Introduction
The coronavirus disease-2019 (COVID-19) pandemic continues to develop across Africa since the first case was reported on the continent in Egypt in February 2020 [Reference Medhat and Kassas1, Reference Rice2]. As of 1 March 2021, over 3.9 million cases with 104 039 deaths have been reported from all 54 countries [3]. This represents a small fraction of the over 113 million cases and 2.5 million deaths reported globally [4]. However, despite the unique challenges presented by the COVID-19 pandemic, African countries are experienced in responding to outbreaks of emerging and re-emerging infectious diseases. The COVID-19 pandemic in many African countries comes in the context of multiple concurrent infectious disease outbreaks, for example Ebola virus disease (EVD), measles and Lassa fever [5] which require robust public health responses to mitigate the adverse impact of these events.
The major outbreak of EVD in West Africa from 2014 to 2016, during which 28 652 infections and 11 325 deaths were recorded, was unprecedented [6]. Three countries, Liberia, Guinea and Sierra Leone were at the epicentre of this outbreak. At the onset of this EVD outbreak, key pillars required for an effective response, such as coordination, surveillance, case management, infection prevention and control and community engagement, were inadequate or fractured [Reference Coltart7, Reference McNamara8]. The inability to rapidly detect, isolate and treat cases to break the chain of transmission was a direct derivative of these weaknesses, resulting in the large-scale transmission of EVD and the associated high mortality rates.
However, with support from the international community, these countries overcame these challenges and the EVD outbreak was ended. At the same time, the countries gained experience and built improved mechanisms for responding to infectious disease outbreaks [Reference Vetter9–Reference Marston11]. A key factor in improving capacity to detect and respond to outbreaks was the revitalisation of the Integrated Disease Surveillance and Response (IDSR) strategy [Reference Nagbe12–Reference Hemingway-Foday14]. The affected countries reported and successfully responded to several outbreaks of other infectious diseases in the aftermath of EVD [Reference Rude15–17]. In addition, joint assessments of the countries’ post-EVD capacities to prevent, detect and respond to public health risks using a multisectoral approach have shown improvements compared to the pre-EVD period, but at the same time have highlighted that health system challenges remain [18–20].
Although EVD and COVID-19 differ in their mode of transmission and pathogenesis, many facets of preparedness and response for outbreaks of these two diseases overlap, and therefore key lessons learned from the response to EVD outbreak are applicable to the response to COVID-19 [Reference Mobula21]. An effective response to COVID-19 also requires the capacity for robust surveillance, rapid case detection, disease confirmation, isolation of suspected and confirmed cases, treatment for moderate and severe cases, and a range of other public health measures to limit or prevent onward transmission.
In this study, we assessed whether the capacities and experience gained after the EVD outbreak in the three affected countries have had an impact on the response to COVID-19. In particular, we analysed the timeliness of case reporting and laboratory confirmation, and how readiness and public health response measures have affected the incidence of COVID-19 during the first 8 months of the pandemic. The findings from these analyses offer valuable lessons on the relevance of sustainably building capacities and strengthening health systems to meet the challenges posed by emerging disease outbreaks.
Methods
Study design and setting
We conducted a retrospective observational cross-sectional study to determine if the COVID-19 readiness and response measures implemented in Guinea, Liberia and Sierra Leone, spanning the period 1 February to 30 September 2020, were derived from the health system strengthening measures put in place as a result of lessons learned from the response to EVD outbreaks. We identified and assessed predictors for timely reporting and laboratory confirmation of cases. All three countries are located in West Africa and together have an estimated population of over 26 million [22].
Key readiness capacities and response measures
We selected seven key readiness capacities covering core thematic areas based on lessons learned from the response to the EVD outbreak as well as World Health Organization (WHO) recommendations to countries. An additional seven response measures implemented by the respective countries were also identified. Table 1 outlines these capacities and measures, their relevance or rationale, and the definitions of their key milestone dates. The time to each milestone for readiness capacities was based on the duration (days) from WHO declaration of COVID-19 as a public health emergency of international concern (PHEIC) to the earliest date on which the readiness capacity was achieved or the measure instituted. For the response measures, the time to each milestone measured the duration in days from confirmation of the first COVID-19 case in the respective countries to the earliest date on which response measures were implemented.
Table 1. Selected COVID-19 readiness capacities and response measures, their rational and definition of key milestone date on which capacities were attained on response measures first implemented

Data sources and measurements
Key milestone dates were obtained from official reports and assessments shared by the respective countries with the WHO Regional Office for Africa (AFRO). In instances where the milestone date was missing, we extracted this information from the official government websites of the respective countries. All milestone dates were cross-checked with the official authorities of the respective countries. The population estimates for the respective countries were obtained from the World Bank database [22].
A line list of confirmed COVID-19 cases reported during the studied period was obtained from each country. We consolidated the line lists from all three countries and selected key variables for our analysis on the timeliness of case reporting and laboratory confirmation. The variables selected were country, date of report, age, sex, date of onset, date of confirmation, place of case detection and outcome (alive or dead). The date of report refers to the earliest date on which a person was notified to the health authorities as being suspected of having COVID-19. The date of onset refers to the earliest known date on which the case was reported to have begun experiencing signs and symptoms associated with COVID-19. The date of confirmation refers to the date on which the earliest laboratory results were released confirming the diagnosis of COVID-19 in each case. We defined the reporting timeliness as the time interval in days from onset of symptoms to case report, while the laboratory confirmation timeliness was defined as the duration from onset of symptoms to laboratory confirmation.
Only confirmed cases were included in our analysis on time to report and confirmation. A confirmed case of COVID-19 was defined as ‘a person with a positive Nucleic Acid Amplification Test (NAAT) or a person with a positive SARS-CoV-2 Antigen-rapid diagnostic test (Ag-RDT) and meeting either the probable case definition or suspected criteria as per the WHO guideline, or an asymptomatic person with a positive SARS-CoV-2 Ag-RDT and who was a contact of a probable or confirmed case’ [23]. We excluded asymptomatic cases, that is, confirmed cases who were reported as not experiencing any sign or symptoms despite being confirmed positive for COVID-19. This was intended to reduce bias because estimating timely reporting among asymptomatic cases may be more complex, without access to data on their likely dates of exposure to their source case and when that case became infectious.
Missing data
Three (0.1%) values were missing for time to report due to missing dates of report, while 133 (3.2%) were missing for time to confirmation due to missing date of confirmation. We performed multiple imputations for the missing data using the Multivariate Imputation by Chained Equations (MICE) package in R [Reference van Buuren and Groothuis-Oudshoorn24]. We performed five iterations using the country, age, sex, outcome and place of detection to impute the missing data.
Data analysis
We plotted a timeline of the key readiness and response milestone dates for the respective countries to show when these capacities or measures were first attained or implemented. Using 30 January 2020 as the date of the WHO declaration of PHEIC, we computed the duration in days to each milestone date for readiness. The number of days to each response measure milestone date was the duration from the first confirmed case in the respective countries. We computed the average number of days to each milestone across all three countries.
We also present key COVID-19 epidemiological parameters such as the number of cases and deaths, the case fatality ratio (CFR), which was defined as the proportion of deaths among all confirmed cases, the cumulative incidence per 100 000 population, and the mortality rates per one million population in the respective countries. We analysed the burden of infection among health workers, which was calculated as the proportion of health worker cases among the total health workforce in the respective countries. Data on testing are presented as the cumulative number of tests performed per 10 000 population.
We computed the median number of days for case reporting and laboratory confirmation for symptomatic cases in the respective countries. The effect of selected covariates on the timeliness of reporting and confirmation were quantified by using two separate multivariable negative binomial regression models. The selected covariates were country, age group, sex and place of detection. Our initial choice of these covariates was based on trends observed in the surveillance reports, a priori knowledge, literature available on this topic, and the extent to which data were available. Based on the age-associated mortality observed in the surveillance data, we categorised the cases into two age groups, <50 years and ⩾50 years. Given the urban nature of the pandemic and the likelihood of access to health services to be skewed towards the capital cities in these countries, we also categorised the place of detection into two groups, those detected in the capital cities and those detected outside the capital cities. The results are presented as an incidence rate ratio (IRR) with a 95% confidence interval (CI). IRR greater than 1 indicates a longer time to detection or confirmation, while less than 1 indicates a shorter time.
Results
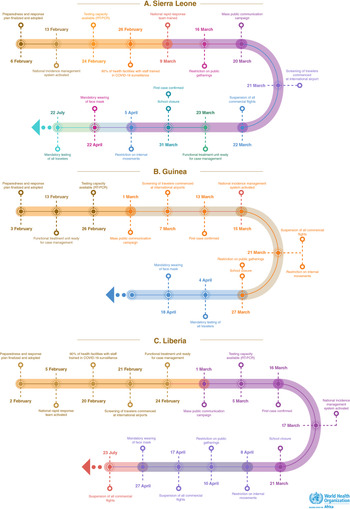
The timeline of key milestones for readiness capacities and response measures are presented in Figure 1. The adoption and finalisation of a COVID-19 national strategic preparedness and response plan was the earliest readiness measure achieved across the three countries, taking an average of 5 days (range 3 days in Liberia to 7 days in Sierra Leone) (Table 2). The screening of all travellers at the respective international airports and the launch of a mass public communication campaign were the last readiness measures implemented, taking an average of 37 days each. It took an average of 29 days for the countries to obtain capacity for performing COVID-19 laboratory testing (range 25 days in Sierra Leone to 35 days in Liberia). Table 2 further shows that the activation of a national incidence management system was the earliest public health response measure implemented, which predated the confirmation of the first case by 15 days on average (range 47 days before the first case in Sierra Leone to 2 days after the first case in Guinea). Results for the timeliness of various readiness and response measures in the respective countries are further shown in Table 2.

Fig. 1. Timeline of selected COVID-19 readiness and response measures in Sierra Leone, Guinea and Liberia, 1 February to 30 September 2020.
Table 2. Duration to attainment or implementation of selected COVID-19 readiness capacities and response measures in Guinea, Liberia and Sierra Leone, 1 February–30 September 2020

N/A means the date on which the milestone was first achieved was not available or could not be determined to compute the number of days to achievement after the declaration of PHEIC by WHO.
A total of 14 227 cases with 220 deaths (CFR 1.5%) was reported from the three countries during the studied period. Guinea was the most affected with the highest case numbers (n = 10 652) and highest cumulative incidence of 82 per 100 000 population (Table 3). Guinea also reported the highest number of health worker cases (n = 513), accounting for an estimated 5.0% of infection among the total health workforce in the country. Liberia reported the highest numbers of deaths (n = 82), CFR (6.1%), and mortality of 16 per million population. Other epidemiological results for the respective countries are shown in Table 3.
Table 3. Key epidemiological features of COVID-19 in Guinea, Liberia, and Sierra Leone, 1 February–30 September 2020

a Population estimates for 2020 sourced from World Bank data.
b Number of tests based on number of persons tested.
c Positivity rate is the proportion of all COVID-19 RT-PCR that tested positive for SARS-CoV-2 infection.
Of 2406 symptomatic cases included in the study, the overall median reporting time was 3 days (interquartile range (IQR) (1–6)) while the laboratory confirmation time was 5 days (IQR (2–8)). When stratified by the respective countries, Liberia had the longest median reporting time of 5 days, while Sierra Leone had the shortest median reporting time of 1 day. The median laboratory confirmation time was also shortest in Sierra Leone (Table 4). Results of the negative binomial regression model showed that country (Liberia and Guinea) and age group (≥50 years) predictors were significantly (P < 0.001) associated with longer reporting and confirmation times compared to Sierra Leone and those below 50 years old, respectively. The place of detection (outside a capital city) was found to be significantly associated with shorter reporting time compared to those within a capital city, although with no influence on the laboratory confirmation time. Table 4 further shows that sex had no influence on the reporting or laboratory confirmation timeliness.
Table 4. Results of negative binomial regression model for reporting and confirmation timeliness of symptomatic COVID-19 cases in Guinea, Liberia and Sierra Leone, 1 February to 30 September 2020

3 IRR, incidence rate ratio.
Discussion
Our results show that all selected readiness measures were instituted across the three countries within the first 2 months of declaration of the PHEIC by WHO. We also found very early response measures implemented across the three countries, with at least one response measure predating the confirmation of the first case. While the incidence of COVID-19 remained relatively low compared to most affected countries globally, our study showed a high CFR, particularly in Liberia and Sierra Leone. Other findings pointed to a high burden of infection among health workers and definite low testing rates compared to many countries globally. The overall median time of 3 days to report and 5 days for laboratory confirmation of symptomatic cases, although with variability among the countries, indicated that this delay could potentially be a key contributor to community transmission of the disease among the local population in the respective countries, despite the response measures instituted.
The relatively rapid readiness and response measures implemented could be attributed in part to the experience gained by these countries and the international community from the devastating EVD outbreak of 2014–2016 [Reference Maxmen25]. It took several months for these countries and the international community to recognise the potential threat and scale of the EVD outbreak, thereby delaying the institution of cogent control measures at national level, as well as slow mobilisation of international assistance [Reference Moon26]. Exposure to a previous major epidemic is associated with faster response, an indication that these countries have learned to move into action early [Reference Tsuei27]. Investments made in strengthening IDSR including laboratory capacities helped these countries to quickly adapt their structures in readiness and response to COVID-19. For example, of the four indicators for real-time surveillance assessed during the joint external evaluation on a scale of 1–5 (1 being no capacity and 5 being sustainable capacity), Liberia and Sierra Leone scored 4 (demonstrated capacity) and Guinea scored 3 (developed capacity) on three of the indicators [18–20]. National laboratory systems were also found to improve with specimen referral systems scoring 3 (developed capacity) across all three countries and testing capacity for priority diseases scoring 4 (demonstrated capacity) in Sierra Leone, 3 (developed capacity) in Guinea, and 2 (limited capacity) in Liberia although capacities in Liberia improved in the aftermath of the evaluation. These existing capacities were leveraged in response to the COVID-19 pandemic, however, the fact that all three countries scored 2 (limited capacity) for an interoperable, interconnected, electronic real-time reporting system as well as 2 (limited capacity) for an effective modern point-of-care and laboratory-based diagnostic indicated major weaknesses in these areas and the need for additional investments or support to effectively respond to a pandemic such as COVID-19. Additionally, the rapid support from WHO and other partner institutions to African countries including Guinea, Liberia and Sierra Leone helped bolster readiness capacities for COVID-19 in key areas, such as in the provision of laboratory reagents and other supplies, technical guidance, training etc [28, 29].
In spite of the rapidity with which measures were taken, it is clear that health systems in these countries remain fragile and underfunded [30], and these constraints can crucially affect health outcomes in a pandemic of this nature. The high CFR in Liberia (6.1%) and Sierra-Leone (3.2%) vs. 0.6% in Guinea and the high proportion of health worker infections in Liberia (2%), Sierra Leone (3.5%) and Guinea (5%) in the early period of the pandemic could in part be attributed to the fragility of the health systems in these countries. In Liberia and Sierra Leone, the implementation of a policy to test all dead bodies for COVID-19 to improve detection, case investigation and contact tracing resulted in the identification of a high number of COVID-19 community deaths, indicating limited use of, access to and quality of health care services in these countries. The relatively lower testing rates across these three countries with an average cumulative test of 71 tests per 10 000 population (45 tests per 10 000 population in Liberia, 52 tests per 10 000 population in Sierra Leone and 93 tests per 10 000 population in Guinea) compared to wealthy countries such as Denmark (6741 tests per 10 000 population), United States of America (3595 tests per 10 000 population) and the United Kingdom (3310 tests per 10 000 population) [Reference Ra31] could also be viewed in the context of resource constraints in low- and middle-income countries to offer mass-based testing to a larger per cent of their population.
The reporting and confirmation timeliness were significantly longer in Guinea and Liberia compared to Sierra Leone. While asymptomatic COVID-19 cases may hold similar transmission potential as symptomatic cases [32], the delay in detection (reporting and confirmation) of cases after the onset of symptoms particularly in the early phase of the pandemic in these countries is an incontestable contributor to the transmission dynamics of the disease among the population. Kieran et al. found that the viral load of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) is highest at or around symptom onset [Reference Walsh33]. A systematic review and meta-analysis involving 79 studies also showed that people are likely to be highly infectious with the SARS-CoV-2 virus in the first week after the onset of symptoms [Reference Cevik34]. These findings mean that delay in the initiation of intervention measures (such as isolation and contact tracing) as a result of the delay in reporting and confirming cases would provide ample time for symptomatic cases to continue transmitting the infection.
Delays in reporting and confirmation among cases ⩾50 years may have led to delay in initiation of treatment, one of several factors which could have resulted in the high CFR in this age group. This finding is consistent with several studies that have shown that people in older age group are at higher risk of complications and deaths from COVID-19, especially when life-saving interventions are delayed [Reference Starke35]. Also, we found it interesting to note that COVID-19 cases were reported earlier among people outside the capital cities compared to those living within the capital cities. Disease surveillance systems are likely to be quickly overwhelmed in high-density population areas as the rate of transmission increases [Reference Lee36]. This could be a result of high population densities in the capital cities of the various countries making surveillance of COVID-19 more challenging. Intense transmission of EVD in the capital cities of the three countries complicated response efforts in the 2014–2016 outbreak [Reference Campbell, Adan and Morgado37].
There are a few limitations to our study. First, although we showed how early these readiness and response measures were implemented, we did not assess the efficiency of their implementation. The early implementation of the restriction measures may have resulted in fatigue among the population, who probably also suffered adverse socio-economic effects. Hence, strict adherence to measures such as wearing a face mask would likely wane over time, potentially resulting in resurgence of cases. Second, most COVID-19 cases are asymptomatic; therefore, the analysis of symptomatic cases may not provide a full picture of the speed with which cases are reported and confirmed in the countries. However, given that mass population-based testing had not been implemented and that most asymptomatic cases were only identified after testing contacts of symptomatic cases, analysis of how rapidly symptomatic cases were identified can provide an estimation of the timeliness of case detection. Third, the statistical power, though not effect, of our analysis, may have been lowered due to omissions or recording errors in onset dates in the line list, leading to the exclusion of potential symptomatic cases. Lastly, we did not account for the effect of contact tracing on reporting and confirmation timeliness because such data were not available. High levels of contacts tracing are more likely to lead to early reporting and confirmation due to the regular monitoring [Reference Keeling, Hollingsworth and Read38].
Despite these limitations, our study has shown that these countries took actions early in the form of readiness and response to avert the negative consequences that they had experienced during the Ebola outbreak of 2014−2016. Using lessons learned from the EVD outbreak as well as capacities gained in its aftermath, the countries were able to take key readiness and response measures early which may have contributed to the low incidence of COVID-19 in these countries, despite the unique challenges posed by COVID-19 given the role of asymptomatic transmission in the context of low level of COVID-19 testing. Strong technical and operational support from WHO and partners has also helped these countries to continue to respond to the pandemic. However, eventual control of the pandemic will require continued implementation of public health measures, along with vaccination campaigns. It would be interesting for future studies to consider assessing the effectiveness of the various response measures implemented and their impact on the COVID-19 pandemic in these countries.
Acknowledgements
We wish to thank all those who contributed to this study. We are particularly grateful to Kwaukuan Yealue of WHO Liberia Country Office, Robert Musoke of WHO Sierra Leone Country Office, and Mamadou Balde of WHO Guinea Country Office for validating the data on COVID-19 response and readiness milestone dates for the respective countries.
Data availability statements
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.