Small-intestinal bacterial overgrowth (SIBO) should always be suspected in elderly subjects that suffer from chronic diarrhoea, anorexia or nausea(Reference Donald, Kitchingmam, Donald and Kupfer1). It has been defined quantitatively as more than 105 colony-forming units/ml of bacteria in the fluid of the proximal small bowel and can be associated with malabsorption and malnutrition in the elderly(Reference Riordan, McIver, Wakefield, Bolin, Duncombe and Thomas2).
The relatively high prevalence of SIBO in the elderly may result from hypochlorhydria caused by atrophic gastritis or by antacid treatments(Reference Saltzman, Kowdley, Pedrosa, Sepe, Golner, Perrone and Russell3). However, mucosal dysmotility, frequently associated with type 2 diabetes, and alterations of the mucosal immune system(Reference Elphick, Chew, Higham, Bird, Ahmad and Sanders4) can also be contributing factors. Besides causing impairment of digestive and absorptive function SIBO is also frequently associated with increased intestinal permeability(Reference Riordan, McIver, Thomas, Duncombe, Bolin and Thomas5). The passage of bacterial products into the intestinal mucosa and the portal vein may play an important role in the altered intestinal digestive/absorptive function, the metabolism, and the low-grade inflammation in the elderly. Administration of probiotic-containing yoghurt may improve the clinical condition by improving gut barrier function and and by its antibacterial, immunomodulatory and/or anti-inflammatory effects(Reference Quigley and Quera6).
The current study examined the effects of an orally administered probiotic-containing yoghurt in an elderly population with a positive glucose/H2 breath test, and as such a suspected SIBO, compared to those with a negative glucose/H2 breath test. More specifically, effects on intestinal colonisation, gut permeability, endotoxin translocation and modification of innate immune functions were assessed.
Methods
Population/study design and product administration
Elderly subjects of both sexes (aged 61–94, mean 76·9 (sd 7·3) years) were recruited from a random sample of 294 elderly subjects that participated in a cross-sectional survey study conducted to determine the prevalence of SIBO based on the excretion in exhaled breath of hydrogen generated by the metabolism of glucose by intestinal bacteria(Reference Romagnuolo, Schiller and Bailey7, Reference Parlesak, Klein, Schecher, Bode and Bode8). Volunteers were not included if they were suffering from viral or bacterial infections, had been under antibiotics in the preceding 3 weeks, or suffered from any acute or chronic disease including malignancies, or had been subjected to any form of gastro-intestinal surgery. The prevalence of SIBO found with the H2 breath test was 15 %(Reference Parlesak, Klein, Schecher, Bode and Bode8).
Twenty-three elderly subjects (eighteen women, five men) with positive breath test (SPH) and thirteen subjects (nine women, four men) with negative breath test (SNH) were included in a follow-up, where the yogurt was administrated (observational study with one treatment group).
Subjects repeated the breath test at the beginning of the study and were asked to consume probiotic yogurt (2 × 150 g) Lactobacillus johnsonii La1 (daily dose of 109colony-forming units) for 4 weeks with a previous restriction of 2 weeks for yoghurt, kefir or butter milk. During the 4-week consumption of the study subjects were not allowed to consume other fermented products. No other advice with regard to diet was given to the participants. They were allowed to continue routine treatment and medication for stable conditions.
Hygrogen breath test
Hydrogen concentration (parts per million; ppm) in exhaled air was determined after ingestion of 50 g glucose as described by Parlesak et al. (Reference Parlesak, Klein, Schecher, Bode and Bode8). A positive result of the H2 test was taken as a rise of at least 10 ppm of expired H2 over baseline within 75 min. The breath test was performed at the baseline (prevalence study(Reference Parlesak, Klein, Schecher, Bode and Bode8)), before probiotic administration (1 week later) and after the 4 weeks of probiotic yoghurt consumption.
Intestinal permeability assessment was performed with polyethylene glycol of different molecular weights (400, 1500, 4000, 10 000) as previously described(Reference Parlesak, Bode and Bode9).
Endotoxin determination was carried out before and after probiotic yoghurt consumption in peripheral venous blood as previously described(Reference Fukui, Brauner, Bode and Bode10, Reference Fukui, Brauner, Bode and Bode11).
Ex vivo phagocytic activity and cytokine production after stimulation with lipopolysaccharide (LPS) (Escherichia coli 0111:B4) was performed on peripheral blood mononuclear cells.
Phagocytosis in human whole blood was performed using opsonised, fluorescein isothiocyanate-labelled E. coli (PHAGO-TEST™; Becton Dickinson, Basel, Switzerland) and flow cytometry as described previously(Reference Donnet-Hughes, Rochat, Serrant, Aeschlimann and Schiffrin12).
For the determination of cytokine release by monocytes peripheral blood mononuclear cells were separated from whole blood by gradient centrifugation with Ficoll©.
Cytokine production by monocytes was determined as follows: monocytes were purified by adhesion to plastic dishes during 1·5 h. Cells were thereafter stimulated by the addition of 10 ng/ml endotoxin from E. coli 0111:B4 (Sigma, Stuttgart, Germany). Cell culture supernatants were collected at 2·5 h for the determination of TNF-α and IL-1β and at 20 h for the determination of IL-10. Cytokine concentrations were measured with commercially available ELISA kits (Pharmingen, Heidelberg, Germany).
Release of reactive oxygen species by neutrophils procedure and evaluation are described in detail in a previous publication(Reference Parlesak, Diedrich, Schafer and Bode13). Neutrophils were isolated from whole blood by gradient centrifugation with Polymorphprep (Nycomed, Oslo, Norway). The chemiluminescence emitted by the LPS-stimulated neutrophils from SPH and SNH groups was assessed as the area under the curve in an assay that covers 1 h post-stimulation with different LPS concentrations.
Statistical analysis
The study presented has to be considered as an exploratory clinical trial. The design consists of one treatment group. The effect of the yogurt was investigated by a pre-post comparison. The observed differences may be due to treatment, but also due to ‘regression to the mean’ as well as due to unknown confounders. Measurements of H2 breath test are not approximately normally distributed. Also log transformation did not achieve the desired distributional characteristics. Consequently summary statistics are presented by median and quartiles and inferential statistics is performed by Wilcoxon sign-rank test. Treatment differences and CI are estimated according to Hodges & Lehmann(Reference Hodges and Lehmann14).
The other outcomes are approximately normally or log-normally distributed. Summary statistics are presented by means and their standard errors. Inferential statistics was performed by the t test. We are aware that our exploratory research may produce also false positive results. Therefore the P values presented throughout this report are interpreted as flags in order to indicate an interesting result. This interpretation is also in agreement with the international conference of harmonisation guideline, ICH E9.
Results
From the 294 subjects, only 279 subjects were further investigated in the cross-sectional screening study (data not shown). In this sample, thirty-eight elderly showed a positive hydrogen breath test (SPH) and 241 showed a negative hydrogen breath test (SNH). The cut point was 10 ppm. SPH elderly, showing compliance, were invited to participate in next stage of the study, resulting in twenty-three SPH participants (eighteen women, five men). Additionally thirteen SNH elderly were enrolled in the study (nine women, four men). The mean age was 78·6 (sd 7·8) and 77·5 (sd 5·8) years for the SPH and SNH subjects, respectively. Both groups were comparable in anthropometric data (weight 66 (sd 15) and 71 (sd 12) kg; BMI 24·0 (sd 3·8) and 25·8 (sd 3·6) kg/m2, for SPH and SNH elderly, respectively).
The H2 breath test was performed three times in each subject, on screening, before and on the last day of yogurt consumption, 4 weeks later.
Development over time of the H2 hydrogen breath test for each observation group (SPH, SNH) is presented in Fig. 1. The SPH subjects are showing a significant decrease ( − 18 ppm; 95 % CI − 34·5, − 7·5; P = 0·006) from screening until start of yogurt intake and then are remaining relatively stable until end of yogurt intake (+1·0 ppm; 95 % CI − 13·5, 12·0; P = 0·87). SNH subjects were consistently negative ( < 10 ppm) in all three determinations and do not show relevant changes from screening until start ( − 0·5 ppm; 95 % CI − 3·0, 2·5; P = 0·75) and until end of yogurt intake ( ± 0·0 ppm; 95 % CI − 2·5, 2·0; P = 0·80).

Fig. 1 Boxplots of hydrogen breath test grouped by negative ( − ) and positive (+) tests at baseline. Base, baseline; Pre, before probiotic intake (1 week after baseline); Post, after 4-week probiotic intake. ●, Median value; ○, outliers; box, interquartile range; whiskers, last outlier not further away than 1·6 times interquartile range (corresponding to 95 % CI for the median); ppm, parts per million; …, the 10 ppm theshold for a normal breath test.
Intestinal permeability
No significant association of increased intestinal permeability to macromolecules (polyethylene glycol) with small bowel intestinal overgrowth was observed, and no change was observed as a result of the probiotic yogurt intake (data not shown).
Endotoxin concentration
Before treatment no differences were observed for endotoxin concentrations in peripheral venous blood between subjects of the different groups (6·49 (sem 8·1) for SPH v. 6·4 (sem 6·3) pg/ml for the SNH group; Fig. 2). After the 4 weeks of probiotic yogurt consumption a trend towards a diminution of endotoxin concentration was observed in the SPH group (6·49 (sem 8·1) v. 4·6 (sem 5·6) pg/ml). While in the SNH group plasma endotoxin was significantly lower after probiotic yoghurt consumption (6·4 (sem 6·3) v. 0·5 (sem 0·0) pg/ml, P = 0·043, before v. after).

Fig. 2 Endotoxin concentrations in plasma of subjects with positive breath test (SPH, □) and subjects with negative breath tests (SNH, ▧) before and at the end of the yoghurt consumption period. Values are means with their standard errors depicted by vertical bars. Mean value was significantly different from that before the yoghurt consumption period: *P = 0·043.
Haematological parameters
Complete blood cell count, erythrocyte sedimentation, total plasma protein, albumin, C-reactive protein and parameters associated to liver, renal and immune functions were analysed before and after the probiotic yogurt consumption. The groups were not different at the start of the ingestion period and no clinically significant changes were observed at the end (data not shown).
Soluble CD14 (sCD14) plays a pivotal role in LPS recognition of LPS-induced cell activation. LPS binding protein (LBP) is a class 1 acute-phase protein with the ability to bind and transfer bacterial LPS to CD14 receptor(Reference Schumann and Zweigner15). sCD14 and LBP were studied only in the SPH group, due to the more restricted size of the SNH group. Plasma levels of soluble sCD14 and LBP showed a significant decrease after the yogurt intake period (3·806 (sem 0·706) v. 3·580 (sem 0·615) μg/ml for sCD14, P = 0·04) and (31 (sem 23) v. 24 (sem 20) μg/ml for LBP, P = 0·03).
Phacocytic activity of monocytes and neutrophils
No differences in the percentage of phagocytes were observed between groups at the beginning of the study and there was no significant change after the 4 weeks of intervention. The mean fluorescence intensity is proportional to the number of incorporated bacteria by individual phagocytes. Mean fluorescence intensity decreased after the consumption of probiotic yogurt in both groups for monocytes and neutrophils (monocytes, P = 0·011 and neutrophils, P = 0·0001 in SPH group; monocytes, P = 0·036 and neutrophils, P = 0·036 in SNH group).
Cytokine release
Before yogurt consumption, monocytes isolated from subjects of the SPH group produced significantly less IL-1β and IL-10 than subjects in the SNH group (199 (sem 359) v. 586 (sem 566) pg/ml, P = 0·035 for IL-1β and 759 (sem 739) v. 1846 (sem 1309) pg/ml, P = 0·009 for IL-10). After 4 weeks of yoghurt consumption monocytes from SPH participants showed an increased capacity to react to endotoxin challenge attaining similar levels to the SNH group.
Release of reactive oxygen species
A trend towards a more elevated release of reactive oxygen species by neutrophils was observed after the yogurt consumption in both groups. The differences were statistically significant at the highest LPS concentration in the SPH group (P = 0·031; see Fig. 3). Reactive oxygen species production by neutrophils from the SNH group (data not shown) showed a similar pattern, higher after probiotic yoghurt consumption, but in this case did not reach statistical significance.
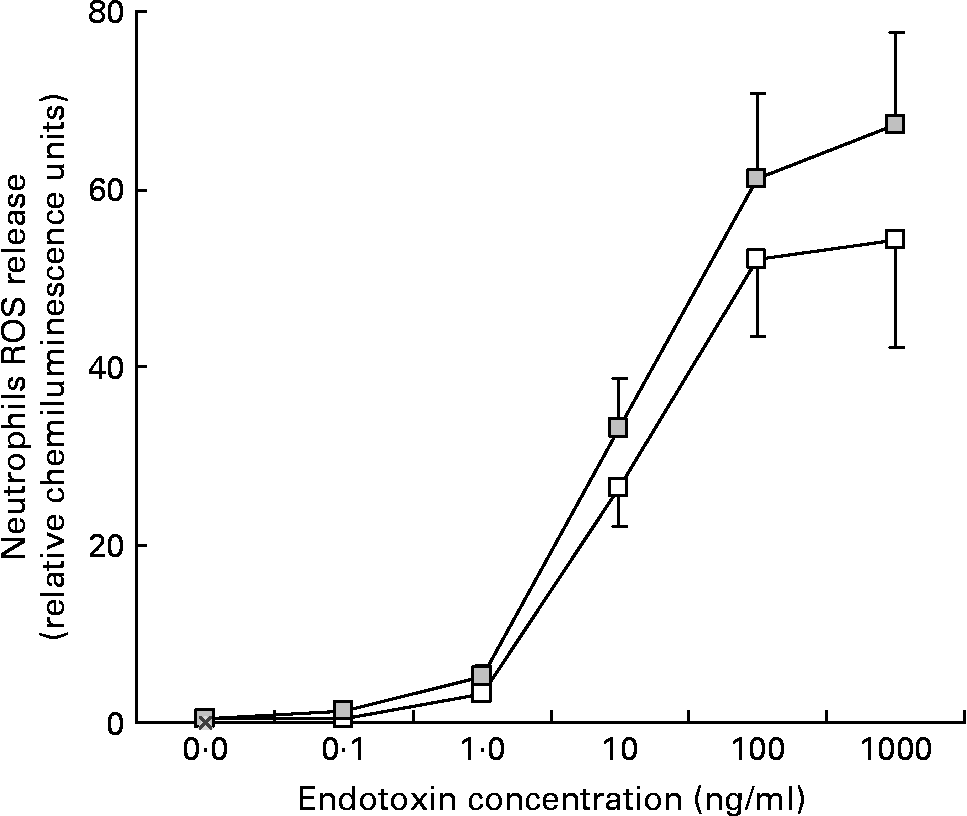
Fig. 3 Release of reactive oxygen species (ROS) by neutrophils isolated from subjects with positive breath test before (□) and at the end (![]() ) of the yoghurt consumption period. (P = 0·0311 for endotoxin highest dose, NS for the rest of concentrations). Values are means with their standard errors depicted by vertical bars.
) of the yoghurt consumption period. (P = 0·0311 for endotoxin highest dose, NS for the rest of concentrations). Values are means with their standard errors depicted by vertical bars.
Discussion
All participants had a good nutritional status and no apparent clinical manifestation of SIBO. Of the SPH subjects enrolled in the present study 43 % did not reach the threshold increment of 10 ppm of H2 in the second breath test before the start of probiotic yoghurt consumption. On the other hand, none of the thirteen SNH subjects had a positive H2 breath test in the second or third breath tests. The spontaneous normalisation of a positive H2 breath test in the SPH group may be due to various factors.
According to most authors, the sensitivity of glucose/H2 breath test is approximately 70 % and the specificity is about 80 %. Thus the apparent discrepancy between the first and the second breath tests performed 1 week apart cannot be attributed to inherent limitations of the test alone(Reference Kerlin and Wong16).
The lack of reproducibility in the test may be due to physiological or pathophysiological variations such as carbohydrate malabsorption, gastrointestinal motor disorders with transit abnormalities(Reference Simren and Stotzer17). The stability of the intestinal bacterial population varies in both the number and type of colonising bacteria. The washout effect of diarrhoeal episodes, the loss of bacterial flora due to antibiotic use, and acidity of the bowel lumen may all contribute to such fluctuations(Reference Quigley and Quera6). Although the study participants were not treated with antibiotics, we cannot discount transient diarrhoeal episodes, changes in bowel motility and evacuation, or other factors which may have contributed to the spontaneous reduction in bacterial overgrowth observed in ten of the twenty-three SPH subjects. Interestingly, seven of these SPH participants are once again positive in the third test after the intervention. None of the SNH subjects had a positive response in either the second or third test. This highly significant difference (7/10 v. 0/13, P = 0·0003) supports the grouping of the participants on the basis of SIBO. At the end of yoghurt consumption no changes in the glucose/H2 breath test were observed for either of the groups.
SIBO can increase intestinal permeability assessed by the lactulose/mannitol test(Reference Riordan, McIver, Thomas, Duncombe, Bolin and Thomas5). Subjects with overgrowth of colonic-like bacteria appear to have more compromised barrier integrity and higher inflammatory response than subjects colonised by other types of microbiota(Reference Riordan, McIver, Thomas, Duncombe, Bolin and Thomas5). In the present study, the SPH and SNH subjects did not differ in their intestinal permeability to polyethylene glycol of different molecular weights. The consumption of probiotic yoghurt for 4 weeks did not influence this permeability.
SIBO can be associated with an increased immune-inflammatory activity of the mucosal immune-competent cells. Increased production of IL-6, higher numbers of intra-epithelial lymphocytes and of IgA-producing cells have been associated with the intestinal bacterial load(Reference Kett, Baklien, Bakken, Kral, Fausa and Brandtzaeg18). The inflammatory/immune reaction seems to be mediated by a coliform predominant microbiota.
In addition to the local effect of SIBO in the mucosal immune compartment, leakage or translocation of bacteria or their components into the body can contribute to visceral toxicity, systemic inflammatory conditions and metabolic changes. SIBO and passage of LPS into the portal vein can be deleterious to the liver and contribute to non-alcoholic liver disease(Reference Angulo19, Reference Wigg, Roberts-Thomson, Dymock, McCarthy, Grose and Cummins20).
It has been postulated that the subclinical inflammatory status observed in a subgroup of elderly may be propagated from components in the intestinal environment as a consequence of a poor mucosal barrier and inadequate mucosal clearance of the abnormally high bacterial challenge(Reference Guigoz, Dore and Schiffrin21).
There is no strong evidence supporting an association between translocation bacteria or their products and increased intestinal permeability(Reference O'Boyle, MacFie, Dave, Sagar, Poon and Mitchell22). Therefore, the unaltered permeability to polyethylene glycol does not rule out a closer interaction of the mucosal immune cells with the abnormally high bacterial load of the small bowel and a concomitant increase in lamina propria cellularity(Reference Welsh, Farmery, MacLennan, Sheridan, Barclay, Guillou and Reynolds23), due to macrophage and lymphocyte infiltration. Increased local IL-6 production is related to the activation of lamina propria mononuclear cells exposed to higher LPS levels. Local mucosal changes may not be detected systemically.
Positive glucose/H2 breath test was not assocaited with higher levels of plasma LPS as both groups had similar concentrations at baseline. After the consumption of the probiotic yoghurt a significant decrease in plasma endotoxin was observed in the SNH group and a similar trend was present in the SPH group (Fig. 2). Since probiotic ingestion did not alter breath test results or permeability to polyethylene glycol, one can assume that the observed cellular changes are due to other mechanisms besides a change in the bacterial load of the small bowel or a compromise of mucosal barrier integrity. An altered small bowel microbiota with lower quantities of coliform endotoxin-producing bacteria may have resulted from probiotic administration. Alternatively probiotic administration may activate the innate macrophage system(Reference Donnet-Hughes, Rochat, Serrant, Aeschlimann and Schiffrin12) in the mucosal compartment and thereby improve LPS clearance. Certainly, removal and clearance of LPS from the body is another of the many functions associated with the intestinal cellular compartment(Reference Ge, Ezzell and Warren24). In experimental conditions lamina propria macrophages and intestinal epithelial cells take up and clear LPS(Reference Ge, Ezzell and Warren24). These cells are probably also involved in the clearance of luminally derived endotoxin and translocating bacteria. Long-term exposure to endotoxin may render phagocytes tolerant to LPS and incapable of mounting an energetic response to further challenge with bacterial products and endotoxin in particular. It is feasible that innate immune cells have a permanently low level of activation but paradoxically, lose their capacity to mount an effective response when needed. This can be clearly observed in the extreme case of liver cirrhosis(Reference Lin, Tsai, Ho, Huang, Lin, Lin, Tseng, Lin, Chen and Sheen25). In the present study the decreased ex vivo phagocytic capacity of unstimulated individual cells after probiotic intake suggests a decrease in basal low-grade cellular activation in the elderly. In contrast, monocytes stimulated ex vivo with endotoxin had a low response of cytokine production that was increased after the consumption of probiotic yoghurt. This change was significant in the SPH group.
The release of reactive oxygen species by neutrophils exposed to endotoxin was similar before and after probiotic intake, but a higher reactivity to increasing concentrations of endotoxin was observed after probiotic administration.
Although we have no direct proof of endotoxin leakage at the gut level there is some evidence to suggest that LBP, sCD14 and possibly other LPS pattern recognition receptors are important parameters for monitoring the host acute-phase reaction secondary to bacterial product leakage into the internal milieu. After the probiotic yoghurt administration a significant decrease in sCD14 and LBP plasma levels was observed.
In conclusion, monocytes and neutrophils exhibited a higher phagocytic capacity before probiotic consumption if no challenge with LPS was performed ex vivo. In contrast, probiotic administration normalised responsiveness to LPS ex vivo and resulted in an increased production of cytokines and free radicals.
A unifying explanation is that probiotic administration probably results in a lower exposure to endotoxin in the mucosal microenvironment and in the body. This lower exposure may be due to a qualitative switch from a Gram-negative endotoxin-producing microbiota to a Gram-positive (less pro-inflammatory) type of microbiota in the proximal small bowel(Reference Guigoz, Dore and Schiffrin21), and/or an activation of the innate immune system by probiotics that results in better clearance of endotoxin and improved immunocompetence.
Probiotics have been extensively studied for their health-promoting activities in a variety of human conditions where altered bacterial ecology seems to play a pathogenetic role. The distal small bowel and the colon are where most intestinal bacteria find their ecological niches and where probiotics may also find a more favourable environment. Here we explored the possibility that probiotics exert effects on abnormal bacterial communities along the entire length of the small bowel. The probiotic effect we observed may be mediated by different mechanisms: (1) competitive exclusion of colonising bacteria that are vying for the same ecological niches with subsequent qualitative changes in the composition of the microbiota; (2) stimulation of immune function and improvement of immune competence for controlling the levels of the bacterial populations; (3) increase in innate immune function and endotoxin clearance; and/or (4) the induction by probiotics of a homeostatic mucosal immune response that can compensate for the pro-inflammatory activity of the colonic-like bacteria. Any one of the aforementioned effects alone or in combination could be of benefit in subjects with SIBO.
The present clinical study suggests that the administration of supplements in the elderly may benefit from the addition of ingredients that improve the composition of the intestinal microbiota. Moreover, the present results indicate that an altered intestinal ecology underlies the low-grade inflammatory status that favours catabolism and loss of lean body mass in the elderly.
Acknowledgements
We are indebted to Mrs A. Bleher and S. Berhard for support in recording the diet histories and instructing the study participants during the breath tests. The work was generously supported by Nestec Ltd. The authors made the following contributions to the study: E. J. S. and A. P.: study concept and design, analysis and interpretation of data, preparation of the manuscript; C. B. and J. C. B.: study concept and design, subject recruitment, acquisition of data; M. A. v. H. and D. G.: analysis and interpretation of data; Y. G.: interpretation of data, preparation of the manuscript. A. P., C. B. and J. C. B. have no conflict of interest. E. J. S., M. A. v. H., D. G. and Y. G. are employees of Nestec Ltd, an affiliate of Nestlé SA, Switzerland.





