Elevated concentrations of fasting TAG may increase the risk of CHD, although whether their contribution to the risk is independent of other concomitant lipid abnormalities remains a matter of debate(Reference Gandotra and Miller1). The Third Report of the National Cholesterol Education Program-Adult Treatment Panel recognised that TAG concentrations ≥ 150 mg/dl become a marker of atherogenic remnant lipoproteins and represent also one of the components of the metabolic syndrome(2). The metabolic syndrome is a complex of interrelated cardiovascular risk factors such as elevated fasting plasma glucose (FPG), high blood pressure (BP), elevated TAG concentrations, low HDL-cholesterol (HDL-C) and central adiposity(Reference Alberti, Eckel and Grundy3). At least three (or more) of these criteria have to be present to establish the diagnosis of the metabolic syndrome. The presence of the metabolic syndrome increases the risk of developing CVD and type 2 diabetes mellitus (T2DM)(2, Reference Galassi, Reynolds and He4).
The treatment of the metabolic syndrome involves treating its individual components. This encompasses lifestyle modifications such as diet, weight loss and exercise as well as pharmacotherapy with antihypertensive, hypoglycaemic and lipid-lowering drugs(Reference Tota-Maharaj, Defilippis and Blumenthal5). Few data exist on whether functional foods may exert beneficial effects on the components of the metabolic syndrome(Reference Rudkowska and Jones6), but recent evidence in mice(Reference Shertzer, Woods and Krishan7) and human subjects(Reference Pal, Ellis and Ho8–Reference Bortolotti, Maiolo and Corazza10) suggests that whey proteins may be among them.
Recently, an innovative industrial process for whole whey fermentation has been developed that results in a malleable protein matrix (MPM), containing whey proteins, peptides, proprietary Lactobacillus kefiranofaciens strain, exopolysaccharides and Ca(Reference Simard, Pilote, Dupont and Bureau11). MPM was shown to stimulate the innate immune defence in healthy animals(Reference Beaulieu, Dubuc and Beaudet12), to exhibit anti-inflammatory effects comparable to those of hydrocortisone(Reference Beaulieu, Dupont and Lemieux13) and to reduce neutrophil infiltration and cytokine and chemokine production (e.g. IL-1β, IL-6 and TNF-α) in murine models(Reference Beaulieu, Girard and Dupont14). Moreover, short-term supplementation with MPM was shown to have TAG- and LDL cholesterol (LDL-C)-lowering properties and to modulate glucose and blood pressure in animal models(Reference Beaulieu, Millette and Trottier15). Our group recently demonstrated in a randomised trial that MPM significantly decreases TAG concentrations in human subjects with hypercholesterolaemia(Reference Berthold, Schulte and Lapointe16).
The purpose of the present clinical trial was to examine the effect of MPM on fasting TAG concentrations in patients with the metabolic syndrome. The effects of MPM on other components of the metabolic syndrome were investigated as secondary outcome measures.
Subjects and methods
Eligible subjects
Those considered eligible for the present study were female (f) and male (m) patients between 18 and 75 years of age and with the metabolic syndrome, diagnosed as: waist circumference>102 cm (m)/>88 cm (f); TAG ≥ 150 mg/dl; HDL-C < 40 mg/dl (m)/ < 50 mg/dl (f); systolic BP (SBP) ≥ 130 mmHg or diastolic BP (DBP) ≥ 85 mmHg; FPG ≥ 100 mg/dl. For the purposes of this trial, the TAG component of the syndrome was mandatory to be present. Subjects having mild forms of the metabolic syndrome were selected by setting maximum threshold values of 400 mg/dl for TAG and < 160/100 mmHg for SBP/DBP (stage 1 hypertension). Moreover, subjects with diabetes were allowed in the study if HbA1c was < 7·0 %. Major exclusion criteria included BMI ≥ 35 kg/m2, a history of CVD (defined as known CHD, myocardial infarction, stroke or peripheral arterial disease), any chronic disease which may impair the subject's ability to participate in the trial (i.e. severe congestive heart failure, history of endocrine disorders, active gastric or duodenal ulcer, etc.), major surgery < 8 weeks before the study, any malignant disease, major weight loss (>10 %) in the last months before the trial, intolerance or allergies to lactose or milk proteins, concomitant treatment with statins or other lipid-lowering drugs or intake of any drug or supplement known to affect lipid metabolism during a period of < 12 weeks before study entry.
Study design and protocol
This was a multi-centre, double-blind, randomised, placebo-controlled trial with two parallel treatment groups, which was conducted between April 2007 and January 2009. The participating centres included one university hospital outpatient lipid clinic, one university sports medicine department and eleven general medical practices. The trial was performed according to the latest version of the Declaration of Helsinki, taking into account the current version of German drug legislation, and it was in accordance with the International Conference on Harmonization guideline for Good Clinical Practice Committee for Proprietary Medical Products/International Conference on Harmonization/135/95. The study protocol was approved by the ethics committee of the German Sport University Cologne and other competent ethics committees. Written informed consent was obtained from all participants. The trial was registrated at European Clinical Trials Database EudraCT (identifier no. 2006-005187-13).
The study design is summarised in Fig. 1. During the initial screening visit, eligible subjects were given dietary and lifestyle recommendations for management of the metabolic syndrome according to the 2005 American Heart Association/National Heart, Lung, and Blood Institute scientific statement(Reference Grundy, Cleeman and Daniels17) and were submitted to a 9-week run-in phase. Before randomisation (visit 2a), subjects were asked to fill in a food diary for three consecutive days (before visit 2) and were asked to keep, as much as possible, the same diet during the whole trial to ensure comparable nutrient intake along the study. At the end of the run-in phase, subjects whose TAG had not reduced by 50 % as compared to the screening visit or were not < 150 mg/dl were randomised to consume twice a day an MPM-supplemented low-fat yoghurt or matching placebo yoghurt with an allocation ratio of 1:1 in a double-blind manner according to a random-permuted block scheme stratified by centre.

Fig. 1 Study design and visit schedule of the double-blind, randomised, placebo-controlled trial. Following a 9-week run-in phase, three visits (V3, 1 month; V4, 2 months; V5, 3 months) were scheduled during treatment to assess the change from baseline in efficacy variables. Treatment was the whey-derived malleable protein matrix (Wheygurt™) or matching placebo twice daily during 3 months. V1 and V2 were screening and baseline visits and V2a was the randomisation visit.
The composition of the whey MPM yoghurt (Wheygurt™) and of the placebo is shown in Table 1. Wheygurt™ provided about 7 g of MPM per serving. A plain low-fat yoghurt supplemented with small amounts of hydrolysed gelatine was used as placebo to match for daily energy, proteins and carbohydrates. All other flavouring ingredients and non-energy sweeteners were used in the same proportion. The trial products were supplied by Technologie Biolactis, Inc. (Laval, QC, Canada) as spoonable yoghurts identically packaged in 150 g servings. Each patient kit was covering a maximum of 15 d of trial product consumption. Subjects were instructed to appropriately refrigerate all trial product supplies and to consume one serving twice daily at meal time (morning and evening). A total of six patient kits were necessary to ensure adequate coverage of trial products for the entire treatment period of 3 months.
Table 1 Composition of the whey malleable protein matrix (MPM) and placebo yoghurts (g/100 g)

* Amount corresponding to a daily consumption of 2 × 150 g servings of either Wheygurt™ or matching placebo yoghurt.
Treatment adherence was calculated at each visit during the treatment phase based on used and unused trial products returned by the study participants from their previous visit. Patients were classified as adherent if they had taken at least 80 %, but not more than 120 % of the trial products. Compliance between 70 and 80 % or 120 and 130 % was rated as minor protocol deviation.
Blood was drawn after an overnight fast for lipid and lipoprotein analysis, other laboratory parameters and safety parameters at the screening visit 1, 2, 3, 4 and 5 as shown in Fig. 1.
Outcome variables
The primary objective of this trial was to investigate the TAG reduction from baseline by MPM compared to placebo. Secondary efficacy variables were changes in total cholesterol (TC), LDL-C, HDL-C, apo-B, FPG and insulin (INS), homeostasis model assessment (HOMA) index, HbA1c, high-sensitivity C-reactive protein, body weight, waist circumference, SBP and DBP.
Safety and tolerability assessments
The safety profile and tolerability were evaluated by recording adverse events (AE) as well as measurement of vital signs, chemistry and haematology variables at each clinic visit. The documentation of AE by the investigators was categorised according to severity (mild, moderate and severe) and causality to the study products (unrelated, unlikely, possible, probable, certain). AE were coded according to the WHO-Adverse Reaction Terminology (www.who-umc.org). The frequencies and incidence rates were calculated on a per-patient basis analysed according to O'Neill(Reference O'Neill18).
Laboratory methods and clinical assessments
TC, HDL-C, TAG (CHOD-PAP and GPO-PAP enzyme assays), FPG (hexokinase/glucose-6-phosphate dehydrogenase method) and high-sensitivity C-reactive protein (immunoturbidimetric latex highly sensitive assay) were determined using commercially available kits and reagents on an Olympus 2700 analyser (Olympus, Hamburg, Germany). LDL-C was determined according to the Friedewald equation (TC − HDL-C − (TAG/5)). INS determination (enzyme immunoassay) was performed on an Immulite® 2000 analyser (Siemens, Eschborn, Germany). Apo-B determination (nephelometric assay) was performed using a BN™ II system (Siemens). HbA1c was determined from EDTA whole blood by HPLC. HOMA index was calculated according to the following formula: (INS (pmol/L) × FPG (mmol/L))/162·32. Biochemical and haematologic safety parameters were determined by standard laboratory methods. All analyses were carried out in a central laboratory that was certified according to the standards of Good Laboratory Practice.
Blood pressure was determined according to the Riva-Rocci method at every visit, with the same blood pressure cuff on the same arm, and if possible, at the same time of the day after at least 5 min rest in a sitting position. The heart rate was measured at rest in a sitting position. Height was determined without shoes using a fixed wall-scale measuring to the nearest 0·1 cm. Weight was measured to the nearest 0·1 kg using an electronic scale that was calibrated before each measurement. Waist circumference was taken with a flexible tape (held horizontally) halfway between the lowest part of the costal arch and the crista iliaca of the hip bone while study participants were standing.
Statistical analysis
The intention-to-treat/full analysis set (ITT/FAS) was defined as those patients who were randomised to one of the trial treatments (all randomised patients) that received at least one dose of the trial product, including placebo, and for which at least one observation visit under the trial product was available. In this trial population, also patients with major protocol violations were included. The ITT/FAS was the primary population for the efficacy analyses in this superiority trial. The per-protocol (PP) set comprised all patients of the full analysis set, who were treated during at least 42 d with trial product and who did not have any major protocol violations. Patients who terminated the trial prematurely (drop-outs) due to inefficacy of the trial treatment and who did not have major protocol violations before trial termination were included in the PP set. The PP set was the secondary population for the efficacy analyses. Before the code was broken, a ‘blind review’ was conducted to decide which protocol violations could be characterised as major. Safety and tolerability were assessed for all patients who had taken at least one dose of the trial product, including placebo.
The primary efficacy parameter was the percentage change of TAG from baseline (visit 2) to 3 months (visit 5) in comparison to placebo. An end-point analysis using the changes from baseline to the last visit was carried out (i.e. visit 5 for completers and last visit available under treatment for drop-outs). The a priori statistical working hypothesis was that a low-fat yoghurt supplemented with MPM is more effective in lowering TAG than the energy/protein matched low-fat yoghurt placebo at study end point. The two-sided level of significance was stipulated at P = 0·05. This was tested initially by means of ANOVA with a full model with effects due to treatment, centre and the treatment-by-centre interaction. Homogeneity of treatment effect across centres was checked with pre-specified pooling criteria defined by either centre size (small centres combined) or medical setting (outpatient clinic v. other practitioners combined). The analysis of all other secondary variables at study end point or at each intermediate visit was done with the same model. Finally, the effect of treatment across study visits was explored by repeated-measures ANOVA with the full model defined with the main effects for treatment, centre and time (study visits 3, 4 and 5) and the interaction terms treatment-by-centre and treatment-by-time.
Sample size estimation was based on the percentage change of TAG from baseline, a level of significance of P < 0·05 (two-sided alternative), a power of 90 % and an expected effect size (Cohen's d) of 0·5. In order to achieve the calculated power, eighty-six evaluable patients were needed in each treatment group. A total of 100 patients per treatment group were planned to be randomised because of an expected drop-out rate of about 15 %.
The statistical package SAS version 8.02 was used for general calculations (SAS, Inc., Cary, NC, USA). Sample size was calculated by using the software ‘Nquery’ version 4.0 (Statistical Solutions, Cork, Ireland). Diet protocols were evaluated using Prodi version 5 Software (Wissenschaftliche Verlagsgesellschaft, Stuttgart, Germany) by NERM (Rheinbach, Germany). Change from baseline to last visit was calculated for total energy, fat (total, monounsaturated, polyunsaturated, saturated), protein, carbohydrates, alcohol, TC and intake of fibre. These variables were compared between the two treatment arms by means of one-way ANOVA.
Results
Subjects disposition, demographics and baseline characteristics
The flow of participants through the trial is presented in Fig. 2. A total of 395 patients were enrolled in thirteen trial centres, of which 197 patients were randomised into the double-blind treatment phase and 180 included in the ITT/FAS population. Of the randomised patients, ninety-nine received MPM and ninety-eight placebo. The reasons for premature termination of the trial were AE, withdrawal of consent and other reasons (see Fig. 2 for details). A total of 171 subjects completed the study with no major protocol deviation and were included in the PP population; eighty-three patients in the MPM and eighty-eight in the placebo group.

Fig. 2 Flow of participants through the trial. ITT, intention-to-treat; PP, per-protocol.
Subject characteristics are provided in Table 2. Demographic data were similar between the two treatment groups. About 53 % of the ITT/FAS analysed population were male. Average age was 53·4 (sd 9·8) years (range, 29–73) and BMI 31·0 (sd 4·1) kg/m2. Most of the patients were Caucasians (n 83 in MPM, n 88 in placebo). Definition of race was by patient self-identification. A history of allergy was reported in forty-one cases. Antihypertensive agents were the most frequently documented concomitant medications. The detailed list of the ten most frequent concomitant medications by Anatomical Therapeutic Classification was as follows: β-blockers (21 %), angiotensin-converting enzyme inhibitors (17 %), thyroid hormones (16 %), angiotensin receptor blockers (9 %), acetic acid derivatives and related substances (9 %), agents for local oral use (8 %), thiazides (8 %), propanoic acid derivatives (7 %), proton pump inhibitors (7 %) and fluoroquinolones (6 %).
Table 2 Demographic characteristics of the study population
(Number of participants, percentages, mean values and standard deviations)

ITT/FAS, intention-to-treat/full analysis set; MPM, malleable protein matrix; NCEP-ATP III, National Cholesterol Education Program-Adult Treatment Panel III.
* Primary population for efficacy analyses.
Overall adherence was 79 (sd 22) % (median 83 %) and 81 (sd 23) % (median 89 %) in the MPM and placebo groups, respectively.
Efficacy analyses
The efficacy analyses were carried out for the ITT/FAS (n 180) and for the PP (n 174) populations. The results of the PP analyses are not shown. In the ITT/FAS group, average treatment duration was 86 (sd 19) and 90 (sd 17) d (MPM or placebo, respectively). Table 3 shows the descriptive results of the changes from baseline to last visit available and to visit 5, after 3 months of treatment, in comparison to placebo. Baseline TAG concentrations were similar in the two groups (MPM: median 218·5 mg/dl, range 142–603 mg/dl; placebo median 203 mg/dl, range 139–565 mg/dl). As the primary outcome, the percentage TAG change from baseline was much greater in the MPM as compared to the placebo group, giving a highly significant relative treatment difference of − 14·0 % at last visit documented (P = 0·007) and − 16·0 % after 3 months (P = 0·004). After 3 months of treatment, TAG decreased by 22·3 mg/dl in the MPM group, while they significantly increased by 19·6 mg/dl in the placebo group. The treatment difference of − 16·6 % (P = 0·023) already reached statistical significance after 2 months (not shown in Table 3). The maximum efficacy in TAG reduction in the MPM group was apparently not reached after 3 months of treatment (Fig. 3). There were no other significant differences in lipids/lipoprotein percentage change from baseline, except a borderline significant difference (P = 0·049) in the ratio of LDL-C:HDL-C at study end point in favour of placebo. However, the respective absolute LDL-C:HDL-C changes of 0·08 in the MPM and − 0·12 in the placebo group were small.
Table 3 Lipid and lipoproteins results
(Number of participants, mean values and standard deviations)

LDL-C, LDL cholesterol; HDL-C, HDL cholesterol.
* P-values were obtained using simple ANOVA.
† To convert the values for TAG to mmol/l, multiply by 0·01129.
‡ To convert the values for cholesterol to mmol/l, multiply by 0·02586.
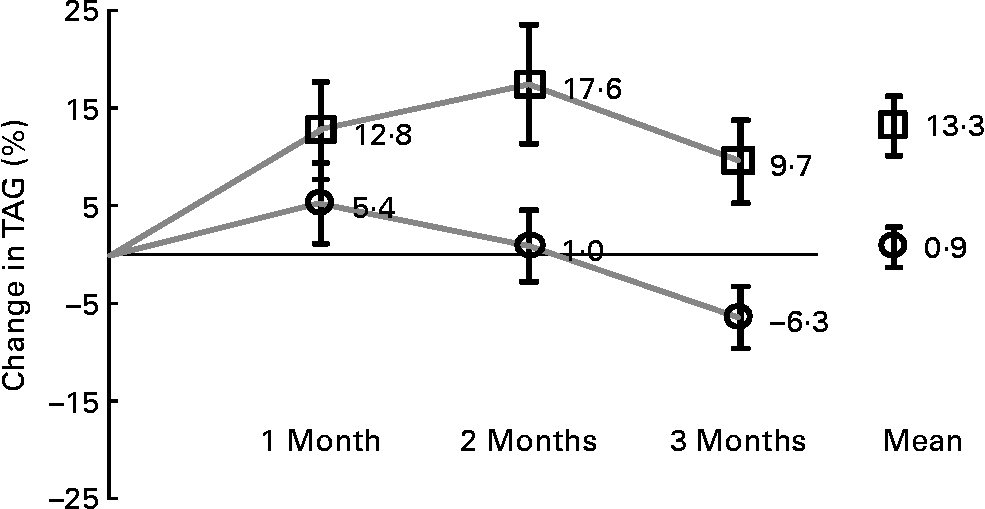
Fig. 3 Percentage change of mean values with their standard error from baseline in fasting TAG concentrations at each study visit and over the study period under treatment. ![]() , Wheygurt;
, Wheygurt; ![]() , placebo.
, placebo.
Among subjects meeting the metabolic syndrome criteria for TAG (n 84/88 in the MPM group; n 82/92 in the placebo group), fifty-nine subjects in the MPM and forty-seven in the placebo group had TAG at baseline greater than 200 mg/dl, defined as high TAG concentrations according to the National Cholesterol Education Program-Adult Treatment Panel categories(2) (see Table 3). In this subset, TAG decreased by 36·8 mg/dl in the MPM group and increased by 16·7 mg/dl in the placebo group, a relative treatment difference effect of − 17·5 % after 3 months (P = 0·005). The treatment difference of − 23·1 % (P = 0·019) also reached statistical significance already after 2 months (not shown in Table 3).
The percentage change from baseline to the end of the study in glucose-related variables is shown in Table 4. For FPG, after 3 months, the relative treatment difference approached but did not reach statistical significance (P = 0·089), while the corresponding absolute treatment difference of − 6·1 mg/dl was significant (P = 0·041). Among subjects meeting the metabolic syndrome criteria for FPG ( ≥ 100 mg/dl), twenty subjects in the MPM and fifteen in the placebo group could be classified as having impaired fasting glucose (IFG) or as having T2DM. In this subset, relative treatment differences in favour of MPM, ranging from − 9·2 % (P = 0·093) at the last visit to − 11·3 % (P = 0·033) after 3 months, were observed. In this subset, five subjects in the MPM and three subjects in the placebo group were taking antidiabetic medications. The medications were unchanged during the course of the trial. No significant changes were observed in fasting INS concentrations or HOMA index.
Table 4 Glucose metabolism results
(Number of participants, mean values and standard deviations)

MPM, malleable protein matrix; FPG, fasting plasma glucose; HOMA, homeostasis model assessment.
* P-values were obtained using simple ANOVA.
† To convert the values for glucose to mmol/l, divide by 18.
‡ HOMA index was calculated as (fasting insulin (μU/ml) × fasting plasma glucose (mg/dl))/405.
Changes in blood pressure and other efficacy variables are shown in Table 5. For BP, no treatment difference was seen when all ITT/FAS subjects were analysed together. Among subjects meeting the metabolic syndrome criteria for BP, fifty-two subjects in the MPM and sixty subjects in the placebo group could be categorised as hypertensive based on the National Heart, Lung and Blood Institute Joint National Committee 7 criteria(Reference Chobanian, Bakris and Black19). There was a significant absolute treatment difference in favour of MPM, achieving − 6·7 mmHg (P = 0·028) and − 5·9 mmHg (P = 0·054) at the last visit and after 3 months, respectively. Antihypertensive medications were documented in 56 % of hypertensive subjects (n 29) and 62 % of hypertensive subjects (n 37) in the MPM and placebo groups, respectively (medications also remained unchanged during the trial).
Table 5 Other efficacy results
(Number of participants, mean values and standard deviations)

MPM, malleable protein matrix; NA, not applicable; hsCRP, high-sensitivity C-reactive protein; SBP, systolic blood pressure; DBP, diastolic blood pressure;
* P-values were obtained using simple ANOVA.
† Mean treatment differences are given as absolute changes in mmHg.
After 3 months, a significant treatment difference in relative weight change from baseline of − 2·1 % (P = 0·015) in favour of MPM was observed. This corresponds to an absolute treatment difference of − 1·7 kg between the two treatment groups. The demographic and baseline characteristics of the subset of participants (n 41/88 in the MPM; n 43/92 in the placebo group) from which weight changes after 3 months were calculated are comparable to the ITT/FAS population. Measurements of anthropometric data (waist, weight) were included in the routine evaluation of participants only after the start of the trial and are therefore not available for the whole ITT/FAS population. No treatment difference in waist change from baseline to the end of the study could be observed.
As shown in Table 6, the absolute number of participants having the metabolic syndrome was not significantly different between the MPM and the placebo groups when comparing baseline and last visit prevalence.
Table 6 Number of subjects and frequency of the various components of the metabolic syndrome at last visit available*

MPM, malleable protein matrix.
* Intention-to-treat/full analysis set.
† Fisher's exact test.
Safety results
A summary of the AE recorded during the trial is documented in Table 7. A total of sixty-seven patients (34·0 %) experienced seven serious AE (SAE) and 129 non-SAE in both treatment groups. In the MPM group, thirty-six (36·4 %) patients had AE/SAE and thirty-one (31·6 %) had AE/SAE in the placebo group. There was no difference between the two groups regarding the number of patients with at least one AE or SAE (Fisher's exact test P = 0·85). The most common AE were gastro-intestinal in nature. There were no relevant differences between the MPM and the placebo groups regarding tolerability assessments.
Table 7 Incidence of adverse events (AE)
(Percentages, number of adverse events and patients)

MPM, malleable protein matrix; SAE, serious adverse event.
* Rated as possible, probable or certain.
Energy and nutrient intake
Food records from the beginning (end of run-in phase) and end of the trial were evaluated using computerised nutrient analysis. There were no significant differences between treatment groups in total energy, fat (total, monounsaturated, polyunsaturated, saturated), protein, carbohydrates, alcohol, TC and fibre intake (Table 8).
Table 8 Results of the 3 d food records at the end of the run-in period (before visit 3) and the end of the treatment period (before visit 5)
(Mean values and standard deviations)

* The reported amounts are exclusive of the trial products.
Discussion
Hypertriacylglycerolaemia is implicated in the pathogenesis of CHD(Reference Gandotra and Miller1, Reference Gouni-Berthold and Krone20). Elevated TAG concentrations ≥ 150 mg/dl are considered to be one of the components of the metabolic syndrome. Any person at high or moderately high cardiovascular risk who has elevated TAG or the metabolic syndrome is a candidate for therapeutic lifestyle changes to modify these risk factors, regardless of LDL-C concentrations(Reference Grundy, Cleeman and Merz21). Non-pharmacological treatment options for elevated TAG are becoming increasingly popular and include various diets (e.g. Mediterranean diet)(Reference Grundy22), n-3 fatty acids(Reference Bays23), soya proteins(Reference Anderson, Johnstone and Cook-Newell24), fibre(Reference Anderson25) and red yeast rice(Reference AbouRjaili, Shtaynberg and Wetz26), with various levels of evidence supporting their efficacy(Reference Nies, Cymbala and Kasten27).
In the present rigorously controlled trial, we show that in subjects with the metabolic syndrome and/or hypertriacylglycerolaemia, the whey-derived MPM decreases fasting TAG concentrations by about 15 % as compared to placebo. Moreover, MPM decreases FPG by approximately 10 % in patients with impaired fasting glucose or T2DM, and SBP by approximately 6 mmHg in subjects with hypertension. MPM consumption is also associated with a decrease in body weight by approximately 1·5 kg over 3 months. MPM was well tolerated and no major safety concerns were observed. The most common adverse effects were of gastro-intestinal nature, such as diarrhoea, flatulence, nausea and upper abdominal discomfort (see Table 7).
These results are in agreement with our recent findings that in patients with hypercholesterolaemia, MPM decreases TAG concentrations by approximately 10 %(Reference Berthold, Schulte and Lapointe16). Supplementation with MPM has previously been shown to have TAG- and LDL-C-lowering properties as well as FPG- and BP-modulating effects in animal models(Reference Beaulieu, Millette and Trottier15).
The BP- and glucose-lowering effects of MPM could be mediated via its anti-inflammatory properties(Reference Beaulieu, Dupont and Lemieux13, Reference Beaulieu, Girard and Dupont14) since inflammation has been associated with both, hypertension and INS resistance(Reference Wang, Gona and Larson28, Reference Libby and Plutzky29). The lipid-lowering effects of MPM could be attributed to specific components of the product (whey proteins and peptides, bacteria-cell debris and exopolysaccharides, vitamins and minerals, such as Ca). In this regard, whey proteins have been shown to decrease de novo cholesterol biosynthesis in the liver(Reference Zhang and Beynen30), to modulate the inhibition of the expression of genes involved in intestinal cholesterol and fatty acid synthesis and absorption(Reference Chen and Reimer31), the inhibition of β-lactoglobulin-mediated cholesterol absorption in the intestine(Reference Nagaoka, Kanamaru and Kuzuya32) and/or to increase faecal sterol excretion(Reference Zhang and Beynen30, Reference Lovati, West and Sirtori33). However, they are most probably due to the synergy among many of these components within MPM(Reference Jacobs, Gross and Tapsell34).
While several studies have investigated in animal models the lipid-lowering ability of whey proteins or specific peptides, the results remain inconclusive(Reference Nagaoka, Kanamaru and Kuzuya32, Reference Nagaoka, Kanamaru and Kuzuya35–Reference Kawase, Hashimoto and Hosoda38). The lipid-lowering properties of whey proteins were investigated till now only in a few clinical trials in human subjects. Kawase et al. (Reference Kawase, Hashimoto and Hosoda38) showed that a 4-week consumption of fermented milk supplemented with a whey protein concentrate, providing around 7 g/d, transiently lowers TAG, but not LDL-C, from baseline (no difference to placebo was reported). However, in the present study, the placebo was given as a non-fermented product, complicating the interpretation of the results. Pal et al. (Reference Pal, Ellis and Dhaliwal9) used a whey protein isolate in overweight and obese subjects (27 g protein/d over 12 weeks) and showed that fasting TAG decreased by 22 %. Bortolotti et al. (Reference Bortolotti, Maiolo and Corazza10) used a whey protein supplement in obese females (60 g/d over 4 weeks) and showed that fasting TAG decreased by 15 %. Our group has recently studied, in a randomised, double-blind, 12-week trial, the effects of MPM (2 × 15 g/d) on lipoprotein concentrations in 161 subjects with hypercholesterolaemia and observed a 10 % decrease in TAG in the MPM group compared to placebo(Reference Berthold, Schulte and Lapointe16). Some studies involving subjects with moderate hypercholesterolaemia report lipid-lowering activities of fermented milk products containing live bacteria such as lactobacilli and bifidobacteria(Reference Agerbaek, Gerdes and Richelsen39–Reference Xiao, Kondo and Takahashi43), but it is unlikely that the observed effect of MPM on lipids come from such probiotic properties since the Lactobacillus strain used in the production of MPM is heat-killed.
In the present trial, we examined the effects of MPM in patients with the metabolic syndrome. Subjects with the metabolic syndrome (and no DM) have an increased risk of all-cause mortality (risk ratio in men 1·4, in women 1·38)(Reference Lakka, Laaksonen and Lakka44) as well as from CVD (risk ratio in men 2·26, in women 2·78)(Reference Mottillo, Filion and Genest45). The prevalence of the metabolic syndrome is high and steadily increasing. Between 1988 and 1994, it was about 25 % and has increased to approximately 35 % between 2003 and 2006, according to the third National Health and Nutrition Examination Survey data(Reference Ervin46). The prevalence of hypertriacylglycerolaemia (about 30 % of US adults have fasting TAG ≥ 150 mg/dl) remained stable over the same period of time(Reference Ervin46, Reference Ford, Li and Zhao47). However, the prevalence of the remaining components of the metabolic syndrome in the same time period increased. Hyperglycaemia increased from 13 to 39 % (at least partly due to the fact that the hyperglycaemia definition was changed from FPG of >110 mg/dl to >100 mg/dl(Reference Grundy, Brewer and Cleeman48, Reference Ford, Giles and Dietz49)), the prevalence of hypertension increased from 34 to 40 % and the one of abdominal obesity from 38·6 to 53 %. Interestingly, the only component of the metabolic syndrome that improved over time is the prevalence of low HDL-C (decrease from 37·1 to 25 %).
Since the metabolic syndrome is a cluster of cardiometabolic risk factors, its management is based on the treatment of its individual components. In clinical practice, lifestyle therapies (weight loss in overweight or obese subjects, increased physical activity and reduction of the intake of saturated fat, trans-fat and cholesterol) are the first-line interventions and produce an improvement in all of the metabolic risk factors simultaneously(Reference Grundy, Cleeman and Daniels50). The next step available would be pharmacotherapy for each individual metabolic syndrome component which, for obvious reasons, should be, if possible, avoided (e.g. costs, side-effects, medicalisation). Use of MPM is unique, because to our knowledge it is the only (non-drug) substance that not only decreases TAG but also positively influences all the components of the metabolic syndrome (decrease in SBP, FPG and body weight), except for one (low HDL-C, where no effect was shown). The multifactorial approach (glucose, lipids and blood pressure) to treatment is of paramount importance, as shown in patients with DM(Reference Gaede, Lund-Andersen and Parving51). The MPM-associated decrease in FPG may be of clinical importance since increased FPG concentrations have been shown to be associated with cardiovascular mortality, even below the diabetes threshold(Reference Coutinho, Gerstein and Wang52). Interestingly, it has been shown recently that whey proteins may also decrease intrahepatocellular lipids in mice and humans(Reference Shertzer, Woods and Krishan7, Reference Bortolotti, Maiolo and Corazza10) and thus may reduce liver steatosis, another element of the metabolic syndrome.
In the present study, only small reductions in the glucose levels in the MPM group were observed, and these mainly in the subgroup of patients with impaired fasting glucose or T2DM. No significant changes were observed in the HOMA index, however, although there was also a decrease in body weight. These seemingly contradictory results may be due to the fact that waist circumference was not decreased in this study. Waist circumference has been established as the best surrogate for visceral adipose tissue(Reference Ho, Chen and Woo53–Reference Jia, Lu and Xiang55), which in turn seems to be a better predictor of INS resistance than body weight(Reference Aekplakorn, Kosulwat and Suriyawongpaisal56, Reference Henderson, Fan and Sharma57). It could be postulated that treatment duration was not long enough to achieve changes in waist circumference. MPM was associated with a significant weight loss of 1·7 kg, an effect that could be at least partially attributed to potential anorexigenic properties of whey protein-based products(Reference Diepvens, Haberer and Westerterp-Plantenga58). Considering that even small decreases in weight (of approximately 2·2 kg) have been shown to influence cardiovascular risk(Reference Wilson, Kannel and Silbershatz59), the MPM-associated weight loss, although numerically small, may be clinically relevant. Moreover, regarding the SBP-lowering effects of MPM (approximately 6 mmHg), it is known that a reduction in SBP of 1 mmHg can decrease the covariate-adjusted CHD mortality by about 2 %(Reference Gerber, Dankner and Chetrit60).
Our present study has some limitations. First, twenty-five out of 197 subjects (ten in the MPM and fifteen in the placebo group), had hypertriacylglycerolaemia but did not meet all the criteria for the diagnosis of the metabolic syndrome. However, it is unlikely that these relatively small numbers, similarly distributed in the two groups, would alter our findings. Second, the treatment duration was 3 months. Therefore, it might be argued that some effects of MPM, as for example, on HbA1c, high-sensitivity C-reactive protein and TAG concentrations, might take longer to fully develop. In this context, it was interesting to note that there was a transient increase in TAG concentrations after treatment initiation (more pronounced in the placebo than in the MPM group), an effect that is also seen with other lipid-lowering therapies(Reference Angelin, Leijd and Hultcrantz61) and which could be explained in the present study by a stimulatory action on hepatic VLDL TAG production by the dietary supplement. The time-course of TAG concentrations (see Fig. 3) indicates that TAG concentrations in the MPM arm were always lower than the ones in the placebo arm and that the maximum potential TAG-lowering effects (by increase in VLDL catabolism) have not been reached after 3 months yet. A double-placebo run-in period before randomisation may have been useful. Third, the daily dosage of Ca provided in the MPM-containing yoghurt formulation was higher than the one of the placebo yoghurt (0·4 v. 0·1 g/100 g, respectively). Since high Ca intake has been suggested to have lipid-lowering effects(Reference Major, Chaput and Ledoux62), an effect of Ca on the observed lipid-lowering properties of MPM cannot be excluded.
Strengths of the study include its design, which is the one generally accepted for studies investigating lipid-lowering agents(Reference Berthold, Unverdorben and Degenhardt63). The trial was of the multi-centre type. All laboratory measurements were done in a central, certified laboratory. The trial consisted of a placebo and diet run-in phase initiated 9 weeks before baseline values were drawn and at the beginning of which instructions to adhere to diet and lifestyle recommendations were given. Diet had no influence on the results, as confirmed by comparison of the 3 d food records at the end of the run-in and the treatment phase.
In conclusion, while diet and exercise remain the fundamental treatment modalities for the metabolic syndrome(Reference Grundy, Cleeman and Daniels50), our findings suggest that daily consumption of the natural whey fermentation product, MPM, significantly improves almost all risk factors of the metabolic syndrome, with the strongest effect being the TAG-lowering, and could thus provide an additional, non-pharmacological therapy, for this prevalent disorder.
Acknowledgements
The present research received financial support from Technologie Biolactis Incorporated, Laval, QC, Canada. Employees of the sponsor participated in the design of the study, but the sponsor had no role in the analysis and interpretation of the data and in the decision of publishing the manuscript. I. G.-B., D. M. S., W. K., H.-G. P. and H. K. B. have no conflicts of interest to declare. J.-F. L. and P. L. are employees of the sponsor. The authors' responsibilities were as follows: H. K. B., J.-F. L. and P. L. designed the research; I. G.-B., D. M. S., W. K. and H.-G. P. conducted the research; H. K. B., I. G.-B., J.-F. L. and P. L. analysed the data; H. K. B., I. G.-B., J.-F. L. and P. L. wrote the manuscript; D. M. S., W. K. and H.-G. P. provided administrative, technical and material support; H. K. B. had primary responsibility for the final content. All authors read and approved the final manuscript.













