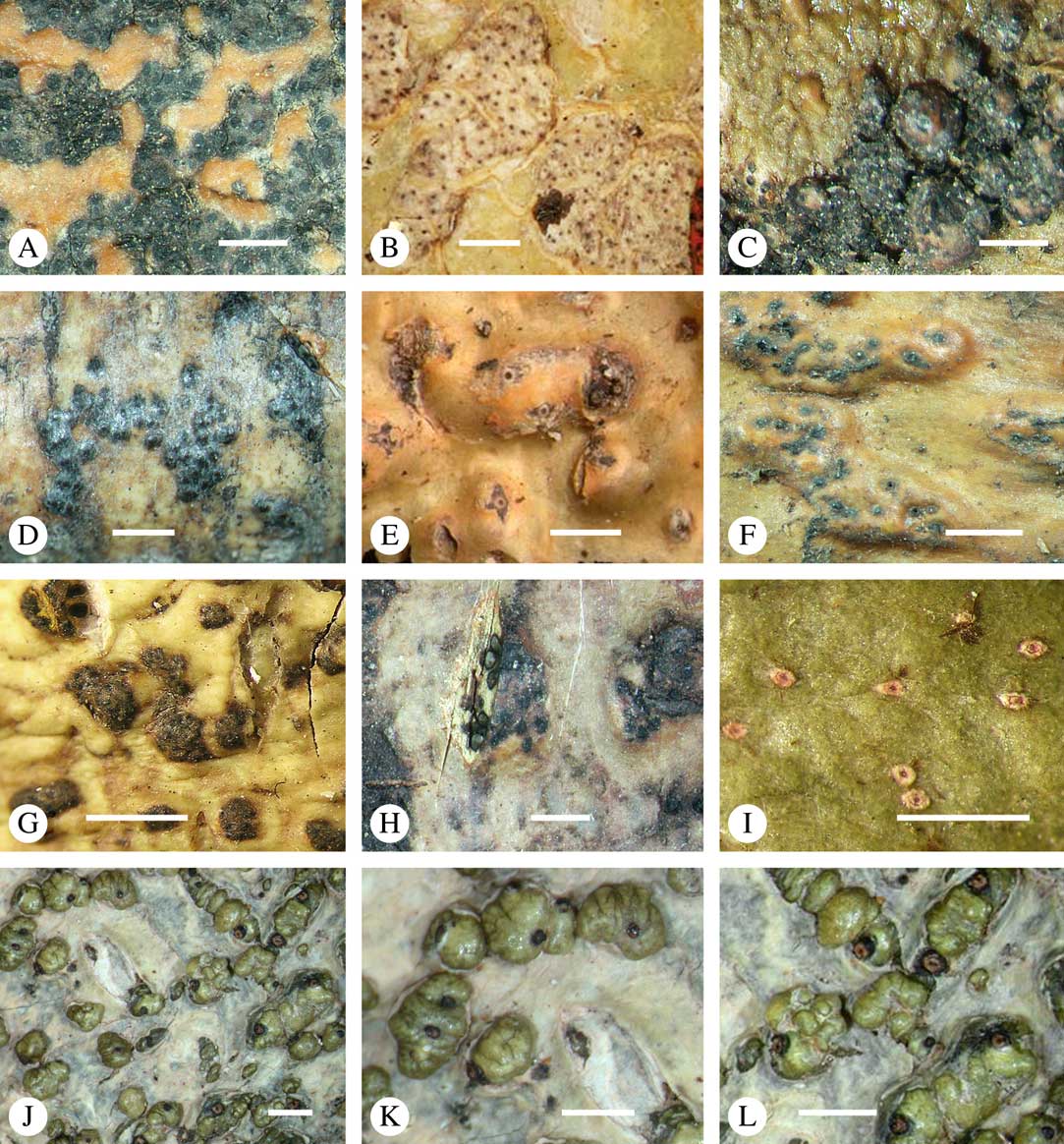
Fig. 17 Habitus of Astrothelium species. A, A. nigrorufum (India, isotype); B, A. pseudodissimulum (Papua New Guinea, holotype); C, A. dissimilum (India, isotype); D. A. aff. dissimilum (South Korea, Aptroot 67444); E, A. decemseptatum (Brazil, holotype); F–H, A. luridum (F, India, isotype of Trypethelium endosulphureum; G, Malaysia, Labuan, isotype of T. endochraceum; H, Japan, Kashiwadani 19781); I, A. calosporum (Cuba, holotype); J–L, A. rimosum (Guyana, holotype). Scales=1 mm.
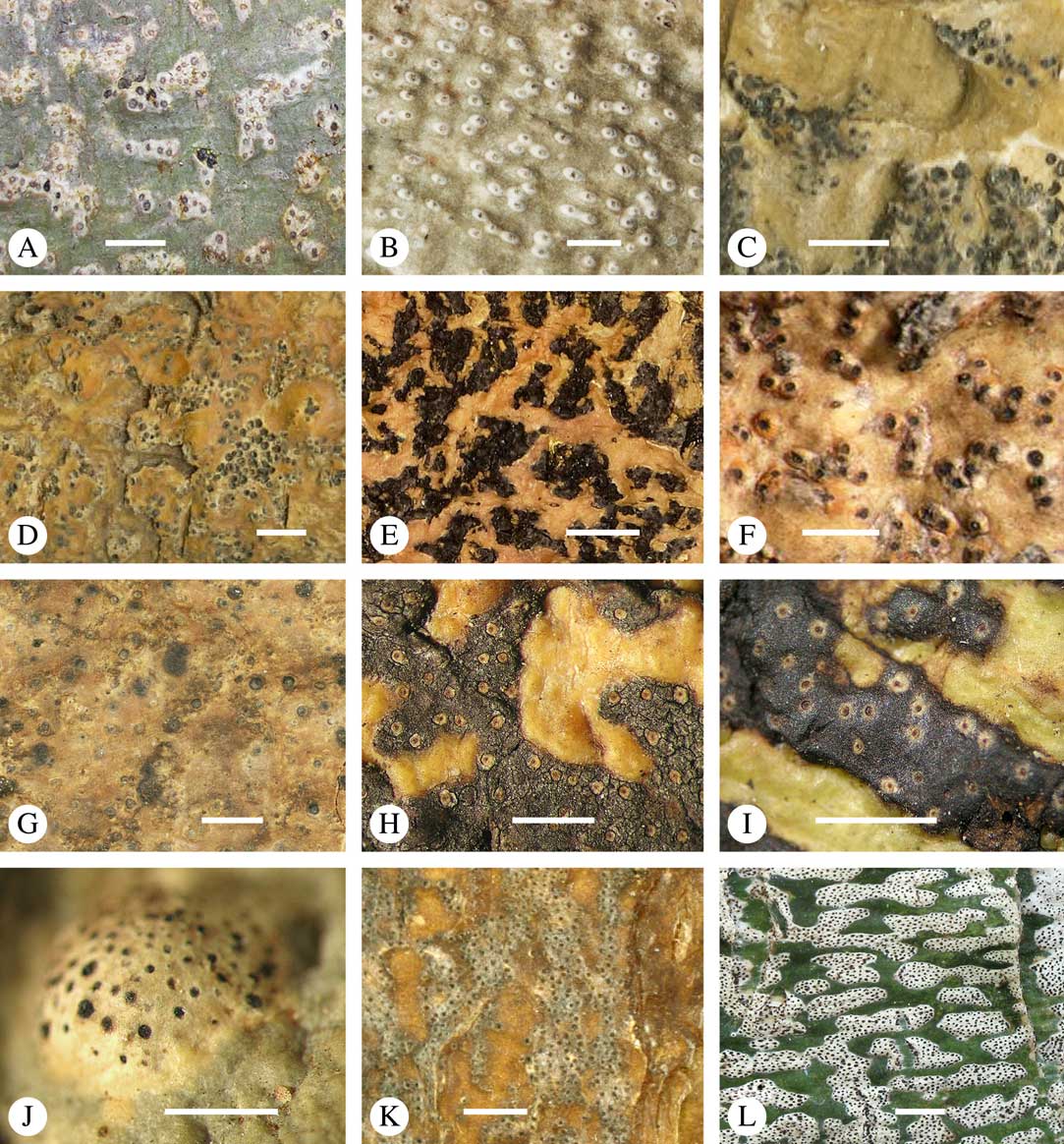
Fig. 18 Habitus of Astrothelium species. A, A. perspersum (Gabon, holotype); B, A. subscoria (Bolivia, holotype); C–E, A. scoria (C, ‘America’, lectotype; D, ‘South America’, holotype of Pyrenula myriocarpa; E, Brazil, isotype of Trypethelium scoria var. sordidius); F, A. aenascens (Papua New Guinea, holotype); G, A. infossum (São Tomé and Príncipe, holotype); H & I, A. infuscatulum (H, Cuba, lectotype; I, Venezuela, Buck 11586); J, A. bicolor (Cuba, lectotype of Trypethelium polychroum); K & L, A. rufescens (K, Cuba, lectotype; L, Peru, Lücking s. n.). Scales=1 mm.
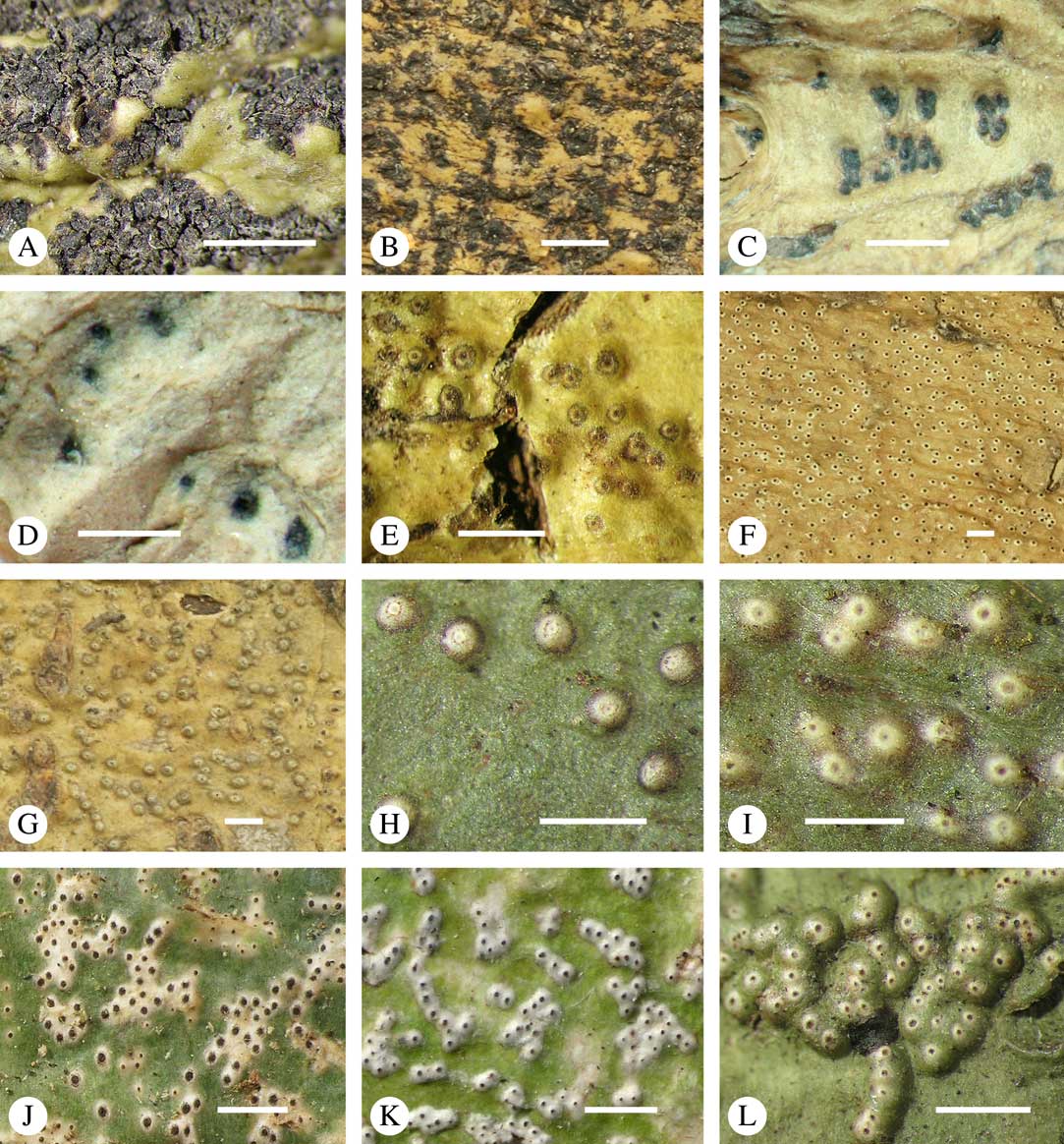
Fig. 19 Habitus of Astrothelium species. A, A. subendochryseum (Brazil, holotype); B, A. nigratum (Cuba, holotype); C & D, A. meiophorum (C, India, isotype of Trypethelium parvicarpum; D, India, isotype of T. flavoalbum); E, A. ceratinum (Peru, lectotype); F–L, A. nitidiusculum (F, Colombia, isotype; G, French Guiana, holotype of Pseudopyrenula neglecta; H, Brazil, Cáceres 438; I, Brazil, Cáceres 437; J, USA, Florida, Lücking 26821; K, USA, Florida, Lücking 26816; L, Brazil, Cáceres 178). Scales=1 mm.

Fig. 20 Habitus of Astrothelium species. A & B, A. floridanum (A, USA, Florida, Harris 30095; B, USA, Florida, Plitt s. n.); C, A. scoriothelium (Guadeloupe; holotype of Pseudopyrenula infuscatula var. tecomae); D, A. disjunctum (Brazil, holotype); E, A. papulosum (Colombia, lectotype); F, A. solitarium (Brazil, holotype); G, A. inspersotuberculosum (Bolivia, holotype); H, A. clypeatum (Vietnam, holotype); I, A. megatropicum (Guyana, holotype); J, A. phaeothelium (Colombia, holotype); K, A. pseudannulare (Ecuador, holotype); L, A. tuberculosum (Guadeloupe, holotype). Scales=1 mm.
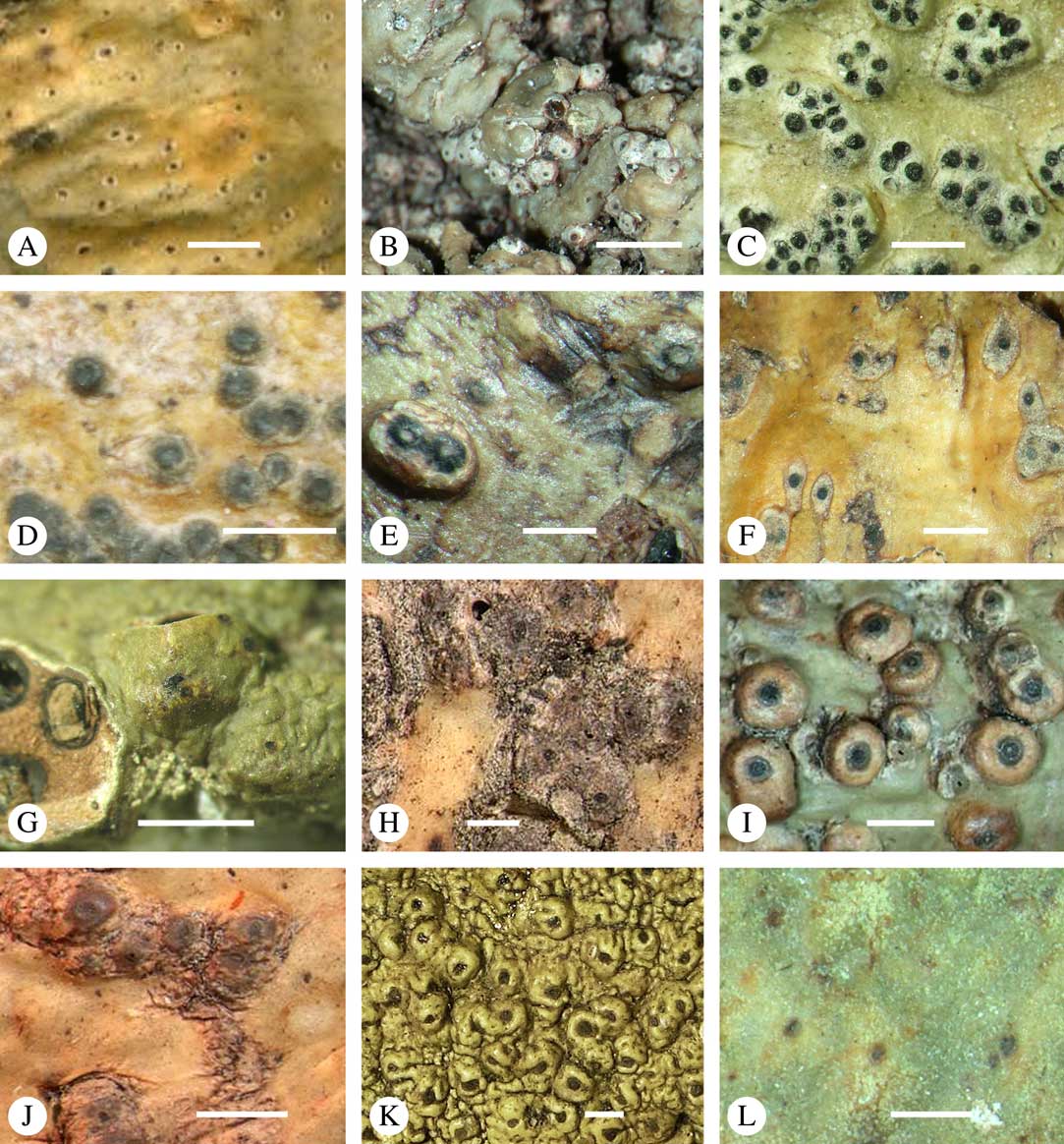
Fig. 21 Habitus of Astrothelium species. A, A. annulare (Colombia, holotype of Verrucaria myriomma); B, A. galligenum (Papua New Guinea, isotype); C, A. ubianense (Philippines, lectotype); D, A. trypethelizans (Malaysia, lectotype); E, A. nitidulum (Sri Lanka, holotype); F, A. macrosporum (India, isotype); G, A. pustulatum (Angola, holotype); H, A. curvisporum (Brazil, holotype); I, A. sipmanii (Guyana, holotype); J, A. pseudomegalophthalmum (Colombia, holotype); K, A. basilicum (Uruguay, holotype); L, A. spectabile (Argentina, isotype). Scales=1 mm.

Fig. 22 Habitus of Astrothelium species. A & B, A. megalophthalmum (Brazil, lectotype); C & D, A. olivaceofuscum (C, ‘South America’, holotype; D, Costa Rica, Aptroot 60454); E, A. dicoloratum (Venezuela, holotype); F, A. inspersogalbineum (Singapore, holotype); G–K, A. macrocarpum (G, Cuba, holotype of Trypethelium ochrothelizum; H, Brazil, lectotype of Astrothelium ochrothelioides; I, Brazil, lectotype of Trypethelium discolor; J, Panama, Mori 4897; K, Costa Rica, Lücking 16309c); L, A. flavocoronatum (Thailand, holotype). Scales=1 mm.

Fig. 23 Habitus of Astrothelium species. A & B, A. ochrothelium (Colombia, lectotype); C, A. versicolor (Puerto Rico, holotype); D, A. aureomaculatum (Brazil, holotype); E, A. grossum (New Caledonia, holotype); F, A. sordithecium (Brazil, holotype); G, A. leucothelium (Colombia, holotype); H & I, A. chrysostomum (H, Malaysia, Borneo, lectotype; I, Malaysia, Borneo, lectotype of Trypethelium leucostomum); J, A. neovariolosum (Thailand, holotype); K & L, A. laevigatum (K, Brazil, holotype; L, Brazil, holotype of Astrothelium simplicatum). Scales=1 mm.
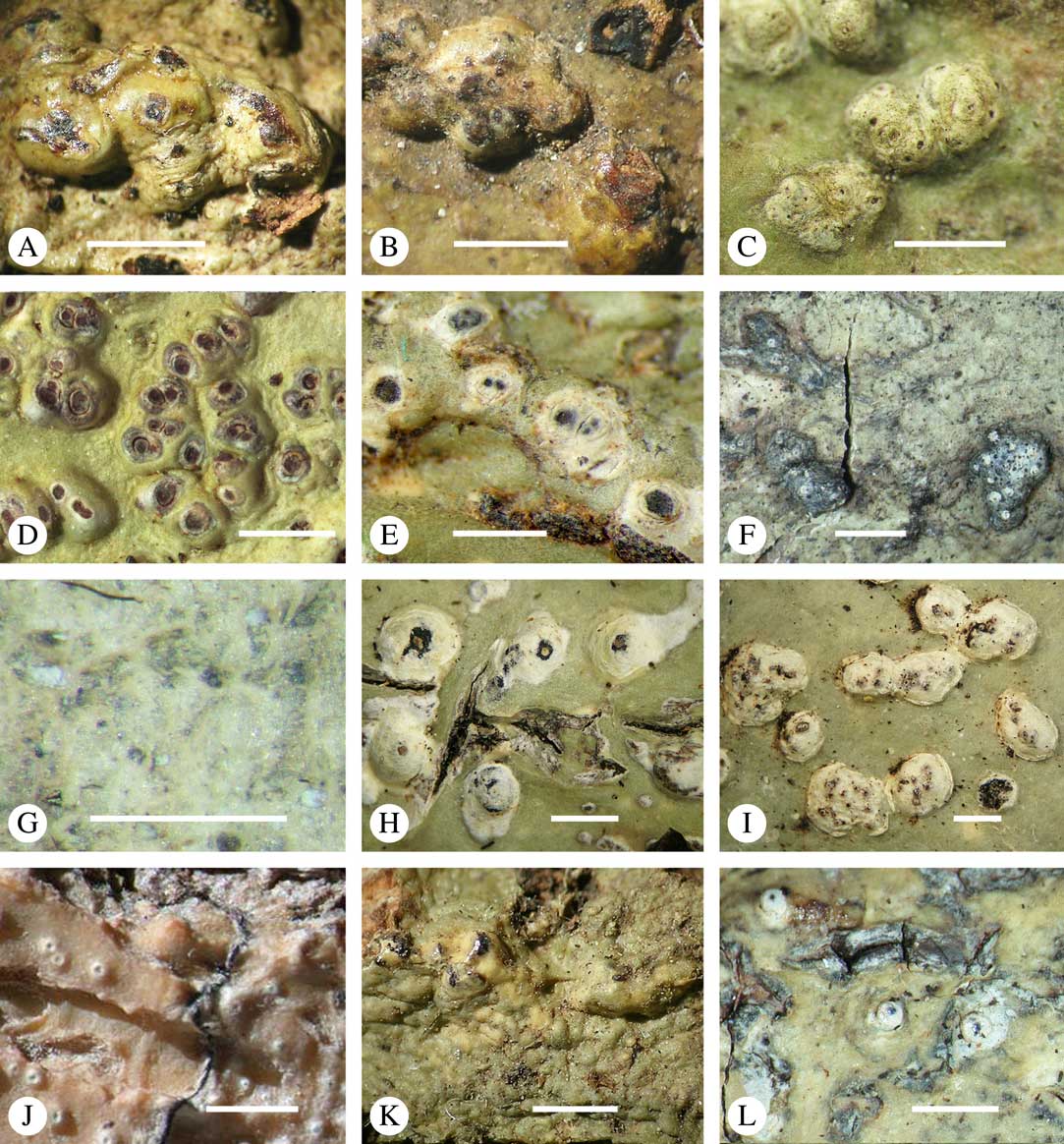
Fig. 24 Habitus of Astrothelium species. A–D, A. variolosum (A, unknown location, holotype; B, Guinea, holotype; C, Brazil, Cáceres 341; D, USA, Florida, Breuss 28962); E, A. subinterjectum (Brazil, holotype); F & G, A. pseudocyphellatum (Guyana, Sipman 40458); H & I, A. interjectum (H, Brazil, isotype; I, Brazil, Samuels 842); J, A. ultralucens (Venezuela, holotype); K & L, A. leucoconicum (K, Brazil, holotype; L, Venezuela, Sipman 27215). Scales=1 mm.
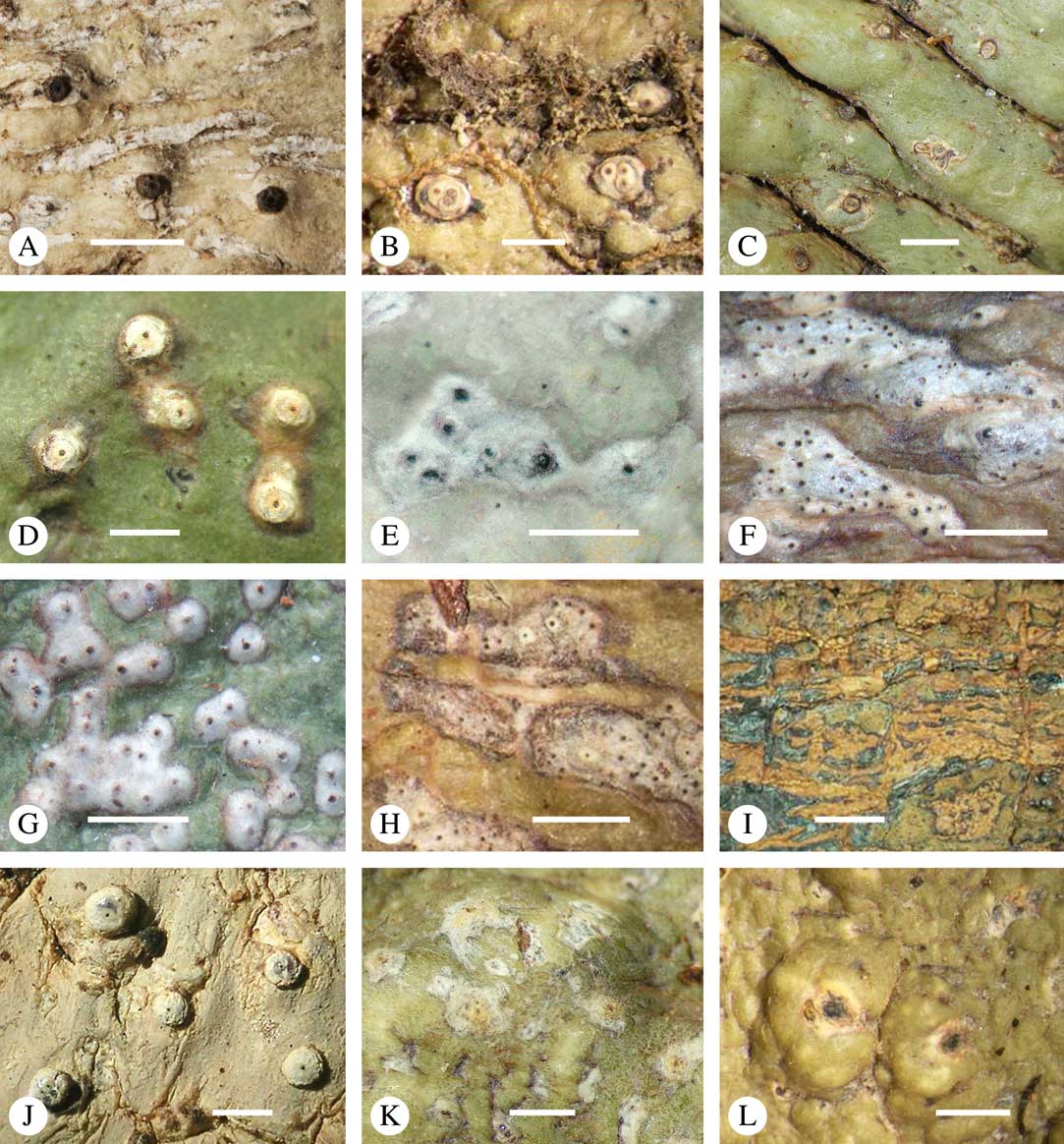
Fig. 25 Habitus of Astrothelium species. A, A. nigrocacuminum (Bolivia, holotype); B, A. sinuosum (Brazil, holotype); C, A. obtectum (Brazil, holotype); D, A. eustomum (Brazil, Cáceres 2076); E, A. neglectum (Thailand, holotype); F, A. sexloculatum (Guyana, holotype); G, A. siamense (Thailand, holotype); H, A. octoseptatum (Brazil, holotype); I, A. septemseptatum (Guyana, holotype); J, A. diplocarpoides (Cuba, holotype); K, A. macrostomoides (Brazil, holotype); L, A. macrostomum (Brazil, holotype). Scales=1 mm.
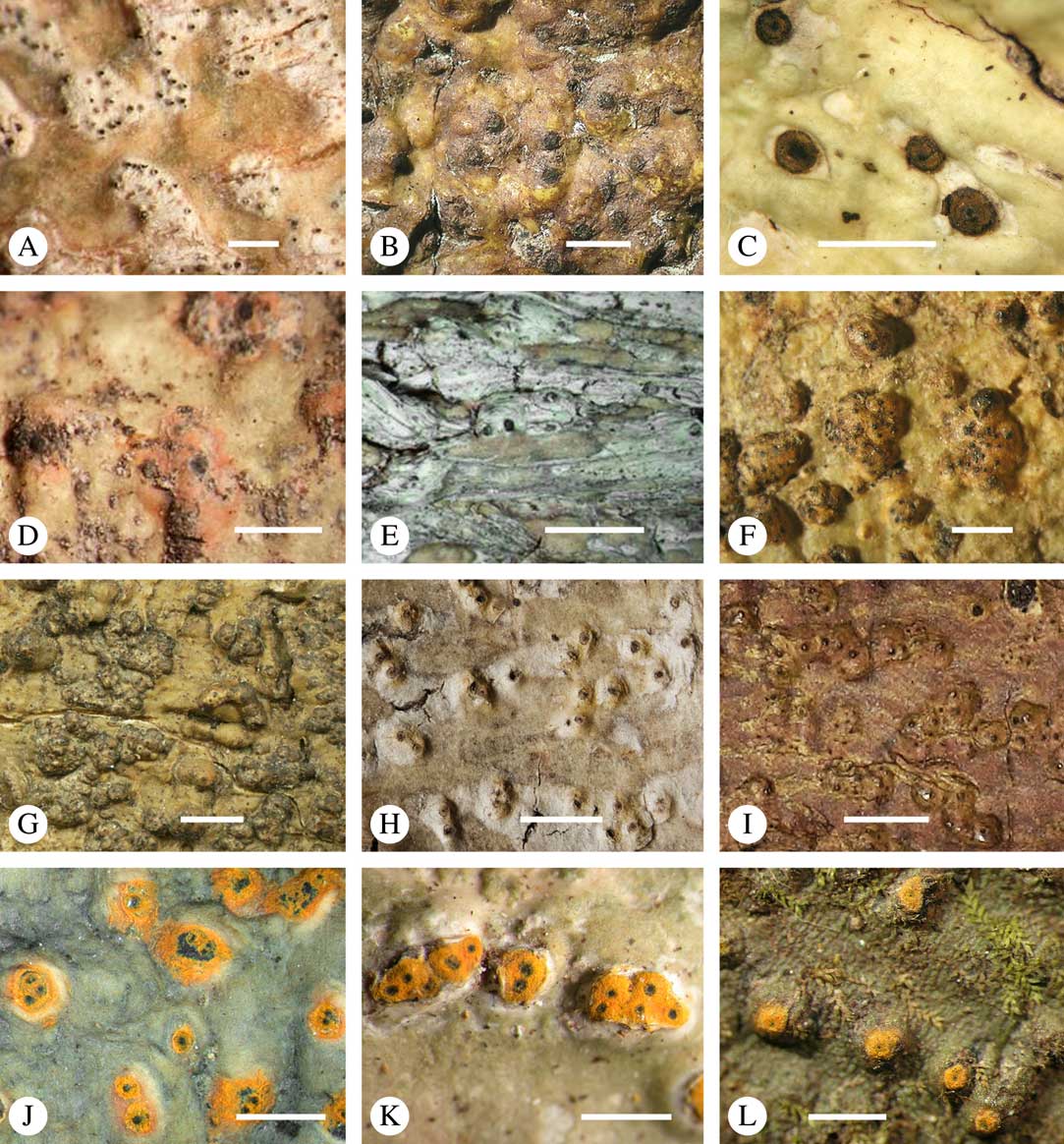
Fig. 26 Habitus of Astrothelium species. A, A. eumultiseptatum (Brazil, isotype); B & C, A. diplocarpum (B, Colombia, holotype; C, USA, Florida, Harris 29938); D, A. bivelum (Brazil, isotype); E, A. pseudoferrugineum (Indonesia, isotype); F & G, A. ferrugineum (F, isolectotype; G, lectotype of Trypethelium ferrugineum var. inornatum); H, A. pallidoflavum (Bolivia, holotype); I–L, A. cinnamomeum (I, Brazil, lectotype; J, Hong Kong, Aptroot 43277; K, Brazil, Cáceres & Aptroot 11061; L, Brazil, Cáceres 312). Scales=1 mm.
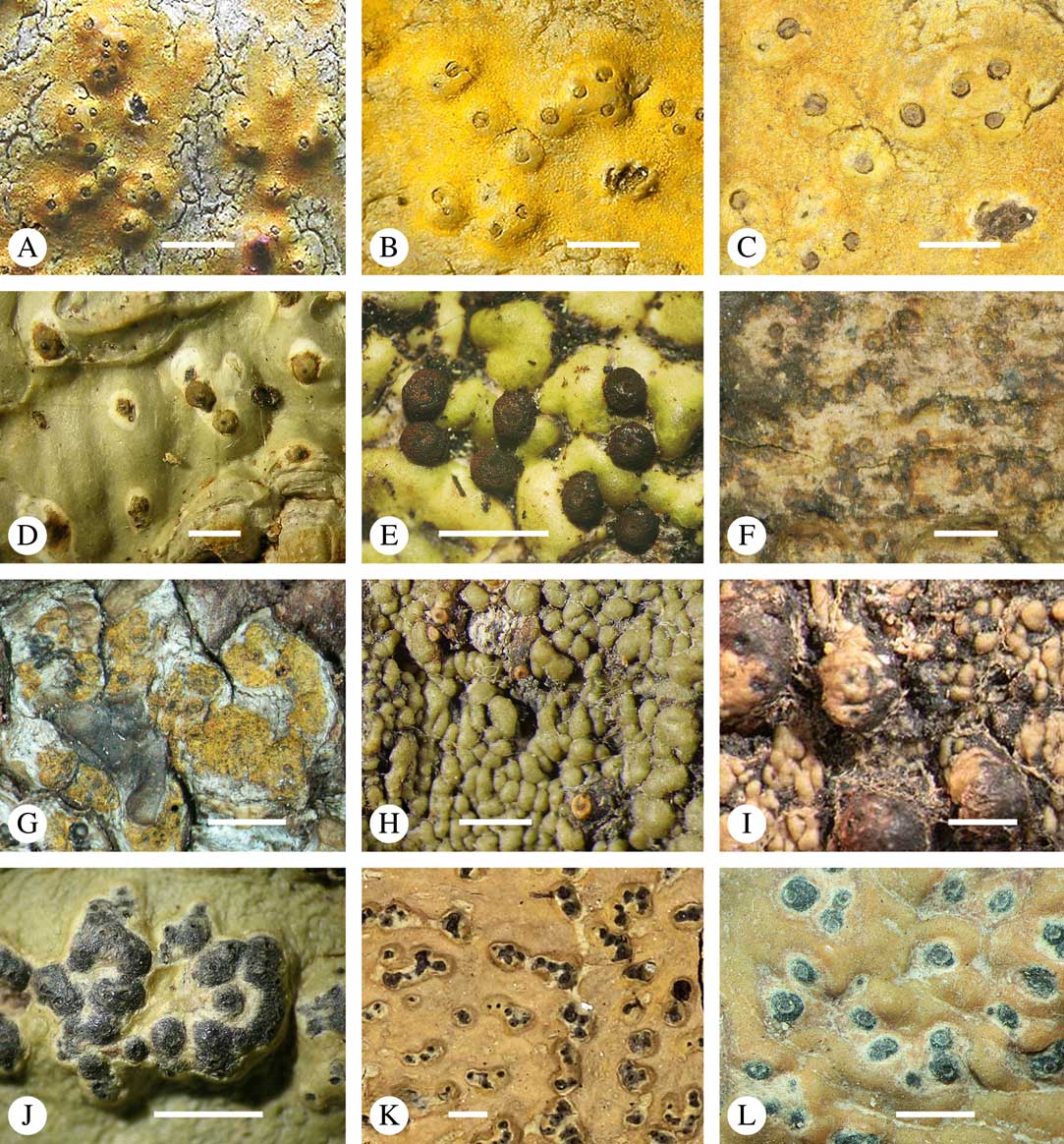
Fig. 27 Habitus of Astrothelium species. A–C, A. croceum (Brazil, holotype); D & E, A. ocellatum (D, Brazil, holotype; E, Costa Rica, Sipman 48120); F & G, A. scorioides (F, Colombia, holotype; G, French Guiana, Aptroot 15442); H, A. simplex (Brazil, isotype); I, A. pseudannulare (Ecuador, holotype); J–L, A. straminicolor (J, Malaysia, Labuan, lectotype; K, Malaysia, Borneo, lectotype of Trypethelium stramineum; L, India, isotype of Astrothelium subvariolosum). Scales=1 mm.

Fig. 28 Habitus of Astrothelium species. A, A. pyrenastrosulphureum (Brazil, holotype); B, A. acrophaeum (Cuba, lectotype); C & D, A. scorizum (C, Cuba, holotype; D, Brazil, Lücking 29814); E, A. peranceps (Malaysia, Borneo, lectotype); F, A. nigricans (Brazil, holotype); G, A. nigrum (Brazil, isotype); H & I, A. subfuscum (H, Singapore, holotype; I, Australia, Aptroot 22236c); J, A. obscurum (Brazil, holotype); K, A. oligocarpum (Australia, lectotype); L, A. subdissocians (Thailand, holotype). Scales=1 mm.
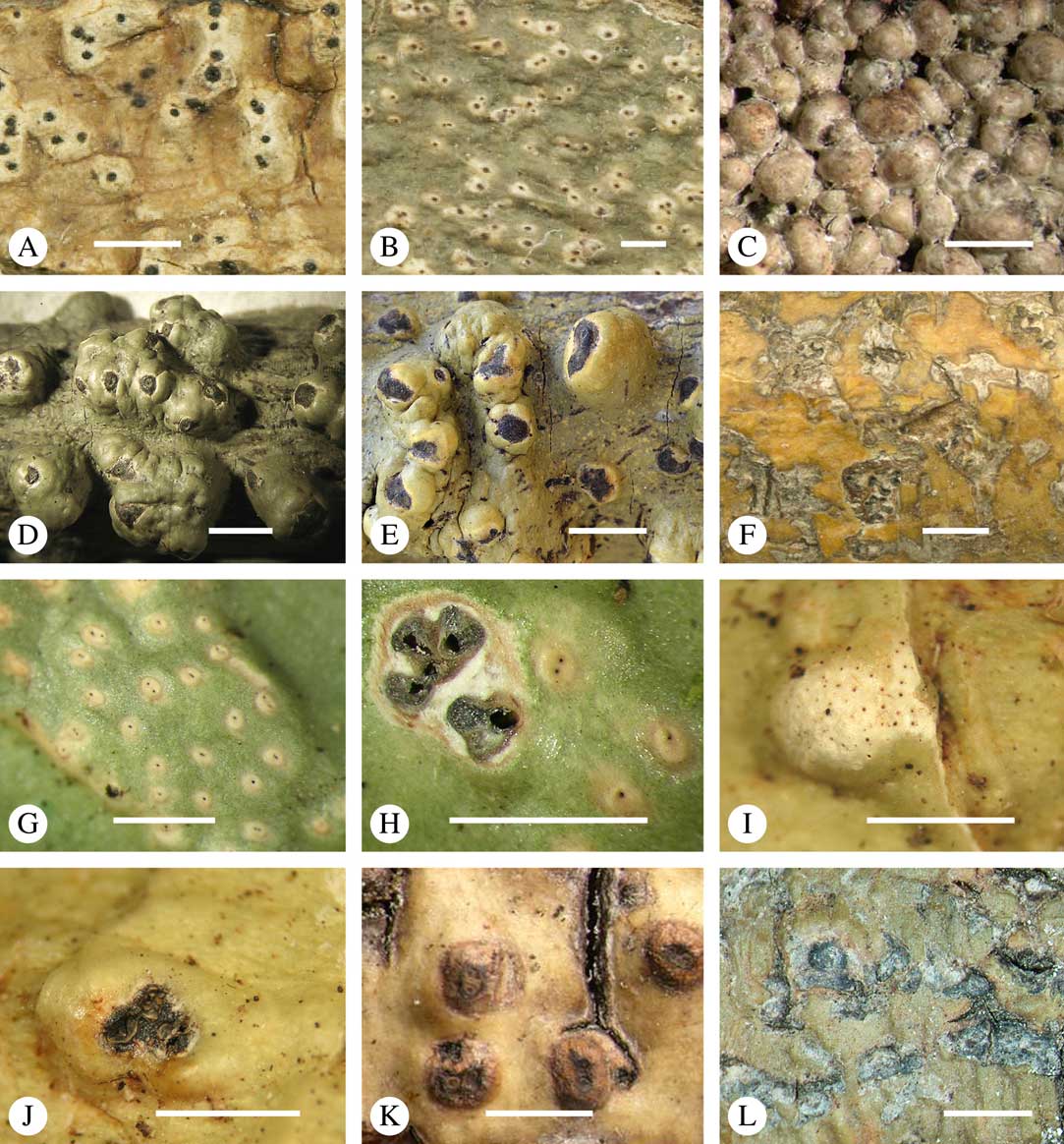
Fig. 29 Habitus of Astrothelium species. A & B, A. crassum (A, ‘South America’, lectotype; B, Colombia, holotype of Astrothelium confusum); C, A. globosum (Brazil, isotype); D & E, A. fallax (D, Sri Lanka, isolectotype; E, Sri Lanka, isolectotype); F, A. marcidum (‘South America’, holotype); G & H, A. intermedium (Costa Rica, holotype); I & J, A. pleiostomum (Brazil, holotype of Trypethelium violascens); K, A. trypethelioides (Venezuela, holotype); L, A. subclandestinum (Brazil, holotype). Scales=1 mm.
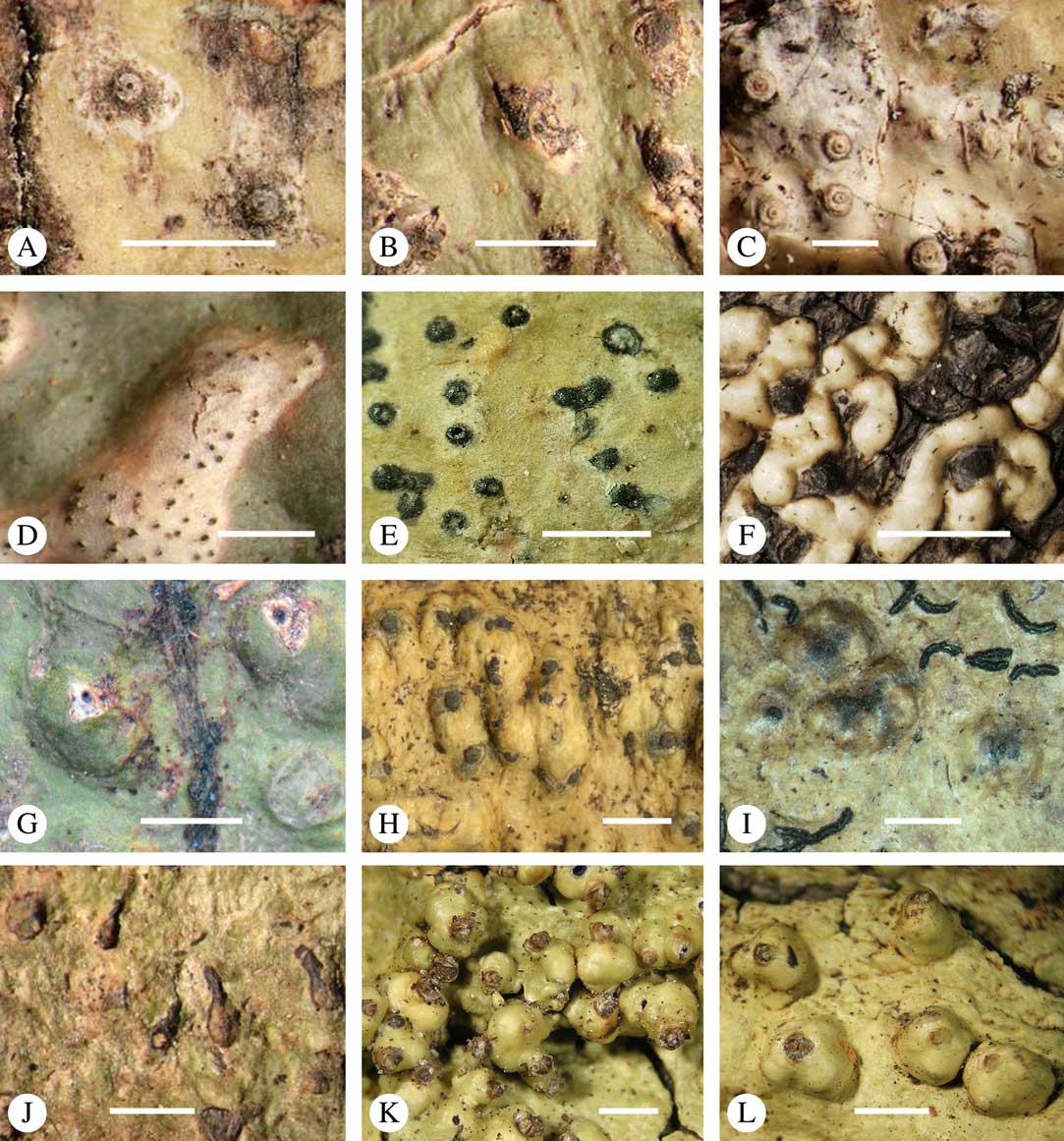
Fig. 30 Habitus of Astrothelium species. A, A. quatuorseptatum (Brazil, isotype); B, A. supraclandestinum (Brazil, isotype); C, A. zebrinum (Guyana, holotype); D, A. novemseptatum (Brazil, isotype); E, A. heterophorum (Philippines, holotype of Pseudopyrenula elmeri); F, A. neodiplocarpum (Bolivia, holotype); G, A. macrostiolatum (Thailand, holotype); H & I, A. robustum (H, Costa Rica, holotype; I, Colombia, Sipman & Velosa 33504); J, A. robustosporum (Brazil, isotype); K & L, A. gigasporum (Brazil, isotype). Scales=1 mm.
Discussion. Type and only species of Phyllothelium Trevis. (Trevisan Reference Trevisan1861). This is a foliose lichen with rhizines below and lichenicolous fungi above, following the description by Trevisan.
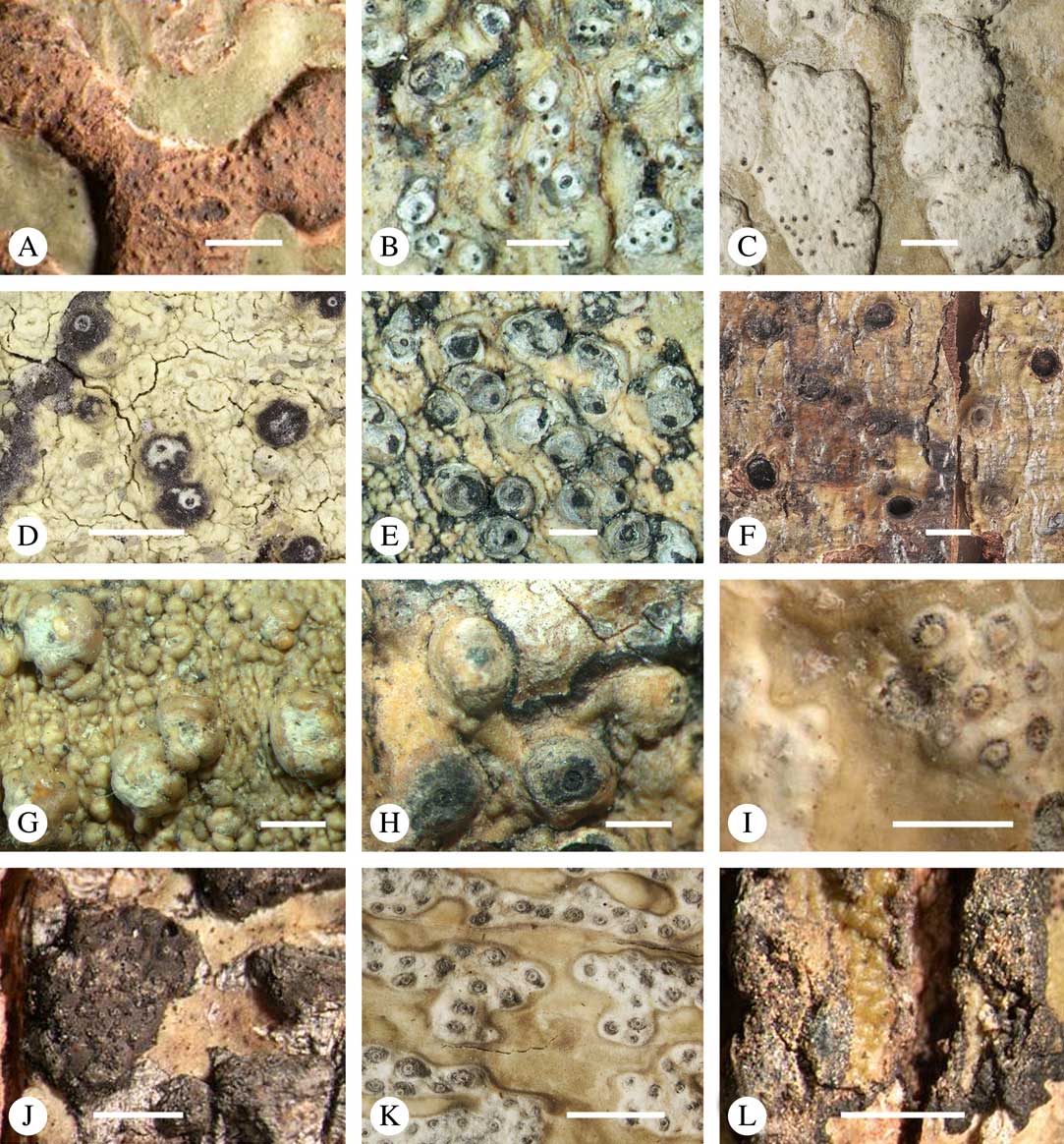
Fig. 31 Habitus of Astrothelium species. A, A. flavoduplex (Brazil, isotype); B, A. sanguineoxanthum (Brazil, holotype); C, A. elixii (Bolivia, holotype); D, A. stramineum (Brazil, holotype); E, A. meristosporoides (Papua New Guinea, Aptroot 19135); F–H, A. meristosporum (F, India, holotype of Verrucaria anoptella; G, India, isotype of Laurera columellata; H, India, isotype of L. indica); I, A. lucidothvallinum (Guyana, holotype); J, A. ochroleucoides (Brazil, isotype); K, A. variabile (Bolivia, holotype); L, A. xanthosuperbum (Brazil, isotype). Scales = 1 mm.
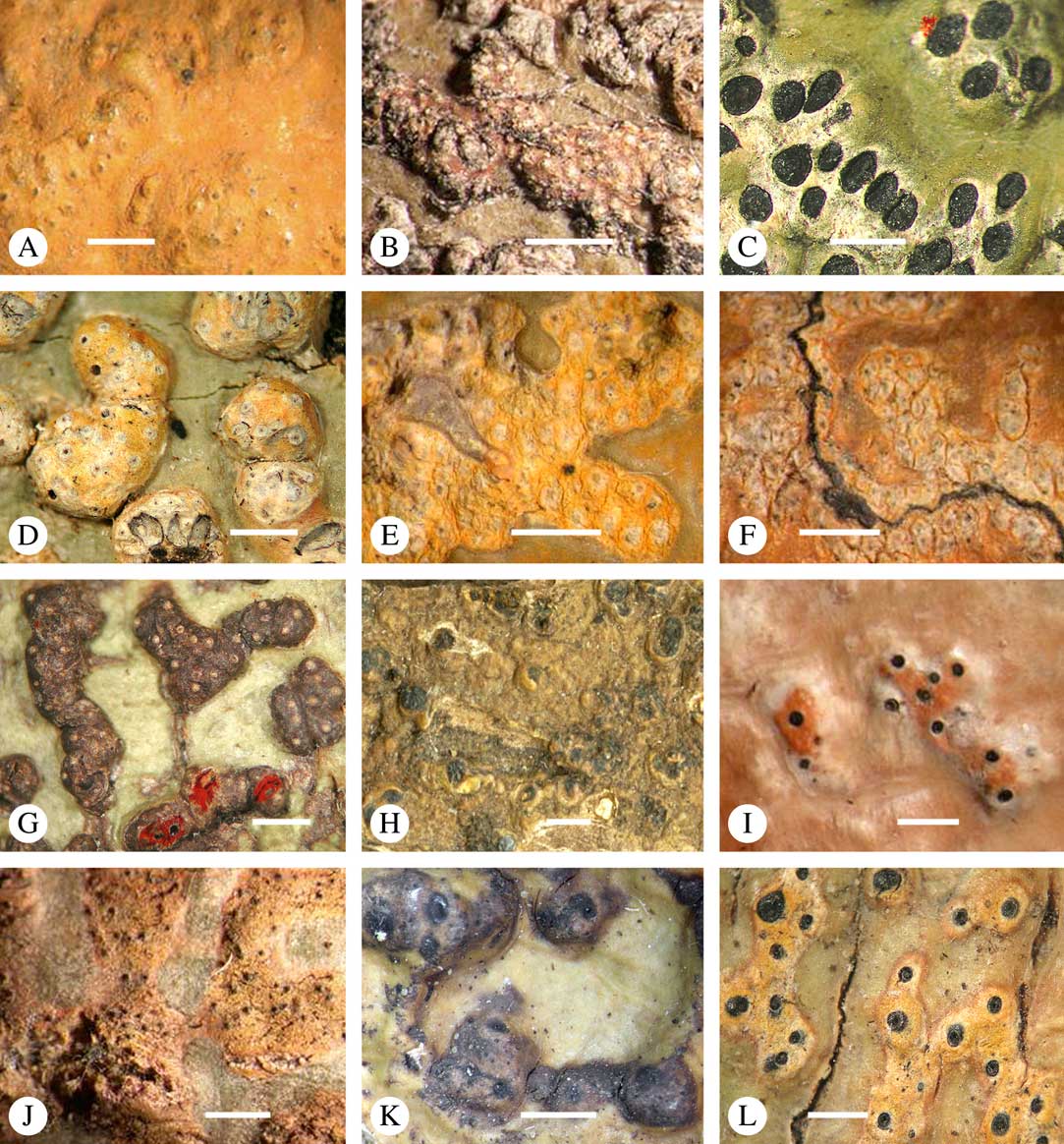
Fig. 32 Habitus of Astrothelium species. A, A. condoricum (Ecuador, holotype); B, A. duplicatum (Brazil, isotype); C, A. sierraleonense (Sierra Leone, holotype); D, A. auratum (Brazil, isotype); E, A. graphicum (Brazil, isotype); F, A. flavomaculatum (Ecuador, holotype); G, A. sanguinarium (Brazil, Brako 7136); H, A. deforme (Malaysia, Borneo, lectotype); I, A. laurerosphaerioides (Guyana, holotype); J, A. mesoduplex (Brazil, isotype); K, A. vezdae (India, holotype); L, A. chrysoglyphum (Dominica, Imshaug 33509A). Scales = 1 mm.
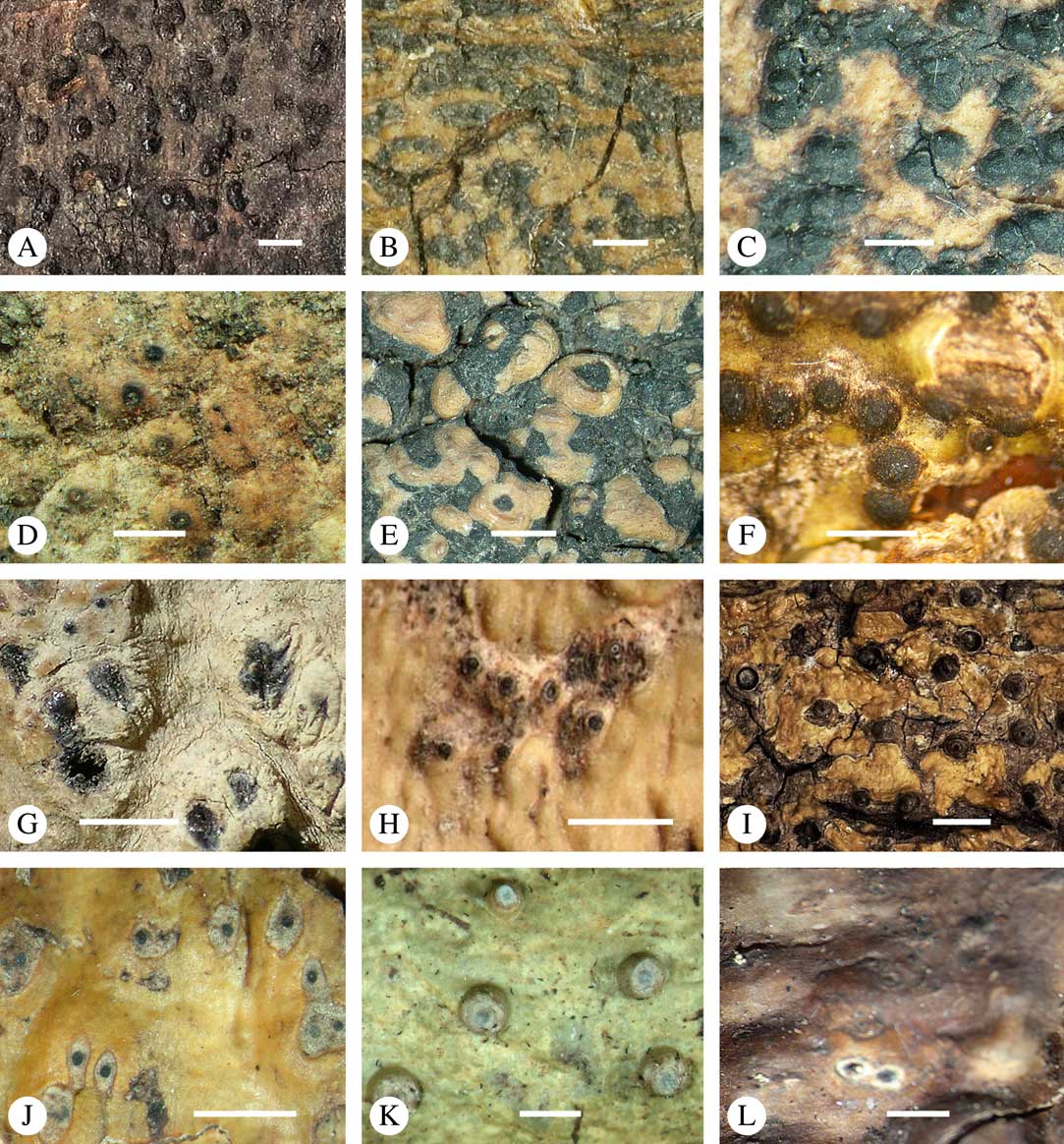
Fig. 33 Habitus of Astrothelium species. A–E, A. subdiscretum (A, India, isotype; B, Cuba, holotype of Bathelium phaeomelodes; C, India, isotype of Laurera verrucoaggregata; D, India, isotype of L. subsphaerioides; E, Netherlands Antilles, van Slageren 8406b); F, A. ambiguum (Brazil, lectotype); G, A. indicum (India, isotype); H, A. colombiense (Colombia, holotype); I, A. megaleium (Malaysia, Sarawak, holotype); J, A. macrosporum (India, isotype); K, A. aurantiacum (Papua New Guinea, Aptroot 18148); L, A. sikkimense (India, isotype). Scales = 1 mm.

Fig. 34 Habitus of Astrothelium species. A, A. alboverrucoides (Indonesia, Sumatra, holotype); B, A. flavomeristosporum (Philippines, holotype); C, A. philippinense (Philippines, holotype); D, A. papillosum (Papua New Guinea, Aptroot 37607); E, A. komposchii (Venezuela, holotype); F, A. irregulare (Brazil, holotype); G, A. flavostiolatum (Ecuador, holotype); H–L, A. megaspermum (H, Angola, isotype of Thelenella fulva; I, Brazil, holotype of Trypethelium ostendatum; J, Sri Lanka, isotype of Laurera megasperma f. immersa; K, São Tomé and Príncipe, holotype of Verrucaria euthelia; L, Costa Rica, Lücking s. n.). Scales=1 mm.

Fig. 35 Habitus of Astrothelium species. A & B, A. variatum (A, Colombia, holotype; B, Cuba, holotype of Heufleria subvariata); C & D, A. gigantosporum (C, Cuba, holotype; D, Puerto Rico, Harris 27863B); E, A. amylosporum (Bolivia, holotype); F, A. tetrasporum (Brazil, isotype); G, A. alboverrucum (India, isotype); H, A. sphaerioides (French Guiana, lectotype); I & J, A. subdisjunctum (I, Cuba, holotype; J, Costa Rica, holotype of Laurera dodgei); K, A. nicaraguense (Nicaragua, Lücking 28546); L, A. exostemmatis (Jamaica, holotype). Scales=1 mm.
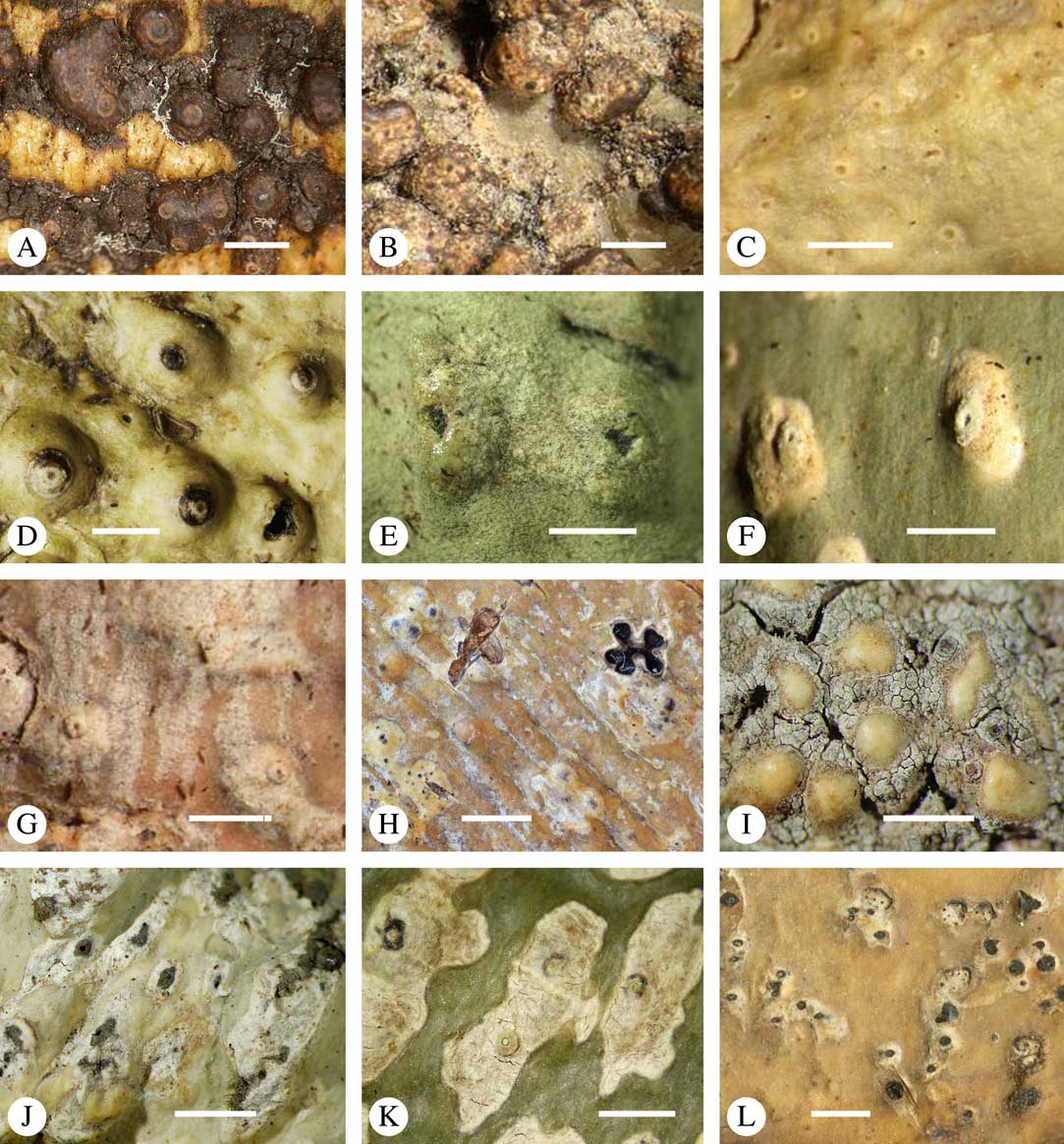
Fig. 36 Habitus of Astrothelium species. A, A. chapadense (Brazil, holotype); B, A. corallinum (Guyana, holotype); C, A. lucidomedullatum (Ecuador, holotype); D, A. carrascoense (Bolivia, holotype); E, A. megalostomum (Brazil, holotype); F, A. eustomurale (Brazil, isotype); G, A. lucidostromum (Guyana, holotype); H, A. sepultum (Peru, isotype of Trypethelium connivens); I, A. cryptolucens (Panama, holotype); J & K, A. norisianum (J, Panama, holotype; K, Venezuela, Guariglia 1485); L, A. consimile (Cuba, holotype). Scales=1 mm.
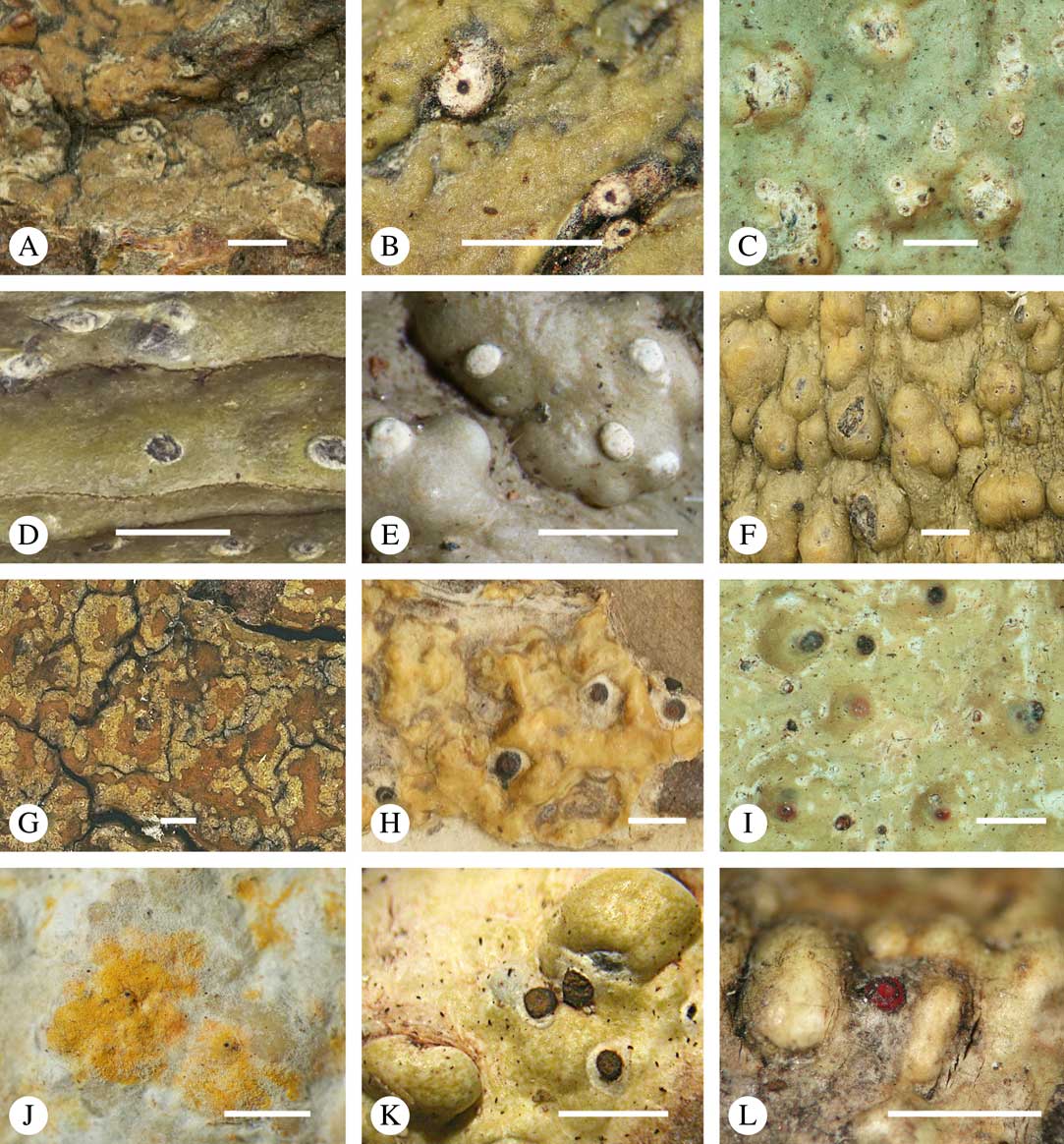
Fig. 37 Habitus of Astrothelium species. A–C, A. defossum (A, French Guiana, holotype; B, French Guiana, Tucker 16330; C, Guyana, Sipman 39596); D, A. octosporum (Brazil, holotype); E, A. megeustomum (Brazil, isotype); F, A. confluens (Brazil, lectotype); G, A. praetervisum (French Guiana, holotype); H, A. subaequans (Cuba, holotype); I, A. amazonum (Brazil, isotype); J, A. carassense (Brazil, holotype); K, A. diplocarpum (Colombia, holotype); L, A. purpurascens (Brazil, isotype of Cryptothelium rhodotitthon). Scales=1 mm.

Fig. 38 Habitus of Astrothelium species. A, A. curvatum (Brazil, isotype); B, A. santessonii (Guyana, Sipman 58142); C & D, A. puiggarii (Brazil, Osorio SM25); E, A. megacrypticum (Panama, holotype); F, A. longisporum (Brazil, isotype); G, A. pyrenuliforme (Bolivia, holotype); H, A. ecuadoriense (Ecuador, holotype); I, A. lugescens (São Tomé and Príncipe, holotype); J, A. andamanicum (India, isotype); K & L, A. tenue (K, Guyana, Bleij s. n.; L, Singapore, Sipman et al. 46164). Scales=1 mm.

Fig. 39 Habitus of Astrothelium species. A, A. guianense (French Guiana, holotype); B, A. bullatum (Bolivia, holotype); C, A. campylocartilagineum (Brazil, holotype); D, A. nicaraguense (Nicaragua, isotype); E, A. testudineum (Brazil, isotype); F, A. mediocrassum (Guyana, holotype); G, A. cinereum (French Guiana, lectotype); H, A. leioplacum (Paraguay, holotype); I, A. saxicola (Brazil, holotype); J, A. flavomurisporum (Brazil, isotype); K & L, A. cecidiogenum (Costa Rica, holotype). Scales=1 mm.
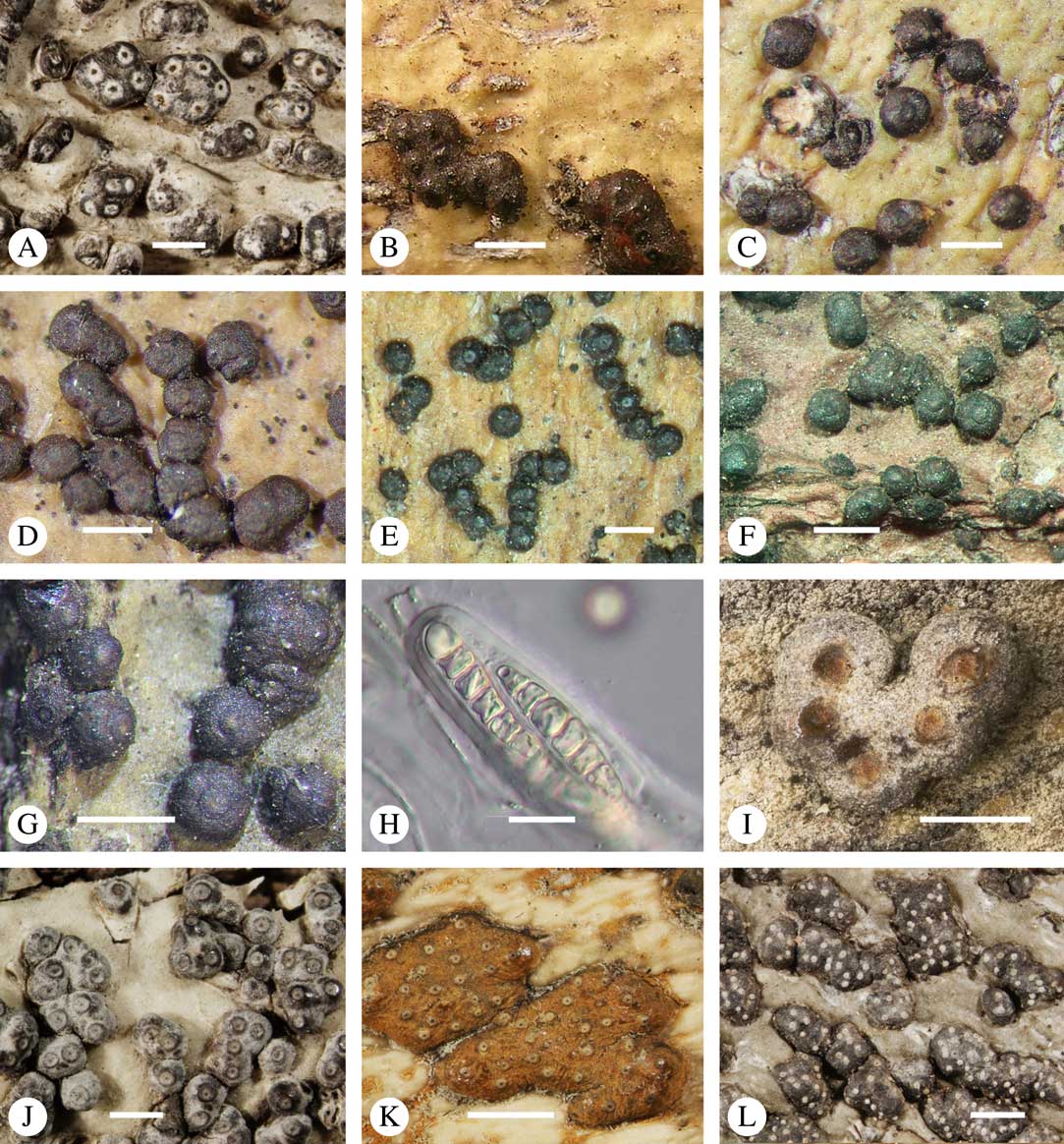
Fig. 40 Habitus and ascospores of Bathelium species. A, B. flavostiolatum (Bolivia, holotype); B, B. carolinianum (USA, South Carolina, isolectotype); C & D, B. porinosporum (Vietnam, holotype); E, B. albidoporum (Papua New Guinea, Aptroot 32903); F–H, B. nigroporum (India, isotype); I, B. pruinolucens (Brazil, lectotype); J, B. pruinosum (Bolivia, holotype); K, B. mirabile (Bolivia, holotype); L, B. boliviense (Bolivia, holotype). Scales: A–G, I–L=1 mm; H=10 µm.

Fig. 41 Habitus and ascospores of Bathelium species. A, B. inspersomastoideum (Bolivia, holotype); B, B. mastoideum (Costa Rica, Buck 43891); C–G, B. madreporiforme (C, Brazil, lectotype; D, South Africa, holotype of Trypethelium marginatum; E, Sierra Leone, holotype of Riddlea papillosa; F, Brazil, Cáceres 258; G, USA, Johnson 6057); H–J, B. tuberculosum (India, Lumbsch 19733j); K, B. lineare (Sierra Leone, isotype); L, B. sphaericum (Sierra Leone, isotype). Scales: A–F, H, K & L=1 mm; G, I & J=10 µm.
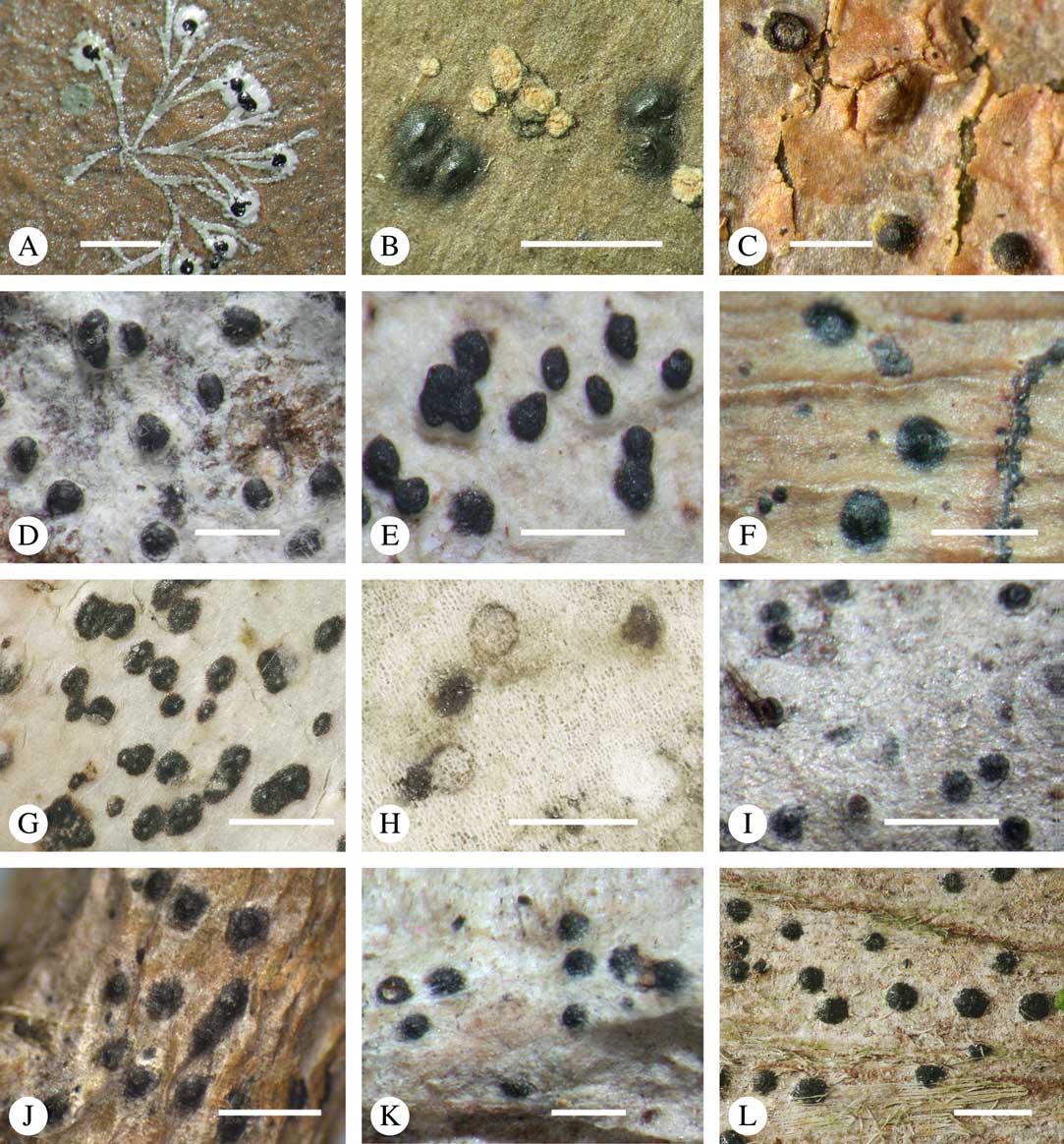
Fig. 42 Habitus of Bogoriella and Novomicrothelia species. A, Bogoriella striguloides (New Zealand, isotype); B, B. collospora (Japan, holotype); C, Novomicrothelia oleosa (Brazil, Cáceres & Aptroot 11967); D, Bogoriella triangularis (Puerto Rico, isotype); E, B. lateralis (Australia, isotype); F, B. punctata (Papua New Guinea, holotype); G, B. socialis (USA, Florida, Lücking 26733); H, B. fumosula (China, isotype); I, B. exigua (Brazil, Menezes s. n.); J, B. minutula (Argentia, Ferraro 8776); K, B. nonensis (Ecuador, Arvidsson 6614); L, B. subfallens (USA, Florida, Lücking 26772). Scales=1 mm.
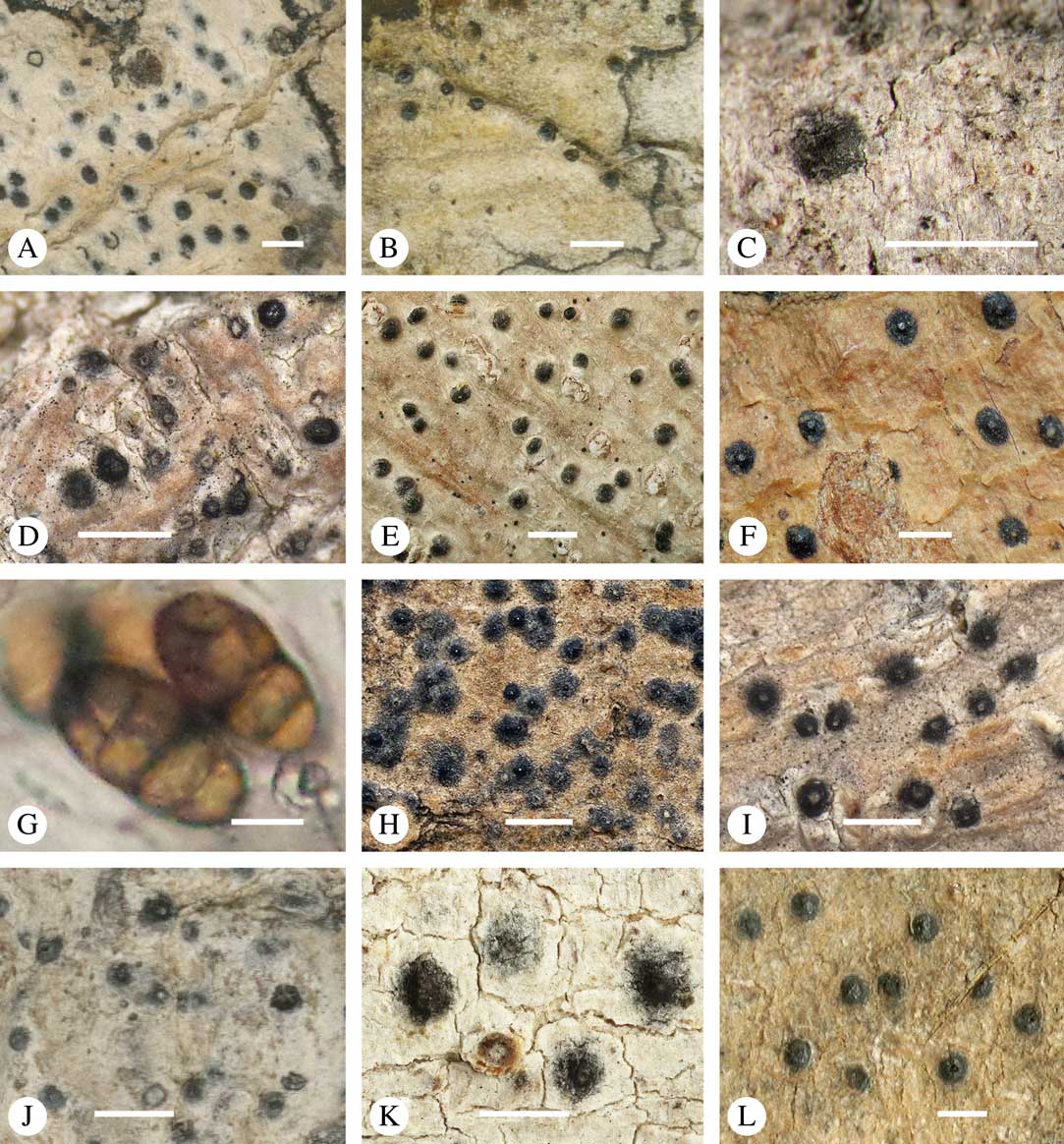
Fig. 43 Habitus and ascospores of Bogoriella species. A, B. miculiformis (Cuba, holotype); B, B. conothelena (India, lectotype); C, B. thelena (Brazil, Cáceres & Aptroot 11903); D, B. captiosa (Argentina, Moncada s. n.); E, B. modesta (Costa Rica, Lücking 15253); F & G, B. decipiens (Indonesia, holotype of Bogoriella subpersicina); H, B. confluens (South Africa, holotype); I, B. hemisphaerica (Argentina, Michlig 544); J, B. apposita (Colombia, holotype); K, B. megaspora (Brazil, Caceres & Aptroot 11920); L, B. queenslandica (Australia, lectotype). Scales: A–F, H–L=1 mm; G=10 µm.
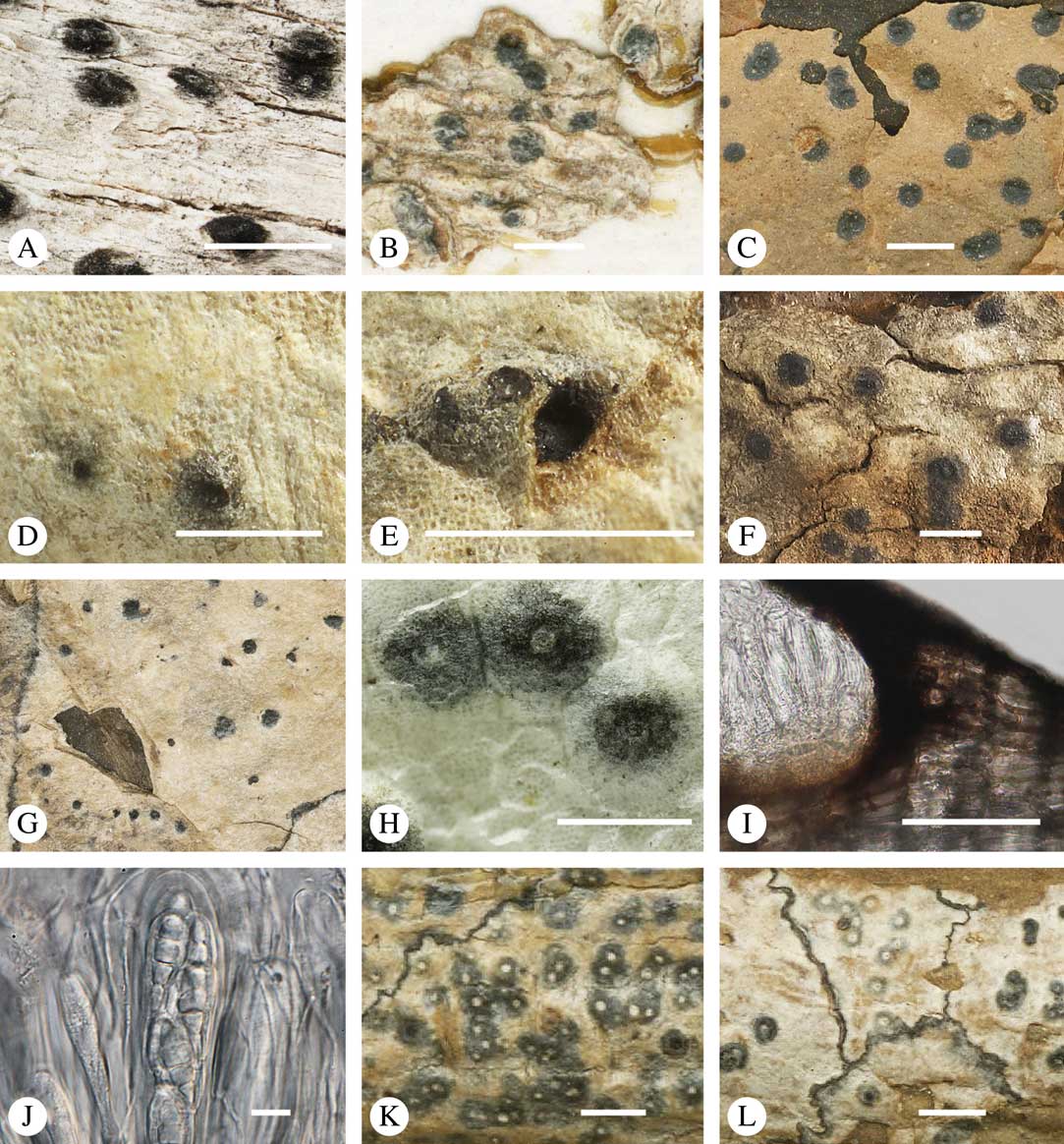
Fig. 44 Habitus of Constrictolumina species. A, C. chiquitana (Bolivia, holotype); B–E, C. malaccitula (B, Singapore, holotype; C, Malaysia, Borneo, lectotype of Didymella gigantea; D & E, Papua New Guinea, Aptroot 19093); F, C. porospora (Dominica, isotype); G–J, C. majuscula (G, Japan, holotype; H–J, Reunion, Schumm & Frahm 15483); K & L, C. leucostoma (unknown location, holotype). Scales: A–H, K & L=1 mm; I=100 µm; J=10 µm. (Images D, E, H–J by F. Schumm).
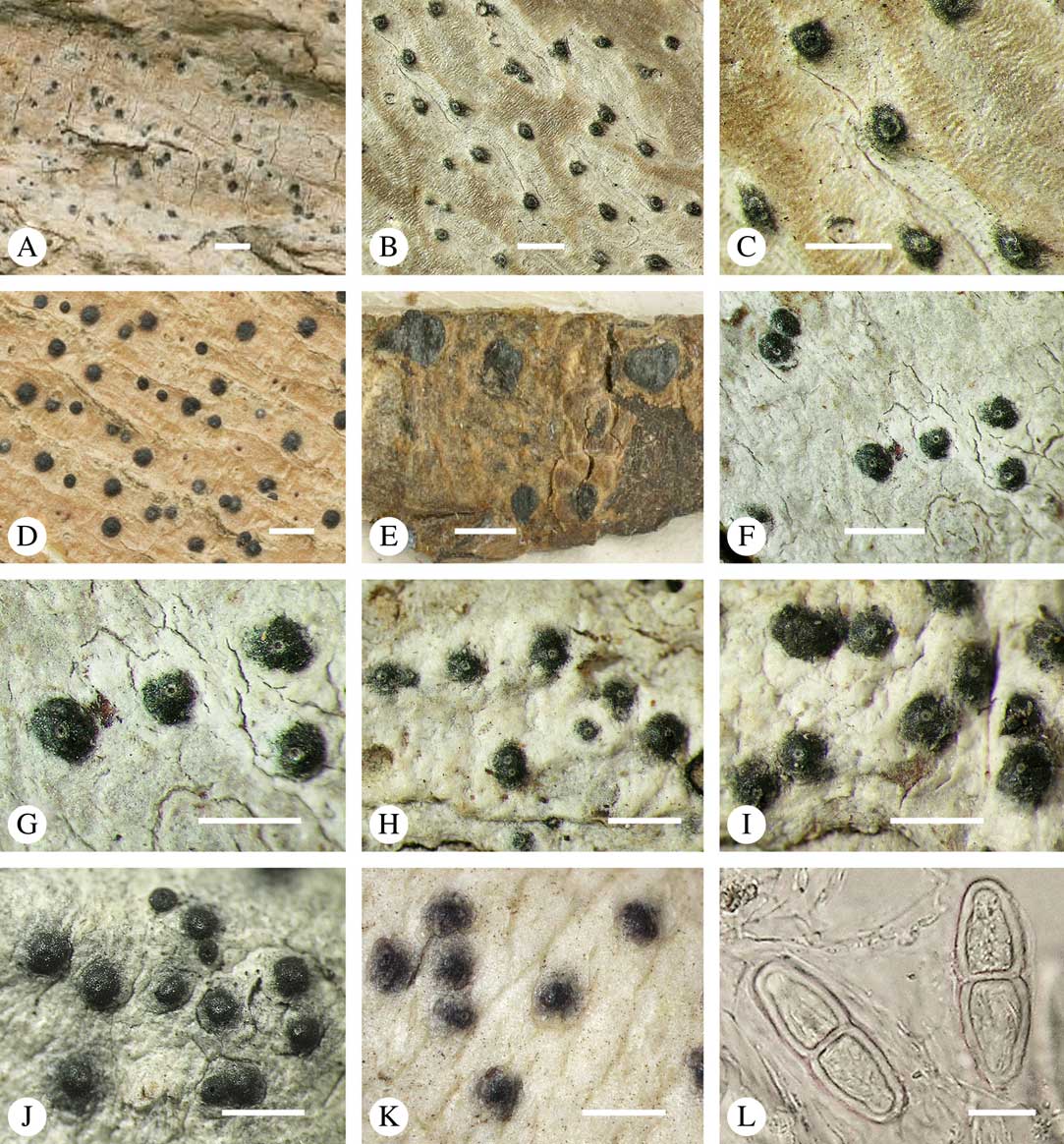
Fig. 45 Habitus and ascospores of Constrictolumina species. A–C, C. lyrata (A, USA, Florida, holotype; B & C, USA, Costa Rica, Lücking s. n.); D, C. planorbis (Cuba, lectotype); E, C. esenbeckiana (‘Antilles’, lectotype); F–L, C. cinchonae (F & G, Costa Rica, Lücking 45; H & I, Costa Rica, Lücking 44; J, Brazil, Cáceres 2106; K, USA, Florida, Lücking 26787; L, Costa Rica, Brenes 188). Scales: A–K=1 mm; L=10 µm.
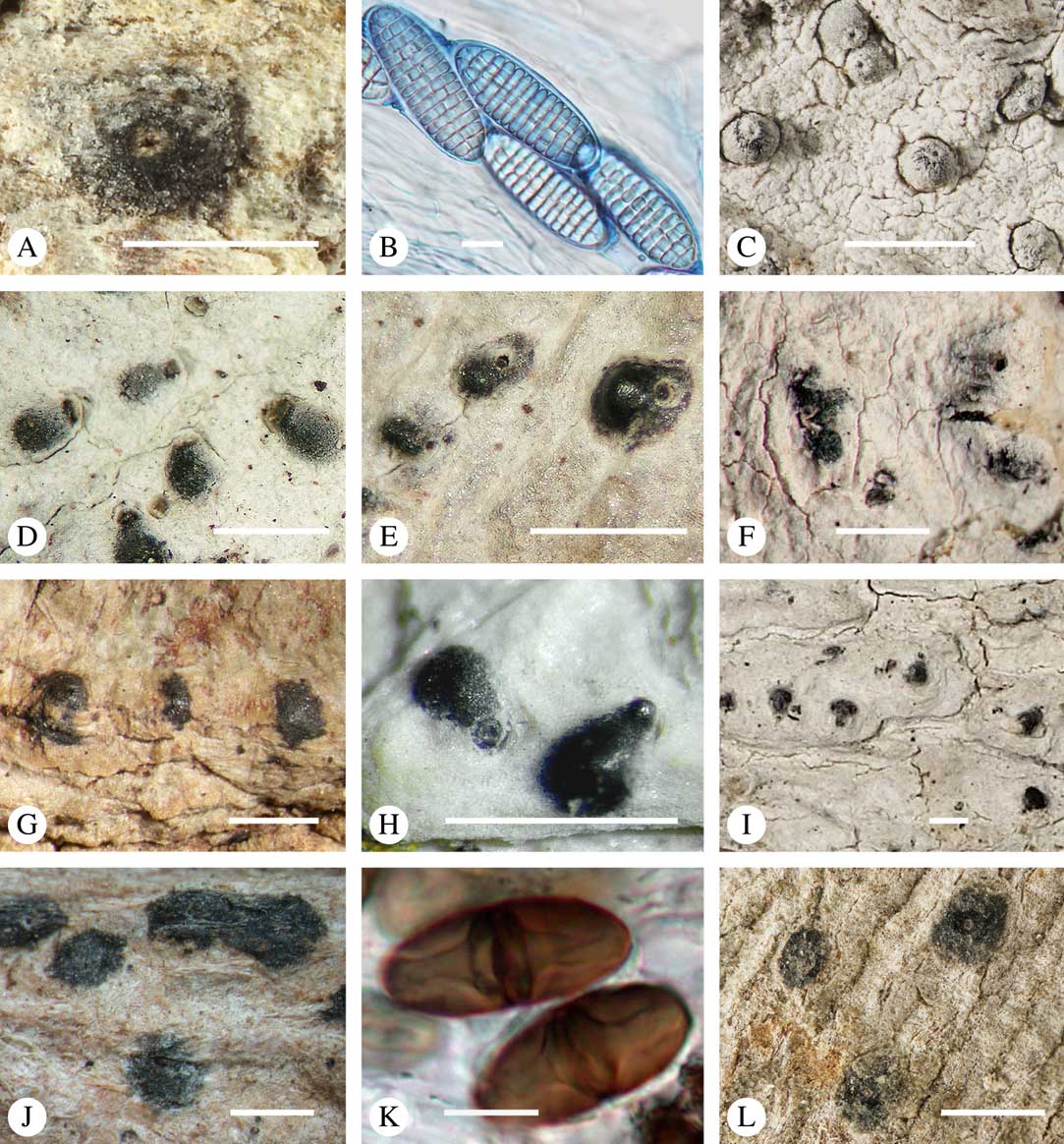
Fig. 46 Habitus and ascospores of Dictyomeridium and Distothelia species. A & B, Dictyomeridium immersum (Brazil, Caceres & Aptroot 11943); C, D. lueckingii (Bolivia, holotype); D & E, D. proponens (D, Cuba, Buck 23544; E, Venezuela, Lópes-Figueiras 22453); F, D. paraproponens (Brazil, isotype); G, D. campylothelioides (Indonesia, Java, Groenhart 5796); H, D. isohypocrellinum (Brazil, holotype); I, D. amylosporum (Brazil, isotype); J & K, Distothelia rubrostoma (Guadeloupe, isotype); L, D. isthmospora (China, isotype). Scales: A=0·5 mm; C–J & L=1 mm; B & K=10 µm. (Images A, B, J–L by F. Schumm).
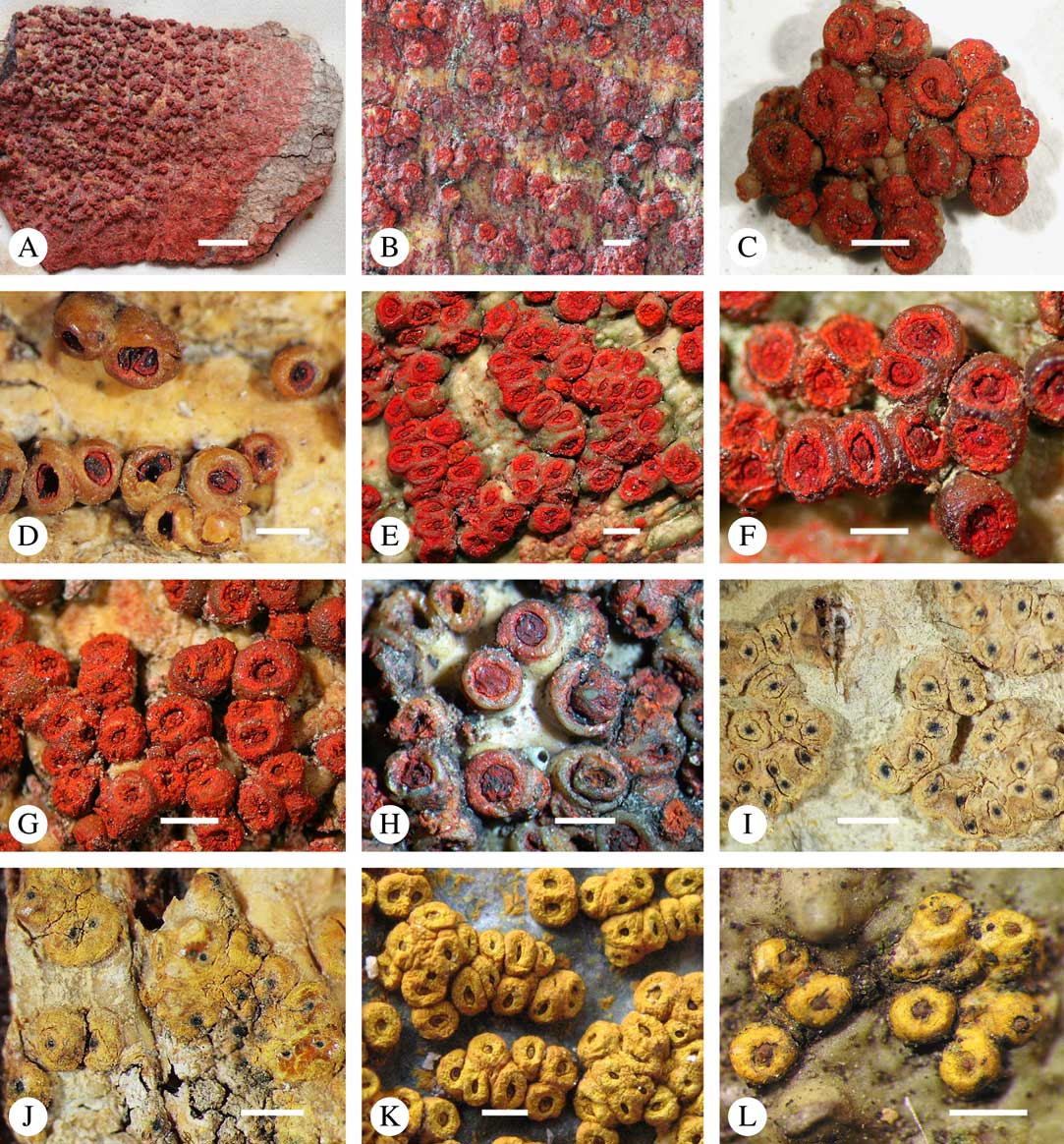
Fig. 47 Habitus of Marcelaria species. A–H, M. purpurina (A & B, Brazil, holotype; C, Brazil, isotype; D, Brazil, holotype of Tremotylium sprucei; E, Colombia, Moncada 3467; F, Brazil, Brako 7381; G, Bolivia, van den Boom 4107; H, DR Congo, Ertz 14840); I & J, M. benguelensis (India, lectotype); K & L, M. cumingii (K, Thailand, Parnmen s. n.; L, Thailand, Polyiam s. n.). Scales=1 mm. (Image K by W. Polyiam).
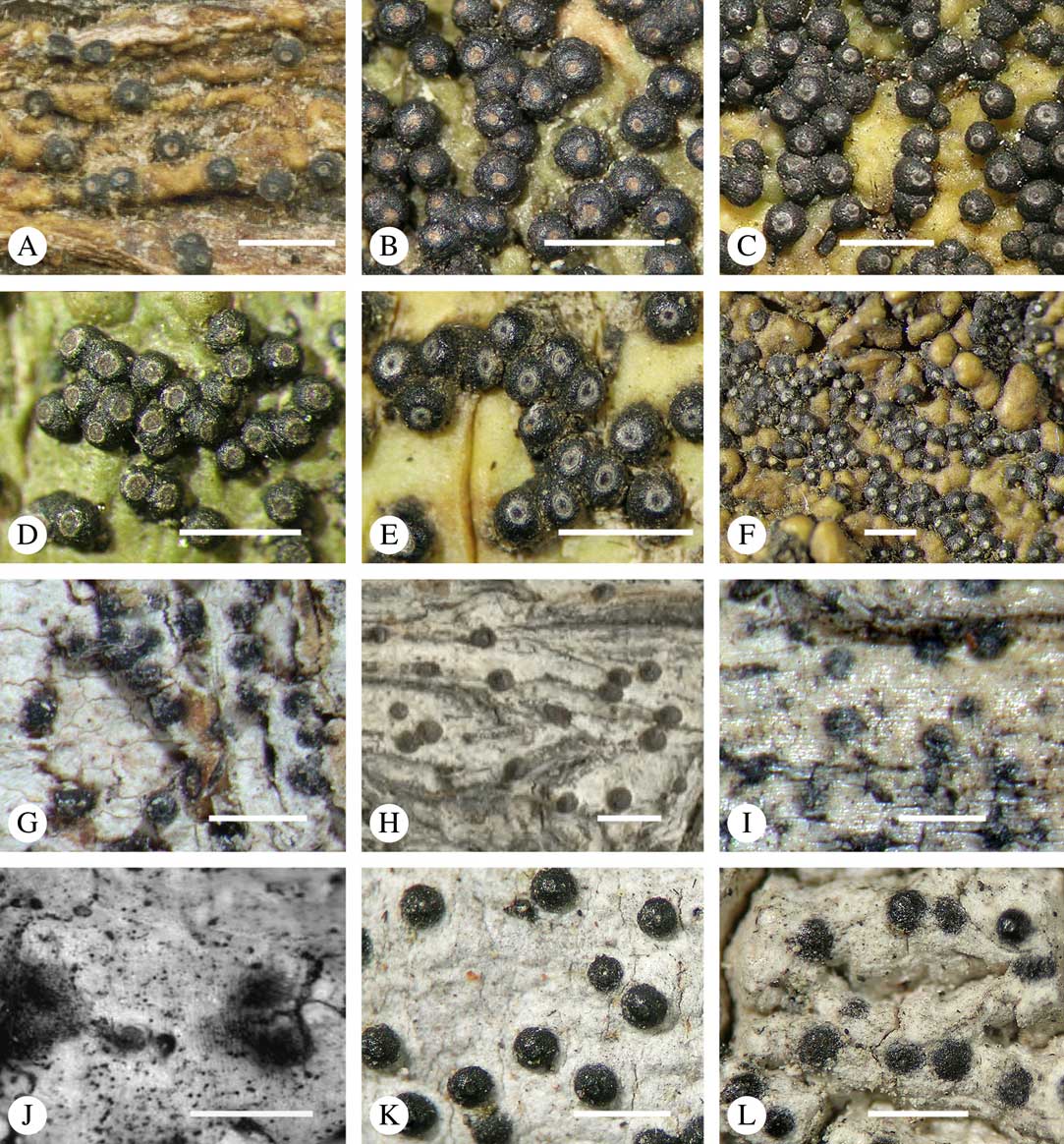
Fig. 48 Habitus of Nigrovothelium and Polymeridium species. A–E, Nigrovothelium tropicum (‘South America’, lectotype); F, N. bullatum (India, holotype); G, Polymeridium subvirescens (Brazil, Cáceres 11110); H, P. pyrenuloides (‘South America’, lectotype); I, P. albidovarians (Indonesia, isotype); J, P. pyrenastroides (Venezuela, holotype); K & L, P. amyloideum (J, Brazil, isotype; K, Brazil, Brako 6028). Scales=1 mm.
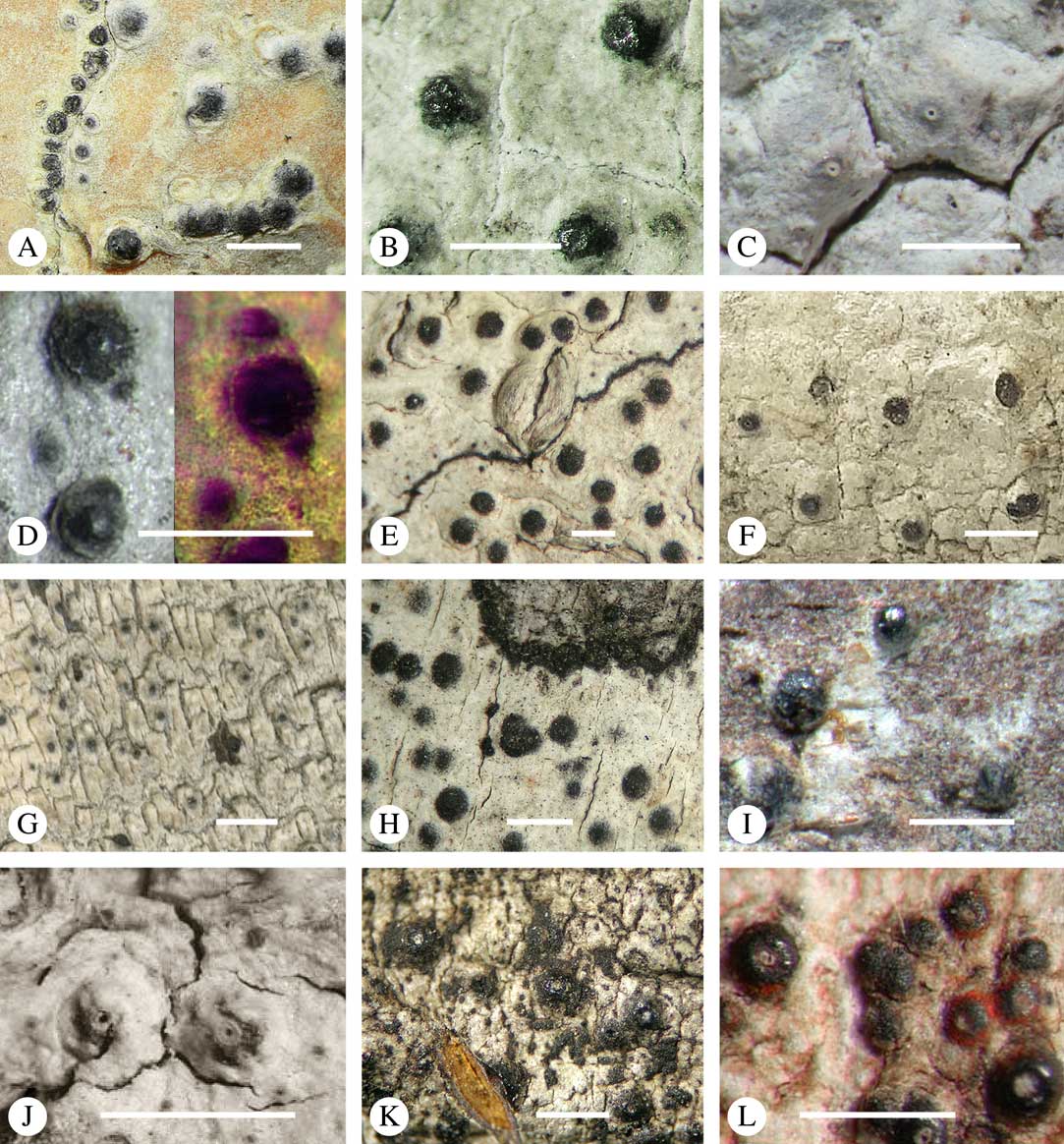
Fig. 49 Habitus of Polymeridium species. A, P. albopruinosum (India, isotype); B, P. bengoanum (Angola, holotype); C, P. xanthoreagens (Australia, isotype); D, P. albidoreagens (Brazil, isotype; right portion under UV light); E, P. catapastum (Bahamas, Britton 6650); F, P. glaucoatrum (Philippines, isotype); G, P. suffusum (New Zealand, isolectotype); H, P. contendens (Brazil, Brako 6107D); I, P. inspersum (Australia, isotype); J, P. tribulationis (Australia, isotype); K, P. endocrocinum (Brazil, holotype); L, P. rhodopruinosum (Puerto Rico, holotype). Scales=1 mm.
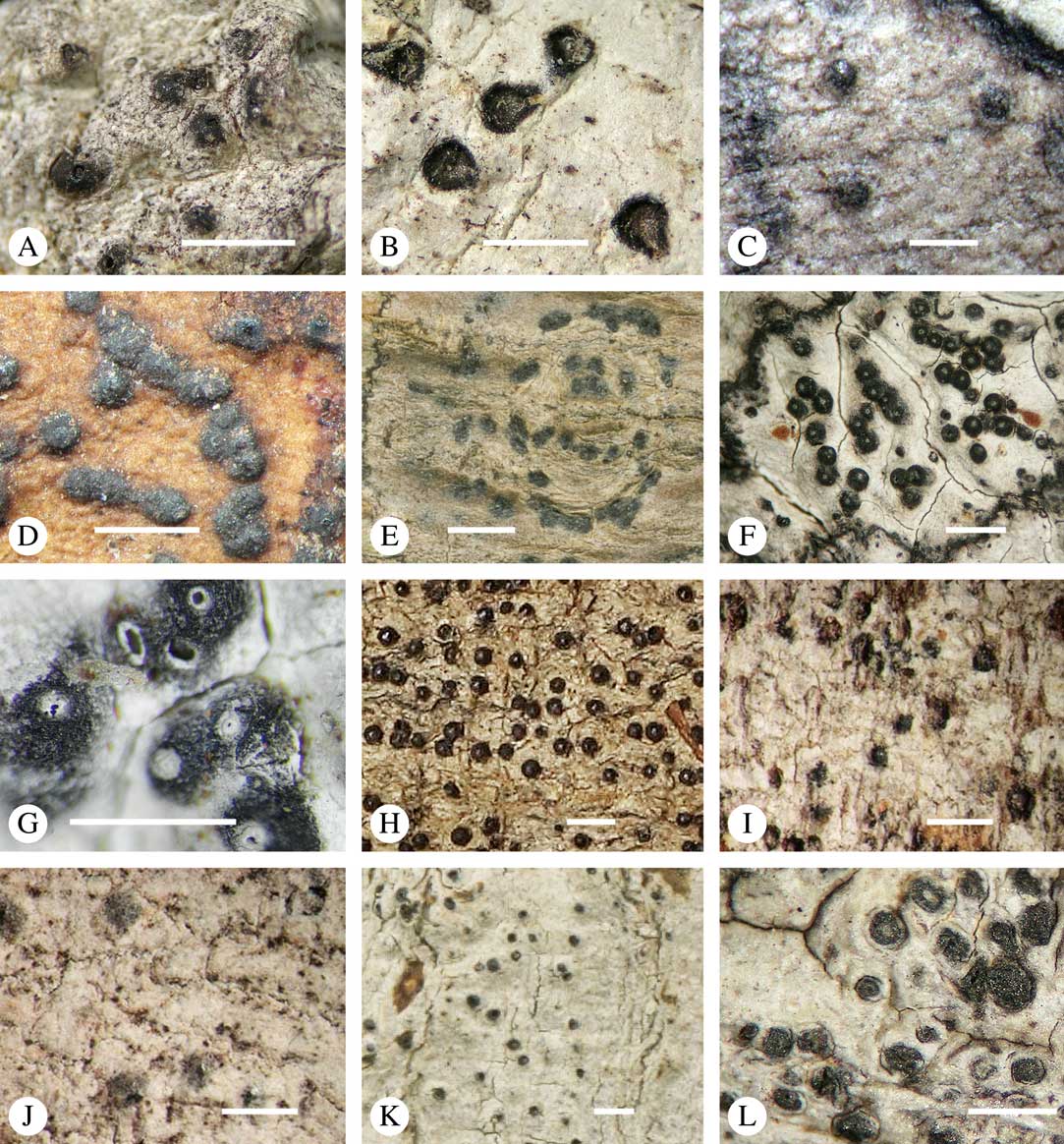
Fig. 50 Habitus of Polymeridium species. A, P. biloculare (Brazil, isotype); B, P. simulans (Brazil, holotype); C, P. catapastoides (Australia, holotype); D, P. microsporum (India, isotype); E & F, P. subcinereum (E, USA, Texas, lectotype; F, Dominican Republic, Harris 19593); G, P. sulphurescens (Australia, Streimann 29632); H, P. albidum (Brazil, holotype); I, P. endoflavens (Brazil, isotype); J, P. longiflavens (Brazil, isotype); K & L, P. chioneum (K, Cuba, holotype of Verrucaria pleiomeroides; L, Brazil, Buck 2980A). Scales=1 mm.
Trypethelium peltigereum G. Merr. (1910); type: Jamaica, on thallus of Peltigera, Cummings 11/111 1905, distributed in Merrill, Lich. Exs. 85 (L!—isotype).
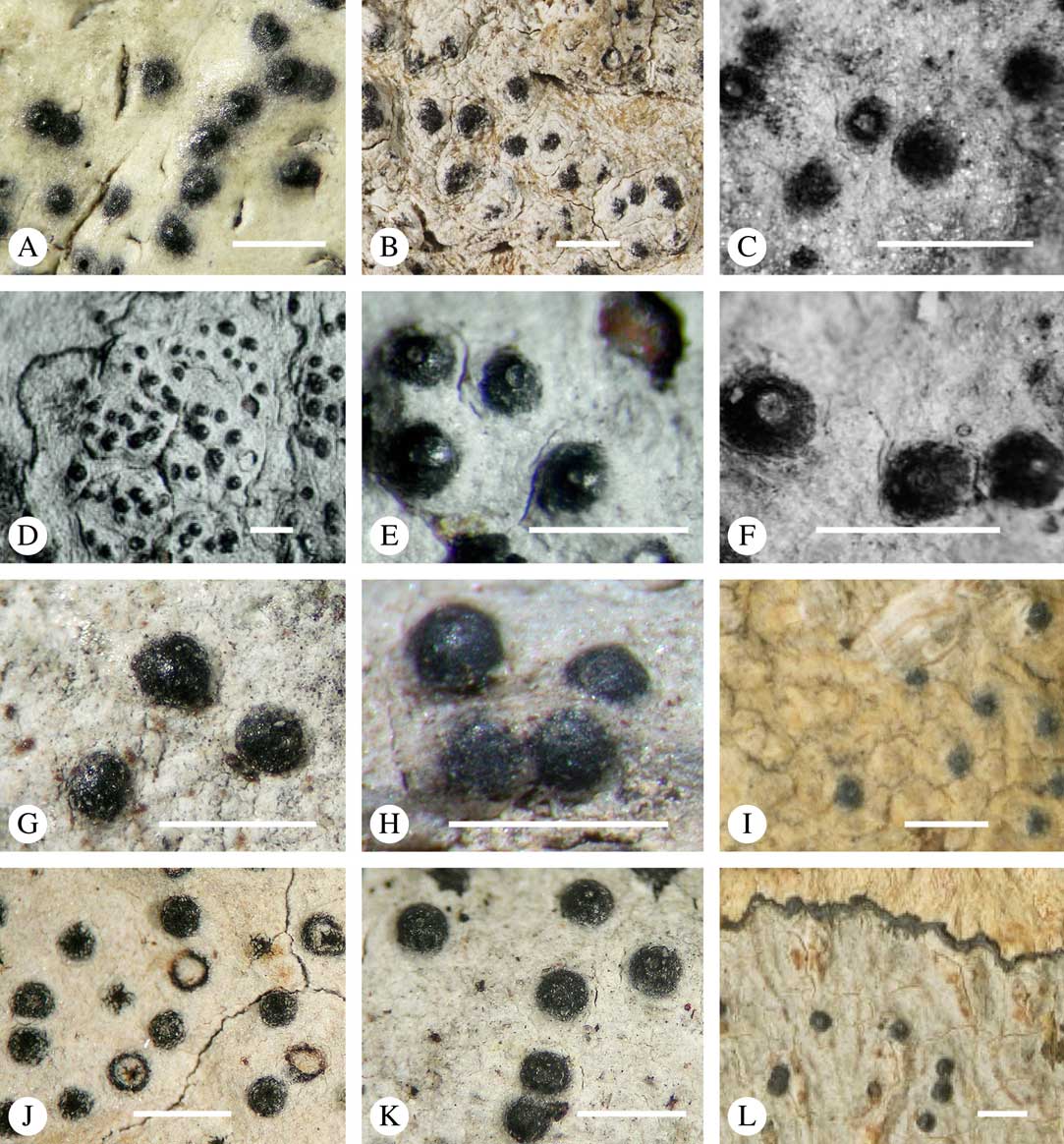
Fig. 51 Habitus of Polymeridium species. A, P. dithecium (Venezuela, holotype); B, P. xanthoexcentricum (Bolivia, holotype); C, P. multiforme (Guyana, isotype); D & E, P. corticatum (Brazil, holotype); F, P. alboflavescens (Venezuela, holotype); G, P. flavothecium (Dominican Republic, holotype); H, P. neuwirthii (Venezuela, isotype); I–K, P. quinqueseptatum (I, USA, South Carolina, isotype; J, Venezuela, López-Figueiras 19405; K, Brazil, Brako 6107); L, P. pleiomerellum (Cuba, holotype). Scales=1 mm.
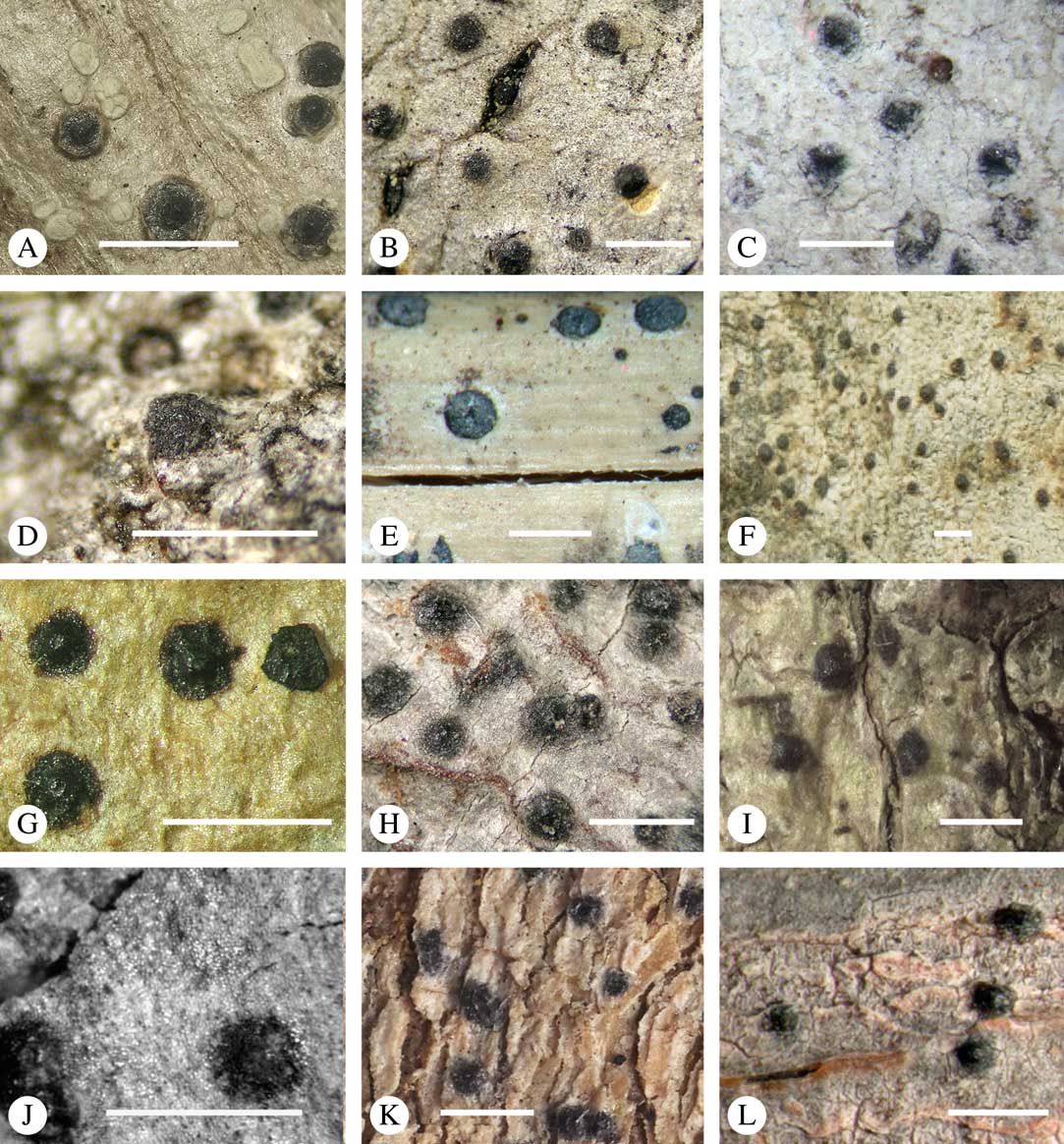
Fig. 52 Habitus of Polymeridium species. A, P. costaricense (Costa Rica, isotype); B & C, P. neblinae (Venezuela, holotype); D, P. pleurothecium (Guyana, holotype); E, P. bambusicola (Argentina, isotype); F–I, P. albocinereum (F, Brazil, isolectotype; G, Philippines, holotype of Pseudopyrenula pentameria; H, Jamaica, Imshaug 13515; I, USA, Florida, Lay 143); J, P. submuriforme (Philippines, holotype); K, P. cinereonigricans (Brazil, isotype); L, P. julelloides (Brazil, isotype). Scales=1 mm.
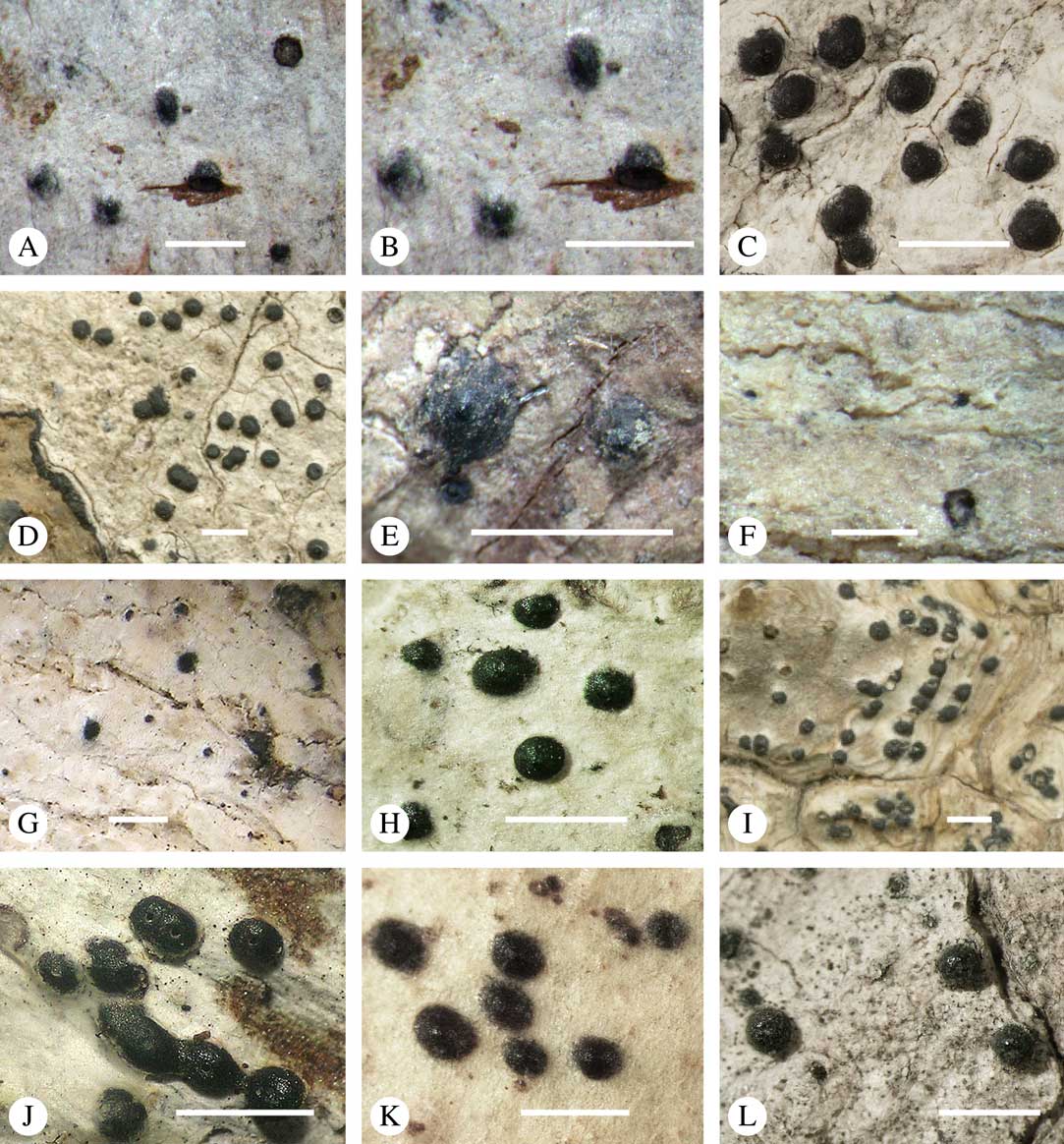
Fig. 53 Habitus of Pseudopyrenula species. A & B, P. flavoreagens (Brazil, isotype); C, P. flavosuperans (Bolivia, holotype); D, P. superans (Cuba, holotype); E. P. papuana (Papua New Guinea, isotype); F, P. serusiauxii (Papua New Guinea, isotype); G, P. thallina (Costa Rica, holotype); H, P. endoxantha (Dominica, holotype); I–K, P. subgregaria (I, Cuba, holotype; J, Costa Rica, Lücking s. n.; K, USA, Florida, Nelsen s. n.); L, P. cerei (Brazil, holotype). Scales=1 mm.
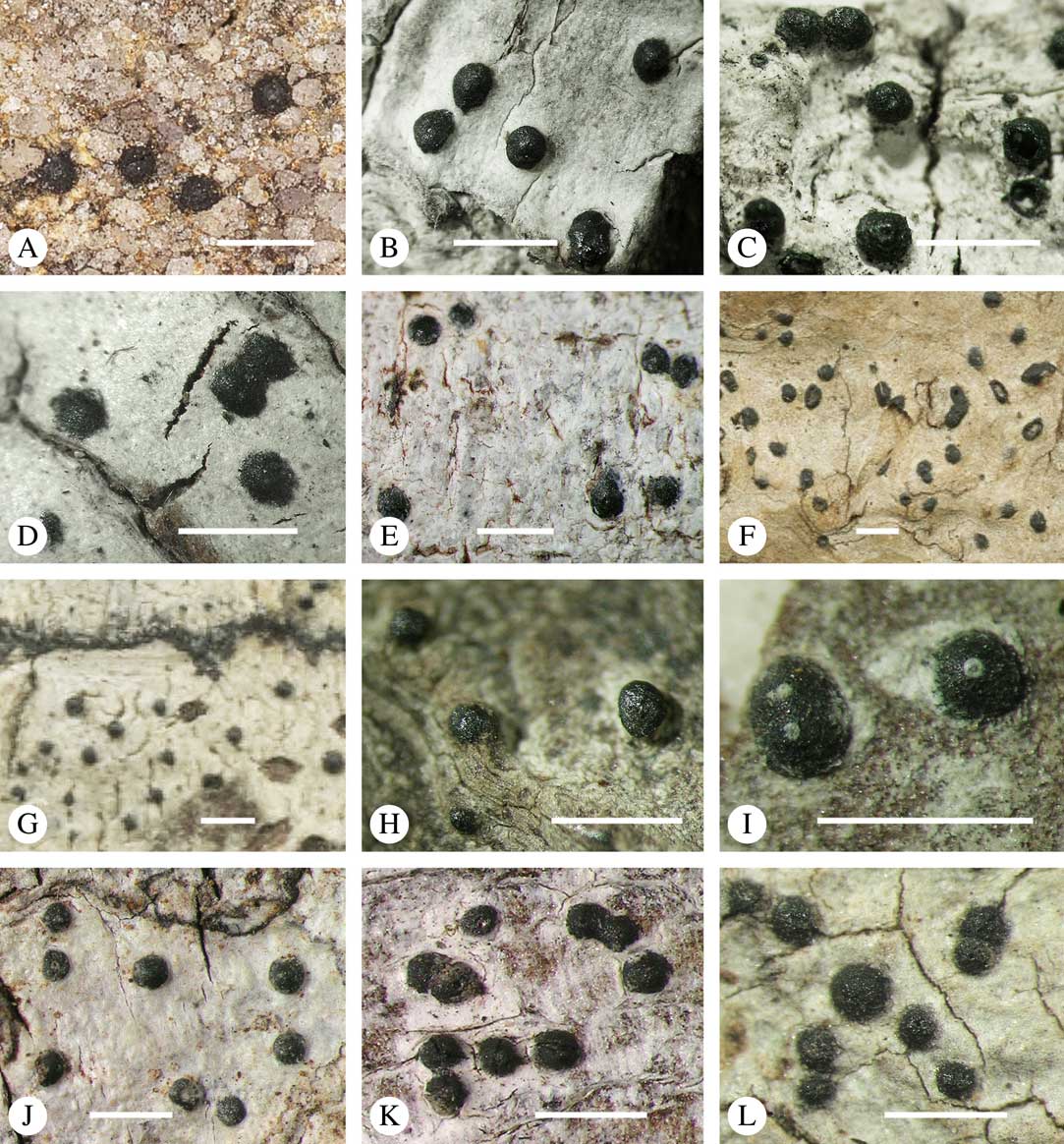
Fig. 54 Habitus of Pseudopyrenula species. A, P. saxicola (Brazil, lectotype); B, P. dubia (Guadeloupe, holotype); C–E, P. diluta (C, Brazil, holotype of P. atroalba; D, Brazil, holotype of P. sitiana; E, Fiji, Lumbsch 19825i); F, P. cubana (Cuba, lectotype); G–L, P. subnudata (G, Brazil, holotype; H, Brazil, holotype of P. araucariae; I, Thailand, holotype of P. diluta var. degenerans; J, Fiji, Lumbsch 19815t; K, Fiji, Lumbsch 19845g; L, Brazil, Cáceres 893). Scales=1 mm.

Fig. 55 Habitus of Trypethelium species. A, T. xanthoplatystomum (Bolivia, holotype); B, T. luteolucidum (Brazil, holotype); C & D, T. regnellii (Paraguay, holotype); E–G, T. krempelhuberi (E & F, Vietnam, holotype; G, Brazil, lectotype of T. platystomum var. denudatum); H & I, T. astroideum (H, Bolivia, holotype; I, Peru, Nelsen s. n.); J–L, T. platystomum (J, French Guiana, isotype; K, Brazil, Cáceres & Aptroot 11485; L, Colombia, Moncada 3267). Scales=1 mm.

Fig. 56 Habitus of Trypethelium species. A, T. infraeluteriae (Vietnam, holotype); B–L, T. eluteriae (B, Indonesia, holotype; C, ‘South America’, lectotype of T. insigne; D, Mexico, isotype of T. pringlei; E & F, Surinam, holotype of T. sprengelii; G, Brazil, holotype of Pseudopyrenula eluteriae ssp. subsulphurea; H, Brazil, Cáceres 134; I, Bahamas, Brace 5049; J, Colombia, Moncada 3399; K, Dominican Republic, Harris 26423; L, Dominican Republic, Harris 26708). Scales=1 mm.

Fig. 57 Habitus and ascospores of Trypethelium species. A–E, T. subeluteriae (A, Brazil, lectotype of T. eluteriae var. polystomum; B, USA, Florida, Nelsen s. n.; C & D, Venezuela, López-Figueiras 21191; E, Costa Rica, Lücking 17611, ascospores); F, T. plicatorimosum (India, isotype); G & H, T. inaequale (G, Peru, holotype; H, India, isolectotype of T. inamoenum); I–L, T. epileucodes (I & J, Malaysia, Labuan, lectotype; K, Australia, lectotype of T. virginicum; L, India, isotype of T. andamanicum). Scales: A–D, F–L=1 mm; E=10 μm.
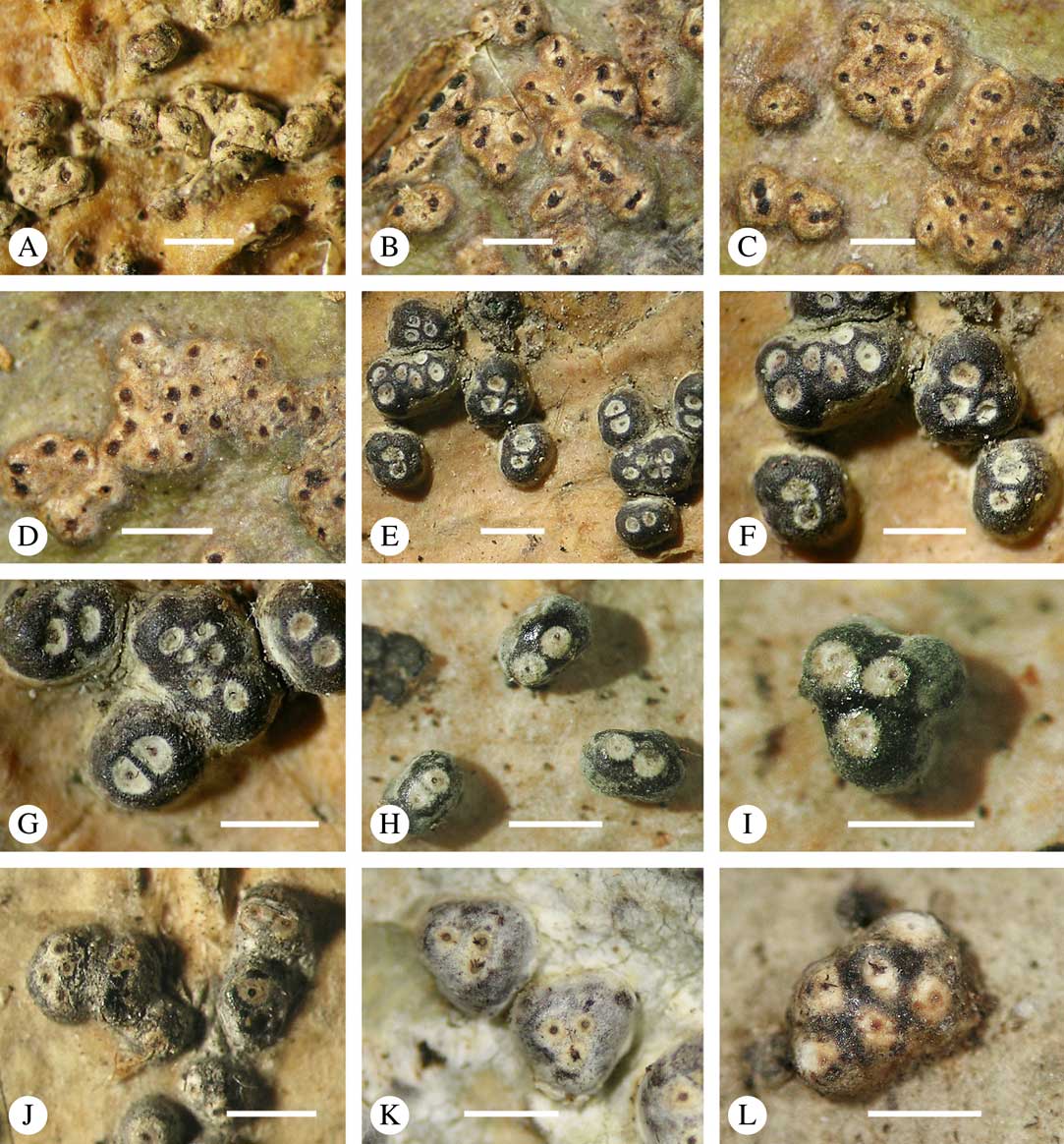
Fig. 58 Habitus of Trypethelium species. A–D, T. foveolatum (A, Brazil, lectotype; B–D, Puerto Rico, Harris 24390); E–I, T. sphaerocephalum (E–G, São Tomé and Príncipe, holotype of T. platystomum f. leucostomum; H & I, Angola, lectotype); J–L, T. ornatum (J, Cuba, lectotype; K, Brazil, Oliveira 351; L, Venezuela, López-Figueiras 16540e). Scales=1 mm.

Fig. 59 Habitus of Viridothelium species. A, V. tricolor (Panama, holotype); B, V. solomonense (Solomon Islands, holotype); C, V. megaspermum (India, isotype); D, V. vonkonratii (Fiji, holotype); E, V. leptoseptatum (Brazil, holotype); F, V. inspersum (Papua New Guinea, holotype); G, V. indutum (Australia, Streimann 45933); H, V. kinabaluense (Malaysia, Sabah, holotype); I–K, V. virens (I, USA, Arkansas, isotype; J, USA, Louisiana, holotype of Trypethelium exocanthum; K, USA, New York, Harris 30395); L, V. cinereoglaucescens (Japan, holotype). Scales=1 mm.
Discussion. This is a new synonym of the lichenicolous fungus Pyrenidium actinellum Nyl. No hamathecium is present. Ascospores are brown, 3-septate, c. 25×10 μm, and constricted at the septa.
Taxa described or placed in genera belonging in Trypetheliaceae but previously excluded from the family at the species level, and their current names
Astrothelium africanum Zahlbr. (1931)=Lithothelium obtectum (Müll. Arg.) Aptroot (Pyrenulaceae).
Astrothelium congregans Eckf. (nom. inval.)=Anthracothecium australiense (Müll. Arg.) Aptroot (Pyrenulaceae).
Astrothelium cryptothelium (Müll. Arg.) Nyl. (1892)=Pyrenula cryptothelia (Müll. Arg.) Aptroot & Etayo (Pyrenulaceae).
Astrothelium epiphyllum R. Sant. (1952)=Trypetheliopsis epiphylla (R. Sant.) Aptroot (Monoblastiaceae).
Astrothelium fugax Müll. Arg. (1895)=Lithothelium fugax (Müll. Arg.) Aptroot (Pyrenulaceae).
Astrothelium interlatens Nyl. (1868)=Anthracothecium sp. (Pyrenulaceae).
Astrothelium interlatens var. nudatum Nyl. (1868)=Anthracothecium sp. (Pyrenulaceae).
Astrothelium isabellinum Vain. (1891), non Eschw.=Lithothelium obtectum (Müll. Arg.) Aptroot (Pyrenulaceae).
Astrothelium ochrocleistum Nyl. (1888)=Pyrenula prostrata (Stirt.) D. J. Galloway (Pyrenulaceae).
Astrothelium parmularia Leight. (1871)=non-lichenized fungus fide Zahlbruckner (Reference Zahlbruckner1922).
Astrothelium prostratum Stirt. (1873).—Heufleridium prostratum (Stirt.) Müll. Arg. (1883).—Parmentaria prostrata (Stirt.) Müll. Arg. (1894)=Pyrenula prostrata (Stirt.) D. J. Galloway (Pyrenulaceae).
Astrothelium pyrenastraeum Nyl. (1890)=Pyrenula septicollaris (Eschw.) R. C. Harris (Pyrenulaceae).
Astrothelium pyrenastroides (C. Knight) C. Knight (1875).—Verrucaria pyrenastroides C. Knight (1860).—Parmentaria pyrenastroides (C. Knight) Müll. Arg. (1894)=Pyrenula pyrenastroides (C. Knight) D. J. Galloway (Pyrenulaceae).
Astrothelium septicollare (Eschw.) Leight. (1866)=Pyrenula septicollaris (Eschw.) R. C. Harris (Pyrenulaceae).
Astrothelium speciosum Zahlbr. (1927, 1928)=typographic error (lapsus) for Anthracothecium speciosum Zahlbr. (Pyrenulaceae).
Astrothelium umbilicatum Fr. (1825) Incorrectly synonymized (fide Aptroot Reference Aptroot1991) with Architrypethelium uberinum by Zahlbruckner (Reference Zahlbruckner1922).
Bathelium epiphyllum Müll. Arg. (1883)=Phyllobathelium epiphyllum (Müll. Arg.) Müll. Arg. (Strigulaceae).
Bathelium madreporiforme var. obscurius (C. Bab. ex Hook. f.) Müll. Arg. (1894)=non-lichenized fungus (Xylariaceae sp.).
Bathelium megaspermum var. tasmanicum Jatta (1911)=Pyrenula ravenelii (Tuck.) R. C. Harris (Pyrenulaceae).
Bathelium pyrenuloides (Mont.) Trevis. (1853)=Pyrenula pyrenuloides (Mont.) R. C. Harris (Pyrenulaceae).
Bathelium velatum Müll. Arg. (1882)=non-lichenized fungus fide Letrouit-Galinou (Reference Letrouit-Galinou1957).
Campylothelium defossum Müll. Arg. (1891)=Graphidaceae or Porinaceae sp.
Heufleria alpina Auersw.=non-lichenized fungus (Rhytismatales).
Laurera astroidella (Vain.) Zahlbr. (1938)=non-lichenized fungus fide Letrouit-Galinou (Reference Letrouit-Galinou1957).
Laurera madreporiformis var. obscurior (C. Bab. ex Hook. f.) Zahlbr. (1922)=non-lichenized fungus (Xylariaceae sp.).
Laurera megasperma var. tasmanica (Jatta) Zahlbr. (1922)=Pyrenula ravenelii (Tuck.) R. C. Harris (Pyrenulaceae).
Laurera ochroleucodes (Vain.) Zahlbr. (1938)=?Cryptothecia sp. fide Letrouit-Galinou (Reference Letrouit-Galinou1957) (Arthoniaceae).
Laurera pauperrima (Müll. Arg.) Zahlbr. (1922)=non-lichenized fungus fide Letrouit-Galinou (Reference Letrouit-Galinou1957).
Laurera velata (Müll. Arg.) Zahlbr. (1922)=non-lichenized fungus fide Letrouit-Galinou (Reference Letrouit-Galinou1957).
Pseudopyrenula awajiensis Vain.=Pyrenula awajiensis (Vain.) Kashiw. (Pyrenulaceae).
Pseudopyrenula balia (Kremp.) Müll. Arg. (1883)=Pyrenula balia (Kremp.) R. C. Harris (Pyrenulaceae).
Pseudopyrenula conica (Müll. Arg.) Müll. Arg. (1883)=non-lichenized fungus (Saccardoella sp.).
Pseudopyrenula galactina Shirley (1893)=Pyrenula dermatodes (Borrer) Schaer. (Pyrenulaceae).
Pseudopyrenula illota (Nyl.) Vain. (1909)=Lithothelium illotum (Nyl.) Aptroot (Pyrenulaceae).
Pseudopyrenula majuscula H. Magn. (1955)=Pyrenula massariospora (Starb.) R. C. Harris (Pyrenulaceae).
Pseudopyrenula obvoluta (Nyl.) Zahlbr. (1922)=Pyrenula obvoluta (Nyl.) R. C. Harris & Aptroot (Pyrenulaceae).
Pseudopyrenula octomera H. Magn. (1955)=non-lichenized fungus (Saccardoella sp.).
Pseudopyrenula quintaria Zahlbr. (1930)=non-lichenized fungus.
Pseudopyrenula ramosii Vain. (1921)=Myriotrema sp. (Graphidaceae).
Pseudopyrenula scoria (Fée) Vain. (1930); nom. illeg.=Pyrenula anomala (Ach.) Vain. (Pyrenulaceae).
Pseudopyrenula subvelata (Nyl.) Müll. Arg. (1883)=Arthopyrenia subvelata (Nyl.) R. C. Harris (Arthopyreniaceae).
Pseudopyrenula tessella (Pers.) P. W. Graff (1917)=non-lichenized fungus [Allantoporthe tessella (Pers.) Petr.].
Trypethelium annulare var. subdepressum Nyl. (1858)=Pyrenula oleagina Fée. (Pyrenulaceae).
Trypethelium anomalum Ach. (1814)=Pyrenula anomala (Ach.) Vain. (Pyrenulaceae).
Trypethelium anomalum var. obscurescens (Vain.) Zahlbr. (1938)=Pyrenula anomala (Ach.) Vain. (Pyrenulaceae).
Trypethelium chiodectonoides Fée (1824)=Pertusaria feeana Zahlbr. (Pertusariaceae).
Trypethelium cinnabarinum C. Knight ex F. M. Bailey (1886)=Pyrenula cruenta (Mont.) Vain. (Pyrenulaceae).
Trypethelium coccinatum Stizenb. (1891)=cf. Pyrenula cruenta (Mont.) Vain. (Pyrenulaceae).
Trypethelium conglobatum Ach. (1814).—Trypethelium eluteriae var. conglobatum (Ach.) Zahlbr. (1922)=non-lichenized fungus on dead lichen.
Trypethelium connivens Stirt. (1873); non Nyl.; Melanotheca connivens Zahlbr.=Pyrenula cruenta (Mont.) Vain. (Pyrenulaceae).
Trypethelium cruentatum Nyl. (1876); nom. nud.=Pyrenula cruenta (Mont.) Vain. (Pyrenulaceae).
Trypethelium cruentulum Nyl. (1876)=Pyrenula aff. cruenta fide Zahlbruckner (Reference Zahlbruckner1922) (Pyrenulaceae).
Trypethelium cruentum Mont. (1837)=Pyrenula cruenta (Mont.) Vain. (Pyrenulaceae).
Trypethelium cruentum var. subdecolor Nyl.=Pyrenula cruenta (Mont.) Vain., based on description in Galloway (Reference Galloway1985) (Pyrenulaceae).
Trypethelium erumpens Stirt. (1875); non Fée.—Melanotheca stirtoniana Müll. Arg. (1894).—Trypethelium stirtonianum (Müll. Arg.) Hellb. (1896)=Pyrenula sp., based on description in Galloway (Reference Galloway1985) (Pyrenulaceae).
Trypethelium fuscum Kremp. (1873).—Melanotheca fusca (Kremp.) Müll. Arg. (1888)=Pyrenula sp. non Pyrenula fusca fide Zahlbruckner (Reference Zahlbruckner1922) (Pyrenulaceae).
Trypethelium gregale C. Knight (1886)=Anthracothecium gregale (C. Knight) Aptroot (Pyrenulaceae).
Trypethelium hemisphaericum Eschw. in Martius (1833)=Porina hemisphaerica (Eschw.) Müll. Arg. (Porinaceae).
Trypethelium inarense Vain. (1883)=Polycoccum sp. (Polycoccaceae).
Trypethelium inconspicuum C. F. W. Meissn. (1831).—Melanotheca inconspicua (C. F. W. Meissn.) Müll. Arg. (1885)=Pyrenula sp. fide Zahlbruckner (Reference Zahlbruckner1922) (Pyrenulaceae).
Trypethelium leucotrypum Nyl. (1867)=Pyrenula leucotrypa (Nyl.) Upreti (Pyrenulaceae).
Trypethelium madreporiforme var. obscurius C. Bab. ex Hook. f. (1855)=non-lichenized fungus (Xylariaceae sp.).
Trypethelium melanophthalmum (Mont.) Nyl. (1857)=Pyrenula melanophthalma (Mont.) Trevis. (Pyrenulaceae).
Trypethelium melanophthalmum f. arctecinctum (Fée) Nyl. (1857)=Pyrenula arctecincta Fée (Pyrenulaceae).
Trypethelium nanosporum C. Knight (1886)=Lithothelium nanosporum (C. Knight) Aptroot (Pyrenulaceae).
Trypethelium nigritulum Nyl. (1863)=Pyrenula sp. fide Zahlbruckner (Reference Zahlbruckner1922) (Pyrenulaceae).
Trypethelium nudum Fée (1837)=Pyrenula sp. fide Zahlbruckner (Reference Zahlbruckner1922) (Pyrenulaceae).
Trypethelium oblitescens Stirt. (1879).—Pyrenastrum oblitescens (Stirt.) Makhija & Patw. (1992)=probably Pyrenula cruenta (Mont.) Vain., based on descriptions (Pyrenulaceae).
Trypethelium ocellatum Zenker (Reference Zenker1827)=Pyrenula mamillana (Ach.) Trevis. fide Zahlbruckner (Reference Zahlbruckner1922) (Pyrenulaceae).
Trypethelium ornatum (Müll. Arg.) Hellb. (1896); non Müll. Arg.; Melanotheca ornata Müll. Arg.=Pyrenula cruenta (Mont.) Vain., based on description in Galloway (Reference Galloway1985) (Pyrenulaceae).
Trypethelium pallidum C. Knight (1886)=Anthracothecium prasinum (Eschw.) R. C. Harris (Pyrenulaceae).
Trypethelium papillatum C. Knight (1886)=Pyrenula quassiaecola Fée (Pyrenulaceae).
Trypethelium paradoxum Ach. (1817)=Sclerophyton seriale (Ach.) Sparrius (Roccellaceae).
Trypethelium pauperrimum Müll. Arg. (1882)=non-lichenized fungus fide Letrouit-Galinou (Reference Letrouit-Galinou1957).
Trypethelium planum C. Knight (1886)=Pyrenula subvariolosa (C. Knight) Aptroot (Pyrenulaceae).
Trypethelium purpurascens (Müll. Arg.) Stizenb. (1895).—Melanotheca purpurascens Müll. Arg. (1895)=Pyrenula sp., probably P. cruenta (Mont.) Vain. fide Zahlbruckner (Reference Zahlbruckner1922) (Pyrenulaceae).
Trypethelium pyrenuloides Mont. (1843)=Pyrenula pyrenuloides (Mont.) R. C. Harris (Pyrenulaceae).
Trypethelium rubescens C. Knight (1889)=Pyrenula cruenta (Mont.) Vain. (Pyrenulaceae).
Trypethelium rubrum C. Knight (1884)=Pyrenula cruenta (Mont.) Vain. (Pyrenulaceae).
Trypethelium schizostomum Leight. (1869)=Thelotrema schizostomoides Zahlbr. fide Zahlbruckner (Reference Zahlbruckner1924) (Graphidaceae).
Trypethelium sclerotium Fée (1824)=Pertusaria sclerotium (Fée) Müll. Arg. fide Zahlbruckner (Reference Zahlbruckner1928) (Pertusariaceae).
Trypethelium scoria Fée (1824); nom. illeg.=Pyrenula anomala (Ach.) Vain. (Pyrenulaceae).
Trypethelium sordidescens Fée (1837)=Leucodecton compunctum A. Massal. (Graphidaceae).
Trypethelium stirtonianum (Müll. Arg.) Hellb. (1896).—Trypethelium erumpens Stirt. (1875) non Fée.—Melanotheca stirtoniana Müll. Arg. (1894)=Pyrenula sp., based on description in Galloway (Reference Galloway1985) (Pyrenulaceae).
Trypethelium subincruentum Nyl. (1890)=Pyrenula cruenta (Mont.) Vain. (Pyrenulaceae).
Trypethelium subplanum C. Knight (1886)=Anthracothecium gregale (C. Knight) Aptroot (Pyrenulaceae).
Trypethelium subumbilicatum C. Knight (1886)=Pyrenula subumbilicata (C. Knight) Aptroot (Pyrenulaceae).
Trypethelium tetrathalamium Fée (1824)=Pertusaria tetrathalamia (Fée) Nyl. (Pertusariaceae).
Trypethelium umbilicatum C. Knight (1886)=Pyrenula subvariolosa (C. Knight) Aptroot (Pyrenulaceae).
Trypethelium verrucarioides Fée (1837).—Chiodecton verrucarioides (Fée) Müll. Arg. (1887)=Enterographa verrucarioides (Fée) Müll. Arg. (1885) (Roccellaceae).
Trypethelium verrucosum Fée (1824)=Pertusaria sp. fide Zahlbruckner (Reference Zahlbruckner1928) (Pertusariaceae).
We are grateful to NSF-DEB 0715660 ‘Neotropical Epiphytic Microlichens – An Innovative Inventory of a Highly Diverse yet Little Known Group of Symbiotic Organisms’ to The Field Museum (PI Robert Lücking) for funding partial aspects of this work. AA thanks the Stichting Hugo de Vries-Fonds for various travel funds. The curators of the herbaria mentioned are warmly thanked for sending specimens and for helping us during visits to their herbaria. Dr Harrie Sipman is especially thanked for placing his huge amount of recent collections at our disposal, and for help with editing the manuscript. Dr Makhija is warmly acknowledged for sending many isotype species of species described from India. Colleagues in Graz placed the collections from the Surumoni crane project at our disposal, and Harald Komposch placed data from an unpublished manuscript on these collections at our disposal. The library staff of Helsinki University are thanked for providing a copy of Acharius’ monograph, which was a very elusive publication. Leo Spier is thanked for performing TLC. Fred Barrie and Paul Kirk assisted in the clarification of nomenclatural questions. Most of the images used in this synopsis were made by the authors; for additional images we thank Felix Schumm (particularly in the general section) and, in selected cases (see Material and Methods), various herbaria contributing to the Global Plants Initiative (GPI), in particular Holger Thüs (BM), Philippe Clerc (G), Soili Stenroos (H), and Arne Anderberg (S), as well as Ek Sangvichien for placing images of new Astrothelium species at our disposal.






























































