Significant outcomes
-
MSRA rs11249969 and rs81442 were significantly associated with ED-related and general psychopathology in the AN patients.
-
Depression was the symptom that showed the strongest association with the identified MSRA haplotypes.
-
Variability in a proximal region in the antioxidant MSRA gene locus profoundly modulated psychopathological symptoms of AN patients.
Limitations
-
The inclusion of more subjects would have been required in the case–control analysis to unequivocally rule out an effect on AN risk.
-
The age of the control subjects was significantly higher than that of the AN patients, which could have partly contributed to the differences in the anthropological parameters.
-
Psychometric scores were not available for the control group.
Introduction
Anorexia Nervosa (AN) is a complex disorder characterised by severe food restriction, fear of weight gain, and body image distortion, all of which leads to the highest mortality rate of any psychiatric disease (Arcelus et al. Reference Arcelus, Mitchell, Wales and Nielsen2011). Traditionally, socio-cultural and family factors have been considered the most important contributors to AN; however, in the last years, twin and family studies have demonstrated the existence of high heritability estimates (Bulik et al. Reference Bulik, Sullivan, Tozzi, Furberg, Lichtenstein and Pedersen2006; Thornton et al. Reference Thornton, Mazzeo and Bulik2011), thus pointing out genetics as a key player in this eating disorder (ED) (Pinheiro et al. Reference Pinheiro, Root and Bulik2009).
The methionine sulfoxide reductase A (MSRA) gene encodes an enzyme (MsrA) that reduces oxidised methionine residues, contributing significantly to the defence against oxidative stress (Kim & Gladyshev Reference Kim and Gladyshev2007). This gene is highly expressed in several areas of the brain and has in fact been related to several psychiatric conditions, such as schizophrenia (Walss-Bass et al. Reference Walss-Bass, Soto-Bernardini, Johnson-Pais, Leach, Ontiveros, Nicolini, Mendoza, Jerez, Dassori, Chavarria-Siles, Escamilla and Raventos2009), bipolar disorder (Ni et al. Reference Ni, Ma, Lin, Lao, Hao, Guan, Li, Jiang, Liu, Ye, Liu, Wang, Zhao, Cao and Li2015) or Alzheimer’s (Gabbita et al. Reference Gabbita, Aksenov, Lovell and Markesbery1999) and Parkinson’s disease (Wassef et al. Reference Wassef, Haenold, Hansel, Brot, Heinemann and Hoshi2007), as well as being associated with a variety of behavioural traits and psychiatric symptoms (Reiterer et al. Reference Reiterer, Schmidt-Kastner and Milton2019). In this regard, various genome-wide association studies (GWAS), replication studies, and meta-analyses (Day et al. Reference Day, Helgason, Chasman, Rose, Loh, Scott, Helgason, Kong, Masson, Magnusson, Gudbjartsson, Thorsteinsdottir, Buring, Ridker, Sulem, Stefansson, Ong and Perry2016; Fan et al. Reference Fan, Wang, Hao, He, Wen, Guo, Wu, Ning, Wang, Wang and Zhang2017; Ma et al. Reference Ma, Deng, Liu, Li, Chen, He, Wang, Wang, Hu, Collier and Li2011; Amare et al. Reference Amare, Schubert, Tekola-Ayele, Hsu, Sangkuhl, Jenkins, Whaley, Barman, Batzler, Altman, Arolt, Brockmoller, Chen, Domschke, Hall-Flavin, Hong, Illi, Ji, Kampman, Kinoshita, Leinonen, Liou, Mushiroda, Nonen, Skime, Wang, Kato, Liu, Praphanphoj, Stingl, Bobo, Tsai, Kubo, Klein, Weinshilboum, Biernacka and Baune2018; Luciano et al. Reference Luciano, Hagenaars, Davies, Hill, Clarke, Shirali, Harris, Marioni, Liewald, Fawns-Ritchie, Adams, Howard, Lewis, Gale, McIntosh and Deary2018; Chu et al. Reference Chu, Liu, Wen, Li, Cheng, Cheng, Zhang, Mei, Qi, Liang, Ye, Kafle, Wu, Wang, Wang, Ning and Zhang2020) have shown that the occurrence of variability in the MSRA gene locus is likely behind the traits and psychiatric conditions observed.
The presence of genetic variants in the MSRA gene has also been linked to obesity. Indeed, three meta-analyses of GWAS have suggested that certain loci in or near this gene are body-mass index (BMI) regulators (Lindgren et al. Reference Lindgren, Heid, Randall, Lamina, Steinthorsdottir, Qi, Speliotes, Thorleifsson, Willer, Herrera, Jackson, Lim, Scheet, Soranzo, Amin, Aulchenko, Chambers, Drong, Luan, Lyon, Rivadeneira, Sanna, Timpson, Zillikens, Zhao, Almgren, Bandinelli, Bennett, Bergman, Bonnycastle, Bumpstead, Chanock, Cherkas, Chines, Coin, Cooper, Crawford, Doering, Dominiczak, Doney, Ebrahim, Elliott, Erdos, Estrada, Ferrucci, Fischer, Forouhi, Gieger, Grallert, Groves, Grundy, Guiducci, Hadley, Hamsten, Havulinna, Hofman, Holle, Holloway, Illig, Isomaa, Jacobs, Jameson, Jousilahti, Karpe, Kuusisto, Laitinen, Lathrop, Lawlor, Mangino, McArdle, Meitinger, Morken, Morris, Munroe, Narisu, Nordstrom, Nordstrom, Oostra, Palmer, Payne, Peden, Prokopenko, Renstrom, Ruokonen, Salomaa, Sandhu, Scott, Scuteri, Silander, Song, Yuan, Stringham, Swift, Tuomi, Uda, Vollenweider, Waeber, Wallace, Walters, Weedon, Witteman, Zhang, Zhang, Caulfield, Collins, Davey Smith, Day, Franks, Hattersley, Hu, Jarvelin, Kong, Kooner, Laakso, Lakatta, Mooser, Morris, Peltonen, Samani, Spector, Strachan, Tanaka, Tuomilehto, Uitterlinden, van Duijn, Wareham, Hugh, Waterworth, Boehnke, Deloukas, Groop, Hunter, Thorsteinsdottir, Schlessinger, Wichmann, Frayling, Abecasis, Hirschhorn, Loos, Stefansson, Mohlke, Barroso and McCarthy2009; Scherag et al. Reference Scherag, Dina, Hinney, Vatin, Scherag, Vogel, Muller, Grallert, Wichmann, Balkau, Heude, Jarvelin, Hartikainen, Levy-Marchal, Weill, Delplanque, Korner, Kiess, Kovacs, Rayner, Prokopenko, McCarthy, Schafer, Jarick, Boeing, Fisher, Reinehr, Heinrich, Rzehak, Berdel, Borte, Biebermann, Krude, Rosskopf, Rimmbach, Rief, Fromme, Klingenspor, Schurmann, Schulz, Nothen, Muhleisen, Erbel, Jockel, Moebus, Boes, Illig, Froguel, Hebebrand and Meyre2010; Dorajoo et al. Reference Dorajoo, Blakemore, Sim, Ong, Ng, Seielstad, Wong, Saw, Froguel, Liu and Tai2012), which has been confirmed in some (Bille et al. Reference Bille, Banasik, Justesen, Sandholt, Sandbaek, Lauritzen, Jorgensen, Witte, Holm, Hansen and Pedersen2011; Albuquerque et al. Reference Albuquerque, Nobrega, Rodriguez-Lopez and Manco2014; Krishnan et al. Reference Krishnan, Thompson, Mitchell, Murphy, McCowan and Shelling2017), but not all (Gonzalez et al. Reference Gonzalez, Estevez, Giralt, Caceres, Perez, Gonzalez-Carpio, Ballester, Sunyer and Rodriguez-Lopez2014; Volckmar et al. Reference Volckmar, Han, Putter, Haas, Vogel, Knoll, Struve, Gobel, Haas, Herrfurth, Jarick, Grallert, Schurmann, Al-Hasani, Hebebrand, Sauer and Hinney2016; Hotta et al. Reference Hotta, Nakamura, Nakamura, Matsuo, Nakata, Kamohara, Miyatake, Kotani, Komatsu, Itoh, Mineo, Wada, Yoneda, Nakajima, Funahashi, Miyazaki, Tokunaga, Kawamoto, Masuzaki, Ueno, Hamaguchi, Tanaka, Yamada, Hanafusa, Oikawa, Yoshimatsu, Nakao, Sakata, Matsuzawa, Nakamura and Kamatani2010) follow-up studies.
There is, therefore, a body of solid evidence supporting a significant role of the MSRA gene in personality traits, psychiatric disorders, and the regulation of BMI; however, its relation to ED remains unexplored. In the present work, we aimed to address this research gap by examining whether the variability in the MSRA gene locus may be associated with ED-related and general psychopathological symptoms that are commonly found in these patients. In addition, by including a group of control subjects, we examined the putative influence of this variability on AN risk.
Patients and methods
Subjects
The patient group consisted of 233 consecutive, Caucasian female patients with AN who were referred to the Eating Disorders Unit (Institute of Mental Disorders, Badajoz, Spain) by their General Practitioner because of symptoms indicative of AN. Patients were interviewed and diagnosed, according to Diagnostic and Statistical Manual of Mental Disorders fifth edition (DSM-5) guidelines, by collaborating clinicians who were blind to genotype. Exclusion criteria included intellectual disability, dementia, schizophrenia, Turner’s syndrome, other neurological disorders, and endocrine pathologies. All patients or their legal guardians gave written consent for their participation in the study, which had been approved by the Bioethics Committee of the University of Extremadura (March 6, 2018), and were carried out in accordance with the Declaration of Helsinki and its subsequent revisions.
The control group consisted of 369 anonymous, female Spanish donors with normal BMI and no records of psychiatric or endocrine disorders. Their genetic material was obtained from the Carlos III DNA National Bank (University of Salamanca, Salamanca, Spain).
Psychometric evaluation
To evaluate ED-related and general psychopathological symptoms, the patients completed the Eating Disorders Inventory Test-2 (EDI-2) and the Symptom Checklist 90 Revised (SCL-90R) self-reported questionnaires, respectively.
The EDI-2 questionnaire gives information about 11 items, namely, Drive for Thinness, Bulimia, Body Dissatisfaction, Ineffectiveness, Perfectionism, Interpersonal Distrust, Interoceptive Awareness and Maturity Fears, Asceticism, Impulse Regulation, and Social Insecurity. This test has been validated in the Spanish population with high internal consistency between the different traits examined (Guimera & Torrubia Reference Guimera and Torrubia1987). SCL-90R, which has also been validated in Spaniards with good results (Derogaitis Reference Derogaitis2002), provides scores obtained for Somatisation, Obsessive Compulsive, Interpersonal Sensitivity, Depression, Anxiety, Hostility, Phobic Anxiety, Paranoid Ideation, Psychoticism, and Additional Items. These scores are summarised in three indexes, namely, Global Severity Index (GSI), Positive Symptom Distress Index (PSDI), and Positive Symptom Total (PST).
Genetic analyses
A 10-ml blood sample was drawn from each patient at the time of their first visit to the ED unit and stored at −80ºC until DNA purification, which was performed with a standard phenol–chloroform extraction method. The coding and adjacent regions of the MSRA gene (ENSG00000175806, HGNC:7377) were retrieved using the GRCh38.p13 genome assembly and tag-SNPs (single-nucleotide polymorphisms that are representative of a certain haplotype) were assigned by Haploview 4.2, setting the minor allele frequency threshold at 10% and the pairwise tagging with a minimum r-square of 0.80. With these conditions, 14 tag-SNPs were identified with minor allele frequencies ranging from 0.129 to 0.491 (Table 1 and Supplementary Figure S1).
Table 1. Tag-SNPs identified in the region encompassing the MSRA gene

HW, Hardy–Weinberg equilibrium; MAF, minor allele frequency; SNP, single-nucleotide polymorphism.
Genotype analyses were carried out by a MassArray system (Agena Bioscience) with iPlex Gold technology at the Spanish Genotyping National Centre (CEGEN-ISCIII, Santiago de Compostela, Spain).
Statistical analyses
Quantitative variables, e.g. psychometric scores, are shown as mean ± standard deviation (SD) values and were compared with the Student’s T/Mann–Whitney or ANOVA/Kruskal–Wallis tests, depending on the normality of the data and the number of groups analyzed. Categorical variables were compared by the Chi-square test and odds ratios (OR) with 95% confidence intervals (CI) were obtained. The assessment of risk associations with SNPs was performed by logistic regression models adjusted for age using the SNPassoc package in the R environment (https://cran.r-project.org/web/packages/SNPassoc/index.html). The same software was used to detect age-adjusted associations between genotypes and quantitative variables by linear regression. With the aim of identifying regions in the MSRA gene locus whose variability might have relevant consequences, we run association analyses with consecutive 3-SNP haplotypes (sliding-window approach) using PLINK v1.07 (Purcell et al. Reference Purcell, Neale, Todd-Brown, Thomas, Ferreira, Bender, Maller, Sklar, de Bakker, Daly and Sham2007) with a haplotype frequency cutoff of 0.1.
After testing all inheritance models in the preliminary genetic analyses, we decided to utilise the dominant model for all the variants considered, because the resulting genotype groups were the more balanced in terms of size and because resulted in far more significant associations.
The statistical power calculation was carried out with Quanto software v. 1.2.4 (University of Southern California) by testing minor allele frequencies with a type-I error of 0.05. With the available sample size, the statistical power to detect differences in the psychometric scores ranged from 0.870 to 0.945, depending on the minor allele frequencies. P values were adjusted for multiple testing by the Benjamini–Hochberg False Discovery Rate (FDR) method, considering 14 markers in the single-marker study and 12 combinations in the haplotype analysis.
Results
Patients with AN had significantly lower BMI (17.32 ± 2.07 vs. 23.39 ± 2.72), weight (44.97 ± 6.86 vs. 63.11 ± 7.68 kg), and height (1.61 ± 0.07 vs.1.64 ± 0.06 m) than healthy females. Mean ± standard deviation global psychometric scores obtained by the AN patients were EDI-2:89.37 ± 46.48, GSI: 1.56 ± 0.81, PST: 61.19 ± 21.57, and PSDI: 2.18 ± 0.59.
Patients with the restrictive (p = 159) or binge–purge (p = 74) anorexia subtypes did not show statistically significant differences in the scores obtained in the psychometric evaluation (Supplementary Table S1), and hence, the AN patients were treated as a whole group in the subsequent analyses.
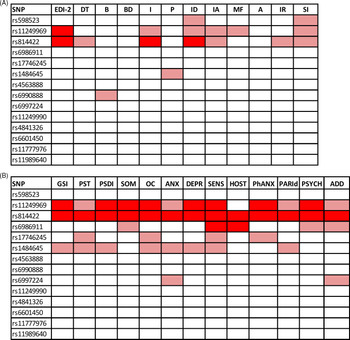
Associations between single markers with ED-related psychopathology
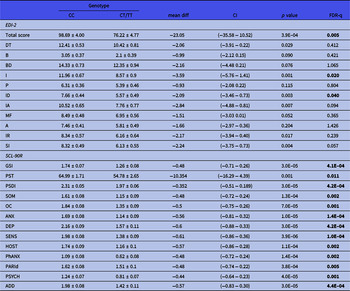
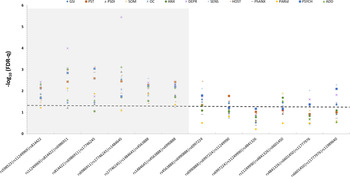
Figure 1(A) depicts the impact of MSRA tag-SNPs on the psychometric evaluation. Two polymorphisms, rs11249969 and rs814422, which were in relative LD (D’ = 0.95, r2 = 0.41), had a remarkable effect on the global score obtained by the AN patients in the psychopathology measured by the EDI-2 test. Thus, carriers of the wild-type rs11249969 GG and rs814422 CC genotypes obtained significantly higher EDI-2 scores than patients with variant alleles in these two loci. Mean (and standard error values) were 100.57 (4.68) vs. 81.84 (4.14), adjusted p value for multiple testing (FDR-q) = 0.020 for rs11249969, and 98.69 (4.00) vs. 76.22 (4.77), FDR-q = 0.040 for rs814422. The wild-type rs814422 CC genotype was also found to be related to higher scores in Ineffectiveness (FDR-q = 0.020) and Interpersonal Distrust [FDR-q = 0.040, Fig. 1(A)].

Fig. 1. Overview of the effect of MSRA tag-SNPs on the scores obtained by the Anorexia Nervosa patients in (A) the EDI-2 and (B) SCL-90R inventories. Light color represents a significant association (p < 0.05). Dark color represents a significant association after correction for multiple testing (FDR-q < 0.05). DT, Drive for thinness; B, Bulimia; BD, Body dissatisfaction; I, Inefficacy; P, Perfectionism; ID, Interpersonal Distrust; IA, Interoceptive awareness; MF, Maturity fears; A, Asceticism; IR, Impulse regulation; SI, Social insecurity; GSI, Global severity index; PST, Positive symptom; PSDI, Positive symptom distress index; SOM, Somatization; OC, Obsessive–compulsive; ANX, Anxiety; DEPR, Depression; SENS, Sensitivity; HOST, Hostility; PhANX, Phobic anxiety; PARId, Paranoid ideation; PSYCH, Psychotism; ADD, additional items.
Associations between single markers with general psychopathology
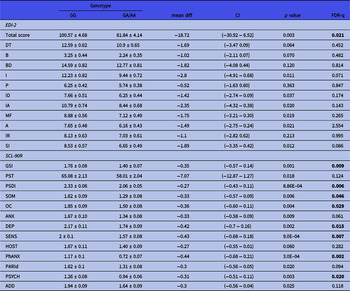
General psychopathological symptoms were assessed by the SCL-90R inventory. Three consecutive tag-SNPs, namely, rs11249969, rs814422, and rs69886911 (spanning positions 10074468 to 10121013 in the MSRA gene), were observed to significantly affect the results after correcting for multiple testing [Fig. 1(B)]. The first two SNPs had already been shown to affect EDI-2 results. The effect of the rs814422 SNP was specially marked, as carriers of the CC genotype scored higher than women with the CC/TT genotypes in all three general indices and the ten individual scales (Table 2). The effect of the rs11249969 SNP was also highly relevant as the wild-type GG genotype increased the scores of all the items, with statistically significant results in eight out of the 13 scales (Table 3). Finally, in comparison with carriers of variant alleles, those patients harbouring the rs6986911 GG wild-type genotype also scored higher in Sensitivity [2.02 (0.11) vs. 1.64 (0.08), FDR-q = 0.037] and Hostility [1.84 (0.14) vs. 1.38 (0.08), FDR-q = 0.018].
Table 2. Influence of rs814422 on EDI-2 and SCL-90R scores obtained by the patients. Mean values ± standard deviation are shown. Significant values after correction for multiple testing are shown in boldface type
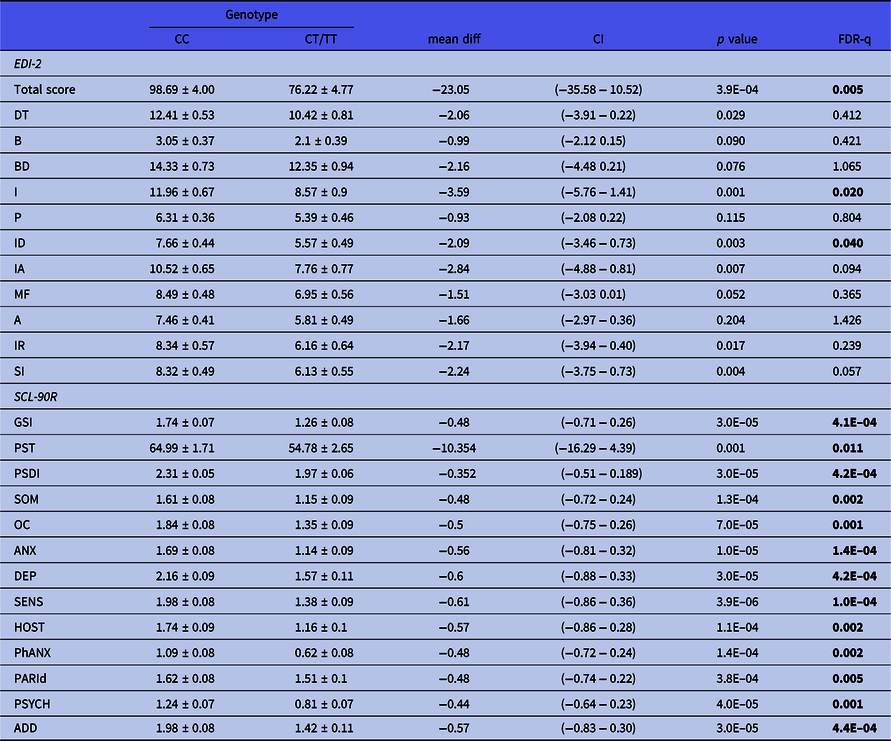
A, Asceticism; ADD, additional items; ANX, Anxiety; B, Bulimia; BD, Body dissatisfaction; DEPR, Depression; DT, Drive for thinness; GSI, Global severity index; HOST, Hostility; I, Inefficacy; IA, Interoceptive awareness; ID, Interpersonal Distrust; IR, Impulse regulation; MF, Maturity fears; OC, Obsessive–compulsive; P, Perfectionism; PARId, Paranoid ideation; PhANX, Phobic anxiety; PSDI, Positive symptom distress index; PST, Positive symptom; PSYCH, Psychotism; SENS, Sensitivity; SI, Social insecurity; SOM, Somatization.
Table 3. Influence of rs11249969 on EDI-2 and SCL-90R scores obtained by the patients. Mean values ± standard deviation are shown. Significant values after correction for multiple testing are shown in boldface type
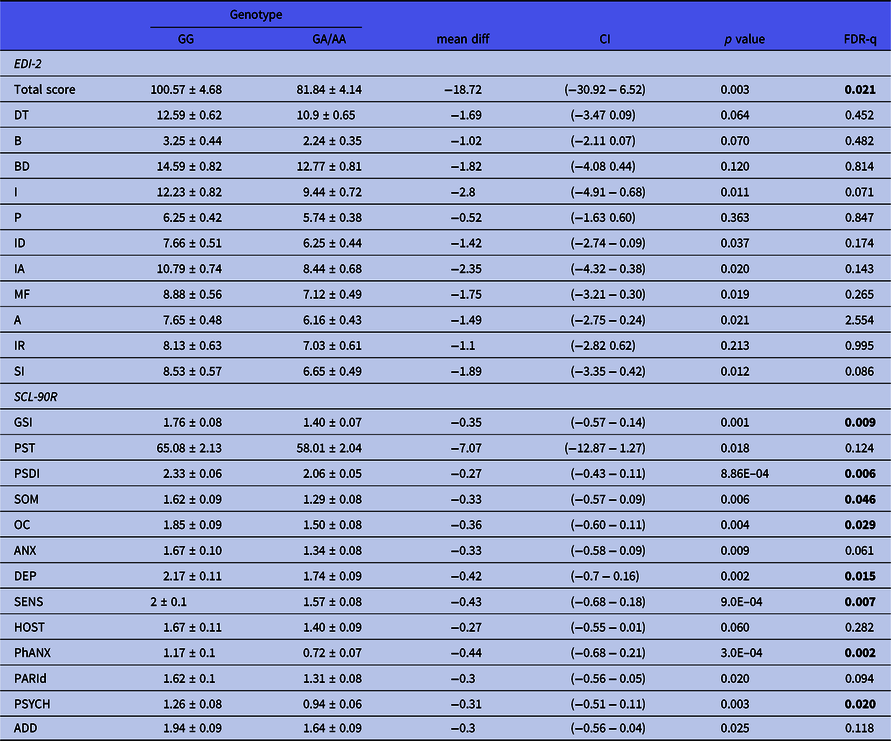
A, Asceticism; ADD, additional items; ANX, Anxiety; B, Bulimia; BD, Body dissatisfaction; DEPR, Depression; DT, Drive for thinness; GSI, Global severity index; HOST, Hostility; I, Inefficacy; IA, Interoceptive awareness; ID, Interpersonal Distrust; IR, Impulse regulation; MF, Maturity fears; OC, Obsessive–compulsive; P, Perfectionism; PARId, Paranoid ideation; PSDI, Positive symptom distress index; PST, Positive symptom; SENS, Sensitivity; SI, Social insecurity; SOM, Somatization; PhANX, Phobic anxiety; PSYCH, Psychotism.
Sliding-window analysis
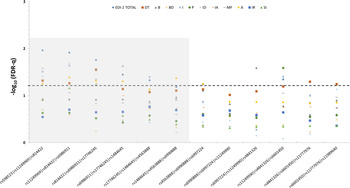
We performed a sliding-window analysis by grouping the variants into 3-SNP adjacent haplotypes and analyzing their influence on the psychometric scores. With regard to the psychopathology measured by the EDI-2 inventory, the results mirrored the results of the single-marker analysis, as the global score was the parameter showing the strongest associations, with the five first haplotypes displaying FDR-q values lower than 0.05 (Fig. 2). Furthermore, in addition to the associations with Ineffectiveness and Interpersonal distrust observed in the single-marker study, several more traits, namely, Drive for thinness, Maturity fears, Asceticism, Bulimia, and Perfectionism, also showed significant associations with at least one haplotype (Fig. 2).

Fig. 2. Summary of the sliding-window analysis for the association of 3-SNP MSRA haplotypes with the scores obtained by the Anorexia Nervosa patients on eating disorders-related psychopathology measured by the EDI-2 inventory. The dotted line represents the 0.05 adjusted q-level of significance. DT, Drive for thinness; B, Bulimia; BD, Body dissatisfaction; I, Inefficacy; P, Perfectionism; ID, Interpersonal Distrust; IA, Interoceptive awareness; MF, Maturity fears; A, Asceticism; IR, Impulse regulation; SI, Social insecurity.
The magnitude of the associations with the SCL-90R results was far greater than that resulting from the EDI-2 scores. The symptom that showed the greatest association with the MSRA haplotypes was Depression, which displayed FDR-q values of 1.03E-04 and 3.60E-06 for haplotypes rs11249969/rs814422/rs6986911 and rs6986911/rs17746245/rs1484645, respectively. In any case, all the SCL-90R scales were significantly associated with at least one but often several haplotypes (Fig. 3).

Fig. 3. Summary of the sliding-window analysis for the association of 3-SNP MSRA haplotypes with the scores obtained by the Anorexia Nervosa patients on general psychopathology measured by the SCL-90R inventory. The dotted line represents the 0.05 adjusted q-level of significance. GSI, Global severity index; PST, Positive symptom; PSDI, Positive symptom distress index; SOM, Somatization; OC, Obsessive–compulsive; ANX, Anxiety; DEPR, Depression; SENS, Sensitivity; HOST, Hostility; PhANX, Phobic anxiety; PARId, Paranoid ideation; PSYCH, Psychotism; ADD, additional items.
In general, variability in a proximal region of the MSRA gene (greyed area) spanning 187.8 Kbp (positions 10061976 to 10249860) was observed to profoundly affect the scores of several scales of the EDI-2 inventory and virtually all of the scales measured by the SCL-90R test (greyed area in Figs. 2 and 3).
Non-psychometric analyses
In contrast to the results obtained in the genetic association analyses with the psychometric scores, there were no significant results regarding the impact of MSRA tag-SNPs on anthropometric parameters, neither in patients nor in controls. Only the rs6601450 GG genotype was found to be associated with higher BMI values (17.81 ± 0.23 vs. 17.13 ± 0.17 for GT/TT genotypes, p = 0.023) in the AN group, but the association lost statistical significance after correcting for multiple comparisons. A summary of the results of the genetic association analyses with BMI, weight, and height in both patients and controls is shown in Supplementary Table S2.
Finally, risk analyses adjusted for age showed that none of the MSRA tag-SNPs or haplotypes analyzed and affected the susceptibility to AN. Odds ratio and p values for all the tag-SNPs studied are depicted in Supplementary Table S3.
Availability of data and materials: The data that support the findings of this study are openly available at Figshare, with DOI: 10.6084/m9.figshare.13621901.
Discussion
We have previously reported that variability in several genes of the central nervous system may contribute to various psychopathological comorbidities in ED (Gamero-Villarroel et al. Reference Gamero-Villarroel, Gordillo, Carrillo, Garcia-Herraiz, Flores, Jimenez, Monge, Rodriguez-Lopez and Gervasini2014; Gamero-Villarroel et al. Reference Gamero-Villarroel, Gonzalez, Gordillo, Carrillo, Garcia-Herraiz, Flores, Rodriguez-Lopez and Gervasini2015; Gervasini and Gamero-Villarroel Reference Gervasini and Gamero-Villarroel2015; Gamero-Villarroel et al. Reference Gamero-Villarroel, Gonzalez, Rodriguez-Lopez, Albuquerque, Carrillo, Garcia-Herraiz, Flores and Gervasini2017; Gervasini et al. Reference Gervasini, Gonzalez, Gamero-Villarroel, Mota-Zamorano, Carrillo, Flores and Garcia-Herraiz2018). These comorbid disorders are very frequent among AN patients and often constitute an unfavourable prognostic characteristic in this ED. Therefore, the systematic evaluation of psychopathology in these patients and the study of the factors behind these symptoms, e.g. genetics, are very important for understanding AN pathogenesis and the implementation of efficient therapies (Salbach-Andrae et al. Reference Salbach-Andrae, Lenz, Simmendinger, Klinkowski, Lehmkuhl and Pfeiffer2008).
Our results show that two tag-SNPs in the MSRA gene, rs11249969 and rs814422, significantly modified the global results obtained by the AN patients in the EDI-2 inventory, which measures ED-related psychopathology. There are not many examples in the literature of studies analyzing the influence of genetics on psychopathological symptoms in AN. Some reports have focused on the influence of dopaminergic and serotonergic genes (Rybakowski et al. Reference Rybakowski, Slopien, Dmitrzak-Weglarz, Czerski, Rajewski and Hauser2006; Bachner-Melman et al. Reference Bachner-Melman, Lerer, Zohar, Kremer, Elizur, Nemanov, Golan, Blank, Gritsenko and Ebstein2007; Steiger et al. Reference Steiger, Richardson, Schmitz, Joober, Israel, Bruce, Gauvin, Dandurand and Anestin2009; Gonzalez et al. Reference Gonzalez, Mota-Zamorano, Garcia-Herraiz, Lopez-Nevado and Gervasini2021), whilst others have looked into hypothalamic genes, such as BDNF, TFAP2B, or KCTD15 (Rybakowski et al. Reference Rybakowski, Dmitrzak-Weglarz, Szczepankiewicz, Skibinska, Slopien, Rajewski and Hauser2007; Gervasini & Gamero-Villarroel Reference Gervasini and Gamero-Villarroel2015; Gamero-Villarroel et al. Reference Gamero-Villarroel, Gonzalez, Rodriguez-Lopez, Albuquerque, Carrillo, Garcia-Herraiz, Flores and Gervasini2017). One GWAS aimed to discover associations with ED-related symptoms, behaviours, and traits also found several variants that showed suggestive evidence for association (Boraska et al. Reference Boraska, Davis, Cherkas, Helder, Harris, Krug, Liao, Treasure, Ntalla, Karhunen, Keski-Rahkonen, Christakopoulou, Raevuori, Shin, Dedoussis, Kaprio, Soranzo, Spector, Collier and Zeggini2012). To the best of our knowledge, there are no previous studies on the involvement of MSRA in ED, let alone studies dealing with associated psychopathology. There are, however, some examples in the general population that reveal associations between MSRA and symptoms that are relevant in ED. For example, several GWAS have identified MSRA variants solidly associated with irritability (Day et al. Reference Day, Helgason, Chasman, Rose, Loh, Scott, Helgason, Kong, Masson, Magnusson, Gudbjartsson, Thorsteinsdottir, Buring, Ridker, Sulem, Stefansson, Ong and Perry2016) and neuroticism (Okbay et al. Reference Okbay, Baselmans, De Neve, Turley, Nivard, Fontana, Meddens, Linner, Rietveld, Derringer, Gratten, Lee, Liu, de Vlaming, Ahluwalia, Buchwald, Cavadino, Frazier-Wood, Furlotte, Garfield, Geisel, Gonzalez, Haitjema, Karlsson, van der Laan, Ladwig, Lahti, van der Lee, Lind, Liu, Matteson, Mihailov, Miller, Minica, Nolte, Mook-Kanamori, van der Most, Oldmeadow, Qian, Raitakari, Rawal, Realo, Rueedi, Schmidt, Smith, Stergiakouli, Tanaka, Taylor, Thorleifsson, Wedenoja, Wellmann, Westra, Willems, Zhao, Amin, Bakshi, Bergmann, Bjornsdottir, Boyle, Cherney, Cox, Davies, Davis, Ding, Direk, Eibich, Emeny, Fatemifar, Faul, Ferrucci, Forstner, Gieger, Gupta, Harris, Harris, Holliday, Hottenga, De Jager, Kaakinen, Kajantie, Karhunen, Kolcic, Kumari, Launer, Franke, Li-Gao, Liewald, Koini, Loukola, Marques-Vidal, Montgomery, Mosing, Paternoster, Pattie, Petrovic, Pulkki-Raback, Quaye, Raikkonen, Rudan, Scott, Smith, Sutin, Trzaskowski, Vinkhuyzen, Yu, Zabaneh, Attia, Bennett, Berger, Bertram, Boomsma, Snieder, Chang, Cucca, Deary, van Duijn, Eriksson, Bultmann, de Geus, Groenen, Gudnason, Hansen, Hartman, Haworth, Hayward, Heath, Hinds, Hypponen, Iacono, Jarvelin, Jockel, Kaprio, Kardia, Keltikangas-Jarvinen, Kraft, Kubzansky, Lehtimaki, Magnusson, Martin, McGue, Metspalu, Mills, de Mutsert, Oldehinkel, Pasterkamp, Pedersen, Plomin, Polasek, Power, Rich, Rosendaal, den Ruijter, Schlessinger, Schmidt, Svento, Schmidt, Alizadeh, Sorensen, Spector, Starr, Stefansson, Steptoe, Terracciano, Thorsteinsdottir, Thurik, Timpson, Tiemeier, Uitterlinden, Vollenweider, Wagner, Weir, Yang, Conley, Smith, Hofman, Johannesson, Laibson, Medland, Meyer, Pickrell, Esko, Krueger, Beauchamp, Koellinger, Benjamin, Bartels and Cesarini2016; Luciano et al. Reference Luciano, Hagenaars, Davies, Hill, Clarke, Shirali, Harris, Marioni, Liewald, Fawns-Ritchie, Adams, Howard, Lewis, Gale, McIntosh and Deary2018; Chu et al. Reference Chu, Liu, Wen, Li, Cheng, Cheng, Zhang, Mei, Qi, Liang, Ye, Kafle, Wu, Wang, Wang, Ning and Zhang2020). Moreover, a replication study analyzing 16 features in over 140 000 participants also demonstrated significant effects of MSRA variants on neuroticism and extraversion (Boutwell et al. Reference Boutwell, Hinds, Tielbeek, Ong, Day and Perry2017). The findings presented herein show that, aside from the impact on the global EDI-2 scores, and after correction for multiple testing, rs814422 was still significantly associated with Ineffectiveness, which assesses feelings of inadequacy, insecurity and worthlessness, and Interpersonal Distrust. Again, there are no previous data linking MSRA with ED-related psychological comorbidities, but a number of studies in the ED setting have reported these two psychological features being modulated by other genes expressed in the brain (Kamakura et al. Reference Kamakura, Ando, Ono and Maekawa2003; Nisoli et al. Reference Nisoli, Brunani, Borgomainerio, Tonello, Dioni, Briscini, Redaelli, Molinari, Cavagnini and Carruba2007; Gonzalez et al. Reference Gonzalez, Mota-Zamorano, Garcia-Herraiz, Lopez-Nevado and Gervasini2021). The effect on Ineffectiveness in particular has been suggested to account for the initial stimulus to take up dieting behaviour, which could evolve to AN should other pathological traits be present (Nisoli et al. Reference Nisoli, Brunani, Borgomainerio, Tonello, Dioni, Briscini, Redaelli, Molinari, Cavagnini and Carruba2007).
The most relevant findings in this work have to do with the general psychopathology assessed by the SCL-90R inventory. It is true that causality has not unequivocally been established yet, but psychiatric comorbidities can be as high as 80% in AN (Hudson et al. Reference Hudson, Hiripi, Pope and Kessler2007; Jaite et al. Reference Jaite, Hoffmann, Glaeske and Bachmann2013; Meczekalski et al. Reference Meczekalski, Podfigurna-Stopa and Katulski2013), with anxiety and depression being the most commonly reported disorders (Preti et al. Reference Preti, Girolamo, Vilagut, Alonso, Graaf, Bruffaerts, Demyttenaere, Pinto-Meza, Haro and Morosini2009; Jaite et al. Reference Jaite, Hoffmann, Glaeske and Bachmann2013). In our population of AN patients, rs11249969 and rs814422, which, interestingly enough, were the same SNPs that modified the EDI-2 results, displayed a profound effect on the scores obtained by the patients in the SCL-90R questionnaire. Most remarkably, the rs814422 CC genotype increased the scores of all the items with statistical significance after correcting for multiple testing. Affected symptoms included, for instance, Depression, a comorbidity with predictive value of AN outcome (Eskild-Jensen et al. Reference Eskild-Jensen, Stoving, Flindt and Sjogren2020; Kahn et al. Reference Kahn, Brunstein-Klomek, Hadas, Snir and Fennig2020); Anxiety, whose prevalence is markedly higher in AN patients as compared to control subjects (Swinbourne & Touyz Reference Swinbourne and Touyz2007; Lloyd et al. Reference Lloyd, Sallis, Verplanken, Haase and Munafo2020); or obsessive–compulsive, a disorder with a considerable overlap with AN that may reflect common psychological, neurobiological, or genetic factors (Serpell et al. Reference Serpell, Livingstone, Neiderman and Lask2002; Yilmaz et al. Reference Yilmaz, Halvorsen, Bryois, Yu, Thornton, Zerwas, Micali, Moessner, Burton, Zai, Erdman, Kas, Arnold, Davis, Knowles, Breen, Scharf, Nestadt, Mathews, Bulik, Mattheisen and Crowley2020). Finally, we should not rule out the possibility that, being rs11249969 and rs814422 in relative linkage disequilibrium (LD), the observed associations might in fact reflect the influence of a single major haplotype.
The sliding-window study confirmed that the results obtained in the single-marker analysis and showed that the variability of a proximal region of the gene was able to markedly modify the psychometric scores obtained by the AN patients. Of special interest were the associations with the symptoms measured by SCL-90R, particularly with depression, which showed two highly significant hits with MSRA haplotypes. This finding is in the same line as those by Chu et al., who recently published a GWAS reporting several MSRA variants related to depressive symptoms in the general population (Chu et al. Reference Chu, Liu, Wen, Li, Cheng, Cheng, Zhang, Mei, Qi, Liang, Ye, Kafle, Wu, Wang, Wang, Ning and Zhang2020). In this regard, the increase of reactive oxygen species (ROS) in the brain has been solidly connected with depression (Bhatt et al. Reference Bhatt, Nagappa and Patil2020). A disruption of MsrA enzyme function, which is part of the antioxidant defence system in the brain (Reiterer et al. Reference Reiterer, Schmidt-Kastner and Milton2019), might, therefore, contribute, at least in part, to the disorder. Indeed, this decreased function has been associated with a variety of psychiatric disorders (Jiang & Moskovitz Reference Jiang and Moskovitz2018). It should be mentioned, though, that the tag-SNPs analyzed in this study were all intronic, hence with no likely relevant consequences for enzyme activity. Their value lies on their role as representative markers of a region in high LD in which relevant mutations must be located.
Finally, we could not find a significant effect of any of the MSRA variants or haplotypes on the anthropometric parameters of AN patients or healthy females. This is in agreement with former studies (Hotta et al. Reference Hotta, Nakamura, Nakamura, Matsuo, Nakata, Kamohara, Miyatake, Kotani, Komatsu, Itoh, Mineo, Wada, Yoneda, Nakajima, Funahashi, Miyazaki, Tokunaga, Kawamoto, Masuzaki, Ueno, Hamaguchi, Tanaka, Yamada, Hanafusa, Oikawa, Yoshimatsu, Nakao, Sakata, Matsuzawa, Nakamura and Kamatani2010; Gonzalez et al. Reference Gonzalez, Estevez, Giralt, Caceres, Perez, Gonzalez-Carpio, Ballester, Sunyer and Rodriguez-Lopez2014; Volckmar et al. Reference Volckmar, Han, Putter, Haas, Vogel, Knoll, Struve, Gobel, Haas, Herrfurth, Jarick, Grallert, Schurmann, Al-Hasani, Hebebrand, Sauer and Hinney2016) that could not replicate the alleged MSRA-BMI connection described in some GWAS (Lindgren et al. Reference Lindgren, Heid, Randall, Lamina, Steinthorsdottir, Qi, Speliotes, Thorleifsson, Willer, Herrera, Jackson, Lim, Scheet, Soranzo, Amin, Aulchenko, Chambers, Drong, Luan, Lyon, Rivadeneira, Sanna, Timpson, Zillikens, Zhao, Almgren, Bandinelli, Bennett, Bergman, Bonnycastle, Bumpstead, Chanock, Cherkas, Chines, Coin, Cooper, Crawford, Doering, Dominiczak, Doney, Ebrahim, Elliott, Erdos, Estrada, Ferrucci, Fischer, Forouhi, Gieger, Grallert, Groves, Grundy, Guiducci, Hadley, Hamsten, Havulinna, Hofman, Holle, Holloway, Illig, Isomaa, Jacobs, Jameson, Jousilahti, Karpe, Kuusisto, Laitinen, Lathrop, Lawlor, Mangino, McArdle, Meitinger, Morken, Morris, Munroe, Narisu, Nordstrom, Nordstrom, Oostra, Palmer, Payne, Peden, Prokopenko, Renstrom, Ruokonen, Salomaa, Sandhu, Scott, Scuteri, Silander, Song, Yuan, Stringham, Swift, Tuomi, Uda, Vollenweider, Waeber, Wallace, Walters, Weedon, Witteman, Zhang, Zhang, Caulfield, Collins, Davey Smith, Day, Franks, Hattersley, Hu, Jarvelin, Kong, Kooner, Laakso, Lakatta, Mooser, Morris, Peltonen, Samani, Spector, Strachan, Tanaka, Tuomilehto, Uitterlinden, van Duijn, Wareham, Hugh, Waterworth, Boehnke, Deloukas, Groop, Hunter, Thorsteinsdottir, Schlessinger, Wichmann, Frayling, Abecasis, Hirschhorn, Loos, Stefansson, Mohlke, Barroso and McCarthy2009; Scherag et al. Reference Scherag, Dina, Hinney, Vatin, Scherag, Vogel, Muller, Grallert, Wichmann, Balkau, Heude, Jarvelin, Hartikainen, Levy-Marchal, Weill, Delplanque, Korner, Kiess, Kovacs, Rayner, Prokopenko, McCarthy, Schafer, Jarick, Boeing, Fisher, Reinehr, Heinrich, Rzehak, Berdel, Borte, Biebermann, Krude, Rosskopf, Rimmbach, Rief, Fromme, Klingenspor, Schurmann, Schulz, Nothen, Muhleisen, Erbel, Jockel, Moebus, Boes, Illig, Froguel, Hebebrand and Meyre2010; Dorajoo et al. Reference Dorajoo, Blakemore, Sim, Ong, Ng, Seielstad, Wong, Saw, Froguel, Liu and Tai2012). In this regard, Scherag et al. showed that at least two of these MSRA loci identified as BMI regulators in adolescents were in fact located between the MSRA and the TNKS gene (Scherag et al. Reference Scherag, Dina, Hinney, Vatin, Scherag, Vogel, Muller, Grallert, Wichmann, Balkau, Heude, Jarvelin, Hartikainen, Levy-Marchal, Weill, Delplanque, Korner, Kiess, Kovacs, Rayner, Prokopenko, McCarthy, Schafer, Jarick, Boeing, Fisher, Reinehr, Heinrich, Rzehak, Berdel, Borte, Biebermann, Krude, Rosskopf, Rimmbach, Rief, Fromme, Klingenspor, Schurmann, Schulz, Nothen, Muhleisen, Erbel, Jockel, Moebus, Boes, Illig, Froguel, Hebebrand and Meyre2010). It could, therefore, be that the association with BMI was more related to TNKS; in this case, our study, focused on MSRA tag-SNPs, would have been unable to detect it.
In the same manner, we could not identify any MSRA variant or haplotype associated with the risk for AN. In our former association studies carried on in the ED setting, we have persistently observed how genetic variability in obesity-related central genes has a negligible-to-weak effect on the risk for the disorder. In contrast, we could generally confirm a remarkable impact on the psychopathology displayed by the patients (Gamero-Villarroel et al. Reference Gamero-Villarroel, Gordillo, Carrillo, Garcia-Herraiz, Flores, Jimenez, Monge, Rodriguez-Lopez and Gervasini2014; Gamero-Villarroel et al. Reference Gamero-Villarroel, Gonzalez, Gordillo, Carrillo, Garcia-Herraiz, Flores, Rodriguez-Lopez and Gervasini2015; Gamero-Villarroel et al. Reference Gamero-Villarroel, Gonzalez, Rodriguez-Lopez, Albuquerque, Carrillo, Garcia-Herraiz, Flores and Gervasini2017). This may suggest that the affected symptoms are likely more important in terms of the severity or persistence of the ED than in terms of susceptibility, where environmental or socio-cultural factors might play a more relevant role (Gervasini & Gamero-Villarroel Reference Gervasini and Gamero-Villarroel2015).
This study has some limitations. First, the sample size generated adequate statistical power to detect differences in the psychometric scores, but the inclusion of more subjects would have been desirable in the case–control analysis to unequivocally rule out an impact on AN risk. Second, the age of the control subjects was significantly higher than that of the AN patients. This obviously did not affect the genetic analyses, but could have partly contributed to the differences in the anthropological parameters reported for the two study groups. Finally, the DNA samples of the control subjects were obtained from an anonymous DNA bank; therefore, psychometric scores were not available for this group. Whilst the main goal of the study, i.e. the assessment of the association of MSRA genetic variability with psychopathology in AN, remained unaltered, having access to these data would have provided with additional, interesting information on the psychological differences between AN patients and healthy women.
This study adds up to the growing evidence that points to genetics as a key factor in the onset or maintenance of psychopathology in ED patients. It is crucial to improve our understanding of these symptoms in AN, as it will help identify at-risk individuals, adopt prevention-oriented solutions and individualise therapies focusing on key elements underlying the ED (Dufresne et al. Reference Dufresne, Bussieres, Bedard, Gingras, Blanchette-Sarrasin and Begin2020). In this work, we have shown, for the first time to the best of our knowledge, that variability in the MSRA gene locus is associated with changes in the psychometric scores of AN patients. In particular, psychological symptoms such as depression are specially affected. A proximal region of the gene seems to be the locus more intimately related to these changes.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/neu.2021.24
Acknowledgements
We would like to thank the DNA repository of the Instituto de Salud Carlos III (www.bancoadn.org) for providing with genetic material from healthy individuals and the members of the Centro Nacional de Genotipado-Instituto de Salud Carlos III (CEGen; www.cegen.org) for their technical assistance.
Authors’ contributions
Guillermo Gervasini designed the study, material preparation was carried out by David Albuquerque and Sonia Mota-Zamorano, data collection was performed by Isalud Flores and Angustias García-Herráiz, and analyses were performed by Luz M González and Guillermo Gervasini. The first draft of the manuscript was written by Guillermo Gervasini, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Financial support
This work has been supported in part by a grant from the Alicia Koplowitz Foundation and by grant GR18007 from Junta de Extremadura, Mérida (Spain) and Fondo Europeo de Desarrollo Regional (FEDER) “Una manera de hacer Europa”. The sponsors did not have any role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Conflict of interest
None.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.