1. Introduction
Fragile X syndrome is the leading cause of inherited mental retardation and developmental disabilities with an incidence of 1 in 4000–6000 (Gantois et al., Reference Gantois, Kooy, Oostra and Meyers2004; Penagarikano et al., Reference Penagarikano, Mulle and Warren2007). Fragile X patients have a significant intellectual disability that may vary from mild to severe. In addition, male patients have a variety of associated characteristics, including physical features such as a long face with prominent chin and enlarged ears, and macroorchidism (Hagerman, Reference Hagerman, Hagerman and Hagerman2002). Behavioural characteristics include hyperactivity, attention deficit disorders, autistic-like behaviour and sensory defensiveness. Females often have a milder intellectual disability and a milder presentation of the physical and behavioural features. The disease is caused by an elongated CGG repeat in the 5′-untranslated region of the fragile X mental retardation gene 1, FMR1 (Verkerk et al., Reference Verkerk, Pieretti, Sutcliffe, Fu, Kuhl, Pizzuti, Reiner, Richards, Victoria, Zhang, Eussen, van Ommen, Blonden, Riggins, Chastain, Kunst, Galjaard, Caskey, Nelson, Oostra and Warren1991). Unaffected individuals have less than 55 CGG repeats. Individuals with 55–200 CGG repeats have a premutation for example, carry an unstable repeat which may expand in future generations, but do not suffer from fragile X syndrome. Elongation of the repeat above a threshold of approximately 200 repeats induces hypermethylation and transcriptional silencing of the gene, preventing synthesis of the FMR1 protein, FMRP. The lack of this specific protein causes fragile X syndrome.
Although the vast majority of fragile X patients share the same fragile X mutation and do not synthesize any FMRP, the disease severity varies enormously from patient to patient even within fragile X families. Allelic variations in the FMR1 gene or environmental factors are not sufficient to explain this variation in disease severity. Therefore, we hypothesized that genetic factors in addition to the lack of FMRP may play an important role in determining the severity of the fragile X phenotype (Errijgers & Kooy, Reference Errijgers and Kooy2004).
In our search for these additional genes, we compared the acoustic startle response (ASR) of fragile X knockout mice bred in different genetic backgrounds. Studies in animal models are often used to guide the search for modifier loci in humans (Nadeau, Reference Nadeau2001; Plappert & Pilz, Reference Plappert and Pilz2001). Many organisms with spontaneous, engineered or induced mutations often vary in subtle or profound ways depending on background genes that act as phenotypic modifiers. The fragile X knockout mouse is a valid animal model to study the human disorder (Bakker & Oostra, Reference Bakker and Oostra2003; Kooy, Reference Kooy2003). Fragile X knockout mice lack Fmrp and show abnormalities compatible with the symptoms observed in human fragile X patients, including mild to severe learning difficulties, macroorchidism and behavioural alterations. To determine how genetic background influences the effect of the Fmr1 knockout mutation in the ASR, we compared ASR of male fragile X knockout mice and control littermates bred in three different genetic backgrounds, including C57BL/6J (C57BL/6J×129P2/OlaHsd) F1 and F2 intercross mice. Startle response is a basic behaviour that has been observed in humans as well as most mammalians, including mice. Startle can be elicited by a sudden, loud acoustic stimulus and is characterized by a coordinated muscle contraction of the eyelid, neck and extremities. It is mediated by a relatively simple neuronal circuit in the lower brainstem (Koch, Reference Koch1999). ASR is used as a behavioural tool to assess the neuronal basis of behavioural plasticity (Plappert & Pilz, Reference Plappert and Pilz2001).
In our study, both increased and decreased ASR measurements were observed in fragile X knockout mice, depending on the genetic background of the mice and the stimulus intensities used.
2. Materials and methods
(i) Animals
All experimental animals were born and raised in the animal facilities at the University of Antwerp. At the time of this study, the mutant Fmr1 mice were backcrossed for more than ten generations in C57BL/6J purchased from Charles River Belgium (Brussels, Belgium). Females heterozygous for the fragile X knockout mutation were crossed with wild-type 129P2/OlaHsd males (Harlan, Oxon, UK) to obtain F1 hybrids. To generate the F2 generation, heterozygous F1 females were intercrossed with male F1 control littermates. Mice were weaned at approximately 4 weeks of age. Only male knockout mice and their male control littermates were used in ASR. Mice were housed in mixed genotype groups in standard mouse cages under conventional laboratory conditions (food and water ad libitum, constant room temperature and humidity, 12 h/12 h light/dark cycle). Genotypes were determined by PCR of tail DNA. Experimenters were blinded as to the genetic status of the animals. All experiments were carried out in compliance with Directive 86/609/EEC of the Council of the European Communities, and the Animal Ethics Committee of the University of Antwerp approved all the protocols.
(ii) Startle apparatus
ASR was measured using the Med Associates Startle Reflex System (Med Associates Inc., St. Albans, VT, USA), which comprised a sound-attenuating chamber equipped with a cylindrical Plexiglas animal enclosure (length=11 cm; inner diameter=3 cm) to prevent bias in ASR measurement from general movements of the animal not elicited by the acoustic stimulus. Ventilation was provided by a small electric fan that also generated a 50 dB background noise. Pure tones were presented by a speaker positioned 12 cm behind the animal enclosure. A linear load cell sensor platform affixed to the animal enclosure frame was used to detect and transduce motion resulting from the animals' response. Pure tone parameters were controlled by a computer and ASR recordings were analysed with the Startle Reflex Version 4.10 software (Med Associates Inc.).
(iii) ASR procedure
A single mouse (aged 8–10 weeks) was placed in the Plexiglas enclosure and was allowed to acclimatize for 5 min. After the acclimatization period, the animal was exposed to three consecutive tests. Each test consisted of various pure tone stimuli – 90, 100 and 110 dB or 100, 110 and 120 dB – presented every 10–20 s, alternated with null stimuli, all arranged in a pseudorandom order. Startle duration was 60 ms.
(iv) Statistical analyses
Each individual mouse was subjected to multiple measurements at each of the three stimulus intensities. For each individual mouse, we calculated mean ASR (i.e. average peak value of amplitude), standard deviation and standard error at each stimulus intensity. This created three data points for each individual mouse. In all calculations, each data point was weighted with the reciprocal of its standard error. If an individual mouse had only been observed once at a particular stimulus intensity, this data point was omitted for the mouse, but the other two data points of this mouse were left in the dataset.
To model the effect of stimulus intensity and genotype on mean ASR within one generation, a multivariate repeated measurements analysis was performed using the Proc Mixed procedure in SAS Version 8.02 (SAS Institute Inc., Cary, NC, USA). In a saturated model for the mean, the covariance structure was determined using Akaike's information criterion. The best-fitting model was determined by likelihood ratio testing.
Comparing the effects of genotype on ASR between generations F1 and F2 was complicated by the fact that the F1 generation was tested at intensities of 90, 100 and 110 dB, whereas the F2 generation was tested at intensities of 100, 110 and 120 dB. Modelling covariances, as described above, is inefficient for such a dataset. Therefore, multiple measurements per individual were modelled through a random effects model using the Proc Mixed procedure in SAS. Intercepts and slopes of each individual mouse were included in the model as random effects. Testing the need to include these random effects was done using a likelihood ratio test, in a saturated model for the mean.
3. Results
In this study, we compared the ASR of male fragile X knockout mice and male control littermates bred in different genetic backgrounds, including C57BL/6J inbreds (C57BL/6J×129P2/OlaHsd) F1 hybrids and F2 intercross mice.
(i) C57BL/6J
For the congenic C57BL/6J strain, ASR measurements at intensities of 90, 100 and 110 dB were available from 13 control and 12 knockout mice. A quadratic regression model, taking into account multiple measurements per individual mouse, was fitted to model the effect of stimulus intensity and genotype on ASR. We observed a significant interaction between stimulus intensity and genotype (P=0·0008). C57BL/6J knockout mice showed a higher ASR at the lowest stimulus intensity of 90 dB and a lower ASR at the highest stimulus intensity of 110 dB when compared with their control littermates (Fig. 1A).
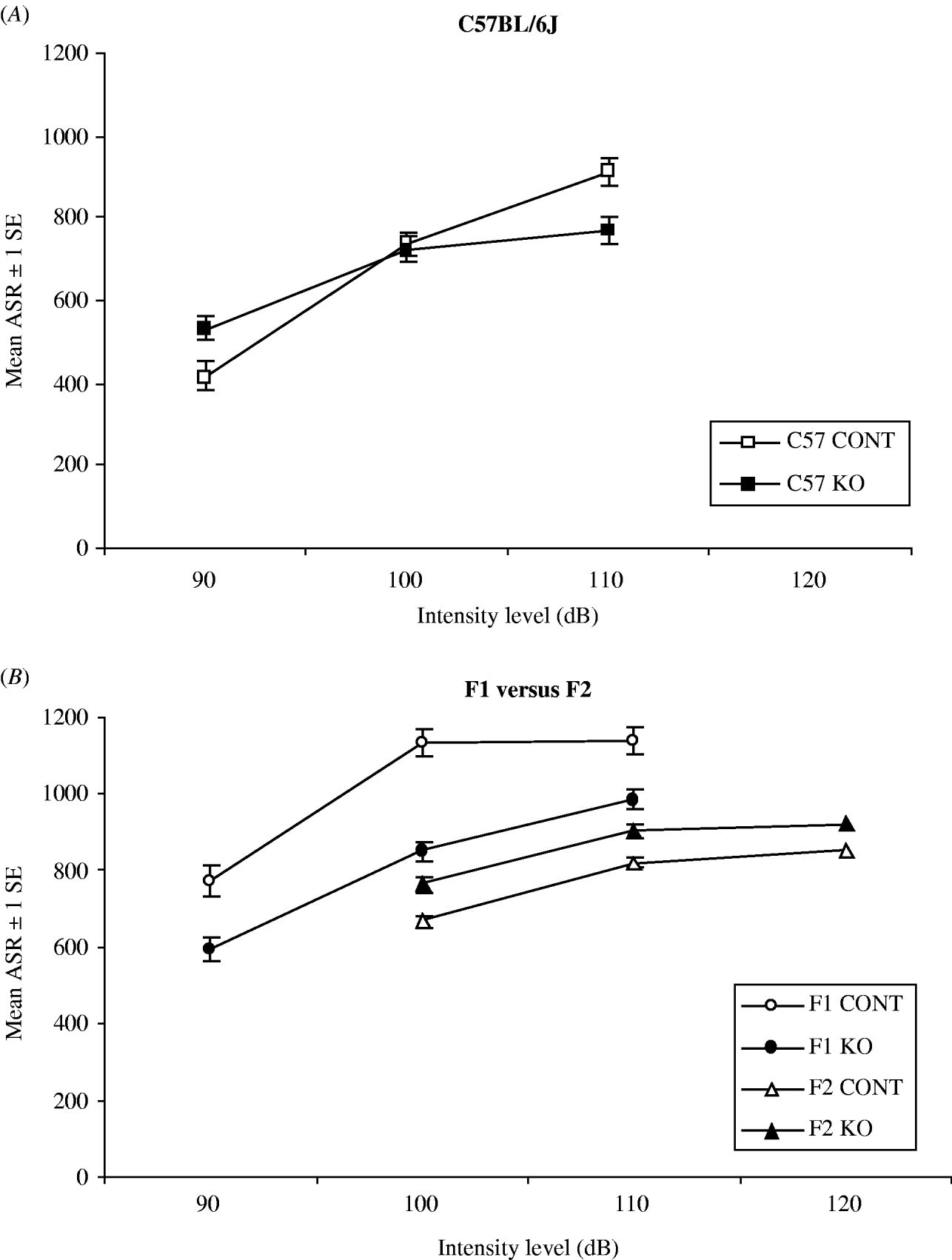
Fig. 1. Error bar chart comparing ASR in fragile X knockouts and controls bred in different genetic backgrounds. Each error bar represents 1 standard error. (A) C57BL/6J knockouts (n=12) showed an increased ASR at 90 dB and a decreased ASR at 110 dB compared with control littermates (n=13). No difference between both genotypes was measured at 100 dB. (B) F1 knockouts (n=18) showed a decreased ASR at 90, 100 and 110 dB compared with control littermates (n=14). The ASR of F2 mice was the opposite of the ASR of F1 mice. F2 knockouts (n=75) showed an increased ASR at 100, 110 and 120 dB compared with control littermates (n=84). Abbreviations: CONT, control; KO, knockout.
(ii) F1 hybrids
In the (C57BL/6J×129P2/OlaHsd) F1 generation, the ASRs of 14 controls and 18 knockouts were compared at stimulus intensities of 90, 100 and 110 dB. The multiple regression model showed a significantly decreased ASR in knockouts compared with controls across all the intensities used (P=0·004) (Fig. 1B). There was no interaction between stimulus intensity and genotype.
(iii) F2 intercross
A total of 84 controls and 75 knockout F2 animals were tested for ASR. The multiple regression model showed a significantly increased ASR in knockouts compared with controls across all the intensities used (P<0·03) (Fig. 1B). No interaction was observed between stimulus intensity and genotype.
Remarkably, the effect of knockout status on ASR in the F2 generation is the opposite of what was observed in the F1 generation (Fig. 1B). In the F1 generation, a decreased ASR was observed, whereas in the F2 generation an increased ASR was measured. To formally compare this effect between the two generations, data from both generations were pooled and a random effects regression model was fitted. The nominal value of the interaction between genotype and generation reached a significance level of P~0·05. In the F1 generation, the estimated difference in average ASR between knockout and controls equals – 114 (95% CI: 64–292) across all the levels of intensity, whereas in the F2 generation the average difference is estimated to be 66 (95% CI: −3–135) across all the levels of intensity.
4. Discussion
The purpose of this experiment was to determine whether genetic factors may influence the Fmr1 knockout phenotype using ASR. A reliable method for assessing genetic influences is to screen inbred mouse strains. Crossing two inbred strains with a different effect on the mutation under study, followed by backcrossing to one of the parental strains or intercrossing the F1 generation, is known to be a successful approach to study the effect of the genetic background on the phenotype (Nadeau, Reference Nadeau2001).
The present study examined startle responses to various intensities of auditory stimuli in F1 and F2 offspring between the congenic C57BL/6J knockout line and the 129P2/OlaHsd inbred strain. C57BL/6J is the most frequently used background in fragile X knockout mouse studies. 129P2/OlaHsd is the donor strain of the E14 stem cell line, used to generate the fragile X knockout mouse (Bakker et al., Reference Bakker, Verheij, Willemsen, van der Helm, Oerlemans, Vermey, Bygrave, Hoogeveen, Oostra, Reyniers, De Boulle, D'Hooge, Cras, van Velzen, Nagels, Martin, De Deyn, Darby and Willems1994). Fragile X knockout mice bred in a 129P2/OlaHsd background are not available, but 129P2/OlaHsd was selected for the F1 hybrids as it has been hypothesized that the presence of 129 genes in the genetic background of the fragile X knockout mice enhances the differences between control and knockout mice (Paradee et al., Reference Paradee, Melikian, Rasmussen, Kenneson, Conn and Warren1999; Errijgers & Kooy, Reference Errijgers and Kooy2004). To obtain F1 hybrids, females heterozygous for the fragile X knockout mutation were crossed with wild-type 129P2/OlaHsd males. Knockouts of the F1 generation showed significantly lower ASRs compared with controls for all three stimulus intensities used. Since all members of the F1 generation are genetically identical, these behavioural alterations appear to be a specific effect of the Fmr1 mutation and resultant loss of Fmrp. Male C57BL/6J knockouts also differed in ASR compared with their control littermates but, as opposed to F1 knockout mice, C57BL/6J knockouts showed an increase in ASR in response to the lowest stimulus of 90 dB and a decrease in ASR in response to the highest stimulus of 110 dB. No difference between both genotypes was measured at a stimulus intensity of 100 dB. Thus, the phenotypic expression of the Fmr1 knockout mutation in the F1 hybrid background appears to be significantly different from the phenotypic expression of the Fmr1 knockout mutation in the C57BL/6J background.
Male F2 progeny were obtained from an intercross between male F1 mice and female F1 hybrids, heterozygous for the fragile X knockout mutation. Surprisingly, F1 and F2 mice showed a remarkable difference in ASR. Knockouts of the F2 generation showed an increased ASR compared with controls, whereas knockouts of the F1 generation showed a decreased ASR. The ASR of F2 mice appears to be the opposite of the ASR of F1 mice. This is especially remarkable as both F1 and F2 mice consist of 50% of the genetic material from the C57BL/6J strain and 50% of the genetic material from the 129P2/OlaHsd strain. However, mice of the F1 generation are genetically homogeneous and consist of one maternal C57BL/6J and one paternal 129P2/OlaHsd chromosome. In contrast, mice of the F2 generation are genetically heterogeneous, due to recombination between C57BL/6J and 129P2/OlaHsd chromosomes during meiosis in the F1 generation. The different distribution of the genetic background of the parental strains seems to be responsible for the difference in ASR between F1 and F2. This opposite ASR in the F1 and F2 generations is unique in behavioural studies and has, to our knowledge, not been previously reported.
The three different genetic backgrounds also differ with regard to baseline performance in controls. Taking the C57BL/6J as a reference, the F1 hybrids show an increased response. This is in accordance with a vast amount of the literature, demonstrating an increased performance of F1 hybrids over inbred strains. The F2 has a lower response than the F1, possibly due to the homozygous C57BL/6J and 129P2/OlaHsd regions present.
In conclusion, fragile X knockouts responded in a different way to various intensities of auditory stimuli compared with control littermates depending on their genetic background. This illustrates the effect of background genes on the phenotypic expression of the fragile X mutation in mice. This interaction between the genetic background and the Fmr1 knockout mutation in ASR of fragile X knockout mice provides further evidence that modifier genes influence the fragile X phenotype. Our results may stimulate further research in order to map and identify such genes.
The work described in this paper was supported by the University of Antwerp Special Research Fund (ASPEO-VIS; NOI-BOF), the Belgian National Fund for Scientific Research – Flanders (FWO) and an agreement between the Institute Born-Bunge and the University of Antwerp.



