Healthcare-associated infections (HAIs) have a significant impact on mortality, length of stay (LOS), and healthcare cost worldwide.Reference Zingg, Holmes and Dettenkofer 1 – 3 In the United States, ∼2 million patients suffer from HAIs annually, nearly 90,000 are estimated to die due to HAIs, and the overall annual direct cost of HAIs to hospitals ranges from $28 billion to $45 billion.Reference Stone 4 The 2008 Annual Epidemiological Report on Communicable Diseases in Europe of the European Centre for Disease Prevention and Control (ECDC) declared that HAIs caused 16 million extra days of hospital stay and 37,000 attributable deaths annually, while the associated annual cost reached 7 billion euros (2019 USD, 7.75 billion). 5
Healthcare-acquired bloodstream infections (HA-BSIs) are the most common HAIs in critically ill pediatric patients of all age groups because central venous catheters (CVCs) are commonly used in these patients.Reference Biwersi, Hepping and Bode 6 – Reference Gaur, Bundy and Werner 9 In the United States, HA-BSIs are associated with the highest number of preventable deaths among HAIs, as well as the highest costs, ranging from $960 million to $18.2 billion annually.Reference Umscheid, Mitchell, Doshi, Agarwal, Williams and Brennan 10 Although strong evidence indicates that most HA-BSIs are preventable,Reference Gaur, Bundy and Werner 9 ,Reference Mobley and Bizzarro11–Reference Sagana and Hyzy16 pediatric and neonatal HA-BSI rates remain far above zero in many countries worldwide, highlighting the need to initiate further national and targeted prevention strategies.Reference Rosenthal, Al-Abdely and El-Kholi 17 – Reference Venturini, Montagnani and Benni 19
Published data regarding pediatric and neonatal HA-BSI outcomes vary significantly, depending on the country of origin, year of publication, and study design.Reference De Angelis, Murthy, Beyersmann and Harbarth 20 Accurate estimates of LOS, cost, and mortality attributed to HA-BSIs are essential for developing cost-effective infection prevention and infection control measures.Reference Manoukian, Stewart and Dancer 21 In the present study, we systematically reviewed the available evidence and estimate attributable LOS, healthcare costs, and mortality rates for pediatric and neonatal HA-BSIs.
Methods
This study was conducted in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. 22 The systematic review protocol is not registered.
Literature search strategy
A systematic search from January 2000 to September 2018 of the PubMed, Cochrane, and CINAHL databases was conducted by 1 researcher (S.K.) using 3 groups of key words related to the terms “bloodstream,” “population,” and “outcome.” These 3 categories were combined using the Boolean “AND” and “OR.” Appendix 1 (online) presents the full search strategy used for MEDLINE, which was adapted for the other databases. Cited references from selected articles were screened to identify additional studies that were not retrieved in the initial search. Conference abstracts were not searched because they do not contain sufficient data for quality assessment.
Selection criteria
Following the literature search, identified studies were checked to exclude duplicates. The remaining articles were independently screened by 2 researchers (S.K. and C.T.) to identify studies that met the predetermined inclusion criteria. The selection process was performed in 2 steps. In the first step, titles and abstracts were evaluated for eligibility against the predetermined criteria. In the second step, the full-text articles were assessed when the information provided in titles/abstracts was insufficient to decide on inclusion or exclusion. Any disagreements between the 2 researchers were discussed and resolved by a third researcher (G.K.).
The study eligibility criteria were selected by applying the PICOS (population, intervention, comparison, outcomes, and setting) question format:
-
Population: Studies referring to neonates and children <18 years of age with HA-BSI were eligible, and those that included both adult and pediatric populations were eligible only if stratified results by age group were presented.
-
Interventions and comparators: Studies including both a group of children with HA-BSI (BSIs) and a group of children without HA-BSI (non-BSIs) were eligible.
-
Outcomes: Studies that provided data on at least 1 of the following factors were included: attributable or excess LOS, or cost, or mortality due to HA-BSIs.
-
Study design: Cohorts or case–control studies were selected.
Cohort or case–control study design was set as a criterion because these study types are reported to measure LOS, cost, and mortality more accurately.Reference Manoukian, Stewart and Dancer 21 Articles that investigated only HA-BSIs caused by specific microorganisms were excluded, as were articles in which the primary outcomes were not the evaluation of attributable or excess LOS, cost, or mortality. Studies that presented mean or median values of the aforementioned attributable outcomes were included in the systematic review; their results are presented separately. However, in the meta-analysis, studies that reported median values of the attributable outcomes were excluded. Finally, only studies with their full text published in English were included.
Data extraction
Data extraction was performed by 1 researcher (S.K.), and the information was recorded in Microsoft Excel tables (Microsoft, Redmond, WA). The following data were noted: first author, year of publication, country, study design, hospital unit type, definition of HA-BSI, number of children with and without infection (BSIs and non-BSIs), and matching criteria (if used). Moreover, LOS, cost, and mortality (separately for each group), as well as attributable LOS, cost, or mortality, along with the corresponding 95% confidence intervals, were recorded. In studies where confidence intervals were not provided, we followed the recommendations of the Cochrane collaboration for calculating them.Reference Higgins, Green, Higgins, Thomas, Chandler, Cumpston, Li, Page and Welch 23 In cases in which these calculations were not feasible, the relevant studies were excluded from the meta-analysis.
For studies that provided separate estimates for >1 hospital unit (eg, the neonatal and the pediatric intensive care units, NICU and PICU), estimates were recorded separately. In 3 studies, the number of children without infection (non-BSI) was calculated by subtracting the BSIs from the total number of pediatric patients included in the studyReference Aviles-Robles, Ojha and Gonzalez 24 ,Reference Allareddy, Rampa and Allareddy25 or by applying the matching ratio.Reference Dramowski, Whitelaw and Cotton 26
Quality assessment
Study quality was evaluated by 2 researchers (S.K. and C.T.) using the Critical Appraisal Skills Programme Tool (CASP) for case–control and prospective cohort and retrospective cohort studies. 27 Disagreements were discussed with a third researcher (G.K.), and all 3 researchers ultimately reached consensus.
Statistical analysis
A meta-analysis was conducted using the STATA commands “metaan” and “metan” to estimate the pooled effect sizes with 95% confidence intervals (CIs) for attributable LOS and attributable mortality, respectively, as well as to graphically present the results in forest plots. The I2 statistic was used to assess heterogeneity among the included studies. An I2 > 75% indicates high heterogeneity among studies, and in such case, a random-effects model was used to obtain the pooled effect sizes. Moreover, sensitivity analysis was conducted by removing 1 study each time to identify the study that most influenced the results. Finally, the Egger test and funnel plots were used to evaluate potential publication bias.
Results
Of the 4,660 papers identified in the literature search, 21 were included in the systematic reviewReference Biwersi, Hepping and Bode 6 ,Reference Aviles-Robles, Ojha and Gonzalez24–Reference Dramowski, Whitelaw and Cotton26,Reference Aiken, Mturi and Njuguna28–Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44 and 13 were included in the meta-analysis (Fig. 1).Reference Atif, Sadaoui and Bezzaoucha 29 ,Reference Duenas, Bran de Casares, Rosenthal and Machuca30,Reference Goudie, Dynan, Brady and Rettiganti32–Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba36,Reference Pessoa-Silva, Miyasaki, de Almeida, Kopelman, Raggio and Wey39–Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44

Fig. 1. Flow diagram of included studies. Note. LOS, length of stay.
The main characteristics of the participating studies are listed in Table 1. Of the included studies, 4 were conducted in a hematology-oncology unit,Reference Biwersi, Hepping and Bode 6 ,Reference Aviles-Robles, Ojha and Gonzalez24,Reference Allareddy, Rampa and Allareddy25,Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44 6 involved NICU patients,Reference Atif, Sadaoui and Bezzaoucha 29 ,Reference Grisaru-Soen, Friedman, Dollberg, Mishali and Carmeli34,Reference Payne, Carpenter, Badger, Horbar and Rogowski38,Reference Pessoa-Silva, Miyasaki, de Almeida, Kopelman, Raggio and Wey39,Reference Rosenthal, Maki and Mehta41,Reference Schwab, Zibell, Piening, Geffers and Gastmeier42 6 involved PICU patients,Reference Duenas, Bran de Casares, Rosenthal and Machuca 30 ,Reference Elward, Hollenbeak, Warren and Fraser31,Reference Gupta, Kapil and Lodha35,Reference Navoa-Ng, Berba and Galapia37,Reference Rosenthal, Jarvis and Jamulitrat40,Reference Slonim, Kurtines, Sprague and Singh43 and 5 involved a mixed pediatric population.Reference Dramowski, Whitelaw and Cotton 26 ,Reference Aiken, Mturi and Njuguna28,Reference Goudie, Dynan, Brady and Rettiganti32,Reference Green, Johnson and Henderson33,Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba36 In most of the studies,Reference Aviles-Robles, Ojha and Gonzalez 24 ,Reference Dramowski, Whitelaw and Cotton26,Reference Aiken, Mturi and Njuguna28–Reference Goudie, Dynan, Brady and Rettiganti32,Reference Grisaru-Soen, Friedman, Dollberg, Mishali and Carmeli34–Reference Navoa-Ng, Berba and Galapia37,Reference Pessoa-Silva, Miyasaki, de Almeida, Kopelman, Raggio and Wey39-Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44 HA-BSI was defined according to CDC/NHSN criteria.Reference Garner, Jarvis, Emori, Horan and Hughes 45 – 47 In 1 studyReference Payne, Carpenter, Badger, Horbar and Rogowski 38 the Vermont Oxford Network (VON) criteria 48 ,49 were used; in another, ICD-9-CM codes were usedReference Allareddy, Rampa and Allareddy 25 ; and in 2 other studies, institution-based criteria were considered.Reference Biwersi, Hepping and Bode 6 ,Reference Green, Johnson and Henderson33 These institution-based criteria were reasonably chosen by the authors, and they were very much similar to the CDC/NHSN criteria. In addition, 10 of the participating studies were characterized as prospective cohort studies,Reference Aviles-Robles, Ojha and Gonzalez 24 ,Reference Dramowski, Whitelaw and Cotton26,Reference Aiken, Mturi and Njuguna28,Reference Duenas, Bran de Casares, Rosenthal and Machuca30,Reference Elward, Hollenbeak, Warren and Fraser31,Reference Gupta, Kapil and Lodha35,Reference Navoa-Ng, Berba and Galapia37,Reference Rosenthal, Jarvis and Jamulitrat40,Reference Rosenthal, Maki and Mehta41,Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44 5 were characterized as retrospective cohort studies,Reference Allareddy, Rampa and Allareddy 25 ,Reference Green, Johnson and Henderson33,Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba36,Reference Payne, Carpenter, Badger, Horbar and Rogowski38,Reference Pessoa-Silva, Miyasaki, de Almeida, Kopelman, Raggio and Wey39 and 6 were characterized as case–control studies.Reference Biwersi, Hepping and Bode 6 ,Reference Atif, Sadaoui and Bezzaoucha29,Reference Goudie, Dynan, Brady and Rettiganti32,Reference Grisaru-Soen, Friedman, Dollberg, Mishali and Carmeli34,Reference Schwab, Zibell, Piening, Geffers and Gastmeier42,Reference Slonim, Kurtines, Sprague and Singh43 However, we decided that the included case–control studies should ultimately be classified as either retrospective cohortsReference Biwersi, Hepping and Bode 6 ,Reference Goudie, Dynan, Brady and Rettiganti32,Reference Grisaru-Soen, Friedman, Dollberg, Mishali and Carmeli34,Reference Schwab, Zibell, Piening, Geffers and Gastmeier42 or as prospective cohortsReference Atif, Sadaoui and Bezzaoucha 29 ,Reference Slonim, Kurtines, Sprague and Singh43 because they actually measured outcomes after prospective or retrospective surveillance of matched BSI and non-BSI children until discharge or death.
Table 1. Main Characteristics of the Included Studies

Note. N/A, not available; BSI, bloodstream infection; CLABSI, central line-associated BSI; HA-BSI, healthcare-acquired BSI; HA-Bacteremia, healthcare-associated bacteremia; CVC-BSI, central venous catheter BSI; LC-BSI or LCBI, laboratory-confirmed BSI; HCAI, healthcare-acquired infection; CVC, central venous catheter; LOS, length of stay; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit; CoNS, coagulase-negative staphylococci; INICC, International Nosocomial Infection Control Consortium; CDC, Centers for Disease Control and Prevention; NHSN, National Healthcare Safety Network.
Finally, with regard to the methodology of the studies that presented attributable HA-BSI LOS and/or cost, 8 studies used a time-fixed statistical approachReference Allareddy, Rampa and Allareddy 25 ,Reference Duenas, Bran de Casares, Rosenthal and Machuca30,Reference Goudie, Dynan, Brady and Rettiganti32,Reference Navoa-Ng, Berba and Galapia37,Reference Payne, Carpenter, Badger, Horbar and Rogowski38,Reference Rosenthal, Maki and Mehta41,Reference Slonim, Kurtines, Sprague and Singh43,Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44 : 7 of these studies presented time-matched outcomes of BSI and non-BSI patientsReference Biwersi, Hepping and Bode 6 ,Reference Aviles-Robles, Ojha and Gonzalez24,Reference Dramowski, Whitelaw and Cotton26,Reference Aiken, Mturi and Njuguna28,Reference Atif, Sadaoui and Bezzaoucha29,Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba36,Reference Pessoa-Silva, Miyasaki, de Almeida, Kopelman, Raggio and Wey39 and only 1 study used multistate modeling to estimate attributable HA-BSI LOS.Reference Green, Johnson and Henderson 33
HA-BSI attributable LOS
In the systematic review, attributable LOS was presented in 17 studies.Reference Biwersi, Hepping and Bode 6 ,Reference Aviles-Robles, Ojha and Gonzalez24–Reference Dramowski, Whitelaw and Cotton26,Reference Aiken, Mturi and Njuguna28–Reference Duenas, Bran de Casares, Rosenthal and Machuca30,Reference Goudie, Dynan, Brady and Rettiganti32,Reference Green, Johnson and Henderson33,Reference Gupta, Kapil and Lodha35–Reference Pessoa-Silva, Miyasaki, de Almeida, Kopelman, Raggio and Wey39,Reference Rosenthal, Maki and Mehta41,Reference Slonim, Kurtines, Sprague and Singh43,Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44 As shown in Table 2, the attributable mean LOS ranged from 4 to 27.8 days, and in the studies in which median LOS values were presented, attributable median LOS ranged from 1.57 to 12 days. For hospital unit, the attributable mean LOS ranged from 11.4 to 21.1 days in PICUs, from 4 to 27.8 days in NICUs, and from 14.24 to 25.1 days in hematology-oncology units.
Table 2. Outcome Data of Included Studies With Regard to Length of Stay (LOS)

Note. N/A, not available; CI, confidence interval; IQR, interquartile range; SEM, standard error of the mean; BSI, bloodstream infection; CLABSI, central line-associated BSI; HA-BSI, healthcare-acquired BSI; CVC, central venous catheter; BW, birth weight; LOS, length of stay; NICU, neonatal intensive care unit; PICU, pediatric intensive care unit; BMTU, bone marrow transplantation unit.
In the meta-analysis, 6 studies were included.Reference Atif, Sadaoui and Bezzaoucha 29 ,Reference Goudie, Dynan, Brady and Rettiganti32,Reference Gupta, Kapil and Lodha35,Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba36,Reference Slonim, Kurtines, Sprague and Singh43,Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44 The pooled mean attributable hospital LOS was 16.91 days (95% CI, 13.70–20.11) (Fig. 2). We detected no heterogeneity amng the studies (I2 = 27.74%) and no publication bias (Egger bias test P = .705) (Appendix 2 online). A subanalysis of 4 studies assessing the attributable LOS of central-line–associated bloodstream infections (CLABSIs),Reference Goudie, Dynan, Brady and Rettiganti 32 ,Reference Gupta, Kapil and Lodha35,Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba36,Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44 resulted in a pooled mean attributable LOS of 18.82 days (95% CI, 15.11–22.54).

Fig. 2. Pooled mean of attributable hospital length of stay (LOS), forest plot. Note. ES, effect size; FE, fixed effects.
For hospital unit, the pooled adjusted mean attributable PICU LOS was 16.4 days (95% CI, 10.1–22.71; I2 = 0) (Fig. 3a) and the pooled mean attributable NICU LOS was 11.37 days (95% CI, 4.85–17.89) (Fig. 3b). No heterogeneity was detected between the PICU studies (I2 = 0%) and NICU studies (I2 = 68.96%). No publication bias was detected either for the PICU studies or the NICU studies: the Egger bias test P statistic was .76 for PICU studies and .07 for NICU studies.

Fig. 3. (a) Pooled mean of attributable hospital length of stay (LOS) in the pediatric intensive care unit (PICU), forest plot; (b) Pooled mean of attributable hospital length of stay (LOS) in the neonatal intensive care unit (NICU), forest plot. Note. ES, effect size; FE, fixed effects.
HA-BSI attributable cost
Attributable healthcare cost was presented in 8 studies,Reference Biwersi, Hepping and Bode 6 ,Reference Allareddy, Rampa and Allareddy25,Reference Atif, Sadaoui and Bezzaoucha29,Reference Elward, Hollenbeak, Warren and Fraser31,Reference Goudie, Dynan, Brady and Rettiganti32,Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba36,Reference Slonim, Kurtines, Sprague and Singh43,Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44 and ranged from $1,642.16 to $160,804 (2019 USD) per patient with HA-BSI. This range refers to the 7 studies that estimated mean (and not median) attributable healthcare cost (Table 3). At this point, providing specific data around cost per hospital unit would be inaccurate because of the small number of referring studies: only 3 studies measured PICU HA-BSI cost,Reference Elward, Hollenbeak, Warren and Fraser 31 ,Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba36,Reference Slonim, Kurtines, Sprague and Singh43 2 studies estimated NICU costs,Reference Atif, Sadaoui and Bezzaoucha 29 ,Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba36 and 4 studies assessed costs for hematology-oncology patients.Reference Biwersi, Hepping and Bode 6 ,Reference Allareddy, Rampa and Allareddy25,Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba36,Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44 All of these studies differ with regard to the corresponding currency and year.
Table 3. Outcome Data of Included Studies With Regard to Cost

Note. N/A, not available; CI, confidence interval; IQR, interquartile range; SEM, standard error of the mean; SE, standard error, BSI, bloodstream infection; CLABSI, central-line–associated BSI; CVC, central venous catheter; BW, birth weight; LOS, length of stay; ICU, intensive care unit; NICU, neonatal ICU; PICU, pediatric ICU; BMTU, bone marrow transplantation unit.
Meta-analysis was not conducted for the attributable HA-BSI cost due to the lack of eligible studies; only 3 of the participating studies estimated standard error of attributable HA-BSI cost, and their study populations presented heterogeneity.Reference Goudie, Dynan, Brady and Rettiganti 32 ,Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba36,Reference Wilson, Rafferty, Deeter, Comito and Hollenbeak44
HA-BSI attributable mortality
Attributable mortality was reported in 8 studiesReference Duenas, Bran de Casares, Rosenthal and Machuca 30 ,Reference Grisaru-Soen, Friedman, Dollberg, Mishali and Carmeli34,Reference Gupta, Kapil and Lodha35,Reference Pessoa-Silva, Miyasaki, de Almeida, Kopelman, Raggio and Wey39–Reference Slonim, Kurtines, Sprague and Singh43 and was calculated in 1 study, using estimates that were provided separately for BSI and non-BSI patients.Reference Green, Johnson and Henderson 33 The attributable mortality rate ranged between 0.01 and 0.24 (Table 4). The attributable mortality rate for the NICU was between 0.01 and 0.24, and for the PICU it was between 0.11 and 0.24.
Table 4. Outcome Data of Included Studies With Regard to Mortality

Note. N/A, not available; CI, confidence interval; BSI, bloodstream infection; HA-BSI, healthcare-acquired BSI; CVC-BSI, central venous catheter BSI; LC-BSI, laboratory confirmed BSI.
In the meta-analysis, 9 studies were included.Reference Duenas, Bran de Casares, Rosenthal and Machuca 30 ,Reference Green, Johnson and Henderson33–Reference Gupta, Kapil and Lodha35,Reference Pessoa-Silva, Miyasaki, de Almeida, Kopelman, Raggio and Wey39–Reference Slonim, Kurtines, Sprague and Singh43 The pooled mortality rate was 0.08 (95% CI, 0.06–0.09) (Fig. 4). We detected no statistically significant heterogeneity among the studies (I2 = 45.3%; P = .067). A subanalysis of 4 studies assessing the attributable mortality of CLABSIsReference Duenas, Bran de Casares, Rosenthal and Machuca 30 ,Reference Gupta, Kapil and Lodha35,Reference Rosenthal, Jarvis and Jamulitrat40,Reference Rosenthal, Maki and Mehta41 resulted in a pooled mean attributable mortality rate of 0.14 (95% CI, 0.08–0.20).
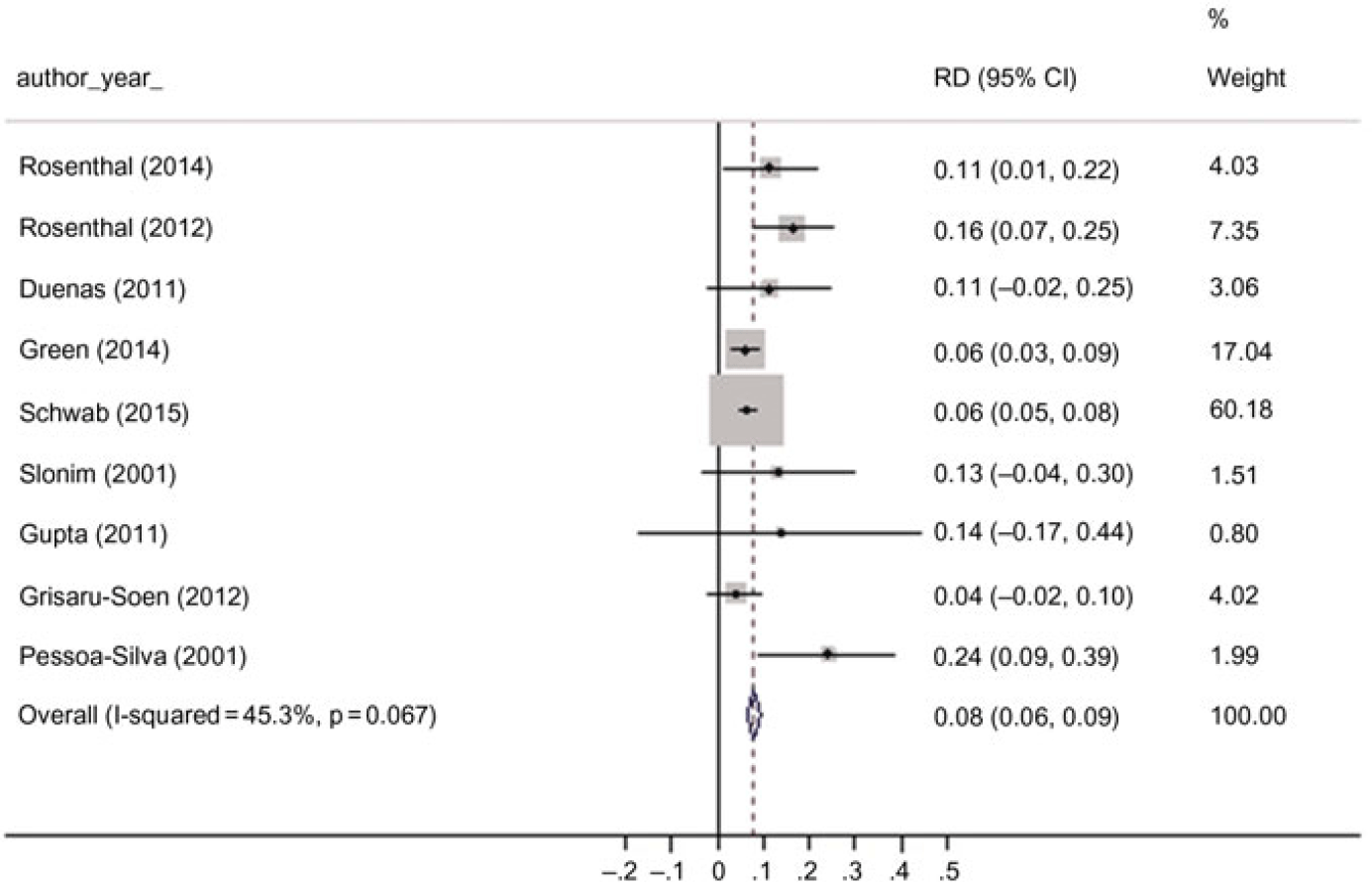
Fig. 4. Pooled overall attributable mortality rate, forest plot. Note. RD, risk difference.
Finally, meta-analysis showed that the pooled attributable mortality rate in NICUs was 0.08 (95% CI, 0.03–0.13) (Fig. 5) and in PICUs this rate was 0.13 (95% CI, 0.07–0.19) (Fig. 5). No statistically significant heterogeneity was detected in either NICUs (I2 = 58.1%; P = .067) or PICUs (I2 = 0%; P = .76). As mentioned, no publication bias was detected for either for PICUs or NICUs.
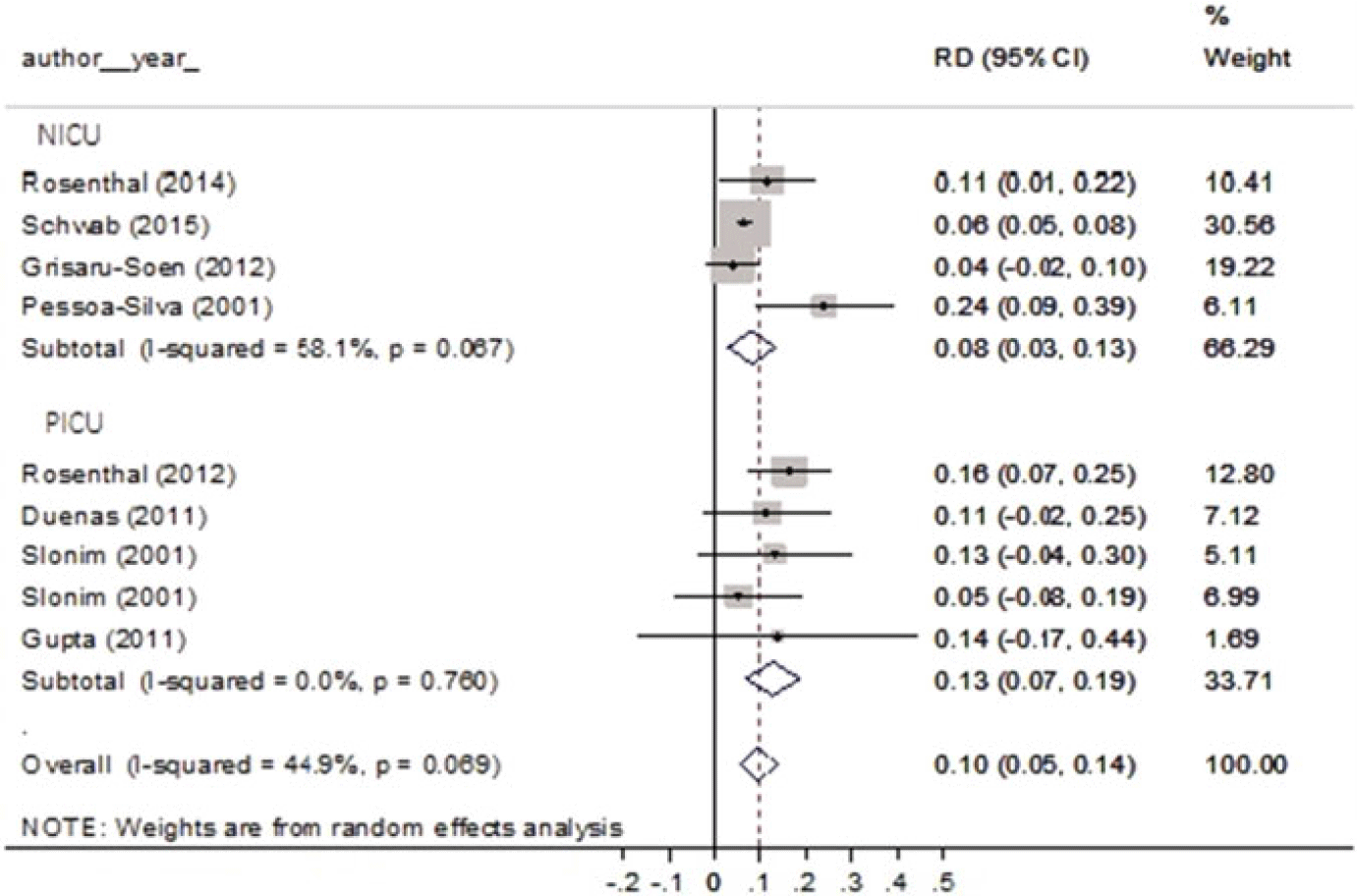
Fig. 5. Pooled attributable mortality rate by unit, forest plot. Note. RD, risk difference.
Discussion
The goal of this systematic review and meta-analysis was to provide evidence around HA-BSI attributable LOS, cost, and mortality among pediatric and neonatal patients, targeting the design and implementation of appropriate and cost-effective prevention strategies. As far as we know, this is the first attempt to synthesize all existing data around HA-BSI outcomes in the pediatric and neonatal population.
The HA-BSI mean attributable LOS ranged from 4 to 27.80 days, and the pooled mean attributable hospital LOS was 16.90 days (95% CI, 13.70–20.11). Stratified results by type of unit revealed a higher impact of HA-BSIs in PICUs (pooled mean attributable LOS, 16.40 days) compared to NICUs (pooled mean attributable LOS, 11.40 days). The results were more consistent in PICUs, with the mean attributable LOS ranging from 11.40 to 21.10 days, whereas in NICUs it ranged between 4 and 27.80 days. However, no statistically significant heterogeneity was observed in PICUs or in NICUs.
As described in the previous section, 7 of the studies that participated in the meta-analysis presented time-matched outcomes of BSI and non-BSI patients with regard to LOS, and only one used a multistate modeling approach to estimate attributable HA-BSI LOS. Previous work by Manoukian et alReference Manoukian, Stewart and Dancer 21 has suggested that excess LOS associated with HA-BSIs in adult populations presents significant variations according to the statistical method employed. More specifically, Manoukian et al suggest that studies using time-fixed methods overestimate the attributable LOS compared to time-varying methods,Reference Manoukian, Stewart and Dancer 21 because they do not take into account the time-dependent bias. Multistate modeling is considered the most accurate statistical method of attributable LOS estimation.Reference De Angelis, Murthy, Beyersmann and Harbarth 20 ,Reference Nelson, Nelson and Khader50 This hypothesis can only be partially confirmed by our study because the only study using multistate modeling among those included in our review provided the lower estimation of excess LOS (1.57 days).Reference Green, Johnson and Henderson 33
Attributable mean healthcare cost ranged from $1,642.16 to $160,804 (2019 USD) per patient with HA-BSI. Previous work by Umscheid et alReference Umscheid, Mitchell, Doshi, Agarwal, Williams and Brennan 10 assessed attributable cost of catheter-associated BSIs (CA-BSIs) in the adult ICU and reported costs from $41,900 to $123,600 (2009 USD).
In general, the large difference observed between reported attributable cost estimates for several HAI types is due to differences in the perspective of cost analysis (ie, hospital or societal), the costing methodology (ie, microcosting approach or not), and the year of costing, as well as differences in clinical practice patterns and healthcare systems among countries (ie, use of novel and expensive technologies in high-income countries, etc).Reference De Angelis, Murthy, Beyersmann and Harbarth 20
Attributable mortality rate ranged between 0.01 and 0.24, and pooled mortality was 0.08 (95% CI, 0.06–0.09). Stratified analysis by type of unit revealed that the pooled mortality rate was higher in PICUs (0.13) compared to NICUs (0.08).
Previous systematic reviews and meta-analyses have presented data indicating that the odds ratio for in-hospital death associated with HA-BSIs in adult patients ranges between 1.96 and 2.75.Reference Ziegler, Pellegrini and Safdar 51 ,Reference Siempos, Kopterides, Tsangaris, Dimopoulou and Armaganidis52 However, attributable HA-BSI mortality rates present significant variations, according to several causative microorganisms and susceptibility patterns.Reference Ziegler, Pellegrini and Safdar 51 ,Reference Zhang, Chen and Huang53
Quantifying excess HA-BSI outcomes is essential for both healthcare providers and policy makers. Improving efficiency with regard to resources and bed days by implementing targeted prevention strategies is crucial to increasing a hospital’s capacity to provide high-quality care to the highest number of patients. Precise measurements of HA-BSI outcomes could guide decision making around investments in infection control.
This study has several limitations. First, we acknowledge the possibility of language bias due to the fact that only studies written in English were incorporated in this review. Practical reasons, namely the difficulty of translating from a variety of languages, led us to the decision to include only English-language studies. Moreover, restricting the search strategy to only electronic databases may have introduced publication bias because this approach is unlikely to identify studies that have not been published in peer-reviewed journals. Because we did not include unpublished studies in our review, it was impossible to assess the potential publication bias by comparing the results of published and unpublished studies. However, we applied the Egger test, which revealed that no publication bias exists in HA-BSI–attributable LOS and mortality studies. We should underscore that the Egger test is inappropriate where there is heterogeneity; the test has low power and is of little use in analyses with few studies.
Finally, another important limitation of this study is the inclusion of studies that used a variety of HA-BSI definitions, and although all of these BSIs were nosocomial, this could result in outcome differences. We tried to overcome this problem by conducting a subanalysis in studies assessing the attributable LOS and mortality of CLABSIs, and the outcomes were presented separately. The heterogeneity in the HAI definitions used by several authors in the literature is a major problem when trying to conduct a qualitative or quantitative synthesis of the available literature data on HAI outcomes.
In conclusion, HA-BSIs in children and neonates are associated with higher mortality, LOS, and healthcare costs than in children and neonates without HA-BSIs. This finding justifies and may enhance efforts to implement HA-BSI prevention strategies. Future research efforts could make better use of existing HAI definitions and evolving statistical methodologies, presenting more accurate, high-quality, and comparable outcome results globally.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2019.353
Financial support
This study was part of the doctoral thesis of the physician K.S., which was financed through a scholarship by the General Secretariat for Research and Technology (GSRT) and the Hellenic Foundation for Research and Innovation (HFRI).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.











