Introduction
Comorbid physical illness complicates the management of depression. This is particularly relevant in late-life depression (LLD), as older adults have higher rates of physical comorbidities than younger adults, and those with LLD have even higher rates of physical comorbidities than their non-depressed counterparts. Potential adverse impacts of physical comorbidities on the course of LLD include lower treatment response rates, higher risk of drug–drug interactions, or adverse effects (Taylor, Reference Taylor2014). Physical comorbidities may also increase risk of related adverse outcomes of LLD, such as development of cognitive impairment (Karameh et al., Reference Karameh2022). Conversely, LLD leads to worse outcomes for physical conditions such as cardiac disease (Jiang et al., Reference Jiang2001) and may increase the risk of developing frailty (Chang et al., Reference Chang2022).
Body composition is one component of physical health that is of potential relevance to health outcomes in older adults. In this context, lean muscle mass has received interest as one potential biomarker and is recognized as a principal component of sarcopenia, a condition of diminished lean muscle mass and strength. Sarcopenia typically develops secondary to aging particularly in the context of chronic medical illnesses including cancer, heart failure, chronic obstructive lung disease, rheumatoid arthritis, and various neurodegenerative disorders (Cesari, et al., Reference Cesari, Nobili and Vitale2016). Risk factors for diminished lean muscle mass and sarcopenia include physical inactivity, malnutrition, hormone deficiency, and chronic inflammation (Budui, et al., Reference Budui, Rossi and Zamboni2015), conditions that have also all been associated with depression. Sarcopenia specifically has been implicated in the risk for overall frailty, cachexia, and osteoporosis, and it increases all-cause mortality risk in older adults (Anderson, et al., Reference Anderson, Liu and Garcia2017; Cruz-Jentoft et al., Reference Cruz-Jentoft2014; Hayashi et al., Reference Hayashi2018; Ohashi et al., Reference Ohashi2018; Yoo et al., Reference Yoo2018). Accordingly, sarcopenia has been recognized as a serious geriatric syndrome, causing disability and increasing health care costs (Norman and Otten, Reference Norman and Otten2019; Tsekoura et al., Reference Tsekoura2017).
Recent research has proposed a link between lean muscle mass or sarcopenia and LLD. However, current clinical evidence regarding this association is conflicting. Diminished lean muscle mass and greater severity of depressive symptoms were found to be correlated in at least two observational studies (Kim et al., Reference Kim2011; Hsu et al., Reference Hsu2014) and one meta-analysis (Chang et al., Reference Chang2017), with one additional study finding a correlation between depressive symptoms and co-occurring osteopenia and sarcopenia (Park et al., Reference Park2021). Conversely, one large population survey did not find such any such effect (Byeon et al., Reference Byeon2016). Some recent evidence has suggested that overall level of frailty is a predictor of treatment resistance in LLD (Brown et al., Reference Brown2021a) and has identified specific components of frailty that are predictive of LLD outcome, such as baseline physical activity level (Brown et al., Reference Brown2021b).
With respect to body composition in treatment-resistant LLD (TR-LLD) specifically, previous work has established an association between older age and less tendency to weight gain following treatment with aripiprazole (Oughli et al., Reference Oughli2019). However, to our knowledge, no study has examined the relationship between lean muscle mass and depressive symptoms in patients with TR-LLD. Assessing this relationship in patients who do not respond to initial treatment is important to identify characteristics that are associated with TR-LLD or may impact illness course and treatment outcomes.
Thus, we conducted a secondary analysis to assess the relationship among lean muscle mass, demographic and clinical characteristics, and depression outcomes in the participants of the Incomplete Response in LLD: Getting to Remission (IRL-GRey) study. We hypothesized that lower lean muscle mass would correlate with older age, higher number of comorbid physical conditions, and higher depressive symptom severity. We also hypothesized that lower lean muscle mass at baseline would be associated with poor treatment outcomes in TR-LLD.
Methods
Overview
The full methods of the IRL-GRey study have been described elsewhere (Lenze et al., Reference Lenze2015). In brief, IRL-GRey was a multicenter study with two phases: in the first phase, patients aged 60 years or older presenting with an acute episode of major depressive disorder (MDD) were treated openly with venlafaxine extended-release (XR) up to 300 mg/day for 12 weeks, with an extra period of up to 12 weeks to establish remission status. Remission was defined as a score of 10 or less for two consecutive assessments on the Montgomery–Åsberg Depression Rating Scale (MADRS). Participants who did not achieve remission were eligible for the second phase during which they were randomized under double-blind conditions to addition of placebo or aripiprazole up to 15 mg/day for 12 weeks.
Sites and participants
IRL-GRey participants were recruited from July 20, 2009, to December 30, 2013, at three academic medical centers (Centre for Addiction and Mental Health, Toronto, ON, Canada; University of Pittsburgh, PA, USA; and Washington University, St. Louis, MO, USA), after approval by local institutional ethics review boards. The main eligibility criteria for IRL-GRey were as follows: 1) age 60 years and older; 2) meeting Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria for MDD and experiencing a current major depressive episode; 3) at least moderately severe symptoms, as defined by a MADRS score of 15 or more; and 4) absence of unstable physical illness or any major neurologic illness.
Of the 181 IRL-GRey participants who did not remit with open treatment with venlafaxine XR and were randomized to aripiprazole or placebo, 178 completed a dual-energy X-ray absorptiometry (DEXA) scans prior to randomization (and 175 also completed a repeat DEXA scan about 12 weeks later). These 178 participants constitute the sample for the analyses described below.
Demographic and clinical measures
Participants were assessed at multiple time points, including at time of enrollment, completion of open treatment with venlafaxine XR (which served as the baseline for the randomized treatment phase and our analyses), and completion of the study.
Evaluation of lean muscle mass with DEXA scans
Participants who were randomized underwent evaluation of body composition via DEXA scans prior to and following the randomized treatment phase (i.e., aripiprazole versus placebo). The DEXA scan is a faster, lower-cost alternative to MRI for body composition analysis (Messina et al., Reference Messina2018) and has been used to assess lean muscle mass and sarcopenia in several studies (Guglielmi et al., Reference Guglielmi2016; Kim et al., Reference Kim2017). Data from DEXA scans were previously used to estimate total body fat in this sample (Oughli et al., Reference Oughli2019).
Muscle mass indices were calculated using the method proposed by Heymsfield et al. (Reference Heymsfield1990). This method divides appendicular skeletal muscle mass (ASM) in kilograms (kg), averaged between the four limbs, by the square of height, in m2, to produce an appendicular skeletal muscle index (ASMI). This method is well established as a means of estimating lean muscle mass in adults (Kim et al., Reference Kim2017).
Statistical analysis
All analyses were conducted using the SAS Enterprise statistical package, version 7.1 (SAS Institute Inc., 2011). First, we described the distribution of lean muscle mass (i.e., ASMI) and estimated the prevalence of sarcopenia, using the cutoffs of a lean muscle mass <7.0 kg/m2 for males and <5.5 kg/m2 for females based on the European Consensus Definition (Cruz-Jentoft et al., Reference Cruz-Jentoft2019). Then, we assessed the cross-sectional relationship between ASMI at baseline and 28 demographic and clinical characteristics using a series of univariate linear regressions. Clinical variables associated with a p value of <0.10 were entered into a multivariate linear regression model. Age and sex were included a priori in the final model. Missing data were addressed using the multiple imputation method.
We next assessed whether lean muscle mass was associated with change in depression scores, using an approach similar to above. Change in depression score from baseline to end of the randomized treatment phase was used as the continuous dependent variable in this multivariate model.
Finally, we explored changes in lean muscle mass over 12 weeks (i.e., between baseline – following open treatment but prior to randomization – and the end of the randomized treatment phase).
Results
Participant characteristics and lean muscle mass
Table 1 summarizes the demographic, clinical characteristics, and baseline lean muscle mass indices of the participants who did not remit with venlafaxine XR and received a DEXA scan prior to entering the randomization treatment phase (n = 178). Figure 1 presents the distribution of baseline lean muscle mass in women (1a) and men (1b) within our TR-LLD sample. The lean muscle mass of 22 (12.4%) of our 178 participants was below the cutoff for sarcopenia at baseline, including 14 women (out of 100, 14.0%) and 8 men (out of 78, 10.4%).
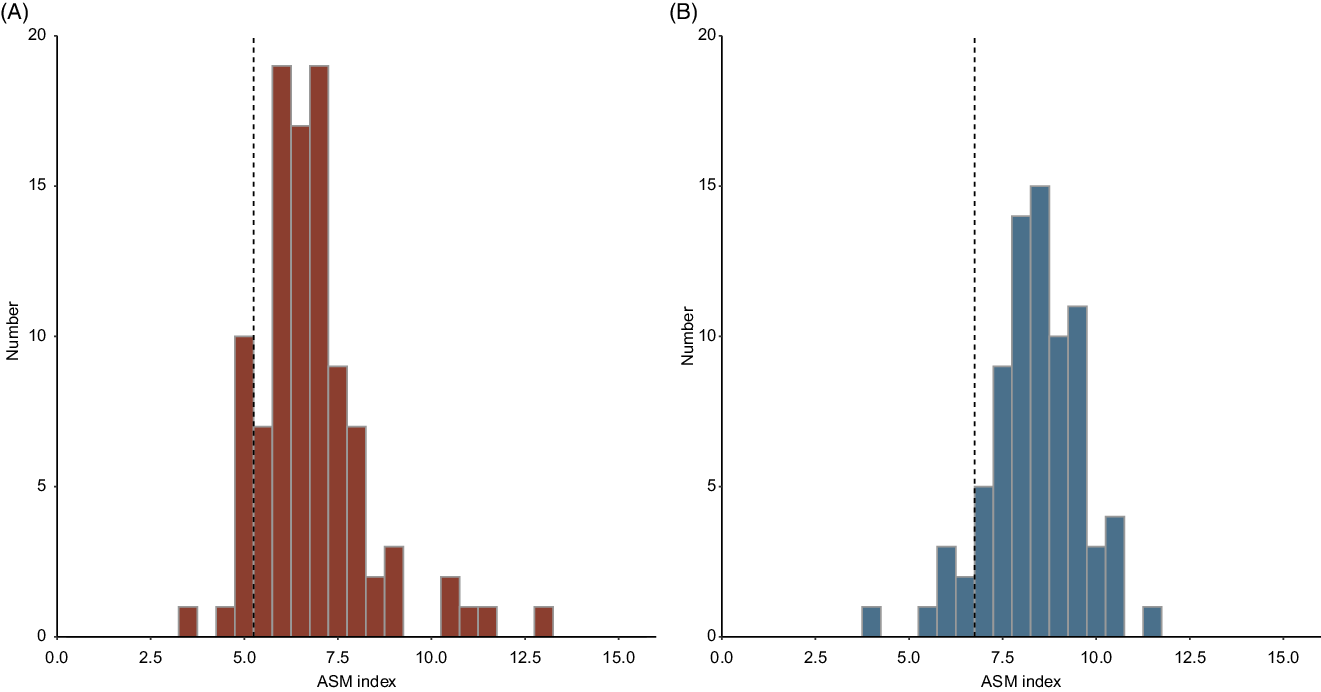
Figure 1. Distribution of lean muscle mass indices in self-reported females (A) and males (B) in our sample. Dotted lines represent the sex-specific muscle-mass cutoffs included in the European Consensus definition of sarcopenia (Cruz-Jentoft et al., Reference Cruz-Jentoft2019). The part of the distribution falling to the left of each cutoff represents participants with lean muscle mass indices indicative of sarcopenia. ASMI: appendicular skeletal mass (ASM) index = ASM, as measured by DEXA scan prior to the randomized treatment phase of the study, divided by height2.
Table 1. Clinical and demographic characteristics of study sample
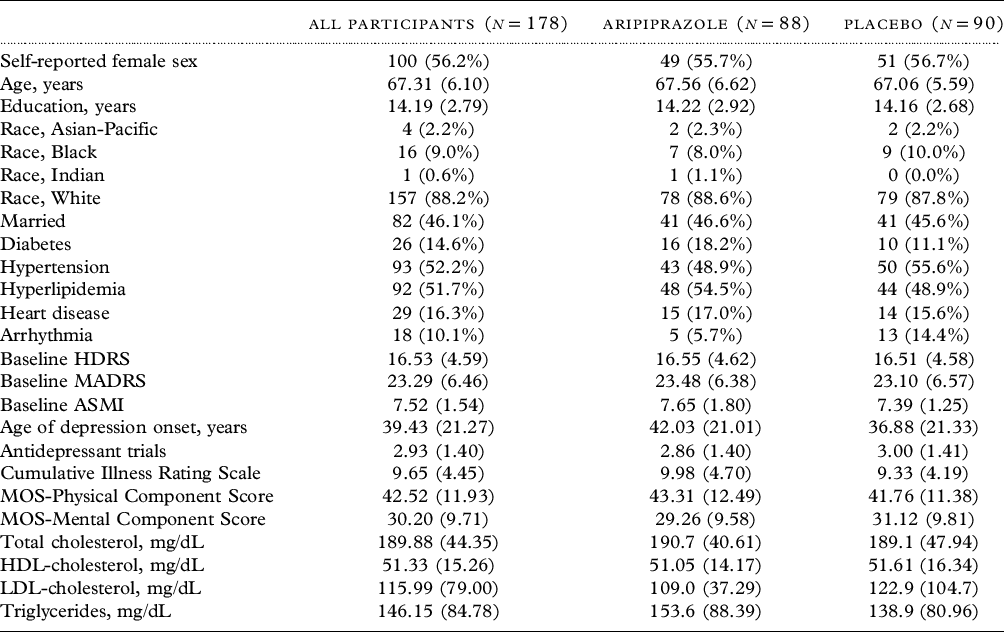
Data are displayed as mean (SD) unless otherwise specified.
HDRS: Hamilton Depression Rating Scale.
MADRS: Montgomery–Åsberg Depression Rating Scale.
ASMI: Appendicular skeletal mass (ASM) index (ASM divided by height2).
MOS: Medical Outcomes Study questionnaire.
Correlates of lean muscle mass (ASMI) in TR-LLD
In the final multivariate regression model, older age and female sex were significantly associated with ASMI at baseline. Severity of depressive symptoms prior to the randomization phase was not found to be significantly associated with baseline ASMI. The final model (Table 2) had a pooled r 2 of 0.41 and was significant (p < 0.0001).
Table 2. Summary of multivariate model for baseline lean muscle mass (ASMI)
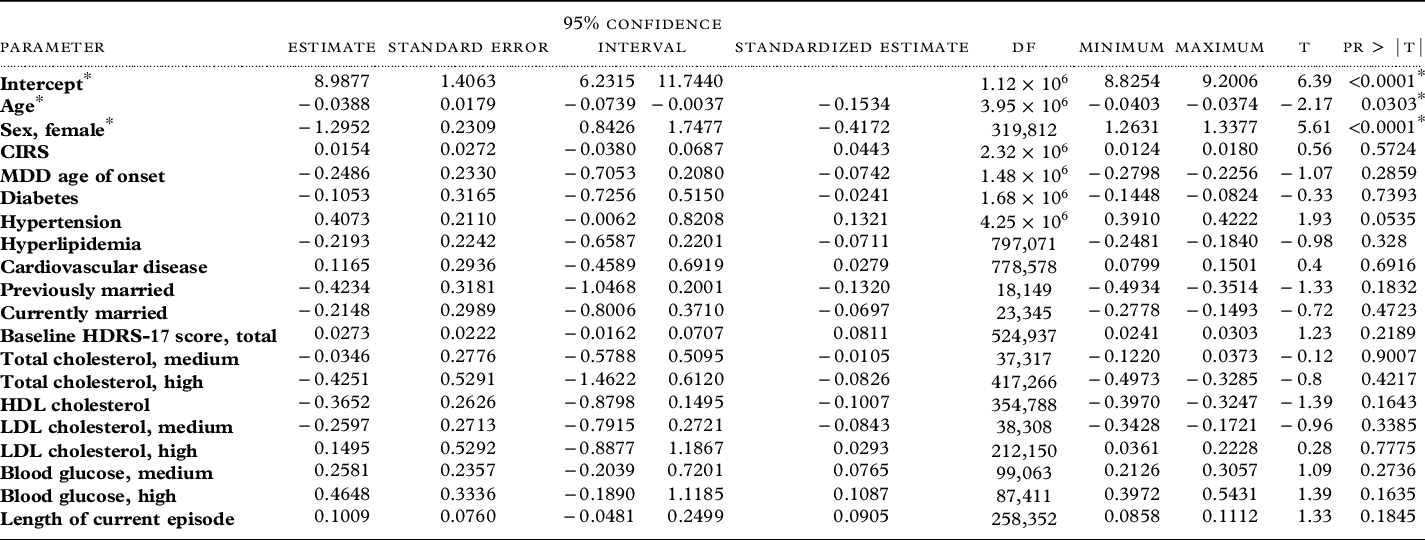
ASMI: Appendicular skeletal mass (ASM) index (ASM divided by height2).
df: Degrees of freedom.
CIRS: Cumulative Illness Rating Scale.
MDD: Major depressive disorder
HDRS: Hamilton Depression Rating Scale.
CIRS: Cumulative Illness Rating Scale.
To explore the possibility of sex-based differences in correlates of ASMI, we stratified our model by sex to analyze self-reported female and male participants separately. Age remained significantly negatively associated with ASMI in female participants. In our male sample, no correlations with ASMI remained significant (see Supplementary Table 1).
Is baseline lean muscle mass (ASMI) associated with outcomes in TR-LLD?
In the final multivariate regression model, marital status (currently or previously married), higher baseline HDRS-17 score, and treatment with aripiprazole were independently associated with decrease in depressive symptoms during the randomized treatment phase. ASMI was not significantly associated with HDRS-17 score change during the randomized treatment phase. The final model (Table 3) had a pooled r 2 of 0.27 and was significant (p < 0.0001).
Table 3. Summary of multivariate model for HDRS-17 score change during the randomized treatment phase
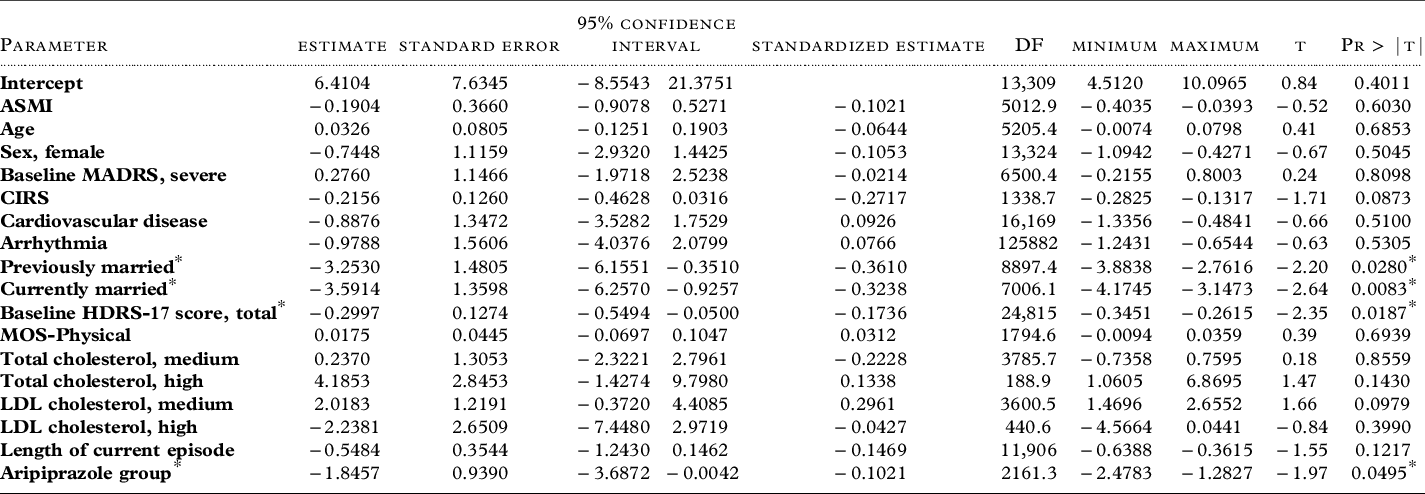
ASMI: Appendicular skeletal mass (ASM) index (ASM divided by height2).
MADRS: Montgomery–Åsberg Depression Rating Scale.
CIRS: Cumulative Illness Rating Scale.
HDRS: Hamilton Depression Rating Scale.
MOS: Medical Outcomes Study questionnaire.
To explore whether an association of ASMI with treatment outcome existed in the aripiprazole treatment group, we stratified our model by treatment group (aripiprazole and placebo). There remained no association between ASMI and HDRS-17 score change in either the aripiprazole or placebo groups when analyzed separately. There were no significant associations between any of the included variables and HDRS-17 change in the placebo group (see Supplementary Table 2).
Lean muscle mass changes during acute treatment of TR-LLD
Figure 2 shows the change in lean muscle mass measurements during the 12- week randomization phase. There was no significant change in ASMI during the randomized treatment phase (Wilcoxon signed rank test, p = 0.392).

Figure 2. Range of ASMI prior to and following the randomized treatment phase. ASMI: appendicular skeletal mass (ASM) index = ASM, as measured by DEXA scan, divided by height2. Box plot upper and lower borders represent the interquartile range (IQR: Q3 – Q1); whisker edges represent values 1.5*IQR above Q3 and below Q1. Bold horizontal lines represent medians. Dots represent outliers. Baseline: DEXA scan performed prior to the randomized treatment phase. End of treatment: DEXA scan performed following the randomized treatment phase.
Discussion
We analyzed the relationship among demographic and clinical characteristics, lean muscle mass (as reflected by the ASMI), and depression outcomes in a sample of older patients with TR-LLD who participated in a 12-week RCT comparing augmentation of venlafaxine XR with aripiprazole versus placebo. As expected, lean muscle mass was associated with some demographic characteristics (i.e., sex and age), with small effect sizes. Although we hypothesized that lean muscle mass would be a proxy for frailty, it was not associated with other physical correlates of frailty in our sample, such as number of medical comorbidities. Contrary to previous findings in other LLD samples (Kim et al., Reference Kim2011; Chang et al., Reference Chang2017; Hsu et al., Reference Hsu2014), lean muscle mass was not associated with the characteristics and outcomes of TR-LLD.
In LLD, some evidence suggests that body composition, in particular low lean muscle mass or sarcopenia, may be a biomarker of depressive symptom severity and treatment outcomes, although evidence is conflicting (Kim et al., Reference Kim2011; Byeon et al., Reference Byeon2016; Hsu et al., Reference Hsu2014; Park et al., Reference Park2021). However, to our knowledge, our study is the first to evaluate this relationship in TR-LLD. We found that only two demographic variables, age and self-reported sex, correlated with lean muscle mass in our sample. Our finding that females had lower muscle mass than males was likely reflective of sex-related differences in lean muscle mass at the population level, rather than representing a pathological finding. However, our finding that the negative association between age and lean muscle mass was only significant in female participants suggests the hypothesis that older females with TR-LLD are more susceptible to loss of muscle mass than older males. Given the small sample sizes in each sex subgroup, this hypothesis needs to be tested in a larger sample to determine whether an interaction exists between sex and age as correlates of muscle mass in TR-LLD.
While lean muscle mass was associated as expected with some demographic features, it was not associated with specific characteristics or outcomes of TR-LLD or a decreased response to aripiprazole. These findings were contrary to our hypothesis that anomalous body composition would be a poor prognostic indicator in TR-LLD. However, given our small sample size, small effect sizes seen, and the exploratory nature of the stratified analysis, our negative findings require some confirmation in a larger sample.
Another possible explanation for the lack of associations with lean muscle mass in our participants is that, because of the lead-in treatment protocol, our participants with TR-LLD were a more homogeneous group than previously studied non-TR LLD samples. A relationship between lean muscle mass and an MDD diagnosis may be more apparent in more heterogeneous samples of patients with LLD. It is also possible that patients with TR-LLD as a whole have physical characteristics distinct from those with LLD who respond to treatment. Whether body composition differs in LLD patients with or without treatment resistance, and the potential implications for their prognosis deserve further study.
Our results, which conflict with prior research in non-TR LLD groups, further contribute to the understanding of the interaction between body composition and psychopharmacotherapy across the lifespan. While aripiprazole is associated with a clinically significant increase in body fat even during a short 12-week treatment course in children and adolescents (Nicol et al., Reference Nicol2018), we did not observe such an increase over the same duration in our trial (Lenze et al., Reference Lenze2015). Extending previous observations of the age-dependent nature of antipsychotic-related weight gain, younger age in our sample was independently associated with measurable treatment-related increase in body fat (Oughli et al., Reference Oughli2019). Our prior results, taken together with these current findings showing that low muscle mass was not associated with poorer response to aripiprazole in TR-LLD, suggest aripiprazole may be a reasonable treatment in older adults with TRD and anomalous body composition. Future studies with a more extensive assessments of sarcopenia (i.e., including measures of muscle strength) will need to confirm this.
Our study has both strengths and limitations. One strength was the use of a sample with treatment resistance that was prospectively defined during protocolized treatment with low dosages of venlafaxine XR (i.e., equivalent to treatment with a selective serotonin reuptake inhibitor) followed by high dosages of venlafaxine XR (i.e., a serotonin-norepinephrine reuptake inhibitor). One limitation of our study was the exploratory approach used, involving 28 potentially relevant variables, which increases the risk of Type I error prior to multivariate modeling. Similarly, the findings from our subgroup analyses stratified by gender and treatment group, given their small sample sizes, should be treated as hypothesis-generating. Another limitation was that we did not measure lean muscle mass in the participants who responded to initial treatment with venlafaxine XR (i.e., those without TR-LLD), which would have permitted direct comparison between the treatment-responsive and treatment-resistant participants in our sample. Another limitation was the relatively short interval (i.e., 12 weeks) between the two DEXA scans, which impeded our ability to detect an impact of treatment response on lean muscle mass. Successful treatment of LLD typically leads to an increase in physical activity (Lindwall, et al., Reference Lindwall, Larsman and Hagger2011), which in turn could lead to an increase in lean muscle mass. However, such an indirect impact of depressive symptom improvement on lean muscle mass would be unlikely to be detected over such a short interval. Our results need to be interpreted in the context of these strengths and limitations.
In conclusion, both LLD and body composition are important clinical entities that impact on health outcomes in late life. Our study provides data in the TR-LLD population that contrast with existing evidence in the general LLD population. The mechanisms linking depression and body composition deserve further investigation in both groups.
Conflict of interest
Dr Ainsworth receives support from the University of British Columbia Clinician Investigator Program. Dr Blumberger receives research support from the Canadian Institutes of Health Research (CIHR), National Institutes of Health – US (NIH), Brain Canada Foundation and the Temerty Family through the CAMH Foundation, and the Campbell Family Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd, and he was the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He received in-kind equipment support from Magventure for investigator-initiated studies. He received medication supplies for an investigator-initiated trial from Indivior. He has participated in an advisory board for Janssen. He has participated in an advisory board for Welcony Inc. Dr Karp has received compensation for development and presentation of a (disease-state, not product-focused) webinar for Otsuka. He has served as consultant (paid) to NightWare and (with potential for equity) to AifredHealth. He receives compensation from Journal of Clinical Psychiatry and American Journal of Geriatric Psychiatry for editorial board service. Dr Lenze receives grant support from the COVID Early Treatment Fund, Mercatus Center Emergent Ventures, the Skoll Foundation, the Taylor Family Institute for Innovative Psychiatric Research, the Center for Brain Research in Mood Disorders, the Patient-Centered Outcomes Research Institute, and Janssen. He receives consulting fees from IngenioRx, Boeringer-Ingelheim, Prodeo, and Pritikin ICR. He has applied for a patent for the use of fluvoxamine in the treatment of COVID-19. Dr Nicol has received grant support from the National Institute of mental health (NIMH), the Health Resources and Services Administration (HRSA), the Barnes Jewish Hospital Foundation, the McDonnell Center for Systems Neuroscience, and the Mallinckrodt Institute of Radiology at Washington University School of medicine, the WUSM Institute for Clinical and Translational Science (ICTS), and Usona Institute (drug only). She has also served as co-investigator on investigator-initiated trials funded by LB Pharmaceuticals, Inc. and Takeda, and has consulted with Alkermes, Inc., Otsuka and Sunovion. Dr Reynolds is paid a stipend by the AAGP for service as editor of the American Journal of Geriatric Psychiatry and receives royalty income from the University of Pittsburgh as co-inventor of the Pittsburgh Sleep Quality Index.
Description of authors’ roles
NJA: study concept and design, analysis, preparation and revision of manuscript; RB: study concept and design, analysis, preparation and revision of manuscript; NG: study concept and design, revision of manuscript; HZ: analysis, revision of manuscript; DMB: study concept and design, recruitment of participants, revision of manuscript; JFK: study concept and design, recruitment of participants, revision of manuscript; EPL: study concept and design, recruitment of participants, revision of manuscript; GEN: study concept and design, revision of manuscript; CFR: study concept and design, recruitment of participants, revision of manuscript; WW: analysis, revision of manuscript; BHM: study concept and design, recruitment of participants, analysis, and revision of manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1041610222000862







