LEARNING OBJECTIVES
-
Delineate the aetiological and epidemiological factors associated with the development of perinatal depression
-
Appreciate the differential diagnosis and assessment of risk in perinatal depression and postnatal psychosis
-
Highlight main pharmacological and psychosocial recommendations for the management of perinatal depression and psychosis
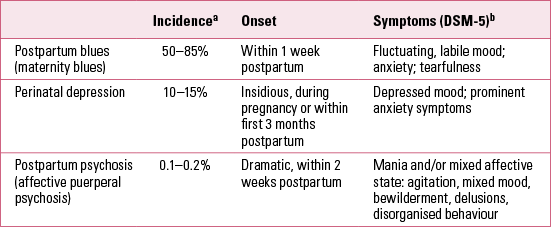
More than 150 years ago, the French psychiatrist Louis Victor Marcé wrote Traité de la folie des femmes enceintes, des nouvelles accouchées et des nourrices (Treatise on the psychoses of pregnant women, and newly delivered and nursing mothers) (Reference MarcéMarcé 1858). Marcé is today considered the founding father of ‘perinatal psychiatry’ and the first to describe postpartum psychosis. A quarter of a century ago, Kendell and colleagues demonstrated that women experience a dramatic increase in their risk of developing severe mental illness in the first 3 months after delivery (Reference Kendell, Chalmers and PlatzKendell 1987). Subsequent studies have corroborated this. During the postpartum period, about 85% of women experience some type of mood disturbance (Reference HenshawHenshaw 2003). For most women the symptoms are mild and short-lived, resolving generally within a week. However, 10–15% of women have clinically significant symptoms of depression or anxiety (Reference Cox, Murray and ChapmanCox 1993). Perinatal mood-related events have typically been divided into three categories: postpartum blues, perinatal depression and postpartum psychosis (Table 1). We will discuss each of these individually later in this article, but first we will consider general diagnostic and aetiological factors relating primarily to perinatal mood disorders.
TABLE 1 Classification and incidence of perinatal/postpartum psychiatric events
Diagnostic classification
Although perinatal depression was initially conceptualised as a disorder specifically related to childbirth and clinically different from other types of depression, more recent evidence suggests that depression occurring during the perinatal period might be clinically indistinguishable from depression at other times in women’s lives (Reference O'Hara, Zekovski and PhilippsO’Hara 1990; Reference Evans, Heron and FrancombEvans 2001; Reference Cooper, Jones and DunnCooper 2007).
Perinatal mental illnesses are not listed separately in DSM-5 (American Psychiatric Association 2013) or in ICD-10 (World Health Organization 2010), but psychiatrists all over the world have retained the terms ‘perinatal depression’ and ‘postpartum psychosis’ to refer to episodes occurring during pregnancy and/or after delivery. The main advantage of such a distinction is that it carries prognostic information concerning risk in future pregnancies. In women with a diagnosis of bipolar illness, those who experience a puerperal (postpartum) psychosis have more than a 60% risk of recurrence of psychosis following a further pregnancy, compared with a risk of 25% for those who do not experience a postpartum episode (Reference Robertson, Jones and HaqueRobertson 2005). An episode of perinatal depression increases the risk of further perinatal depressive episodes with future pregnancies. Furthermore, an episode of depression in the perinatal period confers a higher risk of recurring perinatal depression than does a history of non-perinatal depression (Reference Johnstone, Boyce and HickeyJohnstone 2001; Reference Josefsson, Angelsiöö and BergJosefsson 2002; Reference Forty, Jones and MacgregorForty 2006).
Aetiology of perinatal and postnatal depressive illness
The aetiology of postpartum (maternity) blues is still unknown. However, women with maternity blues are more likely to have a history of premenstrual tension and neuroticism, and to have experienced anxiety and depressed mood during pregnancy (Reference Kennerley and GathKennerley 1989).
Hormonal changes and the HPA axis
It is likely that the massive hormonal re-equilibration process following delivery results in brain changes that lead to the temporary experience of unstable mood (Reference Harris, Lovett and NewcombeHarris 1994, Reference Harris, Lovett and Smith1996). Progesterone is the main gestational steroid and it has been studied in relation to the onset of perinatal depression. There is no difference in either progesterone levels or the rates of decline of this and other hormones post-delivery between women who develop postnatal depression and women who do not (Reference Harris, Lovett and NewcombeHarris 1994, Reference Harris, Lovett and Smith1996). Harris et al did, however, find a difference between cortisol levels during the puerperium and at the onset of depression.
A woman’s hypothalamic–pituitary–adrenal (HPA) axis undergoes gradual changes during pregnancy because of an increasing production of placental corticotrophin-releasing hormone (CRH). The abrupt withdrawal of placental CRH at birth results in a re-equilibration of the HPA axis in the days post-delivery. These changes may be involved in the aetiology of postpartum blues, given the central role of the HPA axis in the aetiology of mood disorders in general, and in perinatal depression in particular (Reference O'Keane, Lightman and MarshO’Keane 2011a).
Depression during pregnancy has also been associated with increased activity of the HPA axis and diminished length of gestation (Reference O'KeaneO’Keane 2008), and may have implications for development of the fetal HPA axis. A pilot study in which one of us was involved (Reference O'Keane, Lightman and MarshO’Keane 2011b) made a prospective comparison of HPA axis variables in depressed and non-depressed pregnant women. The study showed for the first time that the depressed women had higher levels of second-trimester CRH and free evening cortisol than the non-depressed women, results that mirror HPA axis findings in depression outside of pregnancy. Some investigators hypothesise that a subgroup of women are particularly sensitive to the HPA changes that take place during pregnancy and after delivery. These women may be more vulnerable to perinatal depression and to other hormonally driven mood disturbances, such as those occurring during the premenstrual phase of the menstrual cycle or during the perimenopause (Reference Bloch, Schmidt and DanaceauBloch 2000).
Obstetric factors
Various obstetric factors have been considered in relation to perinatal depression. These include pregnancy-related complications (such as preeclampsia, hyperemesis, premature contractions) and delivery-related complications (such as emergency/elective Caesarean, instrumental delivery, premature delivery and excessive bleeding intrapartum). Recent studies, however, have found no overall statistically significant relationship between such factors and perinatal depression (Reference Warner, Appleby and WhittonWarner 1996; Reference Forman, Videbech and HedegaardForman 2000).
Family history of psychiatric illness
A meta-analysis of six studies (involving about 900 women) revealed that there was no association between family history of depression and perinatal depression (Reference O'Hara and SwainO’Hara 1996). However, Reference Johnstone, Boyce and HickeyJohnstone et al (2001) did find an increased risk of perinatal depression in a study of 490 women with a family history of mental illness, personal history of depression or anxiety or a history of depression in the participant’s mother; and Reference Forty, Jones and MacgregorForty et al (2006) found that episodes of depression with onset within 4 weeks of delivery clustered in families.
Depression during or before pregnancy
It has also been found that depressed mood during pregnancy predicts the presence of depression in the postnatal period and that around 50% of episodes of postnatal depression start during pregnancy (Reference Neter, Collins and LobelNeter 1995; Reference Johnstone, Boyce and HickeyJohnstone 2001; Reference Josefsson, Angelsiöö and BergJosefsson 2002). A personal history of recurrent major depression is a very strong risk factor for the occurrence of perinatal depression: around 40% of pregnancies result in relapse (Reference Di Florio, Forty and Gordon-SmithDi Florio 2013).
Other psychological and social risk factors
Other psychological and social risk factors may play a role in the aetiology of perinatal depression. A negative cognitive attributional style, as would be expected, has been more strongly related to high levels of depressive symptomatology assessed through self-report (Reference O'Hara and SwainO’Hara 1996). Life events have also been associated with onset of depression (Reference Brown and HarrisBrown 1978). A meta-analysis found a moderate relationship between perceived life stress and perinatal depression (Reference BeckBeck 2001). This moderate risk has been repeatedly demonstrated to be mediated by poor partner support and/or inadequate social and/or economic supports (Reference Dayan, Creveuil and DreyfusDayan 2010). Stressful life events occurring either during pregnancy or near the time of delivery may increase the likelihood of perinatal depression (Reference BeckBeck 2001). A study conducted by Lee and colleagues showed an association between temporary accommodation, financial difficulties, two or more induced abortions and postnatal depression (Reference Lee, Yip and LeungLee 2000). Two studies have highlighted the importance of perceived social isolation (or lack of social support), finding this to be a strong risk factor for antenatal depressive symptoms (Reference Seguin, Potvin and St DenisSeguin 1999; Reference Forman, Videbech and HedegaardForman 2000). An important recognised risk factor for the development of both ante- and postnatal depression is severe childhood adversity (Reference Dayan, Creveuil and DreyfusDayan 2010).
Postpartum blues
It appears that about 50–85% of women experience postpartum blues during the first week after delivery (Reference HenshawHenshaw 2003). Given how common and self-limiting this type of mood disturbance is, it may be more accurate to consider the blues as a normal experience following childbirth rather than as a psychiatric disorder.
This interesting syndrome was reviewed in great detail by Reference Stein, Brockington and KumarStein (1982). Rather than feelings of sadness, women with what Stein calls the maternity blues more commonly report mood lability (mood can be also elevated temporarily), tearfulness, anxiety or irritability. Crying spells are the hallmark of the maternity blues. These symptoms typically peak on the fourth or fifth day after delivery and may last for a few hours or a few days, remitting spontaneously within 2 weeks after delivery. Although the occurrence of these experiences is sudden, unpredictable and often unsettling, they generally do not interfere with a woman’s ability to function.
Reference Heron, Craddock and JonesHeron et al (2005) reviewed the literature on milder forms of elation occurring in the puerperium. Although these symptoms may often be regarded as clinically insignificant, and part of the maternity blues, there is evidence that the experience of minor puerperal elation may be a predictor of later postpartum depression.
No specific treatment is required for postpartum blues, apart from support and reassurance, owing to the self-limiting nature of the episode (NICE 2007; Royal College of Obstetricians and Gynaecologists 2011; Scottish Intercollegiate Guidelines Network (SIGN) 2012). However, it should be noted that sometimes the blues herald the development of a more significant mood disorder, particularly in women who have a history of depression (Reference Sutter, Leroy and DallaySutter 1997). Studies from various countries demonstrate that severe blues increase the risk of postpartum depression (e.g. Reference Henshaw, Foreman and CoxHenshaw 2004; Reference AdewuyaAdewuya 2006; Reference Watanabe, Wada and SakataWatanabe 2008; Reference Reck, Stehle and ReinigReck 2009).
Perinatal depression
Effects on the mother and child
The effects of depression on the mother, her marital relationship and her children make it an important condition to diagnose, treat and prevent (Reference Robinson, Stewart, Stotland and StewartRobinson 2001). Guidelines on the clinical management of antenatal and postnatal mental health (e.g. NICE 2007; Royal College of Obstetricians and Gynaecologists 2011; SIGN 2012) highlight the importance of detection during pregnancy and the postnatal period, as untreated depression can have long-lasting adverse effects.
For the mother, the episode can be the precursor of chronic recurrent depression (Reference Campbell, Cohn, Murray and CooperCampbell 1997; Reference Pawlby, Hay and SharpPawlby 2009), and suicide resulting from puerperal mental illness is one of the leading causes of death among women during the perinatal period (Confidential Enquiry into Maternal and Child Health 2004, 2007, 2011).
A mother’s ongoing depression can contribute to emotional, behavioural (Reference Hay, Pawlby and SharpHay 2001, Reference Hay, Pawlby and Angold2003), cognitive and interpersonal problems in the later life of the child (Reference Jacobsen and MillerJacobsen 1999). Pawlby and colleagues followed up women with histories of depression during pregnancy/postpartum and their children at 11 and 16 years. They concluded that the children of mothers who met ICD-10 criteria for perinatal depression at 3 months postpartum were four times more likely than those whose mothers had been well in the postnatal period to suffer from a psychiatric disorder themselves at 11 years of age (Reference Pawlby, Sharp and HayPawlby 2008). In a different sample (Reference Pawlby, Hay and SharpPawlby 2009), they found that two-thirds of the women who first suffered antenatal depression during pregnancy experienced further episodes of depression later in life. In addition, 14% of the offspring of the women with antenatal depression were diagnosed with depression at age 16. Most interestingly, every depressed adolescent had been exposed to maternal depression. There was a 4.7-fold greater odds of depression among the 16-year-olds who had been exposed to maternal antenatal depression than among those who were not exposed.
Prevalence of perinatal depression
Perinatal depression is the most common complication of childbearing, affecting about 10–15% of women. Some studies did not find depression to be more common in the perinatal period than at any other stage in life (Reference Cooper, Campbell and DayCooper 1988; Reference O'Hara, Zekovski and PhilippsO’Hara 1990; Reference Cox, Murray and ChapmanCox 1993). However, more recent studies have demonstrated an increased risk of postnatal depression. Using the Danish psychiatric admission and birth registries, Munk-Olsen and colleagues, for example, found a threefold increased risk of admission with unipolar depression on postpartum days 31–60 (Reference Munk-Olsen, Laursen and PedersenMunk-Olsen 2006). The relative risk (RR) for admission with unipolar depression in pregnancy was 0.44 (95% CI 0.31–0.62) compared with 3.53 (95% CI 2.47–5.05) in the postpartum period. For bipolar illness, the increased risk was even more dramatic, with an RR of 0.19 (95% CI 0.04–0.86) for admission during pregnancy and of 23.33 (95% CI 11.5–47.42) for admission during the first month postpartum.
Onset and signs of perinatal depression
Perinatal depression typically begins within 1–3 months of delivery. Some women actually note the onset of milder depressive symptoms during pregnancy, and for others, postpartum blues simply continue and become more severe. In others, a period of well-being after delivery is followed by a gradual onset of depression. However, what is clear is that a large percentage of women who are depressed during pregnancy remain depressed after birth (Reference Leigh and MilgromLeigh 2008), resulting in an increased risk of insecure attachment and impaired development of the child (Reference Champagne and MeaneyChampagne 2006).
Perinatal depression may be clinically indistinguishable from depression occurring at other times during a woman’s life (Reference Wisner, Parry and PiontekWisner 2002), but the content of thinking may focus on the delivery or the baby. Some women worry excessively about the baby’s health or feeding habits and see themselves as ‘bad’, inadequate or unloving mothers (Reference Robinson, Stewart, Stotland and StewartRobinson 2001). Significant anxiety symptoms may also occur. Generalised anxiety is common, but some women also develop panic attacks or hypochondriasis. Postpartum obsessive–compulsive disorder has also been reported, where women have disturbing and intrusive thoughts of harming their infant.
It can be difficult to detect perinatal depression (Box 1), especially in milder cases, because many of the symptoms used to diagnose depression (e.g. sleep and appetite disturbance, fatigue) also occur in postpartum women in the absence of depression. Face-to-face clinical assessment and screening of women who are at risk of perinatal depression is paramount. The Edinburgh Postnatal Depression Scale (EPDS; Reference Cox, Holden and SagovskyCox 1987) is a 10-item questionnaire that may be used to identify/screen women who have perinatal depression. On this scale, a score of 12 or greater, or an affirmative answer on question 10 (presence of suicidal thoughts), raise concern and indicate a need for more thorough evaluation. The current recommendation is that for a score >11 a re-evaluation should take place within 2 weeks, unless there are concerns about suicidal ideation or significant deterioration in mental state, which should prompt to immediate referral to a psychiatrist (Reference Gibson, McKenzie-McHarg and ShakespeareGibson 2009; Reference Hewitt, Gilbody and BrealeyHewitt 2009).
BOX 1 Symptoms of perinatal depression
-
Depressed or low mood
-
Tearfulness
-
Loss of interest in usual activities
-
Feelings of guilt
-
Feelings of worthlessness or incompetence as a mother
-
Fatigue
-
Sleep disturbance
-
Change in appetite
-
Poor concentration
-
Suicidal thoughts
Postpartum psychosis
Hippocrates first described puerperal psychosis in the 5th century BC, thinking that it was the result of breast milk accidentally entering the brain. Postpartum psychosis is the most severe form of perinatal psychiatric illness. It is a rare event that occurs in approximately 1–2 women per 1000 after childbirth (Reference Kendell, Chalmers and PlatzKendell 1987). Its presentation is often dramatic, with onset of symptoms sometimes before delivery, and frequently in the first 48–72 hours after delivery. Around 50% of women who experience postpartum psychosis start to develop symptoms on days 1–3, with 22% of those showing symptoms as early as day 1 (Reference Heron, Robertson Blackmore and McGuinnessHeron 2007). Kendell demonstrated that, in the 3 months following childbirth, women are more than 20 times more likely to be admitted to hospital with a diagnosis of a psychotic disorder than at any other time in their lives (Reference Kendell, Wainwright and HaileyKendell 1976). Terp & Mortensen found that the relative risk of first admission with functional psychosis between days 2 and 28 was 3.21 (Reference Terp and MortensenTerp 1998). More recently, Harlow and colleagues noted that hospital admission was mainly confined to women who had previously had a history of psychosis or bipolar illness, and that most of the postpartum episodes occurred within 4 weeks of delivery (Reference Harlow, Vitonis and SparenHarlow 2007). Munk-Olsen’s group also found that the risk of admission was increased in the first 3 months and was highest for first-time mothers 10–19 days postpartum (Reference Munk-Olsen, Laursen and PedersenMunk-Olsen 2006). Another study has shown that women with bipolar I disorder reported an approximately 50% risk of a perinatal major affective episode per pregnancy/postpartum period (Reference Di Florio, Forty and Gordon-SmithDi Florio 2013). It also found that most perinatal episodes occurred in the first month postpartum, with mania and psychosis having an earlier onset than depression.
Signs of postpartum psychosis
Earliest signs of postpartum psychosis include restlessness, feeling energetic or active, irritability, over-talkativeness and insomnia (Reference Heron, Robertson Blackmore and McGuinnessHeron 2007) (Box 2). Women with the disorder exhibit a rapid shift between depressed and elated mood, disorientation or confusion, and erratic or disorganised behaviour (Reference Heron, McGuinness and Robertson BlackmoreHeron 2008). Delusional beliefs are common and often centre on the infant. Auditory hallucinations that instruct the mother to harm herself or her infant may also occur. The risk of infanticide in postpartum psychosis is low (Reference ApplebyAppleby 1996; Reference Altshuler, Hendrick and CohenAltshuler 1998; Reference SchalekampSchalekamp 2005), as it is for suicide (Reference OatesOates 2003). However, the latest report of the confidential enquiries into maternal deaths in the UK states: ‘The last three Enquiry Reports found that maternal suicide was more common than previously thought and was a leading overall cause of maternal death’, hence the need to screen and identify high-risk cases (Reference Oates and CantwellOates 2011: p. 133).
BOX 2 Early symptoms of postpartum psychosis
-
Restlessness, agitation or perplexity
-
Feeling energetic or active
-
Overtalkativeness
-
Irritability
-
Insomnia
This type of psychotic presentation in the puerperium has reminded many authors of the ‘cycloid psychosis’ and ‘boufée delirante’ described by Reference LeonhardLeonhard (1961), Reference PerrisPerris (1974) and others (Reference KleistKleist 1929; Reference PfuhlmannPfuhlmann 1998). In the words of Reference OrlikovOrlikov (2011), this is an illness of ‘acute onset, cyclic course with full recovery in between, intense involvement of anxiety, psychomotor agitation, affective dysregulation, polymorphous delusions, and hallucinations’. These authors highlighted the favourable prognosis of the illness, the sudden or acute onset and the shifting nature of the symptoms, with opposite polar phases in the same episode (mood swings, confusion, hyperactivity, perplexity, deep feelings of happiness or ecstasy, and particular concern with death or dying).
Prognosis of postpartum psychosis
The prognosis of an acute episode is generally good, with most women making a good recovery and returning to premorbid levels of functioning. However, the risk of further episodes of psychosis both after subsequent pregnancies and at other times is high as mentioned earlier. Garfield et al followed up 66 women during 10 years after hospital admission for puerperal illness. The recurrence rate was 87.2% and the readmission rate was 63.3%, with psychiatric history being the strongest predictor of recurrence (Reference Garfield, Kent and PaykelGarfield 2004). Another very recent study has shown that the risk of readmission for non-puerperal psychosis, although gradually decreasing with time, remains high for many years after postpartum psychosis. Interestingly, their sample was followed-up for 30 years in total (Reference Nager, Szulkin and JohanssonNager 2013).
Preventive measures
The Scottish Intercollegiate Guidelines Network (2012) recommends that all pregnant women be asked about personal history of postpartum psychosis, other psychotic disorders (especially bipolar affective disorder and schizophrenia) and severe depressive disorder. All pregnant women should be asked about family history of bipolar disorder and postpartum psychosis.
Women at high risk of perinatal mental illness should have a detailed plan for their late pregnancy and early postnatal psychiatric management, agreed with the woman and shared with maternity services, the community midwifery team, general practitioner (GP), health visitor, mental health services and the woman herself. With the woman’s agreement, a copy of the plan should be kept in the hand-held records that she takes to antenatal appointments. The plan should identify what support should be in place and who to contact if problems arise, together with their contact details (including out of hours). It should also address decisions on medication management in late pregnancy, the immediate postnatal period and if breastfeeding. Enquiry about depressive symptoms should be made, at minimum, on booking in and postnatally at 4–6 weeks and 3–4 months.
All women of childbearing potential who take psychotropic medication should be made aware of the possible effects of medications in pregnancy. The use of reliable contraceptive methods should be discussed. In view of the risk of early teratogenicity and longer-term neurobehavioural toxicity, valproate (as a mood stabiliser) should not be routinely prescribed to women of childbearing potential.
Assessment of postpartum affective disorder
Postpartum depression and psychosis present as a continuum, and the type of treatment selected is based on the severity and type of symptoms. However, before initiating psychiatric treatment, medical causes for mood disturbance (e.g. thyroid dysfunction, anaemia) must be excluded. Initial evaluation should include a thorough history, physical examination and routine laboratory tests. It is paramount to assess suicidality and infanticidal ideas. Delusions should be clearly defined and their content explored. One study found that 53% of women with severe postpartum mental illness and 78% of those with psychotic postpartum disorders had delusional beliefs about their infant (Reference Chandra, Bhargavarman and RaghunandanChandra 2006). Close observation of mother and baby interaction is very important to elucidate psychopathology. Rating scales can assist the clinician in exploring abnormalities in the mother–infant relationship and bonding (Box 3).
BOX 3 Some rating scales assessing motherinfant relationship
Mother–infant interaction:
-
The Bethlem Mother–Infant Interaction Scale (Reference Kumar and HipwellKumar 1996)
Mother–infant attachment:
-
Screening questionnaire for mother-infant bonding disorders (Reference Brockington, Oates and GeorgeBrockington 2001)
-
Mother-to-Infant Bonding Scale (Reference Taylor, Atkins and KumarTaylor 2005)
In our increasingly multicultural Western society it is important to consider the cultural and ethnic background when assessing the level of support of pregnant and new mothers. Rituals practised within some cultures may be protective against postnatal mental illness because they provide social and practical support for the new mother. However, mental health problems are heavily stigmatised within many cultures, and women and their families may be reluctant to seek help from health professionals, preferring to try to manage the illness with no outside help. Health professionals may be consulted only when the woman is so severely ill that the family can no longer cope (Reference Oates, Cox and NeemaOates 2004).
Psychological interventions for perinatal affective disorder
Guidelines from NICE (2007) and SIGN (2012) recommend that, in non-emergencies, before pharmacological treatment decisions are made, healthcare professionals discuss with the woman the absolute and relative risks associated with treating and not treating the mental disorder during pregnancy and the postnatal period. They should:
-
acknowledge the uncertainty regarding the risks
-
explain the background risk of fetal malformations in pregnant women without a mental disorder
-
describe risks using natural frequencies rather than percentages (e.g. 1 in 10, rather than 10%) and common denominators (for example, 1 in 100 and 25 in 100)
-
if possible, use decision aids in a variety of verbal and visual formats that focus on an individualised view of the risks, and provide written material (preferably individualised) to explain these risks; if possible, audio records of the consultation should be kept.
SIGN suggests that cognitive–behavioural therapies (CBTs) should be considered for treatment of mild to moderate depression in the postnatal period. For a woman who develops mild or moderate depression during pregnancy or the postnatal period, NICE advises the following:
-
self-help strategies: guided self-help, computerised CBT or exercise
-
non-directive counselling delivered at home (listening visits)
-
brief CBT or interpersonal psychotherapy.
A randomised study found short-term CBT to be as effective as treatment with fluoxetine in women with perinatal depression (Reference Appleby, Warner and WhittonAppleby 1997). CBT has also been shown to be effective in preventing further episodes of perinatal depression (Reference Nardi, Laurenzi and di NicoloNardi 2012). Interpersonal psychotherapy is effective not only for mild to moderate depression, but women who receive interpersonal psychotherapy also benefit from significant improvement in the quality of their interpersonal relationships (Reference Reay, Owen and ShadboltReay 2012; Reference StuartStuart 2012). These non-pharmacological interventions may be particularly attractive to patients who are reluctant to use psychotropic medications (e.g. women who are breastfeeding) or for patients with milder forms of depressive illness.
Pharmacological treatment for perinatal affective disorder
Clinicians should be cautious about prescribing during pregnancy and breastfeeding, owing to the possible risks to the fetus and infant. The principles set out in Box 4 should apply.
BOX 4 Principles for prescribing pharmacotherapy in pregnancy and during breastfeeding
-
Establish a clear indication for the pharmacological treatment, involving the woman and her family in the discussion
-
Choose treatments with the lowest known risk
-
Use medication at the lowest effective dose and for the shortest period of time
-
Be aware of potential drug interactions and aim at monotherapy
-
Be aware of the potential effects of pregnancy and childbirth on drug pharmacokinetics and pharmacodynamics
-
Where the risk is known, ensure that fetal screening and monitoring of the neonate are offered
-
Monitor for specific side-effects as well as feeding patterns, growth and development
-
Caution women against sleeping in bed with the baby if they are taking sedative drugs
During pregnancy
For a woman with serious mental illness, at high risk of relapse, discontinuation of treatment may be unwise: relapse may ultimately be more harmful to the mother and child than continuing drug treatment. Nevertheless, clinicians should be aware that antidepressants have been associated with spontaneous abortions, congenital malformations, poor neonatal adaptation, persistent pulmonary hypertension in the newborn and other neurodevelopmental outcomes in the child (Reference Hemels, Einarson and KorenHemels 2005; Reference Lattimore, Donn and KacirotiLattimore 2005; Reference Rahimi, Nikfar and AbdollahiRahimi 2006; Reference Udechuku, Nguyen and HillUdechuku 2010). However, another study (Reference Warburton, Hertzman and OberlanderWarburton 2010) has shown that, controlling for severity of maternal illness, reducing exposure to selective serotonin reuptake inhibitors (SSRIs) at the end of pregnancy had no significant clinical effect on improving neonatal health. These findings have given rise to the possibility that some adverse neonatal outcomes may not be an acute pharmacological condition such as toxicity or withdrawal. SIGN (2012) recommends that paroxetine should not be used as first-line therapy in pregnancy (see also UK Teratology Information Service 2011).
Lithium and anti-epileptic drugs have been associated with congenital malformations, neurological effects on the neonate and other complications (Reference Newport, Viguera and BeachNewport 2005; UK Teratology Information Service 2009; Reference Galbally, Roberts and BuistGalbally 2010; Reference Cunnington, Weil and MessenheimerCunnington 2011). Valproate should not be prescribed in pregnancy (SIGN 2005; Reference Nicolai, Vles and AldenkampNicolai 2008). Women taking antipsychotics during pregnancy should be monitored for alterations in fetal growth and maternal blood glucose levels should be monitored when taking olanzapine or clozapine (Reference GentileGentile 2010; Reference McCauley-Elsom, Gurvich and ElsomMcCauley-Elsom 2010).
In the postnatal period
Depression
Women with more severe postpartum depression may choose to receive pharmacological treatment, either in addition to or instead of non-pharmacological therapies. SSRIs and tricyclic antidepressants (TCAs) may be offered for the treatment of moderate to severe postnatal depression with considerations when breastfeeding (SIGN 2012). To date, only a few studies have systematically assessed the pharmacological treatment of postnatal depression. Conventional antidepressant medications have shown efficacy (Reference Molyneaux, Howard and McGeownMolyneaux 2014) and standard doses seem effective and well tolerated. The choice of an antidepressant should be guided by the patient’s response to previous antidepressant medication and the medication’s side-effect profile. SSRIs are ideal first-line agents, as they are anxiolytic, non-sedating and well tolerated. For women who cannot tolerate SSRIs, bupropion may be an alternative. TCAs are frequently used and, because they tend to be more sedating, may be more appropriate for women who present with prominent sleep disturbance. Given the prevalence of anxiety symptoms in this population, adjunctive use of a benzodiazepine (e.g. clonazepam, lorazepam) may be very helpful.
Postpartum psychosis
Most women with acute psychotic episodes postpartum need to be admitted to hospital, as do those with severe depression or at risk to self or others. Ideally, they should be admitted, with their babies, to specialist perinatal or mother and baby units, where they can recover from the psychotic episode and bond to their infant.
Medication for an acute psychotic episode is not different to that given outside the perinatal period, but the prolactin-elevating properties of potent dopamine-2 receptor blocking drugs should be a consideration for women who have decided not to breastfeed or who are too ill to manage it. Given the well-established relationship between puerperal psychosis and bipolar disorder, postpartum psychosis should be treated as an affective psychosis and a mood stabiliser is indicated. Interestingly, a recent study has recommended initiating prophylaxis with lithium therapy in women with a history of psychosis limited to the postpartum period (Reference Bergink, Bouvy and VervoortBergink 2012). Electroconvulsive therapy (ECT) is well tolerated and rapidly effective for severe postpartum depression and psychosis (Reference Focht and KellnerFocht 2012).
Psychotropic medication and breastfeeding
Clinicians should support women in their choice to breastfeed after clear discussions of the benefits and risks when on psychotropic medication (SIGN 2012). When initiating treatment, consideration should be given to the absolute dose and half-life of the drug. The following should be noted for specific drugs.
-
Doxepine should be avoided if considering breastfeeding (SIGN 2012) and if an SSRI is needed. The Maudsley guidelines recommend paroxetine or sertraline (Reference Taylor, Paton and KapurTaylor 2009).
-
Mothers on lithium should be encouraged not to breastfeed, but if they decide to do it, close monitoring of the infant is necessary, including serum lithium, thyroid and renal functioning.
-
Anti-epileptic drugs are not a contraindication for breastfeeding, but benefits and risks should be discussed with the mother in detail.
-
If a benzodiazepine is required, then short-acting agents should be prescribed and in divided doses. Mothers should be advised not to stop the medication abruptly (because of the risk of discontinuation syndrome) and to seek medical advice if they note sleepiness or poor sucking in their babies. All breastfed infants should be monitored for sedation and extrapyramidal side-effects where mothers are taking antipsychotic medications.
-
Women on clozapine should not breastfeed because of the risk of agranulocytosis.
Conclusions
Mild and short-lived mood disturbance during pregnancy and postpartum is common, but a minority of women develop depressive illness or sudden psychosis. It is important to be vigilant for symptoms of mental illness during both pregnancy and the postpartum period: about half of episodes of so-called postnatal depression start during pregnancy, and some seemingly postpartum psychotic episodes start before delivery. It is essential to keep in mind that untreated antenatal depression can lead to poor outcomes not only for the mother but also for the child.
MCQs
Select the single best option for each stem
-
1 The estimated incidence of postpartum blues is:
-
a 30%
-
b 50–85%
-
c 90%
-
d 10–15%
-
e 35%.
-
-
2 Screening for perinatal depression can best be assisted by the:
-
a Hamilton Rating Scale for Depression (HRSD)
-
b Beck Depression Inventory (BDI)
-
c Childhood Trauma Questionnaire (CTQ)
-
d Edinburgh Postnatal Depression Scale (EPDS)
-
e NEO Personality Inventory.
-
-
3 The development of perinatal depression is not significantly associated with:
-
a life events
-
b maternal age
-
c poor marital support
-
d neuroticism
-
e previous depressive episodes.
-
-
4 As first-line therapy for mild to moderate perinatal depression NICE recommends:
-
a cognitive–behavioural or interpersonal therapy
-
b pharmacotherapy
-
c psychodynamic psychotherapy
-
d solution-focused therapy
-
e compliance therapy.
-
-
5 Most postpartum psychotic episodes happen within:
-
a 8 weeks
-
b 1 week
-
c 2 weeks
-
d 2 days
-
e 1 week.
-
MCQ answers
| 1 | b | 2 | d | 3 | b | 4 | a | 5 | c |




eLetters
No eLetters have been published for this article.