INTRODUCTION
Vitamin D (VitD) deficiency status is associated with tuberculosis (TB) in epidemiological studies that include patients affected by the disease [Reference Davies, Brown and Woodhead1–Reference Ho-Pham4]. However, the results of some clinical trials on the effect of VitD supplements in TB patients are inconclusive [Reference Wejse5–Reference Ralph8]. Moreover, a case-control study [Reference Nielsen9] found that both high and low VitD levels were associated with active TB.
A different approach is the study of VitD deficiency as a risk factor of TB infection conversion (TBIC), the change from non-infection to TB infection without the disease, known as latent TB infection (LTBI). This infection status prior to development of the disease can be measured by immunological tests: tuberculin skin test (TST) and interferon-gamma release assays (IGRAs), including enzyme-linked immunospot (ELISPOT; BD, USA) and ELISA tests such as QuantiFERON-TB Gold In-Tube test (QFT-GIT®; Cellestis Ltd, Australia) [Reference Mack10]. Risk factors associated with TBIC are dependent on the TB status of the index case and include positive bacilli sputum, duration and place of contacts, and characteristics of the exposed subject such as, age, nutritional status, race, and other risk factors [Reference Bailey11]. Recently, a case-control study of contacts of pulmonary TB patients found that sufficient levels of VitD (⩾30 ng/ml) were protective against TBIC [Reference Arnedo-Pena12]. In addition, Ganmaa et al. [Reference Ganmaa13] found that VitD supplements could protect against TBIC in a clinical trial in Mongolian schoolchildren (P = 0·06).
In this context, the aim of this study was to estimate the relationship between baseline VitD status and TBIC in the contacts of pulmonary TB patients, using TST and QFT-GIT tests.
METHODS
The study involved two health departments (Castellon and Vila-real) with a population of 470 000 inhabitants in Valencia Community (Spain), and it was conducted by the Epidemiology Division (ED) of the Public Health Centre in Castellon from December 2010 to December 2012 and the Preventive Medicine department (PM) of La Plana Hospital in Vila-real from December 2010 to August 2011.
The design was a prospective cohort study and the study population was contacts of pulmonary TB patients, who were examined in order to rule out and prevent TB; controls included clinical (TB history and TB symptoms), analytical (complete blood counts and alkaline phosphatase and alanine aminotransferase enzymes), and thorax radiographic examinations. Contacts included household members, other family members, friends, work colleagues, and classmates with exposure to the TB index case. In Castellon ED all these contacts were studied. However, in Vila-real PM only household members of the TB index case were studied when this case was hospitalized, giving a total of 23 contacts screened during the period December 2010–August 2011. The other contacts with residence in Vila-real were studied in Castellon ED. Of the pulmonary TB index cases, 14 were from Vila-real PM and 33 from Castellon ED.
The participants in the cohort were contacts negative by TST/QFT-GIT at the initial examination with a baseline determination of 25-hydroxyvitamin D [25(OH)D] status; children aged <10 years were excluded. After 8–10 weeks, a second examination was performed in order to determine TBIC or TB disease. A LTBI case was defined as having a positive TST/QFT-GIT result and normal clinical, analytical, and thorax radiographic examinations.
Two screening methods were used in the examination to detect TBIC and LTBI. In the first method, TST was used first and QFT-GIT was used for participants positive by TST. In the second method, TST and QFT-GIT were used at the same time. The first method was used in both Castellon ED and Vila-real PM and the second method in Castellon ED only. With the first method (Fig. 1) (December 2010–February 2012), a TST with 2 tuberculin units of PPD (purified protein derivative RT-23) from the Serological Institute of Copenhagen was performed on the first visit. The TST was considered positive in non-bacillus Calmette-Guérin (BCG)-vaccinated participants with an induration of ⩾5 mm, or BCG-vaccinated participants with an induration of ⩾15 mm, or the presentation of vesicles [14]. The BCG vaccination was verified by self-report and clinical examination for BCG scar. If the test was negative, a booster TST was given when considered necessary (BCG vaccination, age>54 years). If the TST was positive, QFT-GIT was administered. After 8–10 weeks, a new TST was administered for contacts with negative TST and/or negative QFT-GIT. If the TST was negative, the contact was considered to be negative for TBIC. TBIC in non-BCG-vaccinated participants was considered as the change from negative to positive TST with an increase of ⩾5 mm in induration, and in BCG participants an increase of ⩾10 mm in induration of the initial TST [Reference Arnedo-Pena12, 14]. If the TST was positive, a QFT-GIT was performed within 72 h of the TST and positive QFT-GIT was defined when QFT-GIT ⩾0·35 IU/ml, and was considered as TBIC. Positive TST and QFT-GIT participants were considered as LTBI if the thorax radiology and other examinations were negative. By the second method (Fig. 2) (March–December 2012), TST and QFT-GIT were administered at the same time, enabling a better comparison and interpretation of the tests. After 8–10 weeks, all contacts presenting as negative by TST and QFT-GIT were screened a second time with TST and QFT-GIT; positive TST and negative QFT-GIT participants were screened a second time with QFT-GIT only. The criteria for TBIC were the same as in the first method. Considering the debate regarding TBIC by QFT-GIT [Reference Zwerling15, Reference Gran, Abmus and Dyrhol-Riise16], an alternative definition of TBIC was considered as follows: a change from negative QFT-GIT in the first test to positive QFT-GIT (⩾0·35 IU/ml) in the second test with an increase of at least 2·6 times the first QFT-GIT test [Reference Zwerling15]. Thorax radiology and other examinations were performed to determine LTBI for all positive QFT-GIT and TST participants. QFT-GIT analyses were performed in the Microbiology Laboratory of Hospital General of Castellon.

Fig. 1. Criteria of tuberculosis (TB) infection conversion (TBIC) by tuberculin skin test (TST) and QuantiFERON-TB Gold In-Tube (QFT-GIT) test from December 2010 to February 2012. The first TST was administered, and if positive, the QFT-GIT was performed 3 days later. For bacillus Calmette-Guérin (BCG)-vaccinated participants or age >54 years, a TST booster was administered if the first TST was negative. First screening: positive TST ⩾5 mm induration, non-BCG-vaccinated participant; TST ⩾15 mm induration, BCG-vaccinated participant. Negative when QFT-GIT <0·35 IU/ml, positive when QFT-GIT ⩾0·35 IU/ml. Second screening: positive TBIC, induration with an increased TST ⩾5 mm, non-BCG-vaccinated participant, or induration with an increased TST ⩾10 mm, BCG-vaccinated participant. Positive TBIC when QFT-GIT ⩾0·35 IU/ml. Latent TB infection (LTBI) was confirmed by thorax radiology.

Fig. 2. Criteria of tuberculosis (TB) infection conversion (TBIC) by tuberculin skin test (TST) and QuantiFERON-TB Gold In-Tube (QFT-GIT) test from March 2012 to December 2012. TST and QFT-GIT tests were performed at the same time. First screening: positive TST ⩾5 mm induration, non-BCG-vaccinated participant; ⩾15 mm induration, BCG-vaccinated participant. Negative when QFT-GIT <0·35 IU/ml, positive when QFT-GIT ⩾0·35 IU/ml. Second screening: positive TBIC, induration with an increased TST ⩾5 mm, non-BCG-vaccinated participant or induration with an increased TST ⩾10 mm, BCG-vaccinated participant. Positive TBIC when QFT-GIT ⩾0·35 IU/ml. Latent TB infection (LTBI) was confirmed by thorax radiology.
The study was completed with an interview with the participants and a self-report questionnaire in order to obtain information about TB risk factors such as exposure to the index case (high exposure was considered to be ⩾6 h/day close contact with the TB patient, medium exposure 1–5 h/day, and low exposure <1 h/day), habits, weight and height, social class, use of VitD supplements and other variables.
During the first examination, 25(OH)D serum level was measured by chemiluminescence immunoassay on an IDS-iSYS automated analyser in the Biochemical Laboratory of Hospital General of Castellon [Reference Cluse17]. Serum 25(OH)D levels of 0–9 ng/ml were considered as very deficient, 10–19 ng/ml as deficient, 20–29 ng/ml as insufficient, and ⩾30 ng/ml as sufficient [Reference Holick18].
Statistical methods
χ 2 and Fisher's tests and the Kruskal–Wallis test were used to compare qualitative and quantitative variables, respectively. Poisson regression models were used to estimate the relative risk (RR) and 95% confidence interval (CI). TBIC was the dependent variable and independent variables were VitD status and other potential risk and protective factors. The independent variables associated with P < 0·05 were included in the final models. In all the Poisson regression models the goodness of fit was not rejected (P > 0·05). An attributable risk (AR) fraction was estimated [Reference Rockhill, Newman and Weinberg19]. The statistical program Stata v. 12 (StataCorp, USA) was used for all calculations.
The study was approved by the Hospital General Ethics Committee of Castellon and signed consent was obtained from all participants or their parents. Children aged <10 years were not included in the study.
RESULTS
A study participant selection flow chart is presented in Figure 3. From 516 contacts of pulmonary TB patients, VitD determinations were performed for 386 contacts (74·8% 386/516). Of the 386 contacts, 90 were positive by TST/QFT-GIT (23·3% 90/386) and were classified as LTBI cases; of the 49 contacts with negative TST/QFT-GIT, only one screening was necessary to rule out LTBI considering that >10 weeks had passed since the last exposure to the TB index case (12·7% 49/386). The initial cohort had 247 contacts of which 198 were screened twice 8–10 weeks after the initial examination, giving a participation rate of 80·2% (198/247). Two new cases of pulmonary TB were detected in the cohort (1·0%, 2/198). Primary prophylaxis for TB was not recommended for any of the participants. Of the 90 positive LTBI contacts, 65, including all the TBIC cases, were recommended chemoprophylaxis against TB.

Fig. 3. Flow of the contacts of pulmonary tuberculosis (TB) patients from December 2010 to December 2012. TST, Tuberculin skin test; QFT-GIT, QuantiFERON-TB Gold In-Tube test; LTBI, latent TB infection.
Table 1 presents the comparison of the 198 participants, the 49 non-participants, and the 130 contacts without measured VitD. These contacts were younger than the participants and they included fewer foreign-born individuals. When participants and non-participants were compared, no significant differences were observed in the studied variables, except the high participation of residents from Vila-real. The participants were older than non-participants and the proportion of foreign-born individuals was lower among participants (21·7% vs. 30·6%). Exposure to TB sputum positive acid-fast bacilli (AFB) smear was higher in participants than in non-participants (77·8% vs. 69·4%). The mean VitD concentration was higher in participants than the mean for non-participants, the percentage of sufficient level of VitD (⩾30 ng/ml) was higher (37·4% vs. 26·5%, respectively), and the percentage of VitD (<20 ng/ml) deficiency was higher in non-participants (42·9% vs. 30·8%). All contacts with deficient VitD status were referred to their physicians with a recommendation to take vitamin D supplements.
Table 1. Description of the cohort of contacts of pulmonary TB patients: participants, non-participants and contacts without measured vitamin D
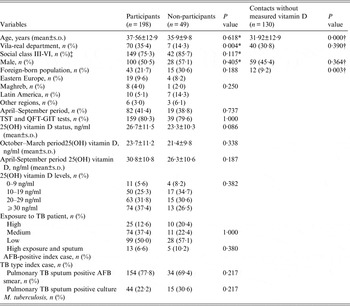
AFB, Acid-fast bacilli; BCG, bacillus Calmette-Guérin; BMI, body mass index; QFT-GIT, QuantiFERON-TB Gold In-Tube test; s.d., standard deviation; TB, tuberculosis; TST, tuberculin skin test.
* Used to compare participants and non-participants.
† Used to compare participants and contacts without measured vitamin D.
‡ Social class: I–II, Professional and managerial and technical; III–VI, Skilled non-manual and manual, partially skilled and unskilled.
There were 18 TBIC cases with a cumulative incidence of 9·1% (18/198). Table 2 shows the characteristics of participants who suffered TBIC, considering the two screening methods used in the study. By the first method, nine TBIC were observed; participant 1·8 was initially TST positive/QFT-GIT negative; the TST was then repeated as an exception to this test and QFT-GIT was then positive. By the second method, seven TBIC cases were observed, all of whom were TST and QFT-GIT positive; two participants presented as TST positive/QFT-GIT negative at the first screening. Three TBIC cases presented a second QFT-GIT between 0·47 and 0·77 IU/ml. Participants 1·7 and 2·17 developed TB disease detected after TBIC.
Table 2. Description of TB infection conversion in the contacts of pulmonary TB patients and vitamin D status. First method (1): TST first only QFT-GIT positive TST; second method (2): TST and QFT-GIT at the same time

AFB, Acid-fast bacilli; BCG, bacillus Calmette-Guérin; QFT-GIT, QuantiFERON-TB Gold In-Tube test; TB, tuberculosis; TST, tuberculin skin test; VitD, vitamin D status.
* Booster TST implemented.
† No booster TST but QFT-GIT.
Table 3 presents the comparison between the TBIC cases and non-cases of the cohort by Poisson regression models. Vila-real residents had a higher TBIC risk (RR 2·87, 95% CI 1·11–7·41) than Castellon residents. Social class V (unskilled occupation) presented higher TBIC risk (RR 2·96, 95% CI 1·15–7·64) than the other social classes. Foreign-born participants presented higher TBIC risk (RR 2·88 95% CI 1·44–7·31) than Spanish-born participants. The mean of VitD in the TBIC cases was lower than for non-cases (20·7±11·9 ng/ml and 27·2±11·4 ng/ml; P = 0·028, respectively). The risk of TBIC decreases significantly from VitD levels ⩾20 ng/ml (P trend = 0·012), and high exposure to TB patients was associated with TBIC (P = 0·001). The exposure to pulmonary TB sputum AFB smear index case was higher in non-cases than in TBIC cases (P = 0·030). The two contacts who developed pulmonary TB presented VitD deficiencies of 6·5 ng/ml and 18·3 ng/ml, respectively.
Table 3. Incidence of tuberculosis infection conversion (TBIC) in the cohort of contacts by Poisson regression

AFB, Acid-fast bacilli; BCG, bacillus Calmette-Guérin; BMI, body mass index; CI, confidence interval; QFT-GIT, QuantiFERON-TB Gold In-Tube test; RR, relative risk; s.d., standard deviation; TB, tuberculosis; TST, tuberculin skin test.
* Social class I–II: Professional and managerial and technical; III–VI: Skilled non-manual and manual, partially skilled and unskilled.
† BMI in 16 cases and 147 non-cases.
‡ Ever smoking in 16 cases and 171 non-cases
§ Use of vitamin D supplements in 17 cases and 154 non-cases.
In a Poisson regression model (Table 4), only VitD levels and high exposure and TB sputum AFB-positive index case showed significant associations with incidence of TBIC. The RR according to VitD levels were: (a) 0–9 ng/ml: 27·3% (3/11), RR 1·00; (b) 10–19 ng/ml: 16·0% (8/50), RR 0·52, 95% CI 0·14–1·99; (c) 20–29 ng/ml: 4·8% (3/63), RR 0·15, 95% CI 0·03–0·77; (d) ⩾30 ng/ml: 5·4% (4/74), RR 0·15, 95% CI 0·03–0·71 (P trend = 0·005). In a Poisson regression model with VitD concentration adjusted for high exposure and TB sputum AFB-positive index case, an increase of 1 ng/ml of VitD decreased the incidence of TBIC by 6% (RR 0·94, 95% CI 0·90–0·99, P = 0·015).
Table 4. Risk and protective factors for TB infection conversion in the cohort of contacts by Poisson regression

AFB, Acid-fast bacilli; CI, confidence interval; RR, relative risk; TB, tuberculosis.
If the alternative definition of QFT-GIT conversion was used [Reference Zwerling15], i.e. an increase of at least 2·6 times between the first and second QFT-GIT tests, two QFT-GIT conversions would not be included (participants 2·15 and 2·18), and the TBIC would be 16 cases, giving a cumulative incidence of 8·2% (16/196). An increase in the inverse association between VitD status and incidence of TBIC was observed. The RRs according to VitD levels, adjusted for high exposure and TB sputum AFB-positive index case, were: (a) 0–9 ng/ml: 27·3% (3/11), RR 1·00; (b) 10–19 ng/ml: 16·0% (8/50), RR 0·50, 95% CI 0·13–1·91; (c) 20–29 ng/ml: 4·8% (3/63), RR 0·14, 95% CI 0·03–0·73; (d) ⩾30 ng/ml: 2·8% (2/72), RR 0·07, 95% CI 0·01–0·44 (trend P = 0·001). Considering the VitD concentration, adjusted for high exposure and TB sputum AFB-positive index case, an increase of 1 ng/ml VitD decreases the incidence of TBIC by 9% (RR 0·91, 95% CI 0·86–0·96).
Considering the deficient VitD status <20 ng/ml to be a risk for TBIC (Table 5), the adjusted RR was 3·96 (95% CI 1·51–10·37) and the AR, adjusted for high exposure and TB sputum AFB-positive index case, was 45·7% (95% CI 20·6–55·2). Therefore, if the association were causal, one out of two TBIC occurring in participants with <20 ng/ml VitD may be attributable to VitD deficiency.
Table 5. Relative risks and attributable risk of tuberculosis infection conversion (TBIC) in the cohort study following vitamin D status by Poisson regression

AFB, Acid-fast bacilli; RR, relative risk; AR, attributable risk; CI, Confidence interval; TB, tuberculosis.
* Formula attributable risk: pd (RR – 1)/RR (where pd÷proportion of cases with vitamin D <20 ng/ml): 11/18 = 0·61.
DISCUSSION
The results of the study indicate that VitD status was associated with TBIC in contacts of pulmonary TB patients. In addition, a considerable proportion of the participants had deficient baseline VitD levels. Deficient and very deficient VitD status was associated with high TBIC.
These results are consistent with studies of VitD status and TBIC. In a case-control study in contacts of TB patients, Arnedo-Pena et al. [Reference Arnedo-Pena12] found that only sufficient VitD levels were protective against TBIC. In addition, the study in Mongolian schoolchildren at risk of TB [Reference Ganmaa13] found that only one TBIC occurred with a VitD concentration of ⩾20 ng/ml vs. eight TBIC with VitD levels of <10 ng/ml (P = 0·05). In a cohort study of contacts of pulmonary TB patients in Pakistan, Talat et al. [Reference Talat20] found significant TB progression when VitD levels were <7 ng/ml compared to higher VitD levels (P = 0·002). In addition, a cohort study of HIV-infected Tanzanian adults found a significant high risk of incident TB when comparing deficient and sufficient VitD status, but risk was not significant when insufficient and sufficient VitD status were compared [Reference Sudfeld21]. On the other hand, an ecological study in Peru found that the midwinter maximum VitD deficiency was followed 6–12 weeks later by increases of TBIC [Reference Wingfield22]. Controversy still remains over what are the adequate levels of VitD concentration [Reference Rosen23]. One study reviewed the guidelines of VitD dietary intake from the Institute of Medicine, and suggested that the adequate VitD level was >20 ng/ml [Reference Aloia24].
The role of VitD status in the development of TB is being studied, but it is not completely understood [Reference Dini and Bianchi25]. VitD acts against M. tuberculosis through the innate and adaptive immune responses and other actions [Reference Hewison26]. The responses include the Toll-like receptor complex [Reference Liu27] that activates the production of cathelicidin and defensin beta 4 against M. tuberculosis, the release of IFN-γ by T cells with autophagy [Reference Fabri28, Reference Liu29], and the balance of Th2 and Th1 cytokine production [Reference Lin and Flynn30]. In addition, VitD is able to inhibit M. tuberculosis directly in culture [Reference Greenstein, Su and Brown31]. On the other hand, we do not know if changes in VitD levels affect the sensitivity and specificity of TST and QFT-GIT, and further work is needed to establish their reliability in VitD-deficient populations.
In our study, the prevalence of LTBI was in line with other studies of contacts of pulmonary TB patients in high-income countries with a range of 24·2–32·4%, [Reference Fox32], but the incidence of new TB was higher. In addition, the incidence of TBIC was situated in the range of other studies of pulmonary TB contacts: 6·1–27% [Reference Cailleaux-Cezar33, Reference Goris-Pereiras34] and lower than the TBIC in the earlier study, 12·5% [Reference Arnedo-Pena12].
The strengths of this study include its prospective design, a higher participation than in other studies of contacts [Reference Goris-Pereiras34, Reference Hill35], exposure to pulmonary TB patients, the seasonal variations of VitD levels during the year, the control of co-variables with Poisson regression models, a valid technique that measures 25(OH)D in blood with determination of vitamin D2 and D3 [Reference Farrell36], and the use of TST and QFT-GIT in order to obtain a better characterization of LTBI and TBIC.
The QFT-GIT is more specific than the TST, and it may reduce false-positive TST conversions. However, the QFT-GIT conversion cut-off is not yet a resolved issue [Reference Nienhaus37], and there are other IGRA tests [Reference Mack10]. In our study, when the alternative definition of QFT-GIT conversion was used, two TBIC cases were excluded, and the protective effect of baseline VitD increased. In addition, our study has some limitations such as the medium size of the sample, and the absence of VitD receptor genetic polymorphism; moreover, VitD was measured on only one occasion and seasonal variations are important in VitD status, and the potential effects of other factors such as poverty and crowding were not considered in this study. Social class VI, analysed as a proxy of poverty, could be insufficient to control for this potential confounding factor. More precise data on these variables should be obtained. Furthermore, of the initial 516 contacts of pulmonary TB patients (Fig. 3), 130 contacts without VitD status measurements and 49 contacts lost to follow-up were not considered (34·7%, 179/516), which is another limitation of this study. Two screening methods were used in the study of TBIC. With the first method, TST was used before QFT-GIT, which is also a limitation. Boosters of TST were administered in order to avoid false-positive TST conversion. However, no difference in TBIC was found when comparing the two methods. On the other hand, a TST administered no more than 3 days before QFT-GIT may not affect this QFT-GIT [Reference Van Zyl-Smit38].
In the future, well designed clinical trials could establish whether or not to recommend the use of VitD in the prevention of TBIC, and knowing the VitD status of contacts of pulmonary TB could be useful in the follow-up monitoring of this population. In addition, more studies of TB contacts are required to improve our knowledge of the effects of VitD status on TB [Reference Verrall39].
CONCLUSION
The results of this study suggest that VitD status is associated with TBIC, and a sufficient VitD level could be a protective factor of TBIC.
ACKNOWLEDGEMENTS
The authors thank the participants for their generous cooperation in making this study possible. The authors are grateful to Antonio Ferrero-Vega, Juan Bellido-Blasco, Concepción Herrero-Carot, Esther Silvestre-Silvestre, Noemi Meseguer-Ferrer, and Joan Puig-Barberá for their contribution in this study. The authors also thank two anonymous reviewers for their comments.
DECLARATION OF INTEREST
None.











