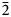Introduction
In the course of a research project dealing with the description and structural characterisation of natural Tl–Ag-sulfides/sulfosalts (i.e. Biagioni et al., Reference Biagioni, Bindi, Nestola, Cannon, Roth and Raber2016; Bindi and Biagioni, Reference Bindi and Biagioni2018; Bindi et al., Reference Bindi, Nestola, Guastoni, Peruzzo, Ecker and Carampin2012a, Reference Bindi, Nestola, Guastoni, Zorzi, Peruzzo and Raber2012b, Reference Bindi, Nestola, De Battisti and Guastoni2013, Reference Bindi, Biagioni, Raber, Roth and Nestola2015a, Reference Bindi, Nestola, Graeser, Tropper and Raber2015b, Reference Bindi, Nespolo, Krivovichev, Chapuis and Biagioni2020), we examined a sample from the Hemlo gold deposit, Marathon, Ontario, Canada (Harris, Reference Harris1989), belonging to the mineralogical collections of the Museo di Storia Naturale of the University of Florence, Italy (catalogue number 46582/G). This is also the type material for the recently approved biagioniite, Tl2SbS2 (IMA2019-120; Bindi and Moëlo, Reference Bindi and Moëlo2020). We were interested in the solution of the crystal structure of criddleite, TlAg2Au3Sb10S10 (Harris et al., Reference Harris, Roberts, Laflamme and Stanley1988), and tested several fragments by single-crystal X-ray diffraction. During this search, a few small grains turned out to be the new mineral thunderbayite, TlAg3Au3Sb7S6.
Thunderbayite was approved as a new mineral by the International Mineralogical Association Commission on New Minerals, Nomenclature and Classification (IMA2020-042, Bindi and Roberts, Reference Bindi and Roberts2020). The mineral name is for the Thunder Bay district, Ontario, Canada in which the Hemlo gold deposit is located. The holotype material is deposited in the mineralogical collection of the Museo di Storia Naturale of the University of Florence, Italy, under catalogue number 46582/G.
Here we report the description of the new mineral thunderbayite, together with its crystal structure.
Material studied
The sample containing thunderbayite, which is preserved in the collections of the Museo di Storia Naturale of the University of Florence, comes from the Hemlo gold deposit. Hemlo is an Archean-aged gold deposit (48°41’41”N, 85°54’13”W) located near the north-east shore of Lake Superior, ~35 km east of Marathon, Ontario, Canada and ~350 km east of Thunder Bay. It is situated in the Hemlo–Schreiber greenstone belt of the Wawa sub-province of the Superior Province (Tomkins et al., Reference Tomkins, Pattison and Zaleski2004, and references therein), is located within a zone of strong deformation that essentially parallels the regional west-northwest trend, and is stratiform within Archean-aged metamorphosed volcano–sedimentary rocks. The deposit has produced more than 21 million oz of gold and consists of several mineralised zones in which the ore minerals were formed from hydrothermal fluids that is related spatially to the shear zone.
The sample consists of tiny thunderbayite grains up to 70 μm across, associated spatially with aurostibite, stibarsen, biagioniite and native gold in a calcite matrix. Thunderbayite was misidentified initially as criddleite.
Physical and optical properties
Thunderbayite occurs as very rare anhedral rims around aurostibite in a calcite matrix (Fig. 1). The mineral exhibits a subhedral to anhedral grain morphology, and shows no inclusions of, or intergrowths with, other minerals. It is black in colour and has a black streak. The mineral is opaque in transmitted light and exhibits a metallic lustre. No cleavage is observed and the fracture is irregular. The calculated density (for Z = 2) for the empirical formula (see below) is 5.693 g/cm3. Unfortunately, the density could not be measured because of the small grain size. The Mohs hardness, estimated with respect to the surrounding calcite (by scratching both minerals), is ~3.
In plane-polarised incident light, thunderbayite is grey in colour, weakly bireflectant and weakly pleochroic from grey–blue to slightly greenish grey–blue. Between crossed polars, thunderbayite is weakly anisotropic with bluish to light-blue rotation tints. Internal reflections are absent and there is no optical evidence of growth zonation.
Reflectance measurements were performed in air by means of a MPM-200 Zeiss microphotometer equipped with a MSP-20 system processor on a Zeiss Axioplan ore microscope. The filament temperature was ~3350 K. An interference filter was adjusted, in turn, to select four wavelengths for measurement (471.1, 548.3, 586.6 and 652.3 nm). Readings were taken for both the specimen and standard (SiC) maintained under the same focus conditions. The diameter of the circular measuring area was 0.04 mm. Reflectance percentages for R min and R max are: 37.9, 38.4 (471.1 nm); 35.3, 36.0 (548.3 nm); 33.9, 34.4 (586.6 nm); and 32.0, 32.5 (652.3 nm), respectively.
In Fig. 2, the reflectance values (measured in air) for criddleite (TlAg2Au3Sb10S10; Harris et al., Reference Harris, Roberts, Laflamme and Stanley1988), vaughanite (TlHgSb4S7; Harris et al., Reference Harris1989) and thunderbayite are compared. Although only a limited set of values (only for the four wavelengths required by the Commission on Ore Mineralogy) have been obtained for thunderbayite, it appears evident that its reflectance is similar to that of criddleite.

Fig. 1. Scanning electron microscopy back-scatter electron image of thunderbayite (grey) associated with aurostibite (white) in a calcite matrix (black). The light grey phase at the bottom centre is thunderbayite with a slightly higher Au/Ag ratio. Type specimen (cat. numb. 46582/G).

Fig. 2. Reflectivity curves for thunderbayite in air (red symbols, the four wavelengths required by the Commission on Ore Mineralogy) compared to criddleite (black squares; Harris et al., Reference Harris, Roberts, Laflamme and Stanley1988) and vaughanite (black triangles; Harris et al., Reference Harris, Roberts and Criddle1989). Filled and open symbols refer to R1 and R2 values, respectively.
Chemical composition
A preliminary chemical analysis using energy dispersive spectrometry, performed on the crystal fragment used for the structural study, did not indicate the presence of elements (Z > 9) other than Ag, Au, Tl, Sb and S. Quantitative electron-microprobe analyses were carried out using a JEOL 8200 microprobe (wavelength dispersive spectroscopy mode, 25 kV, 20 nA, 1 μm beam size, counting times 20 s for peak and 10 s for background). The following lines were used: AgLα, AuMα, TlMα, SbLβ and SKα. The standards employed were: synthetic TlI (Tl), Ag-pure element (Ag), Au-pure element (Au), synthetic Sb2Te3 (Sb) and pyrite (S). The crystal fragment was found to be homogeneous within analytical error. The average chemical compositions (5 analyses on different spots) together with wt.% ranges of elements are reported in Table 1. On the basis of 20 atoms, the empirical formula of thunderbayite is Tl1.00Ag3.01Au3.03Sb7.12S5.84. The ideal formula is TlAg3Au3Sb7S6, which requires Tl 9.45, Ag 14.96, Au 27.31, Sb 39.39, S 8.89, for a total 100 wt.%.
Table 1. Electron-microprobe analysis (wt.% of elements) of thunderbayite.

X-ray crystallography and crystal-structure determination
The same crystal fragment (20 μm × 20 μm × 30 μm) used to obtain the chemical data was selected for single-crystal X-ray diffraction using a Bruker D8 Venture diffractometer equipped with a Photon II CCD detector, and graphite-monochromatised MoKα radiation (λ = 0.71073 Å). Thunderbayite is triclinic, with a = 8.088(3), b = 7.854(3), c = 20.078(8) Å, α = 92.52(3), β = 93.71(3), γ = 90.15(4)°, V = 1271.5(8) Å3 and Z = 2. Systematic absences were consistent with the space groups P1 and P ![]() $\bar{1}$. The statistical tests on the distribution of |E| values (|E 2–1| = 0.729) indicated the absence of an inversion centre and so the P1 space group was chosen. The structure was solved and refined using the program SHELXL (Sheldrick, Reference Sheldrick2008). The occupancy of all the sites was left free to vary (Tl vs. □; Ag vs. □; Au vs. □; Sb vs. □; S vs. □) but all the positions were found to be fully occupied and then fixed in the subsequent refinement cycles. Neutral scattering curves for Tl, Ag, Au, Sb and S were taken from the International Tables for X-ray Crystallography (Wilson, Reference Wilson1992). At the last stage, with anisotropic atomic displacement parameters for all the atoms and no constraints, the residual value settled at R 1 = 0.0220 for 5521 observed reflections [2σ(I) level] and 361 parameters and at R 1 = 0.0245 for all 6799 independent reflections.
$\bar{1}$. The statistical tests on the distribution of |E| values (|E 2–1| = 0.729) indicated the absence of an inversion centre and so the P1 space group was chosen. The structure was solved and refined using the program SHELXL (Sheldrick, Reference Sheldrick2008). The occupancy of all the sites was left free to vary (Tl vs. □; Ag vs. □; Au vs. □; Sb vs. □; S vs. □) but all the positions were found to be fully occupied and then fixed in the subsequent refinement cycles. Neutral scattering curves for Tl, Ag, Au, Sb and S were taken from the International Tables for X-ray Crystallography (Wilson, Reference Wilson1992). At the last stage, with anisotropic atomic displacement parameters for all the atoms and no constraints, the residual value settled at R 1 = 0.0220 for 5521 observed reflections [2σ(I) level] and 361 parameters and at R 1 = 0.0245 for all 6799 independent reflections.
Experimental details and R indices are given in Table 2. Fractional atomic coordinates and atomic displacement parameters are reported in Table 3. Bond distances are given in Table 4. The crystallographic information file has been deposited with the Principal Editor of Mineralogical Magazine and is available as Supplementary material (see below).
Table 2. Crystallographic data and refinement parameters for thunderbayite.

Table 3. Atoms, fractional atom coordinates (Å), and atomic displacement parameters (Å2) for thunderbayite.

Table 4. Selected bond distances (Å) for thunderbayite.

X-ray powder-diffraction data (Table 5) were collected with a Bruker D8 Venture diffractometer equipped with a Photon II CCD detector and using copper radiation (CuKα, λ = 1.54138 Å). The program Apex3 (Bruker, 2016) was used to convert the observed diffraction rings to a conventional powder-diffraction pattern. The least squares refinement gave the following values: a = 8.0882(5), b = 7.8492(5), c = 20.078(1) Å, α = 92.518(5), β = 93.739(5), γ = 90.028(6)° and V = 1270.73(9) Å3.
Table 5. Observed and calculated* X-ray powder-diffraction data (d in Å) for thunderbayite.

*Calculated powder pattern and indexing for thunderbayite on the basis of a = 8.088(3), b = 7.854(3), c = 20.078(8) Å, α = 92.52(3), β = 93.71(3), γ = 90.15(4)° and with the atom coordinates reported in Table 3 (only reflections with I rel ≥ 5 are listed).
Results and discussion
Description of the structure
In the crystal structure of thunderbayite there are two Tl sites, six Ag sites, six Au sites, 15 Sb sites and 12 S sites. Notably, the sites appear chemically pure, with no (or limited, if we consider the refinement uncertainties) substitutions. We cannot apply the classical crystal-chemical description that takes into account the metal–anion coordination polyhedra for thunderbayite, as several metal–metal bonds are present. Thallium atoms are three-fold coordinated by Sb, Ag/Au and S, with an additional contact with Sb at distance > 3.55 Å. By considering a limit <3.4 Å for the coordination environment of Ag, Ag6 is three-fold coordinated, Ag1 and Ag4 are four-fold coordinated, Ag3 and Ag5 are six-fold coordinated, and Ag2 and Ag5 are seven-fold coordinated. Interestingly, all the Ag atoms coordinate Sb and S except Ag6, which shows a short bond with Tl2 equal to 2.803(3) Å. Analogously, taking into account the same limit <3.4 Å for the coordination environment of Au, all the Au atoms coordinate Sb and S except Au1, which shows a short bond with Tl1 equal to 2.920(2) Å. In detail, Au3 and Au4 are five-fold coordinated, Au1, Au5 and Au6 are six-fold coordinated and Au2 is seven-fold coordinated. The Sb atoms show several complex environments ranging from a three-fold to a six-fold coordination with Au/Ag, Sb and S. Only Sb1 and Sb11 make bonds with Tl, with distances varying from 2.889(5) Å (Sb1–Tl2) to 3.392(2) Å (Sb11–Tl1). Sb2, Sb6, Sb9 and Sb13 exhibit a similar coordination environment (five-fold coordination) with mean bond distances of 3.02, 3.08, 3.10 and 2.97 Å, respectively. Sb2 and Sb13 show close values of the mean bond distances as they coordinate to the same set of atoms: 2 Ag, 1 Au, and 2 S. Analogously, Sb6 and Sb9, having an almost identical coordination sphere, coordinate 2 Au, 1 Ag and 2 S atoms.
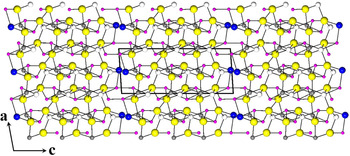
The crystal structure of thunderbayite can be viewed as a strongly deformed pyrite-type structure. It is well known that when half of the S atoms in pyrite-type compounds are replaced by other types of atoms, such as pnictogens, they can form different ordered ternary compounds, such as ullmannite (NiSbS)-type structures. The coordination environment of Ni in ullmannite (considering Sb and S as ‘anions’) closely resembles those observed (on average) for Ag and Au in thunderbayite (Figs 3, 4 and Table 4). If we look at Fig. 4, it appears that the structure of thunderbayite consists of ‘slabs’ stacked along the c axis: three alternate Au and Ag slabs with the Tl atoms terminating the unit cell. The Au slab is very similar to the Ni slab in the crystal structure of ullmannite (Bayliss, Reference Bayliss1977), with several Sb–S short bonds (Table 4). Therefore, thunderbayite might be regarded as an intergrowth structure of ternary pyrite-type slabs and Tl–Sb/S layers stacked along the c axis.

Fig. 3. The crystal structure of thunderbayite down ~[001]. Tl, Ag, Au, Sb and S are given as blue, white, grey, violet and yellow circles, respectively. The unit cell and the orientation of the structure are reported.

Fig. 4. The crystal structure of thunderbayite down ~[010]. Symbols as in Fig. 2. The unit-cell and the orientation of the structure are shown.
Relationships between thunderbayite and criddleite
Thunderbayite shows close similarities with criddleite, TlAg2Au3Sb10S10 (Harris et al., Reference Harris, Roberts, Laflamme and Stanley1988), from an optical, chemical and crystallographic point of view (very similar unit-cell values). As the two minerals come from the same mineral deposit, initially we thought thunderbayite and criddleite to be one and the same phase. However, the strongly different [Tl+Ag+Au]/(Sb+S) ratio (0.54 and 0.30 for thunderbayite and criddleite, respectively) and the differences in the strongest diffraction peaks [4.04(100), 3.92(90), 2.565(50) Å and 2.813(100), 5.63(90), 2.86(70) Å, for thunderbayite and criddleite, respectively] made us confident that they are different mineral species. Table 6 shows a comparison of the powder-diffraction data of thunderbayite and criddleite.
Table 6. X-ray powder-diffraction data (d in Å) for thunderbayite compared to that of criddleite.

*Calculated powder pattern and indexing for thunderbayite on the basis of a = 8.088(3), b = 7.854(3), c = 20.078(8) Å, α = 92.52(3), β = 93.71(3), γ = 90.15(4)° and with the atom coordinates reported in Table 3.
**Observed powder pattern reported for synthetic criddleite (Harris et al., Reference Harris, Roberts, Laflamme and Stanley1988).
Although the crystal structure of criddleite is as yet unknown, it is very likely that it possesses the same metal–metal interactions as observed in thunderbayite. Similarly, the unknown structure of vaughanite, TlHgSb4S7 (Harris et al., Reference Harris, Roberts and Criddle1989), might also show the same features. However, discussions on charge balance, degree of metallic bonding and possible structural models must await the availability of suitable crystals for X-ray investigations.
Acknowledgements
The manuscript benefitted from the review of František Laufek, Paul G. Spry, and an anonymous reviewer. The research was funded by MIUR-PRIN2017, project “TEOREM deciphering geological processes using Terrestrial and Extraterrestrial ORE Minerals”, prot. 2017AK8C32 (PI: Luca Bindi).
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2020.80