Urbanisation, food intake and health
The proportion of people living in large cities has increased dramatically over past decades and is expected to rise even further; in the twentieth century, the proportion of people living in urban environments has grown from 14 to 50 %(Reference Allender, Foster and Hutchinson1). This transition is mostly prevalent in developing countries and these demographic shifts have major impact on public health possibly via effects on sleep and dietary patterns.
This process of urbanisation, the growth of cities on both population and physical size, already started a few centuries ago and was sparked by the invention of electric light in 1879 by Thomas Edison during the Industrial Revolution. Industrialisation, that is, manufacturing in factory settings using machines and labour force with specific, dedicated tasks to increase production, attracted the labour force to move from more rural settings to cities, stimulating the process of urbanisation. Having electric light allowed productivity to increase even further, which also had huge implications on the way people worked and lived. With electric light, it was now possible to work during the night, which previously had not been possible. Thus working in shifts, including night shifts, was introduced and this eventually has led to our 24 h economy. From a public health point of view, the health implications of working in shifts, including night shifts, became apparent many years later. In 2007, the International Agency for Research on Cancer classified shift work with circadian disruption as a probable human carcinogen(Reference Straif, Baan and Grosse2). Circadian disruption refers to the temporal organisation of biological 24 h rhythms which optimise metabolic functioning in our body; this is also called chrono-disruption(Reference Erren and Reiter3) (with chronos meaning time in Greek). Disturbances of circadian rhythms could lead to health problems including fatigue, insomnia, lack of appetite and generally impaired performance(Reference Erren and Reiter3). Light during the ‘biological night’ disturbs the circadian system, alters sleep activity, suppresses melatonin production and deregulates circadian genes, related to metabolic pathways(Reference Erren and Reiter3).
This paper discusses secular trends in sleep patterns and related dietary patterns in the urban setting, introduces basic concepts and mechanisms of chrono-nutrition, and discusses the evidence for the importance of sleep and chrono-nutrition in relation to health outcomes.
Urbanisation and chrono-nutrition
The urban environment comprises many dietary and lifestyle factors that affect health, for example, shift work, sleep, stress, physical activity, age, income, pollution, social jetlag (Fig. 1). The present paper focuses mainly on the impact of urbanisation on dietary factors and in particular the impact of the timing of eating, called chrono-nutrition(Reference Pot, Almoosawi and Stephen4). This relative new area of research studies not only the impact of what we eat but also when we eat. When considering the timing of eating, generally three aspects of time are considered: (1) irregularity (i.e. the inconsistency or inconsistent meal routine), (2) frequency (the number of meals or eating occasions daily) and (3) clock time (actual time of intake), for example, breakfast skipping and consuming meals late at night(Reference Pot, Hardy and Stephen5). All these components could affect our circadian rhythms and thereby have an effect on metabolic health. For example, one of the first epidemiological studies on chrono-nutrition demonstrated that people having a more irregular meal routine had a higher risk of obesity and the metabolic syndrome, despite consuming less energy than those who had a more regular meal routine(Reference Pot, Hardy and Stephen5). The impact of urbanisation on dietary patterns in the present paper is mainly considered in terms of chrono-nutrition, and in particular on the effects of clock time, using an epidemiological approach. As the impact of irregularity and meal frequency have been discussed previously(Reference Pot, Almoosawi and Stephen4, Reference Almoosawi, Vingeliene and Karagounis6), the present paper focuses more on the third aspect of time: clock time.
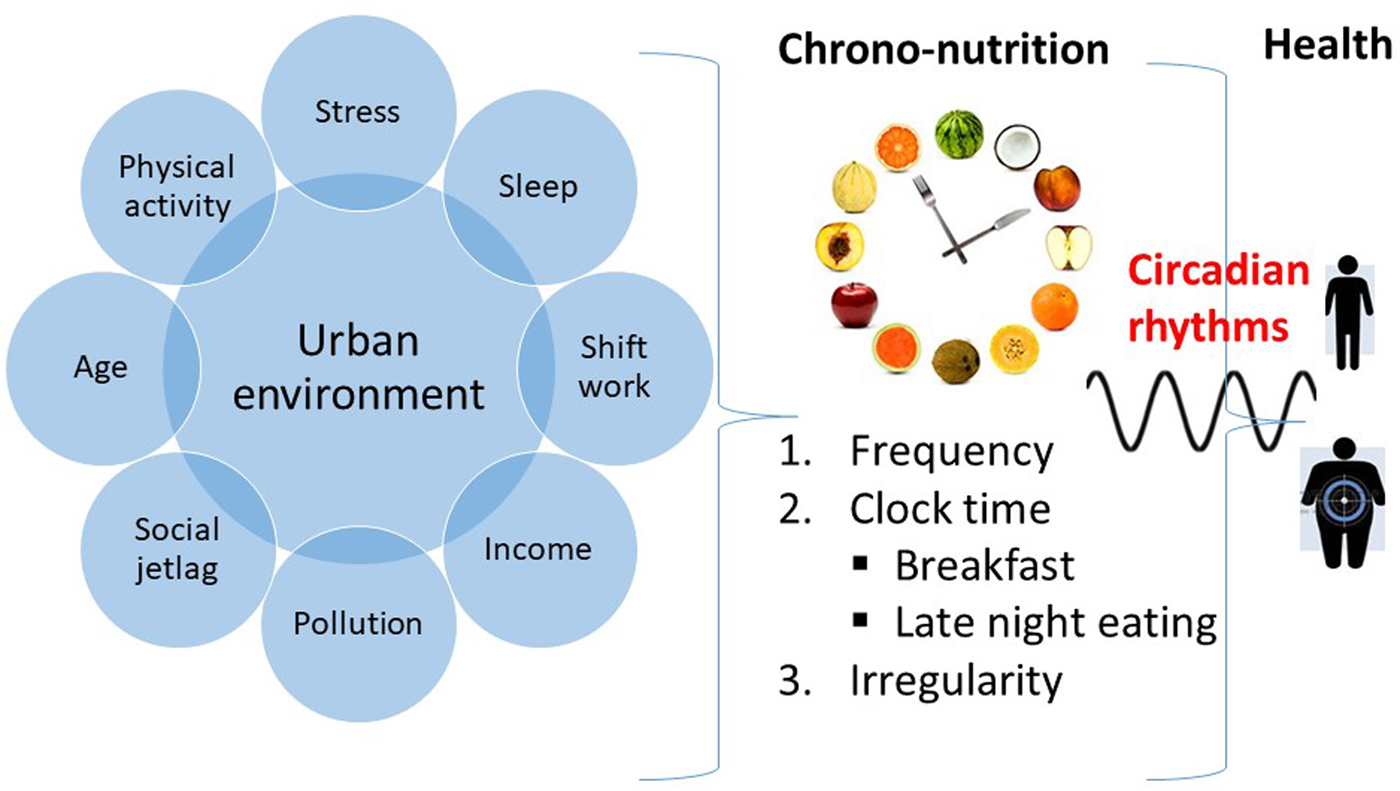
Fig. 1. (Colour online) The urban environment and its effects on chrono-nutrition and health.
The most apparent influence of clock time on our meal patterns can be observed for breakfast and late-night eating, as well in those whose biological clock is disturbed, which will be discussed in more detail.
Trends in sleeping patterns and urbanisation
With urbanisation becoming more prominent in many developed and developing countries over the past century, this has had a huge impact on the way people live their lives, including their daily routines, both work-related as well as related to sleep. The importance of sleep for optimal health, well-being and good functioning has been clearly demonstrated in many studies(Reference Chaput, Despres and Bouchard7, Reference Baron and Reid8). Insufficient sleep has been linked with detrimental health effects(Reference Luyster, Strollo and Zee9) as well as lost productivity and increased accidents and injuries(Reference Nakata, Ikeda and Takahashi10). However, it remains to be investigated which factors exactly affect sleep, as sleep is a multifactorial and complex process. Sleep can be assessed both as sleep duration (number of hours of sleep per night) as well as sleep quality, which also includes slow-wave sleep, rapid eye movement, sleep efficiency and sleep-onset latency (i.e. the time it takes to fall asleep)(Reference Mezick, Wing and McCaffery11). Sleep can be assessed either subjectively via questionnaires or objectively via actigraphy(Reference Kushida, Chang and Gadkary12); in most larger epidemiological studies, sleep is determined subjectively.
Many studies have suggested that sleep duration has declined over past decades(Reference Bixler13–Reference Kornholm, Partonen and Laatikainen15), which parallels other public health trends, including the rise in obesity rates over past decades. However, there is limited empirical evidence to support this claim of declining sleep trends. A review by Bin et al. in 2012 was the first systematic investigation into secular sleep trends in adults(Reference Bin, Marshall and Glozier16). This systematic review included twelve studies from fifteen countries from the 1960s until the 2000s and could not confirm a consistent decrease in sleep duration. They found that self-reported sleep duration in adults had decreased in six countries (Japan, Russia, Finland, Germany, Belgium and Austria) with 0·1–0·6 min/night each year, whereas they found increasing sleep duration in seven countries (Bulgaria, Poland, Canada, France, UK, Korea and the Netherlands) with 0·1–0·7 min/night each year and inconsistent results in the USA and Sweden(Reference Bin, Marshall and Glozier16). As this study was based on self-reported sleep data, which are more prone to bias, a more recent study by Youngstedt et al. in 2015 investigated secular trends in adult sleep duration based on objective sleep assessment(Reference Youngstedt, Goff and Reynolds17). Their systematic review included 168 studies with 257 data points of 6052 adults aged 18–88 years and analyses revealed no significant association of sleep duration with study year(Reference Youngstedt, Goff and Reynolds17), which confirmed the inconsistent trends previously reported by Bin et al.
In children however, secular trends show a clearer picture. A systematic review by Matricciani et al. in 2012 including data from 690 747 children and adolescents from twenty countries from 1905 to 2008 showed consistent rapid declines in sleep duration of children and adolescents(Reference Matricciani, Olds and Petkov18). The sample-weighted median rate of change was −0·75 min nightly per year indicating a decrease of more than 1 h per night over the study period(Reference Matricciani, Olds and Petkov18). The rates of decline did vary per region (Europe, USA, Canada and Asia) and in some regions a small increase was observed (Australia, UK, Scandinavia)(Reference Matricciani, Olds and Petkov18). Another recent review by Keyes et al. in 2015 also reported on alarming declines in sleep duration in US adolescents from 1991 to 2012(Reference Keyes, Maslowsky and Hamilton19).
Sleeping patterns may differ from country to country and variations in demographic structure of a population (e.g. age distribution), economic development, work hours, use of technology and culture may be responsible for some of these observed differences. Older population tend to sleep less(Reference Patel, Blackwell and Redline20). In addition, a strong correlation between socio-economic status is a strong determinant of sleep duration, with short sleep duration more common among those with the lowest household incomes(Reference Stamatakis, Kaplan and Roberts21). It may be the case that sufficient sleep duration for the maintenance of health and well-being has become a luxury in modern society(Reference Stamatakis, Kaplan and Roberts21). However, Keyes et al. reported that racial/ethnic minorities and adolescents of low socio-economic status were more likely to self-report adequate sleep compared with white subjects and those with higher socio-economic status(Reference Keyes, Maslowsky and Hamilton19). Moreover, it has been reported that the sex gap in adequate sleep is widening, with girls less likely to report getting more than 7 h of sleep compared with boys(Reference Keyes, Maslowsky and Hamilton19). Moreover, sex differences in subjective sleep may be amplified with ageing with women showing a stronger impact of ageing(Reference Carrier, Semba and Deurveilher22).
In addition, there may be other factors to consider when investigating the possible decline in sleep duration over past decades. It may be possible that even though the average sleep duration has not declined, the percentage of the population not achieving the sleep recommendations has increased. Moreover, questions also remain whether sleep quality may have declined, and whether sleep duration may have decreased on week or weekend nights. For studies in children, there is also lack of consensus on what optimal sleep is for children(Reference Matricciani, Olds and Petkov18).
A recent study on the determinants of sleep duration, using smartphone data and mathematical modelling, reported on world-wide trends in sleeping schedules and its most important determinants. The study showed that age had the most dominant influence on the timing of mid-sleep and sex has the strongest influence on sleep duration, with women scheduling more hours of sleep by both going to bed earlier as well as rising later(Reference Walch, Cochran and Forger23). The mean sleep duration by country was primarily predicted by bedtime and not wake time suggesting that biological cues around bedtime are either weakened or ignored for societal or environmental reasons, for example, the use of smartphones or other electronic media before sleeping. Participants in the Netherlands and New Zealand reported the longest sleep duration (sleeping on average 8 h 5 min and 8 h 4 min) and participants from Japan and Singapore reported the shortest sleep duration (sleeping on average 7 h 31 min and 7 h 24 min). Country differences were mainly explained by differences in bedtime(Reference Walch, Cochran and Forger23).
Thus, even though these recent systematic reviews have not confirmed a secular decline in sleep duration over past decades in adults, sleep duration has declined in children and sleep quality may have declined in adults and children, especially in urban areas, possibly more on week nights, which warrants further investigation, in different populations including adults, adolescents and children, a wide variety of socio-economic status and both men and women.
Sleep and dietary patterns
As sleep is a pivotal modulator of metabolic functioning, including energy metabolism, glucose regulation and appetite(Reference Koren, O'Sullivan and Mokhlesi24, Reference Van Cauter, Blackman and Roland25), research on the effects of sleep duration and dietary intake has taken off over past years, especially as sleep could possibly present a modifiable risk factor for chronic non-communicable diseases such as obesity.
A recent systematic review by Khatib et al. in 2017 investigated the effects of partial sleep deprivation on energy balance, that is, energy intake and energy expenditure, in five databases(Reference Al Khatib, Harding and Darzi26). From the seventeen included studies in the review, eleven studies were used for meta-analyses including data from 496 study participants and showed that energy intake was significantly increased by 1611 (95% CI 1054, 2163) kJ/d following partial sleep deprivation compared to control subjects. No effect of partial sleep deprivation was found on total energy expenditure or RMR, resulting in a net positive energy balance, which in the long term may contribute to weight gain(Reference Al Khatib, Harding and Darzi26). The observed changes in energy intake were accompanied by significantly higher fat intakes and lower protein intakes but no effect on carbohydrate(Reference Al Khatib, Harding and Darzi26).
Other studies have also investigated the association of sleep duration and nutritional status. A study using data from the National Health and Nutrition Examination Survey (NHANES) in the USA 2007–2008 from 5587 adults found that energy intake varied across very short sleepers (<5 h/night; 8519 kJ), short sleepers (5–6 h/night; 9209 kJ) and long sleepers (>9 h/night; 8058 kJ) relative to normal sleepers (7–8 h/night; 9000 kJ) (P = 0·001)(Reference Grandner, Jackson and Gerstner27). Short sleepers consumed a smaller variety of foods and had lower protein, carbohydrate, fibre and fat intake relative to normal sleepers(Reference Grandner, Jackson and Gerstner27). Furthermore, another study investigating the link between sleep duration and nutritional status including data from NHANES 2005–2006 from 2459 adults aged 20–85 years found inverse associations between serum vitamin B12 and sleep duration, 25-hydroxy vitamin D and sleepiness, and folate and sleep disturbances. Serum total carotenoids concentrations were linked to higher odds of short sleep duration (5–6 h/night) compared with normal sleep duration (7–8 h/night)(Reference Beydoun, Gamaldo and Canas28).
Presently our research group is investigating associations between sleep duration with nutritional intake and status in data from the National Diet and Nutrition Survey Rolling Programme (NDNS-RP). Data from the first 4 years of NDNS-RP including 2075 adults showed that normal sleepers (7–8 h) had higher intakes of vitamin C, fibre and iron, and higher levels of serum total carotenoids, selenium and urinary nitrogen compared with short (≤6 h/night) and long (≥9 h/night)(Reference Pot, Al Khatib and Perowicz29). Presently, we are expanding these analyses with year 5–6 data from NDNS.
Impact of diet on sleep
While there is accumulating evidence that sleep affects dietary intake, diet and specific foods and dietary patterns could also impact sleep and thus far this question has been less investigated. Recently, St-Onge et al. published a review on how diet affects sleep quality(Reference St-Onge, Mikic and Pietrolungo30). Observational studies indicated that higher fat intakes were associated with sleep disorders(Reference Tan, Alén and Cheng31) and that following a Mediterranean diet is associated with less insomnia problems in women(Reference Jaussent, Dauvilliers and Ancelin32). A study in 3129 Japanese female workers showed that skipping breakfast and eating irregularly were strongly associated with poor sleep(Reference Katagiri, Asakura and Kobayashi33). Furthermore, this study in Japanese women showed an association between low intake of vegetables and fish and high intake of confectionary and noodles with poor sleep quality. The evidence from experimental studies that St-Onge et al. collated showed that dietary patterns that favour high carbohydrate intake are associated with better sleep quality (as indicated by reduced sleep-onset latency and slow-wave sleep and increased rapid eye movement), whereas high fat intake is associated with poorer sleep quality (as indicated by lower sleep efficiency and higher slow-wave sleep and arousals). Randomised controlled studies investigating the longer term effects need to confirm these findings. Furthermore, the review by St-Onge et al. described a few specific foods that show sleep-promoting effects, including milk, fish, fruit and vegetables (i.e. kiwi fruit and cherry tart), but studies were too scarce, small and diverse to be able to draw firm conclusions. St-Onge et al. also recommend that further studies should include more participants, both men and women, and could focus on people with sleep disorders. In particular, future studies should also consider the timing of the intake of sleep-promoting foods(Reference St-Onge, Mikic and Pietrolungo30).
Thus, sleep has an impact on diet and diet on sleep, and more research is needed to investigate how sleep could potentially be used as a modifiable factor in the treatment of chronic diseases like obesity.
Trends in dietary patterns and urbanisation
When considering the impact of urbanisation on dietary patterns, the main process by which this occurs is nutrition transition(Reference Popkin, Lu and Zhai34). Nutrition transition was first described by Popkin in 1993 and is the shift in dietary patterns that coincides with economic, demographic and epidemiological changes(Reference Popkin, Lu and Zhai34). This term is mostly used to describe the changes of dietary patterns in developing countries from more traditional diets, usually high in fibre and cereal, to a more Western dietary pattern, characterised by more sugars, fat and animal-source food.
A review study on socio-economic determinants of dietary patterns in low- and middle-income countries by Mayen et al. in 2014 including a total of thirty-three studies from seventeen low- and middle-income countries (thirty-one cross-sectional studies and two longitudinal studies) showed that living in urban areas is associated with overall healthier dietary patterns, including more dietary diversity and more animal food intake(Reference Mayen, Marques-Vidal and Paccaud35). Living in urban areas was generally associated with higher intakes of energy, protein, total fat, cholesterol, polyunsaturated fat, saturated fat, monounsaturated fat, vitamin A and vitamin C, and lower intakes of carbohydrates and fibre(Reference Mayen, Marques-Vidal and Paccaud35). Large numbers of rural inhabitants in many low-income countries, and even some middle-income countries, still rely on unhealthy food preservation methods (e.g. salting or smoking) as access to electricity is limited(Reference Mayen, Marques-Vidal and Paccaud35). Social inequalities in dietary intake exist in low- and middle-income countries.
Other examples of studies specifically investigating eating patterns in urban v. rural populations include a study from Iran that investigated dietary and sleep patterns in urban and rural high school girls(Reference Maddah, Rashidi and Mohammadpour36). This study also showed that sleeping time was significantly related to overweight/obesity, with normal weight girls indicating they usually went to sleep at an earlier hour. In addition, the consumption of food items of low nutrient density as snacks during the school day was common in this population, especially in rural areas. The prevalence of obesity was significantly higher in rural areas (5·2 %) compared with urban areas (4·2 %), and the prevalence of obesity was also significantly higher in those who usually skipped breakfast(Reference Maddah, Rashidi and Mohammadpour36).
A study among 14 039 adults aged 20 years and over of the NHANES 1999–2006 showed that rural adults had a poorer diet as indicated by lower intakes of fibre and fruits and higher intakes of sweetened beverages compared with urban residents, and prevalence of obesity was higher in rural (35·6 %) compared with urban residents (30·4 %)(Reference Trivedi, Liu and Probst37).
Potential mechanisms of the effects of chrono-nutrition on health
When considering the importance of chrono-nutrition on metabolic health, there are several underlying mechanisms to consider (Fig. 2). First, changes in circadian rhythms affect functions of food metabolism, including digestion and absorption of food as well as energy metabolism(Reference Tahara and Shibata38) via the major clock genes (Bmal-1, Clock, Per1/2, Cry1/2)(Reference Bass39). Second, meal timing affects the output of the clock system, for example, skipping breakfast increases obesity risk, whereas regular eating is associated with a reduced risk of obesity(Reference Oike, Oishi and Kobori40). There is some evidence that specific nutrients, such as glucose, ethanol, caffeine, thiamine and retinoic acid can phase-shift (i.e. advance or delay) circadian rhythms(Reference Froy41). Thirdly, sleep is an important determining factor of our internal body clock and can mediate effects through food intake. Decreased sleep is associated with increased food intake, poor diet quality and excess body weight(Reference Chaput42), as well as higher risks of chronic diseases, such as CVD, diabetes and hypertension(Reference Scheer, Hilton and Mantzoros43).
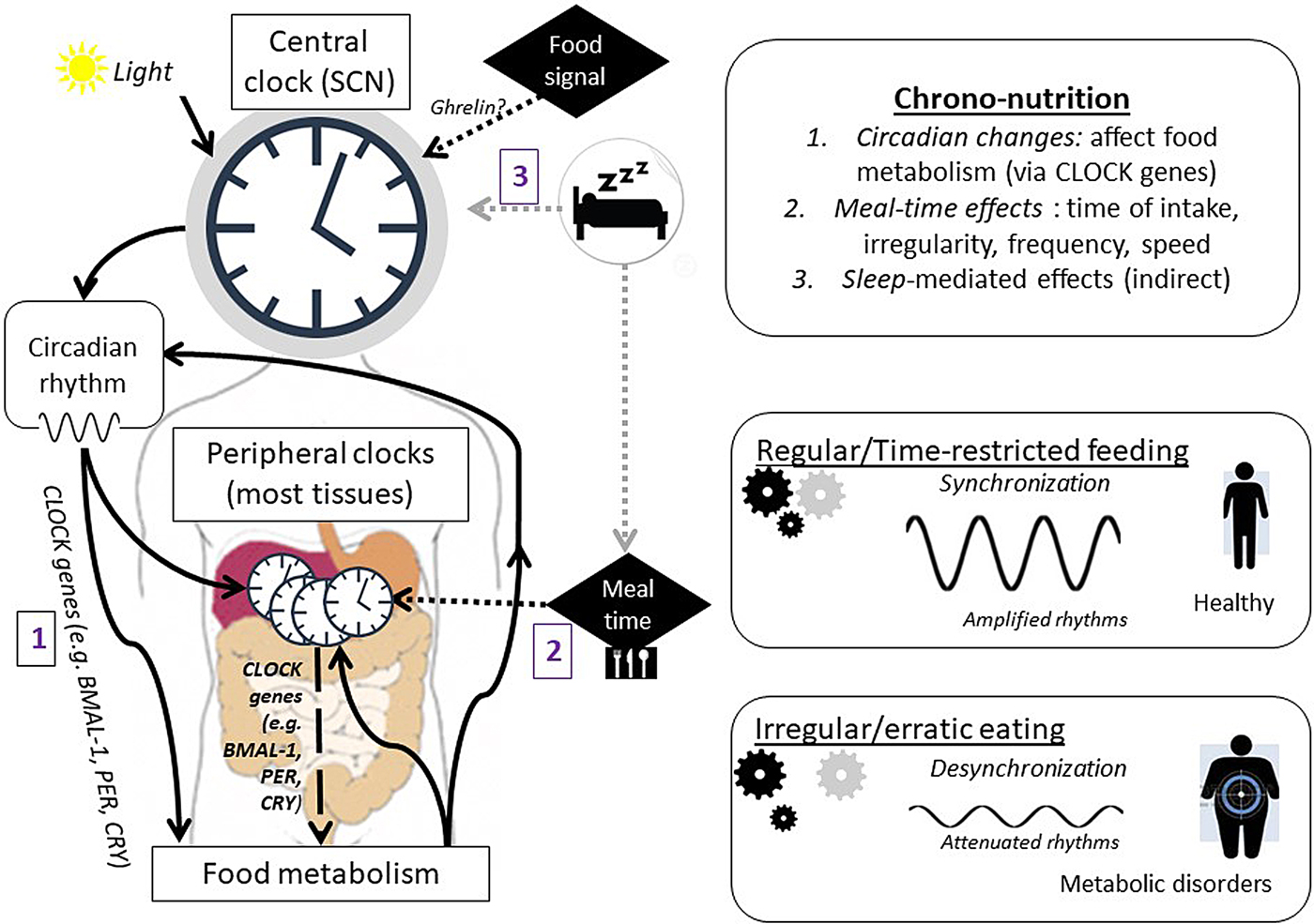
Fig. 2. (Colour online) Proposed mechanisms of how chrono-nutrition affects health (adapted from(Reference Tahara and Shibata38, Reference Oike, Oishi and Kobori40)). CLOCK genes include BMAL-1, PER, CRY. SCN, suprachiasmatic nuclei.
First meal of the day: breakfast
Breakfast is considered the most important meal of the day, though this statement has been subject to much debate. Consuming breakfast is thought to have beneficial effects on diet quality during the rest of the day, health and cognitive and academic performance(Reference Adolphus, Lawton and Champ44). However, breakfast is the meal that is most often skipped, especially by adolescents(Reference Berkey, Rockett and Gillman45). Skipping breakfast does not only affect cognitive and academic performance, it also impacts metabolic health, that is, it is likely to be detrimental for insulin sensitivity(Reference Maki, Phillips-Eakley and Smith46) and could impact weight status(Reference Timlin and Pereira47). A systematic review of sixteen studies in 57 481 children by Szajewska and Ruszczynski in 2010 showed that those children who skipped breakfast had a higher BMI suggesting that eating breakfast is associated with a reduced risk of becoming overweight or obese(Reference Szajewska and Ruszczynski48). However, most of the studies included in the latter review were observational studies, which despite providing interesting information are unable to demonstrate causality.
So far few randomised controlled trials have investigated the effects of breakfast consumption on health and most of these were related to weight gain or weight loss. Evidence from randomised controlled trials suggests that breakfast consumption does not play a large role in weight gain or weight loss; however, specific breakfast strategies, such as consuming more energy in the morning relative to the evening(Reference Almoosawi, Prynne and Hardy49), and certain types of breakfast may produce greater weight loss(Reference Dhurandhar, Dawson and Alcorn50). A review by Timlin and Pereira in 2008 including four randomised controlled trials had suggested that regular meal consumption can potentially reduce the risk of obesity and chronic diseases through mechanisms involved in energy balance and metabolism, including increased satiety and reduced energy intake(Reference Timlin and Pereira47).
A recent randomised controlled trial compared breakfast consumption with fasting until noon and showed no evidence that omission of breakfast had any effect on body weight in twenty-three overweight men and women (average BMI 33·7 (sd 4·9) kg/m2) over 6 weeks(Reference Chowdhury, Richardson and Holman51). Morning fasting resulted in partial dietary compensation (i.e. greater energy intake) later in the day. A similar study by the same research group in twenty-three lean subjects showed that daily breakfast consumption was causally linked with higher physical activity thermogenesis with greater overall dietary energy intake in breakfast consumers and limited subsequent suppression of appetite(Reference Betts, Richardson and Chowdhury52). The regular daily breakfast group maintained a more stable glucose response during the afternoon and evening(Reference Betts, Richardson and Chowdhury52).
A recent review by St-Onge et al. from 2017 on meal timing and frequency and its implications for CVD prevention concluded that on the basis of epidemiological and clinical intervention data, daily breakfast consumption may decrease the risk of adverse effects related to glucose and insulin metabolism(Reference St-Onge, Ard and Baskin53). They found little evidence that breakfast consumption is effective as a weight loss strategy; however, breakfast consumption can contribute to a healthier eating pattern, which can also lead to cardio-metabolic improvements(Reference St-Onge, Ard and Baskin53).
In terms of breakfast composition, a review investigating the effects of breakfast consumption on carbohydrate and metabolic wellness showed that consuming breakfast foods high in whole grains and cereal fibre, while limiting rapidly available carbohydrate, is a promising strategy for metabolic health promotion(Reference Maki, Phillips-Eakley and Smith46). Another recent systematic review by Rosato et al. in 2016 showed that studies on nutrient composition of breakfast on obesity and chronic disease risk have shown inconsistent results. Of the eight included studies, three studies showed that higher amount of carbohydrate and lower amount of fat is significantly related to normal weight in adults(Reference Rosato, Edefonti and Parpinel54). As there were only a few and quite heterogeneous studies, more research is needed.
Adolescents are known to be the most frequent breakfast skippers(Reference Berkey, Rockett and Gillman45). A recent systematic review by Pendergast et al. in 2016 investigated correlates of meal skipping in adolescents. This study showed that breakfast skipping was more common among men, while lunch and dinner were more often skipped by women(Reference Pendergast, Livingstone and Worsley55). Lack of time was consistently reported as the main reason for skipping a meal besides cost and weight control(Reference Pendergast, Livingstone and Worsley55). However, these findings are in contrast with our recent findings investigating the dietary patterns of children in NDNS years 1–4 of breakfast consumers. We found that breakfast was more often skipped by girls aged 11–18 years compared with boys(Reference Coulthard, Palla and Pot56). We also observed that breakfast consumption was associated with an overall healthier dietary pattern over the rest of the day and this even applied for children consuming breakfast on some but not all days.
Overall, there is still much debate about breakfast consumption; however, most research, including longer term clinical intervention studies, still points in the beneficial direction of breakfast consumption, especially in children and adolescents.
Last meal of the day: late-night eating
At the other end of spectrum of eating during the early hours of the day is consuming late at night. Night eating syndrome, when at least 25 % of food intake is consumed after the evening meal or at least two episodes of nocturnal eating per week, was first described in 1955 by Stunkard et al. (Reference Stunkard, Grace and Wolff57). Evidence from European and American studies suggests that night eating syndrome features strongly in populations with severe obesity(Reference Cleator, Abbott and Judd58). Nocturnal eating was added to the definition of night eating syndrome and also patients with nocturnal eating have been reported to have a higher BMI and nocturnal eating was associated with binge eating and breakfast skipping(Reference Striegel-Moore, Rosselli and Wilson59). Several cross-sectional studies have shown an association of late-night eating with a greater risk of poor cardio-metabolic health(Reference St-Onge, Ard and Baskin53). Late-night eating was associated with a higher risk of obesity (odds ratio 1·62 (95 % CI 1·10, 2·39)) compared with those who did not consume late at night in 3610 Swedish men and women(Reference Berg, Lappas and Wolk60). A small cross-sectional study in 239 US men and women demonstrated that individuals who consumed over a third of their total energy intake in the evening had twice the risk of being obese (odds ratio 2·00 (95 % CI 1·03, 3·89))(Reference Wang, Patterson and Ang61). Furthermore, a study among 60 800 Japanese adults showed that the combination of late-night eating and breakfast skipping was associated with a higher risk of the metabolic syndrome (odds ratio 1·17 (95 % CI 1·08, 1·28))(Reference Kutsuma, Nakajima and Suwa62). These cross-sectional studies are limited by their incapacity to demonstrate causality; however, so far, only one prospective study has investigated the association between late-night eating and CVD risk(Reference Cahill, Chiuve and Mekary63). Men who ate late at night (defined as a positive response to the question, Do you consume after going to bed?) had a 55 % higher CVD risk compared with those who did eat late at night (relative risk 1·55 (95 % CI 1·05, 2·29)). However, this association was mediated by BMI and only very few men (n29) reported late-night eating.
A less extreme form of late-night eating that probably occurs in a larger part of the population relates to chrono-type, that is, an attribute that reflects an individual's preferences in the timing of sleep and other behaviours (morningness or eveningness), which may have implications for our dietary patterns. Eveningness has been associated with later eating and a tendency to consume towards fewer and larger meals(Reference Lucassen, Zhao and Rother64). Eveningness was also associated with lower HDL-cholesterol levels, more sleep apnoea and higher stress hormone levels(Reference Lucassen, Zhao and Rother64). Another study found that BMI was strongly associated with night eating(Reference Harb, Levandovski and Oliveira65).
Disturbed biological clocks
To study the impact of timing of meals on disturbed biological clocks more generally, people whose circadian rhythms are changed form an interesting study population. A well-known disorder of circadian rhythms is delayed sleep phase disorder, which is a chronic dysregulation of a person's circadian rhythm in people who are otherwise healthy(Reference Jones, Huang and Ptáček66). This usually develops in early childhood or adolescence. Prevalence among adults is 0·15 %, while this is much higher among adolescents (7–16 %)(Reference Jones, Huang and Ptáček66). Investigating people with delayed sleep phase disorder offers the unique opportunity to evaluate the potential impact of interventions in people with a chrono-biological clock disturbance but otherwise healthy. We have recently started to investigate chrono-nutrition in adolescents with delayed sleep phase disorder to study whether eating patterns are indeed shifted to a later time point compared with controls, which may offer a possible opportunity in the treatment of these patients.
Overall, chrono-nutrition could mediate the effects between sleep, diet and urbanisation, and more research is needed to elucidate the importance of chrono-nutrition for metabolic health and assessing chrono-type could be a useful tool.
Urbanisation, diet and health outcomes
With urbanisation increasing so dramatically over past decades, this is also thought to have had a great influence on public health. People living in rural and remote areas generally have worse health compared with urban residents(Reference Strasser67), with mortality rates increasing with remoteness(Reference Zarocostas68). One of the main public health threats we are presently facing is obesity and related metabolic disorders.
Obesity rates between urban and rural environments show different patterns across the world. In some regions, obesity rates are higher in rural children and adults (USA, Japan, Sweden, Greece)(Reference Trivedi, Liu and Probst37, Reference Johnson and Johnson69–Reference Tambalis, Panagiotakos and Sidossis72) where in more developing countries higher obesity rates are observed in urban environments (India, China, Turkey, Africa, Poland)(Reference Zhang, Zhao and Chu73–Reference Gökler, Buğrul and Metintaş78).
A review by McCormack and Meendering in 2016 looked into differences of diet and physical activity in rural and urban children to possibly explain the higher prevalence of obesity among rural children(Reference McCormack and Meendering79). Their review included five studies on diet between rural and urban children. Of the five studies on diet, two studies showed no difference in dietary intake between rural and urban children. One study showed that rural children consumed more energy, one study showed that urban children consumed fruit and vegetables more frequently and/or more fruit and vegetables(Reference McCormack and Meendering79). More comprehensive dietary information, including multiple 24 h recalls and consistently defined food groups and outcomes, would provide more insight into the dietary patterns, and potential differences therein, of rural and urban youth(Reference McCormack and Meendering79).
Another reason that rural residents have higher risk of disease could be related to their relative distance to health care, which generally tends to be more than urban residents(Reference Strasser67). A systematic review on primary prevention programmes to improve cardio-metabolic risk in non-urban communities by Rodrigues from 2016 showed that barriers to health change behaviour may differ between rural and urban residents and that context is very relevant when designing and implementing effective interventions(Reference Rodrigues, Ball and Ski80).
Considerations and how to move on from here
The findings presented in this narrative review suggest that sleep and dietary habits are important in the urban environment. However, results of the present paper should be interpreted with some caution as the present paper was not attempted to be a systematic review covering all literature. There may have been differences in the definition of urban or rural, as there are also subcategories like suburban. For future studies, it would be recommended that an urban/suburban/rural classification scheme should be developed that more accurately differentiates these areas. This would provide for more robust study of geographical differences in health outcomes(Reference Johnson and Johnson69). Future research could also include suburban children and adults and their behavioural, socio-economic and demographic characteristics affecting them as a third comparison group(Reference Johnson and Johnson69).
This review included data from children, adolescents and adults. Adolescence is a particularly vulnerable period for the emergence of inadequate sleep patterns(Reference Keyes, Maslowsky and Hamilton19). Population estimates have shown that one-quarter to one-third of adolescents get inadequate nightly sleep and there is concern that these rates are rising further. Although the exact reasons for this are unclear, it has been speculated that this could be due to the increased use of internet and social media and increased competitiveness for academic performance. Therefore, future studies should focus on children and adolescents as this is an important time in life when sleep and dietary patterns are formed. When studying dietary patterns in a larger population, it is also valuable to include measures of chrono-type. As differences were observed in sleeping patterns in developed and developing countries(Reference Bin, Marshall and Glozier16, Reference Matricciani, Olds and Petkov18, Reference Walch, Cochran and Forger23), it is also important to consider this wide variety of populations when designing new studies.
Lastly, when studying meal patterns, having clear definitions on what constitutes a meal or eating occasion is imperative(Reference St-Onge, Ard and Baskin53, Reference Berg and Forslund81). The inconsistency in definitions of eating occasions or meals may complicate interpretation and comparisons across studies. The classification of temporal distribution of meals is usually based on cultural norms(Reference Berg and Forslund81, Reference Chiva82) and may not be appropriate for the entire population(Reference St-Onge, Ard and Baskin53).
Conclusion
Sleep and dietary habits in the urban environment have changed vastly over past decades and are both important risk factors to consider for public health. Studying the impact of the timing of eating, chrono-nutrition, across the lifespan as well as in diverse populations across the world, could shed more light on the underlying mechanism how urbanisation impacts public health.
Acknowledgements
I am grateful to researchers and members of the cohorts mentioned in this paper. In addition, I am grateful to Julia Darzi, Haya Al Khatib, Wendy Hall, Janine Coulthard and Suzana Almoosawi, who cooperated with me and carried out some of the research summarised in this paper.
Financial Support
None.
Conflict of interest
None.
Authorship
G. P. was responsible for all aspects of writing this paper.




