Healthcare personnel (HCP) face exposure to severe acute respiratory coronavirus virus 2 (SARS-CoV-2) in community and occupational settings. Reference Celebi, Piskin and Celik Beklevic1 Even though community exposure may account for a larger percentage of HCP exposure, occupational risk remains a concern. Reference Al Maskari, Al Blushi and Khamis2 Within occupational settings, the 2 main sources of exposure are from other HCP and patients.
In a study of HCP in a tertiary-care center in Wuhan, China, HCP infected with SARS-CoV-2 perceived their infection source to mainly come from the hospital setting, mostly from patients. Reference Jin, Huang and Wang3 In a large seroprevalence study in Sweden, HCP who had contact with coronavirus disease 2019 (COVID-19) patients had an increased risk of having antibodies to SARS-CoV-2 compared to HCP who had contact with non–COVID-19 patients. Reference Rudberg, Havervall and Manberg4 Additionally, in a study by Algado-Sellés et al, Reference Algado-Selles, Gras-Valenti and Chico-Sanchez5 HCP with confirmed COVID-19 were 3 times more likely to have a workplace social interaction as the source of infection compared to patient-care–related exposures.
HCP role may also be a factor. In a cross-sectional serosurvey in Chicago among HCP, nurses were more likely to be seropositive than other HCP. Reference Wilkins, Gray and Wallia6 However, serology-based analyses can be challenging to interpret given the lack of clear association with occupational exposures. In a study of HCP who underwent reverse-transcriptase polymerase chain reaction (rtPCR) testing, physicians had a higher likelihood of testing positive than other HCP. Reference Lombardi, Consonni and Carugno7
A significant proportion of HCP may develop asymptomatic infection, though this may occur after community or occupational exposure. Reference Rivett, Sridhar and Sparkes8,Reference Treibel, Manisty and Burton9 In postexposure testing 7 days after exposure in Vermont, 3% of community contacts tested positive, and of these, 35% were asymptomatic. Reference Jones, Fialkowski, Prinzing, Trites, Kelso and Levine10 However, the incidence of asymptomatic infection after a known occupational exposure is not well understood.
Given these discrepancies and gaps in the literature, we evaluated all reported occupational exposures to SARS-CoV-2 at a multistate, tertiary-care medical center to assess factors associated with infection, including HCP role and exposure source,. Additionally, we sought to determine the incidence of asymptomatic infection.
Methods
Study location
This study is a retrospective analysis of all confirmed medium and high-risk occupational exposure incidents to other employees and patients documented in the occupational health service (OHS) COVID-19 database from March 20, 2020, through December 31, 2020, at a large, multisite US academic medical center. Mayo Clinic has main sites in Minnesota, Arizona, Florida, as well as a health system with regional hospitals and clinics in southern Minnesota and Wisconsin. In total, ∼72,000 healthcare workers are employed, and fluctuation regularly occurs due to hiring and retirement. This study was determined to be exempt from review by the Mayo Clinic Institutional Review Board (IRB application no. 20-007051).
Patients were assessed for symptoms of COVID-19 upon entrance to a Mayo Clinic facility. Patients admitted to the hospital were screened for SARS-CoV-2 by reverse-transcription polymerase chain reaction (rtPCR), with positive or suspected cases admitted to designated COVID-19 units with isolation precautions.
Infection prevention and control measures
Universal face mask and eye protection policies for all patient encounters for HCP were instituted on April 17, 2020, and May 15, 2020, respectively. With increased community rates, critical staffing policies were initiated at the Arizona and Florida sites on July 1, 2020, and in Minnesota and Wisconsin on October 1, 2020. This allowed HCP who sustained a non-household exposure to return to work with frequent testing provided that they remained asymptomatic and free of infection.
For patients with confirmed or suspected COVID-19 infection, the recommended PPE for HCP included a surgical face mask, eye protection, gown, and gloves for all encounters. If a procedure with medium or high risk of aerosol generation (ie, an AGP) was performed, use of a respirator instead of a face mask was recommended. HCP were provided training on best practices for using PPE.
HCP were reminded to maintain masking while on campus including when in close contact with other HCP. HCP were also advised to maintain a distance of ∼2 m (6 feet) from others while in situations in which masking was not possible (ie, while eating).
Occupational exposure assessment
Details of exposure reporting, contact tracing and risk assessment have been previously described. Reference Breeher, Boon, Hainy, Murad, Wittich and Swift11 Occupational exposures were identified through contact tracing and through self-reportage. Exposures in all occupational settings were included for evaluation. Employees were educated on appropriate PPE usage and were advised to contact OHS if any concerns for a breach in PPE occurred. Reported exposures to persons with COVID-19 were evaluated by OHS based on guidance from the Centers for Disease Control and Prevention (CDC) (Table 1). Every employee that tested positive for SARS-CoV-2 underwent an assessment for symptoms, source of infection, and secondary exposures to other HCP. Post exposure testing for all symptomatic and asymptomatic employees following exposure was conducted at least three times: at baseline, during days 5–7, and during days 12–14, regardless of work assignment. Staff who had or developed symptoms during the quarantine period had testing coordinated immediately.
Table 1. Exposure Risk Assessment for Exposed Employee to Source Patient by Type

a To qualify as an exposure: Persons must be within 2 m and together cumulatively for >15 minutes when the source is communicable.
b Examples of respirators include fit-tested N95 facemask and powered air purifying respirators (PAPR).
c With an AGP: AGPs require closing the room in which the AGP was performed for an appropriate air clearance time (ACT). During both the AGP and ACT, any amount of time in the room would constitute an exposure.
Data collection
Data were collected from the OHS COVID-19 database, which had demographic data and occupational data, including age during the study period, sex, employment location, role, and SARS-CoV-2 molecular assay results and dates.
The job duties of HCPs were divided into 6 categories: administration staff (ie, administrative office support, business professionals, clinical office support), nurses (ie, LPNs and RNs), providers (ie, physicians, clinical residents and fellows, nurse practitioners, and physician assistants), nonclinical staff (ie, custodial staff and food services, general services, and research personnel), technicians (ie, patient care technicians, laboratory technicians, and ultrasound technicians), and healthcare professionals (ie, paramedics, respiratory therapists, and physical therapists).
Statistical analysis
We presented descriptive statistics by exposure and COVID-19 infection. A 2-level, random intercept, hierarchical logistic regression model was constructed for COVID-19 positive results, accounting for unobserved difference among sources of exposure. The adjusted variables included in the model were age, sex, high-risk exposure, state, and HCP role. Statistical analyses were conducted using Stata version 17.1 software (StataCorp LLC, College Station, TX).
Results
In total, 2,253 confirmed occupational exposures occurred during the study period, and demographic information of exposed HCP is listed in Table 2. Nurses had the most exposures (43%), followed by nonclinical support staff (20.5%). Employees were the source for 1,206 exposures (57.1%), whereas patients were the source for 1,047 exposures (42.9%). Of the employee exposures, 81.3% were high risk, compared to 50.6% of the patient exposures.
Table 2. Demographic characteristics Exposed HCP
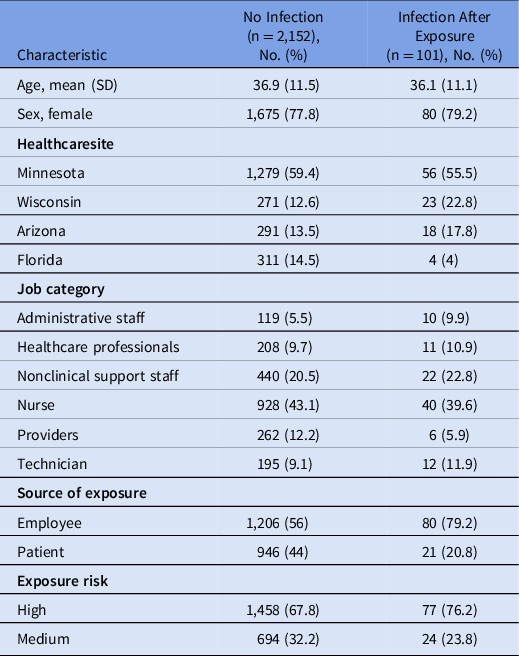
Overall, 101 (4.5%) HCP tested positive during the postexposure period. Of these, 80 (79.1%) had an employee source of exposure and 21 (20.9%) had a patient source of exposure. The infection rate was 6.2% when an employee was the source, compared to 2.2% when a patient was the source. The HCP infection rate after a medium-risk exposure was 3.3%, and 5% after high-risk exposure. Among SARS-CoV-2–positive HCP, employee sources of exposure were more likely to be high risk (83.8%) compared to patient sources (47.6%). Furthermore, 17 HCP (16.8%) were asymptomatic at the time of testing positive.
A temporal trend of exposure was also noted. From March 20 through May 31, 2020, 223 (67.8%) of the 329 occupational exposures were from patients. From June 1 through December 31, 2020, 744 (38.7%) of 1,924 of occupational exposures were from patients (P < .001).
In the multivariate analysis, occupational exposure from an employee source had an increased risk of testing positive compared to a patient source (OR, 3.22; 95% CI, 1.72–6.04). Additionally, we detected location-specific differences. Compared to Minnesota, HCP in Wisconsin had a higher risk of becoming infected after an exposure (OR, 2.48; 95% CI, 1.13–5.45), whereas HCP in Arizona had a lower risk (OR, 0.26; 95% CI, 0.08–0.84). Sex, age, high-risk exposure, and HCP role were not associated with increased risk of testing positive.
Discussion
HCP face exposure risks both in the community and in occupational settings. Multiple studies have shown an increased likelihood of contracting COVID-19 in community settings compared to occupational settings. Reference Al Maskari, Al Blushi and Khamis2,Reference Baker, Nelson and Overton12 Still, occupational exposure remains a concern for HCP. In this cohort of employees with occupational exposures, 4.5% of exposed HCP tested positive after an occupational exposure to a person with SARS-CoV-2, which is consistent with previously reported studies. Reference Maltezou, Dedoukou and Tseroni13 Importantly, significant exposure to an infectious coworker was more common than exposure to a patient, and HCP were 3 times more likely to test positive after exposure to a coworker versus a patient. Several strategies were used to mitigate employee-to-employee transmission, including universal source control masking, opening overflow break rooms for social distancing during meals, and educating personnel and supervisors on best practices for shared workspaces and breakrooms.
Among occupational settings, some studies have reported that patients are a more likely source of infection, Reference Al Maskari, Al Blushi and Khamis2,Reference Lombardi, Consonni and Carugno7 and others have reported increased occupational exposure risk from other HCP. Reference Algado-Selles, Gras-Valenti and Chico-Sanchez5,Reference Fell, Beaudoin and D'Heilly14 Contrary to the study by Zabarsky et al, Reference Zabarsky, Bhullar and Silva15 HCP in our evaluation were 3 times more likely to test positive after an exposure to an employee compared to a patient. This finding is consistent with a study of 5,374 HCP exposures in Minnesota, which reported that 1.3% of HCP tested positive within 14 days of exposure from a patient source, compared to 3.8% from an HCP source. Reference Fell, Beaudoin and D'Heilly14
There may be several reasons for this discrepancy between source of exposure and testing positive. HCP may recognize risks posed from patients with SARS-CoV-2, but they may underestimate the risk from other HCP in settings where masking and distancing may not be feasible, such as breakrooms or other gatherings. The dynamics and risk of HCP exposure may have changed over time because of increased awareness in transmission risk factors for SARS-CoV-2, adequate personnel protective equipment, and enhanced infection prevention and control measures. In an evaluation of 7,050 HCP infections in Ontario, Canada, the HCP infection rate increased nearly 4-fold compared to non-HCP in April 2020, with no difference in September 2020. Reference Schwartz, Achonu and Buchan16 In our evaluation, exposures in April–May 2020 were more likely to come from patient sources, compared to June–December 2020, when coworker sources were more common and PPE requirements including masks and eye protection for patient care were required.
Moreover, hospitalized patients typically present several days after symptom onset, and length of stay ranges widely, from 4 to 19 days. Reference Faes, Abrams and Van Beckhoven17,Reference Rees, Nightingale and Jafari18 Although hospitalized patients may shed virus for prolonged periods of time, much of the transmission occurs early in the disease course, with a progressive decline in the ability to detect virus by viral culture over time. Thus, patients with prolonged hospitalizations may be less likely to be able to transmit virus to HCP compared to asymptomatic or presymptomatic coworkers who may be at work during a more infectious stage of illness. Reference van Kampen, van de Vijver and Fraaij19 The higher incidence of exposures to coworkers compared to patients may also reflect decreased use of PPE with individuals who are not identified as having COVID-19 infection, whereas symptomatic patients are often under isolation precautions, with prominent signage alerting healthcare workers.
We did not find a significant difference in HCP testing positive after a high- versus medium-risk occupational exposure. This finding contrasts with a report by Maltezou et al, Reference Maltezou, Dedoukou and Tseroni13 in which risk of testing positive increased with higher-risk exposures compared to medium or low-risk exposures. Reference Maltezou, Dedoukou and Tseroni13 Many high-risk patient exposures include exposure during an AGP without the use of a respirator or eye protection. Reference Shah, Breeher, Hainy and Swift20 However, in the vast majority of these instances, a surgical face mask was still used, which may have conferred some level of protection. These exposures were deemed to be low or moderate risk in the evaluation by Maltezou et al, Reference Maltezou, Dedoukou and Tseroni13 which may account for the discordance between results. At the time of evaluation, the risk of exposure categorization that was initially proposed by the CDC has been removed. These data align with the consolidated exposure risk assessment outlined by the CDC.
In our evaluation, 17% of HCP who tested positive were asymptomatic at the time of testing. This rate is a slightly lower than those reported in other studies of asymptomatic HCP. Reference Treibel, Manisty and Burton9,Reference Buitrago-Garcia, Egli-Gany and Counotte21 Assessing for asymptomatic infection is critical because these HCP may still be able to transmit the SARS-CoV-2 virus; thus, it is important to isolate these HCP to prevent further occupational transmission.
Although it was a large evaluation of occupational exposures across multiple states, this study had several limitations. Exposures were reported to OHS staff, and lack of reporting or awareness of an exposure is an important limitation. With critical staffing in place during surges in infection rates, asymptomatic HCP may still have been allowed to return to work, and they may have not reported an exposure if they remained asymptomatic. Additionally, a concomitant community exposure cannot be ruled out; however, given a known occupational exposure within the incubation period, evaluation, and testing, the likely source of the infection is occupational. The study period also precedes the availability of vaccination. Widespread vaccination of HCP has likely reduced the absolute risk of infection following exposure, but relative differences in risk or different types of exposure may still occur.
In conclusion, the risk of acquiring COVID-19 following a significant occupational exposure has been relatively low, even in the prevaccination era, likely due to the brevity of unprotected exposure in the healthcare setting. Exposure to an infectious coworker carries a higher risk than exposure to a patient. Continued vigilance and precautions are necessary in healthcare settings, especially within work groups, whereas community transmission of SARS-CoV-2 remains widespread.
Acknowledgments
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.





