Introduction
The seasonally dry areas of the tropics, their ecosystems and vegetation types, have received scant attention from the natural sciences, especially when compared to that given to tropical rain forests (Janzen, Reference Janzen and Wilson1988; Mooney et al., Reference Mooney, Bullock, Medina, Bullock, Mooney and Medina1995). Only in recent times has this tendency been partially reversed (e.g. Pennington et al., Reference Pennington, Lewis and Ratter2006; Dirzo et al., Reference Dirzo, Young, Mooney and Ceballos2011). In the Neotropics, Seasonally Dry Tropical Forests (SDTFs) are floristically and ecologically distinct vegetation types, clearly separated from rain forests (Pennington et al., Reference Pennington, Prado and Pendry2000, Reference Pennington, Lavin and Oliveira-Filho2009; Mayle, Reference Mayle2004) or the Chaco subtropical to temperate dry forests (Prado, Reference Prado1993a, Reference Prado1993b), and they must be considered separately in any biogeographical analyses. For much of the 20th century, the neotropical seasonally dry forests of central South America remained conceptually submerged within other neighbouring vegetation units of the continent, such as the Chaco and Yungas, or amongst the several units of the Amazonian Dominium (e.g. Cabrera, Reference Cabrera1953, Reference Cabrera1971; Rizzini, Reference Rizzini1963; Hueck, Reference Hueck1978; Cabrera & Willink, Reference Cabrera and Willink1980; Hueck & Siebert, Reference Hueck and Siebert1981). This was a result of their spatial discontinuity.
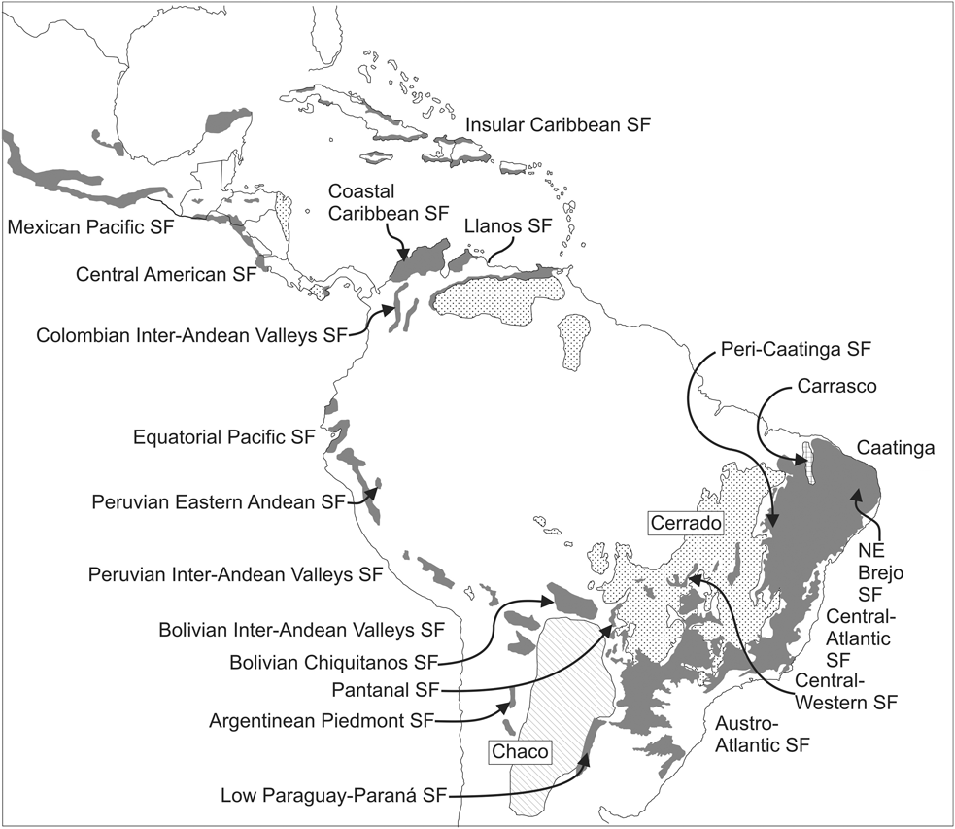
Moreover, for nearly half a century two South American forest and woodland ecosystems were wrongly considered very similar; this confusion apparently stemmed from a misinterpretation of the seminal work by Smith & Johnston (Reference Smith, Johnston and Verdoorn1945). These are the phytogeographical dominia or ‘provinces’ (sensu Takhtajan, Reference Takhtajan1986) of the Chaco and the Caatingas, both dominated by woody species of Mimosoideae (Leguminosae), separated by the extensive Cerrado vegetation of central Brazil (Fig. 1). Based on the high level of endemism in Chaco s.s. (Prado, Reference Prado1993b), the even higher level of endemism of the Caatingas (Prado, Reference Prado, Leal, Tabarelli and Silva2003; Giulietti et al., Reference Giulietti, Conceição and Queiroz2006), and the negligible floristic links between the two ecosystems, this presumed similarity was demonstrated to be incorrect (Prado, Reference Prado1991, Reference Prado2000). Nevertheless, from an analysis of the non-endemic Caatingas woody species, a repeated and consistent disjunct pattern of distribution was established for the neotropical seasonally dry tropical forests.

Fig.1. Distribution of the Seasonally Dry Tropical Forests (SDTFs) in South America. All major forest nuclei are indicated. The Caatinga Nucleus, as here understood, also includes the North-East Brejo and Peri-Caatinga nuclei. The Chaco and savannas (Llanos and Cerrado) are also shown. Figure from Särkinen et al. (Reference Särkinen, Iganci, Linares-Palomino, Simon and Prado2011), modified from Linares-Palomino et al. (Reference Linares-Palomino, Oliveira-Filho, Pennington, Dirzo, Young, Ceballos and Mooney2011). SF: Seasonal Forests.
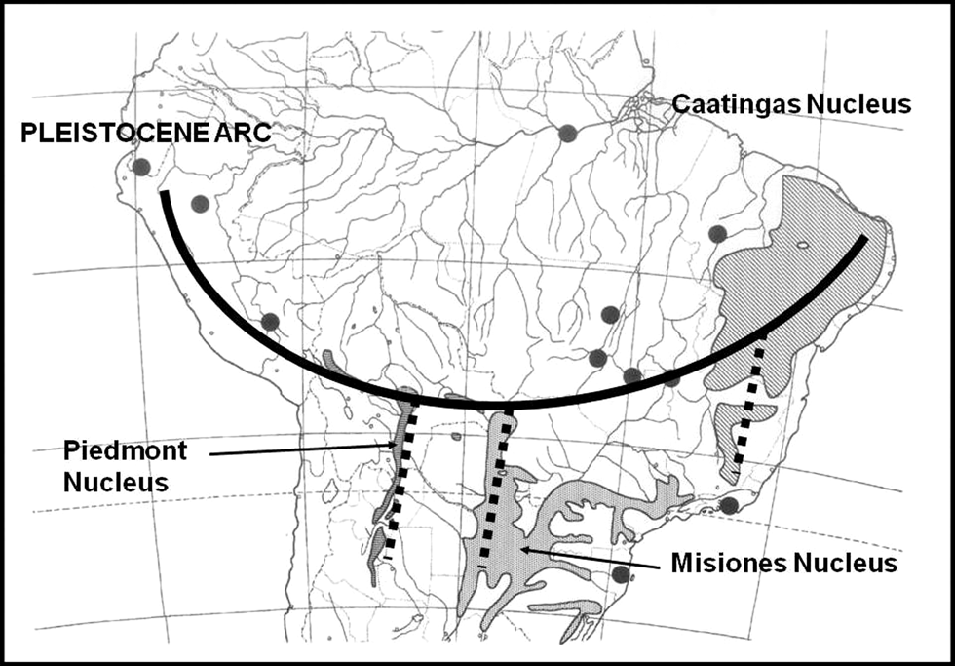
After comprehensive mapping of several woody species of the South American SDTF (Prado, Reference Prado1991) and numerical vegetation analysis, a strongly coincidental phytogeographical distribution pattern was revealed. This pattern goes across the continent in an arc shape (Fig. 2), extending from the Caatingas of north-eastern Brazil (the ‘Caatingas Nucleus’) to the sector formerly occupied by the Jesuit Missions of SE Brazil, eastern Paraguay and NE Argentina (the ‘Misiones Nucleus’), then along the Bolivian Chiquitanía to the area of the sub-Andean foothills of SW Bolivia and NW Argentina (the ‘Piedmont Nucleus’), and finally along the dry inter-Andean valleys of Bolivia, Peru and southern Ecuador (Prado & Gibbs, Reference Prado and Gibbs1993). This fragmentary and disjunct distribution of SDTF has been proposed as a new phytogeographical dominium, taking both varieties of Anadenanthera colubrina (Vell.) Brenan (Mimosoideae, Leguminosae) as its principal indicator tree species. This proposal was based not only on the number of endemic woody plant taxa (both at the species and genus level) but also on the greater similarity of these SDTF fragments to each other than to other surrounding vegetation units (Prado, Reference Prado2000).

Fig.2. The Pleistocene Arc of SDTF of central South America. All major dry forest nuclei are indicated. Modified from Prado (Reference Prado2000).
This new floristic and ecological interpretation of the South American SDTF led to the proposal of the so-called ‘Pleistocene Arc Theory’ (PAT) (Prado & Gibbs, Reference Prado and Gibbs1993). Whereas the alleged Chaco–Caatingas connections proved to be incorrect, the newly emerging distribution pattern across South America gave rise to speculations about the influence of past wet–dry climatic fluctuations on present-day disjunctions of seasonal forests in the continent, with an associated impact on biogeography and paleobiogeography (Pennington et al., Reference Pennington, Prado and Pendry2000; Mayle, Reference Mayle2004). As a possible explanation for the PAT pattern, vicariance has been preferred, given that a single vicariance event is more parsimonious than several independent long-distance dispersals. Today, there is mounting evidence in favour of long-distance dispersal to explain the distribution of the succulent biome on an intercontinental scale (e.g. Davis et al., Reference Davis, Bell, Mathews and Donoghue2002; Lavin et al., Reference Lavin, Schrire, Lewis, Pennington, Delgado-Salinas and Thulin2004; Schrire et al., Reference Schrire, Lavin and Lewis2005a, Reference Schrire, Lewis, Lavin, Lewis, Schrire, Mackinder and Lock2005b, Reference Schrire, Lavin, Barker and Forest2009) as well as that of certain neotropical forest distribution patterns (e.g. Pennington & Dick, Reference Pennington and Dick2004; Dick & Heuertz, Reference Dick and Heuertz2008). Both the latter mainly refer to rain forest species, with the probable single exception of the wide-ranging Cordia alliodora (Ruiz & Pav.) Oken (Rymer et al., Reference Rymer, Dick, Vendramin, Buonamici and Boshier2013). Therefore, it is still possible that these disjunct fragmented patterns of distribution are vestiges of a more continuous SDTF formation that had a wider distribution during the drier glacial periods of the Pleistocene (Ab’Sáber, Reference Ab’Sáber1957, Reference Ab’Sáber1977, Reference Ab’Sáber and Prance1982, Reference Ab’Sáber2000).
The present-day concept of SDTF in South America comprises woody vegetation types related to a strong seasonality, with a well-defined dry season of very variable length. In general terms, these forests are almost completely deciduous during the dry season (Fig. 3), thriving on fertile soils with slightly acid to neutral or somewhat alkaline pH. Floristically they are dominated by Leguminosae (mainly Mimosoideae), frequently accompanied by Anacardiaceae and Bignoniaceae, usually with conspicuous flowers that open near the end of the dry season, later resulting in anemophilous propagules. The understorey comprises numerous species of Cactaceae and Bromeliaceae, and to a lesser degree broad-leaved herbs and Gramineae.

Fig.3. Dry season in SDTF, near Salta, Argentina. Photo: O. G. Martínez. (See online for a colour version of this figure.)
Since the PAT hypothesis is mainly based on comparison of floristic lists, taxonomic revisions and dot mapping of selected woody species, one valid criticism may be insufficient plant collections and incomplete or partial botanical studies, both of which could have led to incomplete or erroneous conclusions. This potential drawback was pointed out in a previous paper (Prado, Reference Prado2000), which postulated that distribution patterns could have simply reflected well-collected areas or routes. In the past 23 years a plethora of new herbarium collections has been made and a sizeable number of monographic taxonomic studies has been carried out, particularly in Leguminosae. Most of the new legume collections and their accompanying information are now easily accessible online. In the present contribution an analysis is made of the distribution patterns of selected woody SDTF species, comparing their distribution based on all currently available collections with their distribution presented at the time of the proposed PAT theory. Also, in a search for new evidence to support or refute the theory, more recent taxonomic studies have been reviewed. The questions we set out to answer are: (i) is the PAT still supported; and (ii) are there any additional legume endemics known from the Chaco and Caatingas?
Material and Methods
Most authors agree that a phytogeographic ‘region’ should contain a number of endemic genera and species, whereas ‘provinces’ (or ‘dominia’) that make up the subdivisions of a region should have a reduced number of monotypic or oligotypic genera but abundant specific endemism (Braun-Blanquet, Reference Braun-Blanquet1919; Cain & Castro, Reference Cain and Castro1959; Takhtajan, Reference Takhtajan1986). Furthermore, Cain & Castro (Reference Cain and Castro1959) emphasised that the only reliable methodology that reduces subjectivity in the designation of floristic territories is the preparation of accurate taxon distribution dot maps for the region being defined. Such maps rely on the availability of a sufficient number of specimens as well as monographic studies. Dot maps were employed in the original formulation of the PAT; some of the original maps from Prado (Reference Prado1991) have been reproduced here for the purpose of visual comparison with updated ones. The original dot maps were based on identification-verified herbarium specimens or citations in reliable taxonomic revisions (solid symbols, Fig. 4A) together with citations in reliable vegetation surveys (open symbols, Fig. 4).

Occurrence data for each legume species analysed were obtained firstly from the original hand-written specimen cards (Prado, Reference Prado1991). A large quantity of additional data was obtained from the GBIF portal (data.gbif.org, March 2013), but also from relevant literature which included information on the set of species under study (Arenas, Reference Arenas1981; Burkart et al., Reference Burkart, Troncoso de Burkart and Bacigalupo1987; Lewis, Reference Lewis1987; Killeen et al., Reference Killeen, García Estigarribia and Beck1993; Barneby & Grimes, Reference Barneby and Grimes1996, Reference Barneby and Grimes1997; Mereles, Reference Mereles1998; Navarro & Maldonado, Reference Navarro and Maldonado2002; Queiroz, Reference Queiroz2009; Lewis et al., Reference Lewis, Wood and Lavin2012). Several web portals were also consulted, including tropicos.org, ipni.org, and the websites ‘Lista de Espécies da Flora do Brasil 2013’ (in floradobrasil.jbrj.gov.br) and ‘Flora del Conosur’ (darwin.edu.ar/Proyectos/FloraArgentina/FA.asp) in order to check synonyms and the area of occupation of species.
The information gleaned from web portals (e.g. Beck et al., Reference Beck, Ballesteros-Mejia, Nagel and Kitching2013) is of variable reliability. To overcome this, all the records obtained from web resources were checked against available taxonomic monographs. In the case of occurrences reported in web sites but not found in the literature, the image of the specimen itself was often consulted (e.g. NYBG, Tropicos, Darwinion, Kew: apps.kew.org/herbcat/navigator.do). If no images were available we only used records whose identification had been made or confirmed by international specialists. This follows the same approach used in another SDTF study (Särkinen et al., Reference Särkinen, Iganci, Linares-Palomino, Simon and Prado2011).
The dataset was compiled and ‘cleaned’ (Hijmans et al., Reference Hijmans, Guarino, Cruz and Rojas2001) by comparing recorded distributions with areas noted in the literature, as well as the field experience of the authors. Obvious distribution outliers were checked and deleted when necessary and cultivated specimens were excluded from the analysis. For specimens lacking geo-reference data, coordinates were estimated using Google Earth, Google Maps and historical maps and gazetteers. DIVA GIS v7.5 (Hijmans et al., Reference Hijmans, Guarino, Cruz and Rojas2001) was used to construct individual and combined distribution maps.
Results and Discussion
Chaco endemics
In South American biogeography the Gran Chaco comprises the thorny vegetation that covers the vast rolling plains of north-central Argentina, western Paraguay and south-eastern Bolivia (Fig. 1), also entering Brazil as a narrow strip following the course of the river Paraguay in Mato Grosso do Sul. It covers approximately 1,000,000 km2 and consists of semi-arid forests, woodlands and occasionally savannas. To confirm the identity of the Chaco as a phytogeographical unit, a revision of its environmental characteristics (geomorphology, soils and climate) was performed (Prado, Reference Prado1993a), together with a search for endemic genera and species, the latter including the preparation of dot maps of several of these taxa (Prado, Reference Prado1991). The Chaco biome usually occurs on saline or alkaline soils with poor drainage and is subject to frequent flooding, particularly in the eastern sector (the so-called Humid Chaco), but decreases to the west where loamy-sandy, xeric soils are more characteristic. Severe winter frosts are very frequent and increase to the west ranging from an average of 0.3 frost days/year and a minimum absolute temperature of –1.5°C in the east to 28.9 or more frost days/year and a minimum absolute temperature of –10°C in the west (Galmarini & Raffo del Campo, Reference Galmarini and Raffo del Campo1964; Conti et al., Reference Conti, Cazenave, Giagnoni and Molina2009). The floristic composition is a distinctive feature of this biome with some typical tree taxa, e.g. several species of Schinopsis Engl. (Anacardiaceae), Aspidosperma quebracho-blanco Schltdl. (Apocynaceae), Tabebuia nodosa (Griseb.) Griseb. (Bignoniaceae), and several species of the genera Prosopis L., Acacia Mill. sensu lato, and Bulnesia Gay (Zygophyllaceae). The Chaco vegetation has strong floristic links with the Prepuna, Monte and some Andean formations (Cabrera, Reference Cabrera1953, Reference Cabrera1971; Pennington et al., Reference Pennington, Prado and Pendry2000; Spichiger et al., Reference Spichiger, Calenge and Bise2004).
With the relatively scant information available in 1991, a total of eight endemic genera and 92 woody or succulent endemic species was noted for the Chaco. Three monotypic endemic legume genera were found (Lophocarpinia Burkart, Mimozyganthus Burkart and Stenodrepanum Harms) and 21 endemic species, eight belonging to Caesalpinioideae, 11 to Mimosoideae and only two to Papilionoideae. The most diverse genus as regards endemic species was Prosopis, with eight endemics: P. elata (Burkart) Burkart, P. fiebrigii Harms, P. hassleri Harms, P. kuntzei Harms, P. pugionata Burkart, P. rojasiana Burkart, P. ruscifolia Griseb. and P. vinalillo Stuck. Almost endemic to the Chaco but of wider distribution is Prosopis nigra (Griseb.) Hieron. that sometimes extends into neighbouring formations (Prado, Reference Prado1991). This high endemism is not surprising, since the centre of diversity of the genus Prosopis includes the Chaco plains (Burkart, Reference Burkart1976a, Reference Burkart1976b; Burghardt & Espert, Reference Burghardt and Espert2007).
The list of Chaco endemics has grown over the past 23 years. There are now four endemic genera of Leguminosae, the additional one, Piptadeniopsis Burkart with its single shrubby species P. lomentifera Burkart, having been overlooked by Prado (Reference Prado1991). This species is restricted to central Paraguay. The phylogenetic affinities of this genus and the partially sympatric sister genus Mimozyganthus (reaffirmed as a Chaco endemic) were studied by Luckow et al. (Reference Luckow, Fortunato, Sede and Livshultz2005), and together with Prosopidastrum Burkart the three genera form a monophyletic group, sister to the Leucaena group. The phylogenetic relationships of the two monotypic caesalpinioids endemic to the Chaco (Lophocarpinia and Stenodrepanum) were also studied and illustrated by Nores et al. (Reference Nores, Simpson, Hick, Anton and Fortunato2012). These two genera are allopatric within the Chaco and are phylogenetically more distant from each other than the mimosoid genera; Lophocarpinia is sister to Haematoxylum L., whereas Stenodrepanum is sister and probably congeneric to the sympatric genus Hoffmannseggia Cav.
At the species level there has been considerable progress in the study of Chaco legumes; the narrow endemic Apurimacia dolichocarpa (Griseb.) Burkart is noteworthy. It was listed by Prado (Reference Prado1991) as a doubtful Chaco endemic and is distributed within an area of less than 400 km2 (Grossi & Funes, Reference Grossi and Funes2011) in Sierra Chaco forests. Otholobium higuerilla (Gillies ex Hook.) J.W.Grimes (= Psoralea higuerilla Gillies ex Hook.) also belongs exclusively in the Sierra Chaco, although much more widely distributed than Apurimacia (Grimes, Reference Grimes1990). Two more endemic Prosopis species are added to the list of Chaco endemics: P. campestris Griseb. (Roig, Reference Roig, Roig, Cavagnaro and Trione1993) and P. nuda Schinini (Antezana et al., Reference Antezana, Atahuachi, Arrázola, Fernández and Navarro2000). In the genus Acacia (in its broad traditional sense), one new Chaco endemic species was described by Fortunato & Cialdella (Reference Fortunato and Cialdella1996): A. emilioana Fortunato & Ciald.; it is restricted to the Chaco of western Paraguay and neighbouring Bolivia. The species is now more correctly referred to Senegalia emilioana (Fortunato & Ciald.) Seigler & Ebinger (Seigler et al., Reference Seigler, Ebinger and Miller2006). Another Chaco endemic, Acacia curvifructa Burkart (Ebinger et al., Reference Ebinger, Seigler and Clarke2000), has been confirmed in a revision of Acacia subgenus Acacia. The accepted name of this species is Vachellia curvifructa (Burkart) Seigler & Ebinger (Seigler & Ebinger, Reference Seigler and Ebinger2005). Also endemic to the Chaco is Chloroleucon chacoënse (Burkart) Barneby & J.W.Grimes (Barneby & Grimes, Reference Barneby and Grimes1996). Finally, Caesalpinia coluteifolia Griseb. is the only entirely Chaco endemic species in the whole Poincianella–Erythrostemon group of Caesalpinia sensu lato (Ulibarri, Reference Ulibarri1996; Lewis, Reference Lewis1998).
Caatingas endemics
In South American biogeography the term Caatingas is employed to indicate the semi-arid vegetation that covers a large part of north-eastern Brazil (Andrade-Lima, Reference Andrade-Lima1981; Velloso et al., Reference Velloso, Sampaio and Pareyn2002), extending from eastern Piauí state, through most of Ceará and Rio Grande do Norte, western Paraíba, Pernambuco, Sergipe, Alagoas, central Bahia, and parts of northern Minas Gerais state. The Caatingas are surrounded and demarcated by the Atlantic Forest to the east and the Cerrado vegetation to the south-west (Fig. 1). They cover more than 850,000 km2 (Queiroz, Reference Queiroz, Pennington, Lewis and Ratter2006). The vegetation consists of forests, woodlands and scrublands (see list of vegetation types in Andrade-Lima, Reference Andrade-Lima1981), physiognomically dominated by thorny deciduous species, mostly legumes. Species with evergreen foliage are scarce, these mainly thriving along seasonally dry riverbeds. As reported for the Chaco, to corroborate the identity of the Caatingas as a true phytogeographical unit, a revision of its environmental characteristics (geomorphology, soils and climate) was performed, together with a search for endemic genera and species and the preparation of taxon dot maps (Prado, Reference Prado1991, Reference Prado, Leal, Tabarelli and Silva2003).
With even more scarce information available for the Caatingas flora in 1991, a total of 13 endemic genera and 183 woody or succulent endemic species was recorded. In the legume family, no endemic Caatingas genera were found. A total of 59 endemic species was recorded, 21 belonging to Caesalpinioideae, 21 to Mimosoideae and 17 to Papilionoideae (Prado, Reference Prado1991). More than 50% of the 59 (32 species) were doubtfully endemic. Critical to establishing the Leguminosae list of Caatingas was the landmark work Legumes of Bahia (Lewis, Reference Lewis1987).
After 23 years, the list of Caatingas endemics has grown greatly. There are now four monotypic legume genera endemic to the Caatingas: Blanchetiodendron Barneby & J.W.Grimes, Mysanthus G.P.Lewis & A.Delgado, Tabaroa L.P.Queiroz, G.P.Lewis & M.F.Wojc., and Goniorrhachis Taub. The genus Blanchetiodendron in the Mimosoideae was proposed by Barneby & Grimes (Reference Barneby and Grimes1996) to accommodate B. blanchetii (Benth.) Barneby & J.W.Grimes as separate from Albizia Durazz., where it had previously been placed by Lewis (Reference Lewis1987) as A. blanchetii (Benth.) G.P.Lewis. Two of the endemic genera belong in the Papilionoideae; the genus Mysanthus was segregated from Phaseolus L. by Lewis & Delgado-Salinas (Reference Lewis and Delgado-Salinas1994) to accommodate its only species M. uleanus (Harms) G.P.Lewis & A.Delgado, which had been of uncertain taxonomic position for a long time. The very narrow endemic Tabaroa caatingicola L.P.Queiroz, G.P.Lewis & M.F.Wojc. is a recently discovered and described, critically endangered taxon occupying an area of less than 12 km2 on inland sandy soils of south-eastern Bahia (Queiroz et al., Reference Queiroz, Lewis and Wojciechowski2010). The caesalpinioid tree Goniorrhachis marginata Taub. may be endemic to the Caatingas. Most collection localities being in arboreal caatinga (Lewis, Reference Lewis1987; Queiroz, Reference Queiroz2009), it is also recorded from ‘seasonal forests’, which sensu lato could refer to Caatingas vegetation.
The remarkable progress made in the last 23 years in neotropical legume taxonomy is evidenced by the increase in endemic taxa, which has almost tripled from the number given by Prado (Reference Prado1991). According to Queiroz (Reference Queiroz, Pennington, Lewis and Ratter2006), of a total of 292 legume taxa native to the Caatingas, some 144 are endemic (almost 50%). It should be pointed out that this is a very high percentage of endemics, comparable to that of isolated oceanic islands, a fact suggested by Pennington et al. (Reference Pennington, Prado and Pendry2000) and later supported by Prado (Reference Prado, Leal, Tabarelli and Silva2003). It is unnecessary to list the new endemic taxa to the Caatingas here; they have been cogently and abundantly studied, analysed, mapped and even ecologically characterised in two exhaustive works by Queiroz (Reference Queiroz, Pennington, Lewis and Ratter2006, Reference Queiroz2009). The latter study permitted an assessment of the endemic character of the 32 doubtful taxa mentioned before and confirmed that most of them (22) are indeed endemic after a comparison of Prado’s list (1991) and Queiroz’s (Reference Queiroz2009) monographic study on the Caatingas legumes.
Nevertheless, the genus Luetzelburgia Harms exemplifies the complex taxonomic history of many legumes of the Caatingas. Luetzelburgia is a genus of beautiful trees rarely seen in flower, fruit and leaf at the same time (Queiroz, Reference Queiroz2009), a phenomenon true of many Caatingas tree species, regardless of plant family. This causes an additional difficulty in fully understanding the SDTF flora. In 1991, at the time of the formulation of the PAT, only three species of Luetzelburgia were known to occur in the Caatingas (Lewis, Reference Lewis1987): L. auriculata (Allemão) Ducke, also occurring in Cerrado vegetation; the caatinga endemic L. andrade-limae H.C.Lima, and L. bahiensis Yakovlev, hesitantly added as a possible endemic by Prado (Reference Prado1991). Today, we know that six species of the genus occur in Caatingas (Queiroz, Reference Queiroz2009), five of which are confirmed endemic, including the two previously listed as such. The three new endemic species were described in a recent publication (Cardoso et al., Reference Cardoso, Queiroz and Lima2008): L. harleyi D.B.O.S.Cardoso, L.P.Queiroz & H.C.Lima, L. neurocarpa D.B.O.S.Cardoso, L.P.Queiroz & H.C.Lima, and L. purpurea D.B.O.S.Cardoso, L.P.Queiroz & H.C.Lima. In addition, and even more recently, the same three authors have described two new species of Luetzelburgia from the SDTF of Bolivia (Cardoso et al., Reference Cardoso, Queiroz and Lima2012), L. andina D.B.O.S.Cardoso, L.P.Queiroz & H.C.Lima and L. sotoi D.B.O.S.Cardoso, L.P.Queiroz & H.C.Lima, thus extending the range of a genus previously only known from Brazil.
Pleistocene Arc Theory (PAT) distribution pattern
The PAT pattern was discovered as a serendipitous by-product of a study of the alleged strong Chaco–Caatingas links. Originally formulated in a PhD thesis (Prado, Reference Prado1991), it was formally published by Prado & Gibbs (Reference Prado and Gibbs1993). According to the theory, a considerable number of SDTF woody species, many of them belonging to the Leguminosae, had probably been involved in the hypothesised expansion–retreat cycles and migrations that led to the current-day SDTF distribution pattern. These species are today known to be members of several tropical and subtropical deciduous forests in South America. These include the extensive Caatingas of north-eastern Brazil and also certain semi-deciduous non-Atlantic Forest formations in the states of São Paulo and Paraná, together with the forests of the Upper Uruguay river valley, most of the forests surrounding the Paraguay-Paraná river system, the Santiago and Chiquitos Sierras of south-eastern Bolivia, and the Piedmont forests in north-western Argentina and south-western Bolivia (stretching southwards from Santa Cruz de la Sierra in Bolivia, to Tucumán and Catamarca in Argentina). Elsewhere these forests are more sparsely scattered in the dry Andean valleys of northern Bolivia, across Peru and occasionally extending into south-western Ecuador. Some of the legume species in these forests are dominant. When all these species are mapped and the maps combined, the resulting compound map shows an unmistakable PAT pattern (see fig. 2 in Prado, Reference Prado2000, and Fig. 2 of this study).
All these units of SDTF, and their constituent species, skirt the impoverished soils of the Brazilian Cerrado (Fig. 1) savanna vegetation (Furley & Ratter, Reference Furley and Ratter1988), even to the extent of ‘island-hopping’ across this region by way of isolated calcareous outcrops (Ratter et al., Reference Ratter, Askew, Montgomery and Gifford1978, Reference Ratter, Pott, Pott, da Cunha and Haridasan1988). This phenomenon has also been highlighted by several other students of the SDTF (Nascimento et al., Reference Nascimento, Felfili and Meirelles2004; Silva & Scariot, Reference Silva and Scariot2004; Pereira et al., Reference Pereira, Venturoli and Carvalho2011). One example is the most iconic tree of the PAT, the mimosoid Anadenanthera colubrina, which comprises two varieties: var. colubrina and var. cebil (Griseb.) Altschul (Altschul, Reference Altschul1964). In the 1991 distribution map of this species (Prado, Reference Prado1991) the varieties were mapped with different symbols (see Fig. 4A (left), open triangles for var. colubrina, solid or open circles for var. cebil), and there was some indication that the two varieties occurred in rather different regions. In the new map (Fig. 4A, right) they are not differentiated because the taxonomic characters traditionally used to distinguish them are almost impossible to apply in the field (e.g. differing leaflet venation, used by Altschul, Reference Altschul1964) and, contrary to the published literature, both varieties coexist in most SDTFs of northern Argentina (i.e. SDTF in Misiones, Corrientes, Formosa, Jujuy, Salta and Tucumán provinces; Martínez et al., Reference Martínez, Barrandeguy, García, Cacharani and Prado2013).
Anadenanthera colubrina, known as ‘angico’ in Brazil, or ‘curupay’, ‘curupaú’ or ‘cebil’ in the rest of the Neotropics, is one of the more common species in the arboreal Caatingas of north-eastern Brazil (Andrade-Lima, Reference Andrade-Lima1981), as witnessed by the large number of collections from this region (Fig. 4A). It is more scattered across the Brazilian Cerrado, but occurs exclusively in mesophilous woodlands or mesotrophic cerradão (Ratter et al., Reference Ratter, Askew, Montgomery and Gifford1978) on calcium-rich soils. The species then extends through the Misiones Nucleus of the Paraguay-Paraná river system from where there are numerous herbarium specimens and observation records, whereas the reasonably well-collected Chaco plains show an impressive absence of the species. On the western border of the Chaco there is a notable concentration of dots on Fig. 4A (right), coinciding with a narrow fringe of SDTF in the foothills of the sub-Andean sierras of north-western Argentina and south-western Bolivia, a vegetation type that has traditionally been submerged in the Yungas province (as ‘Transitional Forest’, Cabrera, Reference Cabrera1971). The distribution of Anadenanthera colubrina then extends in a more scattered pattern along the dry inter-Andean valleys of north-western Bolivia and throughout Peru, reaching its ultimate north-western limit in southern Ecuador.
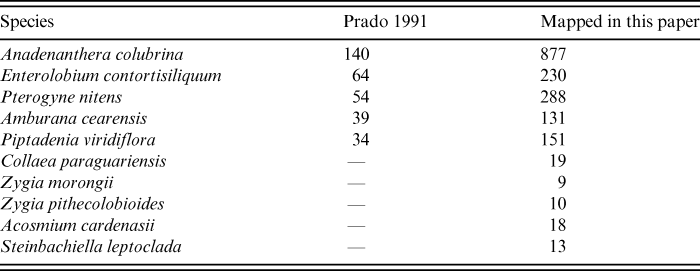
The distribution map (Fig. 4A, right) includes post-1991 collections together with the previously mapped ones (Prado, Reference Prado1991). The original map included only 140 records (Fig. 4A, left) and the updated one includes 877 (Fig. 4A, right; Table 1). Nevertheless, in both maps of Anadenanthera colubrina (Fig. 4A), the species is nearly absent from the Chaco, thus reinforcing the lack of floristic links amongst this region, the Caatingas and the SDTF. The Cerro León area is located within the Chaco of Paraguay (at approximately 20°19′54,30″S, 60°19′58,06″W). This massive Devonian outcrop was scarcely known until relatively recently (Ramella & Spichiger, Reference Ramella and Spichiger1989). Although A. colubrina was mentioned by these authors as very important in the local dry forests (Ramella & Spichiger, Reference Ramella and Spichiger1989), only one literature record of the species was included from the area in the map of Prado (Reference Prado1991). In our new map numerous records of A. colubrina have been added to the Cerro León area and nearby gallery forests (Fig. 4A, right). The slopes of the Cerro León are in fact covered with SDTF vegetation (Ramella & Spichiger, Reference Ramella and Spichiger1989; Pennington et al., Reference Pennington, Prado and Pendry2000).
Table 1. Comparison of number of specimen records (including literature citations) mapped in Prado (Reference Prado1991) vs. in this paper, for a select number of the Leguminosae species

Differences between the original and present-day distribution maps of Anadenanthera colubrina also include increased records from the Cerrado in Brazil, and also in Paraguay, Bolivia and Peru. New records from the Brazilian Cerrado mostly correspond to calcareous rock outcrops (Ratter et al., Reference Ratter, Askew, Montgomery and Gifford1978, and Fig. 4A, right). In central Paraguay there are many new records which include some intrusions into the Humid Chaco on levees with better drained soils. As stated by Spichiger et al. (Reference Spichiger, Calenge and Bise2004, Reference Spichiger, Bise, Calenge, Chatelain, Pennington, Lewis and Ratter2006), Paraguay can be seen as a huge ecotone in South America, at the intersection of the Chaco biome, the São Francisco Basin, and the Paraná Basin vegetation. The Chiquitanía area (Bolivian Chiquitanos SF; Fig. 1) and the north-western Andean slopes in Bolivia (Bolivian Inter-Andean Valleys SF; Fig. 1), together with the SDTF area of northern Peru, close to the frontier with Ecuador (Peruvian Eastern Andean SF; Fig. 1), likewise are now well represented by collections of A. colubrina.
Despite all these new records since 1991, the distribution of Anadenanthera colubrina still closely maps the original PAT pattern presented by Prado & Gibbs (Reference Prado and Gibbs1993). This PAT distribution pattern is repeated, wholly or in part, by a number of other woody species of South American SDTF that have been mapped elsewhere (Prado & Gibbs, Reference Prado and Gibbs1993; Mayle, Reference Mayle2004). These include the leguminous trees Enterolobium contortisiliquum (Vell.) Morong, Pterogyne nitens Tul., Amburana cearensis (Allemão) A.C.Sm., and Piptadenia viridiflora (Kunth) Benth. These species are also presented in the maps of Fig. 4, as they characterise the PAT pattern.
The mimosoid Enterolobium contortisiliquum is a large conspicuous tree with indehiscent peculiarly shaped fruits. Apparently rather scarce in the Caatingas as presented in the 1991 map (Prado, Reference Prado1991; Fig. 4B, left), it had been seen and collected in arboreal Caatinga by Andrade-Lima (Reference Andrade-Lima1975, Reference Andrade-Lima1981) and also recorded infrequently from south-eastern Brazilian forests. It is frequent in the Paraguay-Paraná river system (Reboratti & Neiff, Reference Reboratti and Neiff1986; Prado et al. Reference Prado, Franceschi and Bianchi1989; Oakley et al., Reference Oakley, Prado and Adámoli2005), where it has been used traditionally as a fish poison by indigenous communities (Dujak & Marchi, Reference Dujak and Marchi2010). Known as ‘pacará’ in north-western Argentina and south-western Bolivia, where it is abundant and even dominant in parts of the sub-Andean Piedmont forests, it was not recorded in any dry Andean valleys until 1991 (Fig. 4B, left). It had occasionally been noted within the Chaco plains, though only in gallery forests on alluvial levees along the main rivers (Morello & Adámoli, Reference Morello and Adámoli1974) together with other non-chaquenian elements. In making the updated map (Fig. 4B, right), the collections of Enterolobium timbouva Mart., a species doubtfully accepted as distinct by Barneby & Grimes (Reference Barneby and Grimes1996), have been considered by us as a synonym of E. contortisiliquum in agreement with Lewis (Reference Lewis1987), accepted by Prado (Reference Prado1991), and also suggested by Queiroz (Reference Queiroz2009), who considers them possibly to be two ecotypes of the same species. The number of known records is now nearly four times that presented in the 1991 map (Table 1). This species is almost absent from the Chaco. However, in the following regions there is now a marked increase in records compared with the 1991 map: the Misiones Nucleus (which somewhat equates to the Austro-Atlantic SF; Fig. 1), the Chiquitanía (Bolivian Chiquitanos SF; Fig. 1), in the Bolivian part of the Piedmont Nucleus forests, and remarkably in the north-western Andean slopes in Bolivia (Bolivian Inter-Andean Valleys SF; Fig. 1), from which no records were available on the original 1991 map (Fig. 4B, left).
The case of Pterogyne nitens is rather peculiar, because of its somewhat invasive nature. It also has a similar distribution to the previous species, and had been reported from gallery forests within the Chaco as well (Morello & Adámoli, Reference Morello and Adámoli1974; Prado, Reference Prado1991). The northern half of the Chaco now has numerous records of P. nitens (Fig. 4C, right). In several cases the appearance within the Chaco corresponds to its spontaneous presence in tall grasslands of Elionurus muticus (Spreng.) Kuntze in the Chaco of Paraguay (Ramella & Spichiger, Reference Ramella and Spichiger1989; Mereles & Degen, Reference Mereles and Degen1994; Mereles, Reference Mereles2005). Although native to a number of vegetation types, in some areas the tree is visibly invading new ground, such as open urban spaces in the city of Corumbá, Mato Grosso do Sul, Brazil (authors’ personal observation, 2003), and vacant plots in the city of Asunción in Paraguay, and it is expanding in disturbed rural areas in southern Formosa province, Argentina (authors’ personal observations). Nevertheless, P. nitens is now known to occur as a native species in SDTF of the Bolivian Chiquitanía, but is rare in the northern Bolivian Andean valleys (Fig. 4C).

Fig.4. Distribution maps of some selected woody Leguminosae typical of the South American SDTF. A: Anadenanthera colubrina; B: Enterolobium contortisiliquum; C: Pterogyne nitens; D: Amburana cearensis; E: Piptadenia viridiflora. On the left: as in Prado (Reference Prado1991); on the right, new maps. (See online for a colour version of this figure.)
Amburana cearensis is a well-documented but not abundant species of the PAT in Caatingas vegetation (arboreal and shrubby caatinga; Andrade-Lima, Reference Andrade-Lima1981). In the Piedmont Nucleus, it is rather scarce but a constant SDTF species (Fig. 4D). In both maps it is rare in the Cerrado. The lack of collection records in the 1991 map (Fig. 4D, left) from the southern half of the Misiones Nucleus is now updated (Fig. 4D, right). New records from Rio de Janeiro state in Brazil and the Bolivian Chiquitanía add to the overall distribution pattern. Additionally, A. cearensis is now better known from the north-western Andean slopes in Bolivia (Bolivian Inter-Andean Valleys SF; Fig. 1) and Andean Peru and north-eastern Paraguay (López Villalba et al., Reference López Villalba, Little, Ritz, Rombold and Hahn2002; Oakley & Prado, Reference Oakley and Prado2011). New records from neighbouring Brazil, mainly from the state of Acre, are also noteworthy (Fig. 4D).
The tree species Piptadenia viridiflora was scarcely recorded from north-western Argentina until Burkart (Reference Burkart1952) noted it for the first time. Conversely it has long been a well-known component of the Caatingas (Fig. 4E). Collection records have increased fourfold since 1991. A few scattered new records from coastal south-eastern Brazil and Paraguay add to the 1991 map (Fig. 4E, right). In 1991 the species was hardly known from Bolivian Chiquitanía or more northern areas. Now P. viridiflora is known to be common in the Chiquitanía, as well as the northern dry valleys of Bolivia, and is also known to occur in northern Peruvian SDTF and southern Ecuador.
The original PAT pattern (Prado & Gibbs, Reference Prado and Gibbs1993) is still perfectly recognisable. The rapid growth in collection records in the past 23 years (occurrences in Table 1) has added support to the PAT. Moreover, the PAT distribution pattern is better defined and continues to be very significant because it is repeated, wholly or in part, by a number of other woody species of South American SDTF legumes (e.g. Geoffroea spinosa Jacq., and other plant families; Caetano et al., Reference Caetano, Prado, Pennington, Beck, Oliveira-Filho, Spichiger and Naciri2008).
Endemism in other nuclei
When the PAT theory was proposed as a hypothesis, the authors could not provide a list of endemic taxa in the Misiones Nucleus, nor in the Bolivian Chiquitanía, because of the lack of data from those regions at the time. In the last 23 years, these data gaps have been filled, at least for Leguminosae. For example, in the Misiones Nucleus, a number of endemic taxa have been added from a thorough review of the recent literature and the combined field knowledge of the present authors. These species had previously been overlooked, under-collected or have been described or redefined since 1991; e.g. Arachis correntina (Burkart) Krapov. & W.C.Greg. (Krapovickas & Gregory, Reference Krapovickas and Gregory1994); Collaea paraguariensis (Hassl.) R.C.García (García, Reference García1989) (see Fig. 5); Desmanthus hexapetalus J.F.Macbr. (Luckow, Reference Luckow1993); Sesbania macroptera Micheli (Gómez Sosa, Reference Gómez Sosa2000); Tephrosia hassleri Chodat (Vanni, Reference Vanni1994); Zygia morongii Barneby & J.W.Grimes and Zygia pithecolobioides (Harms) Barneby & J.W.Grimes (Barneby & Grimes, Reference Barneby and Grimes1997) (see Fig. 5). This list of species reinforces the Misiones Nucleus (e.g. Prado & Gibbs, Reference Prado and Gibbs1993; Spichiger et al., Reference Spichiger, Calenge and Bise2004, Reference Spichiger, Bise, Calenge, Chatelain, Pennington, Lewis and Ratter2006) as a biogeographical entity.

Fig.5. Distribution maps of Zygia pithecolobioides and Zygia morongii (left), and Collaea paraguariensis (right), both Misiones Nucleus endemics. (See online for a colour version of this figure.)
For the Chiquitanía, there is also mounting evidence of endemism to support it as a recognisable biogeographical entity. The Chiquitanía Nucleus, as part of the PAT, is now better supported by increased legume collection data. In addition, Pennington et al. (Reference Pennington, Prado and Pendry2000), Navarro & Maldonado (Reference Navarro and Maldonado2002), Killeen et al. (Reference Killeen, Chavez, Peña-Claros, Toledo, Arroyo, Caballero, Pennington, Lewis and Ratter2006), Linares-Palomino et al. (Reference Linares-Palomino, Oliveira-Filho, Pennington, Dirzo, Young, Ceballos and Mooney2011), Sánchez-Azofeifa & Portillo-Quintero (2011) and Särkinen et al. (Reference Särkinen, Iganci, Linares-Palomino, Simon and Prado2011) have either mapped the Chiquitanía as a SDTF centre or provided detailed floristic lists supporting it as a valid biogeographical and floristic entity. Moreover, Werneck et al. (Reference Werneck, Costa, Colli, Prado and Sites2011) demonstrated that the Chiquitano area has been stable for 21,000 years, and served as a refugium during the climatic fluctuations of the Pleistocene. These authors employed recent modelling techniques (e.g. BM/MAXENT) to scrutinise the SDTF paleo-distribution, and compared their results with the available palynological evidence. Comparing analyses of the climatic variables corresponding to the SDTF of today, 6000 years ago and the Last Glacial Maximum (LGM), the models show great stability of the original nuclei proposed for the PAT, and support the Chiquitanía as a fourth nucleus. Overlaying these model results with paleo-palynological data produced similar results, and corroborated a circum-Amazon corridor of SDTF in the recent geological past (Werneck et al., Reference Werneck, Costa, Colli, Prado and Sites2011).
The new Chiquitanía Nucleus presents a relatively reduced number of legume endemics so far; e.g. Acosmium cardenasii H.S.Irwin & Arroyo (Killeen et al., Reference Killeen, Chavez, Peña-Claros, Toledo, Arroyo, Caballero, Pennington, Lewis and Ratter2006; Schütz Rodrigues & Azevedo Tozzi, Reference Schütz Rodrigues and Azevedo Tozzi2007) (see Fig. 6); Luetzelburgia sotoi D.B.O.S.Cardoso, L.P.Queiroz & H.C.Lima (Cardoso et al., Reference Cardoso, Queiroz and Lima2012); Steinbachiella leptoclada Harms (Lewis et al., Reference Lewis, Wood and Lavin2012) (see Fig. 6), and Swartzia jorori Harms (Killeen et al., Reference Killeen, Chavez, Peña-Claros, Toledo, Arroyo, Caballero, Pennington, Lewis and Ratter2006). To these species can be added an endemic species of Cactaceae: Cereus tacuaralensis Cárdenas (Killeen et al., Reference Killeen, Chavez, Peña-Claros, Toledo, Arroyo, Caballero, Pennington, Lewis and Ratter2006), and a species of Malvaceae: Pseudobombax pulchellum Carv.-Sobr. (Carvalho-Sobrinho et al., Reference Carvalho-Sobrinho, De Queiroz and Alverson2013). Another new feature of the Chiquitanía Nucleus inferred from Navarro et al. (Reference Navarro, Molina and Pérez de Molas2006) are similar dry forests cited under the misleading name of ‘cerradones’ in northern Paraguay. Thus, the Chiquitano Nucleus extends further south than previously recorded (Fig. 1).

Fig.6. Distribution maps of Acosmium cardenasii (left) and Steinbachiella leptoclada (right), both Chiquitanía Nucleus endemics. (See online for a colour version of this figure.)
Conclusions
More than 20 years since the formulation of the Pleistocene Arc Theory, the Chaco and the Caatingas provinces are confirmed as distinct phytogeographical entities. The numbers of endemic legume genera and species records in both nuclei have increased. The increase in novelties recorded from the Chaco come mainly from previously under-explored Paraguayan Chaco.
The original PAT distribution pattern (Prado & Gibbs, Reference Prado and Gibbs1993) is still clearly visible and recognisable in the new taxon maps prepared for this study. The PAT pattern has also been independently confirmed by more objective techniques using similar sets of species (Spichiger et al., Reference Spichiger, Calenge and Bise2004, Reference Spichiger, Bise, Calenge, Chatelain, Pennington, Lewis and Ratter2006). A new area of endemism in the Pleistocene Arc is added: the Chiquitanía Nucleus in south-eastern Bolivia and bordering Paraguay, which is supported by recent vegetation modelling (Werneck et al., Reference Werneck, Costa, Colli, Prado and Sites2011; Collevatti et al., Reference Collevatti, Terribile, de Oliveira, Lima-Ribeiro, Nabout, Rangel and Diniz-Filho2013).
Naturally, there are a number of species that do not follow the PAT distribution pattern, which were not included in the present analysis as those additional analyses were beyond the scope of this paper. Prado (2003, p. 50) has described several different distribution patterns for SDTF species, of which the PAT pattern is just one, although one that we stress is very consistent.
Phylogenetic studies of SDTF taxa (particularly at genus level) reviewed by Pennington et al. (Reference Pennington, Lavin, Prado, Pendry, Pell and Butterworth2004) demonstrated that speciation primarily occurred well before or at the beginning of the Pleistocene in South America. Nevertheless, according to these authors this evidence did not refute the possibility of a more widespread SDTF formation. In the case of widespread species (e.g. Anadenanthera colubrina, Enterolobium contortisiliquum) that show a strong PAT pattern, perhaps the period of isolation between the SDTF nuclei has not been long enough to promote speciation, but has been long enough to produce genetic variability. Population genetics studies are still very scarce (e.g. Caetano et al., Reference Caetano, Prado, Pennington, Beck, Oliveira-Filho, Spichiger and Naciri2008; Collevatti et al., Reference Collevatti, Terribile, Lima-Ribeiro, Nabout, de Oliveira, Rangel, Rabelo and Diniz-Filho2012) and the only one published for the Leguminosae to date covers a relatively small part of the total distribution range of Anadenanthera colubrina (Barrandeguy et al., Reference Barrandeguy, García, Prinz, Rivera Pomar and Finkeldey2014).
The vicariance or long-distance dispersal debate to explain the PAT pattern (Pennington et al., Reference Pennington, Prado and Pendry2000 vs. Mayle, Reference Mayle2004) remains. Probably, the historical biogeographical processes that had shaped the SDTFs as we currently know them involved a combination of long-distance dispersal and vicariance in response to past environmental shifts. Recent evidence from phylogeographic and population genetics studies suggests that long-distance dispersal, perhaps by oceanic and wind currents, and/or by bird dispersal (Renner, Reference Renner2004), is much more common than previously noted, and this phenomenon may have an important role in explaining the present distribution of many taxa in neotropical rain forests (Pennington & Dick, Reference Pennington and Dick2004; Dick & Heuertz, Reference Dick and Heuertz2008). In neotropical seasonally dry forests (SDTF), population migration and long-distance dispersal (Mayle, Reference Mayle2004; Linares-Palomino et al., Reference Linares-Palomino, Oliveira-Filho, Pennington, Dirzo, Young, Ceballos and Mooney2011) may have occurred throughout the Cerrado and the Chaco, via smaller peripheral areas of SDTF on calcareous soils, although there is no strong molecular evidence for this.
On the contrary, some works (e.g. Caetano, Reference Caetano2008; Caetano et al., Reference Caetano, Prado, Pennington, Beck, Oliveira-Filho, Spichiger and Naciri2008; Collevatti et al., Reference Collevatti, Terribile, Lima-Ribeiro, Nabout, de Oliveira, Rangel, Rabelo and Diniz-Filho2012; Barrandeguy et al., Reference Barrandeguy, García, Prinz, Rivera Pomar and Finkeldey2014) present results that support, albeit partially, the PAT hypothesis. Moreover, evidence from other SDTF nuclei in the Americas (Fig. 1) provide a strong indication of phylogenetic geographic structure, with sister species cohabiting similar areas, for example in legumes of South American dry inter-Andean valleys (Pennington et al., Reference Pennington, Lavin and Oliveira-Filho2009) and in Mesoamerican Bursera species (De-Nova et al., Reference De-Nova, Medina, Montero, Weeks, Rosell, Olson, Eguiarte and Magallón2012). In addition, a high beta diversity in Bursera (De-Nova et al., Reference De-Nova, Medina, Montero, Weeks, Rosell, Olson, Eguiarte and Magallón2012), and in the woody plant species of the SDTFs as a whole (Linares-Palomino et al., Reference Linares-Palomino, Oliveira-Filho, Pennington, Dirzo, Young, Ceballos and Mooney2011), has been recorded. This high phylogenetic geographic structure is apparently not repeated in Amazonian rain forests (Pennington et al., Reference Pennington, Lavin and Oliveira-Filho2009; Dick & Pennington, Reference Dick and Pennington2012), probably reflecting the fact that dispersal is more important in shaping tropical rain forest communities (Pennington & Dick, Reference Pennington and Dick2004) than fragmented SDTFs (Pennington et al., Reference Pennington, Lavin, Särkinen, Lewis, Klitgaard and Hughes2010; Dick & Pennington, Reference Dick and Pennington2012). The present-day fragmented distribution pattern of SDTF is most likely a vestige of a more continuous SDTF formation and the PAT can still confidently be tested against the long-distance dispersal hypothesis. One way to resolve the vicariance vs. long-distance dispersal debate as regards the present distribution pattern of the South American SDTFs may be to carry out population genetics and phylogeographic studies of the emblematic PAT species that we discuss in this work.
Acknowledgements
The authors thank the National University of Rosario, Argentina, for funding and facilities to perform this work. D. Prado and V. Mogni also thank the Consejo Nacional de Investigaciones Científicas y Técnicas. Particular thanks to the AVE Program (Programa de Ayuda Viajes al Exterior, Docente) of the National University of Rosario, for providing funding to the senior author to travel to Johannesburg to the 6th International Legume Conference. Thanks are due to Agr. Luciano Galetti for his meticulous help in the preparation of legume inventories, and also to Libr. Mariela Mejorada for scanning the maps. We also thank Kyle Dexter and Toby Pennington for critical reading of a previous version of this manuscript, improving it with valuable comments and suggestions, and two anonymous reviewers for their very helpful and constructive comments.