INTRODUCTION
Radiocarbon (14C or carbon-14, half-life 5730 yr) is a key radionuclide in the assessment of the safety of a geological disposal facility (GDF) for radioactive waste in the United Kingdom and the need to better understand the possible importance of gaseous carbon-14 bearing species has been recognized (NDA 2012). Carbon-14 is expected to be released from a GDF over a timescale of several thousand years. A number of radioactive gases will be generated from waste materials within a GDF, with carbon-14 bearing methane (14CH4) likely to be the dominant carbon-14 species transported in the gas phase, potentially reaching the biosphere at low activity concentrations.
The main sources of carbon-14 in UK radioactive wastes are irradiated graphite, irradiated steels, irradiated reactive metals (primarily Magnox and uranium), and spent nuclear fuel. There are 17,700 TBq of carbon-14 in the 2013 UK Derived Inventory of which 7090 TBq is associated with irradiated steel wastes (RWM 2015).
The potential radiological impact of any release of carbon-14 as gas from irradiated steels will be site-specific, depending on the gas migration characteristics of the host rock and the overlying geological formations. A recent generic post-closure assessment of the potential radiological impact of carbon-14 released as gas from a UK GDF found that irradiated steel wastes could be responsible for the majority of the release of gaseous of carbon-14 from a UK GDF at times greater than 500 years after closure (RWM 2016; Swanton et al. Reference Swanton, Swift, Plews and Smart2016). However, in the absence of suitable experimental data, different assumptions about the proportion and speciation of gas phase carbon-14 releases were scoped in the biosphere to assess the potential consequences.
This paper describes work being carried out to measure the rates and speciation of carbon-14 release from irradiated stainless steel during corrosion under simulated disposal conditions to address this key gap in understanding. This work has been performed as part of the European Commision CArbon-14 Source Term, (CAST), project, which started in October 2013 and concluded in March 2018 (Williams and Scourse Reference Williams and Scourse2015). It is one of a series of studies under CAST Work Package 2 concerned with carbon-14 release from steel wastes under conditions of a cement-based GDF (Mibus et al. Reference Mibus, Diomidis, Wieland and Swanton2018). This paper describes the design of leaching experiments to measure the release of carbon-14 to both gas and solution phases from irradiated stainless steel under high-pH, anoxic conditions, and the results obtained during the first year of operation.
EXPERIMENTAL
Samples
Stainless steel samples from the material testing irradiation experiment R268-07 in the High Flux Reactor (HFR) at Petten, were chosen for this study. The samples selected are 316L(N) austenitic stainless steel taken from a single steel plate. The composition of the stainless steel batch used (as obtained from the material data sheet) is given in Table 1. Unirradiated material from the same batch of stainless steel was used in non-active trial and control experiments.
Table 1 Composition of the selected stainless steel samples (wt.%, Fe – balance).
The specimens were irradiated in the HFR up to a nominal target dose level of 2 dpa (displacements per atom) at a target temperature of 80°C. The specimens were in direct contact with the HFR core cooling water. The irradiation was performed over 5 cycles (28 days per cycle) in the years 1996 and 1997. The samples are so-called CT (compact tension) specimens. Each CT specimen has outer dimensions of 30 × 28.8 × 12 mm3, a geometric surface area of 38.14 cm2 and mass of 74 g.
The amount of carbon-14 (and other radionuclides) in the stainless steel specimens has been calculated using the ORIGEN computational code based on the composition given in Table 1. The calculated activities for different samples vary by nearly a factor of two. This is caused by the variations in the thermal neutron fluence, which is dependent on the position of the samples during irradiation (the resulting activation being greater for those samples closer to the centre of the core). Gamma contact dose rates of the samples were estimated from the calculated cobalt-60 activities to be in the range 10–20 Gy hr–1 at the front face (on 1 June 2016).
Leaching Experiments
Three leaching experiments are being performed, two with irradiated stainless steel and one with unirradiated stainless steel as a control. The irradiated specimens were used in the experiments as-stored, with no preparation (e.g. pre-washing) being applied. The steel samples are being leached in a 0.1 mol dm–3 sodium hydroxide solution (pH ∼13) under a nitrogen atmosphere to simulate conditions in a high-pH cementitious near field, as could be present in the engineered barrier system of a GDF for intermediate-level radioactive waste. A sodium hydroxide rather than a calcium hydroxide solution was selected to avoid complications that may arise from the precipitation of any carbon-14 released from the steel in the form of CO2 as calcium carbonate, and has also been used in studies of carbon-14 release from irradiated graphite (Marshall and Baston Reference Marshall and Baston2011; Baston et al. Reference Baston, Marshall, Otlet, Walker, Mather and Williams2012, Reference Baston, Preston, Otlet, Walker, Clacher, Kirkham and Swift2014).
Owing to the high gamma dose rates from the irradiated samples, the experiments are being performed in a shielded cell, the G1 chemical hot cell in NRG’s Hot Cell Laboratories at Petten, which has placed specific demands on the experimental design. For gas phase sampling and analysis, a gaseous carbon-14 capture and analysis methodology developed by Radio Carbon Dating (RCD) Limited for studies of carbon-14 releases from irradiated graphite (Marshall and Baston Reference Marshall and Baston2011; Baston et al. Reference Baston, Marshall, Otlet, Walker, Mather and Williams2012, Reference Baston, Preston, Otlet, Walker, Clacher, Kirkham and Swift2014) has been applied. This method provides high sensitivity for the selective capture and quantification of carbon-14 species released to the gas phase. The RCD method involves the periodic purging of the head-space of the experimental container with nitrogen and the selective oxidation and capture of carbon species from the gas phase as CO2 in a series of soda lime columns. After sampling, the soda lime columns are removed and returned to RCD for analysis. The oxidation catalysts and soda lime columns for carbon-14 capture are built into two sampling rigs, which were manufactured by RCD. To avoid potential cross-contamination, the sampling rigs are positioned in a dedicated glove box adjacent to the shielded cell.
The leaching containers, about 1.5 L in internal volume, consist of an outer vessel with cover, both made from borosilicate glass. The two parts of the vessel join at a flange. The flange seals on an acetylnitrile butadiene rubber (NBR) O-ring, which is known to have good radiation tolerance, and is secured using a clamp. An inert zirconia crucible serves as an inner container to hold the leachant during the experiment. This is to avoid contact between leachant and glass that could potentially affect the experiment; it is known that the presence of silicate ions can reduce the corrosion rate of steels in water (Francis and Mercer Reference Francis and Mercer1985), and the borosilicate glass container would react with the high-pH solution to release silicate species into solution.
The gas sampling system is formed by inlet and outlet valves located on the sides of the container, with inlet connected to the nitrogen supply and outlet to the RCD rigs. Liquid sampling and leachant addition are performed via a sampling tube (dip leg), made of quartz, built into the cover. The valves for gas and liquid sampling are of the HighVac manual stopcock type, made from borosilicate glass. Each stopcock valve has a glass piston fitted with three elastomer (Viton) O-rings. The containers and valves have been modified for remote handling by manipulators.
Each experiment contains three CT specimens: Container 1 with non-irradiated samples; Containers 2 and 3 with irradiated samples. Each CT specimen is hung on a stainless steel hook from a triangle that is placed on the zirconia crucible. The calculated carbon-14 and cobalt-60 contents of Containers 2 and 3 are given in Table 2.
Table 2 Overview of samples in the containers and their calculated activity content on 1 June 2016.

After assembling the experiments, each container was leak tested prior to leachant addition. The experiments were started by the addition of 600 cm3 of de-oxygenated leachant via the dip leg.
The experiments have been sampled after 1, 3, 6, 13, 22, and 59 weeks (except Container 1 where the latest sampling was at 52 weeks). First the gas phase carbon-14 is collected by purging the container head-space with nitrogen for about 7 hr and passing the purge gas through the RCD rigs. Following gas sampling, solution phase samples (about 15 cm3) are drawn via the dip-leg into an evacuated bulb. Final sampling and termination of the experiments is planned after two years.
Analytical Methods
14C Analysis in Gas Samples
The RCD method allows the separation and quantification of (Marshall and Baston Reference Marshall and Baston2011):
Carbon-14 released as CO2;
Carbon-14 released as CO (any volatile oxygen-containing organic species e.g. alcohols, aldehydes and ketones that escape from solution into the gas phase would also be collected in this fraction); and
Carbon-14 released as volatile hydrocarbons, principally CH4, (any other volatile carbon-containing species that have passed through the “CO” collection column would also be collected in this fraction).
The three fractions are collected on separate soda lime columns. Each column is pre-loaded with fossil CO2, made from North Sea gas that contains no carbon-14, to provide a bulk carrier during processing for the small amounts of gaseous carbon species released in the experiments. The CO2 is recovered from each soda lime column by acidification, and is converted to benzene via reaction with lithium to produce lithium carbide, its hydrolysis to acetylene and catalytic trimerisation of the acetylene. The benzene produced is mixed with Opti-Fluor scintillant and the carbon-14 content measured by liquid scintillation counting (LSC) using a Wallac Quantalus counter. Counting times of up to 2000 min allow minimum detectable activities (MDAs) of about 0.04 Bq carbon-14 per sample. Fuller details of the RCD method are provided in Baston et al. (Reference Baston, Preston, Otlet, Walker, Clacher, Kirkham and Swift2014).
Inorganic 14C Analysis of Solution Samples
Solution sub-samples are processed to release inorganic carbon-14 from the solution by acidification with nitric acid, purging by nitrogen and capture of released carbon-14 in Carbosorb E. The carbon-14 is determined by LSC in Instagel using a TriCarb 3180 TR/SL LSC. The samples are analysed in low-level count mode for a period of 60 min. The MDA in this mode is 0.1 Bq per sample. The effectiveness of the separation method has been validated using standard solutions of carbon-14 labeled sodium carbonate, which was recovered near-quantitatively (recovery 93%), and carbon-14 labeled sodium acetate, which remained in the solution phase.
Total 14C Analysis of Solution Samples
A small number of preliminary total dissolved carbon-14 (TD14C) activity measurements were undertaken on solution samples collected after 59 weeks leaching. Prior to analysis, a 1 cm3 volume of 50 g dm−3 sodium carbonate solution was added to each of the solution samples (varying in volume from 7 to 11 cm3) to ensure sufficient bulk carbon-12 was present in the solutions to act as carrier for the separation procedure.
A known mass of solution (approximately 1 cm3) was combusted in a glass boat containing about 0.30g cellulose powder in a two-stage catalytic pyrolyser (Catalytic Pyrolyser-6 Trio, Raddec Limited). The 14CO2 produced was captured in a bubbler containing Carbon-Trap and measured by LSC in CarbonCount. Process blanks were carried out on the furnace before and after every sample run. A 2.0 Bq cm–3 reference sample gave a recovery of 98%. The nominal MDA of the method is 0.01 Bq. The organic fraction of dissolved carbon-14 in the leachate solution is then given by the difference between the total carbon activity and the total inorganic carbon activity.
A fuller set of TD14C analyses is planned on the solution samples collected after two years and on stored samples collected at earlier sampling times.
60Co Analysis of Solution Samples
The Co-60 content of the solution samples was measured on a calibrated HPGe gamma spectrometer. Each sample contains approximately 400 μL solution double-contained in polyethylene (PE) containers. The data have been processed by the NIAGADA (Burgers Reference Burgers1989) program, part of the NEMO package (Van Dijken and Oudshoorn Reference Van Dijken and Oudshoorn2011), with correction for gamma-self-absorption, sample dimensions, the shielding of the double PE containment and the background. The MDA of the detector for 60Co is 1.0 Bq for a counting time of 48 hr.
RESULTS AND DISCUSSIONS
In this paper, the results for up to one year’s leaching are presented. Fuller details can be found in De Visser-Týnová et al. (Reference De Visser-Týnová, Stijkel, Swanton, Otlet and Walker2018).
The cumulative releases of carbon-14 to the gas phase collected in each of the gas fractions over the 59-week duration of the experiments are shown for Containers 2 and 3 in Figure 1. The error bars in these figures are the cumulative errors from addition of the results from each sampling period. These cumulative errors become significantly larger than the errors in individual measurements for sampling periods at longer times.
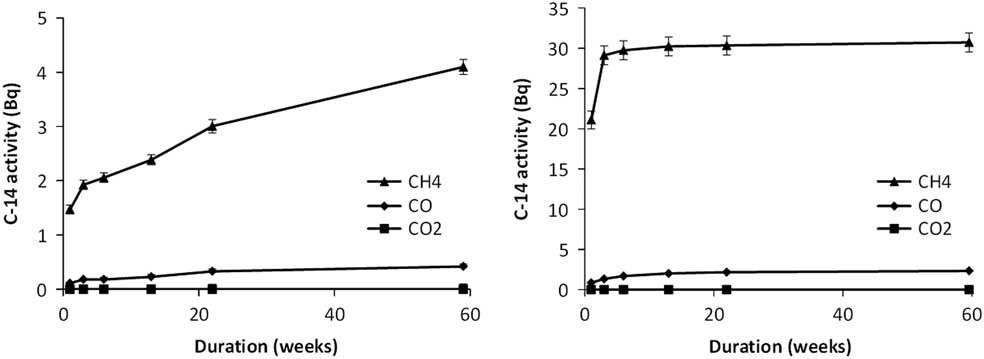
Figure 1 Cumulative gas phase 14C release in Container 2 (left) and Container 3 (right). Note that the error bars on these figures represent the uncertainties in the cumulative releases not in the individual measurements.
The cumulative releases of carbon-14 to the solution phase are shown in Figure 2. These results take account of the solution volumes and inorganic carbon-14 therein removed at each sampling point. In the case of TD14C the cumulative releases have been calculated similarly using an assumption that the ratio of inorganic to organic carbon-14 species released remains constant throughout the experiments.
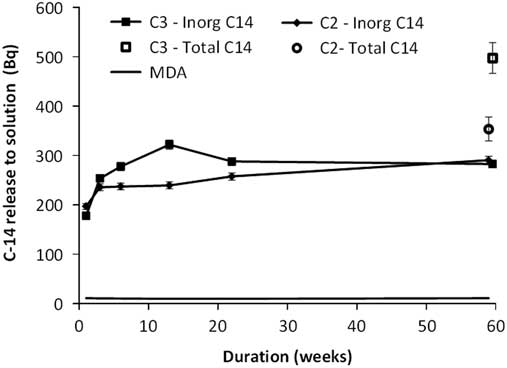
Figure 2 Cumulative 14C releases to solution as inorganic (filled symbols) and as total 14C (TD14C, open symbols) from Containers 2 (C2) and 3 (C3).
Cobalt forms a solid solution with iron, nickel and chromium in austenitic stainless steel. Thus, based on the known cobalt and calculated Co-60 contents of the irradiated steel samples, the release of Co-60 on leaching could potentially provide a measure of the corrosion rate of the steel. This relies on the cobalt oxidised by corrosion processes dissolving into and remaining in solution as the experiments progress. In both Containers 2 and 3, a significant release of cobalt-60 was observed in the first week of leaching. However, after 3 weeks the cobalt-60 activity in both solutions was found to have decreased and continued to drop throughout the tests in Container 2, indicating that cobalt is being removed from solution (e.g. by precipitation or sorption). Thus the measurement of cobalt-60 in solution is not a suitable marker of the corrosion rate of the steel in these two experiments. At the end of the experiments, it is planned to determine the amounts of any precipitated or sorbed cobalt-60.
14C Release in Container 1 – Unirradiated Steel Blank
A small amount of gas phase carbon-14 (0.158 ± 0.009 Bq) was detected on the 14CH4 column for Container 1 at the end of week one. In addition, a small amount of carbon-14 (0.070 ± 0.004 Bq) was detected on the 14CO column at the end of week three. Otherwise, gas phase carbon-14 measurements after 6, 13, 22, and 52 weeks have all been below MDA, as expected.
Given that Container 1 should contain no significant carbon-14, nitrogen is being used as the purge gas, and the air inlet to the RCD rigs is passed through a soda lime column to trap atmospheric CO2, the source of the carbon-14 collected in these two gas samples is uncertain. It is noted that no carbon-14 was detectable in a blank experiment when the RCD samplers were run continuously for 9 days, far longer than the 7 hr used in this experiment.
No inorganic carbon-14 has been detectable in the solution phase in Container 1, as expected (noting that an inorganic carbon-14 release in excess of ∼11 Bq to the 600 cm3 of solution would be required for carbon-14 to be detectable in solution samples by LSC). A small amount of carbon-14 (0.011 ± 0.010 Bq g−1) was detected in the single total carbon-14 measurement performed on the one-year sample. However, the amount is very close to the MDA of 0.009 Bq and taking account of the uncertainty, the result is indistinguishable from it.
14C Release in Containers 2 and 3 – Irradiated Stainless Steel
The results of the preliminary TD14C analysis on the one-year samples combined with the inorganic carbon-14 solution analyses conducted on all 6 solution samples from both experiments, indicate that carbon-14 is present in solution in contact with the irradiated steel in both inorganic and organic forms. The organic fractions of dissolved carbon-14 in the leachates after one year are estimated to be about 18 ± 7% in Container 2 and 43 ± 5% in Container 3. Thus, the periodic measurements of inorganic carbon-14 release to solution prior to one year are likely to underestimate the total carbon-14 release from the steel to the solution phase during the early stages of leaching. Nevertheless, they provide a measure of the leaching behaviour of the irradiated steels and the following discussion is based on the dissolved inorganic carbon-14 results.
In both Containers 2 and 3, there is an initial fast release of carbon-14 during the first week of leaching, followed by a drop in the rate of release at longer times. However, the amounts of carbon-14 are very small with fractional releases of the calculated carbon-14 contents of the experiments over 59 weeks of ∼ 7 × 10–6 and 1.1 × 10–5 for Containers 2 and 3, respectively. About 1% of the measured release over one year occurs to the gas phase in Container 2 (the proportion increasing slightly over time) and about 6% in Container 3. The predominant gas-phase species are hydrocarbons collected on the 14CH4 column. However, there is a small increase in the cumulative fraction of gas phase releases collected on the 14CO column over time. No 14CO2 is detected in the gas phase from either Containers 2 or 3.
Beyond 3 weeks, the rate of carbon-14 release from the steel to both the gas and solution phases in Container 2 drops significantly but remains measurable up to 59 weeks. The ratio of gas phase hydrocarbon species to 14CO collected from Container 2 varies between 13:1 and 6:1 over the six sampling intervals.
For Container 3, between one and six weeks, the release of carbon-14 continues at a higher rate than in Container 2, with a higher proportion of the release being to the gas phase (10–12% based on dissolved carbonate and gaseous species only). The dissolved inorganic carbon-14 activity measurements for Container 3 at 6, 22, and 59 weeks, were the same within measurement uncertainties, although the measurement at 13 weeks was about 15% higher. The reason for this higher result is unclear, but the other 3 measurements suggest that the release of inorganic carbon-14 to solution from the steel samples had decreased to a very low rate beyond six weeks.
The rate of release of carbon-14 to the gas phase in Container 3 was an order of magnitude higher than in Container 2 during the first week. It then decreased in each sampling interval and after 13 weeks the rate was lower than in Container 2. The ratio of gas phase hydrocarbon species to CO collected from Container 3 also decreased with time, dropping from about 25:1 in week 1 to an average of about 4:1 between 3 and 59 weeks.
The amount of organic carbon-14 release to solution over one year is higher in Container 3 than in Container 2 and this correlates with the larger gas-phase release from Container 3. It also correlates with the higher releases collected in the 14CO columns for Container 3; and it is possible that a proportion of the 14CO fraction arises from collection of oxygen-containing volatile organic species that may have partitioned from the solution during gas sampling.
Rates of 14C Release
In most models of carbon-14 release from irradiated stainless steels used to date in safety assessments (e.g. Swanton et al. Reference Swanton, Swift, Plews and Smart2016), it has been assumed that carbon-14 is uniformly distributed through the steel, in a reactive form and its release occurs congruently with steel corrosion. This assumes that the carbon-14 content of the steel arises primarily from uniform thermal neutron activation of the nitrogen-14 impurities within low-carbon reactor steels and there are no other significant sources of carbon-14 contributing to the carbon-14 inventory of steels in a reactor (e.g. by deposition of carbon-14 from activation of reactor coolant gas components on steel surfaces, or from activation of oxygen-17 present in the oxide surface layer). Thus, it is assumed that the carbon-14 is distributed uniformly in solid solution throughout the steel and thus will be exposed to solution, react with water and be released, at a rate controlled by the corrosion rate of the steel. There is uncertainty about these assumptions, depending upon reactor type, the nature of the steels and the location of the steels within the reactor (which affects the uniformity of neutron flux and thus uniformity of activation though the steel). In the case of the 316L(N) specimens used in this study, the potential for additional carbon-14 to arise within or from depositions onto the oxide surface layer during irradiation is uncertain. The uniformity of carbon-14 distribution though the specimens is also uncertain given the peripheral location of the specimens in the HFR during irradiation and the potential for self-shielding through the sample thickness (which may lead to variations in the extent of activation through the samples). Nevertheless, in the absence of more detailed information, the assumption of a uniform carbon-14 distribution serves as a first approximation for interpretation of the experiments.
The results show a fast initial release of carbon-14, within the first week of leaching, followed by a much slower longer-term release. About 200 Bq of carbon-14 (as dissolved carbonate and gaseous species only) is released from the steel during the first week of leaching in both Containers 2 and 3, corresponding to a release fraction of about 4 × 10–6 of the estimated carbon-14 inventory of each experiment. Such a release rate is much higher than the fraction of 6 × 10–9 that can be calculated based on a long-term anaerobic stainless steel corrosion rate of 0.8 nm.yr–1(Swanton et al. Reference Swanton, Baston and Smart2015) and the estimated surface area of each CT specimen of 38.12 cm2, assuming uniform distribution of carbon-14 throughout the sample. This suggests that the initial carbon-14 release is unlikely to be associated solely with anaerobic steel corrosion. These high initial release of carbon-14 are likely to be associated with carbon-14 that is more accessible and/or at higher concentrations than that in bulk material (e.g. sorbed to the surface of the steel or located at more active sites).
After the first three weeks of leaching, a continuing release of carbon-14 from the irradiated steel was measurable to both solution and gas phases in Container 2. The cumulative carbon-14 release (dissolved carbonate and all gaseous species only) was found to continue at a near constant rate over the period from 3 to 59 weeks. A least squares fit to the cumulative carbon-14 release (dissolved carbonate and all gaseous species only) over this period indicates a carbon-14 release rate of about 0.15 ± 0.02 Bq day−1. This is over two orders of magnitude lower than during the first week of leaching. The corresponding least squares fit to the fraction of carbon-14 released as gaseous species between 3 and 59 weeks indicates a mean rate of gas phase release of 0.0063 ± 0.0005 Bq day−1. Thus, gaseous releases contribute about 4% to the cumulative release (dissolved carbonate and all gaseous species only) over this period.
Assuming a uniform distribution of carbon-14 and its congruent release with steel corrosion (and on the proviso that the calculated inventories of the samples are accurate), the rate of cumulative carbon-14 release (as dissolved inorganic and gaseous species) corresponds to an equivalent corrosion rate for the steel of ∼3 nm yr−1. (The inclusion of a small dissolved organic carbon-14 fraction would only have a small effect on this value.) This can be compared with long-term corrosion rates measured recently for unirradiated 18/8 (304-type) stainless steel under alkaline, anoxic conditions in Japan (Swanton et al. Reference Swanton, Baston and Smart2015). The data indicate a mean anaerobic corrosion rate of 0.8 nm yr−1 for the stainless steel at 30°C after two years exposure to a pH 12.5 solution. However, the data also show a decrease of the anaerobic corrosion rate over the two-year duration of the experiments; the corrosion rate measured after about 90 days was ∼1.5 nm yr−1. Thus, the equivalent corrosion rate measured in the Container 2 experiment beyond 3 weeks’ leaching seems reasonable and broadly consistent with the Japanese results. This finding provides a first indication that the longer-term release of carbon-14 may be congruent with corrosion of the steel.
Due to the release of a larger organic carbon-14 fraction to solution than in Container 2 and the high inorganic carbon-14 measurement at 13 weeks, a longer-term rate of carbon-14 release from Container 3 cannot be estimated with confidence at this stage. Additional analyses of Container 3 solution samples for total carbon-14, which are planned, should help to clarify the longer-term release behaviour. The rate of gas phase release from Container 3 decreases with time and between 6 and 59 weeks, the average rate is less than 0.004 Bq day–1.
Comparison with Other Studies
Prior to the CAST project, a small number of leaching experiments on irradiated steels had been undertaken in Japan. These studies have not been published in detail (and so are difficult to evaluate fully), however, the available information has been collated and summarised in Swanton et al. (Reference Swanton, Baston and Smart2015). In one experiment, in which a sample of irradiated steel was leached in a pH 10 cement-equilibrated water, carbon-14 was reported to be released to the solution phase as a mixture of inorganic (25–34%) and organic (66–75%) species. No gas phase measurements were made. In another experiment, a small amount of carbon-14 was reported to be released to the gas phase on leaching irradiated steel in pH 12.5 NaOH solution in an ampoule for 42 months. About 25% of the release was to the gas phase with equal amounts of organic and inorganic carbon-14 measured in solution. Thus, the results from the present study, seem to corroborate these previous observations of a mixture of inorganic and organic carbon-14 species being released on leaching irradiated stainless steels under high pH, anoxic conditions.
As part of CAST, Wieland and Cvetković (Reference Wieland and Cvetković2018) are also undertaking a corrosion experiment with irradiated stainless steel under high-pH anoxic conditions, with results available up to 412 days leaching based on periodic measurements of total organic carbon-14 (TO14C) in solution measured by accelerator mass spectrometry (AMS). Quantification of the dissolved 14C-carbonate fraction or gaseous releases has yet to been undertaken as both methods are still under development. Thus, the results presented by Wieland and Cvetković to date complement the findings of this study but cannot be directly compared. In common with the experiments presented here, Wieland and Cvetković observed a period of fast initial release of carbon-14 to solution over the first two weeks of leaching with a decrease in rate of release up to 93 days followed by a significant drop in TO14C release at longer times. In the 412-day leachate samples, carbon-14 containing acetate, formate and lactate were identified as the the main water-soluble organic carbon-14 containing compounds released from the steel.
Wieland and Cvetković also used conventional analysis techniques (high performance ion exchange chromatography and gas chromatography both coupled with mass spectrometry) to separate and quantify individual carbon-12 compounds (volatile and aqueous species). Methane and ethene were detected in the gas phase while acetate, formate and lactate were the main water-soluble carbon species identified. The gaseous species constitute only a small fraction of the total carbon release compared to the dissolved carboxylate species, similar to the observations from this study, but with the important difference that the dissolved releases are primarily organic.
SUMMARY AND OUTLOOK
The main observations to date on the release of carbon-14 from irradiated 316L(N) stainless steel under anoxic, alkaline conditions can be summarised as follows:
Carbon-14 is released to both solution and the gas phase in small amounts (fractional release ∼ 10–5 over 59 weeks);
There is an initial “fast” release to solution and the gas phase followed by a slower long-term release;
The main release of carbon-14 is to solution;
Between about 1 and 6% of the carbon-14 released is present in the gas phase;
The predominant gas-phase species are hydrocarbons with a smaller fraction of 14CO (which may include some volatile oxygen-containing carbon-species);
Dissolved carbon-14 is present as both carbonate and organic species.
The results have been compared with other results available from CAST WP2 and with previous studies of the speciation of carbon-14 releases from irradiated steels that were reviewed at the start of the CAST project. In general, the results presented here are consistent with those from other experiments.
Final sampling and termination of the experiments is planned after two years, with further measurements of releases to the gas and solution phases. The analysis of the solution phase will include TD14C measurements as well as inorganic carbon-14. Further TD14C analysis is also planned on stored samples collected at earlier sampling times. These measurements will provide further information on the longer-term rates and predominant speciation of carbon-14 releases from irradiated steel under anoxic alkaline conditions. The increased understanding being gained from this study and from CAST WP2 will help to reduce the uncertainties in assessing the radiological risk of carbon-14 releases potentially associated with the UK GDF.
ACKNOWLEDGMENTS
This study is funded by Radioactive Waste Management Limited and the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 604779, the CAST project.






