There are an estimated 700 000 people with dementia in the UK, of whom 400 000 have Alzheimer's disease (The Alzheimer's Disease Society, 2001). Incidence is age dependent, hence current trends in demographic change are likely to result in an increase in the prevalence of the disease and an impact on the overall cost of care. A number of estimates of the costs of caring for dementia in England have been made (Reference Gray and FennGray & Fenn, 1993; Reference Souetre, Qing and VigoureuxSouetre et al, 1995; Reference Bosanquet, May and JohnsonBosanquet et al, 1998) but these have been based on prevalence data and relatively unsophisticated averaging. Little is known of the longitudinal costs of dementia for individuals or groups of patients. These costs depend on two important factors: the setting in which care is given and the progression of dementia, including the range of cognitive and behavioural problems. Several published studies (Reference Ernst, Hay and FennErnst et al, 1997; Reference Hux, Bj and IskedjianHux et al, 1998; Reference Jonsson, Lindgren and WimoJonsson et al, 1999; Kavanagh & Knapp, Reference Kavanagh and Knapp1999, Reference Kavanagh and Knapp2002; Reference McNamee, Gregson and BuckMcNamee et al, 1999; Reference Souetre, Thwaites and YeardleySouetre et al, 1999) have examined the relationship between cognitive function and patient costs but none of these go beyond using cross-sectional analysis and only two of the studies considered behavioural or daily living aspects of disease progression (Reference McNamee, Gregson and BuckMcNamee et al, 1999; Reference Kavanagh and KnappKavanagh & Knapp, 2002).
The objectives of this study were to explore the factors affecting time to institutionalisation and to estimate the relationship between the costs of care and disease progression, measured by cognitive function, behavioural scores and activities of daily living (ADL), using retrospective analysis of a longitudinal data-set for a cohort of 100 patients diagnosed with Alzheimer's disease and/or vascular dementia.
METHOD
Setting and participants
The data for this study were extracted retrospectively from the research data of 100 subjects (51% male) with a clinical diagnosis of dementia recruited to a prospective longitudinal study of behaviour in dementia (Hope et al, Reference Hope, Keene and Fairburn1997a, Reference Hope, Keene and Fairburnb). The diagnosis comprised 51% Alzheimer's disease, 6% Alzheimer's disease/vascular dementia and 2% vascular dementia from pathological diagnosis and 28% Alzheimer's disease, 7% Alzheimer's disease/vascular dementia, 1% vascular dementia and 5% other types of dementia from clinical diagnosis. They were recruited to the study through local general practitioners (GPs), community psychiatric nurses (CPNs) and consultant geriatricians. At the start of the study, the subjects were all living at home with a carer who was able to give detailed information about the subject. All subjects lived in Oxfordshire, UK. The subjects were representative of the general population with regard to the distribution of social class by occupation and their diagnosis (Reference Hope, Keene and FairburnHope et al, 1997b). At four-monthly intervals the subjects were assessed in terms of their cognition and the carers were interviewed about the subjects' behaviour, ADL and all health, social and long-term care services used. Additional information on the carer's attitude to caring and physical ability to cope was collected at the beginning of the study. The date of the first interviews ranged from February 1988 to May 1989. The maximum number of interviews was 33, with the final interview for the final subject taking place in August 1999 (see Fig. 1). This enabled analysis over an 11-year period.
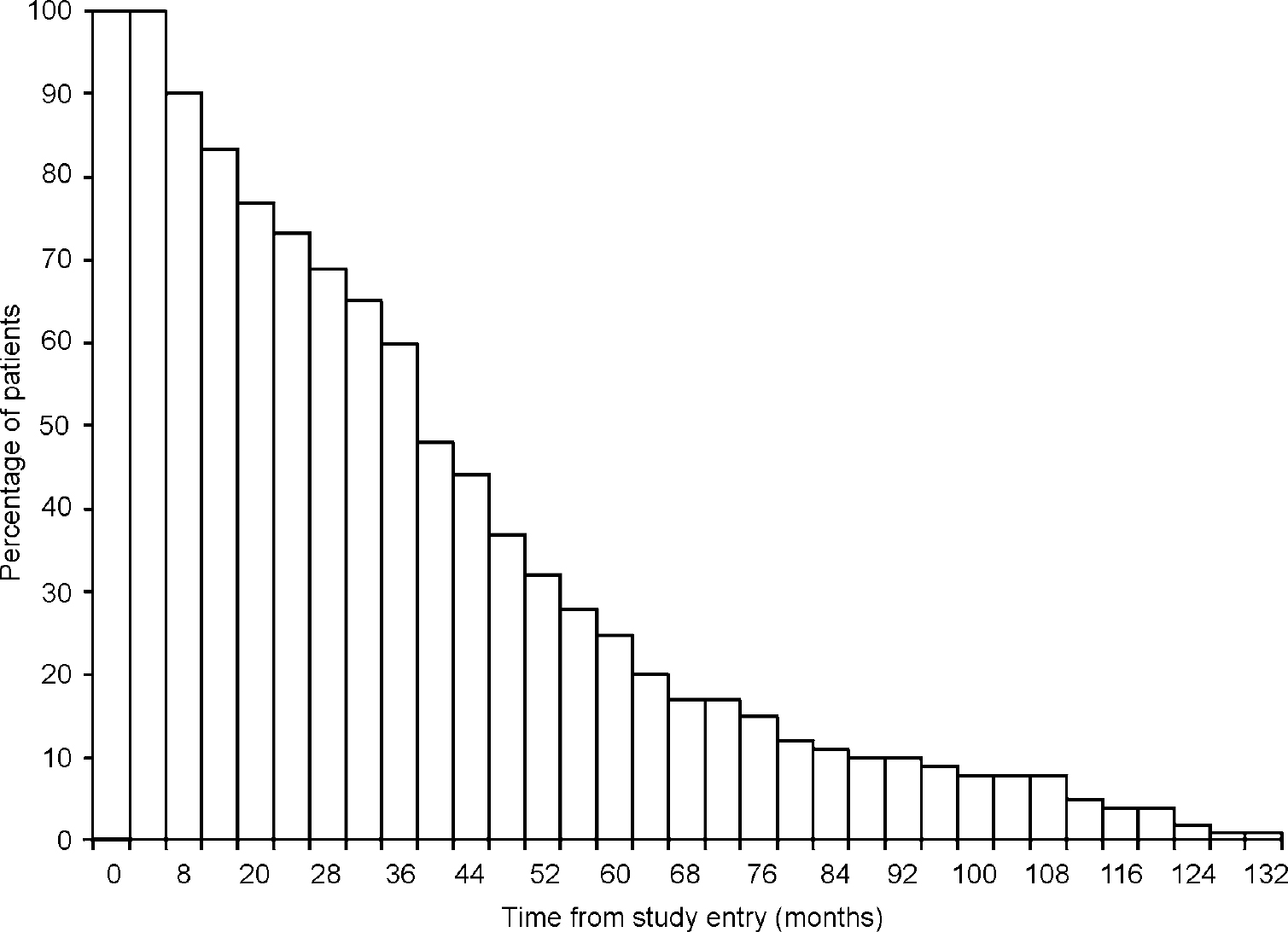
Fig. 1 Percentage of patients available at interview.
Out of the 100 subjects interviewed at study entry, six withdrew from the study and three were still alive at August 1999. The mean age at study entry was 78 years (s.d.=7.0) but, given that all subjects had already been diagnosed with dementia when recruited to the study, the mean age at onset was 73 years (s.d.=7.5).
Resource use and unit costs
A coding frame was designed and information on resource utilisation was extracted from each subject's four-monthly assessment records on the following: number and duration of acute hospitalisations and respite care; number of out-patient visits, day care and home attendances by district nurses, CPNs, home helps or other care assistants; and number of visits by or to the GP or practice nurse. Details on the use of special aids and adaptations such as wheelchairs, bath/bed hoists, incontinence pads and sheets and any special dietary requirements were also recorded. An important aspect of care for a patient with dementia is where the care took place. At each interview it was noted whether the subject still resided at home or had been institutionalised. The point at which subjects were rated as being ‘institutionalised’ was taken as the time when they were admitted to a hospital ward or a nursing home for permanent care (Reference Hope, Keene and FairburnHope et al, 1998).
Unit costs were attached to these cost-generating events (see Table 1), enabling an estimation of patient-specific costs of dementia by the four-month period from study entry to death or censor point. Where appropriate, the unit costs of all hospital admissions and out-patient visits were based on information from financial returns for the specified trusts. The unit costs of home attendances by district nurses, CPNs, home helps or other care assistants and visits by or to the GP or practice nurse were taken from previously published sources (Netten et al, Reference Netten, Dennet and Knight1998, Reference Netten, Dennet and Knight1999). The market price of equipment, consumables and non-structural home modifications was used. Residential and nursing home care costs were based on actual costs of care in each facility. All unit costs were updated to 1998 prices and are reported in UK £ sterling.
Table 1 Unit costs and sources of information

| Type of service | Unit of service | Source | 1998 prices | |
|---|---|---|---|---|
| Hospital admissions1 | General surgery | Per in-patient stay | TFR returns | £ 225.77-285.78 |
| General medicine | Per in-patient stay | TFR returns | £ 125.09-256.28 | |
| Geriatrics | Per in-patient stay | TFR returns | £ 120.01-157.64 | |
| Psychiatric | Per in-patient stay | TFR returns | £ 463.75 | |
| Out-patient visit1 | Per out-patient visit | TFR returns | £ 81.36-110.85 | |
| Psychiatrist | Domiciliary visit | Per hour of patient contact | Netten et al(Reference Netten, Dennet and Knight1999) | £ 238.00 |
| GP | Surgery visit | Per visit (8.4 min) | Netten et al(Reference Netten, Dennet and Knight1998) | £ 14.00 |
| Domiciliary visit | Per visit (13.2 min+12 min travel) | Netten et al(Reference Netten, Dennet and Knight1998) | £ 46.00 | |
| Domiciliary visit, emergency call | Per visit (13.2 min+12 min travel) | Netten et al(Reference Netten, Dennet and Knight1998) | £ 47.00 | |
| GP telephone | 10.8 min | Netten et al(Reference Netten, Dennet and Knight1998) | £ 17.00 | |
| Practice nurse | Surgery visit | Per consultation | Netten et al(Reference Netten, Dennet and Knight1998) | £ 7.29 |
| Respite care | Community hospital | Per in-patient day | TFR returns | £ 125.09 |
| Teaching hospital wards | Per in-patient day | TFR returns | £ 125.09 | |
| Private nursing home | Per in-patient day (based on care package of short-term resident week) | Netten et al(Reference Netten, Dennet and Knight1999) | £ 55.70 | |
| Day care | Per hour | Netten et al(Reference Netten, Dennet and Knight1999) | £ 4.75 | |
| Domiciliary help | District nurse | Per domiciliary visit | Netten et al(Reference Netten, Dennet and Knight1998) | £ 15.00 |
| Chiropodist | Per domiciliary visit | Netten et al(Reference Netten, Dennet and Knight1999) | £ 15.00 | |
| Care assistant | Per domiciliary visit (2-hr session) | CIPFA | £ 25.00 | |
| CPN | Per domiciliary visit | Netten et al(Reference Netten, Dennet and Knight1998) | £ 20.00 | |
| Other helper (e.g. OT, physiotherapist) | Per domiciliary visit | Netten et al(Reference Netten, Dennet and Knight1998) | £ 35.00 | |
| Aids and adaptations | Wheelchair, walking frame, hoist | Per day | Netten et al(Reference Netten, Dennet and Knight1998) | £ 0.40 |
| Incontinence pads | Per pad | Personal communication2 | £ 0.25 | |
| Kylie/incontinence sheets | Per sheet | Personal communication2 | £ 0.75 | |
| Enema | Per enema | Personal communication2 | £ 3.00 | |
| Accommodation | Private household | Per day | Netten (Reference Netten1990) | £ 31.42-48.64 |
| Warden-controlled housing | Per day | Netten et al(Reference Netten, Dennet and Knight1998) | £ 17.29-37.71 | |
| Residential home2 | Per day | Personal communication2(4 homes) | £ 31.42-61.43 | |
| Nursing home2 | Per day | Personal communication2(9 nursing homes) | £ 54.42-82.14 |
Indicators of disease progression
As part of the original cohort study, data were collected every 4 months on the cognitive, behavioural and functional abilities of the patients using the Mini-Mental State Examination (MMSE; Reference Folstein, Folstein and McHughFolstein et al, 1975) and the Present Behavioural Examination (PBE; Reference Hope and FairburnHope & Fairburn, 1992). The MMSE score ranges from 0 to 30, with a score of zero indicating the severest loss of cognitive function. The behavioural and functional data collected using the PBE questionnaire were transcribed onto the Barthel ADL index (Reference Wade and CollinWade & Collin, 1988). For the purpose of this paper it was felt that it was better to transcribe the data collected using the PBE to the Barthel index owing to the complexity of the PBE, the greater familiarity with the Barthel index, to enable comparisons with other studies and the fact that the Barthel index is widely used in long-term care insurance. This index measures functional capabilities such as bowel and bladder continence, toilet use, bathing, feeding, grooming, dressing, mobility and ability to cope with stairs. The score ranges from 0 to 20, again with zero indicating the greatest impairment. The transformed data were believed to have good validity, because the information required to complete the Barthel index was much less than the information collected by the PBE. Two other variables measuring degrees of aggressive behaviour and wakefulness, which are dimensions of behaviour not included in the MMSE or Barthel index, were obtained from the PBE questionnaire and included in the analysis.
Data analysis
The data were entered into SPSS version 10, and primary analysis of the total cost per patient over the whole period was conducted. One-way analysis of variance was used to explore the difference in costs between disease severity scores for the MMSE and Barthel index. For the purpose of further analysis using longitudinal and survival and analysis techniques, the data were transferred to another statistical software package: Stata version 6.
Variations in the measured aggregate cost per four-month period and the impact of covariates such as age, measures of disease progression and care regime were explored using a fixed-effects regression model (Reference GreeneGreene, 1999). In this model, consistent patient-specific differences in costs that are not explained by the covariates are estimated through the regression constants (the ‘fixed effects’) rather than absorbed into the residual. The second analytical technique used in this paper to explore the relationship between time to institutionalisation and covariates such as age, gender, disease progression and domestic circumstances is the Cox proportional hazards regression (Reference Cox and OakesCox & Oakes, 1984). This technique is used to analyse time-to-event data. In this paper it explores the impact of the (potentially time-varying) covariates described above on the hazard of institutionalisation.
RESULTS
Costs
The total cost per patient over the course of the study (mean follow-up was 40 months, range 1-132) averaged £66 697 (s.d.=60 249). Figure 2 shows that institutional care, comprising long-stay nursing home, residential home and long-stay hospitalisations, represents a major component (69%) of the total cost, whereas respite care accounts for 15% of the total cost.
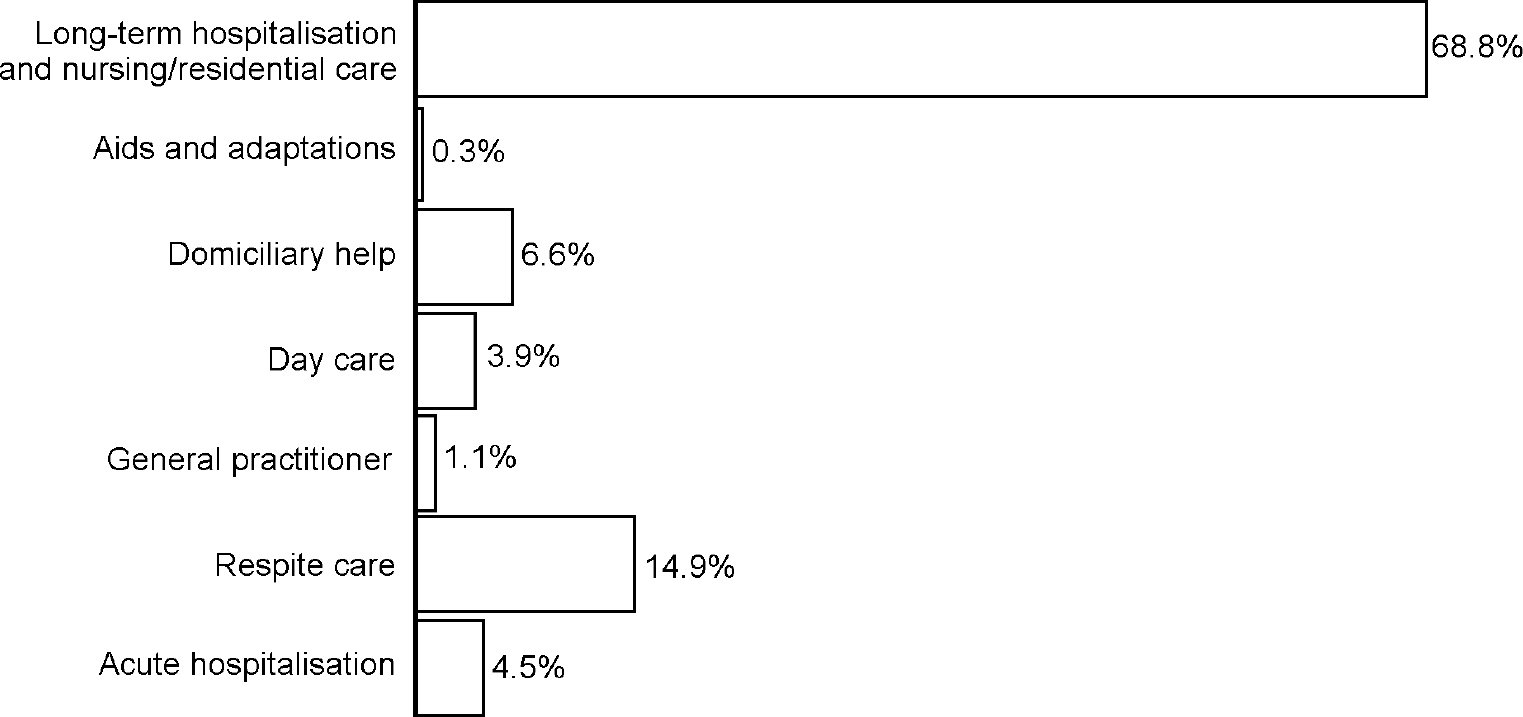
Fig. 2 Components of total cost.
The relationship between the cognitive, functional and behavioural capabilities of the patients and the costs incurred caring for the patients was explored. The MMSE and Barthel scores were separated into disease severity classifications that had been used previously in studies exploring the relationship between cost of disease and disease progression (Reference Hux, Bj and IskedjianHux et al, 1998; Reference Porsdal and BoysenPorsdal & Boysen, 1999). The MMSE score was divided into severe (<10), moderate (10-14), mild to moderate (15-20) and mild (>20) and the Barthel score into severe (0-9), moderate (10-14), slight (15-19) and no disability (20). Figure 3 shows how the average annual cost of care per patient increases significantly with severity of dementia. Focusing on cognition as measured by the MMSE, the annual cost per patient was estimated to be £8312 (s.d.=5602) for mild disease, £ 11 643 (s.d.=7808) for mild to moderate disease, £15 681 (s.d.=9509) for moderate disease and £22 267 (s.d.=14 507) for severe disease (one-way analysis of variance: F=23.17, P<0.001). On the Barthel ADL scale, average annual care cost per patient ranged from £8086 (s.d.=3556) for no disability, £12 752 (s.d.=7632) for slight disability, £23 240 (s.d.=15 638) for moderate disability to £23 516 (s.d.=13 253) for severe disability (one-way analysis of variance: F=38.72, P<0.001). The cost information reported here is on an annual basis. Elsewhere in this paper the costs are based on a four-month period.
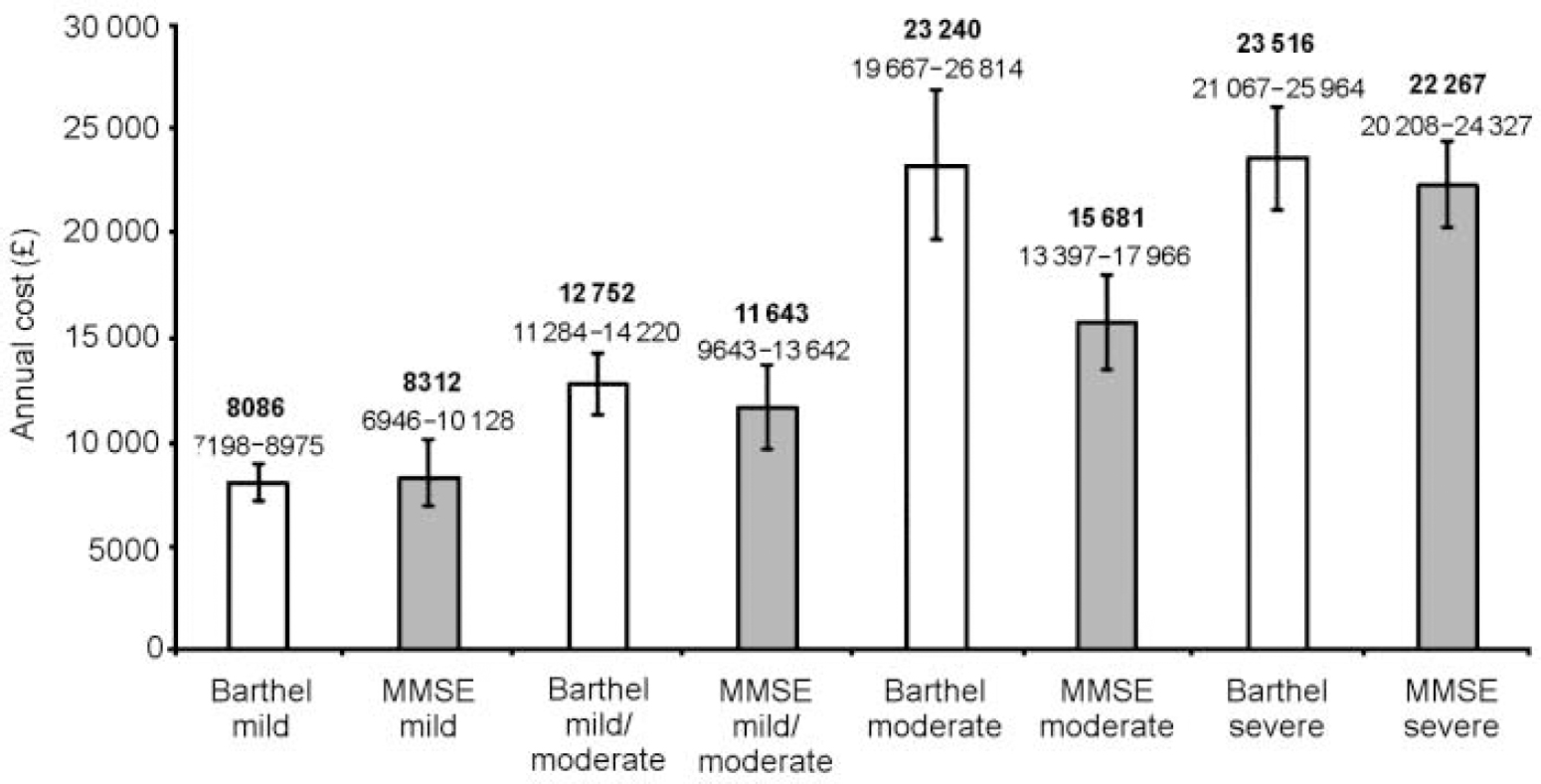
Fig. 3 Mean annual cost of care by Mini-Mental State Examination (MMSE) and Barthel severity scores with 95% confidence intervals.
Cost per period
Given that the data were collected on a longitudinal basis, we explored the impact of the different variables on the total cost of care over time using longitudinal data methods. A list of the variables used in the analyses and their descriptions is displayed in Table 2. The results from the fixed-effects regression model are reported in Table 3. The model is specified so that age, MMSE and Barthel covariates are interacted with a dummy variable indicating whether the patient was in long-term institutional care (Ii=1) or not (Ii=0). The results are discussed for each covariate in turn.
Table 2 Description of variables used in the analyses

| Variable | Description | Frequency (n= 1094) | Mean | s.d. |
|---|---|---|---|---|
| Inst | Institutionalised=1 | 400 | ||
| Not institutionalised=0 | 694 | |||
| age | Patient age | 1092 | 79 | 6.58 |
| mmse | MMSE score, range 0-30 | 1075 | 8.71 | 8.79 |
| barthel | Barthel score, range 0-20 | 1094 | 12.25 | 6.35 |
| gende_0 | Gender=male | 495 | ||
| gende_1 | Gender=female | 599 | ||
| livin_0 | Living alone | 47 | ||
| livin_1 | Living with spouse | 699 | ||
| livin_2 | Living with spouse and others | 74 | ||
| livin_3 | Living with family (excluding spouse) | 214 | ||
| livin_4 | Living with others (not family) | 58 | ||
| care_1 | Resents having to care for patient | 5 | ||
| care_2 | Caring because they feel they ought to | 10 | ||
| care_3 | No resentment to caring | 85 | ||
| cope1 | Not coping with caring | 0 | ||
| cope2 | Is coping but only just | 12 | ||
| cope3 | Moderately fit and able to do all that is necessary | 27 | ||
| cope4 | Physically fit and able to cope easily | 61 | ||
| wake_1 | Wakeful at night due to passing urine | |||
| 1=mild (≤2 times per night) | 601 | |||
| 2=severe (>2 times per night) | 462 | |||
| wake_2 | Wakeful for other reasons | |||
| 1=mild (≤2 times per night) | 193 | |||
| 2=severe (>2 times per night) | 84 | |||
| agg_1 | Physical aggression | |||
| 1=mild | 193 | |||
| 2=severe | 211 | |||
| agg_2 | Aggressive resistance | |||
| 1=mild | 183 | |||
| 2=severe | 277 | |||
| agg>3 | Verbal aggression | |||
| 1=mild | 372 | |||
| 2=severe | 349 |
Table 3 Fixed-effects regression on four-monthly total care cost

| Variable | Coefficient | s.e. | t | P | 95% CI |
|---|---|---|---|---|---|
| Inst | 8390.66 | 4580.67 | 1.832 | 0.067 | -600.33 to 17381.65 |
| age | -129.97 | 104.37 | -1.245 | 0.213 | -334.84 to 74.90 |
| li×age | -147.67 | 56.54 | -2.612 | 0.009 | -258.63 to −36.70 |
| mmse | -56.77 | 26.64 | -2.131 | 0.033 | -109.06 to −4.47 |
| li×mms_1 | 39.93 | 38.01 | -1.051 | 0.294 | -34.67 to 114.53 |
| barthel | -586.16 | 57.08 | -10.269 | 0.000 | -698.21 to −474.12 |
| li×bar_1 | 438.87 | 70.42 | 6.232 | 0.000 | 300.65 to 577.10 |
| wake_1 | -441.62 | 492.03 | -0.898 | 0.370 | -1407.39 to 524.15 |
| wake_2 | -1128.03 | 529.40 | -2.131 | 0.033 | -2167.15 to −88.91 |
| agg_1 | 819.19 | 449.92 | 1.821 | 0.069 | -63.91 to 1702.29 |
| agg_2 | -251.81 | 362.21 | 0.695 | 0.487 | -962.75 to 459.14 |
| agg_3 | -36.43 | 324.41 | -0.112 | 0.911 | -673.19 to 600.33 |
| constant | 24273.25 | 8556.36 | 2.837 | 0.005 | 7478.72 to 41067.79 |
Institutionalisation
As predicted, the patients incur an additional cost of approximately £ 8000 per four-month period when in institutional care, assuming everything else is held constant. This is approximately equivalent to the annual cost of institutional care noted in Table 1.
Age
The model indicates that the age—institutionalised interaction term is significantly inversely associated with cost. When all other factors are held constant, each additional year of age reduces the cost incurred by £ 130 per four-month period, although this is not in itself statistically significant. When cared for in an institution, each additional year of age has the additional effect of significantly reducing four-monthly costs by a further £147 (i.e. £277 in total). These results imply that, having controlled for disease progression, older subjects at home or in an institution are less likely to have health and social care resources committed to them.
Measures of disease progression
The results indicate that changes in both the MMSE and Barthel scores have an independent and significant effect on costs, and that changes in the Barthel index have a larger impact. Each one-point decline in the MMSE score is associated with a £56 increase in four-monthly costs, whereas each one-point fall in the Barthel index is associated with a £586 increase in costs. Even allowing for the shorter range of the Barthel scale (20 points, compared with 30 points in the MMSE scale), it seems that changes in ADL have a much greater impact than cognitive changes on the health and social care resources required by dementia sufferers.
However, looking at the MMSE/Barthel and institutionalisation interaction terms, it appears that the pattern just described holds only for those outside institutional care. Once in institutional care, the results suggest that most costs are fixed and therefore further declines in cognition or ADL have little additional impact on costs (although more detailed information on patient-specific nursing and other care inputs in institutional care would be required to confirm and explain this fully). The variable ‘wakefulness for reasons other than passing urine’ is the only behavioural variable to have a significant impact on the care costs. In fact, this variable significantly reduces costs by over £1000, perhaps because it correlates with other factors that reflect increased independence and therefore may be associated with a delay in the time to institutionalisation.
Time to permanent institutional care
The conditional probability that a patient with dementia is admitted to permanent institutional care is likely to depend on his/her mental, physical and behavioural abilities combined with the support available from carers and community services. Table 4 shows the results for the Cox proportional hazards regression, in which time to institutionalisation is used to determine the way in which these factors affect the hazard of time to admission.
Table 4 Cox regression on time to institutionalisation

| Variable | Coefficient | s.e. | Z | P | 95% CI |
|---|---|---|---|---|---|
| age | 0.069025 | 0.030 | 2.284 | 0.022 | 0.0097842 to 0.1282657 |
| mmse | -0.100454 | 0.035 | -2.910 | 0.004 | -0.1681034 to −0.032804 |
| barthel | -0.159344 | 0.037 | -4.340 | 0.000 | -0.2313129 to −0.087376 |
| gende_1 | -0.894397 | 0.375 | -2.386 | 0.017 | -1.6292 to −0.159595 |
| Livin_1 | -1.514713 | 0.660 | -2.294 | 0.022 | -2.808984 to −0.220443 |
| Livin_2 | -1.814786 | 0.889 | -2.042 | 0.041 | -3.556417 to −0.073156 |
| Livin_3 | -1.820078 | 0.702 | -2.592 | 0.010 | -3.196526 to −0.443630 |
| Livin_4 | -1.324685 | 0.888 | -1.492 | 0.136 | -3.064404 to 0.4150333 |
| care_2 | -1.441768 | 0.835 | -1.727 | 0.084 | -3.078484 to 0.1949474 |
| care_3 | -1.512468 | 0.717 | -2.110 | 0.035 | -2.917154 to −0.107782 |
| cope3 | 0.639001 | 0.489 | 1.307 | 0.191 | -0.3191401 to 1.597141 |
| cope4 | 0.017134 | 0.484 | 0.035 | 0.972 | -0.9316915 to 0.96596 |
| wake_1 | -0.005965 | 0.520 | -0.011 | 0.991 | -1.025691 to 1.013762 |
| wake_2 | 1.193029 | 0.520 | 2.294 | 0.022 | 0.1739289 to 2.212128 |
| agg_1 | 0.065185 | 0.470 | 0.129 | 0.897 | -0.8597688 to 0.980810 |
| agg_2 | -0.665096 | 0.417 | -1.593 | 0.111 | -1.483282 to 0.153090 |
| agg_3 | 0.309782 | 0.375 | 0.825 | 0.409 | -0.4260757 to 1.045639 |
Age
The analysis shows a significant association between the age and the hazard of institutionalisation, indicating that as the individual ages, the hazard of being admitted to institutional care increases: this is indicative of a reduced time to institutionalisation. Because all subjects were living at home at entry to the study and had a mean age of 78 years, this result may have been anticipated, however, it should be noted that this age effect is identified after controlling for disease progression and carer commitment and capability.
Gender
A significant association between gender and the hazard of institutionalisation is also found, with women having a lower hazard than men (approximately 10% lower than men), implying longer times to institutionalisation (see Table 4).
The MMSE, Barthel and other measures of behaviour
As noted earlier, an advantage of this study compared with many others in the area is the presence of more than one measure of disease progression, allowing the opportunity to assess the relative importance of cognitive decline, behavioural change and ADL. The results indicate that both the MMSE score and the Barthel index are negatively associated with the hazard of institutionalisation: as each declines, along with the patient's deterioration, the hazard of being admitted increases and consequently the duration to institutionalisation falls. The inclusion of extra behavioural variables that measure wakefulness and aggressive behaviour that are not captured by the MMSE or Barthel index has no impact on the significant independent association between MMSE, Barthel and the hazard of being admitted to long-term care. This finding — that cognition and ADL have an independent and highly significant (P<0.004) effect on the likelihood of being institutionalised — may have important implications for future study designs and analyses.
Domestic circumstances
Three measures were included relating to domestic circumstances: who the subject lived with, the attitude of the carer to caring at study entry and the physical ability of the carer to cope with caring. The results indicate that, when exploring the impact of who the patient lived with, living with others (including at least one member of the patient's family) significantly reduces the hazard of institutionalisation relative to living alone, and the strongest effects were found where the number of potential carers was highest. Living with others from outside the patient's family also seemed to reduce the hazard of institutionalisation relative to living alone, but this effect was not statistically significant. (Even though subjects were categorised as living alone, they were still closely supervised by family/friends.) The results also indicate that the presence of a carer who has an active preference for caring for the subject at home (as assessed at baseline) significantly reduces the hazard of institutionalisation. Finally, there is no evidence of an independent association between the assessed physical ability of the carer to cope and the hazard of institutionalisation.
DISCUSSION
This is a unique study in that no other has had access to a data-set of patients with dementia employing frequent longitudinal assessment of time-varying covariates such as resource use, residential status, age and disease progression. It illustrates that although there are only 100 patients in this data-set, its longitudinal nature, with a mean follow-up time of 40 months, generates a rich source of information.
Modelling disease progression on the basis of changes in cognition and ADL
One of the key findings from this study is that it may be inappropriate to model disease progression in dementia solely on the basis of measures of cognitive change. It has been suggested elsewhere (Reference Davies, Fernandez and NomerDavies et al, 2000; Reference McDonnell, Redekop and Van der RoerMcDonnell et al, 2001) that changes in scores for ADL and information on behavioural disturbances may be potential indicators of progression to institutional care and costs of care. This study has shown that the MMSE and the Barthel index are independent significant predictors of time to institutionalisation and cost of care, but changes in the levels of ADL seem to be much more important than changes in cognition in predicting costs outside institutional care. It is also interesting to note that despite the inclusion of measures of behavioural disturbances not captured by the MMSE or Barthel index, such as wakefulness and aggression, the MMSE and Barthel index remain independent significant predictors of time to institutionalisation and care costs. This finding may have wide-ranging implications for future studies in this area: for example, pivotal trials of therapies for dementia have placed a strong emphasis on changes in cognition, and almost all the modelling work conducted to date on the cost-effectiveness of dementia interventions uses the impact of therapy on cognition as the linking mechanism to costs of care. The possibility that cognitive changes lead to changes in ADL would require further investigation, but such research goes beyond the bounds of the current study.
Institutionalisation and respite care
The study confirms findings from previous research that institutionalisation represents a significant proportion of the total cost burden for dementia patients. Institutionalisation accounts for 69% of the total care costs and, when all other factors are held constant, it adds an additional cost of approximately £8000 per four-month period. Less expected is the finding that respite care represents the next most important burden of resource use, accounting for 15% of the total care costs for dementia patients. This suggests that more attention should be paid to the collection of information on respite care in prospective studies and that onset of need for respite care should be included in modelling studies.
In contrast with the majority of previous studies — which have assumed costs to be constant once a person with dementia is admitted to institutional care — a feature of this study is that the costs of care in institutions do not consist solely of the flat rate cost per week of the institution, but also include such items as GP consultations, out-patient visits and short-term hospital stays. Some evidence exists on health service use among elderly people in institutional care, but not specifically those with dementia (Reference Kavanagh and KnappKavanagh & Knapp, 1998). A valuable extension to this study would be to explore in more detail the variation in the actual nursing care and other resources used by individuals within an institutional setting: in the absence of such data, there is no clear evidence that disease progression while in institutional care increases costs. However, the finding in this study that age is inversely associated with the cost of care both at home and in institutional care is intriguing, because it suggests the possible existence of age-related rationing: controlling for everything else, a person with dementia appears less likely to get access to health and social care as his/her age increases.
Lifetime costs of dementia
Finally, the empirical estimates of the effects of disease progression on care costs that this study has established should be valuable in assessing more accurately the true lifetime costs of dementia and their association with disease progression and, in future work, modelling the cost-effectiveness of therapeutic interventions.
CLINICAL IMPLICATIONS
-
• It may be inappropriate for economic models of disease progression in dementia to be based solely on measures of cognitive change.
-
• Institutionalisation represents a significant proportion of the total cost burden for patients with dementia.
-
• The empirical estimates of the effects of disease progression on care costs that this study has established are valuable in assessing more accurately the true lifetime costs of dementia and their association with disease progression.
LIMITATIONS
-
• The original data were not collected for the purpose of this particular study.
-
• The same size of 100 subjects is relatively modest and, if solely used on a cross-sectional basis, would have implications for the power of the study.
-
• Although there is a nominal cost included for informal care in this study, it has not been estimated in detail.
Acknowledgements
Our thanks go to: Kathy Gedling, Research Assistant at the Department of Psychiatry, University of Oxford, who collected the data; Sandra Cooper, Administrator at the Department of Psychiatry, University of Oxford, who was involved in the administration of the original clinical study; and Professor Christopher G. Fairburn, Department of Psychiatry, University of Oxford, who was joint grant holder in the original clinical study.










eLetters
No eLetters have been published for this article.