Introduction
Kochia [Bassia scoparia (L.) A.J. Scott] is a summer annual weed in the Chenopodiaceae (goosefoot) family commonly found throughout the United States (Forcella Reference Forcella1985; Friesen et al. Reference Friesen, Beckie, Warwick and Van Acker2009). Throughout North America, B. scoparia is present in arid and semiarid regions of disturbed sites and cropping systems (Friesen et al. Reference Friesen, Beckie, Warwick and Van Acker2009). Bassia scoparia has been documented to be problematic in many major crops grown in the Great Plains such as sugar beet (Beta vulgaris L.), corn (Zea mays L.), and dry bean (Phaseolus vulgaris L.) (Blackshaw Reference Blackshaw1990; Schweizer Reference Schweizer1981; Weatherspoon and Schweizer Reference Weatherspoon and Schweizer1970; Wilson et al. Reference Wilson, Wicks and Fenster1980). It is often one of the first summer annual weed species to emerge in regions where it is naturalized, making it a highly competitive species (Friesen et al. Reference Friesen, Beckie, Warwick and Van Acker2009). Bassia scoparia populations have been documented to begin emerging with as low as 151 accumulated growing degree days (GDD) and have an emergence duration of 556 GDD when a base temperature of 0 C was selected for calculating daily GDD (Kumar et al. Reference Kumar, Jha, Dille and Stahlman2018). In the Northern Great Plains, 151 GDD will typically accumulate by early to mid-April (30-yr average of April 7 in Scottsbluff, NE, and April 12 in Huntley, MT), with an additional 556 GDD accumulating by the end of May.
Seed persistence and dormancy in the soil is relatively short, with more than 95% of B. scoparia seed not persisting for more than 2 yr (Dille et al. Reference Dille, Stahlman, Du, Geier, Riffel, Currie, Wilson, Sbatella, Westra and Kniss2017). Bassia scoparia success in cropping systems is also attributed to rapid growth and prolific seed production, which is further augmented by evolved resistance to numerous herbicide sites of action (Derksen et al. Reference Derksen, Anderson, Blackshaw and Maxwell2002; Evetts and Burnside Reference Evetts and Burnside1972; Friesen et al. Reference Friesen, Morrison, Rashid and Devine1993; Heap and Duke Reference Heap and Duke2018; Nussbaum et al. Reference Nussbaum, Wiese, Crutchfield, Chenault and Lavake1985; Varanasi et al. Reference Varanasi, Godar, Currie, Dille, Thompson, Stahlman and Jugulam2015). Herbicide-resistant weed populations pose economic challenges to growers by rendering once efficacious herbicides useless (Dille et al. Reference Dille, Stahlman, Du, Geier, Riffel, Currie, Wilson, Sbatella, Westra and Kniss2017). In Wyoming, Nebraska, and Montana, B. scoparia populations have evolved resistance to photosystem II inhibitors, enzyme 5-enolpyruvylshikimate 3-phosphate synthase inhibitors, synthetic auxins, and acetolactate synthase (ALS) inhibitors, with some populations exhibiting multiple resistance (Heap Reference Heap2018; Varanasi et al. Reference Varanasi, Godar, Currie, Dille, Thompson, Stahlman and Jugulam2015).
Understanding the effects of crop management practices on B. scoparia emergence, survival, and seed production is imperative in improving long-term weed management strategies, especially for herbicide-resistant weed populations (Walsh et al. Reference Walsh, Broster, Schwartz‐Lazaro, Norsworthy, Davis, Tidemann, Beckie, Lyon, Soni and Neve2018). To better understand how B. scoparia emergence and crop management are related, the impacts of crop canopy and herbicide use pattern on weed seedling emergence, survival, and development need to be examined. Light is commonly a limiting factor in developed crop canopies, as a well-developed crop canopy blocks the necessary stimuli for weed seed germination and seedling emergence (Ballaré and Casal Reference Ballaré and Casal2000; Rajcan and Swanton Reference Rajcan and Swanton2001). Crop canopies associated with higher leaf area, height, and rapid development are more proficient in inhibiting weed seedling emergence (Seavers and Wright Reference Seavers and Wright1999). A study conducted in Kansas found that B. scoparia plants grown in the absence of a surrounding plant canopy had greater seed production than in the presence of a canopy, with grain sorghum [Sorghum bicolor (L.) Moench] and corn inhibiting B. scoparia seed production more than soybean [Glycine max (L.) Merr.] and wheat (Triticum aestivum L.) stubble (Esser Reference Esser2014).
Additional crop management practices such as planting and harvest dates could also impact weed seedling emergence and recruitment (Parish Reference Parish1990). Growers commonly control weeds before planting with tillage or preplant burndown herbicides, which result in reduced competition with weeds as crops emerge and mature (Heatherly and Hodges Reference Heatherly and Hodges1999; Price et al. Reference Price, Wilcut and Cranmer2002). However, if a weed species has an extended emergence pattern or emerges in multiple flushes and grows tall quickly, then preplant weed control will be less efficacious. Therefore, weeds will continue to emerge after crop emergence and may have the ability to grow above the crop canopy (Rajcan and Swanton Reference Rajcan and Swanton2001; Roberts and Potter Reference Roberts and Potter1980; Schwinghamer and Van Acker Reference Schwinghamer and Van Acker2008).
Herbicide use pattern has been extensively shown to impact the evolution of herbicide-resistant weeds. Selection pressure caused by using the same herbicide mechanism of action season after season results in the buildup of resistant weed species (Jasieniuk et al. Reference Jasieniuk, Brûlé-Babel and Morrison1996; Powles et al. Reference Powles, Preston, Bryan and Jutsum1996; Young Reference Young2006). Alternating herbicide chemistries through effective mixtures and, to a lesser extent, herbicide rotation has been documented to reduce the buildup of herbicide-resistant weed populations (Beckie and Reboud Reference Beckie and Reboud2009; Evans et al. Reference Evans, Williams, Hager, Mirsky, Tranel and Davis2018; Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles and Burgos2012). Although all of these management practices are often recommended for herbicide-resistant weed management, to date there is little information available showing how the combined effects of crop rotation, which includes diversity in planting and harvest dates as well as diversity in crop canopy and architecture, paired with herbicide use impact weed emergence and seed production. Understanding how these practices impact weed survival and fecundity is imperative to long-term management of herbicide-resistant weed populations. Such findings could lead to more proactive, long-term approaches to managing weeds while minimizing grower dependence on herbicides. The objective of this study was to quantify the impact of varying crop canopies and ALS-inhibiting herbicide applications on B. scoparia density and seed production under field conditions.
Materials and Methods
Field studies were conducted in 2014 at four different university research sites where B. scoparia was known not to be a management problem in order to ensure low initial density. Study sites included the Sustainable Agriculture Research and Extension Center (42.1°N, 104.4°W at 1,390-m elevation) located near Lingle, WY; the Powell Research and Extension Center (44.8°N, 108.7°W at 1,331-m elevation) in Powell, WY; the Panhandle Research and Extension Center (41.9°N, 103.7°W at 1,199-m elevation) in Scottsbluff, NE; and the Southern Agricultural Research Center (45.9°N, 108.2°W at 914-m elevation) in Huntley, MT. Studies were conducted on a Heldt silty clay (fine, smectitic, mesic Ustertic Haplocambids) with 1.8% organic matter and a pH of 7.8, in Lingle, WY; Garland loam (fine-loamy over sandy or sandy-skeletal, mixed, superactive, mesic Typic Haplargids), with 1.6% organic matter and a pH of 8.2 in Powell, WY; Tripp very fine sandy loam (coarse-silty, mixed, superactive, mesic Aridic Haplustolls) with 0.7% organic matter and a pH of 8.3, in Scottsbluff, NE; and Lohmiller silty clay (fine, smectitic, calcareous, mesic Torrertic Ustifluvents) with 2.8% organic matter and a pH of 8.1, in Huntley, MT. Bassia scoparia seed was collected from different areas in Goshen County, WY, in fall of 2013. Six different seed lots encompassing a variety of field histories were collected, from which a 5% ALS-resistant seed blend was made (Supplementary Table 1). To create the 5% resistant population used in the study, we tested germination rate and ALS-resistant proportion from each seed lot individually by counting the total number of emerged B. scoparia from a subsample of each seed lot. Immediately after counting emergence, each subsample was treated with chlorsulfuron at 35 g ai ha−1. Approximately 10 d after treatment, the number of resistant B. scoparia was estimated by counting the number of live B. scoparia within each subsample. These counts were used to estimate the resistant proportion of seed within each lot. All lots contained relatively low resistant proportions (<20%), with some containing no detectable resistance. Lots were then mixed to create the 5% ALS-resistant seed blend used at all four locations in the study. Bassia scoparia was seeded at a rate of 2,000 g ha−1 to ensure that approximately 8 germinable seeds m−2 were present across each study site (40 germinable seeds g−1). A rotary hand spreader was used to seed B. scoparia in each plot just before each crop was planted. Thus, a uniform population of B. scoparia was used to quantify its response to varying crop canopies and herbicide treatments. Although sites were chosen to minimize existing B. scoparia density, B. scoparia is common in the region and we could not completely exclude preexisting B. scoparia from our study sites.
The experiment was arranged in a split-plot randomized complete block design with four replicates. Crop treatments included corn, dry bean, spring wheat, or sugar beet. Because this study was part of a larger ongoing study, the number of plots for each crop differed (Table 1). Each crop whole plot was split and treated with either an ALS-inhibiting herbicide that effectively controls ALS-susceptible B. scoparia or a non–ALS inhibiting herbicide that is effective in controlling ALS-resistant and ALS-susceptible B. scoparia (Table 2). All herbicides (ALS-inhibiting herbicides and non–ALS inhibiting herbicides) were selected and applied based on grower preference and practice throughout the region. PPI herbicides were applied before planting in dry bean plots. Herbicide treatments for spring wheat consisted of only one POST herbicide application when B. scoparia reached approximately 3 cm in height. Corn and sugar beet were treated with two POST herbicide applications (one early season when B. scoparia was approximately 3-cm tall, and one at least 14 d later once additional B. scoparia emergence was observed or at the latest crop stage allowed by the label). Acetochlor was added to the second POST application in corn and sugar beet to help control other weed species that were present, namely redroot pigweed (Amaranthus retroflexus L.) and hairy nightshade (Solanum physalifolium Rusby). Acetochlor applied POST did not control any emerged B. scoparia. Herbicide treatments for dry bean consisted of only a PRE herbicide followed by one POST herbicide application. EPTC was applied preplant in the ALS-inhibitor treatment at all sites except Lingle to control other weed species; our previous experience has shown that EPTC applied PRE is ineffective for B. scoparia control.
Table 1. Cultural practices for establishing corn, dry bean, spring wheat, and sugar beet, and number of plots at four sites in Huntley, MT, Powell and Lingle, WY, and Scottsbluff, NE, in 2014.

Table 2. Non–ALS inhibitor and ALS inhibitor–only herbicide treatments and rates used for corn, spring wheat, dry bean, and sugar beet at four sites in Huntley, MT, Powell and Lingle, WY, and Scottsbluff, NE, in 2014.

a POST applications in corn and sugar beet were applied twice, with the first application initiated when Bassia scoparia was approximately 3-cm tall, and the second at least 14 d afterward. LAYBY acetochlor was applied with the second POST application to control late-emerging weeds like Amaranthus retroflexus and Solanum physalifolium; this herbicide is not effective for controlling emerged B. scoparia.
b Dicamba, Clarity®, BASF, Research Triangle Park, NC.
c Resolve Q®, DuPont, Wilmington, DE.
d Glyphosate, Roundup PowerMax®, Monsanto Company, St Louis, MO.
e COC, crop oil concentrate, Prime Oil®, WinField United, Arden Hills, MN.
f Warrant®, Monsanto Company, St Louis, MO.
g Huskie®, Bayer CropScience, Research Triangle Park, NC.
h Harmony SG®, DuPont, Wilmington, DE.
i MCPA-ester®, WinField United, Arden Hills, MN.
j NIS, nonionic surfactant, Preference®, WinField United, Arden Hills, MN.
k UAN, urea ammonium nitrate.
l Prowl H2O®, BASF, Research Triangle Park, NC.
m EPTC was incorporated with overhead irrigation; EPTC applied PPI was used to control weeds other than B. scoparia and was expected to provide little to no control of B. scoparia based on previous experience. Eptam® 7E, Gowan Company, Yuma, AZ.
n Basagran®, BASF, Research Triangle Park, NC.
o Raptor®, BASF, Research Triangle Park, NC.
p UpBeet®, DuPont, Wilmington, DE.
Plot lengths were 15.2 m at all sites, but plot width varied by location due to crop row spacing and equipment availability. Split-plot widths were 6.1, 6.7, 3.4, and 3.7 m for Lingle, Powell, Scottsbluff, and Huntley locations, respectively. Throughout the season, plots were kept free of any weed species except B. scoparia by regular hand weeding.
Bassia scoparia density data were recorded midway through crop maturity (at least 14 d after the final herbicide applications had been made) by sampling two random 1-m2 areas per plot and counting the number of B. scoparia plants within them. Bassia scoparia seed production per plant was estimated by placing up to three pollination bags, per plant, on three different B. scoparia plants per plot. Bags were placed on the B. scoparia plants as pollination neared completion but before B. scoparia seed maturity, both determined through field scouting. Bags were tied around the stem to ensure no seed escape. Immediately before crop harvest, the percentage of each plant covered by pollination bags was estimated. All seeds within the three pollination bags per B. scoparia plant were then aggregated and weighed. A 5-g subsample of B. scoparia seed from each plant was removed, placed in a paper envelope, and left to air-dry for approximately 2 mo to mimic quiescence. Subsamples were then planted into 25 by 25 cm trays filled with approximately 2.5-cm-deep potting soil in a greenhouse, where they were watered daily. Seedlings were counted weekly and then removed for approximately 8 wk, and the cumulative number of emerged seedlings was totaled. The number of germinable seeds per B. scoparia plant was then calculated using Equation 1:
 $$\scale 78%\[\frac{{{\rm{Total}}\,{\rm{number}}\,{\rm{of}}\,{\rm{seed}}\,{\rm{germinated}}/({\rm{sub}}\,{\rm{sample}}\,{\rm{seed}}\,{\rm{weight/total}}\,{\rm{seed}}\,{\rm{sample}}\,{\rm{weight}})}}\over\scale 78%{{{\rm{estimated}}\,{\rm{\% }}\,{\rm{plant}}\,{\rm{coverage}}/100}}\]$$
$$\scale 78%\[\frac{{{\rm{Total}}\,{\rm{number}}\,{\rm{of}}\,{\rm{seed}}\,{\rm{germinated}}/({\rm{sub}}\,{\rm{sample}}\,{\rm{seed}}\,{\rm{weight/total}}\,{\rm{seed}}\,{\rm{sample}}\,{\rm{weight}})}}\over\scale 78%{{{\rm{estimated}}\,{\rm{\% }}\,{\rm{plant}}\,{\rm{coverage}}/100}}\]$$
Bassia scoparia seed production per square meter was then calculated by multiplying the mean number of germinable seeds per plant for each plot by the B. scoparia density per plot. The effect of crop and herbicide treatment on B. scoparia density and seed production were analyzed using a linear mixed-effects model appropriate for count data and unbalanced design (Bates et al. Reference Bates, Maechler, Bolker and Walker2015). For all response variables, the effect of site was considered a random effect, and the effects of crop, herbicide, and crop by herbicide interaction were considered fixed effects.
For all response variables, preliminary analysis suggested a log-normal distribution, so a log transformation was used, first adding half of the minimum value for each variable to each observation to account for zeros in the data set. The contribution of crop and herbicide treatments in explaining variance in each response was quantified using mean squares from type III analysis of variance, using Satterthwaite’s method to estimate denominator degrees of freedom (Kuznetsova et al. Reference Kuznetsova, Brockhoff and Christensen2017). For significant fixed effects, estimated marginal means (also sometimes called least-squares means) were calculated using the emmeans package and presented in the back-transformed, original scale (Lenth et al. Reference Lenth, Singmann, Love, Buerkner and Herve2018). Tukey-adjusted pairwise comparisons between treatments were made at the α = 0.05 level and presented using the mean separation algorithm proposed by Piepho (Reference Piepho2004). All data were analyzed using R statistical software v. 3.6.1 (R Core Team, Vienna, Austria, https://www.R-project.org).
Results and Discussion
Bassia scoparia density, seed production per plant, and seed production per square meter differed by location; however, overall treatment trends were generally consistent throughout all four locations of the study (Figure 1).
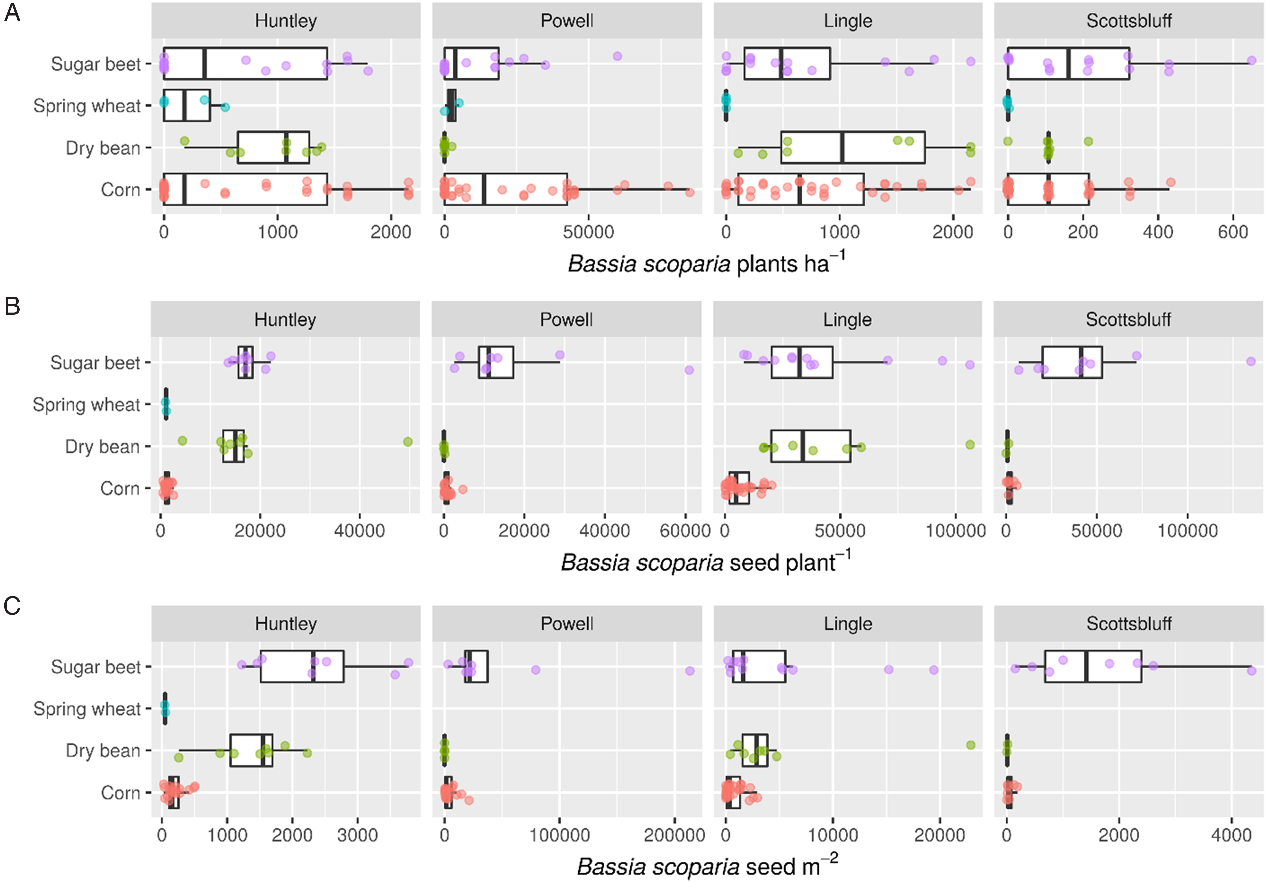
Figure 1. Distribution of Bassia scoparia density per square meter (A), seed production per plant (B), and seeds per square meter (C) as affected by crop in Huntley, MT, Powell and Lingle, WY, and Scottsbluff, NE, in 2014.
Bassia Scoparia Density
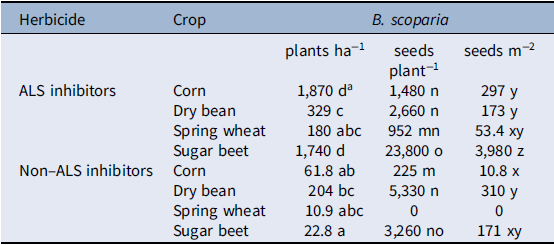
There was a significant herbicide by crop interaction for B. scoparia density (Table 3). After accounting for the interaction, herbicide had a greater effect on B. scoparia density compared with crop choice, as type III mean squares were 13 times greater for herbicide than crop. The non–ALS inhibiting herbicides, which controlled both resistant and susceptible biotypes, resulted in less B. scoparia density compared with the ALS-inhibiting herbicides alone in all crops, although differences were only statistically significant at the 5% level in corn and sugar beet (Table 4). Within the less-effective ALS-inhibiting herbicide treatment, dry bean and spring wheat had significantly less B. scoparia density (180 to 329 plants ha−1) compared with either corn (1,870 plants ha−1) or sugar beet (1,740 plants ha−1). The lowest B. scoparia density in both herbicide treatments was observed in spring wheat, suggesting this crop had the greatest ability to suppress B. scoparia establishment. Even when paired with the less-effective ALS-inhibiting herbicide treatment, spring wheat resulted in B. scoparia density similar to the non–ALS inhibiting herbicide treatments in all crops.
Table 3. ANOVA table showing fixed-effects mean squares and variance components for Bassia scoparia density and seed production.

Table 4. Bassia scoparia density and seed production (estimated marginal means) as affected by ALS-inhibiting herbicide treatment and crop at four locations near Huntley, MT, Powell and Lingle, WY, and Scottsbluff, NE, in 2014.
a Means within a response variable followed by the same letter are not statistically different (Tukey-adjusted pairwise comparisons, α = 0.05).
Bassia Scoparia Seed Production
There was a significant herbicide by crop interaction for B. scoparia seed production per plant, and seed production per square meter (Table 3). Variance in B. scoparia seed production per plant was influenced more by crop (mean squares[df = 3, 121] = 31.35) compared with herbicide (mean squares[df = 1, 122] = 14.78), indicating crop selection is an important factor driving B. scoparia seed production in plants that survive or escape herbicide treatment.
Bassia scoparia seed production was not observed in any replicate at three of four locations where spring wheat was treated with a non–ALS inhibiting herbicide (Table 4), and this treatment was therefore excluded from the statistical analysis. A total lack of seed production in nearly all plots across four experimental sites is a notable result and suggests the combination of spring grains with effective herbicides can be a powerful combination for B. scoparia seedbank management. In treatments where B. scoparia seed production was observed, seed production per plant was lowest in corn treated with non–ALS inhibiting herbicides (225 seeds plant−1), or spring wheat treated with ALS-inhibiting herbicides (952 seeds plant−1). These differences are likely due, in part, to the relative height of corn and wheat compared with other crops in the study. The taller corn and spring wheat are relatively more competitive for light compared with either dry bean or sugar beet (Kasperbauer Reference Kasperbauer1987; Seavers and Wright Reference Seavers and Wright1999; Smith et al. Reference Smith, Casal and Jackson1990). It is also notable that spring wheat, which was the earliest harvested crop, had the lowest B. scoparia seed production per plant within both herbicide treatments. The early harvest date reduced the ability of B. scoparia to produce viable seed, as most of the B. scoparia plants were still in the vegetative or early flowering stages at the time of wheat harvest. Low B. scoparia seed production could also be due to a dense spring wheat crop canopy, which has been shown to suppress weed pressure (Weiner et al. Reference Weiner, Griepentrog and Kristensen2001). Spring wheat was one of the first crops planted in this study (throughout late April), which is similar to the primary emergence window for B. scoparia the region (Dille et al. Reference Dille, Stahlman, Du, Geier, Riffel, Currie, Wilson, Sbatella, Westra and Kniss2017; Kumar et al. Reference Kumar, Jha, Dille and Stahlman2018; Schwinghamer and Van Acker Reference Schwinghamer and Van Acker2008). Because spring wheat was planted before or during the peak emergence time for B. scoparia within the region, this likely put B. scoparia at a disadvantage trying to emerge amongst the densely seeded wheat crop, and likely contributed to low seed production per B. scoparia plant. Sugar beet was planted shortly after spring wheat, but allowed the greatest B. scoparia seed production per plant. Sugar beet is a poor competitor against B. scoparia (Mesbah et al. Reference Mesbah, Miller, Fornstrom and Legg1994; Weatherspoon and Schweizer Reference Weatherspoon and Schweizer1971), in part due to slow early-season canopy development compared with many other crops. In addition, sugar beet was harvested well after B. scoparia had produced viable seed, so harvest operations had little impact on reducing seed production per plant.
Long-term reduction of weed seedbanks will require minimizing the seed produced per unit area, and this is most likely to be obtained through a combination of reducing weed establishment and minimizing viable seed production per plant. In this study, the proportion of variance explained by the main effects of crop and herbicide effects were more similar for B. scoparia seed production per square meter compared with either B. scoparia density or B. scoparia seed production per plant (Table 3), indicating both management tools were influential in impacting germinable B. scoparia seeds produced per unit area. Because no B. scoparia seed production was observed in spring wheat treated with non–ALS inhibiting herbicides, this treatment was once again excluded from statistical analysis (Table 4). The non–ALS inhibiting herbicide reduced B. scoparia seed production per square meter compared with the ALS-inhibiting herbicide in all crops except dry bean. Among the less-effective ALS-inhibiting herbicide treatment, sugar beet resulted in the greatest B. scoparia seed production of nearly 4,000 seeds m−2, compared with the other three crops, which resulted in 53 to 297 seeds m−2. Where non–ALS inhibiting herbicides were used, corn and spring wheat again resulted in the least B. scoparia seed production, at less than 11 seeds m−2.
For spring wheat, low seed production per unit area was due to a combined effect of reduced B. scoparia density plus little to no B. scoparia seed production among surviving plants. Early planting and harvest dates combined with dense crop canopy make spring wheat an effective crop choice for reducing B. scoparia seed production compared with the other crops in this study. Spring wheat was harvested in this study from August to early September. Bassia scoparia has been observed to produce flowers and set seed between mid-July and late September (Anderson and Nielsen Reference Anderson and Nielsen1996; Mickelson et al. Reference Mickelson, Bussan, Davis, Hulting and Dyer2004). As stated by Mickelson et al. (Reference Mickelson, Bussan, Davis, Hulting and Dyer2004), because spring wheat is harvested between mid-July and early August in this region, this could prevent seed set of B. scoparia, eliminating the unwanted plant during harvest before it is able to produce viable seed. Addition of an effective herbicide treatment (the non–ALS inhibiting herbicides) further reduced B. scoparia density and completely eliminated seed production across all four sites. For corn, which also has a competitive crop canopy, the impact of varying herbicide treatments was more evident. Implementation of an efficacious herbicide regime by using non–ALS inhibiting herbicides resulted in nearly 26 times less B. scoparia than treatment with ALS-inhibiting herbicides.
Dry bean and sugar beet typically contained more germinable B. scoparia seed produced per square meter compared with spring wheat. This could again be in part due to cropping practices. Both of these crops were harvested between mid-September and early October in this study. This time frame provided plenty of time for the B. scoparia that emerged and survived herbicide treatments within these plots to produce seed, considering it has been observed to produce flowers and set seed between mid-July and late September (Anderson and Nielsen Reference Anderson and Nielsen1996; Mickelson et al. Reference Mickelson, Bussan, Davis, Hulting and Dyer2004).
Sugar beet, unsurprisingly, allowed both high B. scoparia densities to persist and high seed production, making it the least favorable crop with respect to B. scoparia competition. This is consistent with grower experiences that B. scoparia is among the most troublesome and difficult to control weeds in this crop (Weatherspoon and Schweizer Reference Weatherspoon and Schweizer1969, Reference Weatherspoon and Schweizer1971). As was observed with corn, herbicide regime greatly exacerbated B. scoparia seed production per unit area in sugar beet, with approximately 23 times more germinable B. scoparia seed per unit area being produced when ALS-inhibiting herbicides were used compared with non–ALS inhibiting herbicides.
Long-term weed seedbank management will likely require implementing complementary practices that target different phases of the weed life cycle. This work demonstrates how herbicides and crop rotation interact to influence seed production in B. scoparia. Seed production per unit area, arguably the most important variable in determining the success of long-term weed seedbank management, was influenced by the combined effect of herbicides and crop selection. Herbicides primarily influenced the survival of weeds during the germination and early vegetative stages when the herbicide was applied and had a substantial impact on weed density. Crop choice had a lesser effect on weed density, but was the most important factor in influencing the amount of seed produced per surviving plant due to competitive effects as well as the timing of planting and harvest. This suppression of surviving weed seed production is likely to be especially important at the onset of herbicide-resistant weed evolution, when the herbicide is ineffective and it is important to reduce the buildup of resistant weed seed in the seedbank.
Improved knowledge of the combined effects of crop canopy and herbicide treatment on B. scoparia will aid in long-term management of herbicide-resistant weed populations, which is a major concern for growers in the Northern Great Plains and elsewhere. The results of this research show that combined effects of crop canopy and herbicide treatment can minimize B. scoparia establishment and seed production. Effective herbicides, when paired with a poorly competitive crop like sugar beet, can still allow substantial seed production and, therefore, a net increase in the soil weed seedbank. Conversely, even a relatively ineffective herbicide program may be sufficient if crop competitiveness and harvest dates are optimized, as was observed with spring wheat. Pairing combinations of effective crop rotations with efficacious herbicides will result in the best long-term weed management, and identifying the relative contributions of these management practices under field conditions remains an important area of research.
These findings can be used to implement more proactive, long-term approaches to managing B. scoparia, while minimizing grower dependence on herbicide use. Therefore, future research is needed to address how these management practices would impact B. scoparia density, seed production, and evolution of herbicide resistance after multiple years of implementation and to determine the economic feasibility of integrating cultural weed control with herbicides within the Northern Great Plains.
Acknowledgments
This work was supported by the Controlling Weedy and Invasive Plants program (award no. 2014-67013-21551, project accession no. 1000664), U.S. Department of Agriculture National Institute of Food and Agriculture. Partial funding to support this work was also provided to the University of Wyoming by Monsanto Company. Crop seed and herbicides were provided by Monsanto Company to the University of Wyoming, University of Nebraska, and Montana State University to support this research. University of Wyoming, Montana State University, and University of Nebraska receive grants and gifts to support the research of ARK, PJ, and NCL from various herbicide and seed companies with economic interests in the crops and herbicides used in this research; however, these other funding sources were not involved in this work.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2020.23







