1. Introduction
Nitrous oxide (N2O) is a primarily natural but persistent trace gas in the Earth’s atmosphere. In recent years human activities have increased its global abundance. Still higher concentrations of N2O are expected in the future and they may add to the global warming from the increasing levels of CO2, CH4, and other trace gases. Moreover, N2O may deplete the stratospheric ozone layer, thus further affecting the global environment (see WMO 1986, Ramanathan and others 1985). In this paper we discuss the concentrations of N2O in the pre-industrial atmosphere of several hundred to several thousand years ago, when human activities had not affected its global distribution. These concentrations are deduced from analyses of air trapped in bubbles of polar ice.
All the known sources of nitrous oxide are at the Earth’s surface. The natural sources include microbial processes in the world’s oceans and continents. Burning of fossil fuels and the use of nitrogen fertilizers are among the most significant anthropogenic sources. Once released, nitrous oxide remains in the atmosphere for a long time. Current estimates of its atmospheric lifetime range from 100 to 150 years. It is removed primarily in the stratosphere by photolysis and also by reacting with O(1D) (excited oxygen) atoms. In the pre-industrial atmosphere, we believe that the annual production and destruction of nitrous oxide were nearly balanced for long periods. Now there is an excess of N2O every year, produced by anthropogenic activities. This excess is causing a slow but steady build-up of N2O in the atmosphere. The analysis of polar ice cores to determine the concentration of N2O in the atmosphere over the last several thousand years provides information on its natural annual emissions and the effect of human activities on its cycle and global distribution.
The plan of our paper is to discuss, in section 2, the data on pre-industrial levels of N2O, including an analysis of trends and distribution from 3000 years ago to the present. The global sources and removal of N2O are discussed in section 3, along with the implications of the low concentrations found in the polar ice cores. The conclusions are summarized in section 4.
2. Pre-Industrial Concentrations, Trends, and Distribution
2.1 Methods
We chose samples from the Crête and Camp Century cores from the Arctic and from the Byrd Station core, Antarctica. The ice was kept frozen until analysis. Typical samples were about 15 cm long and the outer 1–3 cm were scraped off to avoid surface contamination. A sample thus prepared was sealed in an internally electro-polished stainless-steel container designed for the experiment. While the ice was still frozen, the chamber was flushed and pressurized with Helium to lOOOTorr. Then the ice was melted and the concentrations of N2O, CH4, N2, O2, CCl3F, and other gases were measured in the air above the melt water. The concentrations of N2O and CCl3F were determined by electron-capture gas chromatography (EC/GC) (Reference Rasmussen, Khalil and AitkinRasmussen and Khalil 1980). A mass-balance model, for amounts in the water and the space above, was used to calculate the concentration of N2O in the air extracted from the ice-core sample. The measurements of CCl3F (F-11) served as an index of contamination, since this chloro-fluorocarbon is entirely man-made and did not exist in the atmosphere at the times represented by our samples. Indeed, in many samples no detectable concentrations of F-11 were found (<5 ppt volume). We have discussed these procedures in detail in our earlier paper describing the measurements of methane in the ice cores (Reference Rasmussen and KhalilRasmussen and Khalil 1984). The measurements of nitrous oxide discussed here and of methane, reported earlier, were made in 1983 on the same samples. The data for N2O are tabulated in the Appendix and can be compared directly with the results reported for methane in our earlier paper (Reference Rasmussen and KhalilRasmussen and Khalil 1984).
The age of the air in the bubbles is less than the age of the ice from which it is extracted, because it takes many years for bubbles to seal after the snow has fallen. Moreover, the air trapped in the bubbles probably represents the average atmospheric concentrations of trace gases over several years, perhaps even decades, depending on the efficiency of the mixing of the atmospheric air and the air deep in the firn layer of the polar regions. These processes make it difficult to estimate accurately the age of the air in the bubbles compared to the age of the surrounding ice, which is better known. We have taken the age of the air to be 90 years less than the surrounding ice, which represents the average time for the transition from firn to ice. This quantity is variable, as shown by the work of Reference Schwander and StaufferSchwander and Stauffer (1984), and making accurate estimates of the firn-to-ice transition is an active area of research; however, the variability of this transition time does not significantly affect our conclusions (see also Appendix, section A.4). The depths are tabulated in the Appendix so that the data may be re-examined if the ages can be estimated more accurately.

Fig. 1. Concentrations of nitrous oxide over the last 3000 years. The ice-core measurements are averaged over 50–400 year periods to estimate mean concentrations and their uncertainties as tabulated in the Appendix, Table A.3. + designates data from the Arctic and • is used for data from the Antarctic. The first panel shows all the data, the next one contains data only for the last 500 years, and the final panel shows results from direct atmospheric measurements over the last decade (Reference Rasmussen and KhalilRasmussen and Khalil 1986). The concentration has risen from about 285 ppb volume in the pre-industrial atmosphere to about 307 ppb volume in 1985.
2.2 Concentrations
The data are quite variable, with only a few measurements in each century; therefore we took averages over 50–400-year periods (see Appendix, Tables A.2–A.3). We estimated the variability of the concentrations as 90% confidence limits of the mean values. The results are shown in Figure 1, which also includes the concentrations measured in the present atmosphere over the last decade (Table A.1). The mean concentrations and 90% confidence limits are plotted at the average time when samples were taken. The figure includes the results of all measurements in the first panel, spanning about 3000 years, then in the next panel we show an expanded view of the last 500 years, and finally of the last 10 years as in Reference Rasmussen and KhalilRasmussen and Khalil (1986).
We estimate that the average concentration of N2O in the pre-industrial atmosphere was about 285 ± 1 ppb volume, which agrees well with the 289 ± 6 ppb volume reported by Pearman and others (1986) for the period between A.D. 1600 and 1800. The two data sets are not from the same locations or periods, which may explain some of the differences. The data of Pearman and others (1986) are from an Antarctic core and span a period as recent as 1940 and go back about 400 years before present, whereas our data are from both poles, but we have almost no data from Antarctica over the last 400 years. The ± values here, and throughout our paper, are 90% confidence limits of the mean.
One Tg of nitrous oxide in the atmosphere is approximately equivalent to 0.1368 ppb volume, assuming that there are about 1044 molecules of air in the atmosphere. The total amount of N2O in the pre-industrial atmosphere therefore turns out to be about 2100 Tg, based on an average concentration of 285 ppb volume, and there are about 2260 Tg in the present atmosphere (307 ppb volume, 1984).
2.3 Trends
No systematic trends were detected in the data spanning the last 150–3000 years. We believe that all our data are from times before human activities could have influenced the cycle of nitrous oxide. Our latest data are five measurements from the Arctic that span the last 160–200 years. The average concentration during this period was 289 ± 7 ppb volume, which is about 5 ± 6 ppb volume greater than the average concentration for the rest of the data from the Arctic ice cores. The difference, however, is not statistically significant. Data from the Antarctic ice core are for much earlier times, in which we found no evidence of trends.
We also analyzed the data of Pearman and others (1986), which, although not as detailed, represent more recent times. We found that there were no significant trends up to about A.D. 1910. The four latest measurements representing atmospheric concentrations around 1940 appeared to be significantly higher (α<0.05) than the rest of the data, suggesting that by 1940 some change in N2O may have occurred; however, we are not able, on the basis of the reported measurements, to make an estimate of the change.
We conclude from our data that there was no significant change in N2O concentration between 3000 years B.P. and at least up to the mid-nineteenth century. From the results of Pearman and others (1986), it appears that the concentration of N2O did not start increasing until some time after the turn of the century. These results agree with the explanation that the present increase in N2O is caused primarily by fossil-fuel combustion and possibly by the use of nitrogen fertilizers (Reference WeissWeiss 1981, Reference Khalil and RasmussenKhalil and Rasmussen 1983, Hao and others 1987). We used a mass-balance equation, to be discussed later, and recent estimates of N2O production from the burning of coal and oil (Hao and others 1987), and calculated that by 1900 the concentration of N2O would have increased by only 1.5 ppb volume, assuming an atmospheric lifetime of between 100 and 150 years. Such a small change would be undetectable in the measurements. By 1940, however, human activities should have caused the concentration to rise by about 5 ppb volume, which is still a small change. The detection of this change will require more precise measurements of N2O in ice cores and many more measurements for recent times than have been reported until now. It is likely that N2O started increasing rapidly only in recent times, with most of the increase taking place over the last 40 years or so.
2.4 Inter-hemispheric gradient
Since nitrous oxide has a long lifetime in the atmosphere and because its sources are distributed all over the Earth’s surface, the inter-hemispheric gradient, which is the difference of mean concentrations in the northern and southern hemispheres, is very small. It is small in both the pre-industrial and the present atmospheres; however, in recent times human activities may have reversed this gradient. In the natural pre-industrial atmosphere the concentration in the Southern Hemisphere may have been slightly higher than in the Northern Hemisphere (Mahlman and others 1986), whereas now there is slightly more in the Northern Hemisphere, The pole-to-pole gradient, which is larger than the average inter-hemispheric gradient, is defined by Equation 1:
where Cn and Cs are the average concentrations in the northern and southern polar regions.

Fig. 2. The frequency distribution of N2O concentrations from Antarctic and Arctic ice cores. The average concentration is 285 ± 1 ppb volume (90% confidence limit of the mean).
The frequency distributions of nitrous oxide concentrations in ice cores from the two polar regions are shown in Figure 2. There appears to be slightly more nitrous oxide in the Southern Hemisphere. However, the data are too variable for the difference to be statistically significant (α ≤ 0.05). We estimate Δ and the 90% confidence limits to be –1.9 ± 2.5 ppb volume. In the present atmosphere Δ is +1.1 ± 0.4 ppb volume. The overall average concentration, as reflected in Figure 2, is 285 ± 1 ppb volume.
The reason for the present difference of concentrations between hemispheres is that anthropogenic industrial (and agricultural) sources are concentrated in the Northern Hemisphere. We estimated a gradient of 0.9 ppb volume (Reference Khalil and RasmussenKhalil and Rasmussen 1983), which is as observed. The reasons for the reversed gradient in the pre-industrial atmosphere are more complicated. Recent simulations of nitrous oxide, using a three-dimensional general-circulation model, showed consistently that there should be somewhat higher concentration near the South Pole, compared to the North Pole, by about 3–4 ppb volume. The conditions under which these experiments were run are more appropriate to the pre-industrial atmosphere than to the present. The cause of this asymmetry was said to be related to asymmetrial dynamical processes in the stratosphere (Mahlman and others 1986). We do not know whether our measurements reflect this phenomenon, especially since the observed asymmetry is not statistically significant and is based on highly variable and limited data.
We turn next to the budget of nitrous oxide to interpret the concentrations found in the ice cores, compared to the higher atmospheric concentrations observed in the atmosphere today.
I. Sources and Emissions of Nitrous Oxide: Tg/year = 1012 g/year.

3. Sources, Sinks, Mass Balances and Future Concentrations
3.1. Sources, emission rates, and lifetime
In spite of more than a decade of research, the global emissions of nitrous oxide are still not accurately known. When there are no trends, the total amount of N2O, or any other trace gas, in the atmosphere, is the product of the annual emission and its lifetime. Since the mid-1960s atmospheric measurements have pinned down the average concentration and hence the total amount of N2O in the atmosphere (Reference WeissWeiss 1981, Reference Bowman and ShawBowman and Shaw 1963, and references in Hahn and Junge 1977). Early work suggested that the lifetime of N2O was of the order of a decade or two, leading to estimates of large annual emissions to account for the observed concentrations (Reference Hahn and JungeHahn and Junge 1977, McElroy and others 1976). Neither large annual emissions nor the processes for removing such amounts were found. Estimates of the annual removal of N2O by the known mechanisms suggested that its lifetime may be very long, probably in the range of 100–150 years, which has led to the present view that the global annual emissions of N2O are only 24–30 Tg/year (1 Tg = 1012 g).
For the analysis of the ice-core data and present trends we will use our earlier estimates of global emissions of N2O, which are shown in Table I, along with a more recent budget by McElroy and Wofsy for comparison (quoted in WMO 1986). The sources are subdivided into natural and anthropogenic, with the natural sources further subdivided into land- and ocean-based components. Anthropogenic sources include biomass combustion, use of nitrogen fertilizers, and burning of fossil fuels, particularly coal and oil. Soils and oceans are natural sources. Our budget was derived from the previous studies, which included the works of Reference WeissWeiss (1981), Elkins and others (1978), McElroy and others (1976), Reference Pierotti and RasmussenPierotti and Rasmussen (1976), and Reference Weiss and CraigWeiss and Craig (1976).
Using photochemical calculations it is estimated that the atmospheric lifetime of N2 is between 100 and 150 years, caused by its reaction with O(1D) and its photolysis in the stratosphere (WMO 1986, Mahlman and others 1986). Here we will assume the lifetime is 100 years, as in our previous paper (Reference Khalil and RasmussenKhalil and Rasmussen 1983).
3.2 Mass-balance calculations of past and present concentrations and trends
Using the sources and the lifetimes discussed earlier we can calculate the expected concentrations of N2O over the last 3000 years and extend these calculations into the future.
The mass balance between sources and sinks can be written as follows:
All the observations, sources, and sinks discussed earlier can be linked together by solving the mass-balance equation. Assuming that the natural sources have not changed over the last several thousand years, the solution of the equation is:
where η = 1/τ and S(t) = Sn + Sa. The first term represents the natural level of N2O (Cn = Snτ). The value of this term is deduced from the ice-core measurements discussed earlier (= 285 ppb volume). The second term reflects the growing influence of human activities, where C1 is the contribution from anthropogenic sources at time t = 0. We describe the anthropogenic source by a logistic equation:
which has the following solution:


where α = BA, K is a dimensionless constant of integration determined entirely by Sp, which represents the present (1987) annual anthropogenic emissions, and by Tp, the present time (= 500 years). This model provides a means for taking present estimates of anthropogenic emissions and calculating the emissions at earlier times until human activities contribute insignificant amounts to the global cycle of N2O, and for extrapolating the anthropogenic emissions into the future. Time “t” in Equations 3–5 is taken to be t = time (in A.D.) – 1487, so that t = zero 500 years ago, it is 500 (tp) at present and is extrapolated into the future to 613, which represents A.D. 2100.
Here is how we estimated the values of the paremeters A, B, and K needed to solve the equation. We assume that A = 3 ppb volume/year. It represents the most that anthropogenic activities can contribute in the future. This limit is suggested by expected population growth (Ehrlich and Others 1977). The value of B is chosen to make the present rate of increase of anthropogenic emissions about 3%/year (see Hao and others 1987). Under this constraint, the value of B is found to be 0.0167/ppb volume from Equation 4. K is calculated from Equation 5b by taking the present (1987) anthropogenic source to be 8.7 Tg/year (1.18 ppb volume/year), which represents a 3%/year increase over the value for 1/1978 given in Table I. The value K turns out to be 9 × 1012. The resulting function describing the emissions agrees well with estimates of the use of coal and oil over the last 50 years or so (see Hao and others 1987).
The solution of Equations 2–5 is plotted in Figure 3, which shows the overall agreement between the observed and calculated concentrations in the first panel, an expanded view in the second panel spanning approximately the last 400 years, a third panel demonstrating that concentrations started increasing recently, and finally a panel giving the atmospheric concentrations over the last decade. The model for the anthropogenic emissions was extended to estimate the build-up of N2O in the future.
3.3 Future concentrations
There is a practical need to estimate the future concentrations of nitrous oxide and other gases so that we can accurately assess their potential effects on the environment. Global warming or depletion of the stratospheric ozone layer depends critically on the build-up of trace gases that has yet to occur. Although there is no certain way to predict future concentrations, a mass-balance model such as the one discussed earlier isolates natural and anthropogenic sources, and then the anthropogenic sources are increased according to expected changes in global industrial, agricultural, economic, or social conditions, or changes in population. We calculated possible future concentrations by extending the source function of Equation 5 to future times.

Fig. 3. Measured concentrations in ice cores and in the present atmosphere are shown; concentrations were calculated with a global mass-balance model assuming an atmospheric lifetime of 100 years, and sources as described by Reference Khalil and RasmussenKhalil and Rasmussen (1983) and in the text. Significant changes appear to have started recently, as shown in the third expanded panel. Other combinations of sources and lifetimes may also explain the observations; however, such combinations are not likely to differ greatly from those discussed here.
We estimate that 50 years from now the concentration of N2O would be about 390 ppb volume, assuming that the lifetime is 100 years and the anthropogenic sources increase according to a logistic equation limited by population, as discussed in the previous section. In our earlier paper (Reference Khalil and RasmussenKhalil and Rasmussen 1983) we had used the same type of model, and for similar rates in increase of present anthropogenic sources we had estimated the concentration to be 370 ppb volume in 509 years (taking the case of α = BA = 0.06, which gives dSa/dt in 1978 = 0.04; see Appendix, section A.4). The previous estimates assumed a smaller anthropogenic source (0.72 ppb volume/year), a lower ultimate limit (A = 2.16 ppb volume/year), and a lower pre-industrial concentration of 282 ppb volume, which explains the somewhat lower estimated future concentrations. Other estimates of future concentrations are discussed by Reference WeissWeiss (1981), who assumed that anthropogenic sources would increase exponentially at 3.5%/year and therefore estimated concentrations of 350 ppb volume by about the year 2020. Exponentially increasing sources cannot be extended for long periods before they result in very high concentrations. Hao and others (1987) assumed that anthropogenic sources would continue to increase linearly at present rates and thus calculated that N2O concentrations would reach levels a little less than 360 ppb volume in 50 years, which they said was a conservative estimate. Both Hao and others (1987) and Weiss (1981) assumed that the atmospheric lifetime of N2O is 150 years. Of the three models to estimate future concentrations, ours is the only one in which growth of anthropogenic sources is limited. The results of these models suggest that 50 years from now the concentration of N2O will be 360–390 ppb volume, or about 16–25% more than the present, which is a change 3–4 times larger than has taken place between now and pre-industrial times.
4. Discussion and Conclusions
We have shown that the pre-industrial levels of N2O were about 285 ppb volume and that there is no evidence of any significant changes until very recent times. During the last decade atmospheric concentrations have risen steadily at about 0.3%/year, and the average concentration in 1984 was about 307 ppb volume. To explain these observations quantitatively we made two assumptions: that our previous estimates of the global annual emissions of N2O from natural and anthropogenic sources were correct and that the atmospheric lifetime of N2O is about 100 years. Next we assumed that the present anthropogenic sources can be extrapolated both backward and forward in time by a logistic growth model. Using the lifetime and the source function we then calculated the expected concentrations, which fully explain the observations shown in Figure 3. The calculated concentrations reproduce the past and present concentrations and current rate of increase. They also show that the changes being observed have taken place over recent times, becoming significant around the 1940s and 1950s.
The calculations provide a consistent framework for describing the observed concentrations of N2O in ice cores and the present atmosphere, the estimated anthropogenic and natural sources, and the atmospheric lifetime. There are, however, other combinations of emissions and lifetimes that may also explain the observations equally well. With more data the range of possibilities can be reduced.
Acknowledgements
We thank Dr P J Fraser (CSIRO-Australia) for discussions. Samples were obtained from the ice-core facility at the State Univesity of New York at Buffalo. Financial support for this work was provided in part by grants from the National Aero nautics and Space Administration (NASA, grant no. NAGW-280) and from the National Science Foundation (NSF, grant no. DPP-8207470). Additional support was provided by the Biospherics Research Corp. and by Andarz Co.
Appendix
A.1. Atmospheric N2O over the last decade
Table A.1 consists of the average concentrations during each year between 1975 and 1984. To obtain the average concentration for the year, we took the average of concentrations measured in January of a given year and the January of the next year (Reference Rasmussen and KhalilRasmussen and Khalil 1986).
A.1. Concentrations of Nitrous Oxide over the Last Decade (ppb volume).

A.2. Ice-core measurements of N2O
Table A.2 consists of the measured concentrations of nitrous oxide in the ice cores. We reported measurements of methane for the same samples in an earlier paper. Other details of the samples and their analyses are provided in the same paper (Reference Rasmussen and KhalilRasmussen and Khalil 1984). The age of the air, reported in the following tables, is assumed to be 90 years less than the age of the ice from which it is extracted. The ages of the air are rounded to the nearest 10 years.
A.2. Pre-Industrial Concentrations of Nitrous Oxide from ICE Cores.

A.3. Average concentrations and confidence limits
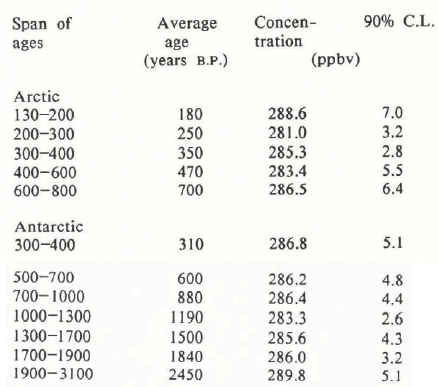
Average concentrations over various lengths of time are reported in Table A.3. The times are rounded to the nearest 10 years. These concentrations are plotted in Figure 1.
A.3 Averaged Concentrations of Nitrous Oxide Over the Last 3000 Years
A.4. Notes on comparisons with our previous publications:
1. Rasmussen R A, Khalil M A K 1984 Atmospheric methane in the recent and ancient atmospheres: concentrations, trends and inter-hemispheric gradient. Journal of Geophysical Research 89(D7): 11599-11605.
In the course of comparing this present paper with our earlier work on methane (Reference Rasmussen and KhalilRasmussen and Khalil 1984), we discovered some errors that could lead to confusion if left uncorrected.
In table A.4 of the CH4 paper, which is analogous to Table A.2 here, for all except the Crête 1974 core, the ages reported are not the ages of the ice as stated in the paper but are 90 years less, which corresponds to our estimated average transition time from firn to ice. In the CH4 paper, for the five measurements of the Crête 1974 core, the reported numbers are the ages of ice and 90 years should be subtracted to arrive at the age of the air. Secondly, in the CH4 paper the formula relating the depth of ice to its age is wrong for the Crête 1974 core although figure A.1 shows the correct relationship. The formula should read: T(age B.p.) = -29 (years) + 3.26 (years/m) Z (depth, m). These errors affect only the appendix of the CH4 paper and not the conclusions of the paper.
In the CH4 paper we used the ages of the ice samples as estimated by Dr C.C. Chou of the Scripps Oceanographie Institution. Over the ranges of depths we investigated, the ages are, to a very good approximation, represented by linear functions of the form: (T(age) = A + BZ(depth), where A and B are constants, as reported in the CH4 paper and corrected above for the Crête 1974 core. In this paper we have used these formulae and not the actual ages. We used A = -293 years and B = 8.67 years/m for the Byrd cores, A = 12 years and B = 3.13 years/m for the Camp Century cores. This introduces small differences, generally around 10 years, between the reported ages of the air in Table A.2 here and in table A.4 of the CH4 paper. For a few samples of deeper ice the discrepancy may be somewhat larger. These differences do not affect our conclusions in the slightest.
Finally it should be noted that there are samples in which we did not measure CH4, and there are others for which no N2O analyses were done; however, for most samples we measured both gases.
2. Khalil M A K, Rasmussen R A 1983 Increase and seasonal cycles of nitrous oxide in the earth’s atmosphere. Tellus 35B: 161-169.
In Equation 5 of our paper in Tellus and in the definition of α, B should be B as it is in Equation 4 of this paper. This typographical error could lead to confusion with B in equation 4 of the Tellus paper, where it represents the anthropogenic emissions at time 0 (1978). In the Tellus paper we extrapolated the concentrations of N2O into the future and therefore assumed time to be 0 in 1978, which is the year when we first took atmospheric measurements.
We did not make it clear in the Tellus paper that a range of values was chosen for a, to make the rate of increase of anthropogenic sources lie between 2%/year and 4%/year at t = 0 (1978). This is particularly confusing since in the figure (fig.4) these calculations were compared with other calculations, using an anthropogenic source increasing exponentially at rates of 3.5 and 6%/year. This comparison was a mistake. The intention was to compare the results of the logistic model with exponentially increasing anthropogenic sources at 2-4%/year rates of increase. Therefore it should be noted that, in the Tellus paper, for α = 0.035 the rate of increase of anthropogenic emissions in 1978, given by 1/Sa(dSa/dt)1t = 0, is 2.3%/year, and for αx = 0.06 it is 4%/year. These rates should be compared with the calculations in this paper, where we adopt the rate of increase of 3%/year for anthropogenic sources in recent years, based on the work of Hao and others (1987). Other matters relevant to comparing the calculations in this paper and those given in the Tellus paper are discussed in the text.






