Introduction
Haemophilus influenzae is a Gram-negative coccobacillus found mainly as a commensal in the human respiratory tract, and occasionally also in the gastrointestinal and genitourinary tracts. From there, the bacteria may disseminate and cause invasive diseases or local infections (meningitis, sepsis, septic arthritis, epiglottitis, pneumonia, otitis media, sinusitis, conjunctivitis, cellulitis, pericarditis, urinary tract infection, peritonitis, etc.) [Reference Murphy, Mandell, Bennett and Dolin1]. Based on the antigenic characteristics of their polysaccharide capsule, encapsulated strains are divided into six serotypes (a–f) [Reference Murphy, Mandell, Bennett and Dolin1]. Strains without a capsule (non-encapsulated) are termed non-typable. Among all the capsular types, H. influenzae serotype b (Hib) is the most significant cause of invasive disease. Before the introduction of the Hib conjugate vaccine, Hib was a major cause of childhood meningitis and other forms of invasive H. influenzae disease [Reference Murphy, Mandell, Bennett and Dolin1]. Since Hib conjugate vaccine was introduced more than two decades ago, the epidemiology of invasive H. influenzae disease has changed markedly [Reference Shapiro and Ward2, Reference Peltola3]. Recent reviews on invasive H. influenzae disease have been published [Reference Ulanova and Tsang4–Reference Agrawal and Murphy6], but none has dealt with the disease in Indigenous peoples although it is known that some Indigenous populations in North America have higher rates of invasive Hib disease compared to the general population. In this review, we examine the current status of invasive H. influenzae disease in Indigenous populations of North America including epidemiology, risk factors for invasive infection, prevention and control measures, and discuss the needs for future research.
Biology of H. influenzae
The capsule of H. influenzae is the most studied virulence factor and protective antigen. Using isogenic mutants which differ only in their capsule structures (and hence their serotypes) to infect infant rats, Zwahlen et al. demonstrated that a mutant with the Hib capsule is the most virulent, followed by H. influenzae serotype a (Hia), which in turn is more virulent than serotypes c–f [Reference Zwahlen7]. Currently licensed vaccines for protection against invasive H. influenzae disease are based on the capsule of Hib. Protein D from non-typable H. influenzae has also been used as a carrier protein in one form of pneumococcal conjugate vaccine (Synflorix™, GlaxoSmithKline Biologicals, Belgium) [Reference Vesikari8]. However, the impact of this vaccine on invasive H. influenzae disease has not been evaluated. Other virulence factors that have been noted in H. influenzae include lipo-oligosaccharide with its endotoxic lipid A, pili as well as other adherence factors, and IgA protease.
The population biology of encapsulated H. influenzae have been studied, and strains can be grouped into two phylogenetic groups, clonal divisions I and II [Reference Musser9]. Most serotypes a–d H. influenzae isolates belong to clonal division I, while small numbers of serotypes a and b, as well as all serotype f isolates belong to clonal division II. Serotype e isolates may belong to an intermediate group between clonal divisions I and II. In addition, each serotype of H. influenzae appears to be clonal with its own unique set of sequence types (STs) with minimal or no mixing between serotypes and their unique sets of clones or STs [Reference Sill10]. In contrast to encapsulated serotypable H. influenzae, non-typable H. influenzae strains have been regarded as genetically diverse and non-clonal [Reference Kaur11].
Another interesting feature of H. influenzae is the plasticity of its genome in part due to the high number of DNA uptake sequences present [Reference Smith12, Reference Hogg13]. How this genetic property of H. influenzae may affect its long-term evolutionary response to vaccine pressure is not known at this time. There are currently two schools of thought: one suggests that strain replacement has taken place and that replacement strains are now causing disease and altering the epidemiology of invasive H. influenzae disease [Reference Adam14], and another suggests that strain replacement has not occurred [Reference Ladhani15].
Epidemiology of invasive H. influenzae disease in the pre-Hib vaccine era
Before the introduction of Hib conjugate vaccines in the late 1980s–early 1990s, the highest incidence rates of invasive H. influenzae disease in the world were found in Indigenous children, i.e. in North American Indians, Eskimo/Inuit in the Arctic, and Australian Aboriginals [Reference Coulehan16–Reference Hanna21] (Table 1). According to population-based surveillance studies, the incidence rates of invasive Hib disease in the USA varied between 40 and 100 cases/100 000 children aged <5 years [Reference Wenger22]. In White Mountain Apache children, the annual incidence of H. influenzae meningitis was eight times higher than in the general US population [Reference Losonsky17]. In Alaska, the incidence of invasive Hib disease was 5·5 times higher in Eskimo than in non-Native children [Reference Ward, Lum and Hall18]. Although Hib was the dominant serotype of H. influenzae causing invasive disease worldwide, Hia was responsible for a number of cases in White Mountain Apache Indians, Australian Aboriginal and Papua New Guinea populations [Reference Losonsky17, Reference Hansman, Hanna and Morey19, Reference Hanna21, Reference Gratten23]. For example, in one White Mountain Apache Indian community, during 1981–1983, Hia strains accounted for 17% of invasive H. influenzae disease cases [Reference Losonsky17].
Table 1. Annual incidence rates of invasive Haemophilus influenzae disease in Indigenous populations before the introduction of Hib conjugate vaccine
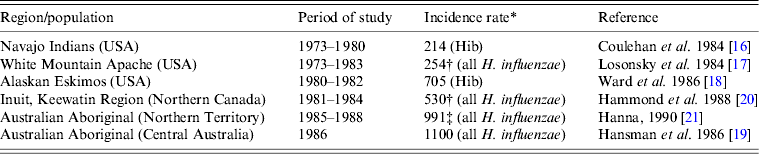
* Per 100 000 for children aged <5 years.
† Meningitis only.
‡ Estimated incidence.
In the pre-Hib vaccine era, invasive H. influenzae disease was a paediatric infection affecting mostly infants and young children. North American Indigenous children were affected at a younger age than their non-Indigenous counterparts, e.g. the peak occurrence of Hib meningitis in Navajo was age 4–8 months [Reference Coulehan16] and in White Mountain Apache children age 4–7 months [Reference Losonsky17]. In comparison, in the general US population, peak rates of invasive Hib disease occurred at age 6–11 months [Reference Wenger22]. A series of studies performed in the 1980s in Alaska found the highest incidence of invasive Hib disease during the first 2 years of life suggesting that an early exposure to Hib was responsible for the high rates of invasive disease in this population [Reference Ward, Lum and Hall18, Reference Ward24]. In Indigenous populations, non-symptomatic healthy carriers of Hib served as the major reservoir of infection and source of disease transmission. Carriage of Hib typically started at a very young age [Reference Jacups, Morris and Leach25]. For example, a high frequency of asymptomatic Hib carriage (18%) was found in Navajo infants on at least one occasion before they reached age 9 months [Reference Coulehan16].
Most cases of invasive Hib disease in North American Indigenous children presented as meningitis, often followed by severe neurological sequelae. In Navajo children, meningitis accounted for 70% of invasive Hib disease [Reference Coulehan16] while in Apache children aged <1 year, all cases of invasive Hib disease presented as meningitis [Reference Losonsky17]. The case-fatality rates for H. influenzae meningitis in Navajo Indians and Alaskan Eskimos were found to be lower than in the general US population, i.e. 4% and 3% vs. 5%, respectively [Reference Coulehan16, Reference Ward, Lum and Hall18, Reference Fraser26]. However, in Navajo children, neurological sequelae occurred in 16% of meningitis survivors [Reference Coulehan16].
Immune response to H. influenzae
Anti-capsular polysaccharide antibodies, which mediate complement-dependent killing and phagocytosis of encapsulated bacteria, are the major immune mechanism against invasive H. influenzae disease [Reference Schreiber27]. The immune response to Hib capsular polysaccharide antigen, polyribosylribitol phosphate (PRP) has been well studied. In response to either natural exposure to Hib or vaccination with PRP, IgG, IgM and IgA antibodies are produced; IgG is the dominant isotype with prevalence of IgG2 followed by IgG1 [Reference Schreiber27, Reference Shackelford28]. An anti-PRP antibody level of ⩾0·15 μg/ml has been considered as an indicator of short-term protection against invasive Hib disease; a level of ⩾1 μg/ml achieved 1 month after immunization correlates with clinical protection for a minimum of 1 year and serves as an indicator of long-term protection [Reference Anderson29]. The role of IgA in defence against invasive H. influenzae disease is uncertain, but secretory IgA may control the spread of bacteria from the nasopharynx to the normally sterile sites of the body.
In the pre-vaccine era, the development of protective immunity against Hib followed the natural history of exposure to this microorganism. Newborn babies and infants during the first months of life are protected by maternal IgG antibodies; infants become susceptible to invasive Hib disease as maternal IgG declines. The carriage of Hib in the nasopharynx usually starts after age 2 months and contributes to the development of natural immunity. Natural antibodies against Hib are also induced by the exposure to some non-pathogenic bacteria that are common in the environment and carry antigens cross-reacting with PRP. With age, an increase in levels of anti-PRP antibodies in serum coincides with a decline in the incidence of invasive Hib disease in the population (summarized in [Reference Kelly, Moxon and Pollard30]). Bacterial capsular polysaccharides induce a T-cell independent antibody response, which is characterized by a delay in the development until age >2 years [Reference Vos31]. As a result, most unvaccinated children have low anti-PRP antibody levels between ages 6 months and 4 years [Reference Mäkelä32].
Studies in some North American Indigenous populations found that neonates were protected against invasive Hib disease by maternal IgG but that they rapidly lost the antibody. Among Navajo neonates, 79% had protective anti-PRP levels (⩾0·15 μg/ml); however, by age 3–8 months, only 14–16% of infants had protective antibody levels. The loss of maternal antibody coincided with the peak occurrence of Hib meningitis in this population at age 4–8 months [Reference Coulehan16]. Similarly, Eskimo neonates were protected against invasive Hib disease by maternal IgG, but did not sustain protective antibody levels between ages 2 and 23 months that corresponded to the highest incidence of invasive Hib disease during the first 2 years of life [Reference Ward24]. Markedly, both Eskimo newborns and children aged >4 years had greater anti-Hib antibody titres compared to children of similar ages in other US populations [Reference Ward24]. Further, Alaska Native infants had higher anti-PRP antibody levels at 2 months compared to 2-month-old infants in California, potentially due to acquisition of larger amounts of maternal antibody [Reference Ward33]. However, 18- and 24-month-old Apache had significantly lower anti-PRP antibody levels than age-matched Caucasian children [Reference Siber34]. These findings suggested that North American Indigenous children contracted Hib disease during the time when maternal antibody declined, but their own production of antibody was still insufficient. Young Eskimo infants often failed to mount antibody in response to invasive Hib disease explaining why they developed recurrent episodes of the disease when re-exposed to the pathogen [Reference Brenneman, Silimperi and Ward35]. In Eskimos of south-western Alaska, pharyngeal carriage of Hib was associated with increases in anti-PRP antibody levels both in carriers and their households [Reference Hall36]. In this study, antibody levels in unvaccinated children significantly increased with age from 6 months to 10 years. Low Hib carriage rates occurring in some villages were coincident with declining levels of anti-PRP antibodies during the observation period, and also with low incidence of invasive Hib disease [Reference Hall36].
Immune response to Hib vaccines in Indigenous children
The first Hib vaccine, which used purified PRP as an antigen, failed to induce protective immunity in children aged <2 years [Reference Ward33, Reference Coulehan37] and was subsequently replaced with Hib conjugate vaccines (PRP covalently linked to a protein carrier). The protein-polysaccharide conjugation results in T-cell dependent response to PRP that is characterized by an early development and the formation of immunological memory [Reference Kelly, Moxon and Pollard30]. Several formulations of Hib conjugate vaccines have been developed that differ in carrier proteins, polysaccharide size, type of linkage with the protein, and exhibit certain differences in immunogenicity and efficacy: PRP conjugates with (1) tetanus toxoid (PRP-T), (2) diphtheria toxoid (PRP-D), (3) non-toxic variant of diphtheria toxin CRM197 (HbOC), and (4) Neisseria meningitidis group B outer membrane protein (PRP-OMP).
Clinical trials of various Hib vaccines demonstrated that some groups of Indigenous children had low antibody response to immunization, although certain vaccine formulations were highly immunogenic in all the populations studied (Table 2) [Reference Ward33, Reference Siber34, Reference Coulehan37–Reference Santosham43]. Anti-PRP response to a single dose of HbOC at age 18 months was lower in Navajo than in Caucasian children. When two doses of HbOC were given together with DTP vaccine to 2- and 4-month-old Navajo infants, their anti-PRP response was lower than in Caucasians, although anti-tetanus antibody concentrations were similar in the two groups suggesting a selective defect in antibody response to polysaccharide, but not protein, antigens [Reference Santosham40]. However, if such a defect existed, it could be overcome either by repeated immunizations or by using PRP-OMP. After a third dose of HbOC at age 7 months, Navajo infants had anti-PRP concentrations similar to those in Caucasians [Reference Santosham40]. Moreover, 2-month-old Navajo and Apache infants developed protective antibody response to a single dose of PRP-OMP [Reference Santosham39, Reference Santosham43]. The use of HbOC instead of PRP-OMP for immunization of Alaska children resulted in the resurgence of invasive Hib disease in 1996–1997 [Reference Galil44]. A combined schedule in which PRP-OMP was used for the first dose and HbOC for the second and third doses was found to be optimal in protection against Hib of American Indian and Alaska Native infants [Reference Bulkow42, Reference Singleton45]. Because this regimen was found to be difficult to implement, the American Academy of Pediatrics currently recommends using PRP-OMP for immunization of this population [Reference Pickering, Baker, Kimberlin and Long46].
Table 2. Immune response to different Haemophilus influenzae type b vaccines in American Aboriginal populations (data are derived from clinical trials)
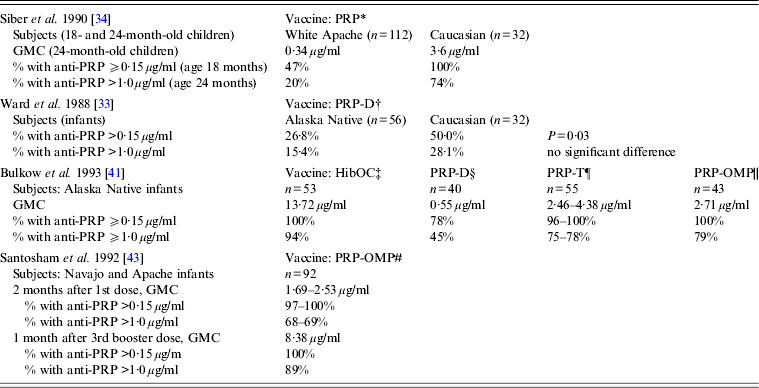
GMC, Geometric mean antibody concentration; HibOC, non-toxic variant of diphtheria toxin CRM197; PRP, Polyribosylribitol phosphate; PRP-D, PRP conjugate with diphtheria toxoid; PRP-T, PRP conjugate with tetanus toxoid; PRP-OMP, Neisseria meningitidis group B outer membrane protein.
* Hib plain polysaccharide vaccine given to 18- to 24-month-old infants; response measured 1 month after immunization.
† PRP-D conjugate vaccine given at ages 2, 4, and 6 months; response measured 3 months after the 3rd dose.
‡ Hib oligosaccharide conjugated to CRM197, given at ages 2, 4, and 6 months; response measured 1 month after the 3rd dose.
§ PRP-D conjugate vaccine given at ages 2, 4, and 6 months; response measured 1 month after the 3rd dose.
¶ PRP-T conjugate vaccine (liquid or lyophilized) given at ages 2, 4, and 6 months; response measured 1 month after the 3rd dose.
|| PRP-Neisseria meningitidis OMP conjugate vaccine, given at ages 2 and 4 months; response measured 2 months after the 2nd dose.
# PRP-Neisseria meningitidis OMP conjugate vaccine, given at ages 6–8 weeks, 4 months, and a booster dose at 12–15 months.
As immunization results in greatly reduced Hib carriage rates even in unvaccinated individuals, the herd effect also indirectly contributes to the protective mechanism of Hib conjugate vaccines at the population level [Reference Kelly, Moxon and Pollard30, Reference Jacups47]. However, the effects of Hib conjugate vaccines on Hib carriage differ between Indigenous and non-Indigenous populations. In children aged 1–5 years in remote south-western Alaska, Hib carriage remained high when HbOC was in routine use (9·3%) although it declined with routine use of PRP-OMP vaccine (1%) [Reference Galil44]. In comparison, infant immunization reduced the oropharyngeal carriage of Hib in children aged 2–5 years in metropolitan Atlanta to 0·17% [Reference Mohle-Boetani48]. Persistent carriage could have been responsible for the re-emergence of invasive Hib disease in the Alaskan Native population after switching to HbOC, which does not induce protective antibody after the first dose, in contrast to PRP-OMP [Reference Galil44]. Because higher anti-PRP IgG levels (⩾5 μg/ml) may be required to prevent pharyngeal colonization compared to invasive disease [Reference Anderson29, Reference Fernandez49] in some Indigenous populations, antibody induced by Hib vaccines may be insufficient to prevent carriage.
Because the anti-PRP antibody repertoire utilizes only a limited number of genes encoding the variable immunoglobulin domains, it is possible that certain genetic factors underlie a decreased response to Hib vaccines in some North American Indigenous populations. Indeed, a polymorphism in the gene encoding 60% of total anti-PRP antibody repertoire was detected in Navajos [Reference Feeney50]. In Alaskan Eskimos, an interaction of two genetic loci, Gm allotype and HLA-DR8, was found to be associated with an increased susceptibility to invasive Hib disease [Reference Petersen51]. Although these associations were reported (in the late 1980s and mid-1990s), no subsequent supporting data were published, and the clinical or population level significance has not been determined for either association. Moreover, the past 20 years’ vaccination experience shows that low responsiveness to PRP can be successfully overcome by repeated immunizations with conjugate vaccines or using a vaccine with a potent adjuvant effect (PRP-OMP). Insufficient antibody response to Hib is age-dependent and does not seem to be present in adults, presumably due to the maturation of the immune system. Multiple environmental and socioeconomic factors may contribute to enhanced carriage as well as high transmission rates of Hib in these populations.
Epidemiology of invasive H. influenzae disease after the introduction of Hib conjugate vaccines
In the pre-Hib conjugate vaccine era, most cases of invasive H. influenzae disease were due to Hib and occurred in children aged <5 years [Reference Shapiro and Ward2, Reference Peltola3, Reference Dajani, Asmar and Thirumoorthi52]. In the post-Hib conjugate vaccine era, the proportion of invasive disease caused by different encapsulated or non-encapsulated H. influenzae has changed significantly. While the proportion of invasive Hib has decreased markedly, the proportions caused by serotypes a and f as well as non-typable strains have increased. In one US study, the authors reported an increase in the incidence of invasive H. influenzae disease in the post-Hib vaccine era, rising from 0·4 to 1·0 cases/100 000 population, and in those aged ⩾65 years the increase was even bigger, i.e. from 1·1 to 3·9 cases/100 000 [Reference Dworkin, Park and Borchardt53]. Non-typable strains were responsible for the largest proportion of cases in almost all age groups examined [Reference Dworkin, Park and Borchardt53]. Most cases of invasive H. influenzae disease in the US general population are now due to non-typable strains (responsible for 68–70% of all cases) [Reference MacNeil54]. Non-Hib encapsulated strains are responsible for about 26% of all cases with over half due to serotype f, and only a small percentage of cases due to Hib (3·6–3·7%) [Reference MacNeil54]. Moreover, most cases are now found in adults (82·9%) and children account for 17·1% only [Reference MacNeil54]. In Canada, similar trends are observed [Reference Adam14] but in regions with high proportions of Indigenous populations, serotype a (Hia) appears to be the most common cause of invasive H. influenzae disease [Reference McConnell55–Reference Kelly57]. The incidence rates of invasive Hia disease in Indigenous populations in different parts of North America are summarized in Table 3 [Reference MacNeil54–Reference Rotondo60]. Although the currently published data show that incidence rates of invasive Hia disease are fractions of what the rates for invasive Hib disease were in the pre-Hib conjugate vaccine era, it is unclear if there will be further increases in Hia infections in the future. While in one study the authors concluded that there was no increase in the rate of invasive Hia disease after the introduction of Hib conjugate vaccine [Reference Millar58], in another study performed in Alaska, it was noted that no Hia disease was identified prior to 2002 [Reference Bruce59]. Interestingly, small outbreaks or clusters of cases of invasive Hia disease have been reported [Reference Hammitt61, Reference Adderson62]. An increased incidence of invasive Hia disease in the post-Hib vaccine era was also documented in Brazil [Reference Ribeiro63], although the published studies do not provide ethnic background of the cases and it is uncertain whether this infection preferentially affects Indigenous people in this country. Recent studies reported high rates of invasive Hia disease in Australian Indigenous children aged <5 years, i.e. an annual average of 11/100 000 population, without evidence of increasing rates over the last years (2001–2011) [Reference Menzies64].
Table 3. Annual incidence rates of invasive Haemophilus influenzae serotype a (Hia) disease in Indigenous populations of North America
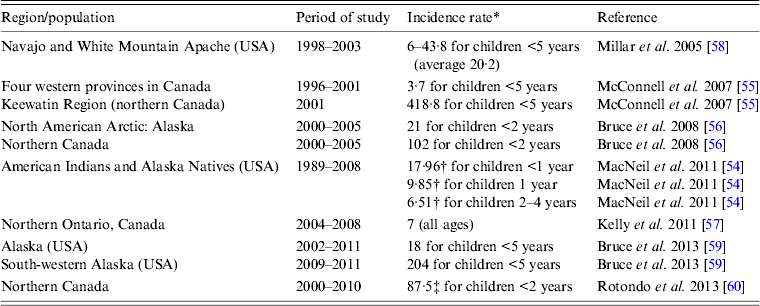
* Per 100 000.
† Estimated incidence.
‡ 91% of the Hia cases in Aboriginal population.
The clinical spectrum and severity of invasive Hia disease resembling that of Hib has also been described [Reference Adderson62]. A virulence marker of invasive Hia strains has been identified in the H. influenzae capsule synthesis operon, involving a partial deletion of its IS1016-bexA gene [Reference Kapogiannis65]. This mutation, involving deletion of parts of the IS1016-bexA genes, appears to stabilize the duplication of the genes involved in capsule synthesis and allow the strains to increase synthesis of capsule materials and hence enhance their virulence [Reference Kroll, Moxon and Loynds66]. Furthermore, a genotype identified by multilocus ST4 containing the IS1016-bexA partial deletion, has been found to cause a higher case-fatality rate compared to another genotype of ST23 and without the IS1016-bexA partial deletion [Reference Lima67]. Most Hia case isolates from Alaska [Reference Bruce59] and Canada [Reference Tsang68] did not have the genotype characterized by presence of the IS1016-bexA partial deletion. The overall population biology of invasive Hia strains in three Canadian provinces has been described and appears to resemble that of Hib involving two genetic populations, each with their own unique STs [Reference Tsang68].
Besides Hia, communities with higher populations of Indigenous people also continue to have higher rates of invasive Hib disease compared to the general population despite high Hib conjugate vaccine coverage [Reference Singleton45]. In 1992–1999, the rate of invasive Hib disease was 22/100 000 Navajo and White Mountain Apache children aged <2 years compared to 2/100 000 in the same age group in the US general population, although 90% of Navajo and White Mountain Apache children have received three doses of Hib vaccine by age 15 months [Reference Millar69]. One potential reason for continued infectious burden is higher oropharyngeal carriage of Hib in these communities despite vaccination. The continuing circulation of Hib in a community may increase the risk of elderly individuals with either waning immunity or certain medical conditions causing secondary immunodeficiency to develop invasive disease due to their inability to maintain a robust immunity to Hib [Reference Nix70].
The role of socioeconomic risk factors in susceptibility to invasive H. influenzae disease
Unfavourable socioeconomic factors have been considered as a reason for an increased susceptibility of Indigenous children to invasive H. influenzae disease and for a higher burden of Hib infection in Indigenous communities. Among young children, greater exposure to the pathogen due to poor housing conditions, such as overcrowding and lack of access to adequate volume of water in the home may cause high transmission rates of H. influenzae [Reference Hennessy71]. Several epidemiological studies have found an association of invasive Hib disease with indicators of low socioeconomic status (low income, single parents, low parental education levels, household crowding, indoor wood heating, rodents in the home), number of children in a family, shared childcare facilities, parental smoking and lack of breastfeeding in Navajo and Alaska Native children [Reference Wenger22, Reference Wolff72]. High burden of chronic conditions, such as obesity, diabetes, chronic obstructive pulmonary disease, tuberculosis as well as a prevalence of smoking, alcohol and substance abuse that have been identified as important health issues in Canadian Indigenous populations [Reference Adelson73] may have a negative effect on natural immunity against H. influenzae in the population and hence contribute to an enhanced circulation of the pathogen in Indigenous communities. In Indigenous populations living in the North American Arctic, multiple factors of socioeconomic deprivation along with high incidence of alcohol and other substance abuse, poor hygiene, overcrowding, and environmental degradation may underlie an emergence of invasive disease caused by serotype a of H. influenzae in the post-Hib vaccine era [Reference Hotez74].
Factors responsible for continuing burden of invasive H. influenzae disease are strikingly similar in different Indigenous populations of the world. Higher rates of invasive Hib disease than in the general population persist in Australian Aboriginal children [Reference Jacups47] as in Alaskan Native children [Reference Singleton45, Reference Singleton and Bulkow75] despite successful vaccination programme delivery. In the Northern Territory (Australia) Hib carriage persists in Indigenous children despite the high level of vaccination coverage that is attributed to unfavourable socioeconomic factors present in the population [Reference Jacups, Morris and Leach25]. Because persistent carriage creates a reservoir for invasive Hib and Hia disease transmission, H. influenzae carriage monitoring in high-risk populations remains a priority in the era of universal anti-Hib immunization [Reference Jacups, Morris and Leach25, Reference Jacups47, Reference Singleton and Bulkow75].
Treatment and post-exposure prophylaxis of invasive H. influenzae disease
Empirical treatment of systemic infection due to H. influenzae should be parenteral administration of cefotaxime or ceftriazone, as recommended for treatment of invasive Hib infections [Reference Pickering, Baker, Kimberlin and Long46]. If antibiotic susceptibility results suggest an ampicillin-sensitive strain, replacement of the third-generation cephalosporin with ampicillin can be implemented. For invasive disease due to non-typable strains, besides the third-generation cephalosporins, other parenteral agents may include ampicillin-sulbactam, fluoroquinolones, and macrolides such as azithromycin or clarithromycin. When a case of invasive Hib disease occurs in a household, childcare facility or nursery, chemoprophylaxis with rifampicin is routinely offered to contacts under the following circumstances. When a household member is aged <4 years and not appropriately immunized or immunocompromised, all household members, regardless of age and Hib immunization status, are recommended to receive chemoprophylaxis. In childcare and pre-school setting with unimmunized or incompletely immunized children, if two cases of invasive Hib disease have occurred within a 60-day period, all children and staff should be offered chemoprophylaxis regardless of age and vaccine status [Reference Pickering, Baker, Kimberlin and Long46, Reference Gkentzi76]. Chemoprophylaxis of contacts will not only prevent secondary cases of invasive disease, but will also eliminate pharyngeal carriage and hence prevent any further transmission. As Hia and Hib have very similar clinical presentations and share similar epidemiology [Reference Ulanova and Tsang77], it is not unreasonable to suggest that chemoprophylaxis given to household contacts of index Hia cases may also prevent secondary cases as well as pharyngeal carriage although no published data are available to support this. Rifampin prophylaxis was given to 'close contacts’ of invasive Hia cases in Alaska [Reference Hammitt, Hennessy, Romero-Steiner and Butler78], but more data on the choice of chemoprophylaxis agents, their dosage, efficacy in elimination of pharyngeal carriage and prevention of secondary cases of Hia are required before any recommendation can be developed.
Research needs and future direction
Although several studies have indicated an increased incidence of invasive Hia disease in Indigenous communities, further population-based studies in more regions are required to provide a true burden of the disease as well as the characteristics of the infection and carriage rate. The true genetic diversity of Hia is also unknown as only limited data are available from selected regions [Reference Bruce59, Reference Lima67]. The genetic relationship of respiratory vs. invasive Hia isolates has not been studied. Further, the clinical disease due to different genetic types has not been studied in detail or using standardized methods for clonal typing. The only known virulence factor for Hia is the polysaccharide capsule [Reference Zwahlen7], and its chemical structure is defined [Reference Branefors-Helander79]. However, little is known if there may be other important virulence factors or superantigens involved. Whole genome sequencing and comparative genomic studies coupled with studies of the proteomes of various encapsulated H. influenzae strains may shed light on how the capsular genes as well as other potential virulence genes may contribute towards the emergence of Hia as an important pathogen. Should the burden of disease suggest a need for a new H. influenzae vaccine, more information is required on the nature of protective immunity and serological correlates of clinical protection.
Conclusion
This review summarized current knowledge of invasive H. influenzae disease in Indigenous peoples of North America. Despite vaccination against Hib and significant reduction in Hib infections, invasive Hib disease has not been eliminated and its rates in Indigenous communities remain higher than in the general North American population. In addition, Hia has emerged as a significant cause of invasive disease in Indigenous populations in North America, similar in severity to invasive Hib disease in the pre-Hib vaccine era. In certain remote regions, the incidence of invasive Hia disease may be approaching levels of invasive Hib disease in the pre-vaccine era. Important lessons learned from decades of research on invasive H. influenzae disease include the following. The epidemiology of invasive H. influenzae disease in the Indigenous population appears to be different from that in the Caucasian population. Indigenous patients with invasive H. influenzae appear to be younger than their Caucasian counterparts, and most often affected by serotype a rather than serotype f, which seems to affects the Caucasian population more. The Indigenous population may respond differently than the Caucasian population to the same Hib conjugate vaccine, and hence special consideration may be required including the choice of the protein carrier for the capsular polysaccharide as well as the schedules of immunization (e.g. Hib-OMP in the Indigenous population vs. Hib-T for the Caucasian population). Finally, these lessons learned from the Hib experience in North America may be applicable to Indigenous populations in other parts of the world such as Australia and South America; as well as to the development of other capsular polysaccharide vaccines for control of invasive encapsulated bacterial diseases, possibly including those caused by Hia.
In view of the changing epidemiology of invasive H. influenzae disease, groundwork to pave the way for a new H. influenzae vaccine that targets both Hia and Hib should be considered. As epidemiological studies suggest that invasive H. influenzae disease in Indigenous peoples is associated with certain unfavourable socioeconomic factors more research is needed to clarify the role of biological vs. environmental and socioeconomic risk factors.
Acknowledgements
This research received no specific grant from any funding agency, commercial or non-for-profit sectors. The findings and conclusions in this review are those of the authors and do not necessarily represent the official position of the Public Health Agency of Canada or the Centers for Disease Control and Prevention.
Declaration of Interest
None.






