Early developmental experiences, including growth and weight gain occurring during critical plasticity periods, have shown to affect health throughout life(Reference Barker1). In this sense, the investigation of weight trajectories could help in the recognition of individuals with a higher risk of being affected by overweight in adulthood(Reference Ward, Long and Resch2). Rapid weight gain intra-utero(Reference Evensen, Emaus and Kokkvoll3), during infancy, early childhood(Reference Munthali, Kagura and Lombard4,Reference Zheng, Lamb and Grimes5) and overall during childhood(Reference Giles, Whitrow and Davies6,Reference Rzehak, Oddy and Mearin7) , has been associated with adiposity later in life. Recently, a study showed that early rapid growth in infancy was associated with the combined effect of greater body mass index (BMI), waist circumference and subcutaneous fat at age 4(Reference Lin, Jiang and Wang8). In addition to the effects on adiposity, accelerated weight gain during childhood seems to increase arterial stiffness in school-age children(Reference Pais, Correia-Costa and Moura9), leads to coronary events(Reference Barker, Osmond and Forsén10) and has a higher risk of all-cause and cancer mortality in adulthood(Reference Von Bonsdorff, Törmäkangas and Rantanen11). In this sense, it is important to identify sensitive periods in time that could lead to the development of overweight/obesity and associated non-communicable diseases.
The association between rapid weight gain early in life and adiposity may be explained by a variety of maternal and child factors. Studies have speculated that the adverse effects of rapid growth on adiposity may be, in part, explained by low birth weight, following a period of growth restriction. Infants with low birth weight are more likely to have higher renal androgen levels, insulin resistance and central fat accumulation, increasing the vulnerability to weight gain(Reference Zheng, Lamb and Grimes5). It has been described that rapid prenatal and postnatal growth seem to program for later obesity by altering hypothalamic function, as observed in animal and human studies(Reference Remmers and Delemarre-van de Waal12). It is also believed that energy balance and its regulation may also be programmed, which may be explained by a variety of factors, such as alterations on the predominant appetite regulation site of the fetus, leading to hyperphagia throughout life(Reference Desai and Ross13), and on the food-related orosensory circuit development (i.e., the stimuli raised from palatable foods and the positive feedback to the brain leading to increases in the rate and amount of food consumed)(Reference Zeltser14). Additionally, the effects of early life growth on appetite hormones need to be mentioned(Reference Jornayvaz, Vollenweider and Bochud15,Reference Önal, Cinaz and Atalay16) . It has been described that intra-uterine growth restriction may affect appetite control; low birth weight has been associated with higher leptin(Reference Jornayvaz, Vollenweider and Bochud15) and ghrelin levels(Reference Önal, Cinaz and Atalay16), and greater odds of feeding difficulties and poorer eating in the first 4–6 months of life(Reference Oliveira, De Lauzon-Guillain and Jones17). One study with 2227 children in Amsterdam showed that conditional weight gain, which was defined as the difference between observed and predicted body size, during early infancy (1–6 months) and preschool years (1–5 years of age) was associated with greater energy intake and lower Satiety Responsiveness at age 5, that is, the ability to reduce food intake to compensate for prior foods/snacks to regulate one’s energy intake(Reference van Deutekom, Chinapaw and Vrijkotte18). In another study, in a small longitudinal sample (209 children in China), those with an early infancy rapid growth showed larger appetite (i.e., greater Food Responsiveness and lower Satiety Responsiveness and Food Fussiness) at age 4 and were more likely to develop higher adiposity, compared to those with steady growth and lower appetite(Reference Lin, Jiang and Wang8). Considerable evidence of a bidirectional effect of child weight and weight gain and eating self-regulation over time has been described in a recent systematic review, suggesting that children with excess weight in early life may be more susceptible to develop food approach traits later in childhood(Reference Grammer, Balantekin and Barch19). However, the effect of weight trajectories in the beginning of life on child eating self-regulation was not accessed in this study and remains unclear. Overall, these results may indicate that early life growth and development could influence later appetitive traits, which may have a mediating role in the development of overweight and non-communicable diseases throughout life.
Lifestyles, such as appetitive traits, screen activity and sleep duration, developed in early childhood are some of the main modifiable factors influencing child weight status(Reference Larqué, Labayen and Flodmark20). As highlighted in recent studies, it seems that greater weight gain and weight status early in life are associated with appetitive traits; however, the effect of weight trajectories, occurring in infancy and early childhood, on later appetitive food approach (namely, Food Responsiveness, Enjoyment of Food, Emotional Overeating and Desire to Drink) and food avoidant traits (namely Food Fussiness, Satiety Responsiveness, Emotional Undereating and Slowness in Eating) in school-age years is not well established. The assessment of a wider range of appetitive traits, common in school-age, and the use of longitudinal designs and larger sample sizes would strengthen previous evidence. To explore this research gap, this study aims to explore the association between weight trajectories from birth to 5 years of age and appetitive traits at 7. We expect to find an association between an accelerated weight gain in the first years of life and food approaching appetitive traits in school-age.
Materials and methods
Study population
This study includes children from the population-based birth cohort study Generation XXI. Mothers were invited to participate in the study within 72 hours after delivery in all public maternities of Porto, Northern Portugal. Of all eligible mothers, 91 % agreed to participate (8495 mothers and 8647 children at baseline). All families were invited for follow-ups at 4 years old (year) (n 7459) and 7 years (n 6889). At baseline, mothers were mainly married or living with a partner (94·0 %); approximately 72 % were employed at delivery and nearly a third of the participants had a monthly income above 1500 € per month. Additional details can be found elsewhere(Reference Alves, Correia and Barros21,Reference Larsen, Kamper-Jørgensen and Adamson22) . The present investigation included 3855 children (study flow chart in Fig. 1). When comparing child and maternal characteristics from the study sample (n 3855) with excluded participants (n 4792), we found that participating mothers were slightly older (mean 29·89 (sd 5·15) years v. 28·36 (sd 6·34), tWelch = 12·43, P < 0·001) and more educated (median ± interquartile range (IQR), 12·0 ± 8·0 completed years of education v. 9·0 ± 6·0, Mann–Whitney z = 15·68, P < 0·001). No differences were found regarding maternal pre-pregnancy BMI, child sex and child BMI z-scores (BMIz) at 4 and 7 years. According to Cohen’s effect size values(Reference Cohen23), the magnitude of differences was not high for maternal age and education (Cohen’s d = 0·26 and 0·35, respectively), suggesting that these differences were due to the large sample size rather than due to differences between participants’ characteristics.
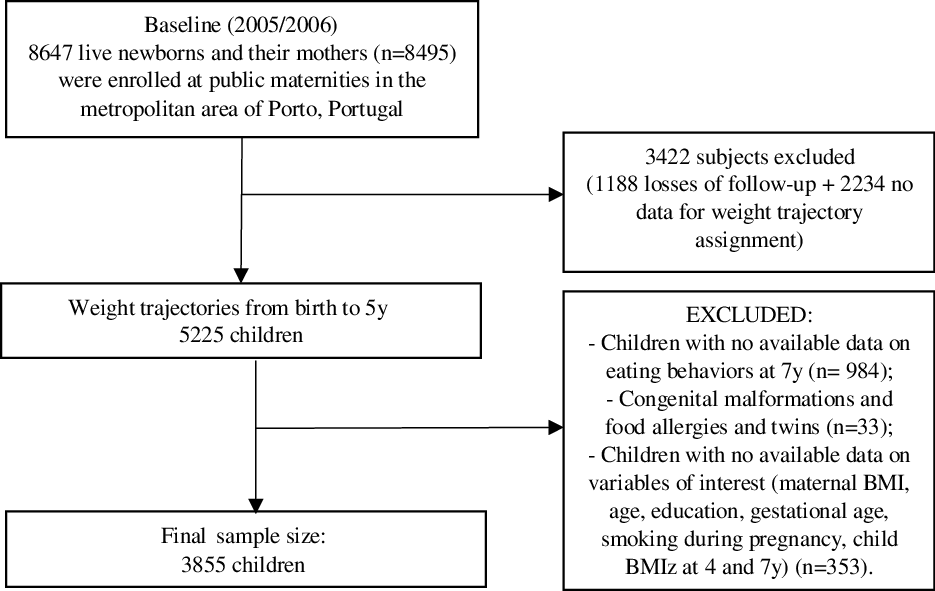
Fig. 1. Study flow chart of participants from the Generation XXI birth cohort.
Growth trajectories
At the 4-year follow-up, mothers were asked to bring their child health book, that is, child anthropometric measurements record performed and filled out by health professionals at every medical visit, to extract data on the child’s weight measurements from birth to current age. Even though follow-up started at 4 years, child age varied from 3·6 to 6·4 years. Each child had, in median, sixteen records. Weight trajectories were defined, in previous studies within Generation XXI, by the intercept, slope, quadratic and cubic random terms estimated by a mixed model (normal mixture modelling for model-based clustering)(Reference Pais, Correia-Costa and Moura9,Reference Fonseca, Durão and Lopes24) . Although growth trajectories are sex-specific, in a sensitivity analysis, fixed effect terms by sex were added to the model, but no significant interaction was found; thus, growth trajectories were modelled without sex stratification. As described by Fonseca et al., weight trajectories for both sexes together were identified in 5225 participants(Reference Fonseca, Durão and Lopes24). Models with the lowest Bayesian information criterion values, allowing for the most homogeneous grouping of individual pattern of growth, were considered appropriate(Reference Pais, Correia-Costa and Moura9,Reference Fonseca, Durão and Lopes24) . Four weight trajectories were identified (Fig. 2). The denomination for ‘normal weight gain’ (trajectory A) was chosen because the average weight in this weight trajectory closely overlapped the 50th percentile in the weight-for-age curve(Reference Fonseca, Durão and Lopes24), according to the World Health Organization (WHO)(Reference Pais, Correia-Costa and Moura9,25) . ‘Weight gain during infancy’ (trajectory B) included children who were born with low birth weight and gained weight mainly during infancy, showing a catch-up growth in the first year of life, ‘weight gain during childhood’ (trajectory C), included those children with continuous weight gain since birth and ‘persistent weight gain’ (trajectory D), included those children who always showed higher weight than the average(Reference Fonseca, Durão and Lopes24).

Fig. 2. Weight trajectories from birth (month 0) to 70 months in the Generation XXI birth cohort. Lines represent ‘normal weight gain’ trajectory (A); ‘weight gain during infancy’ trajectory (B); ‘weight gain during childhood’ trajectory (C) and ‘persistent weight gain’ trajectory (D), as described previously(Reference Pais, Correia-Costa and Moura9,Reference Fonseca, Durão and Lopes24) .
Child appetitive traits
Appetitive traits were assessed at the 7-year follow-up, using a parent-report questionnaire, the Children’s Eating Behaviour Questionnaire(Reference Wardle, Guthrie and Sanderson26), mainly responded to by mothers (88·4 %). This questionnaire was previously validated in this same cohort(Reference Albuquerque, Severo and Oliveira27) and includes thirty-five items, divided into eight subscales: four of these subscales, namely Enjoyment of Food, Food Responsiveness, Desire to Drink and Emotional Overeating, assess children’s general appetite and interest for food and drinks and are therefore labelled as ‘food approach behaviours’. The remaining subscales, Satiety Responsiveness, Slowness in Eating, Food Fussiness and Emotional Undereating, assess child avoidance and lack of interest towards foods and are therefore labelled as ‘food avoidant behaviours’. Responses are given in a 5-point Likert scale, ranging from ‘Never’ to ‘Always’. Higher subscale mean scores indicate greater levels of each behaviour. In accordance to the original scale(Reference Wardle, Guthrie and Sanderson26), five of the items were reverse-coded due to opposite phrasing. In questionnaires with < 50 % of missing items (approximately 3 %), data were imputed. Adequate psychometric properties of the Children’s Eating Behaviour Questionnaire were observed at 7 years (Cronbach’s α = 0·74–0·85)(Reference Albuquerque, Severo and Oliveira27).
Other maternal and child characteristics
At 4 and 7 years, anthropometric measurements were performed by trained examiners according to standard procedures(Reference Gibson28). BMI was calculated and age- and sex-adjusted BMIz were generated, following the WHO (for descriptive purposes only)(25,Reference de Onis, Onyango and Borghi29) . For those children younger than 5 years, BMIz values below –2 sd were considered as ‘underweight’, between ≥ −2 sd and ≤ 1 sd as ‘normal weight’, between > 1 sd and ≤ 2 sd as ‘at risk of overweight’, between > 2 sd and ≤ 3 sd ‘overweight’ and BMIz values > 3 sd as having ‘obesity’(25). For children above 5 years, ‘underweight‘ was defined for z-scores < −2 sd, ‘normal weight’ for z-scores ≥ −2 sd and ≤ 1 sd, ‘overweight’ for z-scores >1 and ≤ 2 sd and ‘obesity’ for z-scores above 2 sd (Reference de Onis, Onyango and Borghi29). Prematurity was calculated based on the gestational weeks at delivery, and premature children were defined as those born with less than 37 weeks. Children born with less than 1500 g were defined as having ‘very low birth weight’, between 1500 g and less than 2500 g as ‘low birth weight’, and equal and above 2500 g as normal birth weight(30).
Maternal age, education, smoking habits during pregnancy, pre-pregnancy BMI and household monthly income were obtained by face-to-face interviews conducted by trained researchers. Mother’s pre-pregnancy weight status was classified as follows: BMI < 18·5 kg/m2 for ‘underweight’, between ≥ 18·5 and < 25·0 kg/m2 for ‘normal weight’, between ≥ 25·0 and < 30·0 kg/m2 for ‘overweight’ and ≥ 30·0 kg/m2 for ‘obesity’(31). Smoking habits during pregnancy were dichotomised in ‘never smoked’ and ‘smoked’ and were also included in the adjustments.
Statistical analysis
Continuous data were expressed as mean and standard deviations or median and interquartile ranges, and frequency distributions (n (%)) were expressed for categorical data. Distribution of variables was evaluated using Kolmogorov–Smirnov test and Q-Q plots. The first approach to test associations between weight trajectories (independent variable/exposure) and appetitive traits at 7 (outcome variable) was through one-way ANOVA for each appetitive trait. Crude means of each behaviour and 95 % confidence interval (CI) in each weight trajectory were described. Homogeneity was investigated through the Levene’s test(Reference Levene and Olkin32), and Welch corrections(Reference Welch33) were performed when unequal variances were identified. Lastly, Dunnett’s post-hoc testing was performed in order to identify significant differences between weight trajectories and ‘normal weight gain’ trajectory. A second approach was through adjusted generalised linear models, with the calculation of β-regression coefficients and the respective confidence intervals.
The inclusion of confounders was based on literature review on factors possibly associated with both child early life growth(Reference Munthali, Kagura and Lombard4,Reference Giles, Whitrow and Davies6,Reference Mattsson, Maher and Boland34,Reference Walton, Daniel and Mahood35) and appetitive traits at school-age(Reference Carnell, Pryor and Mais36,Reference Fildes, Mallan and Cooke37) . Since crude models were very similar to the first adjusted models, these are presented as supplementary material only (online Supplementary Table 1). Model 1 was adjusted for maternal characteristics only: pre-pregnancy BMI, maternal smoking during pregnancy, age, and education. Model 2 was further adjusted for child characteristics: child sex and prematurity. Finally, the final adjusted model further included child height (in cm) at the time of appetitive trait measurement (i.e. 7 years). As in the first approach, the ‘normal weight gain’ trajectory was considered the reference category. Since we hypothesised that child prenatal life could affect the development of appetitive traits(Reference Albuquerque, Severo and Oliveira27), and based on the P-interaction terms among maternal pre-pregnancy BMI and the weight trajectories (e.g. weight gain during childhood trajectory × pre-pregnancy normal weight and obesity) (P = 0·030 and 0·39 in Children’s Eating Behaviour Questionnaire-Emotional Overeating model), we performed a sensitivity analysis by stratifying for maternal pre-pregnancy BMI (underweight × normal weight × overweight/obesity). Models were adjusted for maternal age, maternal smoking during pregnancy, education, child sex and prematurity (model 1) and child height at 7 years (model 2) (online Supplementary Tables 2a-c).
Linear regression assumption checks were performed, by testing homoscedasticity, residuals’ symmetric distribution through Q-Q plots and Cook’s distance, and multicollinearity between independent variables using variance inflation factor. Bonferroni’s correction, which is the most common method used to adjust for multiple comparisons by adapting the significant threshold for the number of tests that are performed (i.e. α (0·05)/tests for eight appetitive traits)(Reference Vasilopoulos, Morey and Dhatariya38), was used and an α of 0·006 was considered for statistical significance in the multivariable models. Statistical analyses were carried out using Stata/SE version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. StataCorp LLC).
Ethical approval
Generation XXI was approved by the University of Porto Medical School/São João Hospital Centre Ethics Committee (27 April 2005) and by the Portuguese Data Protection Authority (Protocol code 5833, approved on 30 May 2011). All the phases of the study complied with the Ethical Principles for Medical Research Involving Human Subjects expressed in the Declaration of Helsinki. Accordingly, written informed consent from the parents (or legal substitute) and oral assent from the children were obtained at each evaluation.
Results
Mother and child characteristics are described in Table 1. Unadjusted mean differences and the respective 95 % CI between the different weight trajectories are shown graphically in Fig. 3. Compared with the ‘normal weight gain trajectory’, children with ‘weight gain during infancy’, ‘weight gain during childhood’ and ‘persistent weight gain’ in the first years of life showed significantly higher scores on the food approach behaviours Enjoyment of Food (P < 0·001) and Food Responsiveness (P < 0·001) at 7 years. Higher scores on Emotional Overeating at 7 years, compared with the normal weight gain trajectory, were observed among those with ‘weight gain during infancy’ and ‘weight gain during childhood’ (P < 0·001). Regarding food avoidant behaviours and compared with the ‘normal weight gain’ trajectory, children with ‘weight gain during infancy’, ‘weight gain during childhood’ and ‘persistent weight gain’ scored less on Satiety Responsiveness (P < 0·001) and Slowness in Eating (P < 0·001) at 7 years. Compared with children in the ‘normal weight gain’ trajectory, those children gaining weight during infancy and childhood showed lower scores of Food Fussiness at 7 years (P < 0·001). Those children with ‘weight gain during infancy’ also showed lower scores on Emotional Undereating, compared to those with normal weight gain (P < 0·001).
Table 1. Mother and child characteristics at baseline, 4 and 7 years of age, Generation XXI birth cohort (n 3855) (Numbers and percentages; mean values and standard deviations; medians and interquartile ranges)

Md, median; IQR, interquartile range; CEBQ, Children’s Eating Behaviour Questionnaire.
* Values are counts and percentages for categorical variables, means and standard deviations for continuous normally distributed variables, medians and interquartile ranges) for continuous, non-normally distributed variables.
† Mother weight status was calculated according to WHO.
‡ Birth weight was defined according to WHO.
§ Child weight status was calculated according to WHO. ‘At risk of overweight’ is only calculated for children below 5 years of age.
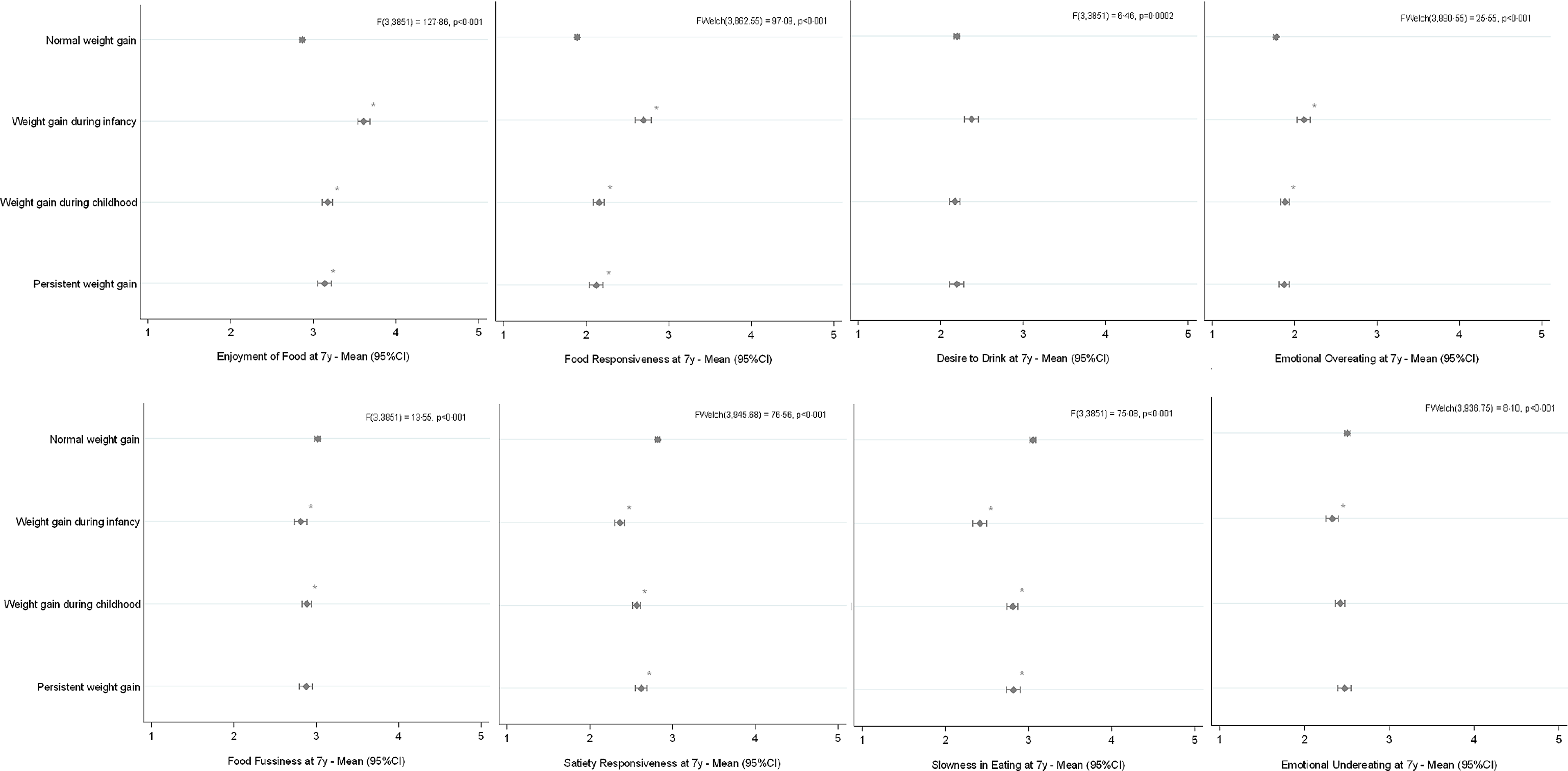
Fig. 3. Crude mean scores of each child appetitive trait at 7 years, according to weight trajectories from birth to 5 years (n 3855). Asterisks indicate statistically significant differences, compared with ‘normal weight gain’, according to Dunnett’s post-hoc testing.
In the final adjusted model of the multivariable linear regression analyses, children in the ‘weight gain during childhood’, ‘persistent weight gain’ and especially those in the ‘weight gain during infancy’ trajectory, compared with those in the ‘normal weight gain’, showed significantly lower scores on Slowness in Eating, that is, ate faster at 7 years, and lower on Satiety Responsiveness, that is, were less able to compensate for prior food intake at 7 years, and higher scores on Food Responsiveness and Enjoyment of Food, indicating a greater interest towards foods (Table 2). Gaining weight during infancy was also significantly associated with lower scores on Food Fussiness and Emotional Undereating and higher scores on Emotional Overeating at 7 years. Those children gaining weight during infancy showed also higher scores in Desire to Drink at 7 years (models 1 and 2); however, the association lost significance when further adjusting the model for child height at 7 years.
Table 2. Generalised linear regression analyses for the associations between weight trajectories from birth to 5 years, and appetitive traits at age 7 (n 3855) (Beta-coefficients and 99·4 % confidence intervals)

* CI with Bonferroni’s correction,
† P < 0.006.
Model 1 is adjusted for maternal BMI, maternal smoking during pregnancy, age and education; model 2 is adjusted for model 1 plus child sex and prematurity; model 3 is adjusted for model 2 plus child height at 7 years.
In the sensitivity analyses, we found that among mothers with underweight before pregnancy (n 144) and whose children gained weight during infancy, compared with those in the normal weight gain trajectory, children scored higher on Food Responsiveness at age 7 (online Supplementary Table 2a). Those children gaining weight during infancy, childhood and persistently showed greater scores on Food Responsiveness and Enjoyment of Food, and lower scores on Satiety Responsiveness and Slowness in Eating among mothers with normal weight before pregnancy (n 2497), after adjusting for confounders (online Supplementary Table 2b). The previously found association between gaining weight during infancy and higher scores on Emotional Overeating was also maintained significant in the stratified analyses, among mothers with normal weight before pregnancy. In addition, being less responsive to internal satiety cues (i.e. Satiety Responsiveness) and eating faster (i.e. lower scores on Slowness in Eating) at 7 years was found among those mothers with excess weight before pregnancy (n 1214) and whose children gained weight mainly during infancy and childhood, compared with those in the normal weight gain trajectory. Regarding food approach behaviours, those children gaining weight during infancy and childhood showed higher scores on Food Responsiveness and Enjoyment of Food at 7. Finally, gaining weight mainly during infancy was associated with greater scores on Emotional Overeating, compared with those in the normal weight gain trajectory (online Supplementary Table 2c).
Discussion
Overall, we found that weight trajectories during the first 5 years, deviating from normal weight gain, were associated with a more avid appetite in school-age years. Associations were stronger if greater weight gain occurred during infancy, rather than during childhood or persistently across the first 5 years; however, for all weight trajectories, significant effects were found for food approach and food avoidant behaviours. For those children gaining weight during infancy, significant lower scores for Food Fussiness and Emotional Undereating, and higher scores for Emotional Overeating were found, indicating that these children tend to eat more due to negative emotional states and be less fussy, independently of confounders.
To our knowledge, only a few studies investigated the association between weight trajectories in the first years of life and later appetitive traits. Deutekom and colleagues(Reference van Deutekom, Chinapaw and Vrijkotte18) described that conditional weight gain (i.e. the difference between observed and predicted body size) in the first months of life (1–3 and 3–6 months) and from 1 to 5 years was associated with an increased daily energetic intake and decreased Satiety Responsiveness at 5 years, even after adjusting for child BMIz. Our results corroborate with these findings, since we also showed an association between weight gain during infancy, childhood and persistent weight gain and lower scores on Satiety Responsiveness at 7 years. Since this study only investigated the effect on Satiety Responsiveness, we are not able to compare results with the other appetitive traits. Besides this study, in a recent study in China(Reference Lin, Jiang and Wang8), researchers found that those children that showed a rapid growth during early infancy (using weight-for-age z-score) had higher BMI, waist circumference and subcutaneous fat at age of 4 and, additionally, higher scores on Food Responsiveness and Enjoyment of Food and lower scores on Satiety Responsiveness and Food Fussiness, corroborating with our results in this younger age group. We observed that the effect on Food Fussiness was only observed among children gaining weight during infancy. Parents’ perception of a child Food Fussiness may, therefore, be because children are perceived as being thin(Reference Oliveira, De Lauzon-Guillain and Jones17); however, more studies to assess this relationship are necessary. Additionally, the lower scores on Satiety Responsiveness, which is an inhibitory mechanism driven by interoceptive satiety cues, and higher food cue responsiveness (Enjoyment of Food, Food Responsiveness and Emotional Overeating (among those gaining weight in infancy)), which are product of both genetic and environmental influences, at age 7 may be a risk factor for a higher weight in the future, as shown by others(Reference Boutelle, Manzano and Eichen39).
One possible mechanism that could explain the association between weight trajectories and appetite is fetal programming. Prenatal undernutrition and growth restriction (leading to low birth weight) have shown to increase the release of dopamine and serotonin leading to hyperphagia, greater consumption of palatable foods(Reference Laureano, Alves and Miguel40) and weight gain(Reference Manuel-Apolinar, Rocha and Damasio41) in animal studies. In humans, individuals that experienced intra-uterine growth restriction showed altered food preferences in infancy and adulthood, mainly driven by hedonic eating, with increased dopamine signalling(Reference Silveira, Pokhvisneva and Gaudreau42), greater preference for sweet tastes(Reference Silveira, Pokhvisneva and Gaudreau42) and/or high-fat foods(Reference Lussana, Painter and Ocke43). This phenomenon can be explained by fetal programming that occurs in utero as an attempt of the fetus to adapt to poor intra-uterine conditions. This attempt may be beneficial if the prenatal conditions would prevail in postnatal life; however, they become detrimental in the case of postnatal life differing from the prenatal life(Reference Hales and Barker44), increasing the risk of weight gain and overeating(Reference Dalle Molle, Bischoff and Portella45). As premature children often experience challenging eating behaviours and eating difficulties in childhood(Reference Walton, Daniel and Mahood35), child prematurity was also tested in the adjustment of models, together with child sex. However, we believe that since a little percentage of children were born premature in the current sample (6·84 %), effects between weight trajectories and child appetitive traits at 7 years did not change significantly. Additional research with greater sample of preterm children is necessary to confirm the mechanism of fetal programming and later appetite in childhood.
Another possible mechanism is the effect of appetite regulatory hormones, as described briefly above, which may be programmed in early postnatal life and could influence later appetitive traits. Weight gain during infancy (first 9 months of life) was shown to be negatively related to ghrelin corrected to adiposity and adiponectin concentrations (i.e. hormone concentration/body fat and body fat percentage) at 17 years, but no effect was observed on leptin levels(Reference Larnkjær, Schack-Nielsen and Mølgaard46). It has also been suggested that children born with low weight might develop high leptin levels during catch-up growth, leading to leptin resistance later in life(Reference Jaquet, Leger and Tabone47). Leptin resistance would, in this sense, produce a weakened anorexigenic effect of leptin and thus promote a heightened food approach and lower food avoidant behaviours, as also previously observed by our research group(Reference Warkentin, Carnell and Oliveira48). However, our study aims did not include the investigation of hormones in the association between weight trajectories and appetitive traits. Future research is necessary aiming to examine the associations between appetitive hormones and appetitive traits from early infancy on weight trajectories later in life.
Lastly, it is worth mentioning the potential effect of genetic predisposition on appetitive traits. The behavioural susceptibility to obesity theory(Reference Carnell and Wardle49) proposes a gene–environment interaction, in which individuals with greater obesogenic appetitive traits, such as higher Food Responsiveness and lower Satiety Responsiveness, are more likely to overeat in situations when high palatable foods are available, thus increasing weight gain. Therefore, it is plausible to assume that children who experience a rapid weight gain in early life live in more obesogenic environments, for example, having parents with overweight(Reference Griffiths, Hawkins and Cole50), a high consumption of unhealthy foods(Reference Guerrero, Mao and Fuller51) and lower breast-feeding duration(Reference Carling, Demment and Kjolhede52), and this fact would trigger the expression of genes on these appetitive traits(Reference Llewellyn and Wardle53). As described in the sensitivity analysis, maternal pre-pregnancy BMI seem to influence the development of later appetitive traits, especially food approaching behaviours.
Limitations of this study include the assessment of child appetitive traits through parent-reports, which may introduce measurement error due to its subjectivity and social desirability bias. However, the Children’s Eating Behaviour Questionnaire has demonstrated good internal consistency and reliability in this sample of 7-year-olds(Reference Albuquerque, Severo and Oliveira27) and good correspondence with objective behavioural measures of child’s eating(Reference Carnell and Wardle54). Weight measurement procedures recorded in health books could be subjected to some variability, since these were performed by different health professionals, in different health units, which may have increased measurement errors(Reference Fonseca, Durão and Lopes24). On the other hand, weight measurements were performed by a health professional or a trained examiner, which eliminates recall bias(Reference Fonseca, Durão and Lopes24). The current study also has a large proportion of missing data due to losses of follow-up and no data for weight trajectory assignment, and weight trajectories from analysed children were significantly different from those excluded (χ2(3) = 19·77, P < 0·001)). Additionally, although we have adjusted associations for several maternal characteristics, we did not have data on maternal own eating behaviours, which would be interesting to include in the adjustment of models as a possible confounder on child eating behaviours. Finally, our study findings may not be generalised to other populations with different socio-economic and cultural backgrounds. Strengths include the population-based prospective design, which allowed several weight measurements from birth to age 5 years, comprising a large number of repeated observations. In addition, we were able to adjust for many potential confounders. To our knowledge, this is the first study that investigated, using a large population-based birth cohort, the associations of weight trajectories at the beginning of life on appetitive traits in school-age years.
In sum, our results suggest that trajectories of weight during infancy and childhood play a role in the development of later appetitive traits, at school-age among Portuguese children. Gaining weight during infancy, childhood and persistently were associated with both food approach (Enjoyment of Food, Food Responsiveness) and food avoidant traits (Satiety Responsiveness and Slowness in Eating), at age 7, independently of maternal and child characteristics. Additionally, children gaining weight during infancy showed to be less fussy and had lower scores on Emotional Undereating but eat more in response to negative emotions (Emotional Overeating) at age 7. Finally, current results suggest that maternal pre-pregnancy BMI may influence appetitive traits in childhood, for example, decreasing responsiveness towards foods among those children whose mothers had excess weight before pregnancy. Intra-utero life and early infancy seem to be a sensitive period in the development of later appetitive traits, given the high susceptibility for environmental cues of the hypothalamic centres at the beginning of life. The control of rapid growth during infancy, in addition to strategies focused on the overall environment where children are living during pre-school and school-age years, period in which children are becoming more autonomous eaters and have a greater social life(Reference Rahill, Kennedy and Kearney55) are warranted. More prospective studies are necessary in order to replicate these findings, determine whether the noted appetitive traits track throughout life along with specific weight trajectories and support the hypotheses that appetitive traits that followed low birth weight and catch-up growth during infancy may be mediating the well-known effect between prenatal and postnatal growth and the development of excessive weight and non-communicable diseases in adulthood.
Acknowledgements
The authors gratefully acknowledge the families enrolled in Generation XXI for their kindness, all members of the research team for their enthusiasm and perseverance and the participating hospitals and their staff for their help and support.
Generation XXI was funded by the Health Operational Programme – Saúde XXI, Community Support Framework III and the Regional Department of Ministry of Health. This study was supported through FEDER from the Operational Programme Factors of Competitiveness – COMPETE and through national funding from the Foundation for Science and Technology – FCT (Portuguese Ministry of Education and Science) under the projects ‘Appetite regulation and obesity in childhood: a comprehensive approach towards understanding genetic and behavioural influences’ (PTDC/SAU-EPI/30334/2017/ POCI-01-0145- FEDER-030334), ‘Appetite and adiposity – evidence for gene-environment interplay in children’ (IF/01350/2015 – Andreia Oliveira) and ‘Pathways from early life to cardiometabolic risk during childhood’ (IF/01060/2015 – Ana Cristina Santos). This research was also supported by the Calouste Gulbenkian Foundation, Portugal. The authors would also stand out the support of the Epidemiology Research Unit (EPI-Unit: UIDB/04750/2020; LA/P/0064/2020; POCI-01-0145-FEDER-006862).
S. W. was responsible for study concept, data analysis and interpretation; drafting of the manuscript and final approval of the version to be published. A. C. S. was responsible for management of data collection; interpretation of data; critical revision of the manuscript and final approval of the version to be published. A. O. was co-responsible for study concept; interpretation of data; critical revision of the manuscript and final approval of the version to be published. All authors read and approved the final manuscript.
There are no conflicts of interest.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114523000272.








