Implications
How the gastrointestinal tract senses the arrival of dietary nutrients and non-nutrients (e.g. toxins) has a tremendous impact on the hunger–satiety cycle. Nutrient sensing is mediated by the activation of taste receptors or other sensors/transporters present in the intestinal epithelium associated with the enteroendocrine system. For example, the excess of specific non-limiting dietary amino acids in pigs has the capacity to strongly trigger satiating signals through chemosensory mechanisms (Muller and Roura, unpublished). In the future, standard feed formulation guidelines in farm animals (including pigs) will have to include not only essential-limiting amino acids but the wider array of dietary amino acids as well. A full understanding of these mechanisms is essential to develop dietary strategies to optimize feed intake in farm animals such as the pig.
Introduction
Nutritional chemosensing is the scientific discipline studying how nutrients are perceived in biological systems including genomic, metabolic, physiological and behavioural mechanisms (Roura et al., Reference Roura, Koopmans, Lallès, Le Huerou-Luron, de Jager, Schuurman and Val-Laillet2016). The molecular mechanisms of oral nutrient and non-nutrient sensing involve a large repertoire of receptors including taste receptors (TRs). The activation of TRs trigger the depolarization of the sensory cell in the tongue and the stimulation of the gustatory cortex of the brain mediated by the signalling of the cranial nerves VII, IX and X (Barretto et al., Reference Barretto, Gillis-Smith, Chandrashekar, Yarmolinsky, Schnitzer, Ryba and Zuker2015). In addition, the mechanisms of nutrient perception discovered in the oral cavity have also been described outside the oral cavity as part of the enteroendocrine system (EES) mediating the hunger–satiety cycle (reviewed by Steensels and Depoortere, Reference Steensels and Depoortere2018). In the intestinal epithelium, there are several cell types, such as enterocytes, enteroendocrine cells (EECs), tuft, Paneth, goblet, microfold and cup cells, which play a key role reporting the luminal content to the brain (Depoortere, Reference Depoortere2014). These mechanisms were originally studied in humans and laboratory rodents; however, in recent reports homologous mechanisms have been uncovered in pigs (reviewed by Roura and Fu, Reference Roura and Fu2017; Roura and Foster, Reference Roura and Foster2018).
In addition, some nutrient transporters seem to play a dual role meaning that the main role of transporting might be coupled to nutrient-sensing signalling. These transporters have been referred to as ‘transceptors’ (Reimann et al., Reference Reimann, Habib, Tolhurst, Parker, Rogers and Gribble2008; Poncet and Taylor, Reference Poncet and Taylor2013). Sensory functions of nutrient transporters in and outside the oral cavity are increasingly being recognized in mammalian species (Diallinas, Reference Diallinas2017; Roura and Foster, Reference Roura and Foster2018; Steensels and Depoortere, Reference Steensels and Depoortere2018). The molecular mechanisms of oral nutrient sensing and transporters are complex and imply a high degree of specificity to each nutrient type. This review aims at summarizing the current knowledge on nutrient (carbohydrates, proteins and lipids) and non-nutrient (bitter or bacterial compounds) chemosensing and the mediation of appetite-regulating gut peptides in pigs, presented using the progress obtained in humans and laboratory rodents as a reference. Novel research avenues on ‘microbial and parasite sensing’ have been highlighted in the ‘Microbial and parasite sensing…’ section of the review.
Carbohydrate sensing
Carbohydrate sensing has been related to two taste-like types referred as sweet and starchy tastes in humans (Aji et al., Reference Aji, Warren and Roura2019). On the one hand, sweet taste has evolved around mono-, di- and tri-saccharides (simple sugars), not only in humans but also in other mammalian species including pigs (Sclafani, Reference Sclafani1987; Glaser et al., Reference Glaser, Wanner, Tinti and Nofre2000; Lapis et al., Reference Lapis, Penner and Lim2014; Low et al., Reference Low, Lacy, McBride and Keast2017; Roura and Fu, Reference Roura and Fu2017). On the other hand, starch is the primary carbohydrate source in pigs. In recent years, starch-related sweet taste has gained relevance as part of the dietary nutrient-sensing mechanisms in the oral cavity. Despite a short contact time with starch in the mouth, salivary alpha-amylase has the potential to elicit sweet taste in humans by releasing maltose and maltotriose (Aji et al., Reference Aji, Warren and Roura2019).
However, the sensing of complex carbohydrates is independent of sweet taste as described in laboratory rodents (Sclafani, Reference Sclafani1987). Lapis et al. (Reference Lapis, Penner and Lim2014) demonstrated the taste of glucose was correlated with sucrose but not with the sensing of complex carbohydrates. Pigs were also reported to sense complex carbohydrates from hydrolysed corn starch (Roura et al., Reference Roura, Shrestha and Diffey2013).
Carbohydrate sensors and transceptors in the gastrointestinal tract
Most mammalian species (except strict carnivores) have a very conserved mechanism of simple sugar perception (related to sweet taste in humans). Table 1 summarizes the main receptors and transporters known to be involved in sensing sugars in the gastrointestinal tract (GIT) in humans and pigs. Among other potential receptors, simple carbohydrates sensing involves a heterodimer of two G-protein-coupled receptors (GPCRs) known as taste receptor type 1 member 2 and member 3 (TAS1R2 and TAS1R3, respectively) (Bachmanov and Beauchamp, Reference Bachmanov and Beauchamp2007). In addition, a TAS1R2/TAS1R3-independent sensing of monosaccharides (e.g. glucose and fructose) has been recently described in the oral cavity. The system was related to the glucose transporters/co-transporters (GLUTs) and sodium-dependent glucose transporter type 1 and 2 (SGLT1/2), and the brush border enzymes present in the apical membrane of some taste sensory cells (Glendinning et al., Reference Glendinning, Stano, Holter, Azenkot, Goldman, Margolskee, Vasselli and Sclafani2015; Sukumaran et al. Reference Sukumaran, Yee, Iwata, Kotha, Quezada-Calvillo, Nichols, Mohan, Pinto, Shigemura and Ninomiya2016). An analogous system has been previously described in the small intestine (Cheng et al., Reference Cheng, Chegeni, Kim, Zhang, Benmoussa, Quezada-Calvillo, Nichols and Hamaker2014; Zhang et al., Reference Zhang, Hasek, Lee and Hamaker2015). In addition, the stimulation of the TAS1R sweet receptor dimer seemed to upregulate SGLT1 to facilitate glucose uptake in the intestine (Mace et al., Reference Mace, Affleck, Patel and Kellett2007; Margolskee et al., Reference Margolskee, Dyer, Kokrashvili, Salmon, Ilegems, Daly, Maillet, Ninomiya, Mosinger and Shirazi-Beechey2007). GLUT5 has also been reported to influence glucagon-like peptide-1 (GLP-1) release from enteroendocrine K-cells (Douard and Ferraris, Reference Douard and Ferraris2008). However, potential dual roles for other sugar transporters/sensors known to be expressed in the GIT (i.e. KATP channel, SGLT2, GLUT2 or GLUT5) have not been reported to date (Table 1). Some of these molecular mechanisms have also been described in pigs (Roura and Fu, Reference Roura and Fu2017). The identification of putative receptors responsible for the sensing of starch and glucose polymers remains elusive to date in mammalian species.
Table 1. Main simple carbohydrates receptors and transporters known to be involved in GIT sensing in humans and pigs1
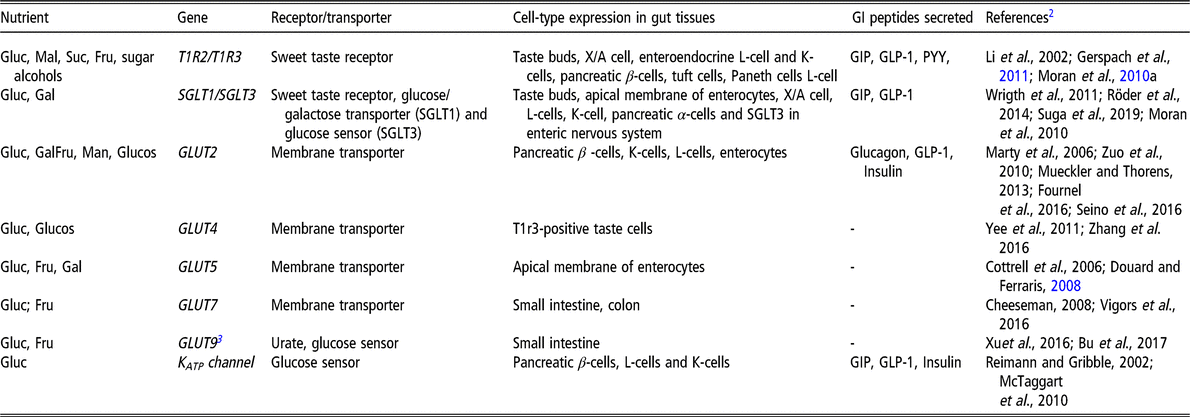
GIT = gastrointestinal tract; GI = gastrointestinal; GIP = glucose insulinotropic peptide; GLUT = glucose transporter; PYY = peptide YY; SGLT = sodium–glucose cotransporter 1; T1R = taste receptor family 1; KATP channel = ATP-sensitive K+ channel; GLP-1 = glucagon-like peptide 1; Fru = fructose; Gluc = glucose; Gal = galactose; Man = mannose; Mal = maltose; Suc = sucrose; Glucos = glucosamine.
1 All the receptors and transporters presented in the table are relevant to humans and pigs except if noted with the superscript 3.
2 Table references are provided in Supplementary Material S1. Note: some references to laboratory rodent research have been used to illustrate the discovery or proof of the GIT-related function of some genes.
3 No literature evidence of the functionality of this gene has been found in pigs.
Carbohydrates sensors and the enteroendocrine system
The presence of simple sugars in the GIT activates the expression and stimulation of TAS1Rs in EECs which, in turn, release gut peptides relevant to the orchestration of the hunger–satiety cycle (Rozengurt et al., Reference Rozengurt2006). The main hormones involved in this response include cholecystokinin (CCK), peptide tyrosine tyrosine (PYY) and GLP-1 (Badman and Flier, Reference Badman and Flier2005). These hormones are known to regulate energy and glucose metabolism by modulating the homoeostatic and food reward systems in the brain implicated in hunger and satiety (Berridge and Robinson, Reference Berridge and Robinson1998). In particular, carbohydrate sensing mediated by TAS1R2/TAS1R3, SGLT1 and/or the KATP has been described on L-cells and K-cells known to secrete GLP-1 and glucose insulinotropic peptide (GIP), respectively (Steensels and Depoortere, Reference Steensels and Depoortere2018). The expression and co-localization of TAS1R2, TAS1R3 and transceptor SGLT1 in L-cells has been related to GLP-1 secretion in humans and rodents (Jang et al., Reference Jang, Kokrashvili, Theodorakis, Carlson, Kim, Zhou, Kim, Xu, Chan, Juhaszova, Bernier, Mosinger, Margolskee and Egan2007; Steinert et al., Reference Steinert, Gerspach, Gutmann, Asarian, Drewe and Beglinger2011a; Gerspach et al., Reference Gerspach, Steinert, Schonenberger, Graber-Maier and Beglinger2011). In addition, sugar sensors are found in human stomach, expressed in endocrine P/D1 cells (also referred to X/A cells in lab rodents) and inhibit the release of the hunger hormone ghrelin (Wang et al., Reference Wang, Liszt, Deloose, Canovai, Thijs, Farre, Ceulemans, Lannoo, Tack and Depoortere2019). However, the effect of glucose on GLP-1 and PYY release could be overruled or potentiated by other nutrients such as proteins or fats (Gerspach et al., Reference Gerspach, Steinert, Schonenberger, Graber-Maier and Beglinger2011). Interestingly, artificial sweeteners showed no effect on GLP-1 in vivo in rodents and humans, suggesting that they may not induce physiological effects in the GIT (Steinert et al., 2011b; Steensels et al., Reference Steensels, Cools, Avau, Vancleef, Farre, Verbeke and Depoortere2016).
In pigs, Moran et al. (Reference Moran, Al-Rammahi, Arora, Batchelor, Coulter, Daly, Ionescu, Bravo and Shirazi-Beechey2010a and Reference Moran, Al-Rammahi, Arora, Batchelor, Coulter, Ionescu, Bravo and Shirazi-Beechey2010b) found that dietary carbohydrates or saccharin enhanced SGLT1 expression in small intestine epithelial cells including L and K cells resulting in an increased glucose absorption. In addition, L and K cells co-expressed pTas1r2/pTas1r3, SGLT1 and GIP and GLP-1. Thus, SGLT1 was shown to be the main route of absorption of dietary sugars and that the increased expression of SGLT1 in epithelial cells was mediated by the stimulation of pTas1rs in pigs (Moran et al., Reference Moran, Al-Rammahi, Arora, Batchelor, Coulter, Ionescu, Bravo and Shirazi-Beechey2010b).
Gene polymorphisms in carbohydrate sensing
Based on population genomic analyses, 18 single-nucleotide polymorphisms (SNPs) (of which 10 were non-synonymous – ns – that is, causing a change in the amino acid (AA) sequence of the receptor) have been identified in TAS1R2 (Kim et al., Reference Kim, Wooding, Riaz, Jorde and Drayna2006). TAS1R2 variants have been associated with higher sucrose taste thresholds and dietary sugar intake (Eny et al., Reference Eny, Wolever, Corey and El-Sohemy2010) or to lower carbohydrate intake (Ramos-Lopez et al., Reference Ramos-Lopez, Panduro, Martinez-Lopez and Roman2016). In addition, Dias et al. (Reference Dias, Eny, Cockburn, Chiu, Nielsen, Duizer and El-Sohemy2015) found that the functional impact of another TAS1R2 polymorphism was body mass index (BMI) dependent – that is, high sucrose thresholds and sugar intake found in overweight individuals (BMI>25) but not in normal-weight individuals (BMI<25). Furthermore, low compared to high sweet taste sensitivity volunteers consumed a higher amount of energy from a buffet meal, implying a strong involvement of TAS1R2 allelic variants on food choices (Han et al., Reference Han, Keast and Roura2017). In the same study, low sweet sensitivity was related to high salivary leptin. Similarly, a high oral sensitivity to the taste of complex carbohydrates (maltodextrin and oligofructose) was associated with higher consumption of energy and starch and waist circumference (Low et al., Reference Low, Lacy, McBride and Keast2017). Regarding genetic polymorphisms in pigs, the studies conducted to date have not reported potential pTas1r2 variants because the gene was not annotated in the pig genome at the time the studies were conducted (Da Silva et al., Reference Da Silva, De Jager, Burgos, Reverter, Perez-Enciso and Roura2014; Clop et al., Reference Clop, Sharaf, Castello, Ramos-Onsins, Cirera, Mercade, Derdak, Huisman, Fredholm, van As and Sanchez2016).
Protein/amino acid sensing
Dietary protein, as a source of AA, plays a fundamental role in growth and development. Of the 20 proteinogenic AAs needed for protein synthesis in eukaryotic cells, a few cannot be metabolically synthesized ‘de novo’ from other carbon and nitrogen sources within the cells, and need to be consumed as part of the diet. Thus, optimal growth and development in pigs requires a balanced supply of these so-called dietary essential AAs; one of the key aspects in current pig feed formulation practices. Failure to supply a balanced diet in terms of essential AA results in deficient growth and development and ultimately death. Thus, it is not surprising that a wide array of AAs and peptide sensors exist in mammalian species. In humans, the oral sensing of dietary AA was originally related to glutamate (and aspartate) and defined as the umami taste (Ikeda, Reference Ikeda1909). Other AAs sensed include aromatic AA (e.g. L-Phe), basic AA (L-Arg) and dietary peptides (Zhang et al., Reference Zhang, Huang, Jiang, Mulpuri, Wei, Hamelberg, Brown and Yang2014). However, in other mammals such as laboratory rodents and pigs, the oral/umami sensing of AA involves several L-AAs (Tinti et al., Reference Tinti, Glaser, Wanner and Nofre2000; Roura et al., Reference Roura, Humphrey, Klasing and Swart2011).
Amino acid sensors and transceptors in the gastrointestinal tract
Table 2 shows the main receptors and transporters known to be involved in AA sensing in the GIT in humans and pigs. The umami taste receptor is a GPCR heterodimer: TAS1R1/TAS1R3 (Nelson et al., Reference Nelson, Chandrashekar, Hoon, Feng, Zhao, Ryba and Zuker2002). In addition, the metabotropic glutamate receptors (particularly mGluR1 and mGluR4) have also been related to glutamate sensing in humans, in and outside the oral cavity (San Gabriel and Uneyama, Reference San Gabriel and Uneyama2013). Other AA sensors have been identified including the calcium sensing receptor (CaSR, sensing basic AA and Ca2+ as a heterotrophic cooperative enhancer) and GPRC6A (sensing aromatic AA) (Zhang et al., Reference Zhang, Huang, Jiang, Mulpuri, Wei, Hamelberg, Brown and Yang2014; Steensels and Depoortere, Reference Steensels and Depoortere2018). CaSR acts in concert with GPRC6A and are found expressed in D-, G- and L-cells (Haid et al., Reference Haid, Jordan-Biegger, Widmayer and Breer2012). Finally, di- and tripeptides are sensed by GPR92/93. Similar to previous receptors, AA sensors are also widely expressed throughout the GIT in humans, lab rodents and pigs (Wellendorph et al., Reference Wellendorph, Johansen and Brauner-Osborne2010; Roura and Foster, Reference Roura and Foster2018) (Table 2).
Table 2. Main AA receptors and transceptors known to be involved in GIT sensing in humans and pigs1
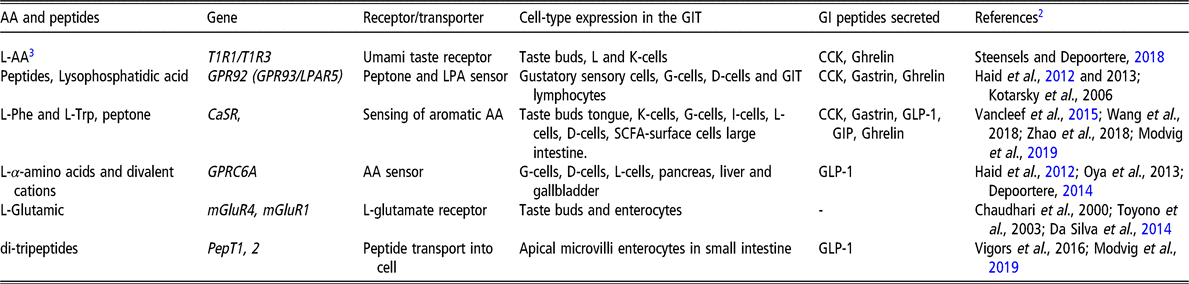
AA = amino acid; GIT = gastrointestinal tract; GI = gastrointestinal; LPA = lysophoshatidic acid; CaSR = calcium-sensor receptor; CCK = cholecystokinin; L-AA = L isomer of amino acids; GPCRs = G-protein-coupled receptors; SCFA = short-chain fatty acid; GLP-1 = glucagon-like peptide 1; GIP = glucose-dependent insulinotropic peptide; mGluRs = metabotropic glutamate receptors; PepT1,2: peptide transporter 1 and 2; T1R = taste receptor family 1.
1 All the receptors and transporters presented in the table are relevant to humans and pigs.
2 Table references are provided in Supplementary Material S1. Note: some references to laboratory rodent research have been added to illustrate the discovery or proof of the GIT-related function of some genes.
3 Refers to L-AA stimulating the umami taste receptor dimer in humans (glutamic and aspartic acids) or pigs (Ala, Asn, Asp, Glu, Gln, Pro, Ser and Thr) (source: Roura et al., Reference Roura, Humphrey, Klasing and Swart2011).
There is a complex and highly specific network of AA and peptide intestinal transporters belonging to the solute carrier (SLC) family. A detailed description of these transporters can be found elsewhere (Broer, Reference Broer2008). However, the evidence of any of these transporters to function as AA sensors remains to be fully studied.
Amino acids and the enteroendocrine system
In the GIT, the stimulation of the umami heterodimer and the CaSR have been associated with the secretion of CCK, ghrelin and GLP-1 (Liou et al., Reference Liou, Sei, Zhao, Feng, Lu, Thomas, Raybould and Wank2011a; Diakogiannaki et al., Reference Diakogiannaki, Pais, Tolhurst, Parker, Horscroft, Horscroft, Rauscher, Zietek, Daniel, Gribble and Reinmann2013; Vancleef et al., Reference Vancleef, Van Den Broeck, Thijs, Steensels, Briand, Tack and Depoortere2015). In addition, GPR92/93 has been reported in stomach G-cells and STC-1 cells responding to a protein hydrolysate by releasing CCK (Choi et al., Reference Choi, Lee, Shiu, Yo, Halldén and Aponte2007; Rettenberger et al., Reference Rettenberger, Schulze, Breer and Haid2015).
Similar to the TAS1R-independent mechanisms of sweet taste perception, the AA sensing also seems to partially rely on AA transceptors as an alternative pathway to signal responses through EEC. Di/tripeptide uptake in L cells occurs via peptide transporter 1 (PEPT1) and results in subsequent basolateral activation of the CaSR and GLP-1 release (Diakogiannaki et al., Reference Diakogiannaki, Pais, Tolhurst, Parker, Horscroft, Horscroft, Rauscher, Zietek, Daniel, Gribble and Reinmann2013; Daniel and Zietek, Reference Daniel and Zietek2015; Modvig et al., Reference Modvig, Kuhre and Holst2019). Another potential example of AA transceptor is the sodium-dependent neutral AAs transporter 2 (SNAT2) involved in GLP-1 secretion (Reimann et al., Reference Reimann, Ward and Gribble2006; Young et al., Reference Young, Rey, Sternini and Rozengurt2010). A large number of additional AA transporters (e.g. the SLC family) are known to be expressed in the GIT but, as indicated previously, their potential role as transceptors has not been fully described (Broer, Reference Broer2008). In pigs, the first fully functional taste receptor gene to be sequenced, cloned and expressed in a cell reporter system was the umami heterodimer pTas1r1/pTas1r3 (Humphrey et al., Reference Humphrey, Tedó, Klasing and Roura2009; Tedo Perez, Reference Tedo Perez2009; Roura et al., Reference Roura, Humphrey, Klasing and Swart2011). The results indicated that the umami taste in pigs was tuned to 8 L-AA (Ala, Asn, Asp, Glu, Gln, Pro, Ser and Thr) (Roura et al., Reference Roura, Humphrey, Klasing and Swart2011). The expressions of the porcine metabotropic glutamate receptors (mGluR1 and mGluR4) and other AA and peptone receptors (i.e. CaSR, GPRC6A and GPR92) have also been reported more recently in pig tongue and stomach epithelia (Haid et al., Reference Haid, Jordan-Biegger, Widmayer and Breer2012; Da Silva et al., Reference Da Silva, De Jager, Burgos, Reverter, Perez-Enciso and Roura2014). In addition, the AA receptors involved in sensing protein breakdown products were identified in G-cells and D-cells in pigs (Haid et al., Reference Haid, Jordan-Biegger, Widmayer and Breer2012). Finally, several AA transporters of the SLC family have been identified in the pig GIT; however their potential role as sensors has not been addressed (Vigors et al., Reference Vigors, Sweeney, O’Shea, Browne and O’Doherty2014).
Gene polymorphisms in amino acid sensing
In humans, 17 SNPs (14 ns) and 12 SNPs (6 ns) were reported for TAS1R1 and TAS1R3, respectively (Kim et al., Reference Kim, Wooding, Riaz, Jorde and Drayna2006). These polymorphisms have been associated with a lower ability to taste glutamate (Chen et al., Reference Chen, Alarcon, Tharp, Ahmed, Estrella, Greene, Rucker and Breslin2009) and with specific food choices (Han et al., Reference Han, Keast and Roura2018). In particular, the research published from Han et al. (Reference Han, Keast and Roura2018) reported that human carriers of one of the TAS1R1 SNPs consumed more fat and calories from a buffet meal. In addition, Raliou et al. (Reference Raliou, Wiencis, Pillias, Planchais, Eloit, Boucher, Trotier, Montmayeur and Faurion2009) showed that mGluR1 polymorphisms contributed to a lack of sensitivity to glutamate. Genetic variants in other AA sensors (i.e. CaSR and GPRC6A) have also been reported; however, the physiological impact of this variation is currently unknown.
In pigs, an SNP analysis of 79 pig genomes (belonging to 14 different breeds) revealed 13 (5 ns and 1 stop-lost) and 9 (1 ns) polymorphisms in pTasS1r1 and pTas1r3, respectively (Da Silva et al., Reference Da Silva, De Jager, Burgos, Reverter, Perez-Enciso and Roura2014). The research also showed several SNPs for the other AA sensors: 22 (2 ns), 6 (3 ns), 16 (1 ns) and 28 (2 ns) for CaSR, GPRC6A, mGluR1 and mGluR4, respectively. Clop et al. (Reference Clop, Sharaf, Castello, Ramos-Onsins, Cirera, Mercade, Derdak, Huisman, Fredholm, van As and Sanchez2016) identified 31 (including 1 splice, 1 stop-gained and 1 stop lost, 3 frame shifts and 4 moderate impact) pTas1r1 variants and 14 (including 1 stop gained and 1 moderate impact) pTas1r3 variants. In addition, they identified an mGluR1 SNP linked to umami taste, feed intake and growth. However, the incidence of SNP in AA sensors compared to the bitter sensing system was very low (Da Silva et al., Reference Da Silva, De Jager, Burgos, Reverter, Perez-Enciso and Roura2014). This limited number of ns SNPs may indicate that AA receptor/transceptor functions are highly conserved across individuals and across pig breeds.
Lipid/fatty acid sensing
Fats are an essential dietary energy source that play a key role in gut hormone release (Hara et al., Reference Hara, Kashihara, Ichimura, Kimura, Tsujimoto and Hirasawa2014). Triglycerides, the main dietary fat source, are digested by lipases releasing free fatty acids (FFAs) and monoacylglycerides.
Fatty acid sensors and transceptors in the gastrointestinal tract
The chemosensory system for fats has evolved mainly around the sensing of FFAs and consists of an array of nine receptors (FFARs) and transceptors featuring a degree of specificity based on chain length (Table 3). In particular, the main ligands for FFAR2 and FFAR3 and olfactory receptor OLFR78 are short-chain fatty acids (SCFAs). The receptors FFAR1 and GPR84 showed the highest affinity for medium-chain fatty acids (MCFAs)(Wang et al. Reference Wang, Wu, Simonavicius, Tian and Ling2006; Liu et al., Reference Liu, Costanzo, Evans, Archer, Nowson, Duesing and Keast2018), whereas FFAR4 (also kwon as GPR120) and fatty acid (FA)-binding protein 2 (FABP2), FA transport protein 4 (FATP4) and cluster of differentiation 36 (CD36) have been characterized as receptors for long-chain fatty acids (LCFAs) (Bachmanov and Beauchamp, Reference Bachmanov and Beauchamp2007; Mattes, Reference Mattes2011). In addition, GPR119 has been proposed as a putative receptor for endogenous lipids containing oleic acid (e.g. oeloylethanolamide) and 2-monoacylglycerol (Hansen et al., Reference Hansen, Rosenkilde, Holst and Schwartz2012).
Table 3. Main FFA receptors and transceptors known to be involved in GIT sensing in humans and pigs1

FFA = free fatty acids; GIT = gastrointestinal tract; GI = gastrointestinal; FFARs = free fatty acid receptors; CCK = cholecystokinin; CD36 = cluster of differentiation 36; FABP2 = fatty acid binding protein 2; FATP4 = fatty acid transporter 4; SLC16A1 = solute carrier family 16 member; GLP-1 = glucagon-like peptide 1; GIP = glucose insulinotropic peptide; GLP-1 = glucagon-like peptide 1; GPBAR1 = G-protein-coupled bile receptor; TGR5 = Takeda G-protein-coupled receptor 5; GPR = G-protein-coupled receptor; MCT1 = monocarboxylate transporter 1; OLFR78 = olfactory receptor 78; PYY = peptide YY
1 All the receptors and transporters presented in the table are relevant to humans and pigs except if noted with the superscript 4.
2 Table references are provided in Supplementary Material S1.
3 Refers to the oral sensation elicited by free fatty acids (Note: to date, the FFA sensing has not achieved full recognition as a primary taste type by the sensory science community).
4 No literature evidence of the existence or functionality of these genes has been found in pigs.
Fatty acids and the enteroendocrine system
The receptors FFAR1 and FFAR4 are present throughout the GIT found in EECs. The uptake of dietary FFAs is slow (compared to sugars and AA) and requires bile acids secreted in the duodenum. In contrast, FFAR2, FFAR3 and OLFR78 are preferentially expressed in the colon, where abundant SCFAs are produced resulting from bacterial fermentation (Canfora et al. Reference Canfora, Jocken and Blaak2015; Fleischer et al., Reference Fleischer, Bumbalo, Bautze, Strotmann and Breer2015). GPR84 has been reported in mouse gastric mucosa (Widmayer et al., Reference Widmayer, Kusumakshi, Hägele, Boehm and Breer2017). GPR119 expression has been associated with EECs (L-cells) and pancreatic cells (Overton et al. Reference Overton, Fyfe and Reynet2008; Lan et al., Reference Lan, Vassileva, Corona, Liu, Baker, Golovko, Abbondanzo, Hu, Yang, Ning, Del Vecchio, Poulet, Laverty, Gustafson, Hedrick and Kowalski2009; Hansen et al., Reference Hansen, Rosenkilde, Holst and Schwartz2012).
On the one hand, intragastric administration of dietary oral gavage of LCFA has been reported to increase the orexigenic (appetite) hormone ghrelin secretion presumably through the stimulation of FFAR4 (Janssen et al. Reference Janssen, Laermans, Iwakura, Tack and Depoortere2012). In addition, the activation of FFAR2-expressing gastric X/A-cells by SCFA inhibited ghrelin (Engelstoft et al. Reference Engelstoft, Park, Sakata, Kristensen, Husted, Osborne-Lawrence, Piper, Walker, Pedersen, Nøhr, Pan, Sinz, Carrington, Akiyama, Jones, Tang, Ahmed, Offermanns, Egerod, Zigman and Schwartz2013). Short-chain fatty acid can reach the stomach through the portal vein (Morrison and Preston, Reference Morrison and Preston2016). This may be indicative of an excessive fermentation occurring in the lower GIT which is consistent with an anorexegenic (satiating) response. On the other hand, some FFARs have also been related to anorexigenic events associated with CCK and/or GLP-1 and GIP. An acute oral dose of butyrate increased GLP-1 and PYY levels in mice, presumably through FFAR3 (Lin et al. Reference Lin, Frassetto, Kowalik, Nawrocki, Lu, Kosinski, Hubert, Szeto, Yao, Forrest and Marsh2012). The expression of GPR84 in X/A-like ghrelin cells and surface cells suggests an important role of MCFA in the developing gastric mucosa of suckling mice (Widmayer et al., Reference Widmayer, Kusumakshi, Hägele, Boehm and Breer2017). In addition, SCFA olfactory receptor OLFR78 and GLP-1 and PYY co-express in murine colonic L-cells (Pluznick, Reference Pluznick2014; Fleischer et al., Reference Fleischer, Bumbalo, Bautze, Strotmann and Breer2015). Furthermore, GPR119 ligands (i.e. monoglycerides) triggered GLP-1 secretion from intestinal primary cultures, particularly from colon (Moss et al., Reference Moss, Glass, Diakogiannaki, Pais, Lenaghan, Smith, Wedin, Bohlooly-Y, Gribble and Reimann2016). Fatty acid transceptors CD36 and FATP4 have been also reported to mediate lipid-induced gut hormone secretion (Sundaresan et al., Reference Sundaresan, Shahid, Riehl, Chandra, Nassir, Stenson, Liddle and Abumrad2013; Poreba et al., Reference Poreba, Dong, Li, Stahl, Miner and Brubaker2012).
In pigs, De Jager et al. (Reference De Jager, Zhan, Rzepus and Roura2013) reported the expression of FFAR1, FFAR2, FFAR3, FFAR4 and GPR84 in circumvallate papillae. In addition, Da Silva et al. (Reference Da Silva, De Jager, Burgos, Reverter, Perez-Enciso and Roura2014) revealed a very low incidence of allelic variants across FFARs and GPR84 compared to other TR genes such as the TAS2R family (bitter taste) indicating that FFARs were highly conserved in pigs.
The FFARs expression pattern described in pigs evidenced some differences compared to humans. In particular, FFAR2 and FFAR3 were predominantly found in the distal small intestine (Haenen et al., Reference Haenen, Zhang, Souza da Silva, Bosch, van der Meer, van Arkel, van den Borne, Perez Gutierrez, Smidt, Kemp, Muller and Hooiveld2013) while FFAR4 in colon (Colombo et al., Reference Colombo, Trevisi, Gandolfi and Bosi2012; van der Wielen et al., Reference van der Wielen, van Avesaat, de Wit, Vogels, Troost, Masclee, Koopmans, van der Meulen, Boekschoten, Muller, Hendriks, Witkamp and Meijerink2014). It is tempting to speculate that these findings may be related to the higher fermentative capacity of the hindgut of the adult pigs compared to humans (Stevens, Reference Stevens1988). In contrast, FFAR2 and FFAR3 were found expressed in colonic enteroendocrine L-cells responding to increased levels of SCFA (i.e. butyrate) released after high inclusion of resistant starch (Haenen et al., Reference Haenen, Zhang, Souza da Silva, Bosch, van der Meer, van Arkel, van den Borne, Perez Gutierrez, Smidt, Kemp, Muller and Hooiveld2013). In addition, a co-expression pattern was uncovered between FFAR2 and FFAR3 with PYY, GLP-1 and serotonin in pig colon (Weatherburn, Reference Weatherburn2015).
Gene polymorphisms in fatty acid sensing
The ability to sense fats has been associated with an increased consumption of fatty foods, higher BMI and obesity (Stewart et al., Reference Stewart, Newman and Keast2011; Ichimura et al., Reference Ichimura, Hirasawa, Poulain-Godefroy, Bonnefond, Hara, Yengo, Kimura, Leloire, Liu, Iida, Choquet, Besnard, Lecoeur, Vivequin, Ayukawa, Takeuchi, Ozawa, Tauber, Maffeis, Morandi, Buzzetti, Elliott, Pouta, Jarvelin, Körner, Kiess, Pigeyre, Caiazzo, Van Hul, Van Gaal, Horber, Balkau, Lévy-Marchal, Rouskas, Kouvatsi, Hebebrand, Hinney, Scherag, Pattou, Meyre, Koshimizu, Wolowczuk, Tsujimoto and Froguel2012). FFA4 gene variants have been found to have a significant impact on receptor responses (Hudson et al., Reference Hudson, Murdoch and Milligan2013). In addition, the FFAR4 mutation was found to increase the risk of obesity, demonstrating the key role in fat sensing and the control of energy balance in humans and rodents (Ichimura et al., Reference Ichimura, Hirasawa, Poulain-Godefroy, Bonnefond, Hara, Yengo, Kimura, Leloire, Liu, Iida, Choquet, Besnard, Lecoeur, Vivequin, Ayukawa, Takeuchi, Ozawa, Tauber, Maffeis, Morandi, Buzzetti, Elliott, Pouta, Jarvelin, Körner, Kiess, Pigeyre, Caiazzo, Van Hul, Van Gaal, Horber, Balkau, Lévy-Marchal, Rouskas, Kouvatsi, Hebebrand, Hinney, Scherag, Pattou, Meyre, Koshimizu, Wolowczuk, Tsujimoto and Froguel2012). In addition, FA transporter CD36 was shown to play a crucial role in oral fat sensing as well (Pepino et al., Reference Pepino, Love-Gregory, Klein and Abumrad2012). Genetic CD36 variants were associated with the taste intensity of oleic acid and triolein, total dietary fat and energy intake, and the development of obesity in teenagers (Toguri, Reference Toguri2008; Pepino et al., Reference Pepino, Love-Gregory, Klein and Abumrad2012; Keller et al., Reference Keller, Liang, Sakimura, May, van Belle, Breen, Driggin, Tepper, Lanzano, Deng and Chung2012; Daoudi et al., Reference Daoudi, Plesník, Sayed, Šerý, Rouabah, Rouabah and Khan2015; Mrizak et al., Reference Mrizak, Sery, Plesnik, Arfa, Fekih, Bouslema, Zaouali, Tabka and Khan2015). In addition, CD36 gene variants have also been implicated in obesity, type 2 diabetes, the metabolic syndrome, hypertension and coronary heart disease (Precone et al., Reference Precone, Beccari, Stuppia, Baglivo, Paolacci, Manara, Miggiano, Falsini, Trifirò, Zanlari, Herbst, Unfer and Bertelli2019).
In pigs Da Silva et al. (Reference Da Silva, De Jager, Burgos, Reverter, Perez-Enciso and Roura2014) revealed a low incidence of polymorphisms in FFARs genes when comparing to bitter taste sensors. In particular, the total number of SNP for FFAR1, FFAR2, FFAR3, and FFAR4 were 8 (4 ns), 11 (1 ns), 11 (2 ns) and 1 (0 ns), respectively (Da Silva et al., Reference Da Silva, De Jager, Burgos, Reverter, Perez-Enciso and Roura2014). In addition, the results published from the genomic analysis in pigs by Clop et al. (Reference Clop, Sharaf, Castello, Ramos-Onsins, Cirera, Mercade, Derdak, Huisman, Fredholm, van As and Sanchez2016) identified three CD36 variants associated with growth and fat deposition. Finally, significant differences in allele frequencies of FFAR4 were observed between two extreme pig groups based on growth rates (Fontanesi et al., Reference Fontanesi, Bertolini, Scotti, Schiavo, Colombo, Trevisi, Riban, Buttazzoni, Russo and Dall’Olio2015).
Bitter sensing
Bitter sensing has been associated with harmful contaminants, toxic compounds and general synthetic chemicals such as pharmaceuticals present in foods/feeds (Nelson and Sanregret, Reference Nelson and Sanregret1997; Meyerhof et al., Reference Meyerhof, Batram, Kuhn, Brockhoff, Chudoba, Bufe, Appendino and Behrens2009). These compounds cause defensive and protective responses in the host including food aversion, vomiting, and inhibition of gastric motility and activation of efflux from enterocytes accompanied by an increase in satiation and satiety (Sarkadi et al., Reference Sarkadi, Homolya, Szakacs and Varadi2006; Jeon et al., Reference Jeon, Seo and Osborne2011; Avau et al., Reference Avau, Rotondo, Thijs, Andrews, Janssen, Tack and Depoortere2015; Deloose et al., Reference Deloose, Corsetti, Van Oudenhove, Depoortere and Tack2017a and Reference Deloose, Janssen, Corsetti, Biesiejiersji, Masuy, Rotondo, Van Oudenhove, Depoortere and Tack2017b). In contrast, some non-toxic plant-derived compounds (such as polyphenols) may also elicit bitter taste (Soares et al., Reference Soares, Silva, Garcia-Estevez, GroBmann, Bras, Brandao, Mateus, de Freitas, Behrens and Meyerfof2018). Overall, close to 1000 compounds are known to be bitter to humans while 81 to laboratory rodents and 27 to pigs (Wang et al., Reference Wang, Fu, Navarro and Roura2017; Roura and Fu, Reference Roura and Fu2017; Dagan-Wiener et al., Reference Dagan-Wiener, Di Pizio, Nissim, Bahia, Dubovski, Margulis and Masha2019).
Bitter sensors in the gastrointestinal tract
Bitterants activate the TAS2R family, which consists of 25 functional genes in humans (Meyerhof et al., Reference Meyerhof, Batram, Kuhn, Brockhoff, Chudoba, Bufe, Appendino and Behrens2009). The size of the bitter taste receptor (TAS2R) repertoire is species specific, ranging from the 36 genes in the rat to none in carnivorous marine mammals (Roura and Foster, Reference Roura and Foster2018). The sensitivity of pigs to bitterness has been widely reported in the literature (Nelson and Sanregret, Reference Nelson and Sanregret1997; Danilova et al., Reference Danilova, Roberts and Hellekant1999; Roura et al., Reference Roura, Humphrey, Tedó and Ipharragerre2008; Roura and Navarro, Reference Roura and Navarro2018). The porcine pTas2r repertoire was recently characterized consisting of 16 functional genes and 3 pseudogenes (Colombo et al., Reference Colombo, Trevisi, Gandolfi and Bosi2012; Roura, et al., Reference Roura, Koopmans, Lallès, Le Huerou-Luron, de Jager, Schuurman and Val-Laillet2016; Roura and Fu, Reference Roura and Fu2017).
Bitter sensing and the enteroendocrine system
TAS2R transcripts have been observed in the oral and GIT mucosa of several mammalian species including humans and pigs (Rozengurt, Reference Rozengurt2006; Da Silva et al., Reference Da Silva, De Jager, Burgos, Reverter, Perez-Enciso and Roura2014). In humans, TAS2R5 and TAS2R38 have been co-localized with GLP-1-, CCK- and PYY-labelled EECs in the human small intestine and colon and TAS2R10 with ghrelin cells in the human stomach (Park et al., Reference Park, Kim, Kim, Lee, Jeong, Kim and Jang2015; Latorre et al., Reference Latorre, Huynh, Mazzoni, Gupta, Bonora, Clavenzani, Chang, Mayer, De Giorgio and Sternini2016; Wang et al., Reference Wang, Liszt, Deloose, Canovai, Thijs, Farre, Ceulemans, Lannoo, Tack and Depoortere2019). Bitter herbal medicines were shown to affect GLP-1 and CCK release in EEC lines (Avau and Depoortere, Reference Avau and Depoortere2016). However, the active compounds of the medicinal extracts studied remain to be identified. Finally, in tuft cells, bitter agonist denatonium benzoate elicited a paracrine activation of enterocytes presumably following the release of acetylcholine (Schutz et al., Reference Schutz, Jurastow, Bader, Ringer, von Engelhardt, Chubanov, Gudermann, Diener, Kunner, Krasteva-Christ and Weihe2015).
The presence of pTas2r in the porcine GIT has been reported by several groups (Colombo et al., Reference Colombo, Trevisi, Gandolfi and Bosi2012; Da Silva et al., Reference Da Silva, De Jager, Burgos, Reverter, Perez-Enciso and Roura2014; Ribani et al., Reference Ribani, Bertolini, Schiavo, Scotti, Utzeri, Dall’Olio, Trevisi, Bosi and Fontanesi2017; Clop et al., Reference Clop, Sharaf, Castello, Ramos-Onsins, Cirera, Mercade, Derdak, Huisman, Fredholm, van As and Sanchez2016). However, little is known to date about the function, except that dietary quinine and caffeine increased plasma insulin and GLP-1 (Fu et al., Reference Fu, Val-Laillet, Guerin and Roura2018).
Gene polymorphisms in bitter sensing
TAS2R38 variants determine the sensitivity to bitter substance phenylthiocarbamide in humans (Sandell and Breslin, Reference Sandell and Breslin2006; Risso et al., Reference Risso, Mezzavilla, Pagani, Robino, Morini, Tofanelli, Carrai, Campa, Barale, Caradonna, Gasparini, Luiselli, Wooding and Drayna2016) and have been associated with food preferences (Sandell and Breslin, Reference Sandell and Breslin2006), alcohol intake (Duffy et al., Reference Duffy, Davidson, Kidd, Kidd, Speed, Pakstis, Reed, Snyder and Bartoshuk2004), obesity (Tepper et al., Reference Tepper, Koelliker, Zhao, Ullrich, Lanzara, D’Adam, Ferrara, Ulivi, Esposito and Gasparini2008) and susceptibility to respiratory pathogens (Lee et al., Reference Lee, Xiong, Kofonow, Chen, Lysenko, Jiang, Abraham, Doghramji, Adappa, Palmer, Kennedy, Beauchamp, Doulias, Ischiropoulos, Kreindler, Reed and Cohen2012). Similarly, other gene variants of TAS2R14 and TAS2R50 have been associated with human diseases such as cancer and cardiovascular disease, respectively (Campa et al., Reference Campa, Vodicka, Pardini, Naccarati, Carrai, Vodickova, Novotny, Hemminki, Försti, Barale and Canzian2010; Akao et al., Reference Akao, Polisecki, Kajinami, Trompet, Robertson, Ford, Jukema, de Craen, Westendorp, Shepherd, Packard, Buckley and Schaefer2012). In addition, TAS2R16 variants appear to have had an evolutionary role to prevent consumption of dangerous raw foods (Valente et al., Reference Valente, Alvarez, Marques, Gusmão, Amorim, Seixas and Prata2018). Other genetic TAS2Rs have been related to the perception of bitterness in coffee (TAS2R2, TAS2R4 and TAS2R5), alcohol consumption (TAS2R13) and grapefruit liking (TAS2R19) (Hayes et al., Reference Hayes, Feeney and Alle2013).
The porcine bitter taste system presented a high incidence of allelic variants compared with the non-bitter taste genes, suggesting a potential role for these genes in ecological adaptation in pigs (Da Silva et al., Reference Da Silva, De Jager, Burgos, Reverter, Perez-Enciso and Roura2014). This high variability within and between species of the TAS2R gene repertoire seems to reflect an adaptive nature to survive in specific/novel ecological niches particularly to avoid plant-derived toxins. In addition, three phenotype–genotype studies reported SNPs with functional significance on the porcine bitter receptors pTas2r38, pTas2r39 (Clop et al., Reference Clop, Sharaf, Castello, Ramos-Onsins, Cirera, Mercade, Derdak, Huisman, Fredholm, van As and Sanchez2016; Ribani et al., Reference Ribani, Bertolini, Schiavo, Scotti, Utzeri, Dall’Olio, Trevisi, Bosi and Fontanesi2017) and pTas2r40 (Herrero-Medrano et al., Reference Herrero-Medrano, Megens, Groenen, Bose, Pérez-Enciso and Crooijmans2014). The associations reflected the impact of the fixed alleles on pig growth, fat deposition and environmental adaptation.
Microbial and parasite sensing in the gastrointestinal tract
While the role of nutrient receptors and transceptors has been mostly linked to exogenous or dietary nutrients and potential harmful compounds, recent findings indicate that this sensors may also respond to compounds produced within the intestinal tract. For example, products of the microbial population in the GIT, such as SCFA and MCFA, have the capacity to affect the chemosensory system. Similarly, metabolites produced in the GIT by parasitic or protozoan infections may also be able to activate some of the receptors and transceptors.
Microbial metabolites
SCFA and MCFA resulting from bacterial fermentation in the GIT affect the expression of nutrient sensors and gut peptides in EECs (Steensels and Depoortere, Reference Steensels and Depoortere2018). A decrease in FA sensors (FFAR1, FFAR4 and CD36), together with an increase in glucose and AA sensors (TAS1R3 and SGLT1), were reported in germ-free mice (Duca et al., Reference Duca, Swartz, Sakar and Covasa2012; Swartz et al., Reference Swartz, Duca, de Wouters, Sakar and Covasa2012). These changes were associated with reduced CCK, GLP-1 and PYY. In addition, bacterial endotoxins activate the toll-like receptors which are co-localized in CCK, PYY and serotonin secreting EECs (Bogunovic et al., Reference Bogunovic, Dave, Tilstra, Chang, Harpaz, Xiong, Mayer and Plevy2007; Larraufie et al., Reference Larraufie, Dore, Lapaque and Blottiere2017).
Commensal bacteria have evolved to produce metabolites that chemically mimic mammalian agonists and trigger eukaryotic cellular responses (Cohen et al., Reference Cohen, Kang, Chu, Huang, Gordon, Reddy, Ternei, Craig and Brady2015). Bacterial N-acyl amides showed high affinity to host GPR119 functioning to regulate GIT physiology, gut hormones and glucose homeostasis (Cohen et al., Reference Cohen, Esterhazy, Kim, Lemetre, Aguilar, Gordon, Pickard, Cross, Emiliano, Han, Chu, Vila-Farres, Kaplitt, Rogoz, Calle, Hunter, Bitok and Brady2017). Sung et al. (Reference Sung, Kim, Denou, Soltys, Hamza, Byme, Masson, Park, Wishart, Madsen, Shertzer and Dyck2017) replicated the positive effect of oral resveratrol by fecal microbiome transplants to obese (but naive to dietary resveratrol) mice. In addition, Clostridium coli and Escherichia coli were shown to affect intestinal motility by modulating serotonin synthesis from enterochromaffin cells (Cao et al., Reference Cao, Liu, An, Zhou, Liu, Xu, Dong, Wang, Yan, Jiang and Wang2017). Taken together, robust evidences are accumulating, showing that gut microbes have evolved to interact and modulate animal host GIT physiology.
Parasites
Parasitic worms and protozoan infections initiate a signalling cascade in tuft cells mediated by TAS1Rs and/or TAS2Rs (Gerbe et al., Reference Gerbe, Sidot, Smyth, Ohmoto, Matsumoto, Dardalhon, Cesses, Garnier, Pouzolles, Brulin, Bruschi, Harcus, Zimmermann, Taylor, Maizels and Jay2016; Howitt et al., Reference Howitt, Lavoie, Michaud, Blum, Tran, Weinstock, Gallini, Redding, Margolskee, Osborne, Artis and Garrett2016; von Moltke et al., Reference von Moltke, Ji, Liang and Locksley2016). Tuft cells orchestrate type 2 cell-mediated immunity in a process where TR sensing signals mediate the differentiation of epithelial crypt progenitors to tuft cells and goblet cells. Tuft and goblet cells hyperplasia is instrumental to achieve worm clearance (Zhao et al., Reference Zhao, Urban, Anthony, Sun, Stiltz, van Rooijen, Wynn, Gause and Shea-Donohue2008). Furthermore, the succinate receptor and TAS2Rs are expressed on tuft cells to detect the metabolites secreted by the parasites (Nadjsombati et al., Reference Nadjsombati, McGinty, Lyons-Cohen, Jaffe, DiPeso, Schneider, Miller, Pollack, Nagana Gowda, Fontana, Erle, Anderson, Locksley, Raftery and von Moltke2018; Luo et al., Reference Luo, Chen, Xue, Zhao, Lu, Li, Li, Du, Liu, Wang, Liu and Huang2019).
Conclusions
The nutrient and non-nutrient sensing in the GIT tract has evolved as a continuum function necessary to orchestrate ingestion, digestion, absorption, metabolism and neutralization of harmful substances. The mechanisms related to the sensing of carbohydrates, AAs, FAs, bitter compounds and microbial and parasite metabolites involve specialized cells in the enteric mucosa (i.e. EEC) that elicit hormonal responses (i.e. CCK, GLP-1, PYY, ghrelin, etc.) which, in turn, mediate changes in passage rate and appetite. Gene variations have been related to food choices in humans while in pigs to ecological adaptations particularly regarding the bitter taste receptor repertoire. In addition, genetic mutations have the potential to lead to the development of novel nutritional strategies in pigs, for example, regarding FFA sensing. In addition, our understanding on the impact of gut microbiome on the host’s gut-brain communications has started to unfold.
Acknowledgements
None.
Eugeni Roura 0000-0002-9073-9946
Declaration of interest
The authors declare no conflicts of interest.
Ethics statement
Not applicable.
Software and data repository resources
Not applicable.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1751731119001794





