Lycopene is an acyclic C40 non-polar carotenoid, present in several dietary sources such as tomato, watermelon, guava and apricot(Reference Boileau, Boileau and Erdmea1). Although lycopene is not a provitamin A, studies have shown multiple biological activities including decreased risk of prostate cancer(Reference Gann, Ma and Giovanucci2), inhibition of cell proliferation, migration and invasion in breast, endometrial and liver carcinoma cells(Reference Blaner, Olson, Sporin, Roberts and Goodman3–Reference Nahum, Zeller and Danilenko8), and prevention of mutagenesis and chromosome instability(Reference Liu, Russell and Wang9, Reference Russell10). In addition, a variety of epidemiological trials indicated that high intakes of lycopene-containing foods (primarily tomato products) or blood lycopene concentrations are associated with decreased risk of CVD and prostate cancer(Reference Rao11–Reference Erdman, Ford and Lindshield15).
Lycopene has eleven conjugated double bonds and each of them could be either in an E or Z configuration. All-E-lycopene is the predominant isomer in plants, representing about 80–97 % of total lycopene in tomatoes and related products(Reference Shi and Le Maguer16). In human body fluids and tissues such as plasma, breast milk, prostate, testis and skin, 25–70 % of lycopene is found in various Z forms(Reference Alien, Smith and Clinton17–Reference Wu, Schwartz and Platz24). The high concentrations of Z-isomers in vivo triggered the hypothesis that they may be more bioavailable and/or have a different specific bioactivity. In terms of bioefficacy, Shi & Le Maguer(Reference Shi and Le Maguer16) indicated that the biological potency of Z-lycopene isomers is different from that of the all-E form. Böhm et al. (Reference Böhm, Puspitasari-Nienaber and Ferruzzi25) found that some Z-isomers had a stronger in vitro antioxidant activity than the all-E form. For these reasons, Z-lycopene isomers are regarded as offering potentially better health benefits than the all-E-isomer.
Although Z-lycopene is the main component in human plasma and tissues, it is still not known if Z-isomers are absorbed preferentially from the food and/or if isomerisation occurs in humans. According to Tyssandier et al. (Reference Tyssandier, Reboul and Dumas26), lycopene appears in plasma chylomicrons mostly as all-E-lycopene after consumption of a tomato-rich meal. In contrast, Gustin et al. (Reference Gustin, Rodvold and Sosman27) and Unlu et al. (Reference Unlu, Bohn and Francis28) reported that about 40 % of lycopene is present in chylomicrons as Z-lycopene isomers, indicating that lycopene isomerisation could take place during absorption. When a tomato-based meal rich in the all-E-isomer is consumed daily over a few weeks, plasma lycopene concentration increases, where 60 % is as Z-lycopene isomers and 40 % is as all-E-lycopene(Reference Richelle, Bortlik and Liardet29, Reference Burri, Chapman and Neidlinger30).
In the present study, we investigated the isomerisation of lycopene during the absorption process. This evaluation has been performed at different stages of absorption: under conditions in the gastrointestinal tract using simple in vitro models such as gastric and duodenal juices, incorporation into mixed micelles and using the more sophisticated gastrointestinal model (TIM-1), under conditions found in enterocytes using Caco-2 cells as a model of human intestinal epithelium, and in vivo, in chylomicrons released during the postprandial phase in humans.
Materials and methods
Tomato products
Tomato paste, used for the TIM-1 model and for the human clinical trial, was obtained from Thomy (Vevey, Switzerland). It contained 0·0751 g lycopene per 100 g. Tomato oleoresin, used for the in vitro experiments, was purchased from Indena (Milan, Italy). It was prepared by ethyl acetate extraction of tomatoes and its lycopene content was 8·8 g per 100 g. Both tomato extracts had an identical profile of lycopene isomers consisting of about 94 % all-E- and 5 % 5-Z-isomers.
In addition to lycopene, these tomato extracts contained phytoene, phytofluene and β-carotene, but in lower amounts. Typically, in tomato paste lycopene, phytoene, phytofluene and β-carotene contributed 79, 12, 6 and 3 % of total carotenoids, respectively.
Gastric and duodenal juices
Gastric juice (100 ml) was prepared using 0·3 g pepsin and 0·5 g NaCl dissolved in 80 ml distilled water; pH was adjusted with 1 m-HCl. The solution was transferred into a volumetric flask and the volume adjusted to 100 ml. Before every experiment, gastric juice was mixed with sodium chloride (0·5 %) with a ratio 20:6 (v/v). Final pH was in the range of 2–4.
Duodenal juice was prepared by mixing a water solution of 4·9 % porcine bile with a solution of 2·42 % pancreatin (in water) and sodium phosphate buffer of pH 7·0 (39 mm-NaH2PO4 and 62·5 mm-Na2HPO4H2O) with a ratio of 1:1:7 (by vol.). All the chemicals and digestive enzymes used to prepare the gastric and duodenal juices were from Sigma (Basel, Switzerland).
For every test on gastric or duodenal juice, 2 mg tomato oleoresin were weighed into a dark Pyrex tube and incubated with 0·8 g groundnut oil under agitation at room temperature for 90 min. After this period, 4 ml gastric juice or 4 ml duodenal juice or a combination of 3 ml of each were added to the tube and incubated at 37°C. Samples were collected after 15 min, 30 min, 1 h and 2 h for the gastric juice and after 30 min, 1 h and 2 h for the duodenal juice and stored at − 80°C until analysis of their total lycopene content and their lycopene isomer profile.
Mixed micelles preparation
Artificial mixed micelles were used to screen for isomerisation within the gastrointestinal tract and also to deliver lycopene to Caco-2 cells. Tomato oleoresin, as the lycopene source, was dissolved in dichloromethane. The preparation of mixed micelles was performed according to Sugawara et al. (Reference Sugawara, Kushiro and Zhang31).
Dynamic gastrointestinal model
The dynamic gastrointestinal model (TIM-1) was from TNO (Wageningen, The Netherlands) as described in detail by Minekus et al. (Reference Minekus, Marteau and Havenaar32). The digestive enzymes used with this model are pepsin, porcine bile, trypsin and (-amylase from Sigma (Basel, Switzerland), Pancrex V powder from Paines & Byrne (Staines, Middlesex, UK) and lipase from Amano (Chipping Norton, Oxon, UK). Tomato paste (5 g) present in a 125 g portion of the standard meal was tested in duplicate. The standard meal consisted of semolina (70 g) cooked in 200 ml hot water, white bread (40 g), egg whites (60 g), groundnut oil (40 g), natural yoghurt (125 g), sugar (5 g) and water (330 g) and was of the same composition as the one used in the human intervention study.
Tissue culture
Caco-2 cell monolayers are a model of the human intestinal epithelium. Caco-2 cells spontaneously differentiate into polarised absorptive cell monolayers and, after differentiation, display morphological and biochemical characteristics similar to human enterocytes. This model gives reproducible values that closely correlate with in vivo data and has been used to study the molecular mechanisms involved in the absorption of carotenoids(Reference Reboul, Abou and Mikail33–Reference Moussa, Landrier and Reboul35). For the present study we used three different Caco-2 cell clones: Caco-2 SM, a generous gift of Dr Shubha Murthy (University of Iowa, USA), Caco-2 and Caco-2 HTB37 which were obtained from the America Type Culture Collection (ATCC; Rockville, MD, USA). For maintenance, Caco-2 and Caco-2 HTB37 cells were seeded at a density of 40 × 103 cells/cm2, and cultured in a humidified incubator at 10 % CO2 and 37°C in Dulbecco's modified Eagle's medium containing 4·5 g glucose/l, 20 % fetal bovine serum, 1 % non-essential amino acids, gentamicin (150 μg/ml), fungizon (1 μg/ml), penicillin (100 U/ml) and streptomycin (100 mg/ml). Caco-2 SM cells were seeded at a density of 6·7 × 103 cells/cm2 and cultured in a humidified incubator at 5 % CO2 and 37°C in Dulbecco's modified Eagle's medium containing 4·5 g glucose/l, 10 % fetal bovine serum, gentamicin (150 μg/ml), penicillin (100 U/ml), streptomycin (100 mg/ml) and 2 mm-l-glutamine. The medium was changed every 2 d. In order to obtain differentiated monolayers, Caco-2 and Caco-2 HTB37 cells were seeded at a density of 1 × 106 cells/well and cultured for 21 d. Caco-2 SM cells were seeded at a density of 1·5 × 105 cells/well and cultured for 14 d.
Cellular lycopene uptake
Medium (2 ml) containing lycopene-rich mixed micelles (2 μm of total lycopene) was incubated with the different Caco-2 cell clones for periods of 2 or 6 h. After the incubation, the supernatant fraction of the wells was collected on dry ice and stored at − 80°C pending analysis of total lycopene content as well as lycopene isomer profile. The cell monolayers were washed twice with PBS and cells were lysed with 5 % sodium dodecyl sulfate. The lysate was also collected on dry ice and stored at − 80°C pending analysis of the total lycopene content and isomer profile.
Human intervention study
Subjects
There were thirty healthy men enrolled in the study. A total of twenty-seven subjects, aged 24 (sem 1) years, completed the study. Their mean starting body weight was 70 (sem 1) kg and BMI was 22·5 (sem 0·3) kg/m2. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the ethical committee of Marseille (Marseille, France). All subjects received information on the background and design of the study and gave written informed consent before participation. They were free to withdraw from the study at any time.
Study design
Subjects were asked to refrain for 48 h before the postprandial test from eating tomato (fresh or sauce including ketchup and harissa), pizza, ratatouille, lasagna, pasta including tomato sauce, watermelon, pink grapefruits and guava. In addition to this dietary restriction, the subjects ate a standard meal the evening before the postprandial test consisting of green vegetables, a source of cereals (paste, bread or rice), lean meat or fish, one low-fat natural yoghurt, one fruit and one mineral water. They should have consumed this dinner in the evening between 19.00 and 20.00 hours. These recommendations were checked by the investigator on the postprandial test day. After an overnight fast, each subject consumed a standard meal (as previously described in the TIM section). This meal was consumed within 30 min. No other food was allowed over the following 6 h, but the subjects were allowed to drink bottled water. Blood samples were drawn before administration of the standard meal as well as at 2, 3, 4, 5 and 6 h post-absorption and introduced into evacuated tubes containing K-EDTA. The tubes were immediately placed into an ice-water-bath.
TAG-rich lipoprotein isolation
On the test day, plasma (6 ml) was overlaid with 0·9 % NaCl solution and centrifuged for 28 min at 32 000 rpm at 10°C in an SW41Ti rotor (Beckman, Fullerton, CA, USA) in an L7 ultracentrifuge (Beckman). The upper phase containing TAG-rich lipoproteins (TRL), i.e. mainly chylomicrons with low amounts of VLDL, was collected. Immediately after recovery, TRL were divided into samples and immediately stored at − 80°C. Total lycopene content and lycopene isomer profile were determined within 10 d.
Lycopene determination
Total lycopene content and the profile of lycopene isomers were determined according to the method described previously by Schierle et al. (Reference Schierle, Bretzel and Bühler21) for lycopene products (Fig. 1) and in vitro models, and according to the method of Ferruzzi et al. (Reference Ferruzzi, Nguyen and Sander36) for TRL. The main lycopene isomers identified are 5-Z-, 9-Z-, 13-Z- and all-E-lycopene. Minor compounds (shown with an asterisk in Fig. 1) consisted of other Z-lycopene isomers. They were characterised as Z-lycopene with liquid chromatography (LC)–MS/MS using an Applied Biosystems APIC 4000 LC–MS/MS (Foster City, CA, USA): isocratic flow 1 ml/min; declustering potential, 130 V; 60 psi (414 kPa) N2; capillary voltage 22 V; vaporiser temperature, 400°C; corona needle 5 μA; the fragmentation conditions used were as described by dos Anjos Ferreira et al. (Reference dos Anjos Ferreira, Yeum and Russell22). The peak areas of unidentified Z-lycopene isomers were summed and reported as x-Z-lycopene (Table 1).

Fig. 1 Representative HPLC chromatogram of lycopene isomers. * Presence of other Z-lycopene isomers as confirmed by liquid chromatography–MS/MS.
Table 1 Lycopene isomer profiles measured in tomato paste and tomato oleoresin, in gastrointestinal milieu (TIM-1), in mixed micelles, in Caco-2 cells as well as in human TAG-rich lipoproteins (TRL) secreted postprandially*
(Mean values and standard deviations or standard errors)
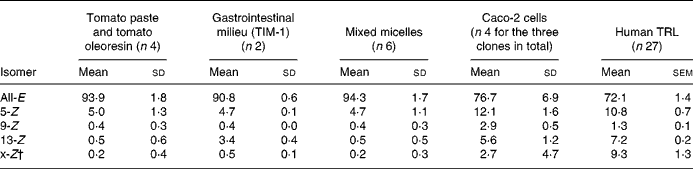
* Results are expressed as percentage of total lycopene.
† x-Z = sum of unidentified Z-lycopene isomers.
Expression of the results
Total lycopene is the sum of all lycopene isomers, i.e. all-E- and Z-isomers. Results of in vitro experiments are expressed as mean values and standard deviations. Results of TRL from the human intervention study are expressed as mean values with their standard errors.
Results
A simplified scheme of the absorption process of carotenoids in humans is depicted in Fig. 2. We used several models (in vitro and in vivo) to determine at which stage lycopene can undergo isomerisation: (1) within the lumen of the gastrointestinal tract; (2) incorporation into mixed micelles; (3) within the small intestine using three Caco-2 cell clones and (4) during release from enterocytes in vivo into human blood plasma (chylomicrons or TRL).

Fig. 2 Simplified scheme of the absorption of lycopene in human subjects. The in vitro and in vivo model systems mimicking different stages of lycopene digestion and absorption that were used during the work are indicated as 1–4.
Lycopene isomerisation within the gastrointestinal tract
Lycopene in either tomato paste or tomato oleoresin consists of 94 % all-E- and 5 % 5-Z-isomers.
Step 1 in Fig. 2
We assessed lycopene isomerisation within the gastrointestinal tract using various in vitro models. Lycopene did not undergo Z isomerisation in the presence of gastric juice at pH values between 2 and 4, nor in the presence of duodenal juice at pH 7 and also not when incubated sequentially in both conditions (data not shown).
Lycopene isomerisation within the intestine
Step 3 in Fig. 2
The next step in the lycopene absorption process is the transfer from the mixed micelles into human enterocytes. As a model for human enterocytes, differentiated Caco-2 cells (Caco-2, Caco-2 SM and Caco-2 HTB37) were incubated with mixed micelles containing lycopene (2 μm). The content as well as the isomer profile of lycopene present in mixed micelles were stable for at least 6 h under cell-culture conditions in the absence of cells.
Cells efficiently took up lycopene from mixed micelles in a time-dependent manner leading to a 13 % transfer of lycopene from the mixed micelles into Caco-2 or Caco-2 SM cells at the end of the 6 h incubation, while this transfer was about four times lower (3 %) for the Caco-2 HTB37 clone. The intracellular lycopene isomer profile was similar in the three cell clones and consisted of a significantly higher proportion of Z-isomers than in the starting material: 5-Z (12·1 (sd 1·6) % total lycopene)>13-Z (5·6 (sd 1·2) % total lycopene)>9-Z (2·9 (sd 0·5) % total lycopene)>x-Z (2·7 (sd 4·7) % total lycopene). The lycopene isomer profile in the cells was similar after 2 and 6 h incubation (data not shown), even though the total lycopene content was lower after 2 h. The absence of Z-lycopene isomers in the incubation medium indicates that lycopene isomerisation took place within the cells.
Lycopene isomerisation during absorption in humans
Step 1 to step 4 in Fig. 2
We performed a postprandial test in healthy males to allow characterisation of the lycopene appearing in intestinal secreted lipoproteins, i.e. chylomicrons. Subjects consumed a standard meal containing lycopene present in tomato paste, i.e. providing mainly all-E-lycopene (Table 1). This standard meal contained 40 g groundnut oil to ensure that subjects would secrete chylomicrons into the blood circulation. Consumption of the standard meal led to a marked production of chylomicron particles in TRL as characterised by the increase of plasma TAG concentration (Fig. 3(a)). Lycopene was efficiently absorbed and secreted in chylomicrons as shown by the rise of TRL-lycopene concentration (Fig. 3(b)). The profile of lycopene isomers appearing in TRL consisted of 29 % of Z-lycopene isomers with the proportion decreasing in the following order: 5-Z (10·8 (sd 0·7) %); x-Z (9·3 (sd 1·3) %); 13-Z (7·2 (sd 0·2) %); 9-Z (1·3 (sd 0·1) %). This profile of Z-lycopene isomers was similar between 2 and 6 h post-absorption. In contrast to the 23-fold inter-individual variation observed for total lycopene bioavailability, the proportion of Z-lycopene isomers in TRL only showed an inter-subject variation of 4 (Fig. 4). This indicates that ‘low’ and ‘high’ lycopene absorbers(Reference Hadley, Clinton and Schwartz19–Reference Schierle, Bretzel and Bühler21) exhibit quite similar ability to isomerise lycopene.

Fig. 3 TAG (a) and lycopene isomer (b) concentrations in TAG-rich lipoproteins (TRL)-containing chylomicrons of subjects having consumed a standard meal. Results are expressed in mm for TAG and in nm for lycopene. (–⋄–), All-E-lycopene; (–![]() –), 5-Z-lycopene; (–
–), 5-Z-lycopene; (–![]() –), 9-Z-lycopene; (–
–), 9-Z-lycopene; (–![]() –), 13-Z-lycopene; (–■–), sum of unidentified Z-lycopene isomers. Values are means (n 27), with standard errors represented by vertical bars.
–), 13-Z-lycopene; (–■–), sum of unidentified Z-lycopene isomers. Values are means (n 27), with standard errors represented by vertical bars.

Fig. 4 Inter-individual variation: total lycopene concentration (a) and percentage of all-Z-lycopene isomers (b) in TAG-rich lipoproteins (TRL) of each individual subject having consumed a standard meal containing lycopene (n 27). Results are expressed in nm for total lycopene and as percentage of total lycopene for the all-Z-lycopene isomers. The all-Z-lycopene isomers consist of the sum of 5-Z-, 9-Z-, 13-Z- and unidentified Z-lycopene isomers. AUC, area under the curve.
Discussion
The absorption of lycopene requires lycopene partitioning into bile salt micelles during small intestine digestion, uptake across the brush-border membrane of enterocytes and incorporation into chylomicron particles for secretion into lymph (Fig. 2).
Lycopene isomerisation did not take place in the gastrointestinal lumen either in the stomach, in the duodenum, or during transfer into mixed micelles, as assessed with various in vitro models. In contrast, all-E-lycopene was isomerised into various Z-lycopene isomers when in contact with various clones of Caco-2 cells. Lycopene absorption with a meal led to the secretion of chylomicrons present in TRL containing about 29 % of lycopene as Z-lycopene isomers. The proportion of individual lycopene isomers in Caco-2 cell monolayers, and in human TRL, was quite consistent, and decreased in the following order: 5-Z>13-Z = x-Z>9-Z. The presence of 9-Z-, 13-Z- and x-Z-isomers indicates lycopene isomerisation rather than preferential uptake of Z-lycopene, since these lycopene isomers were absent in the test lycopene preparations which contained about 94 % all-E- and about 5 % 5-Z-lycopene. However, this does not exclude a preferential uptake of 5-Z-lycopene by cells, since its proportion was higher in the Caco-2 cell monolayers and TRL than in the lycopene preparation.
Given that isomerisation of all-E-lycopene into Z-isomers is promoted by contact with acids(Reference Rodriguez-Amaya37), our first goal was to assess whether there was any isomerisation of lycopene in the acidic environment of the stomach and the small intestine. Our finding showing an absence of lycopene isomerisation in the stomach is in agreement with results obtained in vitro (Reference Failla, Chitchumroonchokchai and Ishida38) and in healthy subjects(Reference Tyssandier, Reboul and Dumas26). However, they disagree with in vitro results showing an isomerisation of lycopene that has been reported in vitro in a ‘gastric milieu’ set at pH 1·6(Reference Tyssandier, Reboul and Dumas26, Reference Moraru and Lee39). This discrepancy might be due to the use of a low pH in this in vitro model, which mimics the stomach pH in the fasting stage only. Indeed, the stomach pH, which is about 1·8 in the fasting state, sharply increases to 5·4–6·2 after intake of a meal, and then continuously decreases to reach 1·8–2·9 after 3 h digestion. The duodenal pH, which is about 5 in the fasting state, increases to pH 6·1–6·6 after intake of a meal and remains constant during digestion. In the present results, we did not observe isomerisation of lycopene in the in vitro model mimicking this duodenal condition. In the gastrointestinal lumen, the final step before absorption is the transfer of lycopene into mixed micelles. The isomer profile of lycopene in the mixed micelles was similar to the one of the tomato product, which indicates no lycopene isomerisation, but also no preferential transfer of Z-lycopene (in our particular case, 5-Z-lycopene). This is in disagreement with results of Boileau et al. (Reference Boileau, Merchen and Wasson40) showing a higher incorporation of Z-lycopene into mixed micelles than the all-E counterpart. Using the more sophisticated TIM-1 model, which is an in vitro model that allows the closest simulation of in vivo dynamic physiological processes occurring in the lumen of the stomach currently available, we confirm that lycopene does not undergo isomerisation in the gastrointestinal tract. These results are in agreement with those recently reported by Blanquet-Diot et al. (Reference Blanquet-Diot, Soufi and Rambeau41). Although the main parameters of digestion, such as pH, body temperature, peristaltic mixing and transport, gastric, biliary and pancreatic secretions and passive absorption of small molecules and water are reproduced, the actual limitations of the TIM-1 model reside in the absence of a cellular system and a feedback mechanism.
To mimic enterocyte transport and metabolism, we used three clones of Caco-2 cells. Cell culture condition did not affect either lycopene content or isomer profile. Lycopene uptake by either Caco-2 or Caco-2 SM was similar, but markedly lower by Caco-2 HTB37 cells. However, lycopene isomerised in all these three cell clones and the profile of lycopene isomers were similar. The results in Caco-2 cells matched in vivo results. Lycopene appearing in chylomicrons (TRL) and in Caco-2 cells consisted of 29 and 24 %, respectively, as a mixture of Z-lycopene isomers. This is in agreement with results described by Gustin et al. (Reference Gustin, Rodvold and Sosman27) and Unlu et al. (Reference Unlu, Bohn and Francis28). In contrast, Tyssandier et al. (Reference Tyssandier, Reboul and Dumas26) did not detect Z-lycopene isomers in chylomicrons isolated from plasma from most individuals. Although a large inter-individual variation of lycopene bioavailability is in agreement with data reported by several authors(Reference Borel, Grolier and Mekki42–Reference O'Neill and Thurnham44) and is characteristic of the presence of low and high absorbers in the population, a low inter-individual variation of the proportion of Z- to E-lycopene is noteworthy. These results indicate that whatever the lycopene absorption efficiency, lycopene isomerisation is quite similar and suggest that the factors participating in lycopene absorption are different from those involved in lycopene isomerisation.
In most human tissues, more than 50 % of the lycopene is a mixture of Z-isomers(Reference Stahl and Sies23, Reference Unlu, Bohn and Francis28, Reference O'Neill and Thurnham44, Reference Stahl, Schwarz and Sundquist45). The major Z-isomers in plasma were, in decreasing order of abundance: 5-Z>13-Z>9-Z. In humans, the Z-lycopene concentration is high in the liver, adrenal glands, testes, skin and prostate(Reference Clinton, Emenhiser and Schwartz18, Reference Clinton46, Reference Khachik, Carvalho and Bernstein47). The 29 % Z-lycopene in human chylomicrons present in TRL suggests that, in the human body, additional mechanisms are involved which increase markedly the proportion of Z-lycopene by processes such as isomerisation, preferential uptake or reduced catabolism of the Z-lycopene isomers. The high proportion of Z-lycopene isomers in human tissues could conceivably be a conversion to a more biologically effective form. It is not feasible to compare the efficacy of Z- and E-lycopene in vivo, owing to the metabolic conversion of one form to another. However, Böhm et al. (Reference Böhm, Puspitasari-Nienaber and Ferruzzi25) demonstrated that Z-lycopene isomers exhibit higher antioxidant capacity than their all-E counterpart in vitro. Thus it is tempting to speculate that the E- to Z-lycopene conversion in vivo is a metabolic activation of this carotenoid.
Acknowledgements
We thank Dr Karen Cooper for organising the in vitro gastrointestinal TIM-1 trials at TNO and Dr Francesca Giuffrida for the LC–MS/MS identification of the Z-isomers of lycopene.
The present study was funded in full by the Nestlé Research Center, Lausanne, Switzerland.
M. R., B. S., P. L., K. B. and G. W. conceived of and designed the present study. B. S., I. T., P. L. and K. B. achieved the design and production of formulations of the lycopene products. M. R. coordinated the trial and supervised the analytic aspects. B. S., I. T., P. L. and K. B. contributed to the development of analytic methods, lycopene analysis and data collection. M. R. wrote the manuscript, and all authors were involved in interpreting the results and in critical revision of the paper.
No author has any advisory board affiliations. There is no conflict of interest.







