Introduction
Control of blood-borne infections such as HIV and hepatitis C virus (HCV) among persons who inject drugs (PWID) has traditionally focused on reducing the number of unsafe injections per person through syringe exchange and medically assisted treatments of methadone and buprenorphine. Recent advancements in drug therapies for treatment of HIV and HCV infection – the latter in the form of direct-acting antivirals that are now pangenotypic and nearly completely curative [Reference Deterding1, Reference Feld2] – have spurred interest in novel HCV control strategies [3]. Evidence of the preventive impact of antiviral treatment on infectiousness [Reference Cohen4] has motivated investigation of strategies to slow transmission by reducing the size of the infectious population through treatment [Reference Granich and Williams5, Reference Grebely and Dore6]. The appeal of these treatment-based approaches lie in their ability to appropriate drugs already in use for therapeutic treatment and exploit them for their population-level preventive effects. Critics have questioned the feasibility, however, of achieving the ambitious treatment coverage levels required by such strategies given the social–structural treatment barriers faced by PWID that often result in decadal delays in treatment [Reference Bruggmann and Grebely7]. Particularly in the case of HCV, high drug costs are forcing tough decisions, with a prioritisation of treatment for those with advanced disease [Reference Gornall, Hoey and Ozieranski8], a proposal that some fear overlooks opportunities to avert eventually costly complications and that also miss opportunities to treated asymptomatic PWID who may inadvertently be contributing to further new infections [Reference Innes9]. Informed targeting of a finite resource of antiviral drugs must therefore balance ethical questions of meeting clinical demand with what is known about the potential for treatment to block transmission.
Mixing matrices quantify how people with similar or different demographic and other characteristics make contact with each other in a population. Together with information on behavioural and disease distributions across subgroups, matrices can illustrate epidemiologically salient patterns of contact and identify potential points of treatment-based prevention strategies. Cluster randomised trials are exploring the efficacy of treatment to prevent HIV transmission in PWID (e.g. Integrated Care Centers to Improve HIV Outcomes in Vulnerable Indian Populations (ClinicalTrials.gov Identifier: NCT01686750) or the Integrated Treatment and Prevention for People Who Inject Drugs (ClinicalTrials.gov Identifier: NCT02935296)), but an equivalent strategy for HCV remains largely theoretical [Reference Martin10, Reference Martin, Vickerman and Hickman11], with several exploratory studies underway in Australia (ClinicalTrials.gov Identifier: NCT02363517 and NCT02102451). In the absence of data, most such models assume that mixing among PWID occurs randomly and homogeneously, with probabilities of partnership formations based solely on relative group size. A notable exception involves a series of empirically grounded network models using data from the ‘Networks 2’ study, a sociometric network of PWID in Melbourne [Reference Miller12]. These models provide key insights into how disease spreads through social networks, although none describe mixing patterns within the observed population. Given the rarity of truly random mixing in human populations [Reference Newman13], as well as the substantial impact of mixing assumptions on model outcomes [Reference Garnett and Anderson14, Reference Anderson15], empirical evidence of PWID mixing patterns – and in a directly useable form for future modelling efforts – is clearly needed.
A better understanding of mixing patterns can also inform our understanding of population-level transmission dynamics by providing insight into the extent to which disease is transmitted among and across population subgroups. Highly assortative populations can often more resemble a series of separate networks within which most of the transmission circulates [Reference Newman13]. The incidence and prevalence of infection in each such sub-network of an assortatively mixed population are therefore shaped less by the amount of disease present in other subgroups as by factors such as early epidemic seeding patterns or group-specific risk behaviours. Well-mixed populations, by contrast, experience broader diffusion of disease throughout groups. Particularly in the case of HCV, treatment delays due to long asymptomatic periods, barriers to care commonly faced by PWID and rapid acquisition of blood-borne diseases in new initiates to injection drug use [Reference Thomas16–Reference Garfein18] all create substantial age gaps between those at greatest risk of transmitting and those with symptomatic disease. These dynamic patterns, together with information regarding mixing patterns and relative distribution of disease across subgroups, can provide guidance of targeting treatment-based strategies to reduce HCV transmission [Reference Read, Eames and Edmunds19].
Here we present empirical data to describe the patterns by which PWID in Baltimore, Maryland share drug injection equipment with partners according to age, gender and race. Findings are intended to provide a better understanding of mixing patterns crucial for informed design and implementation of treatment-based prevention strategies in PWID.
Material and methods
Study population
Our analysis uses data on PWID and their drug using networks from the STEP into Action (STEP) study, an HIV prevention intervention among PWID in Baltimore, Maryland [Reference Tobin20]. Data were collected semi-annually over 18 months from April 2005 through September 2007 in four consecutive surveys. Participants were recruited through targeted street outreach, word-of-mouth and posted advertisement in communities with documented prevalence of illicit drug use. Enrolees were eligible to take part as primary participants if they were 18 years or older, had injected drugs in the prior 6 months, resided in Baltimore, had not participated in HIV or network study in the past year and were willing to provide written informed consent.
Study procedures
Consenting individuals completed interviews that included both interviewer-administered sections and Audio Computer-Assisted Self-Interview (ACASI) sections for items pertaining to drug use and sexual behaviours. A personal support network inventory was used to solicit names of network members including those with whom respondents shared drug injection equipment in the previous 6 months with the following naming stimulus, ‘Think back to the last time that you shared a cooker/needle. Who are the people that you shared with?’ Respondents then provided the first name and initial of the last name for each member of their social network with whom they had shared injection equipment provided they had known this person for at least a month. Specific information was further collected for each listed name, including demographic characteristics, nature of the relationship and recent sharing behaviours between the respondent and sharing partner. No limits were placed on the number of partners that could be named. Contact with listed partners was differentiated by the type of sharing reported (i.e. sharing needles, sharing cookers or sharing both). This information was collapsed into a single measure (i.e. “any sharing”) after confirmation no substantial differences if analysed separately.
Protocols were approved by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board prior to study implementation.
Statistical analysis
Characteristics of drug equipment-sharing partnerships were explored by pooling dyads reported across the four surveys. Sharing dyads were constructed on the basis of characteristics (age, gender, race) reported by the participants for both themselves as well as for their sharing partners.
Patterns of contact between each subgroup were estimated as the ratio of observed shares between each age group relative to the expected number of shares between them. The probabilities were adjusted for the availability of shares in each age group, which was expressed as the product of the average number of partnerships in each subgroup and the size of each subgroup, which in this case was estimated from the AIDS Linked to IntraVenous Experiences (ALIVE) study. The ALIVE cohort is one of the largest and longest standing (since 1988) PWID cohorts in the country. Its diverse recruitment methods including community outreach at drug treatment centres, shelters for the homeless, local emergency departments and areas of the city with heavy drug using activity is thought to draw distinct sample of PWID from the same underlying population as the STEP study [Reference Celentano21]. Summary baseline characteristics of both populations are provided in Table 1.
Table 1. Age, gender and racial distributions in the baseline studies of the AIDS Linked to IntraVenous Experience (ALIVE) and STEP into Action (STEP) study cohorts, both conducted in Baltimore, MD

ALIVE data represent the subset of participants who were enrolled in 2005 and 2006, the same period of time as the STEP study.
a Recent behaviours of ALIVE participants correspond to those reported most recently in 2005 or 2006.
The observed intensity of sharing between each subgroup combination was then calculated as:
where c i,j represents the average number of sharing partnerships between groups i and j, N i represents the total number of individuals in group i in the STEP study, and ![]() $N_i^* $ represents the total number of individuals in group i in the ALIVE study. The same value was calculated under the assumption of proportional mixing; that is, expected sharing patterns if partner choice was dictated only by the availability of sharing partners in each age group, in the following way:
$N_i^* $ represents the total number of individuals in group i in the ALIVE study. The same value was calculated under the assumption of proportional mixing; that is, expected sharing patterns if partner choice was dictated only by the availability of sharing partners in each age group, in the following way:
 $$E_{i,j} = \displaystyle{{c_iN_i^{^\ast} c_jN_j^{^\ast}} \over {\mathop \sum \nolimits_{i,j} c_kN_k^{^\ast}}}, $$
$$E_{i,j} = \displaystyle{{c_iN_i^{^\ast} c_jN_j^{^\ast}} \over {\mathop \sum \nolimits_{i,j} c_kN_k^{^\ast}}}, $$
where c i and c j represent the average number of sharing partners reported by groups i and j, respectively, and ![]() $N_i^* $ and
$N_i^* $ and ![]() $N_j^* $ represent the total number of individuals in groups i and j in the ALIVE study, respectively. The denominator provides the total number of available contacts in the population, assuming the age distributions in the ALIVE study are representative of the true population. The ratio of these two values, O i,j/Ei,j, therefore represent the extent to which the sharing observed in the STEP data departs from the assumption of proportionate mixing if the observed population had the same age, gender and race distribution as in the ALIVE study.
$N_j^* $ represent the total number of individuals in groups i and j in the ALIVE study, respectively. The denominator provides the total number of available contacts in the population, assuming the age distributions in the ALIVE study are representative of the true population. The ratio of these two values, O i,j/Ei,j, therefore represent the extent to which the sharing observed in the STEP data departs from the assumption of proportionate mixing if the observed population had the same age, gender and race distribution as in the ALIVE study.
Calculated ratios were then used to estimate the relative preference of each subgroup to share with partners of every subgroup over and above the chance of doing so if partner choice were driven solely by subgroup size alone (i.e. proportional mixing). Ratio values were also used to populate contact matrices, which showed the relative intensity of mixing across every subgroup combination, providing a summary of population-level assortativity. Bootstrapped confidence intervals (CI) were estimated by sampling individuals with replacement over 1000 iterations. The statistical strength of results of the statistics was indicated in terms of whether cell values departed from expectation based on proportionate mixing in 95% or more bootstrap-replicated datasets from our data. This was indicated in the matrix figures using shading to indicate where the departure of the expectation was statistically significant (darker shades) and where it was not (lighter shades).
The extent of assortativity observed in our study was then compared with the past reports of mixing using various methods. To compare our findings with the only known study to date describing drug equipment-sharing patterns by Williams et al. [Reference Williams22], we estimated the proportions of reported partnerships formed among members of the same age, race or gender group, as in the original study. We also compared assortativity observed in our study to other forms of mixing including patterns of casual contact patterns observed in a multi-national influenza study in Europe (the Polymod study) [Reference Mossong23] and sexual mixing in behavioural surveys from Finland [Reference Haavio-Mannila and Kontula24]. The availability of matrix data for these two studies allowed us to compare assortativity using a simple measure of diagonality, defined as the proportion of partnerships between members of the same group relative to all partnerships in the network. Diagonality measures range from a minimum value of zero to a maximum of 100%, in which the minimum value would indicate that no contacts were made between any members of the same attribute (i.e. age group, gender, race) and the maximum value would indicate that contacts take place exclusively between partners of the same attribute. Where data allowed, 95% CI for diagonality measures were constructed using 1000 sample redraws with replacement.
Results
Of the 1025 individuals recruited into the study, 70 (6.8%) reported at every visit that they only ever injected alone, and another 308 (30.0%) reported that they had not injected drugs in the past 6 months. The analysis was therefore restricted to the remaining 647 (63.1%) who collectively reported 2651 partnerships over the course of four study visits, an average of 4.1 partners per participant. Table 2 details participant characteristics, stratified by the number of sharing partners they reported at their baseline visit. Those with one sharing partner in the past 6 months made up the largest group (47.6%; 95% CI 43.8–51.5), while those with over four members in the past 6 months made up the smallest (8.8%; 95% CI 6.6–11). Size of sharing network differed significantly by relationship status, with single PWID more likely to report sharing with only one partner (67.9%; 95% CI 62.6–73.1%) than their non-single counterparts (32.1%; 95% CI 26.9–37.4%), a pattern which was reversed as network size increased.
Table 2. Demographics and baseline network characteristics of the 647 STEP participants with at least one drug-sharing partner
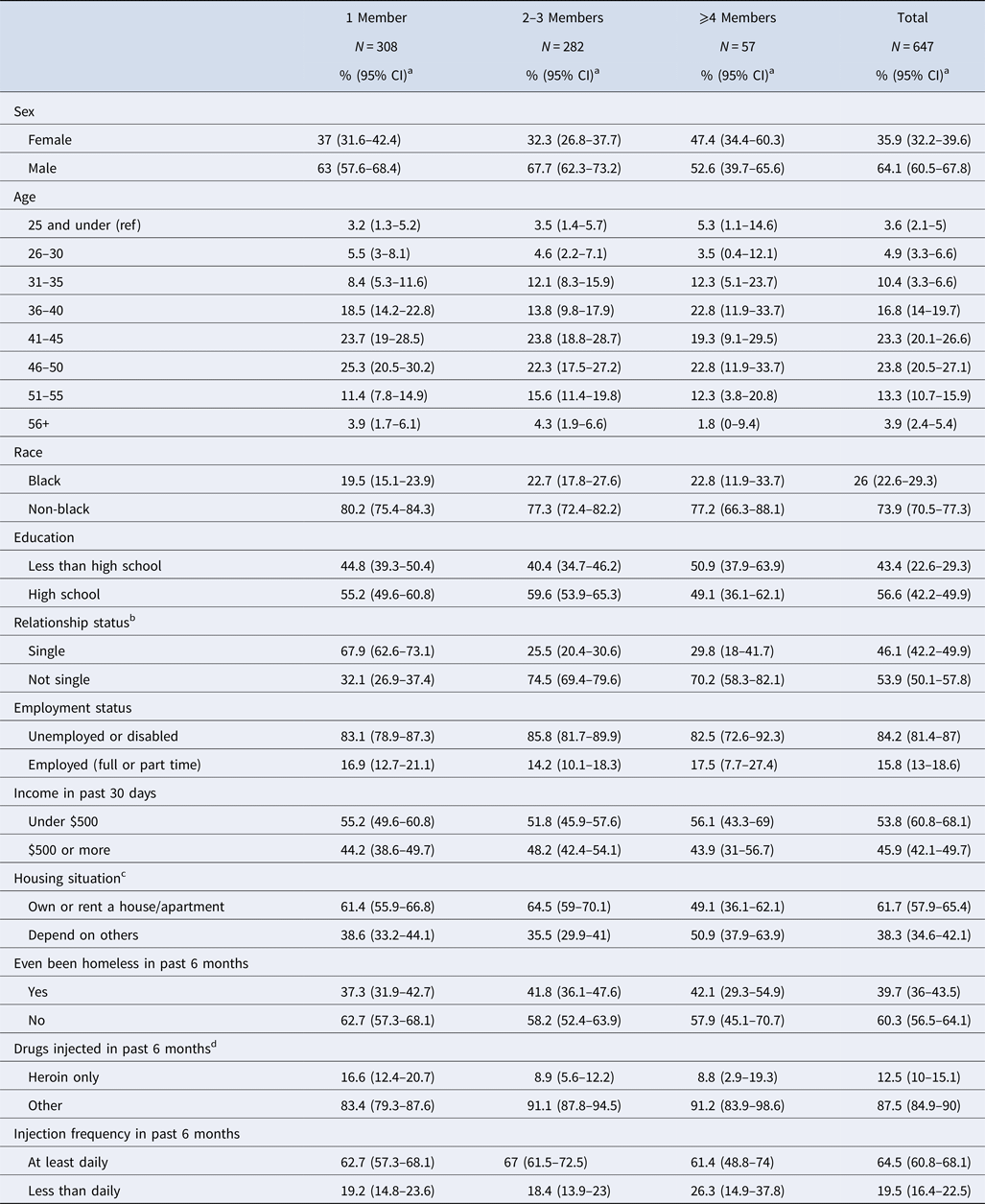
a Wald (normal approximation) confidence intervals for proportions were calculated with an α level of 5%, except for any variables with cell counts <5, for which Clopper–Pearson confidence intervals were calculated.
b Relationship status: ‘not single’ includes ‘married’ and ‘in committed relationship’; single includes ‘divorced’, ‘separated’ or ‘widowed’.
c Housing: ‘depend on others’ includes renting from others, staying with someone for free or living on the street or in more than two locations; ‘own or rent a house/apartment’ includes those reporting living in a house or apartment that they themselves own.
d Drugs injected: ‘heroin’ refers to injection of heroin, as opposed to snorting or sniffing it. ‘Other’ drugs that could be injected included, but were not limited to, speedball, cocaine.
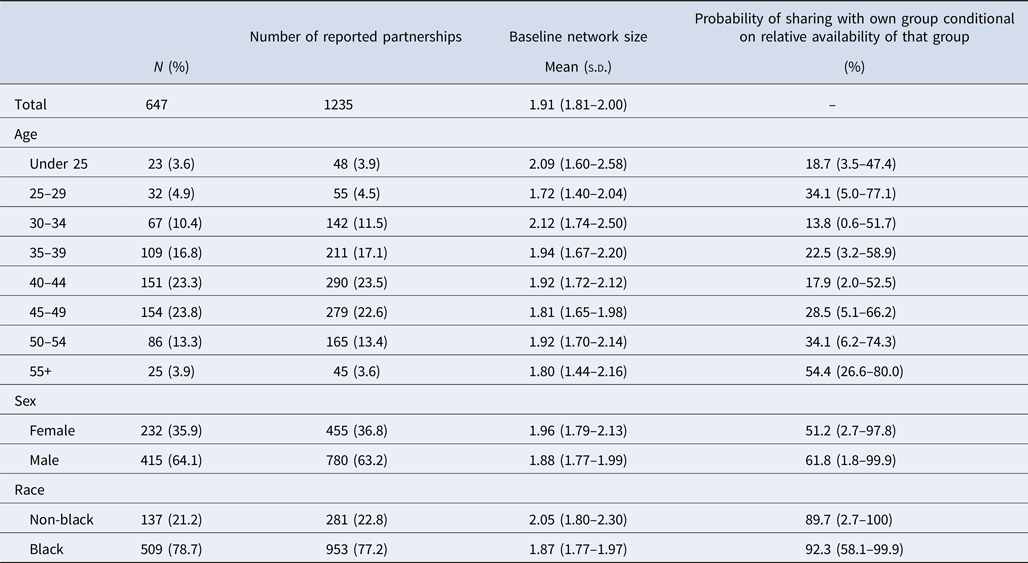
Summary network characteristics in Table 3 show that baseline network sizes were on average larger among younger, female and non-black respondents. Baseline network sizes varied by age group, with those in the 30–34 years old age group reporting the largest number of partners (2.12; 95% CI 1.74–2.50) and those in the 25–29 years old category reporting the fewest (1.72; 95% CI 1.40–2.04). Female PWID reported larger networks than their male counterparts (1.96; 95% CI 1.79–2.13 vs. 1.88; 95% CI 1.77–1.99) and non-black PWID reported larger networks than black PWID (2.05; 95% CI 1.80–2.30 vs. 1.87; 95% CI 1.77–1.97). Overlapping CI across all subgroups, however, suggest that differences were not statistically significant. Adjusted probabilities of the propensity to share with one's own age group indicate that those 55 and older were the most likely to share with their own age group (54.4%; 95% CI 26.6–80.0%), and those in the 30–34 years age group the least likely (13.8%; 95% CI 0.6–51.7%); however, the relationship between selectiveness and age did not appear to be linear. With respect to gender, men had a slightly higher propensity to share with their own gender as compared with women (61.8%; 95% CI 1.8–99.9% vs. 51.2%; 2.7–97.8%), and black PWID had a slightly higher propensity to share with their own racial group than non-black PWID (92.3%; 95% CI 51.8–99.9 vs. 89.7%; 95% CI 2.7–100%), though differences reported were not statistically significant.
Table 3. Network characteristics by age, gender and race among the 647 STEP participants with at least one drug-sharing partner in the baseline visit
Age-based mixing matrices demonstrate that mixing in this population is assortative by age, as indicated by higher rates of sharing among partners in the diagonal or near-diagonal cells (i.e. those of the same or similar ages) relative to frequencies expected that were mixing completely proportionate (Fig. 1). The average of the values among partners in the same age group (along diagonal line) was 2.86, indicating the average extent to which sharing between partners of the same age group departed from that which would be observed under the assumption of proportionate mixing. Among partners who differed by one age category (average of cells adjacent to either side of the diagonal line), this same average value fell to 2.00. Lastly the average across partners differed by more than one age category (values of all remaining cells) was estimated to be 0.69, representing the average extent to which sharing among age groups who differed by more than one category departed from the proportionate mixing assumption. Most reported partnerships were with the partners of the same age group or younger, as indicated by the higher ratio values in the off-diagonal cells of the lower right quadrant (average 1.43) compared with the upper left (average 0.59). Raw data for the age-based mixing matrix are provided in Supplementary materials.
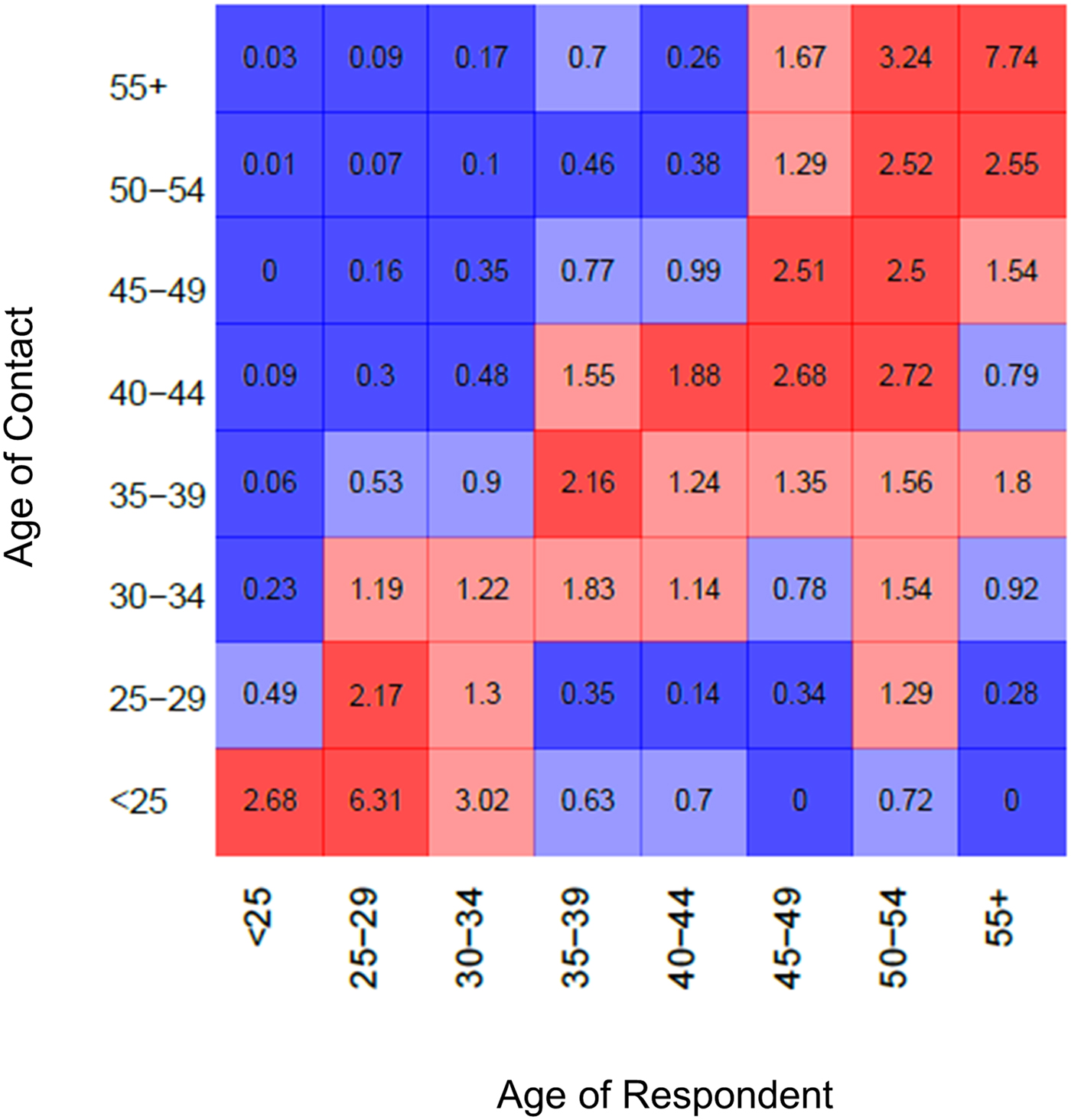
Fig. 1. Age-dependent mixing. Values are ratios of expected numbers of sharing partnerships between PWID of ages i and j under the observed patterns, vs. number of shares under the proportionate mixing assumption. Blue colours indicate less mixing between age groups than expected under the proportionate mixing assumption; red colours indicate more mixing than expected. Lighter shades indicate ratio values whose 95% bootstrapped confidence intervals include the null value.
Matrices based on gender (Fig. 2a) also showed that mixing by partner sex was assortative, indicated by the higher average ratio of observed-to-expected ratio of sharing with one's own sex (1.16), as compared with the lower average of mixed-sex partnerships (0.93). We also observed more sharing between women and men as reported by women than was expected by proportionate mixing. Mixing based on race (Fig. 2b) showed strong assortativity, with a far higher average ratio of observed-to-expected mixing within the same race (5.09) than with a partner of a different race (0.44).
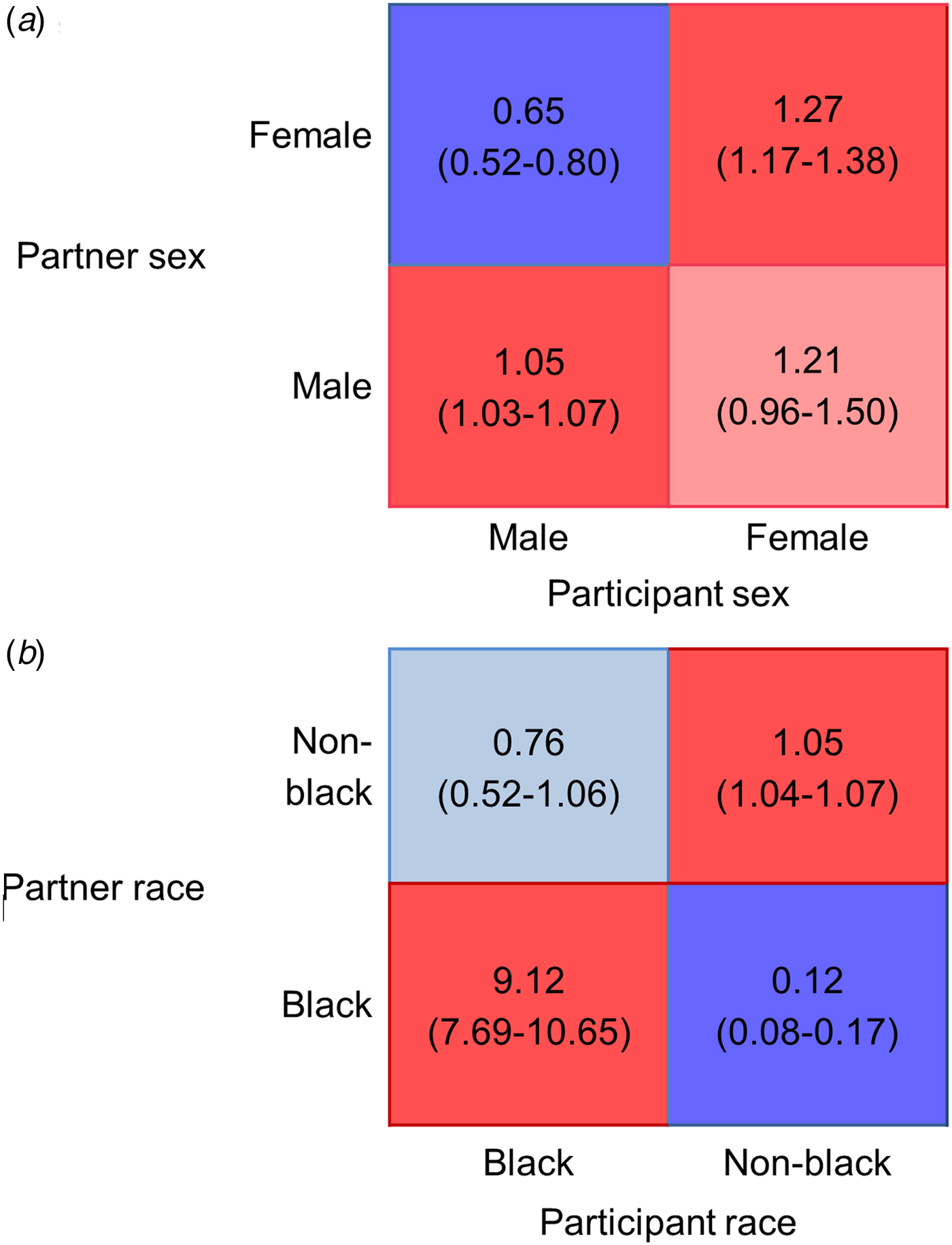
Fig. 2. Gender (a) and race (b) based mixing matrices for total number of sharing partnerships. Blue colours indicate less mixing between age groups than expected under the proportionate mixing assumption; red colours indicate more mixing than expected. Lighter shades indicate ratio values whose 95% bootstrapped confidence intervals include the null value.
Comparisons with the Williams et al.’s study on PWID networks in three US cities [Reference Williams22] showed that assortativity in terms of groups of age (10-year blocs), gender and race, measured as a proportion of respondents who shared within their own subgroup, was greater among STEP respondents than in the other three cities (Table 4). Age assortativity was used to compare mixing observed in the STEP study with other types of mixing, which found that age-based mixing for drug equipment sharing has similarly low assortativity as for casual mixing (comparison with the Polymod study), whereas sexual mixing exhibited far greater age assortativity (comparison with the Finnish sexual survey).
Table 4. Proportions of reported drug equipment-sharing partnerships within and without respondents’ own age, gender and racial group among PWID in three US cities as reported by Williams et al. and in Baltimore as reported by STEP study respondents
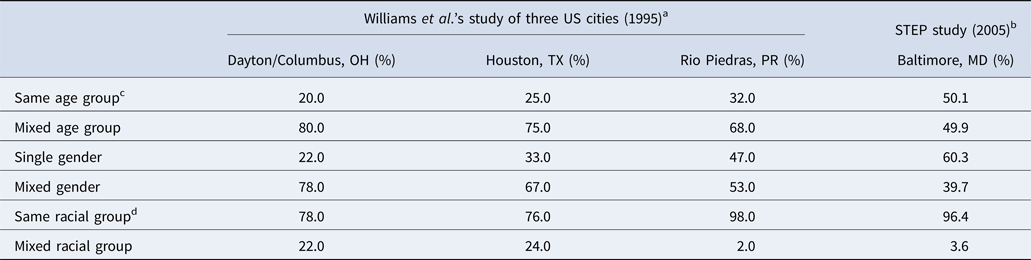
a Sharing network members were defined as those with whom the respondent had shared needles or cookers in the past 30 days. A total of 275 dyads were reported by 192 respondents in a single cross-sectional survey. Information on participant recruitment methods or survey year was not provided, but study was published in 1995.
b Sharing network members were defined as those with whom the respondent had shared needles and/or cookers in the past 6 months. A total of 2651 dyads were reported by 647 respondents over the course of four biannual surveys from 2005 to 2006.
c Age groups were divided into 10-year age categories, with the youngest group being those 30 or under, and the oldest those over 50.
d Racial groups in Williams et al. were categorised as African American, white and Hispanic.
Discussion
Egocentric drug network data from a cohort of PWID in Baltimore shows assortative mixing according to age, gender and race. In our assessment of age-based mixing, comparisons of observed sharing rates to those expected under the assumption of random mixing found that in the majority of age combinations, partner choice was driven more by the similarity of a partner to oneself rather than the availability of that type of partner (i.e. in 36 out of the 64 cells of the Fig. 1 matrix, sharing rates were statistically different from our expectation based on 95% of bootstrap replicates). In terms of gender-based mixing, we found that female participants preferentially shared with both men and women, although estimates for sharing in female–female partnerships did not significantly differ from our proportionate mixing expectation based on bootstrapping results. Notable in our assessment of race-based mixing was the large ratio by which black PWID reported sharing with black partners.
The fact that PWID in this study shared with those more similar to them in terms of age and race but less so in terms of gender expands on existing evidence of sharing dynamics reported by Williams et al. [Reference Williams22], which found that PWID were mainly assortative in terms of race. This underscores the possible salience of race as a determinant of PWID partnering practices. In terms of age, however, STEP respondents were far more assortative than those of the Williams et al.’s study, possibly due to the fact that age-based mixing is heavily impacted by the relative availability of partnerships in various age groups, for which metrics in the Williams et al.’s study are not adjusted. Comparisons of these two studies are also best made in light of significantly different sample sizes (192 vs. 647), geographic variation (three cities vs. one) and a 10-year time gap in study periods. We also used measures of diagonality to compare age-specific assortativity observed in our sample to other types of mixing including casual [Reference Mossong23, Reference Read25–Reference Horby27] and sexual [Reference Barnabas28, Reference Aral29], which have long been used to inform influenza and sexually transmitted infection control. The fact that drug-sharing and casual contact patterns were relatively less age assortative than sexual mixing (Table 5) may be due to factors other than personal preference that drive partner selection, such as limited pools of partners, residential segregation or the additional roles played by some partners who may supply drugs, or for newer initiates, assist them with injection [Reference Bailey30, Reference Becker Buxton31].
Table 5. Background survey information for the Finnish sexual survey [Reference Haavio-Mannila and Kontula24], the Polymod study [Reference Mossong23] and the STEP study used to estimate the measures of diagonality for age-based mixing of sexual, casual and drug-sharing contacts

a Although the survey explored questions of multiple sexual partnerships, ages of sexual partners were only available for those described as ‘steady’ partners.
CI, confidence interval.
Observed mixing patterns can also inform our understanding ongoing trends in the HIV epidemic among PWID. High age assortativity observed in our data, for example, suggests that by consistently partnering with other PWID in their own age group rather than with older PWID from whom most new infections have been traditionally acquired [Reference Neaigus32, Reference Garfein33], younger Baltimore PWID may be experiencing lower forces of infection than in the past. This hypothesis is at least partially supported by the increasing average age of PWID newly diagnosed with HIV between 2001 and 2010 [Reference Flynn34]. Second, our observed race-specific assortativity suggests that rising HCV prevalence in young white injectors [Reference Page35] is unlikely to result in a concomitant resurgence in their black counterparts given the minimal contact between these two groups. Although the lack of laboratory testing for HIV or HCV in this study limits our ability to directly measure the impact of contact patterns on observed epidemiological phenomena, insights on population-level mixing are useful for generating hypotheses about epidemic trends and for comparing mixing patterns across settings and populations.
A better understanding of PWID mixing patterns can also inform the design and evaluation of future treatment as prevention interventions. In settings like ours where age-specific and race-specific assortativity is high, for example, prevention benefits conferred by treatment scale-up may get trapped within subgroups with better access to treatment – namely, PWID who are older, are white or are male [Reference Gornall, Hoey and Ozieranski8, Reference Harris, Albers and Swan36–Reference Stimson39]. Unless special measures are taken to ensure meaningful treatment access among younger PWID (who are more likely to still actively use drugs) as well as female PWID and PWID of colour, intervention effects cannot be expected to reach those beyond those directly treated. Mixing patterns can therefore inform design of treatment as prevention programmes by considering the extent to which treatment of PWID with traditionally better healthcare access could confer indirect benefits to their partners in more hard-to-reach segments of the population. Subgroup-specific disease prevalence and the extent of mixing across age groups should therefore be one of the primary considerations for the design and implementation of treatment-based prevention in PWID.
Findings reported here must be interpreted in light of several limitations. Foremost among these is the time frame of our survey data, which were collected between 2005 and 2007. The consistency of assortativity patterns observed both in our data and that of the Williams et al.’s study collected at least a decade earlier [Reference Williams22] may indicate, however, that our findings are far from obsolete. Our findings also reflect the complex interplay of sociological factors thought to underlie decisions about partners’ choice in PWID [Reference De40]. Recent changes in the HCV and HIV epidemics among PWID in the USA [Reference Cicero41] underscore the importance of ongoing research in this area to update our understandings. A second limitation is in the fact that biased sampling of our study population in terms of age, gender or race could over-represent the types of partnerships reported by sampled individuals. To mitigate the effects of this bias, our matrices measured contact frequencies relative to expected patterns under population distributions observed in the ALIVE study, thought to be a more representative sample drawn of the same population. Comparable distributions in terms of all attributes between the STEP and ALIVE data suggest that sampling bias was likely minimal; although even with careful sampling, the outsized role of a few individuals who share widely across different age groups could possibly distort results. Third, differential reporting bias across participant attributes may also undermine our assumption of mutuality of reported partnerships (e.g. a partnership reported by individual A with partner B would be just as likely reported by individual B had he or she been sampled), though it is not possible to assess the potential extent of this bias. Differential recall or reporting bias across subgroups could also affect our mutuality reporting assumption, such as in situations where respondents of certain ages might more reliably report the ages of partners closer to them in age as compared with those much older or younger. This type of bias may have been exacerbated by our study requirement that participants report only partners they had known for at least a month, a factor that could potentially be interpreted differentially across subgroups. This phenomenon of non-mutual reporting may at least partially explain the asymmetric results, for example, those observed in our gender-based mixing matrix (Fig. 2a), in which women reported partnerships with men more than men did with women. Lastly, by pooling of network information reported across the four survey periods, we assumed that evolving network structures or changes in participants’ reporting habits over time had negligible impact on the representativeness of the data.
Insights gained from this analysis nevertheless provide needed knowledge regarding mixing patterns salient to the spread of blood-borne disease among PWID. The results show that assortativity can vary greatly by attribute, highlighting potential subgroups of demographically isolated clusters for whom targeted interventions may be necessary, as the indirect benefits of generalised health interventions may have limited penetration into these groups. Findings presented here fill a long-standing gap in our understanding of PWID networks, which has been limited due to the challenge of collecting detailed data in stigmatised and marginalised populations. This study also provides a template to guide future studies seeking to generate much needed empirical data on PWID contact networks. Further investigations into PWID networks should carefully consider contextually appropriate attributes important for identifying the types of subgroups who may be playing outsized roles in either transmission or acquisition of disease.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818002042.
Acknowledgements
The authors thank the participants and staff of the STEP study for their contributions to this analysis.
Financial support
This study was supported by the National Institute on Drug Abuse (NIDA) at the National Institutes of Health (R01DA012568 and U01DA036297). DATC is supported by the National Institute on Allergies an Infectious Diseases (R01AI114703, R21AI116296) and by the National Center for Emerging and Zoonotic Infectious Diseases at the Centers for Disease Control (U01CK000337); SHM is supported by NIDA (R01DA012568, R01DA026727, R01DA04134 and R01DA32059) as is CAL (R01DA031030, R01DA032217 and R01DA040488); MKS is supported by NIAID (T32AI102623) and MG by the Bill and Melinda Gates Foundation (OPP1106427).
Conflict of interest
None.










