Flavonoids are polyphenolic secondary metabolites that occur ubiquitously in higher plants. Their abundance in the human diet has led to increasing interest in their bioavailability and biological properties. Flavonoids exert biological activities that are mainly related to their ability to influence various enzymes and/or to their antioxidant properties (Middleton et al. Reference Middleton, Kandaswami and Theoharides2000). Detailed assessment of the bioavailability of putatively beneficial polyphenolic food constituents is vital for the evaluation of health effects in man and animals (Manach et al. Reference Manach, Mazur and Scalbert2005). The flavonol quercetin is one of the major representatives of flavonoids in many edible plants (Herrmann, Reference Herrmann1988; Hertog et al. Reference Hertog, Hollman and Katan1992) and also one of the most studied.
Our group has been investigating the bioavailability of quercetin in pigs for several years. The gastrointestinal as well as the circulatory system in this species is similar to that in man; thus, the pig is considered a relevant model species (Almond, Reference Almond1996). In a previous study, the oral bioavailability of quercetin aglycone from a test meal was enhanced by 57 % by the addition of 15 % (w/w) fat to a low-fat standard pig diet (Lesser et al. Reference Lesser, Cermak and Wolffram2004). We speculated whether the enhancement of quercetin bioavailability was due to the better solubility of the relatively lipophilic quercetin aglycone in the intestinal tract in the presence of fat (Azuma et al. Reference Azuma, Ippoushi, Ito, Higashio and Terao2002). In this case, an increased absorption from fat-containing diets should be largely independent of the type of dietary fat. Another possible explanation could be the generation of chylomicrons in the intestinal mucosa. Dietary fat mainly comprises triacylglycerols with long-chain fatty acids (chain length >C12). After luminal hydrolysis, mucosal uptake and resynthesis of triacylglycerols, long-chain fatty acid triacylglycerols (LCT) are incorporated into chylomicrons and are consecutively transported via the lymph. By contrast, medium-chain fatty acid triacylglycerols (MCT, chain length C8–C12) are hydrolysed and predominantly exported from the enterocytes as NEFA into the mesenterical blood; they reach the liver through the portal vein (Bloom et al. Reference Bloom, Chaikoff and Reinhardt1951; Hashim et al. Reference Hashim, Bergen, Krell and Van Itallie1964; for review, see Harkins & Sarett, Reference Harkins and Sarett1968). Transport together with LCT within chylomicrons via the lymphatic vessels (Murota & Terao, Reference Murota and Terao2005) could enhance quercetin bioavailability by bypassing the liver, thereby reducing biliary elimination during the first liver passage (the first-pass effect). If this was the case, mainly fats containing LCT should increase the bioavailability of flavonols but not fats comprising MCT because of the different handling of LCT and MCT during intestinal absorption (Harkins & Sarett, Reference Harkins and Sarett1968). To differentiate between these two possibilities (i.e. better solubility v. bypassing of the liver), we fed quercetin (30 μmol/kg body weight (BW)) to pigs with test meals consisting of a low-fat pig diet or of the same diet enriched with either 15 g MCT or LCT fat/100 g diet. The quantity of quercetin we administered with the test meals is similar to the amount that can be ingested by man in a meal rich in onions or apples (Hollman et al. Reference Hollman, van Trijp, Buysman, van der Gaag, Mengelers, de Vries and Katan1997).
Materials and methods
Animals and diets
Cross-bred (Deutsche Landrasse × Deutsches Edelschwein) growing male castrated pigs (n 6) with a BW of 30–35 kg were purchased from a local farmer. The pigs were surgically equipped with permanent catheters (Cook Deutschland GmbH, Mönchengladbach, Germany) placed in the left jugular vein. The pigs were restrictively fed (80 % of voluntary feed intake) with a commercial pig diet composed mainly of wheat, barley and defatted soybean meal (Plambeck Kraftfutter, Brügge, Germany). The composition of this diet (designated as standard diet) is shown in Table 1. Test meals were isoenergetic and consisted of the standard diet or of the same diet enriched with either 15 g lard or synthetic MCT oil/100 g diet (w/w; designated as LCT or MCT diet, respectively). The fatty acids of the MCT oil consisted solely of octanoic and decanoic acid with a mean chain length of C8·8. The lard was composed of LCT with a mean chain length of C17·3, as determined from the saponification number. Predominant fatty acids in lard are C18 : 1 (43 g/100 g), C16 : 0 (24 g/100 g), C18 : 0 (14 g/100 g) and C18 : 2 (9 g/100 g), with no fatty acids of less than 14 carbon atoms (Belitz & Grosch, Reference Belitz and Grosch1992). Commercial lard was obtained from Fischermanns GmbH (Duisburg, Germany), and the MCT oil was a kind gift from Unilever (Vlaardingen, the Netherlands). Vitamins and minerals where supplemented according to the recommendations of the German Society of Nutritional Physiology (Ausschuss für Bedarfsnormen der Gesellschaft für Ernährungsphysiologie, 1987). Water was supplied ad libitum for intake by nipple drinkers.
Table 1 Composition of diets*

LCT, long-chain fatty acid triacylglycerols; MCT, medium-chain fatty acid triacylglycerols; NDF, neutral detergent fibre; NFC, non-fibre carbohydrates.
* Isoenergetic amounts of each diet were fed: 200 g of the standard diet, and 162 g each of LCT and MCT diets.
† Composed of 90 % MCT and 10 % LCT.
Additionally, eighteen male CD rats (Charles River, Sulzfeld, Germany) with a mean BW of approximately 250 g were used to investigate gastric emptying after consumption of the specific diets. The rats were housed individually and kept on a 12 h light/dark cycle. They were accustomed to eating the standard pig diet for one week before the experiment.
The experiments were approved by the Animal Welfare Officer of the University of Kiel and by the relevant legal authorities (Ministry of Agriculture and Environment of Schleswig-Holstein).
Experimental procedure
Bioavailability in pigs
Each pig received a dose of 30 μmol quercetin/kg BW that was mixed into the respective test meal directly before offering the test meal. The animals consumed the meal within 5–10 min. Test meals were isoenergetic. Fifteen blood samples (8 ml each) were collected from each pig over a period of 24 h after intake of the test meal. A wash-out period of 24 h was placed between the last sampling point and the application of the following test meal. Thus, the three different quercetin-containing test meals were fed consecutively at intervals of 48 h.
Gastric emptying in rats
Two days before the experiment, the rats were allocated to three groups (six per group) and trained to eat meals of 5 g of the respective test diets (standard, LCT or MCT) within 15 min twice a day (at the beginning and end of the dark period). No further food was provided during this period. The BW did not differ between the groups (253·4 (sem 5·0), 256·3 (sem 6·3) and 254·5 (sem 4·5) g for the standard, LCT and MCT groups, respectively). After fasting for 11 h (water allowed), the rats were fed the respective test meals (5 g containing 0·5 mmol quercetin/100 g diet, equimolar to the concentration in the standard diet pig test meal). The rats were anaesthetized with CO2 1 h after intake of the meals and immediately decapitated. Blood was collected, the abdominal cavity opened, and the stomach proximally and distally ligated and removed. The stomach content was collected by rinsing the stomach with 2 ml of pure water. The gastric content was dried (106°C) to a constant weight. Dry matter was related to the dry matter of the respective diet ingested.
Processing of plasma samples and HPLC analysis
Blood samples from pigs and rats were drawn into heparinized containers and centrifuged immediately (1500 g, 10 min, 4°C). Plasma was stored at − 70°C until analysis by HPLC as described previously (Hollman et al. Reference Hollman, van Trijp and Buysman1996; Ader et al. Reference Ader, Wessmann and Wolffram2000; Cermak et al. Reference Cermak, Landgraf and Wolffram2003). Extraction of flavonols from plasma samples was performed according to Morand et al. (Reference Morand, Crespy, Manach, Besson, Demigné and Rémésy1998). An aliquot (980 μl) of the plasma sample was spiked with 20 μl rhamnetin (internal standard, 50 μg/ml in methanol), acidified (pH 5) with 100 μl acetic acid (0·583 mol/l), and subsequently treated with a mixture of 104 units β-glucuronidase/4 × 102 units sulfatase (crude extract from Helix pomatia, Sigma Aldrich). After incubation for 1 h at 37°C, the flavonol aglyca quercetin, kaempferol, isorhamnetin and tamarixetin were extracted simultaneously with 5·5 ml acetone. The mixture was centrifuged at 3700 g for 45 min and the supernatant evaporated under partial vacuum at 45°C to dryness. The residue was resolved in 200 μl methanol, 77·5 μl nanopure H2O and 22·5 μl HCl (10 mol/l). For HPLC analysis 30 μl were injected onto a C-18 Kromasil 100 column (dimensions: 250 × 4 mm, particle size 5 μm) guarded by a C-18 Inertsil ODS-2 precolumn (Jasco, Groß-Umstadt, Germany). The eluent (1 ml/min) was composed of 0·025 mol/l NaH2PO4, pH 2·4, acetonitrile and methanol (68 : 27:5 v/v/v). The column effluent was mixed with 0·4 ml/min 1·0 mol/l Al(NO3)3 in methanol containing 7·5 % (v/v) acetic acid in a post-column reactor. The column and the reactor were placed in a column oven at 30°C. The fluorescence of the ensuing flavonol–metal complex was measured at 485 nm using a fluorescence detector with an excitation wavelength of 422 nm. The limit of detection was 5–7 nmol/l. Inter-analysis and inter-day variances were within 5 %. Standards were prepared with pure flavonols (Carl Roth GmbH, Karlsruhe, Germany) and treated as described earlier. Identification of peaks obtained in plasma samples was performed using the retention times of the pure flavonols (standards).
Statistical analyses
Data are presented as means with their standard errors (sem). The area under the plasma concentration–time curve (AUC) was determined according to the linear trapezoidal rule. For each pig and treatment, total bioavailability (AUCtotal) was calculated by summing the AUC values of quercetin and its metabolites with an intact flavonol structure (isorhamnetin and tamarixetin). AUC data, maximum plasma concentration (c max) and time at maximum plasma concentration (t max) were analysed using repeated measures one-way ANOVA. The amount of dry matter from the gastric content and the plasma flavonol concentrations of the rats were analysed using one-way ANOVA. Tukey's multiple comparison was applied as post-test (Graphpad Prism 4, Graphpad Software Inc., San Diego, CA, USA). A P value < 0·05 was considered significant.
Results
Bioavailability in pigs
After administration of quercetin with the test meals to pigs, metabolites with an intact flavonol structure appeared in plasma within 30 min. Plasma levels of flavonols decreased below the detection limit within 24 h. In accordance with previous studies (Cermak et al. Reference Cermak, Landgraf and Wolffram2003; Lesser et al. Reference Lesser, Cermak and Wolffram2004), the main metabolite after βglucuronidase/sulfatase treatment of the pig plasma samples was quercetin, while isorhamnetin (3′-O-methyl quercetin) and tamarixetin (4′-O-methyl quercetin) were found in a proportion of approximately 10 % each, irrespective of the dietary treatment.
After intake of quercetin with the standard diet, the mean peak plasma concentration (c max) was reached 95 min (t max) after intake (Table 2). Thereafter, quercetin levels with the standard diet decreased continuously until they were below the detection limit after 24 h (Fig. 1).
Table 2 Pharmacokinetic parameters and relative bioavailability of quercetin in pigs after intake of quercetin in test meals differing in their fat content and/or fatty acid pattern*

c max, maximum plasma concentration of quercetin; t max, time between administration of test meal and the appearance of maximum plasma concentration of quercetin; AUCtotal, area under the plasma concentration–time curve from 0 to 24 h for the sum of quercetin and its metabolites isorhamnetin and tamarixetin; LCT, long-chain fatty acid triacylglycerols; MCT, medium-chain fatty acid triacylglycerols.
a,b Mean values within a column with unlike superscript letters were significantly different (P < 0·05).
* Values are for means of six pigs for each dietary treatment.
† For composition of diets, see Table 1.
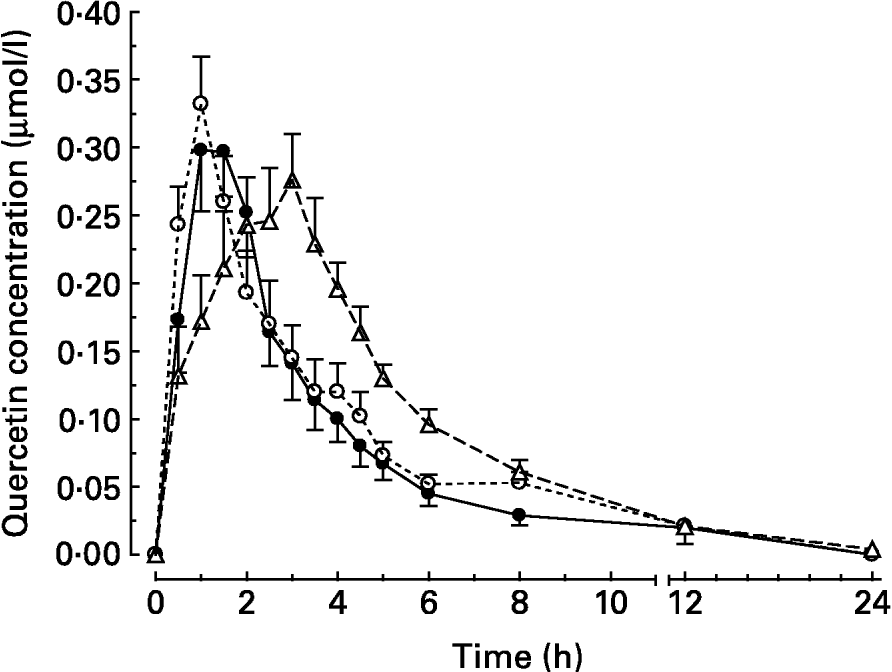
Fig. 1 Plasma concentration–time curves of the main metabolite quercetin after oral administration of quercetin (30 μmol/kg body weight) to pigs in test meals that differed in their fat content and/or fatty acid pattern. Values are means for six pigs, with standard errors of the mean represented by vertical bars. For composition of diets, see Table 1. (●) Standard diet; (○) long-chain fatty acid triacylglycerol (LCT) diet; (Δ) medium-chain fatty acid triacylglycerol (MCT) diet.
When the flavonol was administered together with the LCT diet, the plasma concentrations of quercetin peaked after 65 min. This was not, however, significantly different from the intake with the standard diet (Table 2). At 8 h after meal intake, the plasma quercetin concentrations were 0·053 (sem 0·008) and 0·029 (sem 0·007) μmol/l for the LCT and standard diets, respectively.
After administration of quercetin together with the MCT diet, flavonol plasma concentrations rose steadily over 170 min until peak level (Table 2), and thereafter declined continuously. At 8 h after intake, the mean plasma concentration was still higher (0·061 (sem 0·009) μmol/l) than after intake with the standard diet (P < 0·05). At 12 h after meal intake, the mean plasma concentrations in all groups were low (Fig. 1). The maximum plasma concentrations of quercetin after intake with the three different diets were not significantly different. The total oral bioavailability (AUCtotal) of quercetin, however, was significantly higher after intake of quercetin with the MCT diet than after intake with the standard and LCT diets (Table 2).
Gastric emptying in rats
Within 15 min, the rats consumed 5 g of the experimental diets, which contained the same dietary concentration of quercetin as in the original pig test meals. This resulted in an intake of about 100 μmol quercetin/kg BW. One hour after consumption of the meals, blood samples and gastric contents were collected. Dry matter of the gastric contents did not differ in weight (Fig. 2(a)). About 80 % of dry matter consumed remained in the stomach 1 h after meal intake.
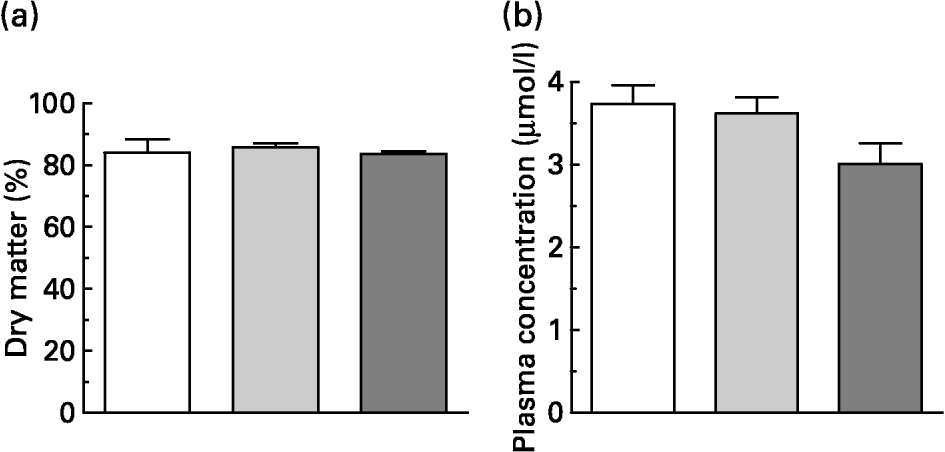
Fig. 2 (a) Gastric content (dry matter expressed as percentage of intake) and (b) flavonol plasma concentration (i.e. quercetin, isorhamnetin and tamarixetin) of rats 1 h after administration of 5 g test meals that differed in their fat content and/or fatty acid pattern. All test meals contained approximately 100 μmol quercetin/kg body weight. Values are means for six rats, with standard errors of the mean represented by vertical bars. For composition of diets, see Table 1. (□) Standard diet; (![]() ) long-chain fatty acid triacylglycerol (LCT) diet; (
) long-chain fatty acid triacylglycerol (LCT) diet; (![]() ) medium-chain fatty acid triacylglycerols (MCT) diet.
) medium-chain fatty acid triacylglycerols (MCT) diet.
The flavonol plasma concentrations (sum of quercetin and methylated metabolites) 1 h after intake of quercetin tended to be lower after intake with the MCT diet (Fig. 2(b); P = 0·073). In rat plasma the pattern of deconjugated quercetin metabolites differed from that found in pigs. In rats, the relative proportions of quercetin, isorhamnetin and tamarixetin in plasma were 52·3 (sem 1·3), 45·0 (sem 1·4) and 4·9 (sem 0·1) %, respectively (n 18). As observed in pigs, however, dietary treatment did not influence the proportions of methylated metabolites.
Discussion
In previous experiments, the addition of fat to a low-fat diet increased quercetin bioavailability from quercetin-3-O-glucoside as well as from quercetin aglycone (Lesser et al. Reference Lesser, Cermak and Wolffram2004). Research during the past decade has shown that any quercetin glycosides present in food have to be deglycosylated prior to absorption into the circulation (Cermak et al. Reference Cermak, Landgraf and Wolffram2003; Murota & Terao, Reference Murota and Terao2003). Thus, we considered that the aglycone that was used in the present study is a representative flavonol of other nutritionally relevant quercetin glucosides that are absorbed in the small intestine, like quercetin-3-O-glucoside. Effects of dietary co-ingestion of either LCT or MCT compared to a low-fat diet on the relative systemic availability of quercetin aglycone were investigated. In principle, the results obtained agree with the observations in the previous study (Lesser et al. Reference Lesser, Cermak and Wolffram2004). Again, the co-administration of dietary fat enhanced the bioavailability of quercetin in pigs. Compared to the previous study, however, the stimulatory effect of the LCT diet was less pronounced and failed to meet the level of significance. The AUC obtained for quercetin applied with MCT fat was significantly higher than with the LCT or standard diet. Quercetin absorption from the test meal containing MCT was substantially delayed, indicated by the relatively slow rise in plasma levels (Fig. 1) and the late t max (Table 2).
One possible cause for this delay in quercetin absorption from the MCT diet could have been a delayed gastric emptying of the MCT diet. In the literature, the mode of action of MCT fat on gastric emptying is controversial. Some studies found that gastric emptying after intake of MCT or medium-chain fatty acids was more rapid than after intake of LCT or long-chain fatty acids in human subjects (Hunt & Knox, Reference Hunt and Knox1968) and in rats (Harkins et al. Reference Harkins, Longenecker and Sarett1964). Another study observed no difference in gastric emptying between LCT and MCT in rats (Maggio & Koopmans, Reference Maggio and Koopmans1987), while other groups reported a retardation of gastric emptying by MCT in comparison to LCT. In rats, the gastric emptying of tridecanoylglycerol was found to be significantly slower than that of cocoa butter or rapeseed oil (Porsgaard et al. Reference Porsgaard, Straarup and Hoy2003). Pirk & Skala (Reference Pirk and Skala1970) observed retarded gastric emptying in both rats and man by X-ray observation after administration of an MCT mixture compared to a lard mixture. They also found a delay in complete evacuation time of the small intestine after administration of MCT.
We investigated gastric emptying in rats after consumption of specific experimental diets. Rats are a frequently used animal model for investigations on gastric emptying, as mentioned earlier, and were chosen in this experiment because of the easier handling of the greater number of animals required (here n 18) compared to pigs. Regulation of gastric emptying in rats and pigs in response to dietary fat seems to function similarly. Plasma levels of cholecystokinin (CCK), a major mediator in the complex interplay of nutritional, hormonal and neural factors regulating gastric emptying, were shown to increase, among other triggers, in response to ingestion or intraduodenal administration of LCT or long-chain fatty acids in rats and pigs (Lilja et al. Reference Lilja, Wiener, Inoue, Fried, Greeley and Thompson1984; Cuber et al. Reference Cuber, Bernard, Levenez and Chayvialle1990; Lewis & Williams, Reference Lewis and Williams1990; Hölzer et al. Reference Hölzer, Turkelson, Solomon and Raybould1994). Intravenous (i.v.) injection of active CCK-8 peptide dose-dependently evokes pylorus contraction and reduces intragastric pressure in rats, thus slowing down gastric emptying (Adelson et al. Reference Adelson, Million, Kanamoto, Palanca and Taché2004). In pigs, gastric emptying was also retarded in response to i.v. application of CCK-8 (Gregory et al. Reference Gregory, McFadyen and Rayner1995), and i.v. application of a specific CCK peripheral receptor antagonist reduced inhibition of gastric emptying triggered by duodenal fat infusion (Rayner & Miller, Reference Rayner and Miller1993).
At 1 h after intake of the test meals in the present investigation, that is at a time point where plasma levels of quercetin metabolites were distinctly different between pigs that had ingested the LCT or MCT diet, no diet-related differences in gastric emptying were observed. The results obtained at this specific time point do not allow conclusions to be drawn regarding the impact of MCT v. LCT diet on gastric emptying in general. However, they do indicate that the delayed quercetin absorption from the MCT diet is probably not explained by slower gastric emptying. Some authors (Hopman et al. Reference Hopman, Jansen, Rosenbusch and Lamers1984; Vu et al. Reference Vu, Verkijk, Muller, Biemond, Lamers and Masclee1999) observed that, in contrast to LCT, dietary MCT do not induce CCK release in man. Because CCK inhibits gastric motility, a decrease in CCK secretion would result in enhanced gastric emptying and thus cannot explain the results obtained in the present study.
We also analysed the plasma flavonol levels at 1 h after meal intake and found that flavonol levels in rat plasma tended to be lower with the MCT diet. This was in accordance with the respective 1 h values in pigs, where we observed a significant difference (Fig. 1; P < 0·05). This might indicate that absorption of quercetin was also delayed from the MCT diet in rats, even though quercetin absorption may already have occurred from rat stomach (Crespy et al. Reference Crespy, Morand, Besson, Manach, Demigné and Rémésy2002).
Although the MCT and LCT fat differ somewhat regarding their melting temperature (lard 26–39°C, C8 : 0 16·3°C, C10 : 0 31·3°C), it can be assumed that both fats are fluid at body temperature. Conjugated bile acids are essential for the formation of mixed micelles, which enhance the absorption of fat-soluble substances, including molecules deriving from luminal triacylglycerol hydrolysis. The fairly lipophilic quercetin molecule might dissolve in the lipid phase of the chyme and mucosal uptake seems to be promoted in the presence of micelles (Azuma et al. Reference Azuma, Ippoushi, Ito, Higashio and Terao2002), as indicated presumably by the accelerated absorption of quercetin from a lard-containing diet, compared with a low-fat diet, found in the previous study (Lesser et al. Reference Lesser, Cermak and Wolffram2004). As mentioned earlier, some authors (Hopman et al. Reference Hopman, Jansen, Rosenbusch and Lamers1984; Vu et al. Reference Vu, Verkijk, Muller, Biemond, Lamers and Masclee1999) observed that, in contrast to LCT, dietary MCT do not induce CCK release with subsequent gallbladder contractions in man. Thus, it can be assumed that in pigs, after ingestion of the MCT-containing diet, an emulsion without formation of mixed micelles will be present in the small intestinal contents. This might be one reason for the delayed absorption of quercetin from the MCT diet compared with the LCT-containing meal. It is known from certain drugs that their solubilization differs during lipolysis of LCT v. MCT (Christensen et al. Reference Christensen, Schultz, Mollgaard, Kristensen and Mullertz2004). However, we do not know how quercetin behaves in this respect. In another study, the hydrophobic drug cyclosporin A was dissolved in a solvent containing either MCT or LCT, which was infused intrajejunally in dogs. The appearance of this drug in portalvenous blood was significantly delayed when it was administered together with MCT, probably because no mixed micelles were formed, in contrast to LCT (Behrens et al. Reference Behrens, Fricker, Bodoky, Drewe, Harder and Heberer1996). However, total absorption of cyclosporin A was significantly decreased with MCT, whereas in the present study the bioavailability of quercetin was increased when administered with the MCT diet.
An explanation for the delayed absorption of quercetin from the MCT diet compared to the LCT or standard diet could be a shift in quercetin absorption from the duodenum to more distal parts of the small intestine, due to physico-chemical factors. This hypothesis is in contrast to the general opinion that MCT are digested and absorbed faster than LCT (Metges & Wolfram, Reference Metges and Wolfram1991). However, some studies reported an acceleration of duodenocecal transit time during administration of MCT fat (Ledeboer et al. Reference Ledeboer, Masclee, Jansen and Lamers1995; Verkijk et al. Reference Verkijk, Vecht, Gielkens, Lamers and Masclee1997). Vu et al. (Reference Vu, Verkijk, Muller, Biemond, Lamers and Masclee1999) suggested that MCT, rather than LCT, might not be rapidly absorbed in the proximal gut but probably reached the ileocolonic region. An accelerated transit of quercetin-containing chyme through the duodenum and proximal jejunum after ingestion with the MCT diet, combined with a lower absorption from the proximal small intestine (due to solution in the lipophilic phase), could have shifted absorption of the flavonol to more distal segments of the small intestine. Assuming a more lipophilic nature of the chyme in the distal small intestine after MCT intake, a prolonged and intensified absorption of quercetin that resulted in higher accumulation in plasma could be feasible. In this context it is of interest that another lipid-soluble substance, vitamin E, showed an enhanced bioavailability when administered with MCT in comparison to LCT (Gallo-Torres et al. Reference Gallo-Torres, Ludorf and Brin1978).
Although the rats ingested a quercetin dosage only about three times higher than that applied to pigs, plasma levels after 1 h were at least ten times higher in rats. In addition, the pattern of methylated metabolites was also different between the two species. The higher proportion of isorhamnetin found in rat plasma in comparison to pig plasma might be due to a higher methylation activity exerted by catechol-O-methyltransferase (EC 2.1.1.6). These observations are in good agreement with findings from other groups (Manach et al. Reference Manach, Texier, Régérat, Agullo, Demigné and Rémésy1996; de Boer et al. Reference de Boer, Dihal, van der Woude, Arts, Wolffram, Alink, Rietjens, Keijer and Hollman2005). Thus, the methylation pattern of quercetin metabolites in pigs seems to be more similar to that in man (Hubbard et al. Reference Hubbard, Wolffram, Lovegrove and Gibbins2003) than in rats.
Although the unexpected finding of a delayed quercetin absorption with the MCT diet somewhat hampered the interpretation of the data with regard to the impact of either solubility within the chyme or integration into chylomicrons on quercetin bioavailability, we observed an enhanced bioavailability of the flavonol quercetin when administered with a diet containing moderate amounts of fat (either MCT of LCT). In general, however, human diets, especially those of a western type, contain by far enough fat to support the absorption of flavonols. Thus, only in the case of diets or meals extremely low in dietary fat might a moderate fat supply improve the bioavailability of quercetin.
Acknowledgements
We are grateful to the German Research Foundation (DFG) for funding this study by grant no. WO 763/2-3, and for supporting S.L. with a fellowship at the Research Training Group GRK820 at the University of Kiel. We are also grateful to Dr Silvia Wein, Maike Jürgensen, Dr Judith Ringel and Clemens Benthin for their support.






